Introduction
In the late 20th century, there was significant
progress in stem cell research when mesenchymal stem cells (MSCs)
were first identified and named (1,2).
MSCs are fibroblast-like pluripotent adult cells, which possess the
following characteristics making them ideal candidates for
cell-based therapy: i) Readily expanded from adult and fetal
tissues; ii) multilineage capabilities (plasticity); iii) immune
privileged; iv) has immunomodulatory abilities; v) releases trophic
factors; and vi) exhibits homing to damaged sites (3). MSCs have been successfully isolated
from various tissues, including bone marrow (BM), umbilical cord
(UC), adipose tissue, placenta, skin, muscle and tonsil tissues
(3–6).
Adult tissue-derived MSCs, for example BM-MSCs, can
have limitations in cell number and increase the risk of
age-related changes, whereas younger tissue-derived MSCs, for
example UC-MSCs, are increasing in popularity for clinical use due
to their faster rate of self-renewal and higher differentiation
potential. In addition, UC-MSCs share a similar gene expression
profile to embryonic stem cells, but without the ethical issues and
risk of tumorigenesis (7–10). The isolation of UC-MSCs has been
performed in humans (3), horses
(11), dogs (12), pigs (13), rats (14) and mice (9), of which mouse UC-derived MSCs
(mUC-MSCs) have been reported only once.
An increasing number of studies have verified the
therapeutic effects of UC-MSCs in clinical trials (15,16)
and preclinical studies, amongst which animal models are crucial in
translating the in vitro properties of MSCs into therapeutic
applications and examining mechanisms of efficacy. There is
encouraging evidence from various experimental models indicating
that UC-MSCs function across species barriers, including in renal
ischemia-reperfusion injury (17),
diabetes (18), Huntington's
disease (12) and mammary
carcinoma (14). However, studies
have reported conflicting results in cross-species models, with
xenogeneic MSCs showing detrimental effects (19). MSCs from different tissues may
exhibit different intrinsic properties. Mice and humans are the
most commonly used recipient and donnor species, however, certain
key effector molecules are divergent between murine MSCs (mMSCs)
and human MSCs (hMSCs), including nitric oxide and indoleamine
2,3-dioxygenase (20). Therefore,
allogeneic MSC therapy may be more suitable for preclinical
studies.
Toll-like receptors (TLRs) are considered to be an
important family of conserved receptors, which mediate immune
responses upon activation by pathogen components or endogenous
molecules. Accumulating evidence indicates that MSCs from different
species (mouse and human) express functional TLRs, and that their
activation affects MSC functions, including proliferation,
migration, differentiation and immunomodulation (21,22).
In addition, Waterman et al (23,24)
described MSC polarization into two phenotypes by TLR signaling.
Specifically, TLR4-primed hMSCs (MSC1) exhibited a pro-inflammatory
profile and attenuated tumor growth, whereas TLR3-primed MSCs
(MSC2) expressed immunosuppressive mediators and promoted tumor
growth. Few studies have investigated mMSCs and TLRs together, and
whether TLR3 or TLR4 with specific ligands control mMSC functions
remains to be elucidated.
To date, the most commonly used mMSCs in mouse
models are mBM-MSCs. The establishment of mUC-MSCs, novel members
of the mMSC bank and promising candidates for allogeneic cell
therapy, may be an important platform for elucidating the cellular
and molecular mechanisms of diseases (9). In the present study, mUC-MSCs derived
from Kunming mice were successfully isolated and expanded using a
novel method and culture system. The isolated cells were
characterized in comparison with mBM-MSCs, and the effects of TLR3
on the expression of stemness-related proteins and cytokines in the
mUC-MSCs were investigated. The results may provide novel clues for
selecting ideal mMSCs in various mouse models and offer novel
insights into the role of TLR3 as a regulator of mUC-MSCs.
Materials and methods
Mice
Breeding pairs of Kunming mice were purchased from
the Laboratory Animal Research Center of Jiangsu University
(Jiangsu, China). The total number of mice used in the present
study was 12 and the ratio of males to females was 3:1 per cage
(age, 8 weeks old; weight, 21 g; 9 males; 3 females). All animals
were housed at 20–26°C with a relative humidity of 40–70%;
the feeding box was 1–2°C higher than the environment, with 5–10%
humidity. A 12-h light/dark cycle was also used and they had free
access to food and water. Experimental procedures involving animals
were approved by the Institutional Animal Care Committee of Jiangsu
University and performed in strict accordance with the guidelines
and regulations.
mUC-MSC isolation and expansion
Fresh mouse UCs were aseptically collected from
Kunming mice at a gestational age of 15–19 days. The collected UCs
were rinsed with phosphate-buffered saline (PBS) containing
penicillin and streptomycin. The washed tissues were mechanically
cut into small sections (0.5–1.0 mm3) and suspended in
Dulbecco's modified Eagle's medium: F-12 nutrient mixture
(F12/DMEM; Hyclone Laboratories; GE Healthcare Life Sciences,
Logan, UT, USA) containing 15% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U penicillin
and streptomycin. The tissue sections, together with culture
medium, were seeded into 3.5 mm cell culture dishes (Corning
Incorporated, Corning, NY, USA) and incubated at 37°C in humidified
air with 5% CO2. The medium was replaced every 3 days,
and the non-adherent tissues and cells were removed. When
well-developed colonies of heterogeneous primary cells had formed
at 9–12 days, the attached cells were trypsinized using 0.25%
trypsin-EDTA (Invitrogen; Thermo Fisher Scientific, Inc.) and
passaged. The first passage was performed at a ratio of 4:1-2:1,
followed by successive passages at a ratio of 1:1-1:2 for early
passages (passages 3–5), with medium being replaced every 2 days.
The homogenously fibroblast-like cell populations appeared
following five passages. mUC-MSCs at passage numbers 7–12 were used
for the follow-up experiments.
Isolation and expansion of
mBM-MSCs
The mBM-MSCs were isolated and cultured using
protocols recommended previously (4). Briefly, 4–6 week old male mice
(weight, ~30 g) were sacrificed and fresh bone marrow cells were
harvested by flushing the femurs with PBS, and cultured in low
glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 15%
FBS at 37°C in humid air with 5% CO2. Following the
initial 3 days, culture medium replacement was performed once every
3 days. On reaching confluence, the cells in colonies were
trypsinized and subcultured. MSCs at passages 15–18 were used for
biological characterization.
Flow cytometric analysis
For surface marker analysis, the MSCs were detached
and stained with anti-mouse monoclonal antibodies for 30 min at
4°C, including PE-conjugated CD11b (cat no. 12-0112-82;
eBioscience, San Diego, CA, USA), CD44 (cat no. 553134), CD45 (cat
no. 553081); FITC-conjugated CD29 (cat no. 561796), and CD34 (cat
no. 560238; BD Pharmingen, San Diego, CA, USA). The labeled cells
were analyzed using flow cytometry (FACSCalibur; BD Biosciences,
San Jose, CA, USA). PE-IgG and FITC-IgG were used as controls; for
all antibodies, 1 µl antibody was added to 2.5×105 cell
suspension in 200 µl PBS. The analysis of the DNA content of the
MSCs was performed as described above. The cell pellets were
incubated in propidium iodide on ice for 30 min. The percentages of
cells in the G0/G1, G2/M and S phases were analyzed using flow
cytometry using BD CellQuest™ Pro software (BD Biosciences).
Growth curve analysis
The MSCs were seeded in 24-well plates
(5.0×103 cells/well) during the logarithmic growth
phase. The number of cells per well was counted for 7 days using an
inverted microscope (Nikon Corporation, Tokyo, Japan), and the
procedure on each day was repeated three times.
Osteogenic and adipogenic
differentiation in vitro
To induce osteogenic differentiation, the MSCs were
seeded at 4×104 cells in 3.5 cm culture dishes, and
cultured in osteogenic induction medium consisting of F12/DMEM or
low glucose-DMEM with 15% FBS and osteogenic supplements
(10−8 M dexamethasone, 10 mM β-glycerophosphate and 50
mg/l ascorbic acid; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) at 37°C, with the medium replaced every 3 days. The cells
were induced for four cycles, and then subjected to alkaline
phosphatase staining, which was observed using a light microscope
(Nikon Corporation).
To evaluate adipogenic potential, MSCs at a density
of 2×105 cells/dish were cultured in adipogenic
induction medium A and B consisting of F12/DMEM or low glucose-DMEM
with 15% FBS. Medium A was supplemented with 1 µM dexamethasone,
200 µM indomethacin and 2 µM insulin (Sigma-Aldrich; Merck
Millipore) whereas medium B was supplemented with 2 µM insulin.
Medium A was replaced every 3 days, followed by maintaining in
medium B for 1 day. The cells were induced for two cycles, and then
identified using Oil red O staining, which was observed using a
light microscope (Nikon Corporation).
Polyinosinic:polycytidylic acid
[poly(I:C)] treatment of mUC-MSCs cell cultures
The TLR3-specific ligand, poly(I:C) was purchased
from Sigma-Aldrich; Merck Millipore (cat no. P1530) and prepared
according to the manufacturer's protocol. The cells were seeded at
a density of 1.2×105 cells or 1.0×105
cells/well, respectively, in 6-well plates. The following day,
medium was added either with or without poly(I:C). The treatment
was continued for 24 h (1.2×105 cells) or 48 h
(1.0×105 cells). The concentrations of poly(I:C) used
were 0, 10, 25 and 50 µg/ml.
RNA extraction, reverse
transcription-polymerase chain reaction (RT-PCR) and
RT-quantitative PCR (qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA using the HiScript First-Strand cDNA
Synthesis kit (cat no. R122-01; HiScript® Q RT SuperMix;
Vazyme, Piscataway, NJ, USA) according to the manufacturer's
protocol. Total RNA (0.5 µg) was mixed with 2 µl of 5X qRT
SuperMix, and the thermocycling conditions were as follows: 25°C
for 10 min, 50°C for 30 min and 85°C for 5 min. The RT-qPCR mixture
contained 10 µl of the SYBR-Green® Master Mix (2X;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) and 1 µl of cDNA;
the total volume was increased to 20 µl with ddH2O. The
primer powder was fixed to the bottom of a 96-well plate and 20 µl
of the PCR mixture was added to each well. RT-qPCR was performed
using the CFX96 real-time instrument (Bio-Rad Laboratories, Inc.)
and the thermocycling conditions were as follows: 95°C for 5 min,
followed by 40 cycles of 95°C for 15 sec, annealing for 15 sec and
72°C for 20 sec, and then a 65–95°C drawing dissociation curve. The
sequences of the specific primers and the annealing temperature
used for each are listed in Table
I. The expression of each gene was defined from the threshold
cycle and the melting temperatures were recorded. The relative
changes in mRNA expression were analyzed using the
2−ΔΔCq method (25).
β-actin was used as an internal control.
 | Table I.Primer sequences of target genes. |
Table I.
Primer sequences of target genes.
| Gene | Primer sequence
(5′-3′) | Fragment size
(bp) | Annealing
temperature (°C) | mRNA accession
no. |
|---|
| TLR3 | F:
GAGGTTGACGCACCTGTTCT | 281 | 60 | NM_126166 |
|
| R:
GCTGCAGTCAGCTACGTTGT |
|
|
|
| Runx2 | F:
AGCCTCTTCAGCGCAGTGAC | 183 | 63 | XM_006523541 |
|
| R:
TGTTGTTGCTGCTGCTGTTG |
|
|
|
| Adiponectin | F:
AGGAGATGCAGGTCTTCTTG | 146 | 58 | NM_009605 |
|
| R:
CACTGAACGCTGAGCGATAC |
|
|
|
| IL-6 | F:
AAGTCCGGAGAGGAGACTTC | 487 | 58 | NM_031168 |
|
| R:
TGGATGGTCTTGGTCCTTAG |
|
|
|
| IL-8 | F:
GGCTGTCCTTAACCTAGGCATCT | 257 | 63 | NM_011339 |
|
| R:
GGTCCTCAGGTAGGAACCTGTTAGT |
|
|
|
| CCL5 | F:
CATATGGCTCGGACACCAC | 145 | 62 | NM_013653 |
|
| R:
CACACTTGGCGGTTCCTTC |
|
|
|
| CXCL10 | F:
GCTGCAACTGCATCCATA | 113 | 57 | NM_021274 |
|
| Rev:
CATCGTGGCAATGATCTC |
|
|
|
| β-actin | F:
CACGAAACTACCTTCAACTCC | 265 | 56 | NM_001017992 |
|
| R:
CATACTCCTGCTTGCTGATC |
|
|
|
Western blot analysis
The cells were lysed and homogenized in RIPA buffer
supplemented with protease inhibitors. Protein concentration was
determined using a Bicinchoninic Acid Protein Quantification kit
(CWBIO, Beijing, China), according to the manufacturer's
instructions. Total proteins (50 µg) were loaded on a 12% SDS-PAGE
gel, followed by electrophoresis and transfer onto a PVDF membrane
(EMD Millipore, Billerica, MA, USA). The sources and dilution
ratios of primary antibodies were as follows: GAPDH (1:2,000; cat
no. CW0100A; CWBIO), sex determining region Y-box 2 (Sox2; 1:500;
EMD Millipore), octamer-binding transcription factor 4 (Oct4;
1:200; cat no. sc-101534), Nanog (1:500; cat no. sc-293121) (both
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
Spalt-like transcription factor 4 (Sall4; 1:500; cat no. ab31968;
Abcam, Cambridge, UK). All primary antibodies were incubated
overnight at 4°C. The goat-anti-rabbit IgG (for Sox2) and
goat-anti-mouse IgG (for GAPDH, Oct4, Nanog and Sall4) horseradish
peroxidase-conjugated secondary antibodies were purchased from
CWBIO (dilution 1:2,000; cat nos. CW0103 and CW0102, respectively).
All secondary antibodies were incubated for 1 h at 37°C. The
antigen-antibody complex was visualized using Immobilon Western
Chemiluminescent HRP substrate (EMD Millipore).
Luminex assay
Following pre-treatment with poly(I:C) for 24 or 48
h, the mUC-MSCs were washed and sequentially cultured in complete
medium for another 48 h. The supernatants were collected and
centrifuged at 500 × g and 4°C for 10 min to remove cell debris. A
mouse cytokine/chemokine magnetic bead panel kit (cat no.
MCYTOMAG-70K-12; EMD Millipore) was used to detect granulocyte
colony stimulating factor (G-CSF), interferon-γ (IFN-γ),
interleukin (IL)-1β, IL-6, IL-10, IL-15, IL-17, chemokine (C-X-C
motif) ligand 10 (CXCL10), vascular endothelial growth factor
(VEGF), and tumor necrosis factor-α (TNF-α) in the prepared
supernatant. All procedures were performed according to the
manufacturer's protocol. The final detection and analysis were
performed using the Luminex 200 system (EMD Millipore).
Statistical analysis
All data are presented as the mean ± standard
deviation. The statistical significances between two groups were
analyzed using Student's t-test, whereas the differences among
multiple groups were determined using one-way analysis of variance
with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
A Tukey's post hoc test was used for multiple comparisons using
SPSS version 20 (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Isolation of mUC-MSCs from mouse
UC
During the initial 24–48 h, the primary cells
migrated out of the explants and adhered to the plastic surface,
exhibiting heterogeneous morphology. At ~3–5 days post-seeding, the
cells began to form colonies (Fig.
1Aa). The well-developed colonies were trypsinized and passaged
at a high density into a new cell culture dish. Following serial
subculturing, a relatively homogeneous population of mUC-MSCs was
observed at passages 5–7 when the purified cells exhibited spindle-
or polygonal-shaped appearance (Fig.
1Ab), which was similar to that of mBM-MSCs (Fig. 1Ac). No morphological changes were
observed within 20 passages.
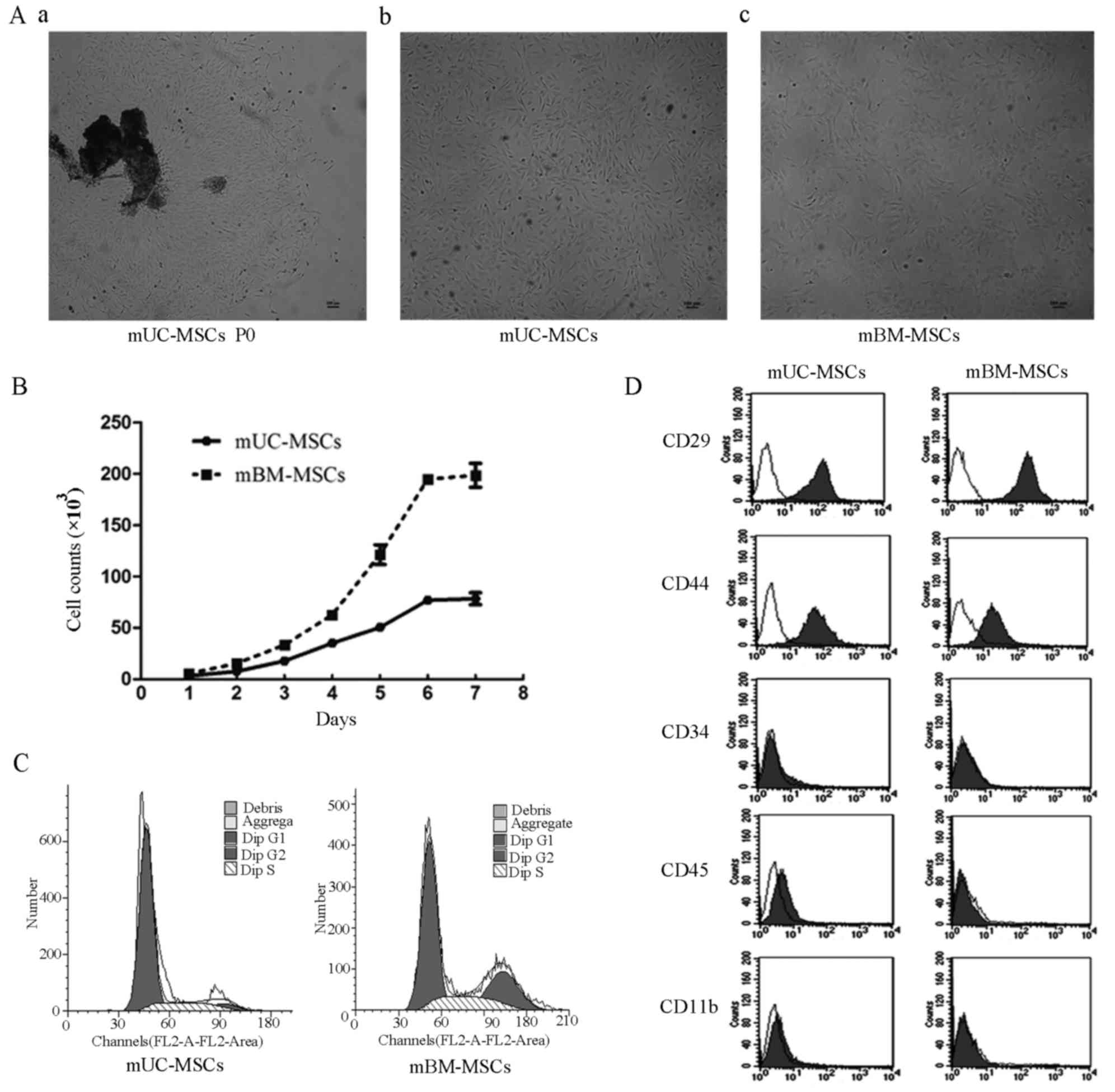 | Figure 1.Morphology, growth, surface antigens
and cell cycle of MSCs derived from mUC-MSCs and mBM-MSCs. (A)
Appearance of mUC-MSCs and mBM-MSCs at passages (a) 0, (b) 10 and
(c) 17 (magnification, ×40; scale bar, 100 µm. (B) Growth curves of
mUC-MSCs and mBM-MSCs. (C) Flow cytometric analysis of surface
markers CD29, CD44, CD34, CD45 and CD11b in mUC-MSCs and mBM-MSCs.
(D) DNA contents of mUC-MSCs and mBM-MSCs. mUC, mouse umbilical
cord; mBM, mouse bone marrow; MSCs, mesenchymal stem cells; CD,
cluster of differentiation. |
Comparison of growth characteristics
between mUC-MSCs and mBM-MSCs
The mUC-MSCs and mBM-MSCs showed S-shaped growth
curves (Fig. 1B). During the
successive 7 days, the two types of cell exhibited a lag phase for
1 day and then moved into the logarithmic phase, when cells
expanded rapidly. On day 6, the cell counts of the two cell types
reached their peak, followed by a plateau phase. The number of
mUC-MSCs increased at a slower rate, compared with that of the
mBM-MSCs. Similar results was observed in the cell cycle analysis.
The DNA contents showed that the population of proliferating cells
in the mUC-MSCs (S+G2/M; 21.38%) was smaller compared with that in
the mBM-MSCs (S+G2/M; 43.56%), although the subset of quiescent
cells in the mUC-MSCs and mBM-MSCs (G0/G1; 78.62 and 56.44%,
respectively) accounted for the predominant population (Fig. 1C). Taken together, the mUC-MSCs
possessed lower proliferative potential, compared with the
mBM-MSCs.
Expression of surface antigens and
stemness-related proteins in mUC-MSCs and mBM-MSCs
The results of the flow cytometric analysis revealed
that the mUC-MSCs were positive for CD29 and CD44, but negative for
CD34, CD45 and CD11b, similar to mBM-MSCs (Fig. 1D). To evaluate the potential of
mUC-MSCs for long-term culture, the cells were subjected to
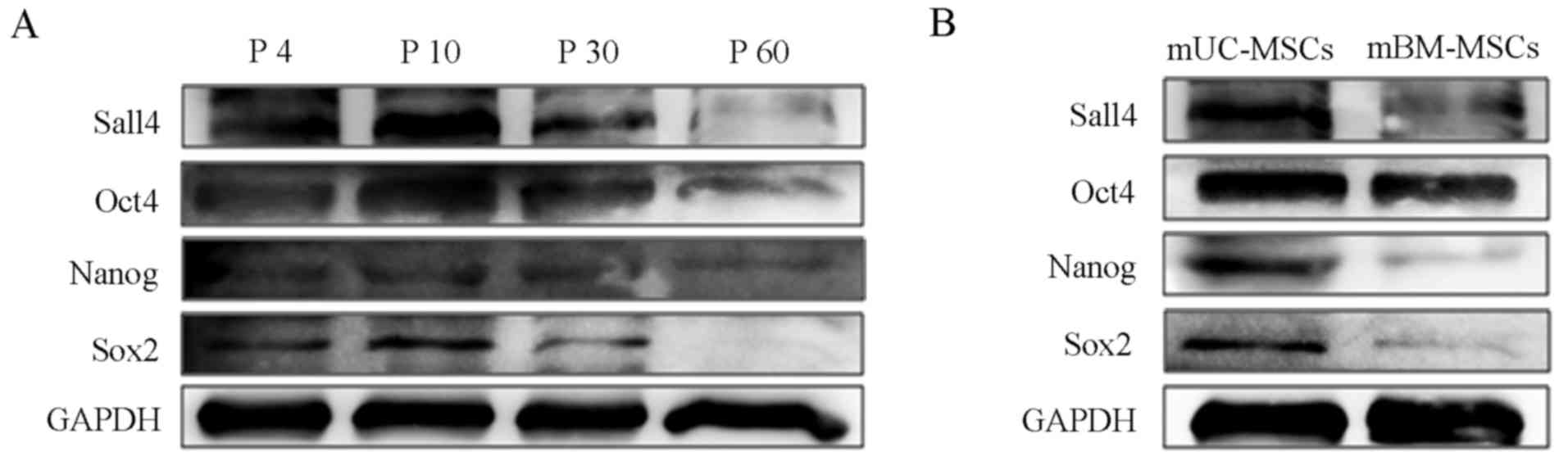
continuous subculture 60 times. The cells of passages 4, 10, 30 and
60 were selected for evaluating the changes in protein expression
levels of Sox2, Nanog, Oct4 and Sall4. The western blot analysis
showed that the expression of these proteins markedly increased as
the cells were purified (P10) and then decreased progressively with
passages, indicating that purified mUC-MSCs of <30 passages were
more suitable for further investigations (Fig. 2A). The mUC-MSCs exhibited higher
expression levels of stemness-related proteins (Sox2, Nanog, Oct4
and Sall4), compared with the mBM-MSCs (Fig. 2B).
Differentiation potential of mUC-MSCs
and mBM-MSCs
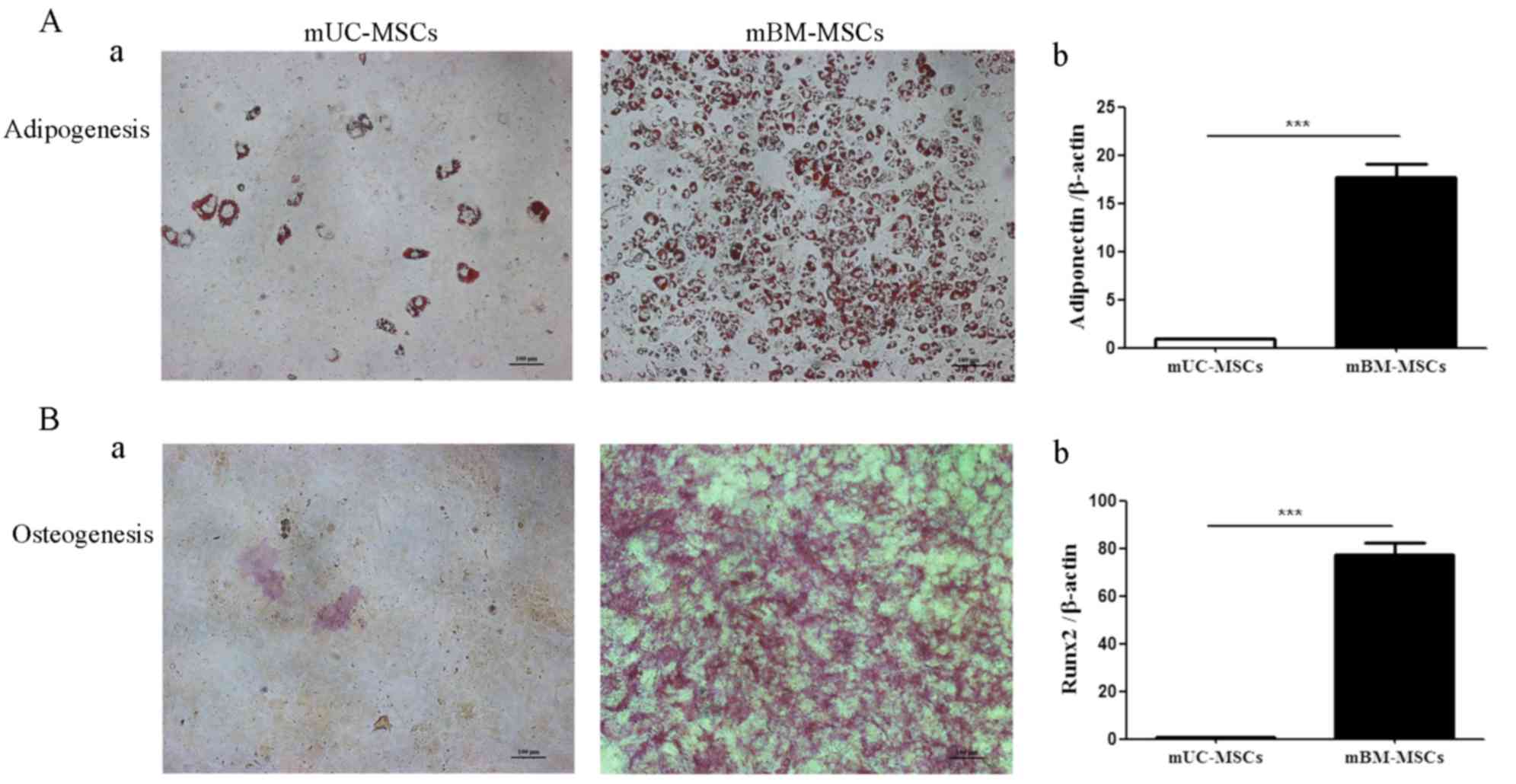
As the mUC-MSCs exhibited significant differences in
stemness-related proteins, compared with mBM-MSCs, the present
study hypothesized that the two cell lines may have different
differentiation potentials, particularly in adipogenic and
osteogenic differentiation. Notably, the induction periods were
shortened due to the higher efficiency of mBM-MSCs. Although both
the mUC-MSCs and mBM-MSCs were capable of differentiation into
adipocytes or osteocytes, the former exhibited markedly lower
potential for adipogenesis, as shown by positive staining of Oil
red O (Fig. 3Aa) and the RT-qPCR
analysis showing significantly lower mRNA levels of adiponectin in
the differentiated mUC-MSCs, compared with those in the
differentiated mBM-MSC (Fig. 3Ab).
The same was true for osteogenesis, as shown by Oil red O (Fig. 3Ba) and the mRNA expression of
runt-related transcription factor 2 (Runx2; Fig. 3Bb) being significantly lower in the
differentiated mUC-MSCs, compared with those in the differentiated
mBM-MSCs. These data indicated that mUC-MSCs possessed lower
differentiation potential, compared with the mBM-MSCs.
Poly(I:C) enhances the expression of
Sox2, Oct4 and Nanog in mUC-MSCs
It has been reported that poly(I:C) treatment can
affect the migration, cytokine induction and immunosuppressive
function of human MSCs (26,27).
However, the effect of poly(I:C) on mUC-MSCs remains to be
elucidated. In the present study, the results of the RT-PCR
analysis confirmed the expression of TLR3 in mUC-MSCs (Fig. 4A). To examine the effect of
poly(I:C) on the levels of stemness-related proteins of mUC-MSCs,
the cells were cultured with different doses of poly(I:C) for 24 or
48 h. As shown in Fig. 4B, the
mUC-MSCs in groups containing 10 or 25 µg/ml poly(I:C) exhibited
higher levels of Sox2, Oct4 and Nanog, compared with those in the
control, whereas poly(I:C) at 50 µg/ml either failed to enhance the
expression of these proteins (24 h) or increased their expression
to a lesser extent (48 h).
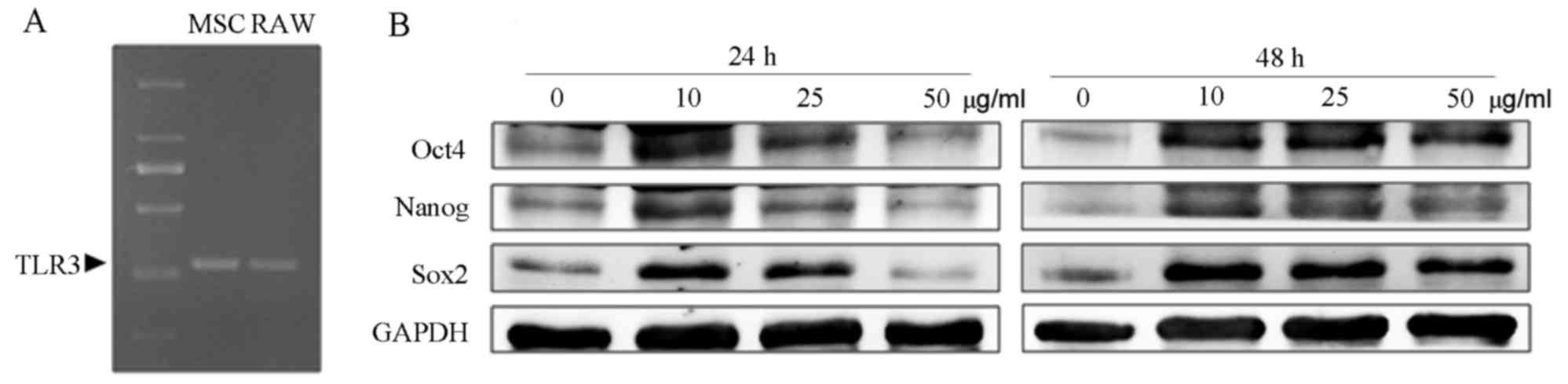 | Figure 4.TLR3-specific ligand poly(I:C)
increased the expression of stemness-related proteins in mUC-MSCs.
(A) Reverse transcription-polymerase chain reaction analysis of the
expression of TLR3 in mUC-MSCs. (B) Western blot analysis for the
expression levels of Sox2, Nanog and Oct4 in mUC-MSCs pre-treated
with 0, 10, 25 and 50 µg/ml poly(I:C) for 24 or 48 h. mUC, mouse
umbilical cord; mBM, mouse bone marrow; MSCs, mesenchymal stem
cells; poly(I:C), polyinosinic:polycytidylic acid; TLR3, Toll-like
receptor 3; Sox2, Sex determining region Y-box 2; Oct4,
octamer-binding transcription factor 4. |
Poly(I:C) promotes the expression and
secretion of inflammatory cytokines in mUC-MSCs
Previous studies have indicated that TLR activation
in MSCs can trigger the production of downstream cytokines.
Therefore, a luminex assay was performed in the present study to
determine the content of several inflammatory cytokines in the
supernatants from mUC-MSCs pre-treated with poly(I:C), termed
poly(I:C)-mUC-MSCs, including G-CSF, IFN-γ, IL-1β, IL-6, IL-10,
IL-15, IL-17, CXCL10, VEGF and TNF-α. As shown in Fig. 5A (24 h) and Fig. 5B (48 h), IL-6 and CXCL10 were
secreted at high levels and upregulated in the supernatants from
the poly(I:C)-mUC-MSCs. In terms of concentration, 50 µg/ml
poly(I:C) pre-treatment markedly increased the secretion of CXCL10,
compared with that in the control, whereas IL-6 was altered to a
lesser extent. To further confirm the enhanced production of IL-6
and CXCL10, RT-qPCR analysis was used to determine the mRNA levels.
It was found that the upregulation of IL-6 and CXCL10 were in
accordance with the luminex assay data. CCL5 showed the most marked
upregulation in mUC-MSCs pre-treated with 50 µg/ml poly(I:C); IL-8
was increased only in the mUC-MSCs pre-treated for 48 h (Fig. 5C).
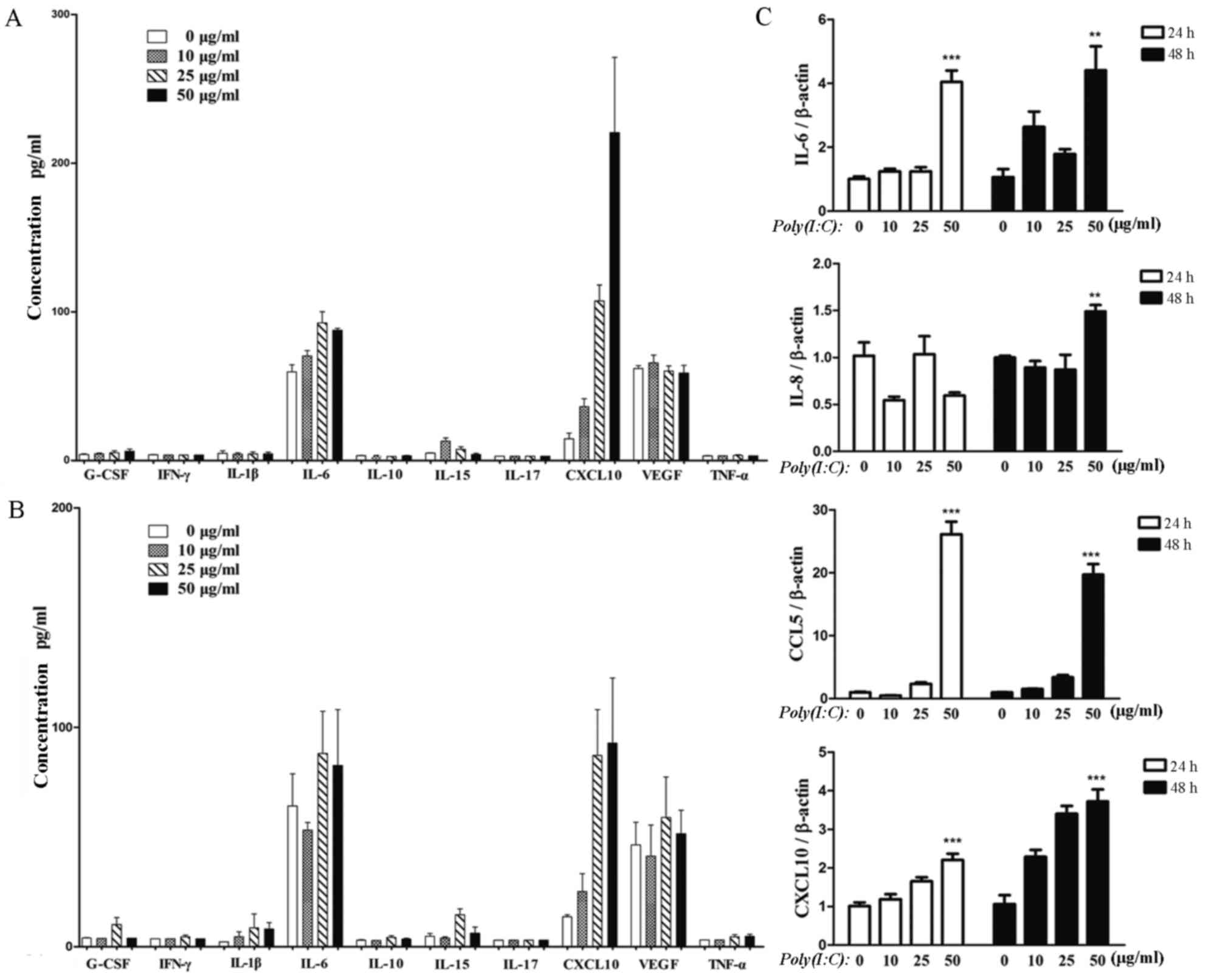 | Figure 5.Poly(I:C) induces cytokine production
in mUC-MSCs. A luminex assay of the levels of 10 cytokines in the
supernatants from mUC-MSCs pre-treated with poly(I:C) for (A) 24 h
or (B) 48 h, including G-CSF, IFN-γ, IL-1β, IL-6, IL-10, IL-15,
IL-17, CXCL10, VEGF and TNF-α. (C) Reverse
transcription-quantitative polymerase chain reaction analysis of
mRNA levels of IL-6, IL-8, CCL5 and CXCL10 in mUC-MSCs treated with
poly(I:C) for 24 or 48 h. ***P<0.001 and **P<0.01, vs.
control group. mUC-MSCs, mouse umbilical cord mesenchymal stem
cells; G-CSF, granulocyte colony stimulating factor; IFN-γ,
interferon-γ; IL, interleukin; CXCL10, chemokine (C-X-C motif)
ligand 10; VEGF, vascular endothelial growth factor; TNF-α, tumor
necrosis factor-α; CCL5, chemokine (C-C motif) ligand 5; poly(I:C),
polyinosinic:polycytidylic acid. |
Discussion
MSCs are considered the primary cell source for stem
cell-based therapeutics. The use of UC-derived MSCs are increasing
in popularity due to their unique properties. In preclinical
studies, mMSCs have been most commonly used to establish
experimental models, and transplantation of cross-species MSCs
(i.e. human MSCs) remains the primary therapeutic method. However,
xenogeneic immune responses may complicate the host cell responses
(9) and further challenge the
ability of MSCs to function across species barriers. In
consideration of allogeneic transplantation mimicry and mMSC
deficiency, the present study successfully isolated MSCs from mUC
using a novel method.
There are two primary methods to harvest MSCs,
either from the whole UC or their compartments, including Wharton's
jelly, cord lining, umbilical blood vessels and the perivascular
region. These are the classic explant method and the enzymatic
digestion method (7). Although the
comparison between these two methods remains controversial, the
former has been shown to be superior, with which plastic-adherent
MSC-like cells were isolated from human gastric cancer tissues and
adjacent non-cancerous tissues, and from hUC-MSCs (28,29).
In the present study, an MSC strain was obtained from the whole UC
using an modified explant procedure and F12-containing media, which
were referred to as mUC-MSCs according to previous reports
(7,10). The purified cells, which met the
criteria of the International Society for Cellular Therapy
(30), exhibited a fibroblast-like
appearance, expressed typical surface antigens, differentiated into
osteoblasts or adipocytes, and were maintained in a quiescent
state, as indicated by the high proportion of cells in the G0/G1
phase. In addition, the expression of stemness-related proteins,
including Sox2, Nanog, Oct4 and Sall4, were enhanced in purified
mUC-MSCs and gradually decreased with passages, indicating their
stemness maintenance ability during long-term culture. The
abovementioned data suggested that the modified method used in the
present study to isolate and culture mUC-MSCs, free from chemical
injury and with the addition of nutritional factors, is efficient,
reliable and cost-saving.
Although mMSCs can be derived from various tissues
(4), BM remains the most commonly
used tissue source. MSCs from BM are limited in terms of cell
number, whereas MSCs account for the predominant stem cell
population in the UC (10).
Therefore, investigation of the characteristics of mUC-MSCs
relative to the ‘gold standard’ cell source of mBM-MSCs was
required. The present study found that mBM-MSCs under the adherent
method required a longer duration for purification, of at least 13
passages. The two cell populations presented with similar
morphology and, to a certain extent, surface antigen profile. With
the exception of the five essential markers, it has been reported
that the differential expression of SSEA-4, LNGFR, CD56 and CD146
can distinguish hUC-MSCs from hBM-MSCs (31). The growth curve assay and DNA
content analysis in the present study indicated that mUC-MSCs
possessed a lower proliferative potential, compared with the
mBM-MSCs. However, similar comparison within hMSCs showed the
opposite result, namely that hUC-MSC showed optimal proliferation,
which may due to certain abundant genes involved in cell
proliferation (7,32,33).
To compare the protein expression levels between mUC-MSCs and
mBM-MSCs, the present study focused on stemness-related proteins,
including Sox2, Nanog, Oct4 and Sall4. The results revealed that
the levels of these proteins were higher in the mUC-MSCs,
suggesting that the cells were primitive stem cells (10,33).
The present study found that mUC-MSCs presented with markedly lower
adipogenic and osteogenic differentiation efficiencies, which was
in accordance with previous reports involving human and enquine
MSCs (11,32–34).
The present study is the first, to the best of our knowledge, to
report on the similarities and differences between mUC-MSCs and
mBM-MSCs, and indicated that the reasonable selection of mMSCs be
made based on their own advantages and the relevant models.
Considering the differences, further investigations are required to
clarify the respective mechanisms.
Previous studies have confirmed that MSCs express
functional TLR3 and that its activation regulates MSC functions
(22–26). The present study confirmed the
presence of TLR3 in mUC-MSCs, in accordance with mBM-MSCs (22). Subsequently, a preliminary
experiment was performed to determine the effect of poly(I:C), the
TLR3 agonist, on mUC-MSCs. The enhanced expression of
stemness-related proteins (Sox2, Oct4 and Nanog) was observed in
the poly(I:C)-treated mUC-MSCs, which was similar to the data from
a previous study suggesting the potential involvement of TLR3 in
stemness maintenance of MSCs (35). As TLR signaling is closely
associated with inflammatory cytokines, the secretion and mRNA
levels of several cytokines were detected in the mUC-MSCs treated
with poly(I:C). The results showed that the levels of IL-6, CXCL10,
CCL5 and IL-8 were markedly upregulated. According to previous
repots, these cytokines may be involved in immune modulation and
cancer progression (23,24).
In conclusion, the present study focused on a novel
cell line of MSCs from mouse UC using a novel method. This, to the
best of our knowledge, is the first demonstration of comparative
analysis of mUC-MSCs and their responses to TLR3 activation. It was
found that the mUC-MSCs exhibited MSC-like characteristics and
shared similarities with mBM-MS, however, they exhibited
differences in purification, proliferation, stem cell markers and
differentiation. Poly(I:C) increased the expression of
stemness-related proteins and inflammatory cytokines. Further
investigations are required to confirm the manifestation and
investigate the underlying mechanisms. The results of the present
study provide novel evidence for the selection of mMSCs and offer
further insight into the role of TLR3 in the regulation of
mMSCs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 31340040, 81272481,
81270214 and 81572075), the Jiangsu Province for Outstanding
Sci-tech Innovation Team in Colleges and Universities (grant no.
SJK2013-10), the Jiangsu Province's Outstanding Medical Academic
Leader and Sci-tech Innovation Team Program (grant no. LJ201117)
and the Special Funded Projects of National Postdoctoral Fund
(grant no. 2017T100337).
References
|
1
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kinet. 3:393–403. 1970.PubMed/NCBI
|
|
2
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frausin S, Viventi S, Falzacappa Verga L,
Quattromani MJ, Leanza G, Tommasini A and Valencic E: Wharton's
jelly derived mesenchymal stromal cells: Biological properties,
induction of neuronal phenotype and current applications in
neurodegeneration research. Acta Histochem. 117:329–338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iser IC, Bracco PA, Goncalves CE, Zanin
RF, Nardi NB, Lenz G, Battastini AM and Wink MR: Mesenchymal stem
cells from different murine tissues have differential capacity to
metabolize extracellular nucleotides. J Cell Biochem.
115:1673–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung J, Choi JH, Lee Y, Park JW, Oh IH,
Hwang SG, Kim KS and Kim GJ: Human placenta-derived mesenchymal
stem cells promote hepatic regeneration in CCl4 -injured rat liver
model via increased autophagic mechanism. Stem Cells. 31:1584–1596.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim
HS, Park YS, Jo I, Park JW, Jung SC, et al: Tonsil-derived
mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in
mice via autophagy activation. Sci Rep. 5:86162015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagamura-Inoue T and He H: Umbilical
cord-derived mesenchymal stem cells: Their advantages and potential
clinical utility. World J Stem Cells. 6:195–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harris DT: Umbilical cord tissue
mesenchymal stem cells: Characterization and clinical applications.
Curr Stem Cell Res Ther. 8:394–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li WW, Wei YH, Li H, Lai DM and Lin TN:
Isolation and characterization of a novel strain of mesenchymal
stem cells from mouse umbilical cord: Potential application in
cell-based therapy. PLoS One. 8:e744782013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bongso A and Fong CY: The therapeutic
potential, challenges and future clinical directions of stem cells
from the wharton's jelly of the human umbilical cord. Stem Cell
Rev. 9:226–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barberini DJ, Freitas NP, Magnoni MS, Maia
L, Listoni AJ, Heckler MC, Sudano MJ, Golim MA, da Cruz
Landim-Alvarenga F and Amorim RM: Equine mesenchymal stem cells
from bone marrow, adipose tissue and umbilical cord:
Immunophenotypic characterization and differentiation potential.
Stem Cell Res Ther. 5:252014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fink KD, Rossignol J, Crane AT, Davis KK,
Bombard MC, Bavar AM, Clerc S, Lowrance SA, Song C, Lescaudron L
and Dunbar GL: Transplantation of umbilical cord-derived
mesenchymal stem cells into the striata of R6/2 mice: Behavioral
and neuropathological analysis. Stem Cell Res Ther. 4:1302013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitchell KE, Weiss ML, Mitchell BM, Martin
P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K,
Hildreth T, et al: Matrix cells from wharton's jelly form neurons
and glia. Stem Cells. 21:50–60. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ganta C, Chiyo D, Ayuzawa R, Rachakatla R,
Pyle M, Andrews G, Weiss M, Tamura M and Troyer D: Rat umbilical
cord stem cells completely abolish rat mammary carcinomas with no
evidence of metastasis or recurrence 100 days post-tumor cell
inoculation. Cancer Res. 69:1815–1820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang J, Gu F, Wang H, Hua B, Hou Y, Shi
S, Lu L and Sun L: Mesenchymal stem cell transplantation for
diffuse alveolar hemorrhage in SLE. Nat Rev Rheumatol. 6:486–489.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lindenmair A, Hatlapatka T, Kollwig G,
Hennerbichler S, Gabriel C, Wolbank S, Redl H and Kasper C:
Mesenchymal stem or stromal cells from amnion and umbilical cord
tissue and their potential for clinical applications. Cells.
1:1061–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Zhang Q, Wang M, Wu H, Mao F, Zhang
B, Ji R, Gao S, Sun Z, Zhu W, et al: Macrophages are involved in
the protective role of human umbilical cord-derived stromal cells
in renal ischemia-reperfusion injury. Stem Cell Res. 10:405–416.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Qiu X, Ni P, Qiu X, Lin X, Wu W,
Xie L, Lin L, Min J, Lai X, et al: Immunological characteristics of
human umbilical cord mesenchymal stem cells and the therapeutic
effects of their transplantion on hyperglycemia in diabetic rats.
Int J Mol Med. 33:263–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Ezzelarab MB and Cooper DK: Do
mesenchymal stem cells function across species barriers? Relevance
for xenotransplantation. Xenotransplantation. 19:273–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bernardo ME and Fibbe WE: Mesenchymal
stromal cells: Sensors and switchers of inflammation. Cell Stem
Cell. 13:392–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DelaRosa O and Lombardo E: Modulation of
adult mesenchymal stem cells activity by toll-like receptors:
Implications on therapeutic potential. Mediators Inflamm.
2010:8656012010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pevsner-Fischer M, Morad V, Cohen-Sfady M,
Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR and Zipori D:
Toll-like receptors and their ligands control mesenchymal stem cell
functions. Blood. 109:1422–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Waterman RS, Tomchuck SL, Henkle SL and
Betancourt AM: A new mesenchymal stem cell (MSC) paradigm:
Polarization into a pro-inflammatory MSC1 or an immunosuppressive
MSC2 phenotype. PLoS One. 5:e100882010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Waterman RS, Henkle SL and Betancourt AM:
Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor
growth whereas MSC2-treatment promotes tumor growth and metastasis.
PLoS One. 7:e455902012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tomchuck SL, Zwezdaryk KJ, Coffelt SB,
Waterman RS, Danka ES and Scandurro AB: Toll-like receptors on
human mesenchymal stem cells drive their migration and
immunomodulating responses. Stem Cells. 26:99–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao X, Liu D, Gong W, Zhao G, Liu L, Yang
L and Hou Y: The toll-like receptor 3 ligand, poly(I:C), improves
immunosuppressive function and therapeutic effect of mesenchymal
stem cells on sepsis via inhibiting miR-143. Stem Cells.
32:521–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu X, Zhang X, Wang S, Qian H, Zhu W, Cao
H, Wang M, Chen Y and Xu W: Isolation and comparison of mesenchymal
stem-like cells from human gastric cancer and adjacent
non-cancerous tissues. J Cancer Res Clin Oncol. 137:495–504. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q,
Jiang R, Yan Y, Mao F, Yang H, et al: Human mesenchymal stem cells
isolated from the umbilical cord. Cell Biol Int. 32:8–15. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeddou M, Relic B and Malaise MG:
Umbilical cord fibroblasts: Could they be considered as mesenchymal
stem cells. World J Stem Cells. 6:367–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsieh JY, Fu YS, Chang SJ, Tsuang YH and
Wang HW: Functional module analysis reveals differential osteogenic
and stemness potentials in human mesenchymal stem cells from bone
marrow and Wharton's jelly of umbilical cord. Stem Cells Dev.
19:1895–1910. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hua J, Gong J, Meng H, Xu B, Yao L, Qian
M, He Z, Zou S, Zhou B and Song Z: Comparison of different methods
for the isolation of mesenchymal stem cells from umbilical cord
matrix: Proliferation and multilineage differentiation as compared
to mesenchymal stem cells from umbilical cord blood and bone
marrow. Cell Biol Int. Oct 7–2013.(Epub ahead of print). doi:
10.1002/cbin.10188. PubMed/NCBI
|
|
34
|
Ding DC, Chang YH, Shyu WC and Lin SZ:
Human umbilical cord mesenchymal stem cells: A new era for stem
cell therapy. Cell Transplant. 24:339–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Liu D, Pu D, Wang Y, Li L, He Y,
Li Y, Li L, Qiu Z, Zhao S and Li W: The role of toll-like receptor
3 and 4 in regulating the function of mesenchymal stem cells
isolated from umbilical cord. Int J Mol Med. 35:1003–1010. 2015.
View Article : Google Scholar : PubMed/NCBI
|