Introduction
Oral hygiene is an important measure for the
prevention of oral diseases (dental caries and periodontitis) and a
prophylactic treatment for aspiration pneumonia, type 2 diabetes
mellitus (1) and cardiovascular
diseases (2). Although dental
caries is a worldwide health concern (3), it is preventable through appropriate
interventions, especially at the early stage of life (4). However, certain groups cannot easily
perform regular oral hygiene care (tooth brushing or flossing) by
themselves, including critically ill patients, elderly people, and
evacuees following disasters. In addition, many individuals lack
the knowledge and motivation for oral hygiene care (5). An interruption of oral hygiene care
results in the formation of microbial biofilms called dental plaque
on the tooth surface. Biofilm formation on tooth surfaces by
cariogenic bacterial communities is the initial step in the
development of dental caries.
Streptococcus mutans is a primary etiological
agent of dental caries (6). The
major virulence traits associated with S. mutans
cariogenicity are acid production from fermentable dietary
carbohydrates, acid tolerance and exopolysaccharide (EPS) formation
(7). Acid production promotes
demineralization of tooth enamel and acid tolerance confers
survival under the low pH environment within dental plaques. EPS
encourages the formation of acidogenic biofilms on the tooth
surface, which are bioaggregates resistant to mechanical tooth
brushing. Although fluoride-based preparations protect tooth
surfaces from acid attacks, their effects are limited unless they
are combined with dental plaque control (8). The use of bactericidal compounds for
the eradication of cariogenic bacteria is controversial because
these compounds disturb the healthy oral microflora and may lead to
the development of multidrug-resistant bacteria. The development of
specific regulatory measures for cariogenic bacteria is expected to
reduce dental plaque formation (9). A number of trials have been performed
to determine whether S. mutans growth and adhesion are
inhibited by various natural products, such as green tea catechins
(10), cranberry constituents
(11), citrus lemon oil (12) and mushroom extract (13). However, these studies did not
evaluate biofilm formation.
Sugar alcohols (polyols) are alternative candidates
used for the prevention of dental caries (14). Of these, xylitol is the most
evaluated polyol that reduces the risk of caries (14,15).
Xylitol disturbs the metabolic processes in bacteria and has a
bacteriostatic effect on S. mutans by forcing the uptake and
efflux of this non-cariogenic sugar alcohol (16). However, the effect of xylitol on
S. mutans is reduced in the presence of other fermentable
sugars (17).
Rare sugars are generally monosaccharides and their
derivatives are infrequently found in nature (18). Recently, rare sugars are the focus
of attention as health-supporting sugar substitutes because of
their equivalent sweetness but much lower caloric content than
sucrose (19). These sugars are
expected to reduce calorie intake, thereby decreasing the risk of
type 2 diabetes mellitus and obesity (20). The ketohexose D-tagatose has 92% of
the sweetness but 38% of the calories of sucrose. D-Tagatose is not
a preferential substrate for bacterial fermentation, and it has
been reported that D-tagatose is not easily catabolized by many
lactic acid bacteria or by pathogens including Escherichia
coli O157:H7, Salmonella enterica serovar Typhimurium,
Staphylococcus aureus, Bacillus cereus, and
Yersinia enterocolitica (21). D-Tagatose has been demonstrated to
suppress the growth of aerobic and lactic acid bacteria in chopped
and formed ham, thereby extending the shelf life of these products
by 7–10 days (21). These findings
indicate that D-tagatose-containing foods may suppress cariogenic
oral bacteria. In fact, D-tagatose has recently been reported to
inhibit the acid production, growth, and water-insoluble glucan
production of S. mutans GS-5 in the presence of sucrose
(22).
In the present study, the effect of D-tagatose on
S. mutans growth and biofilm formation was evaluated. The
results revealed that D-tagatose retards S. mutans growth
and reduces its biofilm formation by interfering with its sucrose
utilization.
Materials and methods
Bacterial strains and growth
conditions
S. mutans GS-5 (23) isolated from dental caries was used
in the present study. This strain belongs to serogroup C, which is
the most prevalent serogroup in the human oral cavity. A glycerol
stock of S. mutans GS-5 stored at −80°C was streaked onto
Brain-Heart Infusion (BHI; Difco; BD Biosciences, Franklin Lakes,
NJ, USA) agar plates and cultured anaerobically at 37°C. Anaerobic
cultivation was performed in an anaerobic chamber conditioned with
mixed gas (N2, 80%; H2, 10%; CO2,
10%) or in an Anaeropack system using anaerobic jars (Mitsubishi
Gas Chemical Company, Inc., Tokyo, Japan).
Monitoring the growth and pH of S.
mutans GS-5 cultures
A fresh S. mutans GS-5 colony from a BHI agar
plate was inoculated into liquid BHI and anaerobically incubated at
37°C for 24–48 h. BHI broth containing the test sugars [1 or 4%
(w/v) sucrose, D-glucose, D-fructose, xylitol, D-tagatose, or their
combination] were sterilized by filtration through a 0.22-µm pore
filter. D-Tagatose was supplied by the Rare Sugar Center of Kagawa
University (Kagawa, Japan). The 48 h S. mutans GS-5 culture
was then added at 4% (v/v, 0.4 ml) to 10 ml sugar-supplemented BHI
broth. Triplicate cultures were prepared for each test group, and
the tubes were incubated at 37°C in an anaerobic chamber. The
optical densities at 590 nm (OD590) and the pH values of
the cultures were measured every 3 h for 24 h. Vortexing was
performed prior to the OD590 measurement to produce a
homogenous suspension. Since S. mutans GS-5 forms hard and
granular EPS aggregates during growth in sucrose-supplemented BHI,
these cultures were incubated with a rotating motion in the
anaerobic chamber to prevent firm glucan adherence onto the glass
tubes. This modification did not hamper EPS production in S.
mutans GS-5. A portion of each culture (0.5 ml) was collected
to check the pH using a micro-volume pH meter (LAQUA-twin Compact
pH Meter; HORIBA Ltd., Kyoto, Japan). The initial pH and
OD590 immediately following incubation were also
measured.
Biofilm assay
S. mutans GS-5 pre-cultures were prepared by
inoculating a fresh colony into 2 ml liquid BHI and incubating it
at 37°C for 48 h. The test sugars (D-glucose, xylitol, or
D-tagatose) were dissolved in liquid BHI containing 1% sucrose at
0.5–4.0% (w/v). As controls, liquid BHI containing 1% sucrose alone
and liquid BHI without any added sugars were also prepared. The 48
h S. mutans GS-5 culture (40 µl) was mixed with 2 ml sugar
solutions and 200 µl each of the mixtures was dispensed into 96
well plates. Eight wells were used for each sample. The plate was
anaerobically incubated at 37°C for 72 h, then the culture media
were discarded, and the layers of biofilm adhered to the bottoms of
the wells were washed three times with 0.2 ml phosphate-buffered
saline [PBS, (pH 7.4)]. The remaining biofilm was stained with 0.1
ml 0.01% crystal violet for 20 min at room temperature and then
washed four times with 0.2 ml PBS. The remaining crystal violet was
eluted with 0.2 ml 33% acetic acid with gentle agitation for 20 min
at room temperature. The absorbance of the eluents was measured at
550 nm.
Scanning electron microscopy (SEM)
examination
The effects of D-tagatose on S. mutans GS-5
biofilm formation were examined by SEM. Sterile plastic discs (Cell
Desk, 13.5 mm in diameter; Sumitomo Bakelite Co., Ltd., Tokyo,
Japan) were set in 24 well microtitre plates. The 48 h S.
mutans GS-5 culture (30 µl) was mixed with 1 ml BHI containing
1% sucrose with or without xylitol (1 or 4%) or D-tagatose (1 or
4%) and 1 ml transferred into a well. Following 72 h anaerobic
incubation, the biofilms formed on the discs were fixed with 2.5%
glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4). Following
fixation, they were dehydrated in a graded ethanol series and dried
in a Hitachi PCP-2 critical point drying apparatus (Hitachi, Ltd.,
Tokyo, Japan). The discs were coated with platinum/palladium in a
Hitachi E-102 sputter coater (Hitachi, Ltd.) and examined with a
JEOL JCM-6000 scanning electron microscope (JEOL, Ltd., Tokyo,
Japan). Biofilm areas were measured as described by Somayaji et
al (24) using the
auto-selection tool based on colour in Photoshop CS6 (Adobe
Systems, Inc., San Jose, CA, USA), and the ratios to the total
observation field were calculated. At least five randomly selected
fields were examined.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mid-logarithmic phase
cultures (OD590=0.4–0.6) of S. mutans GS-5 using
the hot phenol method (25). RNA
was further purified using an RNeasy CleanUp Kit (Qiagen GmbH,
Hilden, Germany) and treated with TURBO DNA-free (Ambion;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) to remove
contaminating DNA. Total RNA was reverse transcribed using a
PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan) with
random hexamers at 37°C for 15 min. Reverse transcription was
terminated by heating the mixtures at 85°C for 5 sec. The cDNA
products were subsequently amplified using SYBR Premix Ex-Taq II
(Takara Bio, Inc.) under the following conditions in a StepOne plus
apparatus (Applied Biosystems; Thermo Fisher Scientific, Inc.):
Preheating at 95°C for 10 sec, followed by 40 cycles of 95°C for 5
sec and 60°C for 34 sec. All of the samples were run in triplicate.
Threshold cycle values were normalized to the levels of 16S
ribosomal RNA gene transcripts, and changes in gene expression were
calculated using the 2−∆∆Cq method (26). The oligonucleotide sequences of the
primers used for each target gene are listed in Table I.
 | Table I.Oligonucleotide primers used for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Oligonucleotide primers used for
reverse transcription-quantitative polymerase chain reaction.
| Target gene | Coding
function | Primer name | Sequence
(5′-3′) | Amplicon (bp) |
|---|
| SMUGS5_04450 | Glucosyltransferase
B | GtfB-F |
agcaatgcagccaatctacaaat | 96 |
|
|
| GtfB-R |
acgaactttgccgttattgtca |
|
| SMUGS5_09145 |
Fructosyltransferase | Ftf-F |
aaatatgaaggcggctacaacg | 101 |
|
|
| Ftf-R |
cttcaccagtcttagcatcctgaa |
|
| SMUGS5_08805 |
Fructose/Mannose-specific PTS subunit
IIC | EII-fru/man-F |
aagactctctttacggtgggttc | 111 |
|
|
| EII-fru/man-R |
agtgaaaccaacaaagaatggaa |
|
| SMUGS5_08440 |
Glucose/Mannose-specific PTS subunit
IIAB | EII-glu/man-F |
ctaaagctgaccgtatcattgttg | 111 |
|
|
| EII-glu/man-R |
attggtacaacattggccttgaca |
|
| SMUGS5_09220 |
Maltose/Glucose-specific PTS subunit
IIABC | EII-mal/glu-F |
acacaatatctttggcggtaaga | 105 |
|
|
| EII-mal/glu-R |
cgaaaccagagataataccaacg |
|
| SMUGS5_03875 | Fructose-specific
PTS subunit IIABC | EII-fru-F |
tgatacttttaaggctgggatca | 116 |
|
|
| EII-fru-R |
aaaagaaccgttgcttcctttac |
|
| SMUGS5_08275 | Sucrose-specific
PTS subunit IIABC | EII-sucF |
ggtgtctttggtgctctgtattc | 113 |
|
|
| EII-suc-R |
gtcaccatgaccagtaccatttt |
|
| SMUGS5_R09892 | 16S ribosomal
RNA | 16SF |
cctacgggaggcagcagtag | 101 |
|
|
| 16SR |
caacagagctttacgatccgaaa |
|
Preparation of cell-associated
glucosyltransferase (GTF)
Cell-associated GTFs of S. mutans GS-5 were
purified as previously described (27). S. mutans GS-5 was cultured
in 800 ml BHI broth. Following 48 h incubation, cells were
collected by centrifugation at 12,000 × g for 20 min at 4°C. The
bacterial pellet was washed with 10 mM potassium phosphate buffer
[KPB (pH 6.0)]. The cell-bound GTF was extracted with 5 ml, 8 M
urea. The cell suspension in urea was incubated at 25°C for 1 h
with periodic agitation. The supernatant was collected following
centrifugation at 12,000 × g for 20 min at 4°C and dialysed for 24
h at 4°C against 10 mM KPB (pH 6.0). Following dialysis, the enzyme
preparations were stored at −20°C until use.
Effects of D-tagatose on
cell-associated GTF
The effects of D-tagatose on S. mutans GS-5
GTF activity were assessed in vitro by quantifying the
water-insoluble glucan produced in the mixture of sucrose and the
cell-associated crude enzyme prepared as described. A total of 2 ml
0.1 M KPB (pH 6.0) containing 0.1 M sucrose and 0.01% sodium azide
with or without 4% D-tagatose was mixed with 1 ml enzyme
preparation and incubated anaerobically at 37°C for 48 h. The
water-insoluble glucan produced in the mixture was quantified by
the phenol-sulfuric acid method (28). Following vortexing for 1 min, the
mixtures were centrifuged (7,500 × g for 10 min at 4°C), then the
pellets were washed to remove the water-soluble glucan with 1 ml
PBS and subjected to a further 5 washes with distilled water
(dH2O). Subsequently, the pellets were dissolved in 1
ml, 1 N NaOH and centrifuged again. The supernatant (0.5 ml) was
mixed with an equal volume of 5% phenol followed by 2.5 ml sulfuric
acid. After standing at room temperature for 20 min, the
OD490 of the mixture was measured with a UV-VIS
spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The
quantity of water-insoluble glucan was calculated from the standard
curve of known concentrations of D-mannose.
Statistical analysis
Statistical analysis of the data was performed using
StatFlex software (version 6.0; Artech Co., Ltd., Tokyo). Analysis
of variance was used to compare the means of all groups, and
Tukey's post hoc test was used to compare the means of each of the
two groups. Dunnett's test was applied to compare the test groups
with the control group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of D-tagatose on growth of S.
mutans GS-5
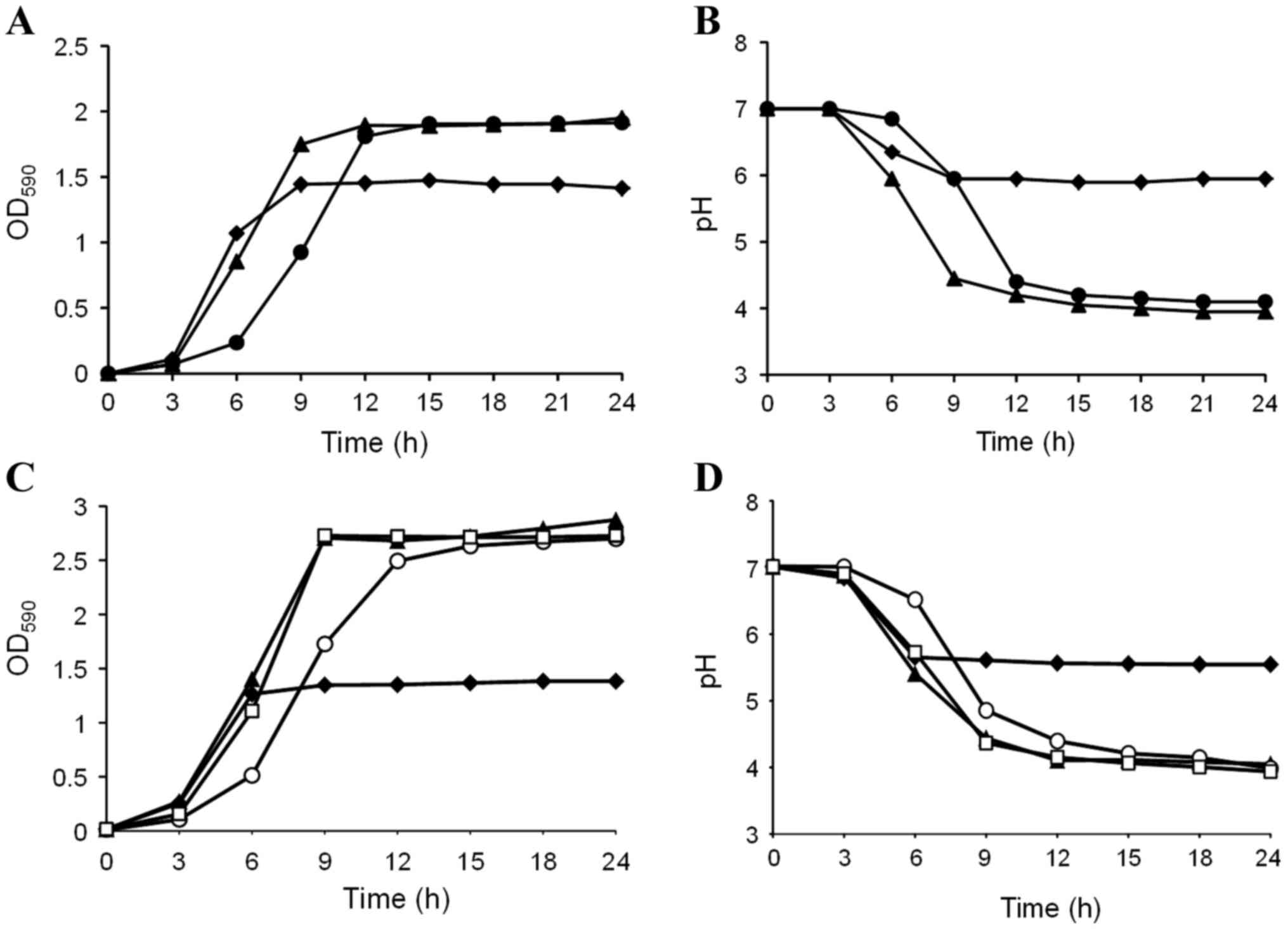
The effects of D-tagatose on S. mutans GS-5
growth were examined in BHI containing 1% sucrose. Sucrose enhanced
S. mutans GS-5 growth compared with BHI alone (Fig. 1A), and the pH of the culture with
sucrose decreased to less than 5.0 following 9 h incubation
(Fig. 1B). Interestingly,
D-tagatose delayed the transition of S. mutans growth to the
logarithmic phase despite the presence of sucrose (Fig. 1A). Correspondingly, the pH decline
of the culture was also delayed by D-tagatose compared with that in
sucrose alone (Fig. 1B). This
growth retardation became more evident, and entry into the
stationary phase was delayed by 6 h when the D-tagatose
concentration was increased to 4%; however, significant differences
were not observed in the final OD590 following 24 h of
cultivation (data not shown). As demonstrated in Fig. 1C and D, the S. mutans GS-5
growth delay and pH decline induced by D-tagatose was abolished
when 1% D-fructose, but not D-glucose, was added to BHI containing
1% sucrose. These results indicate that the rare sugar D-tagatose
inhibits sucrose catabolism in S. mutans GS-5.
Effects of D-tagatose on in vitro S.
mutans GS-5 biofilm formation
Since sucrose metabolism is important for production
of water-insoluble glucan, which is required for biofilm formation,
D-tagatose was predicted to inhibit S. mutans biofilm
formation. The effects of D-glucose, xylitol, and D-tagatose on
in vitro S. mutans GS-5 biofilm formation were, therefore,
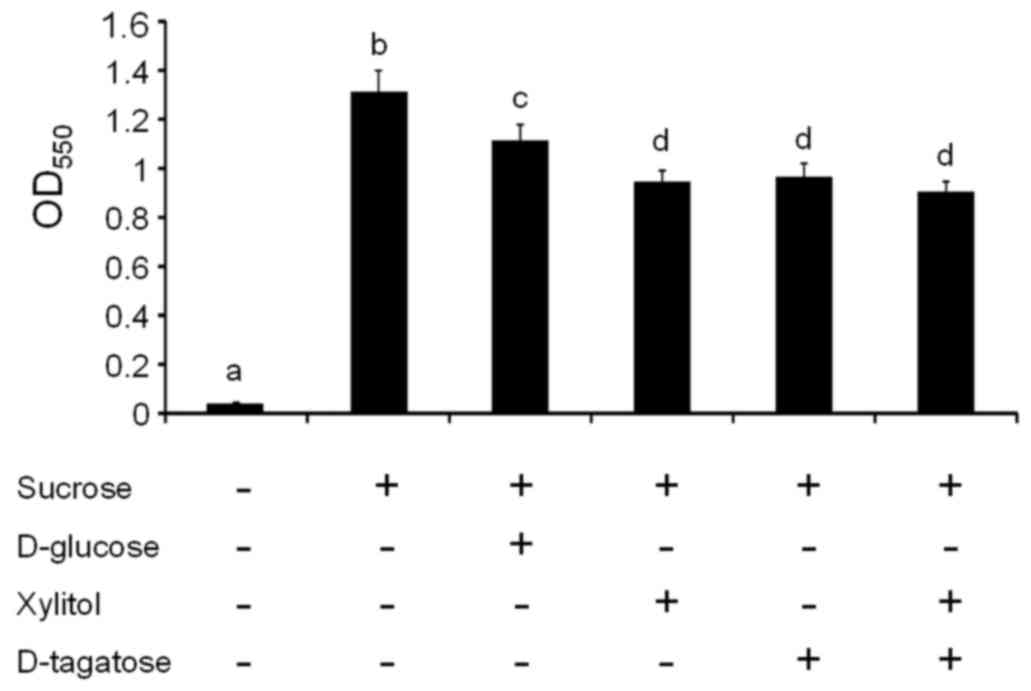
evaluated. The addition of 1% sucrose to growth media significantly
increased biofilm formation by S. mutans GS-5 compared with
unsupplemented media (P<0.05, a vs. b; Fig. 2), which is consistent with many
previous reports (29).
Supplementation with D-glucose and sucrose slightly reduced S.
mutans biofilm formation compared with supplementation with
sucrose alone (P<0.05, b vs. c; Fig. 2). Supplementation with xylitol,
D-tagatose, and their combination significantly reduced S.
mutans GS-5 biofilm formation compared with supplementation
with sucrose alone (P<0.05, b vs. d; Fig. 2) or sucrose and glucose (P<0.05,
c vs. d; Fig. 2).
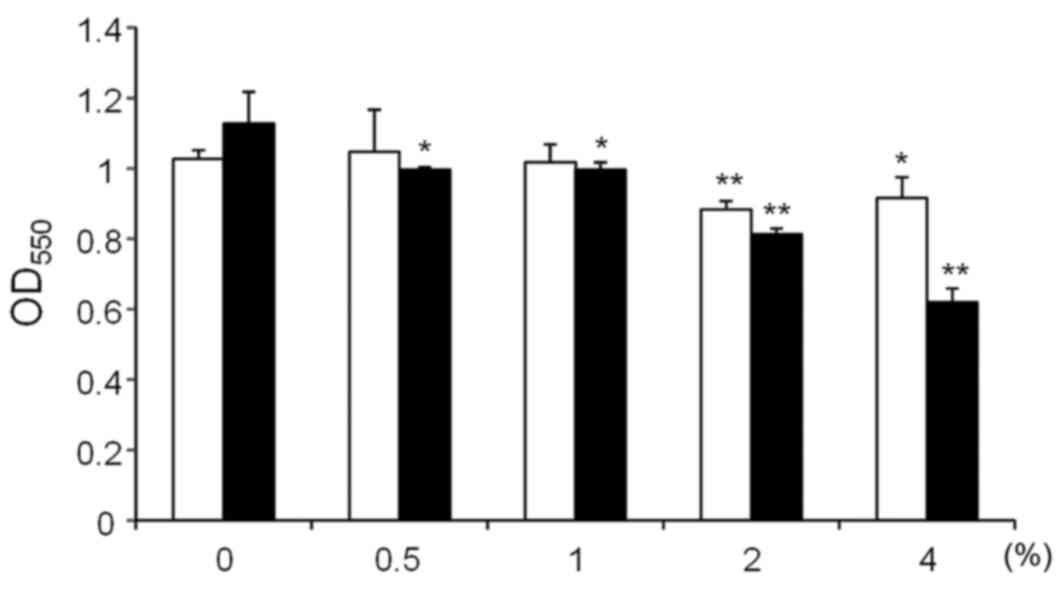
The effects on S. mutans GS-5 biofilm
formation of xylitol and D-tagatose at varying concentrations (0.5,
1, 2 and 4%) were then examined (Fig.
3). Xylitol inhibited S. mutans biofilm formation at 2
and 4%, although an additional effect was not observed for
concentrations >2% (white bars; Fig. 3). By contrast, D-tagatose showed
clear dose-dependent inhibition of S. mutans GS-5 biofilm
formation (black bars; Fig. 3). To
determine whether the effects on biofilm formation were caused by
high osmolality, S. mutans GS-5 biofilm formation was
compared in the presence of 1% and 5% sucrose. The biofilm mass in
the culture with 5% sucrose was significantly smaller compared with
the 1% sucrose (OD550 1.27±0.05 vs. OD550
1.09±0.07, respectively; P<0.01; data not shown). However,
addition of 4% D-tagatose to the culture with 1% sucrose decreased
the biofilm mass to the nearly half of that in the culture with 5%
sucrose (OD550 0.57±0.06 vs. OD550 1.09±0.07,
P<0.01; data not shown). These results indicate that the effects
of high osmolality were limited under the conditions used in this
study.
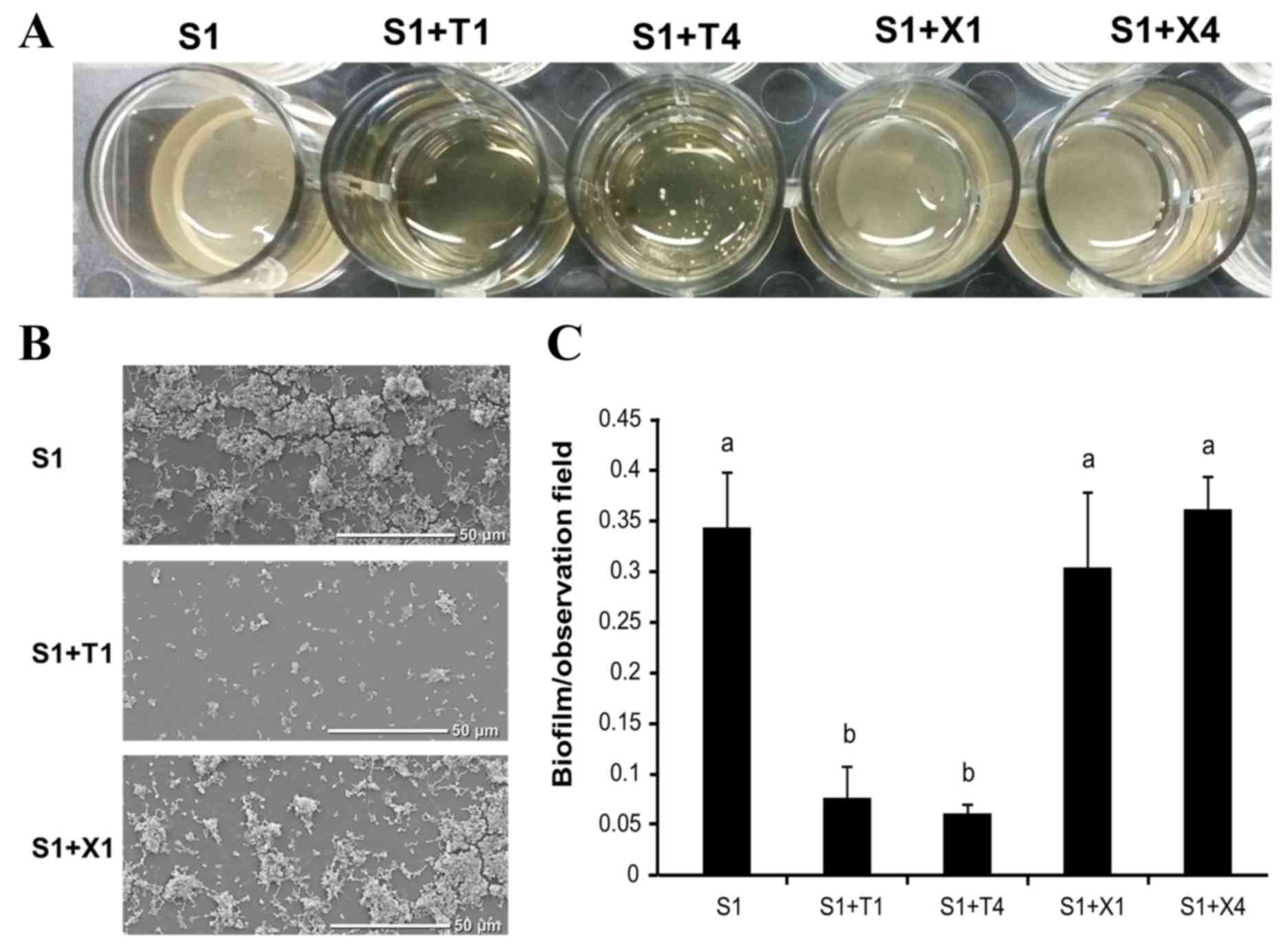
Scanning electron microscopy
examination of S. mutans GS-5 biofilms
S. mutans GS-5 was cultured in 1 ml of BHI
containing 1% sucrose with or without 1 or 4% each of xylitol or
D-tagatose in 24-well plates with plastic disc inserts; the plates
were incubated anaerobically at 37°C for 72 h, and the biofilms
formed on the plastic discs were compared (Fig. 4A). S. mutans GS-5 grew
equally in all of the media tested. However less biofilm formed on
the discs in the cultures containing D-tagatose than on those in
other media (1% sucrose alone or 1% sucrose plus 1 or 4% xylitol;
Fig. 4A). Notably, multiple S.
mutans GS-5 cell aggregates were observed in the cultures
containing D-tagatose, particularly at the higher concentration,
whereas homogeneous biofilms formed on the discs in the other 1%
sucrose-containing cultures tested (Fig. 4A).
SEM examination of the discs also revealed that
there was less biofilm on the discs in the culture with D-tagatose
(Fig. 4B). Quantification of the
S. mutans GS-5 biofilms on the discs showed a significant
reduction in the presence of D-tagatose (P<0.05; Fig. 4C).
Effects of D-tagatose on the
expression of sucrose metabolism genes in S. mutans GS-5
Sucrose increases expression of the gene that
encodes insoluble-glucan-forming glucosyltransferase B
(gtfB), which is a major virulence factor of S.
mutans. The present study confirmed that 1% sucrose
significantly increased the expression of gtfB (P<0.01)
but significantly decreased the expression of the
fructosyltransferase gene (ftf) (P<0.01) compared with
bacteria cultured in BHI alone (Fig.
5). By contrast, the induction of gtfB expression by
sucrose was strongly inhibited by D-tagatose (P<0.01; Fig. 5). The expression of
exo-β-fructosidase (fruA), which releases free D-fructose
from FTF-producing water-soluble fructan, was also reduced in the
presence of D-tagatose and sucrose compared with sucrose only
(P<0.01; Fig. 5). Among the
phosphotransferase system (PTS) genes involved in metabolizing
sucrose or sucrose-derived monosaccharides (D-glucose and
D-fructose), the expression levels of ptsfru,
ptsfru/man and ptsglu/man were
significantly increased by 1% sucrose compared with bacteria
cultured in BHI alone (P<0.01; Fig.
5), although this increase was inhibited by D-tagatose
(P<0.01, P<0.01 and P<0.05, respectively, S1 vs. S1 + T1;
Fig. 5). GtfB releases D-fructose
during glucan synthesis, and these insoluble glucans are degraded
by dextranase and utilized as a glucose source. The fruA
gene product releases free D-fructose from the fructan synthesized
by FTF. The repression of these genes, in addition to the fructose
or glucose pts genes, indicates that D-tagatose limits the
ability of S. mutans GS-5 to access sucrose-derived
monosaccharides, particularly D-fructose. Fig. 6 demonstrates that D-fructose is a
powerful inducer of gtfB expression, and sucrose strongly
increases the expression of fructose PTS, thus indicating that
D-fructose is a key sugar required for S. mutans growth and
biofilm formation.
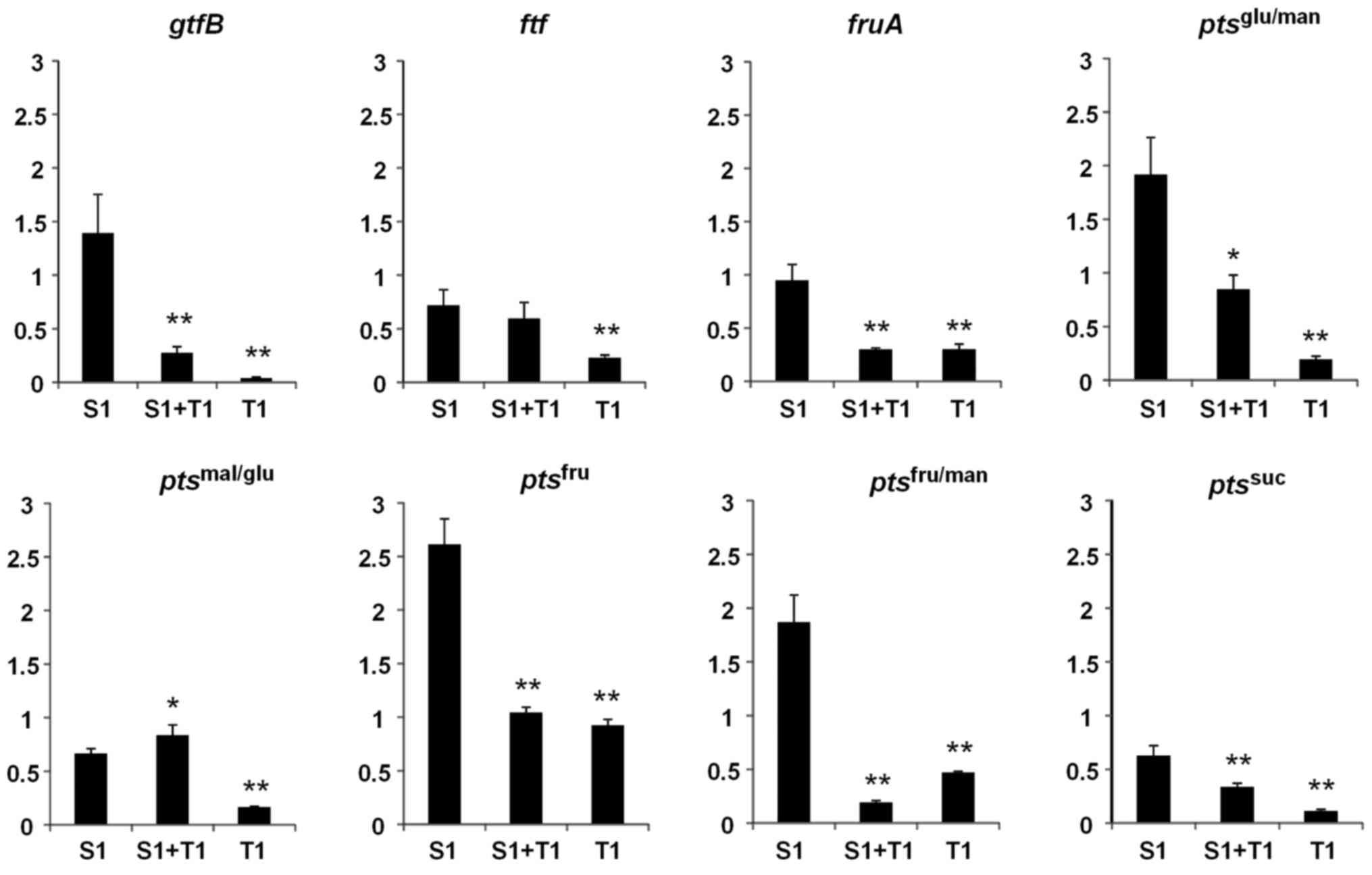 | Figure 5.Effects of D-tagatose on the
expression of sucrose metabolism genes in Streptococcus
mutans GS-5. The relative expression levels of the genes
encoding gtfB, ftf, fruA and the EII
components of the pts specific for suc, fru, fru/man,
mal/glu and glu/man were evaluated by reverse
transcription-quantitative polymerase chain reaction. Gene
expression levels were normalized to the 16S rDNA expression. The
fold change in expression levels relative to that in Brain-Heart
Infusion media alone. Data are expressed as the mean ± standard
deviation of three repeats. *P<0.05 and **P<0.01 vs. S1. S1,
1% sucrose alone; S1+T1, 1% sucrose plus 1% D-tagatose; T1, 1%
D-tagatose alone; gtfB, glucosyltransferase; ftf,
fructosyltransferase; fruA, exo-β-fructosidase; pts,
phosphotransferase system; suc, sucrose, fru, D-fructose; fru/man,
D-fructose/D-mannose; mal/glu, D-maltose/D-glucose; glu/man,
D-glucose/D-mannose. |
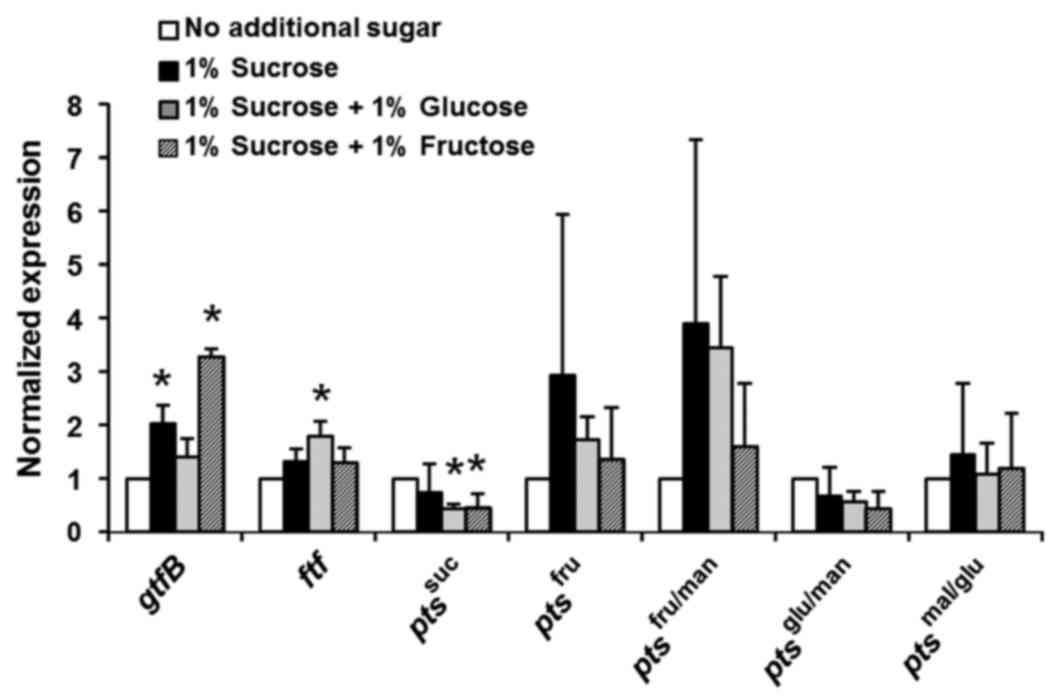 | Figure 6.Effects of sucrose and D-fructose on
Streptococcus mutans GS-5 gtfB and fructose-specific
pts genes. The relative expression levels of the genes
encoding gtfB, ftf and the EII components of the
pts specific for suc, fru, fru/man, mal/glu and glu/man were
evaluated by reverse transcription-quantitative polymerase chain
reaction. Gene expression levels were normalized to the 16S rDNA
expression. The fold change in expression levels relative to that
in Brain-Heart Infusion media alone is shown. The data are
expressed as the mean ± standard deviation of two repeats.
*P<0.05 vs. no additional sugar group. gtfB,
glucosyltransferase; ftf, fructosyltransferase; pts,
phosphotransferase system; suc, sucrose, fru, D-fructose; fru/man,
D-fructose/D-mannose; mal/glu, D-maltose/D-glucose; glu/man,
D-glucose/D-mannose. |
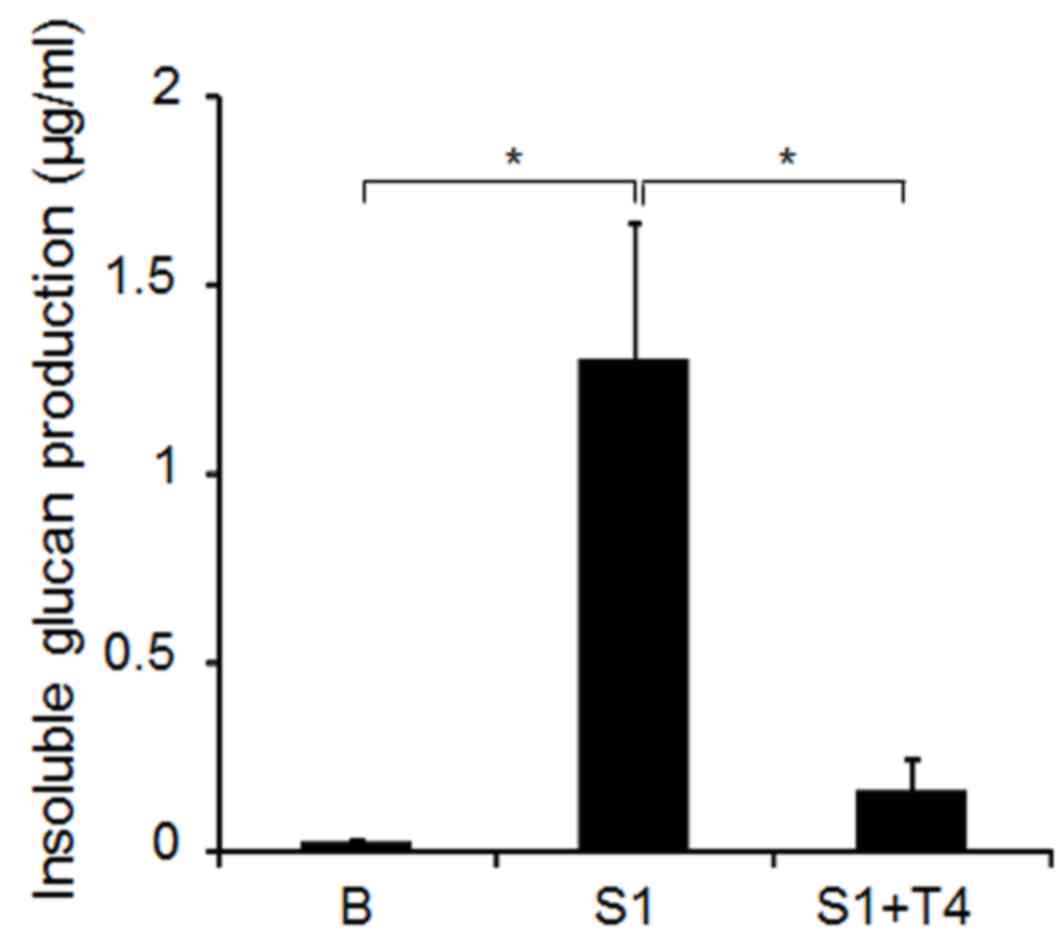
Effects of D-tagatose on S. mutans
GS-5 GTF activity
S. mutans GTFs B and C, which produce
water-insoluble glucan, are known to be cell-associated. To examine
whether D-tagatose directly inhibits the activity of GTF in S.
mutans GS-5, the cell-associated proteins were extracted with
urea from S. mutans GS-5 cells cultured in BHI. The
water-insoluble glucan production was compared among the renatured
enzymes in media with 0.1 M sucrose in the presence or absence of
4% D-tagatose. As demonstrated in Fig.
7, the addition of 4.0% D-tagatose significantly decreased the
water-insoluble glucan production compared with sucrose alone,
indicating that the sugar directly inhibits the activity of S.
mutans GS-5 cell-associated GTF.
Discussion
The use of non-cariogenic sweeteners represents a
method of preventing dental caries, and sugar alcohols such as
xylitol are widely used in chewing gum. D-Tagatose is also
recognized as a tooth-friendly sweetener, and it is not fermented
by cariogenic dental plaque bacteria. Consistent with previous
studies (22), D-tagatose was
demonstrated to be a non-fermentable sugar for S. mutans
GS-5, and the results of gas chromatography-mass spectrometry
analysis revealed that 81.6% of the D-tagatose added to the culture
media was retained, even after 48 h of S. mutans GS-5
culture (data not shown). Although the addition of 1% D-tagatose to
the culture medium retarded the growth of S. mutans GS-5,
the final growth yield did not change compared with the cultures
without the sugar. Nevertheless, D-tagatose inhibited S.
mutans GS-5 biofilm formation, indicating that the effect is
caused by a mechanism other than growth inhibition.
As demonstrated in Fig.
7, D-tagatose inhibits cell-associated GTF activities, which
results in the reduced release of D-fructose from sucrose.
D-Fructose (and sucrose) appears to be a powerful inducer of
gtfB expression (Fig. 6).
The addition of 1% sucrose to the media induced the expression of
D-fructose-specific PTS as well as gtfB, indicating that the
glucan production and energy metabolism pathways that utilize
D-fructose are tightly coordinated in S. mutans. This
finding is consistent with the report by Shemesh et al
(30), who demonstrated that
D-fructose induces higher levels of gtfB expression than
D-glucose in the early exponential phase. Therefore, the
suppression of gtfB expression by D-tagatose may be
partially caused by a decrease in the D-fructose supply.
Furthermore, genes encoding the EII component for the
D-fructose-specific PTS genes (ptsfru and
ptsfru/man) were also downregulated in the
presence of D-tagatose (Fig. 5).
The growth retardation of S. mutans GS-5 by D-tagatose may
also be due to the limited D-fructose supply resulting from GTF
inhibition since the D-tagatose-induced growth retardation was
reversed with D-fructose supplementation (Fig. 1C and D). Alterations in the
availability of this monosaccharide are, therefore, hypothesized to
be responsible for the prolongation of the lag-phase of S.
mutans GS-5 growth by D-tagatose.
By contrast, ftf expression levels were not
changed by D-tagatose in the presence of 1% sucrose. FTF produces
water-soluble inulin-type fructan in S. mutans. Since
fructan is digested by FruA into D-fructose, this fructose polymer
is considered to serve as energy storage for S. mutans. The
downregulation of fruA by D-tagatose is hypothesized to
limit the D-fructose supply for S. mutans. This alteration
in monosaccharide availability might affect fruA expression,
which is known to be sensitive to the control of carbon catabolite
repression via the central regulatory protein CcpA (31).
As mentioned above, D-tagatose appears to inhibit
S. mutans GS-5 GTFs, and the inhibition of cell-associated
GTFs B or C, which produce water-insoluble glucan from sucrose, is
considered to be a primary mechanism underlying the biofilm
inhibition as well as the growth retardation of S. mutans
GS-5 by D-tagatose. Since D-tagatose is a D-fructose epimer at the
C-4 position, its structural similarity to D-fructose might
interfere with the binding or catalysis of sucrose by the GTFs. In
addition, the S. mutans GS-5 biofilm that formed in the
presence of D-tagatose was granular, whereas the biofilm formed in
the culture with sucrose alone or sucrose plus xylitol was uniform
(Fig. 4A). This difference may be
related to the glucan/fructan imbalance caused by D-tagatose.
Bautista et al (21) reported that many human pathogens
are unable to utilize D-tagatose and demonstrated that the sugar is
metabolized by a limited member of lactobacilli. Probiotic
lactobacilli have been reported to suppress the growth of
cariogenic bacteria and prevent dental caries (32). Based on the finding in the present
study, that S. mutans GS-5 did not preferentially ferment
D-tagatose, this sugar is anticipated to prevent S. mutans
colonization on tooth surfaces by promoting the ability of
probiotic oral lactobacilli to resist colonization.
Xylitol is widely used for the prevention of dental
caries, although its effects in clinical trials remain
controversial (33). S.
mutans transports xylitol via a fructose-specific PTS and
xylitol-resistant S. mutans strains lacking this PTS
activity have emerged (34). In
addition, the presence of fermentable sugars, such as sucrose,
attenuates the effects of xylitol. Therefore, alternative
prophylactic treatments for dental caries are required. Xylitol is
a non-fermentable sugar for S. mutans and exhibits a toxic
effect by causing the expenditure of energy for the uptake and
export of this non-cariogenic sugar alcohol (35). The mechanism by which xylitol
suppresses S. mutans appears to be different from that of
D-tagatose as described here; therefore, a synergistic effect might
be expected for their combination. However, a synergistic effect
was not evident in the inhibition of S. mutans GS-5 biofilm
formation (Fig. 2), which might
have been related to interference with xylitol uptake since
D-tagatose downregulates the D-fructose-specific PTS genes,
ptsfru and ptsfru/man (Fig. 5).
In conclusion, D-tagatose appears to inhibit S.
mutans GS-5 growth and biofilm formation by interfering with
GTF activity. This effect may be useful in the prevention of dental
caries. Based on the findings obtained from the present study,
foods or preparations containing D-tagatose could be useful tools
for improving oral hygiene. D-Tagatose may be able to suppress the
intermittent growth of S. mutans between oral care
activities. In addition, S. mutans produces a granular
biofilm in the presence of D-tagatose, which might facilitate the
removal of the biofilm by mechanical brushing compared with
homogeneous biofilms. Ongoing clinical examinations to evaluate the
effectiveness of chewing gum containing D-tagatose as a
prophylactic for dental caries in a clinical trial are being
performed by our research consortium.
Acknowledgements
The present study was supported by a Grant-in-Aid
from the Japan Society for the Promotion of Science KAKEN (grant
no. 25463248) and the Adaptable and Seamless Technology Transfer
Program through Target-driven R&D, Japan Science and Technology
Agency (Study of the effects on bacteria in the oral cavity of
D-tagatose: Grant no. AS242Z03885Q).
References
|
1
|
Gurav A and Jadhav V: Periodontitis and
risk of diabetes mellitus. J Diabetes. 3:21–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holmlund A, Holm G and Lind L: Severity of
periodontal disease and number of remaining teeth are related to
the prevalence of myocardial infarction and hypertension in a study
based on 4,254 subjects. J Periodontol. 77:1173–1178. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selwitz RH, Ismail AI and Pitts NB: Dental
caries. Lancet. 369:51–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jackson RJ, Newman HN, Smart GJ, Stokes E,
Hogan JI, Brown C and Seres J: The effects of a supervised
toothbrushing programme on the caries increment of primary school
children, initially aged 5–6 years. Caries Res. 39:108–115. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cummins D: Dental caries: A disease which
remains a public health concern in the 21st century-the exploration
of a breakthrough technology for caries prevention. J Clin Dent 24
Spec no A. A1–14. 2013.
|
|
6
|
Loesche WJ: Role of Streptococcus mutans
in human dental decay. Microbiol Rev. 50:353–380. 1986.PubMed/NCBI
|
|
7
|
Banas JA: Virulence properties of
Streptococcus mutans. Front Biosci. 9:1267–1277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fejerskov O: Changing paradigms in
concepts on dental caries: Consequences for oral health care.
Caries Res. 38:182–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marsh PD, Head DA and Devine DA:
Ecological approaches to oral biofilms: control without killing.
Caries Res. 49 Suppl 1:S46–S54. 2015. View Article : Google Scholar
|
|
10
|
Hirasawa M, Takada K and Otake S:
Inhibition of acid production in dental plaque bacteria by green
tea catechins. Caries Res. 40:265–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koo H, Duarte S, Murata RM, Scott-Anne K,
Gregoire S, Watson GE, Singh AP and Vorsa N: Influence of cranberry
proanthocyanidins on formation of biofilms by Streptococcus mutans
on saliva-coated apatitic surface and on dental caries development
in vivo. Caries Res. 44:116–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Zhang X, Wang Y, Chen F, Yu Z, Wang
L, Chen S and Guo M: Effect of citrus lemon oil on growth and
adherence of Streptococcus mutans. World J Microbiol Biotechnol.
29:1161–1167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yano A, Kikuchi S, Yamashita Y, Sakamoto
Y, Nakagawa Y and Yoshida Y: The inhibitory effects of mushroom
extracts on sucrose-dependent oral biofilm formation. Appl
Microbiol Biotechnol. 86:615–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mäkinen KK: Sugar alcohols, caries
incidence, and remineralization of caries lesions: A literature
review. Int J Dent. 2010:9810722010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Milgrom P, Söderling EM, Nelson S, Chi DL
and Nakai Y: Clinical evidence for polyol efficacy. Adv Dent Res.
24:112–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trahan L: Xylitol: A review of its action
on mutans streptococci and dental plaque-its clinical significance.
Int Dent J. 45 1 Suppl 1:S77–S92. 1995.
|
|
17
|
Gauthier L, Vadeboncoeur C and Mayrand D:
Loss of sensitivity to xylitol by Streptococcus mutans LG-1. Caries
Res. 18:289–295. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Izumori K: Bioproduction strategies for
rare hexose sugars. Naturwissenschaften. 89:120–124. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chattopadhyay S, Raychaudhuri U and
Chakraborty R: Artificial sweeteners-a review. J Food Sci Technol.
51:611–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hossain A, Yamaguchi F, Matsuo T,
Tsukamoto I, Toyoda Y, Ogawa M, Nagata Y and Tokuda M: Rare sugar
D-allulose: Potential role and therapeutic monitoring in
maintaining obesity and type 2 diabetes mellitus. Pharmacol Ther.
155:49–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bautista DA, Pegg RB and Shand PJ: Effect
of L-glucose and D-tagatose on bacterial growth in media and a
cooked cured ham product. J Food Prot. 63:71–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sawada D, Ogawa T, Miyake M, Hasui Y,
Yamaguchi F, Izumori K and Tokuda M: Potent inhibitory effects of
D-tagatose on the acid production and water-insoluble glucan
synthesis of Streptococcus mutans GS5 in the presence of sucrose.
Acta Med Okayama. 69:105–111. 2015.PubMed/NCBI
|
|
23
|
Gibbons RJ, Berman KS, Knoettner P and
Kapsimalis B: Dental caries and alveolar bone loss in gnotobiotic
rats infected with capsule forming streptococci of human origin.
Arch Oral Biol. 11:549–560. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Somayaji K, Acharya SR, Bairy I, Prakash
PY, Rao MS and Ballal NV: In vitro scanning electron microscopic
study on the effect of doxycycline and vancomycin on enterococcal
induced biofilm. Iran Endod J. 5:53–58. 2010.PubMed/NCBI
|
|
25
|
Nuyts S, Van Mellaert L, Lambin P and Anné
J: Efficient isolation of total RNA from Clostridium without DNA
contamination. J Microbiol Methods. 44:235–238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiater A, Choma A and Szczodrak J:
Insoluble glucans synthesized by cariogenic streptococci: A
structural study. J Basic Microbiol. 39:265–273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dubois M, Gilles K, Hamilton JK, Rebers PA
and Smith F: A colorimetric method for the determination of sugars.
Nature. 168:1671951. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Decker EM, Klein C, Schwindt D and von
Ohle C: Metabolic activity of Streptococcus mutans biofilms and
gene expression during exposure to xylitol and sucrose. Int J Oral
Sci. 6:195–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shemesh M, Tam A, Feldman M and Steinberg
D: Differential expression profiles of Streptococcus mutans ftf,
gtf and vicR genes in the presence of dietary carbohydrates at
early and late exponential growth phases. Carbohydr Res.
341:2090–2097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng L, Wen ZT and Burne RA: A novel
signal transduction system and feedback loop regulate fructan
hydrolase gene expression in Streptococcus mutans. Mol Microbiol.
62:187–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bizzini B, Pizzo G, Scapagnini G, Nuzzo D
and Vasto S: Probiotics and oral health. Curr Pharm Des.
18:5522–5531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riley P, Moore D, Ahmed F, Sharif MO and
Worthington HV: Xylitol-containing products for preventing dental
caries in children and adults. Cochrane Database Syst Rev.
3:CD0107432015.
|
|
34
|
Trahan L, Bourgeau G and Breton R:
Emergence of multiple xylitol-resistant (fructose PTS-) mutants
from human isolates of mutans streptococci during growth on dietary
sugars in the presence of xylitol. J Dent Res. 75:1892–1900. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nayak PA, Nayak UA and Khandelwal V: The
effect of xylitol on dental caries and oral flora. Clin Cosmet
Investig Dent. 6:89–94. 2014. View Article : Google Scholar : PubMed/NCBI
|