Introduction
Glucocorticoids (GCs) are anti-inflammatory agents
used in the treatment of various diseases, such as asthma,
rheumatoid arthritis, and systemic lupus erythematosus (1–4).
Although GCs have been prescribed for many years, their potential
side effects (growth retardation, osteopenia, adrenal
insufficiency, etc.), can prevent their long-term use (5,6).
Significantly, GC-induced osteoporosis (GIO) is thought as the most
severe one of these side effects because of the increased fracture
risk (7–9). GIO resulting from osteopenia has been
described as the most predictable and debilitating complication of
long-term GCs therapy (5,6). Therefore, the development of
medications that prevent GIO is of clinical significance.
Polygonum multiflorum Thunb. (PM, He-Shou-Wu)
is a kind of traditional Chinese medicine (10). PM and its extracts can be used to
improve the health of blood and blood vessels, blacken hair,
strengthen bones, neurosis and other diseases commonly associated
with aging (11–16). Based on previous evidence in our
team, we found that PM and its extracts exert beneficial effects in
the prevention and treatment of osteoporosis, which have already
been applied for China patents (ZL 00101246.0) (17). Furthermore, we have investigated
the effects of main components [(emodin and
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside (TSG)] of PM in
vitro. The results showed that emodin and TSG can promote Bone
Marrow Mesenchymal Stem Cells (MSCs) to differentiate into
osteoblasts. Moreover, emodin can inhibit MSCs differentiate into
adipocytes (18–21). The underlying mechanism of TSG may
be related to regulation of Wnt signaling pathway (22). However, the exact signaling
mechanism by which PM rescued impaired bone formation induced by GC
has not yet been investigated.
The Wnt/β-catenin signaling is an important pathway
that is required by the growth, development and maintenance of
skeletal tissue (9,23). Wnt signals are extracellularly
regulated by several secreted antagonists including secreted
frizzled-related protein (sFRP), Wnt inhibitory factor-1 (WIF1),
and dickkopf-1 (DKK1) (24).
Previous evidence showed that GCs can promote the expression of
DKK1 in cultured human osteoblasts (25). It is known that the cell fate of
pre-osteoblasts is mainly determined by the Wnt/β-catenin signal
pathway (26). Therefore, the
molecules which induce the activation of Wnt/β-catenin signaling
are beneficial for the treatment of osteoporosis. PM has been
demonstrated to exert a simulatory effect on Wnt/β-catenin
signaling pathway in vivo and in vitro (23,27).
Whether the extracts of PM can increase the bone mass or not in the
GIO model characteristic of decreased bone formation? If the
extracts could prevent GIO, and what's the mechanism of PM on bone
metabolism? Considering the above questions, this study aims to
observe the effect and the mechanism of PM underlying bone loss in
GIO rats.
Materials and methods
Preparation of PM extract
The dried roots of PM were purchased in Yulin
Xiang Sheng Chinese Herbal Medicine Co., Ltd. (Henan, China), and
were authenticated by Professor Yuyu Liu. A voucher specimen was
deposited at the herbarium of Guangdong Key Laboratory for Research
and Development of Natural Drugs, Guangdong Medical University
(Guangdong, China). Air-dried roots of PM (56.0 kg) was extracted
by 75% ethanol at 50~60°C, followed by rinsing with cyclohexane.
The organic solvent of PMRF was acquired by evaporation under a
vacuum at 55°C. The PMRF dissolved in water was absorbed by
macroporous resin D-101, and then eluted with H2O, 10,
20, 30, 40, 50, 60, 70, 80 and 90% ethanol successively, and PMR30
was prepared by the collection and concentration of 30% ethanol
elution (28).
Animal experiments
This study was carried out in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of Guangdong Laboratory Animal Monitoring Institute, under
the National Laboratory Animal Monitoring Institute of People's
Republic of China (29). The
experiments have been conducted according to protocols approved for
Specific Pathogen-Free animal care of the Animal Center of
Guangdong Medical University, and approved by the Academic
Committee on the Ethics of Animal Experiments of the Guangdong
Medical University [permit no. SYXK (Guangdong) 2008-0008;
Zhanjiang, China].
The Sprague Dawley (SD) female rats were acclimated
to local vivarium conditions (temperature: 24–28°C, humidity: 60%)
and under specific pathogen-free conditions. Rats were allowed free
access to water and diet.
Experimental protocols
Six-month-old female SD rats weighing (190–210 g,
n=90) were randomly divided into ten groups by weight: basic group,
control (normal saline) group, prednisone (GC, 6
mg·kg−1·d−1, model) group, GC plus PMR30 (H)
(400 mg·kg−1·d−1) group, GC plus PMR30 (M)
(200 mg·kg−1·d−1) group, GC plus PMR30 (L)
(100 mg·kg−1·d−1) group, GC plus PMRF (H)
(400 mg·kg−1·d−1) group, GC plus PMRF (M)
(200 mg·kg−1·d−1) group, GC plus PMRF (L)
(100 mg·kg−1·d−1) group, GC plus calcitriol
(CAL) (0.045 µg·kg−1·d−1) (positive group).
Rats were administered intragastrically with prednisone and/or the
extracts mentioned above for 120 days, and weighed once per week.
Rats were injected subcutaneously with calcein on the 3rd, 4, 13,
and 14th day before killed for the purpose of double labeling in
vivo, respectively (30).
Rats were sacrified by caridiac puncture under
sodium pentobarbital anesthesia at the experimental endpoint. The
serum was separated for testing biochemical markers. The left tibia
was used for bone histomorphometry analysis. The right tibia was
prepared for H&E staining. The left femur was used to test
protein expression of DKK1, WIF1 and SFRP4 using western blotting
assay (31).
Serum markers assay
Blood was collected in specimen tubes and kept at
25°C for 40–50 min in a vertical position for completely clotting.
And then the serum was separated by centrifuging at 1,000 × g for
10 min and stored at −80°C for biochemical markers assays. The
serum was separated for testing biochemical markers, including
Bone-specific alkaline phosphatase (BAP), Hydroxyl-terminal
propeptide of type I procollagen (PICP), tartrate resistant acid
phosphatase-5b (TRACP-5b), Cross-linked Carboxy-terminal
telopeptide of type I collagen (CTX-I), and DKK1. BAP and OCN, as
serum markers of bone formation, and OPG, sRANKL, and TRAP5b, as
the markers of bone resorption, were measured in rats using
commercially available ELISAs (Tuokeda Bio-Tech, Guangzhou,
China).
Bone histomorphometry assay
For histomorphometric analysis, the left tibia was
removed, dissected, and cut. The proximal tibial metaphysis (PTM)
was opened to expose the bone marrow cavity with an Isomet
low-speed saw (Buehler, Lake Bluff, IL, USA) and then fixed in 70%
ethanol after they had been placed in 10% formalin for 24 h,
dehydrated in increasing concentrations of ethanol, defatted in
xylene, and then embedded without decalcification in methyl
methacrylate (32). Frontal
sections of the PTM were cut at thicknesses of 5 and 8 µm. The
region of interest in the PTM was located between 1 and 4 mm distal
to the growth plate-epiphyseal junction, not including cortical
bone. A semiautomatic digitizing image analysis system
(OsteoMetrics, Inc., Decatur, GA, USA) was used for static and
dynamic histomorphometry measurements. For static histomorphometry
measurements with Masson-Goldner trichrome staining (5-µm
sections), the total tissue area, trabecular area, trabecular
perimeter, and osteoclast number (Oc.N) were measured and used to
calculate the percentage of trabecular bone volume (BV/TV),
trabecular number (Tb.N), trabecular separation (Tb.Sp), number of
osteoclasts (N.Oc), percent osteoclast surface perimeter (Oc.S.Pm),
and percent osteoblast surface perimeter (Ob.S.Pm). For dynamic
analyses (8-µm sections), single-labeled perimeter, double-labeled
perimeter, interlabeled width, percent of labeled perimeter
(%L.Pm), mineral apposition rate (MAR), and bone formation rate per
unit of bone surface (BFR/BS), bone formation rate per unit of bone
volume (BFR/BV), and bone formation rate per unit of bone tissue
area (BFR/TV) were measured and calculated. For the tibial shaft
(40-µm sections), the cortical area (Ct.Ar), percent Ct.Ar
(%Ct.Ar), percent marrow area, percent periosteal labeled perimeter
(%P-L.Pm), periosteal MAR, periosteal BFR per unit of bone surface
(P-BFR/BS), percent endocortical labeled perimeter, endocortical
MAR, and endocortical BFR per unit of bone surface (E-BFR/BS) were
calculated from the measured parameters (33).
Western blotting assay
Left femurs were stored at −80°C before they were
used. The whole proteins were extracted by the method previously
described with a Total Protein Extraction kit (Applygen
Technologies, Inc., Beijing, China) (33). Sixty micrograms of total protein
extracts was separated on sodium dodecyl sulfate-polyacrylamide
gels and transferred to poly (vinylene difluoride) membranes. The
membranes were blocked with 5% skimmed milk for 2 h at room
temperature, and were incubated overnight at 4°C with rabbit
anti-DKK1 monoclonal antibody (ab109416), WIF1 antibody (ab155101),
and sFRP4 (ab154167) (all from Abcam, Cambridge, MA, USA) at a
dilution of 1:300. This was followed by incubation with the
corresponding secondary antibodies and goat anti-rabbit IgG
antibodies (Beyotime Institute of Biotechnology, Haimen, China).
Protein expression was visualized using a BeoECL Plus instrument
(Beyotime Institute of Biotechnology). GADPH rabbit mAb (CST, USA)
was used to normalize the sample loading. The images of bands were
quantified with Image-Pro Plus 6.0.
Statistical analysis
Data were described as the means ± standard
deviation (mean ± SD) and analyzed statistically with SPSS, version
13.0. One-way ANOVA was used to detect the differences in changes
between the groups of various treatments after establishing if the
data were normally distributed and equivalency of variances.
P-value (the probabilities) <0.05 were considered statistically
significant.
Results
Effects of PM on body weight
The alterations of body weight of rats were no
statistically significant difference between each group during the
initial stage of experiment, while the weight of rats in prednisone
group decreased significantly from the fourth week compared with
the CON group (P<0.05) (Fig.
1). Reversely, the changes of body weight of rats in PMR30 (M)
and PMRF (H) groups increased compared with the prednisone group
(P<0.05). These results indicated that PMR30 (M) and PMRF (H)
groups can improve the slow growth of GIO rats.
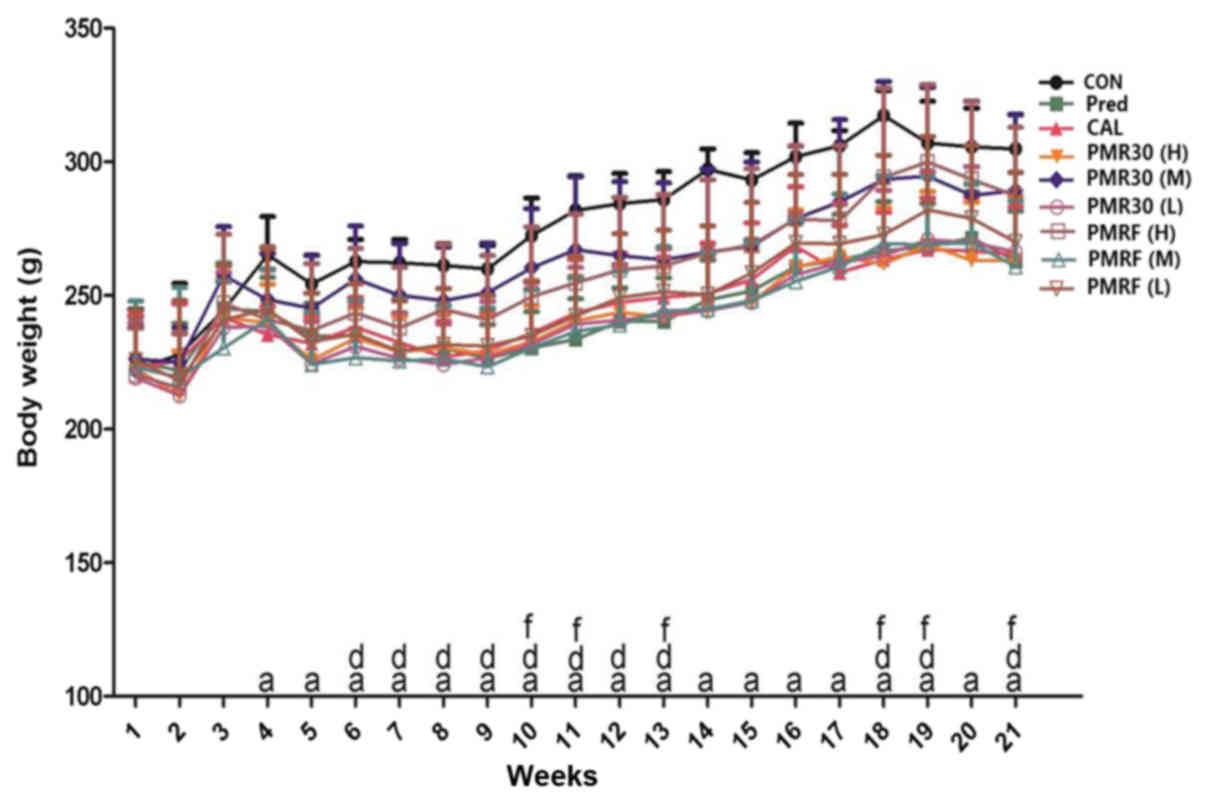 | Figure 1.Body weight (g) changes during the
experimental period. Body weight measurements from vehicle-treated
controls (CON), prednisone 6 mg.kg−1.d−1
(Pre), calcitriol 0.045 µg.kg−1.d−1 (CAL),
PMR30 (H) 400 mg.kg−1.d−1, PMR30 (M) 200
mg.kg−1.d−1, PMR30 (L) 100
mg.kg−1.d−1, PMRF (H) 400
mg.kg−1.d−1, PMRF (M) 200
mg.kg−1.d−1, and PMRF (L) 100
mg.kg−1.d−1 treated rats. aP<0.05 vs CON,
dP<0.05 PMR30 (M) vs. Pre, fP<0.05 PMRF (H) vs. Pre. |
Effects of PM on biomarkers of bone
turnover
We confirmed that GC resulted in the decrease of
biomarkers of the bone formation, including serum bone specific
alkaline phosphatase (BAP) and serum Hydroxyl-terminal propeptide
of type I procollagen (PICP) (Fig.
2 and Table I). Contrarily, GC
stimulated the increase of the biomarkers related to bone
resorption including serum Tartrate-resistant acid phosphatase-5b
(TRAP-5b), DKK1 and C-terminal telopeptides of I collagen (CTX-1)
level of bone tissue (Fig. 2 and
Table I). Encouragingly, PMR30 (M,
L) and PMRF (H) groups showed a capacity to reverse the deleterious
impacts of bone turnover elicited by GC, as effectively as CAL.
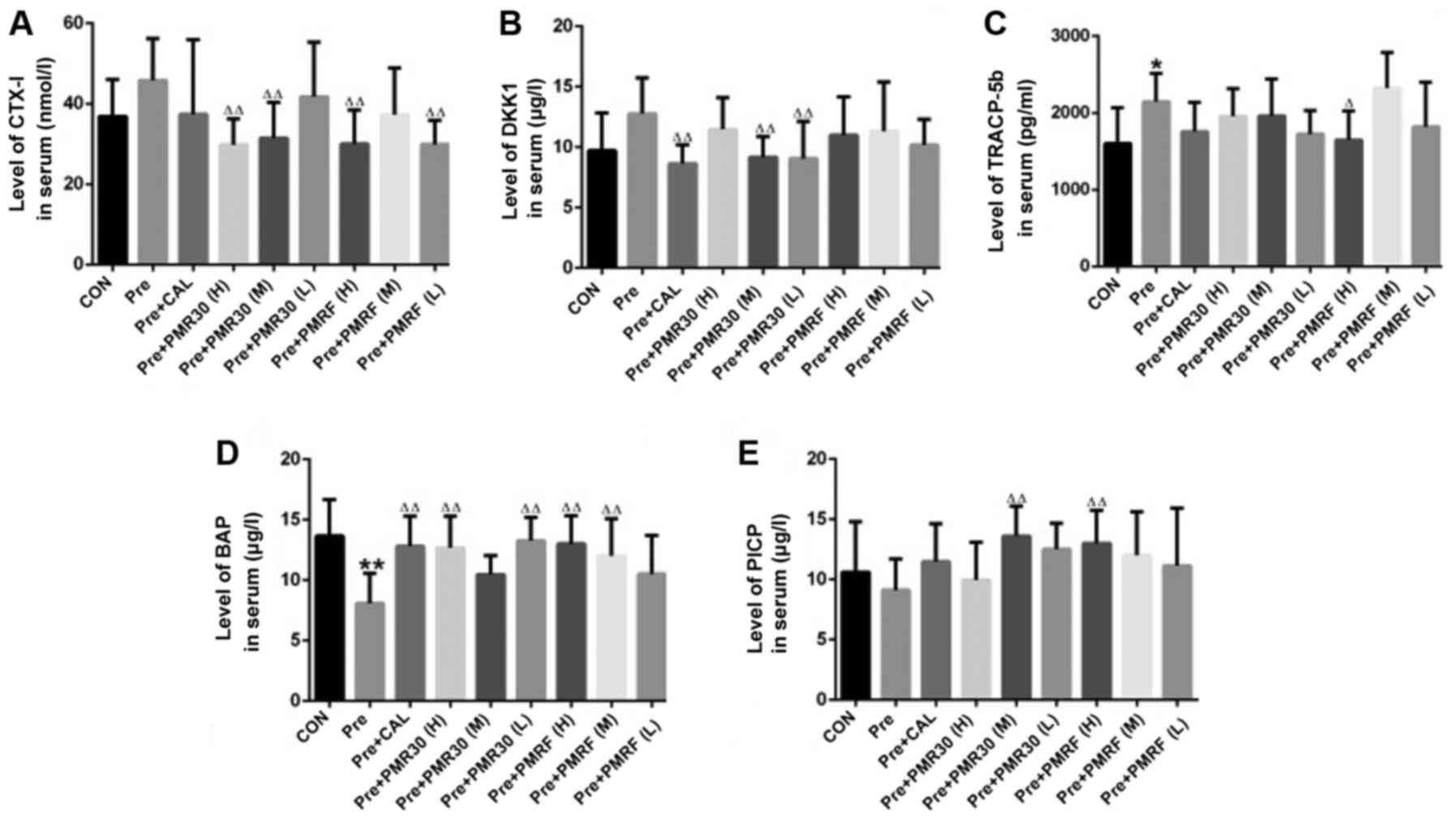 | Figure 2.Endpoint levels of serum biochemical
markers (A) CTX-I, (B) DKK1, (C) TRACP-5b, (D) BAP, (E) PICP in the
rats treated with vehicle (CON), prednisone (Pre), calcitriol
(CAL), and various PMR (30, F) dose levels. *P<0.05, **P<0.01
vs. CON; ∆P<0.05, ∆∆P<0.01 vs. Pred;
#P<0.05, ##P<0.01 vs. CAL. |
 | Table I.Serum biochemical indices of bone
marker in rats (x− ± s). |
Table I.
Serum biochemical indices of bone
marker in rats (x− ± s).
| Group | PICP (µg/l) | BAP (µg/l) | CTX-I (nmol/l) | TRACP-5b
(pg/ml) | DKK1 (µg/l) |
|---|
| CON |
10.59±4.17 |
13.64±3.00 |
36.88±9.18 |
1600±467 |
9.70±3.11 |
| Pre |
9.10±2.58 |
8.06±2.47b |
45.88±10.37 |
2148±368a |
12.76±2.96 |
| CAL |
11.47±3.14 |
12.80±2.46d |
37.40±18.51 |
1758±379 |
8.62±1.55d |
| PMR30(H) |
9.94±3.13 |
12.65±2.61d |
29.84±6.43d |
1959±360 |
11.45±2.62 |
| PMR30(M) |
13.58±2.50d |
10.45±1.56 |
31.46±8.84d |
1960±482 |
9.15±1.68d |
| PMR30(L) |
12.50±2.14 |
13.25±1.93d |
41.77±13.59 |
1726±306 |
9.03±3.07d |
| PMRF(H) |
12.99±2.72d |
13.01±2.31d |
30.03±8.42d |
1649±378c |
10.97±3.16 |
| PMRF(M) |
12.01±3.60 |
12.00±3.06d |
37.28±11.64 |
2325±463 |
11.31±4.07 |
| PMRF(L) |
11.12±4.77 |
10.50±3.17 |
29.91±5.96d |
1820±583 |
10.17±2.08 |
Effects of PM on the parameters of the
two-dimensional histomorphometry of the proximal tibial
metaphysis
The changes of the two-dimensional histomorphometry
of the proximal tibial metaphysis: Compared with the control group,
%Tb.Ar, percent labeled perimeter (%L.Pm), mineral apposition
(MAR), BFR/TV and %Ob.S.Pm were decreased (P<0.05), while
percentage of osteoclast surface perimeter (%Oc.S.Pm) and number of
osteoclast per millimeter (Oc.N) were increased (P<0.05) in
prednisone group. Compared with the prednisone group, %Tb.Ar and
BFR/TV were increased (P<0.05); and %Oc.S.Pm was decreased
(P<0.05) in CAL group. Compared with the prednisone group, %L.Pm
and new bone formation rate per unit of bone surface (BFR/BS) were
increased in PMR30 (H) group (P<0.05). Compared with the
prednisone group, number of osteoclast per millimeter (Oc.N) was
decreased (P<0.05) in PMR30 (H, M, L) groups. Compared with the
prednisone group, %Tb.Ar and BFR/TV were increased (P<0.05) in
PMRF (H) group. Compared with the prednisone group, percentage of
osteoclast surface perimeter (%Oc.S.Pm) was decreased (P<0.05)
in PMRF (H, M, L) groups (Tables
II–IV and Fig. 3). These results indicated that
PMR30 (M, L) and PMRF (H) groups can improve the bone loss and
decreased activity of osteoclast in GIO rats.
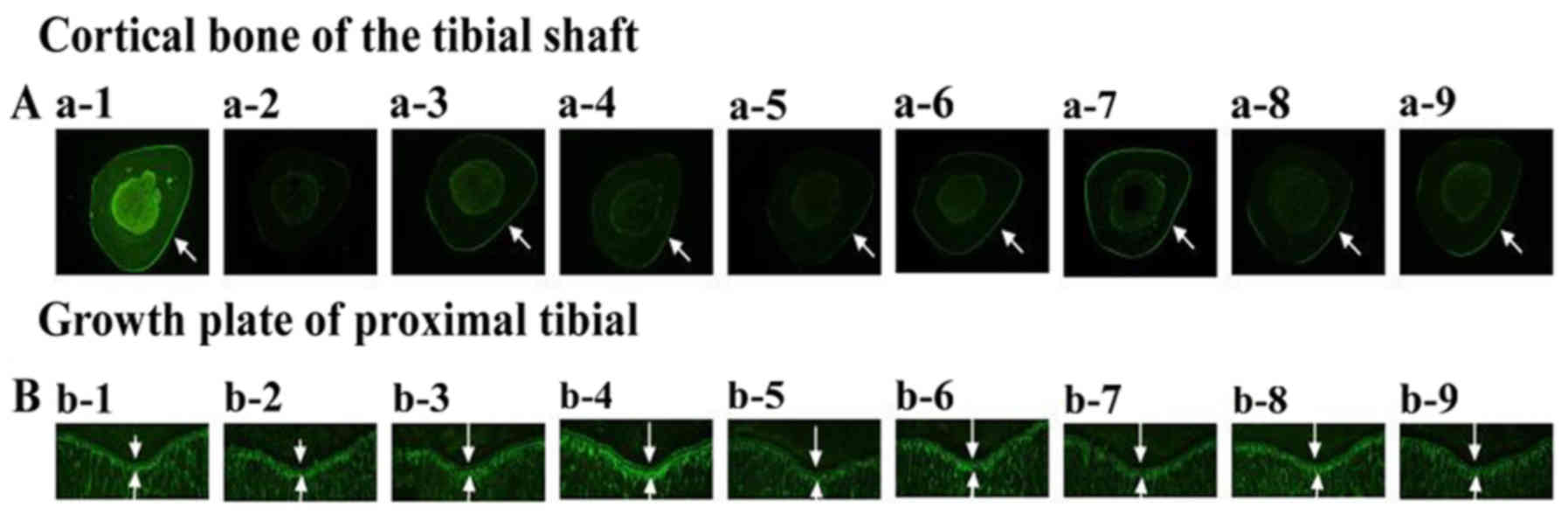
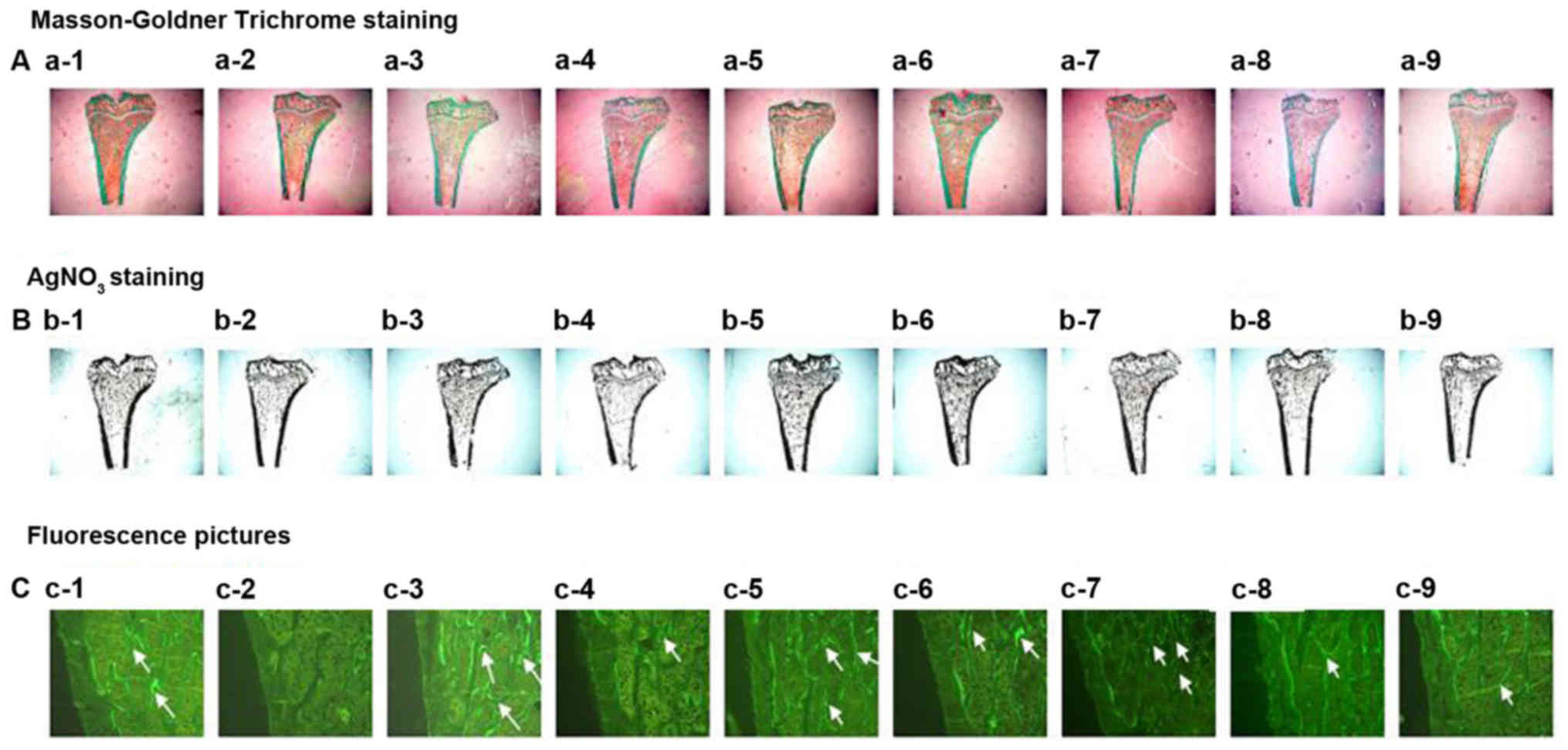 | Figure 3.Effects of vehicle (CON), prednisone
(Pre), calcitriol (CAL), and various PMR (30, F) dose treatments on
the proximal tibial metaphysis (PTM) bone structure and mineral
bone formation. Arrows point to the tetracycline and calcein
labeling. Quantitative measurements of histomorphometric parameters
of PTM are showed in Tables II
and III. (A) Goldner's Trichrome
stain, (B) AgNO3 stain,(C) fluorescence images of
undecalcified sections a-1, b-1, c-1:CON; a-2, b-2, c-2:Pre (6
mg.−1.d−1); a-3, b-3, c-3:Pre+CAL (0.045
µg.kg−1.d−1); a-4, b-4, c-4:Pre+PMR30 (H)
(400 mg.kg−1.d−1); a-5, b-5, c-5:Pre+PMR30
(M) (200 mg.kg−1.d−1); a-6, b-6,
c-6:Pre+PMR30 (L) (100 mg.kg−1.d−1); a-7,
b-7, c-7:Pre+PMRF (H) (400 mg.kg−1.d−1); a-8,
b-8.c-8:Pre+PMRF (M) (200 mg.kg−1.d−1); a-9,
b-9, c-9: Pre+PMRF (L) (100 mg.kg−1.d−1). |
 | Table II.Histomorphometric static parameters
of proximal tibial of rats (x− ± s). |
Table II.
Histomorphometric static parameters
of proximal tibial of rats (x− ± s).
| Group | %Tb.Ar (%) | Tb.Th (µm) | Tb.N (no./mm) | Tb.Sp (µm) |
|---|
| CON |
29.20±5.19 |
52.86±7.60 |
5.58±0.90 |
131.90±32.59 |
| Pre |
18.81±6.60b |
38.21±11.44 |
4.91±0.81 |
172.31±49.13 |
| CAL |
25.82±5.94c |
50.64±6.07 |
5.10±1.02 |
153.21±45.76 |
| PMR30(H) |
20.52±6.25 |
88.84±116.84c,e |
4.33±2.15 |
409.06±597.48 |
| PMR30(M) |
23.25±61.7 |
46.80±8.21 |
4.93±0.82 |
162.48±49.65 |
| PMR30(L) |
22.66±4.64 |
48.76±5.29 |
4.64±0.84 |
173.24±43.53 |
| PMRF(H) |
26.76±7.35c |
52.84±4.65 |
5.01±0.98 |
153.45±43.48 |
| PMRF(M) |
23.62±5.63 |
47.09±9.69 |
5.01±0.53 |
154.57±26.18 |
| PMRF(L) |
19.83±3.89f,g |
47.03±3.75 |
4.23±0.86 |
197.42±46.87 |
 | Table IV.Oc and Ob parameters of proximal
tibial of rats (x− ± s). |
Table IV.
Oc and Ob parameters of proximal
tibial of rats (x− ± s).
| Group | Oc.N (no./mm) | %Oc.S. Pm (%) | %Ob.S. Pm (%) |
|---|
| CON |
0.10±0.06 |
0.31±0.14 |
0.20±0.12 |
| Pre |
0.20±0.09a |
0.87±0.54b |
0.09±0.06 |
| CAL |
0.12±0.06 |
0.19±0.11d |
0.08±0.04 |
| PMR30(H) |
0.19±0.22 |
0.39±0.49c |
0.16±0.28e |
| PMR30(M) |
0.08±0.04d,g |
0.19±0.11d |
0.08±0.04 |
| PMR30(L) |
0.10±0.02c,g |
0.18±0.05c |
0.10±0.06 |
| PMRF(H) |
0.08±0.03d |
0.12±0.06c |
0.23±0.14 |
| PMRF(M) |
0.06±0.05d,h |
0.13±0.14 |
0.13±0.06 |
| PMRF(L) |
0.07±0.03d |
0.12±0.06c |
0.14±0.10 |
Effects of PM on the parameters of
two-dimensional histomorphometry of the tibial shaft
The changes of the two-dimensional histomorphometry
of the tibial shaft: Compared with the control group, Ct.Ar, %Ct.Ar
and P-MAR were decreased (P<0.05) while %Ma.Ar and E-BFR/BS were
increased (P<0.05) in prednisone group. Compared with the
prednisone group, %Ct.Ar and %P-L.Pm were showed a trend toward
increased (P>0.05) while %Ma.Ar and E-BFR/BS were showed a trend
toward decreased (P>0.05) in CAL, PMR30 and PMRF groups
(Fig. 4 and Tables V and VI). These results indicated that PMR30
and PMRF groups can improve the thickness of cortical bone in GIO
rats.
 | Table V.Histomorphometric static parameters
of tibial shaft in rats (x− ± s). |
Table V.
Histomorphometric static parameters
of tibial shaft in rats (x− ± s).
| Group | Ct.Ar
(mm2) | %Ct.Ar (%) | %Ma.Ar (%) |
|---|
| CON |
4.59±0.34 |
78.22±1.77 |
21.77±1.77 |
| Pre |
4.05±0.50a |
75.32±2.72a |
24.68±2.72a |
| CAL |
4.07±0.62 |
75.37±5.58 |
24.63±5.58 |
| PMR30(H) |
4.17±0.39 |
77.02±3.56 |
22.98±3.56 |
| PMR30(M) |
4.21±0.44 |
76.43±4.76 |
23.57±4.76 |
| PMR30(L) |
4.28±0.39 |
75.61±5.99 |
24.39±5.99 |
| PMRF(H) |
4.40±0.32 |
76.38±2.18 |
23.62±2.18 |
| PMRF(M) |
4.49±0.33 |
75.46±2.46 |
24.54±2.46 |
| PMRF(L) |
4.29±0.47 |
76.85±2.67 |
23.15±2.67 |
 | Table VI.Histomorphometric dynamic parameters
of tibial shaft in rats (x− ± s). |
Table VI.
Histomorphometric dynamic parameters
of tibial shaft in rats (x− ± s).
| Group | %P-L. Pm (%) | P-MAR (µm/d) | P-BFR/BS
(%/year) | %E-L. Pm (%) | E-MAR (µm/d) | E-BFR/BS
(%/year) |
|---|
| CON |
27.40±6.73 |
0.89±0.23 |
24.66±10.51 |
14.62±9.11 |
0.41±0.19 |
5.38±3.98 |
| Pre |
29.18±7.72 |
0.65±0.05b |
19.27±5.62 |
20.35±8.95 |
0.59±0.23 |
11.70±6.63a |
| CAL |
34.49±6.81 |
0.91±0.13d |
28.72±8.48c |
16.35±6.83 |
0.52±0.09 |
7.96±3.30 |
| PMR30(H) |
30.67±13.05 |
0.78±0.31 |
21.21±7.80 |
19.94±4.77 |
0.72±0.25e |
11.38±5.87 |
| PMR30(M) |
33.97±7.33 |
0.47±0.27 |
16.81±13.47e |
19.08±5.00 |
0.31±0.24c,e |
6.68±6.04 |
| PMR30(L) |
26.64±11.30 |
0.83±0.23 |
19.25±6.68e |
14.85±4.91 |
0.45±0.19 |
6.83±5.04 |
| PMRF(H) |
38.56±6.11c |
0.65±0.17 |
23.33±7.90 |
18.90±5.22 |
0.42±0.22 |
8.38±5.32 |
| PMRF(M) |
29.02±10.05 |
0.73±0.11 |
21.35±8.49 |
17.37±4.59 |
0.40±0.12 |
6.42±3.02 |
| PMRF(L) |
27.16±4.26 |
0.49±0.16 |
14.52±7.39f |
18.26±8.16 |
0.51±0.16 |
8.58±3.34 |
Effects of PM on the growing
epiphyseal plate of proximal tibial
The results of growth plate, compared with the
control group, the growth plate was become thinner in prednisone
group. Compared with the prednisone group, the growth plate was
become more thicken in CAL, PMRF (M, L) and PMR30 (M, L) groups
(Fig. 4). It is indicated that
PM may be stimulate growth hormone secretion of rat.
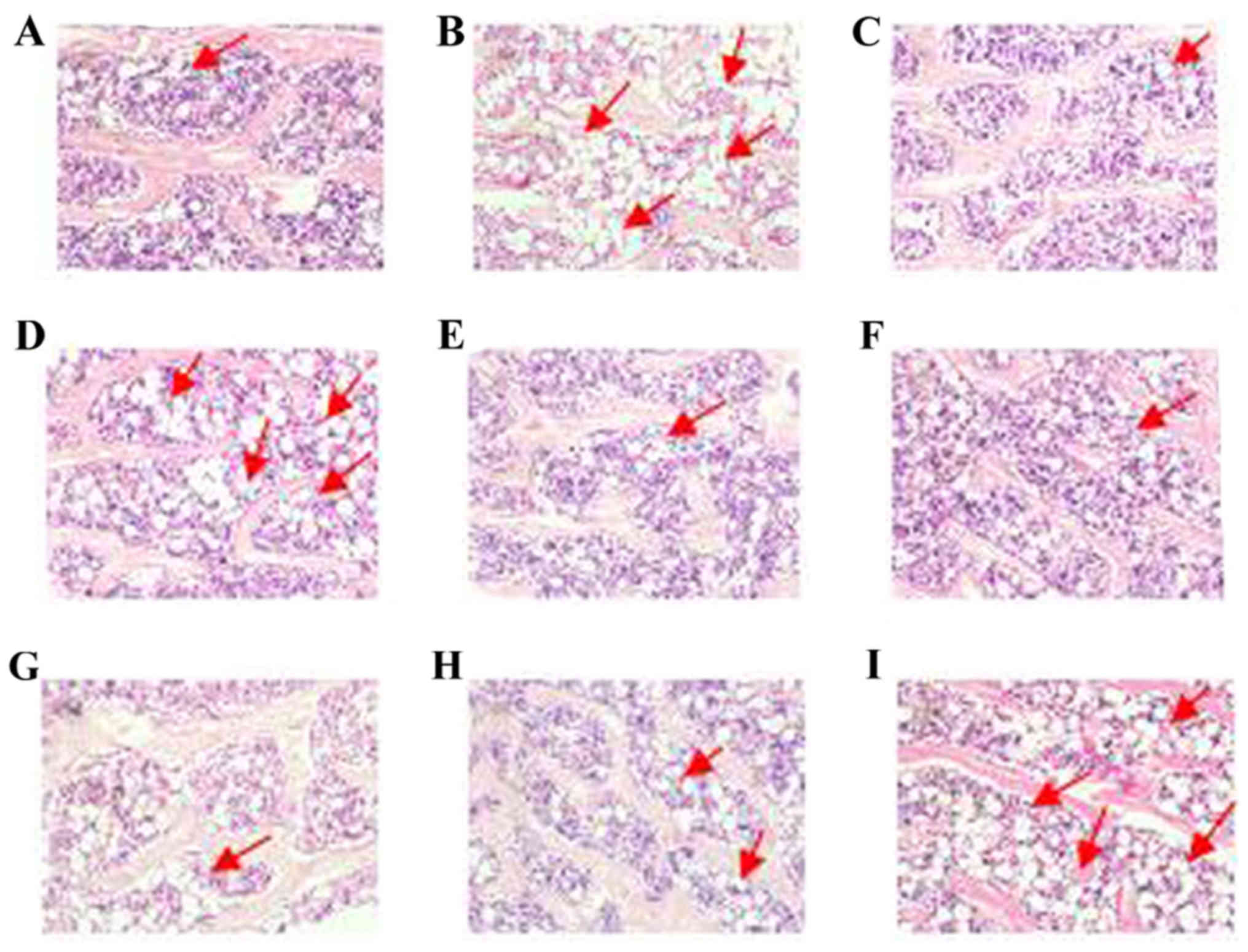
Effects of PM on marrow fat tissue
deposistion of the proximal tibial metaphysis
Compared with the control group, the number of
adipocytes was become more and dense in prednisone group. Compared
with the prednisone group, the number of adipocytes were became
less in CAL, PMR30 (M, L) and PMRF (H, M) groups (Fig. 5). These results indicated that
PMR30 (M, L) and PMRF (H, M) groups can decrease the number of
adipocytes in GIO rats. It is prompted that PM can inhibit
the differentiation of bone marrow stromal cells into
adipocytes.
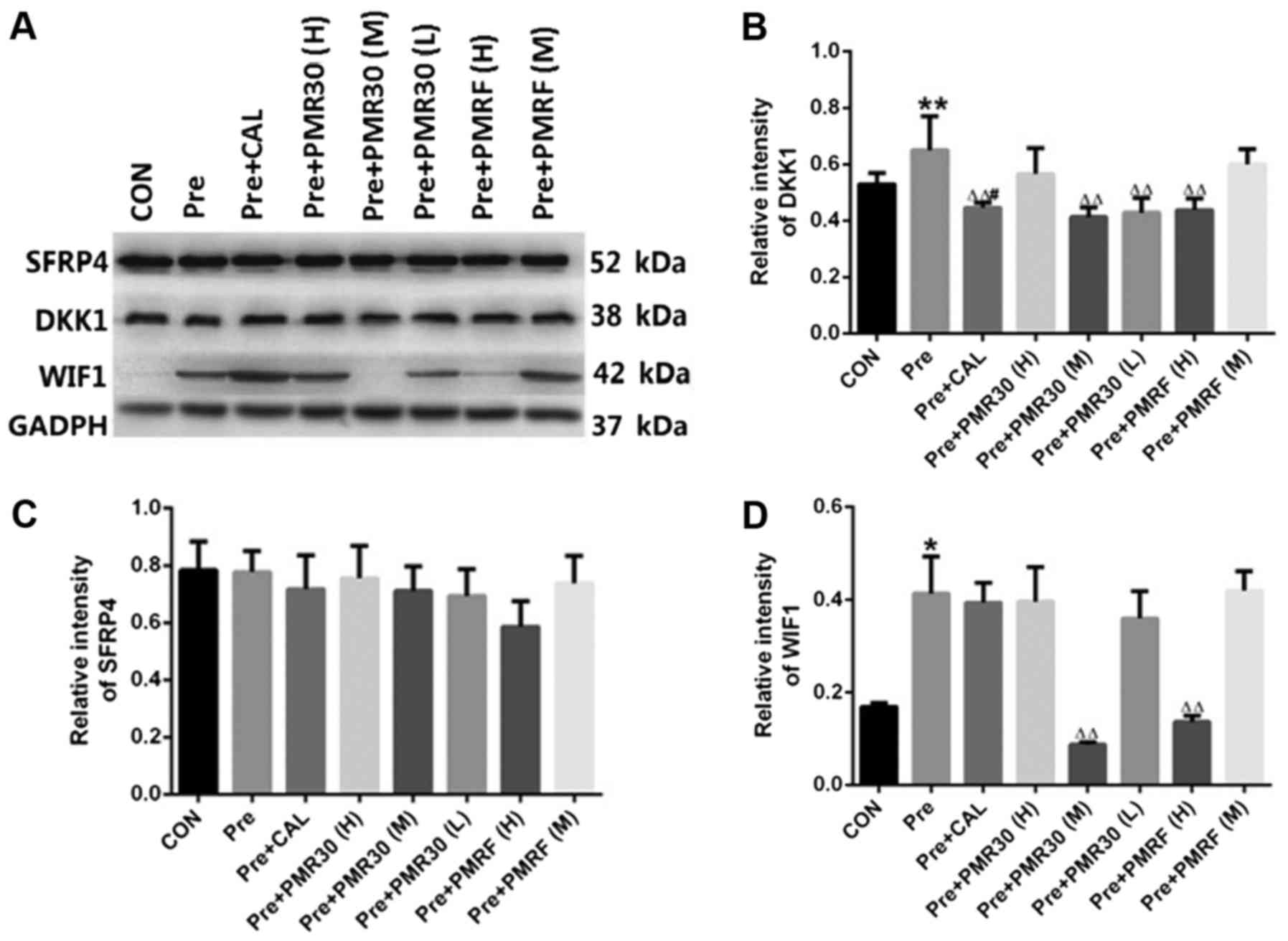
Effects of PM on the Wnt signaling
pathway
Compared with control group, the expression of DKK1
and WIF1 were increased in prednisone group (P<0.05). Compared
with prednisone group, the expression of DKK1 were decreased
(P<0.05) in CAL, PMR30 (M, L) and PMRF (H) groups. Compared with
prednisone group, the expression of WIF1 was decreased (P<0.05)
significantly in PMR30 (M) and PMRF (F) groups (Fig. 6). These results indicated that
PMR30 (M) and PMRF (F) groups can increase the expression of DKK1
and WIF1 aim to regulate the Wnt signaling pathway in GIO rats.
Discussion
It is widely known that GC treatment induces
osteoporosis. In our previous study, GC exerts a series of
deleterious actions on bone tissue in both male and female rats.
Furthermore, previous evidence demonstrated that extracts of PM
exhibits a pretective effect on ovariectomized rats (20). In the present study, extracts of PM
(PMR30 and PMRF) were exhibits a pretective effect on bone loss in
GIO rats, owing to restoration of bone micro-architecture and the
serum levels of biomarkers related to bone formation. In addition,
Polygonum Multiflorm alleviates GIO may be through
regulation of Wnt signaling pathway to protect the bone.
Physiologically, the secretion of endogenous GC was
regulated by hypothalamus-hypophysis-adrenal cortex system
(30), thus inhibited the
secretion of growth hormone (GH) (34). However, excessive exposure to
excessive exogenous clinically GC could induce rapidly bone loss
resulting to the increase of fracture risk. In the present study,
we found that long-term excessive administration of prednisone
caused a decrease of bone formation parameters (MAR and BFR/TV) in
the trabecular bone area and a decrease of growth of longitudinal
bone, and an increase of bone resorption (Oc.S/BS) (Figs. 1 and 4). It has generally been thought that GIO
results from impaired bone formation as well as exaggerated bone
resorption. Possible pathological mechanisms of GIO are listed in
the following: (1) impairing
osteoblast or osteoclast function directly, and (2) secondary hyperparathyroidism, due to
the increased renal excretion and decreased intestinal absorption
of calcium (35).
In this study, PMR30 (M, L) and PMRF (H) groups were
found to be effective at attenuating GIO in vivo, as
evidenced through its restoration of %Tb.Ar, Tb.Th, %L.Pm, BFR/BS,
and BFR/TV (Tables II–IV and Fig.
3). This indicates that the compressive strength and mechanical
properties of the bone tissue and the activity of bone formation
were enhanced. Furthermore, it would promote bone formation and
increase bone turnover rate. This is benefit to replace bone matrix
between old and new, and self-repair the bone micro-structure, so
that fighting brittle fracture by long-term use of prednisone.
PMR30 and PMRF can increase the parameters of the outer membrane
and decrease the inner membrane of tibial shaft in different degree
(Tables V–VII and Fig. 4). This shows that the PM can
promote the periosteal bone formation of the outer membrane and
inhibit endosteal bone resorption of the inner membrane, decrease
bone turnover, and increase the bone mass and thickness of cortical
bone, against long-term use of GC-induced %Ma.Ar increase, then
make cortical become thinner, thereby increasing the quality and
bone biomechanical properties to avoid fracture. Of these results
we also found the ratio of bone conversion are different proximal
tibia and tibia shaft. The conversion rate of proximal tibia was
faster than tibia shaft, this may related to blood vessel is rich
in proximal tibial (37).
Interestingly, we found that longitudinal growth
plate of proximal tibial were increased in PMR30 (H, M, L) and PMRF
(H) groups (Fig. 4), compared with
prednisone group. This may be related to PM inducing the growth
hormone release. Lo has been reported that an emodin derivatives
isolated from PM, this emodin derivative, tentatively named
emoghrelin, was demonstrated to stimulate growth hormone secretion
of rat primary anterior pituitary cells, presumably via the same
molecular mechanism of GHSR activation (38). In the present study, the content of
combined anthraquinone (CAQ) in the sample of PMR30 was over than
PMRF, that the longitudinal growth plate of proximal tibial were
increased in PMR30 (H, M, L) over than PMRF (H) groups. The
deficiency is not to determine the longitudinal growth rate
(LGR).
The disruption of the bone formation-resorption
balance plays a key role in osteoporosis (39). Serum BAP is known as a marker of
bone formation, and TRACP-5b is known as a marker of bone
resorption (35). In the present
study, we found that PM recovered the serum BAP and PICP levels and
significantly reversed the prednisone-induced decrease. We have
also found that the serum of DKK1 and TRACP-5b level were increased
in GC group which were consistent with the histomophometric data
(Tables I–VI). TRACP-5b mainly exists in bone
tissue, which is mainly derived from osteoclasts, and presents the
activity of osteoclasts and its function of bone resorption
(36). The level of TRACP-5b was
increased in prednisone group, suggesting that long-term use of GCs
increased osteoclast activity. Our study data indicated that PM can
promote bone formation and inhibit bone resorption.
Futhermore, it has been reported that high level of
DKK1 which suppresses the Wnt signal of bone formation in
osteoblasts, resulted from activation of transcription through GRE
in the DKK1 gene promoter. In the present study, DKK1 was increased
expression in prednisone group (Fig.
6). The Wnt/β-catenin signaling pathway was also found to be
re-activated by PM, which may be related to the bone-protective
effects of PM. These results indicate that PM exerts protective
effects against GIO. In previous studies, GC-treated animals also
exhibited decreased BMD and bone mineral content (BMC) (27). In addition, in the previous
experiments, we verified that TSG, as a major constituent in
PM Thunb, showed anti-osteoporosis activity in vitro
and in vivo (22). These
data suggest that the bone-protective effects of PM are mediated
through the regulation of Wnt signaling pathway. Wnt binds with
specific cell-surface receptors Frizzled and LRP5/6, thereby
leading to binding with Axin, which in turn mediates the
proteolysis of β-catenin. DKK1 is also known to inhibit Wnt
signaling by binding to LRP5/6 (41). DKK1 are receptor inhibitors which
play a key role in the regulation of the Wnt signaling pathway in
bone formation (42). As expected,
treatment with PM recovered the activity of this signaling pathway.
These results suggest that the Wnt/β-catenin signaling pathway is
involved in the bone-protective effects of PM against
prednisone-induced osteoporosis.
In conclusion, we demonstrated that PM can attenuate
GIO and the mechanism of the preventive effect on GIO may be linked
to direct up-regulation of canonical Wnt/β-catenin pathway.
Acknowledgements
This research was supported by grants from National
Natural Science Foundation of China (no. 81102450 and 81673814), by
the Open Fund Project of Key Laboratory of Guangdong Province (no.
4CX16010G), by the Characteristic Innovation Project (Natural
Science) of Education Department of Guangdong Province (no.
2014KTSCX084), by the Science and Technology Plan of Guangdong
Province (no. 2016ZC0178 and 2016A020215148), and by the Open
Foundation of Guangdong Key Laboratory for Research and
Development of Natural Drugs (TRYW201603).
References
|
1
|
McLaughlin F, Mackintosh J, Hayes BP,
McLaren A, Uings IJ, Salmon P, Humphreys J, Meldrum E and Farrow
SN: Glucocorticoid-induced osteopenia in the mouse as assessed by
histomorphometry, microcomputed tomography, and biochemical
markers. Bone. 30:924–930. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogoshi T, Hagino H, Fukata S, Tanishima S,
Okano T and Teshima R: Influence of glucocorticoid on bone in 3-,
6- and 12-month-old rats as determined by bone mass and
histomorphometry. Mod Rheumatol. 18:552–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buttgereit F, Burmester GR and Lipworth
BJ: Optimised glucocorticoid therapy: The sharpening of an old
spear. Lancet. 365:801–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
den Uyl D, Bultink IE and Lems WF:
Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol
Rep. 13:233–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schäcke H, Döcke WD and Asadullah K:
Mechanisms involved in the side effects of glucocorticoids.
Pharmacol Ther. 96:23–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Humphrey EL, Williams JHH, Davie MW and
Marshall MJ: Effects of dissociated glucocorticoids on OPG and
RANKL in osteoblastic cells. Bone. 38:652–661. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saag KG: Low-dose corticosteroid therapy
in rheumatoid arthritis: Balancing the evidence. Amat J Med.
103:31S–39S. 1997. View Article : Google Scholar
|
|
8
|
Adachi JD, Bensen WG and Cividino A:
Corticosteroid induced osteoporosis. J Am Med Womens Assoc (1972).
53(25–30): 401998.
|
|
9
|
Chen ZG, Xue JQ, She T, Mu SA and Fu Q:
Curcumin alleviates glucocorticoid-induced osteoporosis through the
regulation of the Wnt signaling pathway. Int J Mol Med. 37:329–338.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling S and Xu JW: Biological activities of
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside in antiaging and
antiaging-related disease treatments. Oxid Med Cell Longev.
2016:49732392016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao W, Gu C, Shao H, Meng G, Wang H, Jing
X and Zhang W: Tetrahydroxystilbene glucoside improves
TNF-α-induced endothelial dysfunction: Involvement of TGFβ/Smad
pathway and inhibition of vimentin expression. Am J Chin Med.
43:183–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan YC, Cheng FC and Wang MF: Beneficial
effects of different Polygonum multiflorum Thunb. Extracts on
memory and hippocampus morphology. J Nutr Sci Vitaminol (Tokyo).
48:491–497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan YC, Wang MF and Chang HC: Polygonum
multiflorum extracts improve cognitive performance in senescence
accelerated mice. Am J Chin Med. 31:171–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu QL, Xiao JH, Ma R, Ban Y and Wang JL:
Effect of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside on
lipoprotein oxidation and proliferation of coronary arterial smooth
cells. J Asian Nat Prod Res. 9:689–697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Um MY, Choi WH, Aan JY, Kim SR and Ha TY:
Protective effect of Polygonum multiflorum Thunb on amyloid
beta-peptide 25–35 induced cognitive deficits in mice. J
Ethnopharmacol. 104:144–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang PY, Almofti MR, Lu L, Kang H, Zhang
J, Li TJ, Rui YC, Sun LN and Chen WS: Reduction of atherosclerosis
in cholesterol-fed rabbits and decrease of expressions of
intracellular adhesion molecule-1 and vascular endothelial growth
factor in foam cells by a watersoluble fraction of Polygonum
multiflorum. J Pharmacol. 99:294–300. 2005.
|
|
17
|
Huang LF, Wu T, Xie H and Liao JM: The
prevention and treatment on bone loss in ovariectomized rats of
Polygonum decoction. Chinese J Gerontology. 25:709–710. 2005.(In
Chinese).
|
|
18
|
Liu YY, Cui L, Wu T and Yao WM: Effects of
emodin on the proliferation and differentiation of osteoblast
isolated from neonatal rat calvarium in vitro. Chin Pharmacol Bull.
21:235–240. 2005.(In Chinese).
|
|
19
|
Liu YY, Cui L, Wu T and Yao WM: Effects of
emodin on adipogenesis of marrow stromal cells in vitro. Chin
Pharmacol Bull. 21:842–846. 2005.(In Chinese).
|
|
20
|
Liu YY, Yao WM, Ai CM and Xu BL: Effects
of emodin on differentiation of bone marrow stroma cell into
osteoblast in rats in vitro. Chin J Clin Pharmacol Ther.
10:191–195. 2005.(In Chinese).
|
|
21
|
Liu YY, Yao WM, Ai CM and Xu BL: Effects
of emodin on osteoblast in vitro. Chin Pharmacol Bull.
21:1473–1477. 2005.(In Chinese).
|
|
22
|
Zheng YY: Seperation and purification of
the Polygonum multiflorum extract and its effect on
anti-osteoporosis. Guangdong Med Univ. 2014.(In Chinese).
|
|
23
|
Ohnaka K, Tanabe M, Kawate H, Nawata H and
Takayanagi R: Glucocorticoid suppresses the canonical Wnt signal in
cultured human osteoblasts. Biochem Biophys Res Commun.
329:177–181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signaling pathway. J Cell. 116:2627–2634. 2003.
|
|
25
|
Ohnaka K, Taniguchi H, Kawate H, Nawata H
and Takayanagi R: Glucocorticoid enhances the expression of
dickkopf-1 in human osteoblasts: Novel mechanism of
glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun.
318:259–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song L, Liu M, Ono N, Bringhurst FR,
Kronenberg HM and Guo J: Loss of wnt/β-catenin signaling causes
cell fate shift of preosteoblasts from osteoblasts to adipocytes. J
Bone Miner Res. 27:2344–2358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou MR, Li J, Wu JK, Zeng XB, Chen JF,
Cui L and Liu YY: The preventive effect of Polygonum multiflorum on
the changes of micro-structural and biomechanical by prednisone.
Chin Pharmacol Bull. 31:1273–1279. 2015.(In Chinese).
|
|
28
|
Zhou MR, Li J, Wu JK, Yang YJ, Zeng XB, Lv
XH, Cui L, Yao WM and Liu YY: Preventive effects of Polygonum
multiflorum on glucocorticoid induced osteoporosis in rats. Exp
Ther Med. 14:2445–2460. 2017.PubMed/NCBI
|
|
29
|
Liu YZ, Cui Y, Chen Y, Gao X, Su YJ and
Cui L: Effects of dexamethasone, celecoxib, and methotrexate on the
histology and metabolism of bone tissue in healthy sprague Dawley
rats. Clin Interv Aging. 10:1245–1253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin S, Huang J, Zheng L, Liu Y, Liu G, Li
N, Wang K, Zou L, Wu T, Qin L, et al: Glucocorticoid-Induced
Osteoporosis in Growing Rats. Calcif Tissue Int. 95:362–373. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Su Y, Wang D, Chen Y, Liu Y, Luo
S, Wu T and Cui L: Tanshinol rescues the impaired bone formation
elicited by glucocorticoid involved in KLF15 pathway. Oxid Med Cell
Longev. 2016:10927462016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cui L, Li T, Liu Y, Zhou L, Li P, Xu B,
Huang L, Chen Y, Liu Y, Tian X, et al: Salvianolic acid B prevents
bone loss in prednisone-treated rats through stimulation of
osteogenesis and bone marrow angiogenesis. PLoS One. 7:e346472012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Q, Xiong X, Zhang X, Lu J, Zhang X,
Chen W, Wu T, Cui L, Liu Y and Xu B: Secondary osteoporosis in
collagen-induced arthritis rats. J Bone Miner Metab. 34:500–516.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong F and Ren J: Insulin-like growth
factors (IGFs) and IGF-binding proteins in nephrotic syndrome
children on glucocorticoid. Pharmacol Res. 48:319–323. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sasaki N, Kusano E, Takahashi H, Ando Y,
Yano K, Tsuda E and Asano Y: Vitamin K2 inhibits
glucocorticoid-induced bone loss partly by preventing the reduction
of osteoprotegerin (OPG). J Bone Miner Metab. 23:41–47. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang SJ, Meikle MC, MacLaine JK, Wong RW
and Rabie BM: Altered serum levels of the osteoclast-specific TRACP
5b isoform in Chinese children undergoing orthodontic treatment.
Eur J Orthod. 35:169–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cui L, Liu YY, Wu T, Ai CM and Chen HQ:
Osteogenic effects of D+beta-3, 4-dihydroxyphenyl lactic acid
(salvianic acid A, SAA) on osteoblasts and bone marrow stromal
cells of intact and prednisone-treated rats. Acta Pharmacol Sin.
30:321–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lo YH, Chen YJ, Chung TY, Lin NH, Chen WY,
Chen CY, Lee MR, Chou CC and Tzen JT: Emoghrelin, a unique emodin
derivative in Heshouwu, stimulates growth hormone secretion via
activation of the ghrelin receptor. J Ethnopharmacol. 159:1–8.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim HJ, Zhao H, Kitaura H, Bhattacharyya
S, Brewer JA, Muglia LJ, Ross FP and Teitelbaum SL: Glucocorticoids
suppress bone formation via the osteoclast. J Clin Invest.
116:2152–2160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ehrlich PJ and Lanyon LE: Mechanical
strain and bone cell function: A review. Osteoporos Int.
13:688–700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bafico A, Liu G, Yaniv A, Gazit A and
Aaronson SA: Novel mechanism of Wnt signalling inhibition mediated
by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol.
3:683–686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rossini M, Gatti D and Adami S:
Involvement of WNT/β-catenin signaling in the treatment of
osteoporosis. Calcif Tissue Int. 93:121–132. 2013. View Article : Google Scholar : PubMed/NCBI
|