Introduction
Central serous chorioretinopathy (CSCR) is
characterized by serous detachment of posterior pole retinal
sensory layer from the pigment epithelium, which results in the
leakage of retinal pigment epithelium, with or without pigment
epithelium detachment (1). Fundus
angiography is an important method for the diagnosis of CSCR.
Previous studies using indocyanine green angiography (ICGA) showed
that CSCR is mainly associated with choroid perfusion
abnormalities, while the retinal pigment epithelium (RPE) lesions
and accumulation of subretinal fluid are secondary changes
(2), suggesting that CSCR may be
caused by choroid microvascular lesions. It is well known that CSCR
is a self-limiting disease, and the majority of patients generally
self heal within 3–6 months, but chronic and recurrent occurrence
can eventually lead to permanent loss of vision (3). However, the exact pathogenesis of
CSCR remains unclear. As a result, it is important to discover a
method to determine whether patients with early stage can self
heal, and provide guidelines for early intervention to avoid
permanent loss of vision caused by CSCR for patients who can not
self heal.
Optical coherence tomography (OCT) plays a very
important role in the diagnosis of CSCR. The fundus image OCT can
evaluate the subretinal fluid volume and location of RPE lesion,
and provide information for the diagnosis and treatment of CSCR.
However, it does not provide clear clues for choroidal and retinal
vasculature (4). In recent years,
the optical coherence tomography angiography (OCTA) has
successfully solved this problem. It is a new noninvasive imaging
technique compared with the traditional OCT. The most prominent
advantage of OCTA system is the application of the SSADA algorithm
(5) and En-face scanning. The
SSADA algorithm can reduce the artifacts and noise to a certain
extent, and improve the signal to noise ratio. The application of
En-face can obtain three-dimensional image data, displaying all
aspects of information, such as the retinal nerve fiber layer, RPE,
and choroid. In addition, OCTA is not affected over time, since it
is a non-invasive method without injection of contrast agent
(5). OCTA provides a detailed view
of the retinal blood vessels, and can accurately describe retinal
microvascular abnormalities and vascular occlusion. It can also
help to quantify vascular damage.
Combined with previous studies, it has been shown
that the pathological changes of CSCR are mainly choroidal
microvascular abnormalities, and whether there is a change in the
microvessels in the macular region remains unexplored. In this
study, OCTA was used to quantify the retinal microvascular density,
and to explore the changes of retinal microvascular network in
patients with CSCR.
Materials and methods
Research subjects
To ensure the maximum comparability between the
experimental group and the control group, we conducted a
prospective randomized controlled study. To minimize the effects of
non-experimental factors, such as age and course of disease, we
recruited 15 CSCR patients (all right eye) with symptoms duration
less than 1 months according to the principle of minimum
distribution imbalance index from the Affiliated Eye Hospital and
First Affiliated Hospital of Nanchang University from 2016 to 2017,
and 15 age and gender matched healthy participants (all right eye)
as control group. The subjects completed a comprehensive
examination of the ocular surface and OCTA. The clinical
manifestations of CSCR included decreased visual acuity, blurred
vision, visual distortion, light shadow occlusion, dim vision, dark
landscape and so on.
Recruitment criteria
For all patients, diagnosis was based on the
best-corrected visual acuity (BCVA), routine eye examination, and
fundus fluorescein angiography (FFA) examination (6). In accordance with CSCR diagnostic
criteria, clinical presentation of the disease included: i)
clinical manifestations of visual impairment, floating shadows or
central scotoma (blind spot), visual darkening, discoloration,
deformation, narrowing; ii) FFA examination showing macular edema
or discoid anti-halo, with or without yellow/white punctiform
exudation or old exudative spots; iii) FFA examination showing
either the typical or atypical punctate pigment epithelial leakage
on the posterior pole and smoke-like or ink-like stains on the
macular area.
Exclusion criteria
The exclusion criteria were: i) eyelid disease and
other ocular surface diseases and intraocular diseases that were
not cured; ii) eye surgery within six months; iii) rheumatoid
arthritis, systemic lupus erythematosus, Sjogren syndrome and other
systemic autoimmune disease; iv) long period administration of anti
hypertension and anti depressants drugs; v) pregnant or lactating
patients.
Ethical considerations
The present study was conducted in accordance with
the principles of the declaration of Helsinki, according to the
requirements of the two hospitals' ethics committee. A detailed
explanation of the method and content of the study was given to
each patient, and the consent was signed by the patient.
OCTA
OCTA image was taken using the RTVue Avanti XR
system (Optovue, Fremont, CA, USA). This device has recently been
introduced into clinical practice for simultaneous visualization of
retinal cross sections and blood vessels. The instrument scanned at
70000 A-per second, including a super light emitting diode, with a
central wavelength of 840 nm and a bandwidth of 45 nm (6). It provided an axial resolution of 5
µm within the tissue, and the retinal plane spot diameter was 22 µm
(horizontal resolution). A series of B scans were obtained over a
6×6 mm area focused on the concave obtained choroidal OCTA images
(Fig. 1 A-C). Each B scan
contained 216 A-scans (along the X axis) and five consecutive B
scans were captured at each of the 216 locations (along the Y
axis). With a speed of 270 frames per second, a total of 1080 B
scans (216 y-positions × 5 positions) were obtained. 6×6 mm OCTA
image acquisition was created by a set of four volume scans,
including a total of two horizontal and a set of two vertical
gratings (a total of 933 120 A-scans). Kraus et al (7) has shown that it is based on the use
of an algorithm for orthogonal scan alignment to correct motion
artifacts.
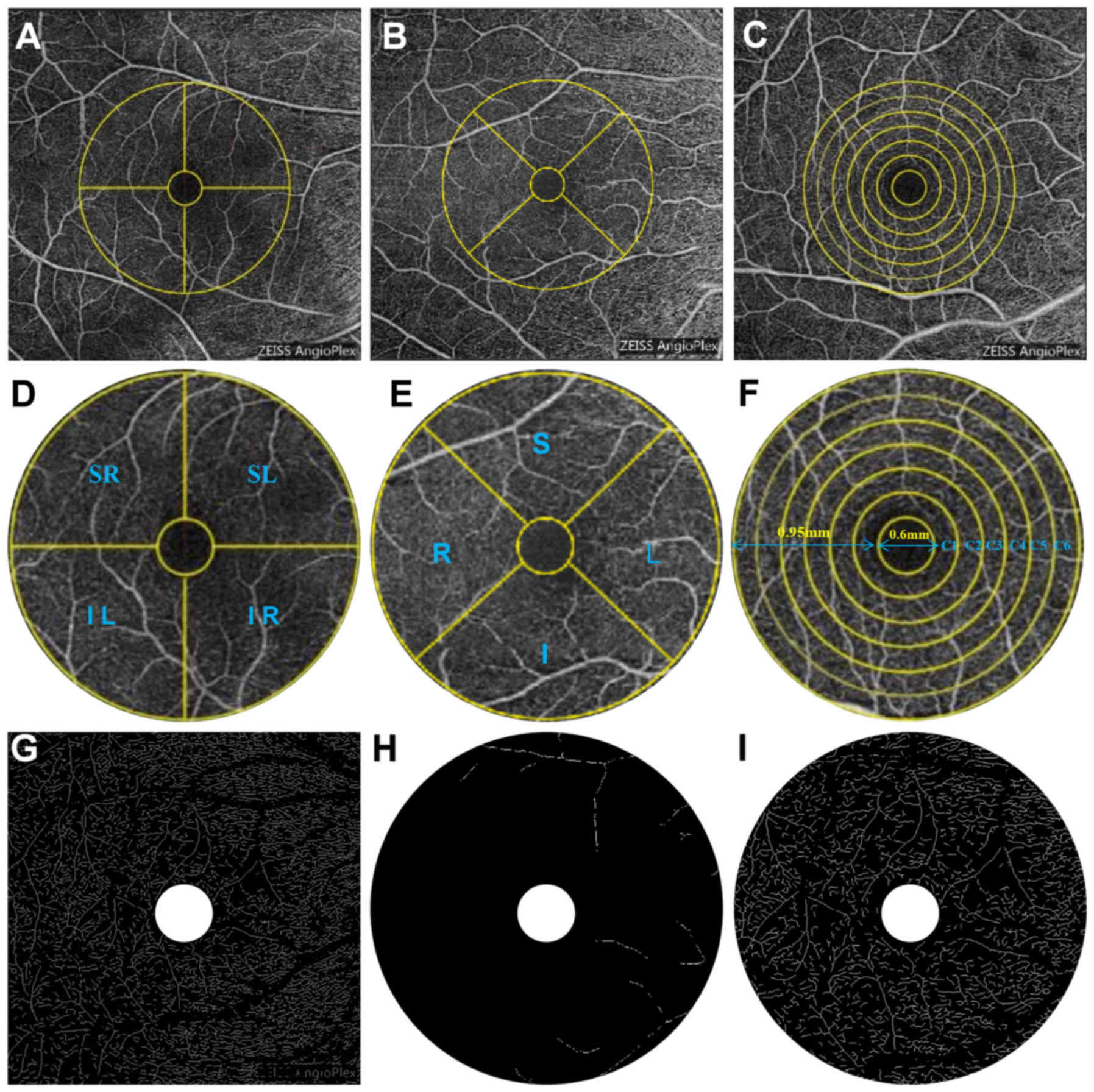 | Figure 1.OCTA scans of the 6×6 mm shallow image
of the macular region of the retina. (A) Hemispheric partition
method: The image is divided into 4 quadrants of vertical and
horizontal regions, followed by R, S, L and I. (B, E) Early
Treatment Diabetic Retinopathy Study ring area is divided into 4
quadrants by the diagonal of the two quadrants. (C) The algorithm
searches from the center to the periphery of the macular 6×6 mm
image with intensity gradient detection software to identify the
foveal avascular zone center (FAZ). (D, E) Customized segmentation
image processing program divided images into (D) SR, SL, IL and IR
and (E) S, L, and R quadrants, and included a series of actions
such as inverting, balancing, and removal of the background noise
and non-vessel structure to create a binary image, and retained the
individual microvascular skeletonization image with diameter larger
than 25 mm and large vessels remained after the removal of small
vessels. (F) After removal of the avascular zone (0.6 mm diameter
of the fovea), a circular region of 0.6 to 2.5 mm in diameter is
defined as the ring with bandwidth of 0.95 mm. The annular region
is divided into 6 thin rings with a bandwidth of 0.16 mm. (G) The
skeletonized image of superficial total microvascular: 6×6 mm
macular area. (H) The skeletonized image of superficial macrovessel
ring: 3 mm macular ring area. (I) The skeletonized image of
superficial microvessel ring: 3 mm macular ring area. I, inferior;
IL, inferior left; IR, inferior right; L, left; R, right; S,
superior; SC, superficial central annuli; SL, superior left; SMAR,
superficial macrovascular ring; SMIR, superficial microvessel ring;
SR, superior right; STMI, superficial total microvascular. |
The system used RTVue XR to create the OCTA images
(8). Simply put, it is an
algorithm for computing the correlation of the amplitude of a
series of spots from the same pixel at the same location. The
correlation in the speckle amplitude can be related to the cardiac
cycle from the motion of the blood vessels or the pulsating motion
of the whole retina. In contrast to the OCT beam moving toward the
vertical moving blood cells, the eye pulsations occur along the
axial direction. The resolution of the asymmetric OCT system, in
which the axial resolution is typically 3 to 5 times higher than
the lateral resolution, results in higher sensitivity to tissue
motion than the axial resolution. In order to improve the
signal-to-noise ratio of detection, the RTVue XR Avanti system
utilizes four different frequency bandwidths of OCT to reduce the
noise of the axial motion. This modification creates four voxel
data sets with the same resolution (20×20×20 M instead of 20×20×5 M
in full spectrum light source). Decorrelation of each pixel is
performed at each frequency band to calculate the average increased
traffic signal. High-resolution correlation is the value of low
correlated speckle amplitude derived from blood flow, tissue motion
and background noise. Decorrelation signal reduces the outliers
(too high decorrelation) and the zero correlation value of the
arbitrarily assigned voxel backscatter amplitude is lower than the
threshold value (8). Because of
the influence of liquid in the macular area of CSCR patient, the
deep layer intra-retinal of OCTA image was not clear.
Statistical analysis
All data were analyzed with a statistical package
(Statistica, v7.1; StatSoft, Inc, Tulsa, OK, USA), MedCalc software
(v10; MedCalc Software, Mariakerke, Belgium). Continuous variables
were presented as mean ± standard deviation (SD). One-way analysis
of variance (ANOVA) was used to analyze superficial vessel density
in sectors between groups. Least significant difference post hoc
tests were used to assess the pairwise difference. P<0.05 was
considered to indicate a statistically significant difference. The
G *Power 3.0.10 software was used for the power calculation, which
has statistical power (n=15) in the present study. The receiver
operating characteristic (ROC) curve was plotted for the proposed
superficial retinal vessel density in differentiating between
healthy and diseased subjects.
Results
Patients before treatment
Study population statistics are shown in Table I. CSCR group (n=15) and control
group (n=15) were not statistically significant (P>0.05) in
gender, age, axial length, diastolic blood pressure, systolic blood
pressure, heart rate, but there was significant difference in
macular edema diameter (P<0.05).
 | Table I.Baseline characteristics of patients
in the study. |
Table I.
Baseline characteristics of patients
in the study.
| Variables | HCs | CSCR |
|---|
| Age (range,
years) | 49±6 | 48±8 |
| Sex ratio,
male:female | 5:10 | 7:8 |
| RE (range,
diopters) | 1.00±0.90 | 5.00±1.00 |
|
| 0~-2.75 | −3~-5.75 |
| AL (mm) | 23.54 | 24.01 |
| SBP (mmHg) | 119±8 | 117±12 |
| DBP (mmHg) | 78±9 | 80±11 |
| HR | 78±12 | 76±14 |
| Macular volume | 0 | 1.18±0.31 |
Macular vascular density
Firstly, we compared the density of superficial
microvascular (SMIR), superficial macrovascular ring (SMAR) and
superficial total microvascular (STMI) in both groups. We found
that compared with the control group, the density of SMIR and STMI
was significantly decreased in CSCR patients (P<0.05), while the
density of SMAR was not significantly changed (Fig. 2A). Secondly, we used the
hemispheric partition (Fig. 1C and
F) and Early Treatment Diabetic Retinopathy Study (ETDRS)
method (Fig. 1B and E) to compare
the density of SMIR, and we found that the two partition methods
were not significant different (P>0.05, Fig. 2B and C), indicating there was no
quadrant changes in the density of SMIR. Lastly, we used the ring
partition method (Fig. 1A and D)
to compare the microvascular density. The results showed that there
was a significant decrease in the density of blood vessels in C1
partition (P<0.05, Fig. 2D).
There was also a decreasing trend in other partitions, but not
statistically significant (P>0.05).
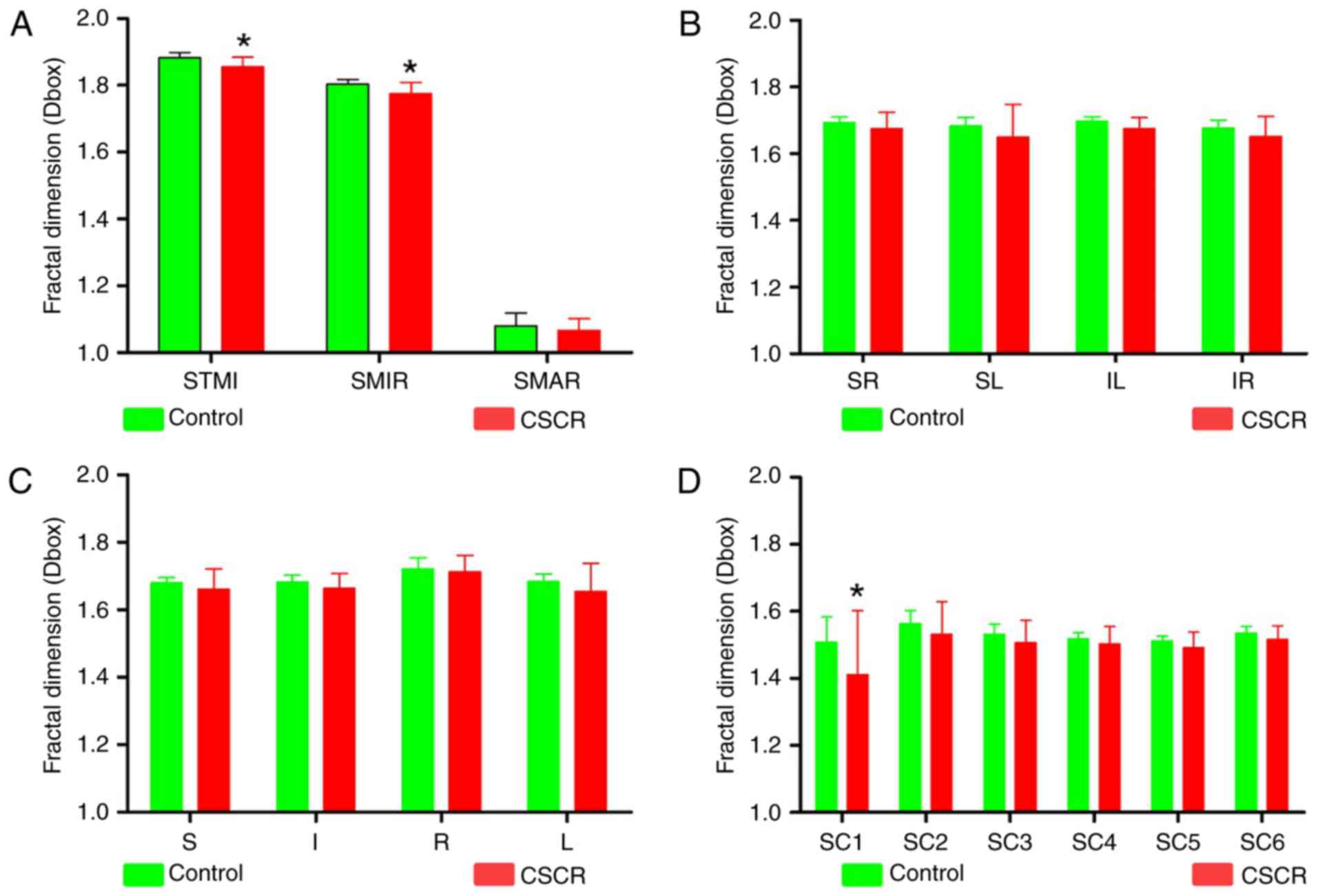 | Figure 2.Comparisons of macula retinal vessel
density (D box) between CSCR and control subjects in the
superficial layer. (A) Compared with the control group, there was a
significant difference in the density of superficial total
microvascular and superficial microvascular ring in macular region
of CSCR patients (P<0.05). (B) The density of superficial
microvessels in the CSCR group was not significantly changed
compared to the control group for all quadrantal methods like the
(B) Hemispheric partition method and (C) ETDRS method about the
superficial retinal layers (all P>0.05). (D) In the CSCR group,
except for the Cl partition of the superior retinal layer
(P<0.05), the microvessel density was not significantly changed
compared to the control group for all quadrantal zones of the
superficial retinal layers (all P>0.05). *P<0.05 in control
vs. CSCR. I, inferior; IL, inferior left; IR, inferior right; L,
left; R, right; S, superior; SC, superficial central annuli; SL,
superior left; SMAR, superficial macrovascular ring; SMIR,
superficial microvascular ring; SR, superior right; STMI,
superficial total microvascular' CSCR, central serous
chorioretinopathy. |
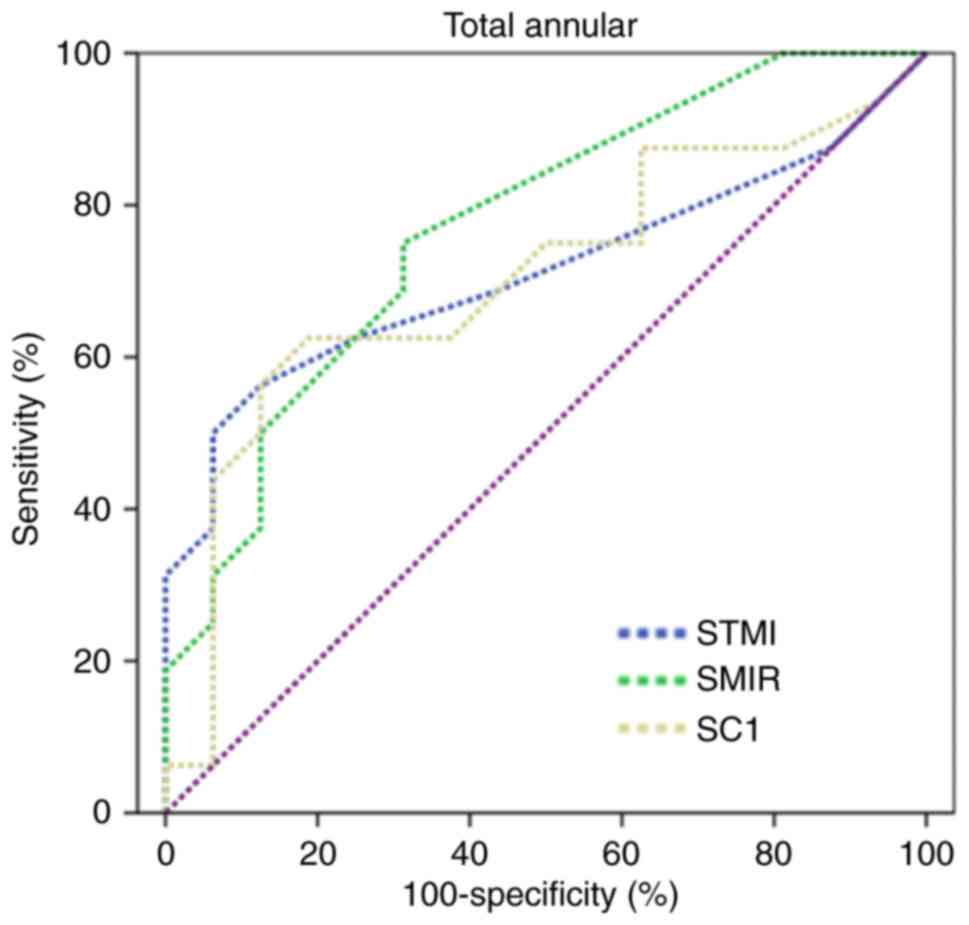
ROC analysis of superficial retinal
microvessel density
The vessel density of the superficial retina
provided by OCTA had the best sensitivity-specificity pairs for
differentiating CSCR from controls. The density of SMIR had the
highest positive likelihood ratios in the CSCR group, whereas the
C1 density of the superficial retina had the lowest negative
likelihood ratio by the annular method. The largest area under the
ROC curves was 0.77 (Fig. 3).
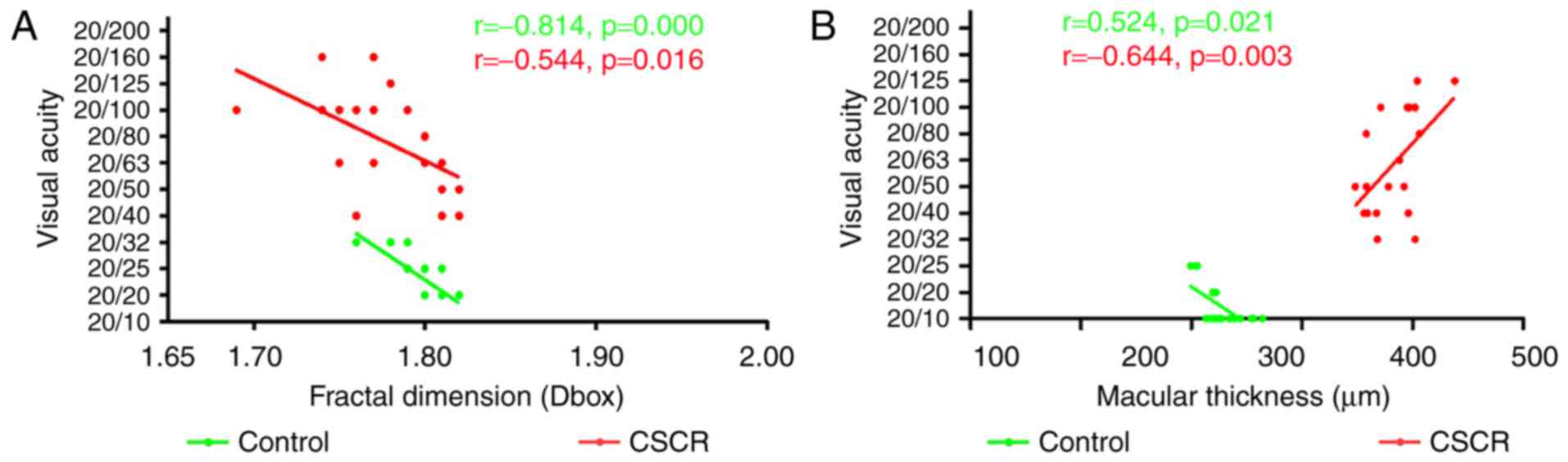
Correlation between macular blood
vessel density, foveal thickness and visual acuity
We analyzed the correlation between SMIR, SMAR, STMI
and macular thickness (Fig. 4).
There was no correlation between the density of SMAR, STMI and
visual acuity in the superficial retinal layer (P>0.05, data not
shown). The correlation index of the SMIR density and visual acuity
in the CSCR group was −0.544 (Fig.
4A). These results suggested that reduced SMIR density in the
macular area could affect the visual acuity. The correlation index
of macular thickness and visual acuity in the CSCR group was −0.644
(P<0.05) (Fig. 4B), indicating
that the changes in the macular thickness would have an affect on
visual acuity.
Discussion
CSCR usually affects young and middle-aged people.
Most patients can self heal, but chronic CSCR is persistent, prone
to recurring, and has long duration. It can also cause irreversible
damage to the patient's vision (9). The current studies mainly believe
that the original CSCR lesions occur in the choroid, leakage of RPE
blood retinal barrier of blood vessels, leading to the occurrence
of CSCR (10). This is the first
study showing reduced retinal superficial microvessel density in
the macular regions of the CSCR patients, providing evidence for a
more comprehensive understanding of CSCR.
In patients with CSCR, there are usually one or a
few small areas of serous retinal pigment epithelial detachment in
the macular region. Prunte et al found that choroidal
capillary lobules and their venous blood retention were related to
the pigment epithelium detachment and leakage (11). ICGA found that CSC patients had not
only changes of RPE leakage, but also delayed perfusion of the
choroid, dilation of choroidal capillary and vein, increased
choroidal vascular permeability, pigment epithelial detachment
(PED) and recessive pigment epithelial detachment (12). According to the pathogenesis of
CSCR proposed by Caccavale: Many factors lead to vasoconstriction
and capillary congestion, causing decreased vascular bed, increased
resistance to blood flow and blood viscosity, and further vascular
bed hypoperfusion and increased cavity pressure. These result in
increased intraluminal pressure in the stroma of serum and small
molecule leakage, further blocking the blood vessels, eventually
increasing permeability of the choroid capillary. The exudation by
RPE leaks into the subretinal space, causing RPE decompensation and
detachment, and finally the formation of CSCR (13). We observed that total macular
retinal microvessel density and microvessel density was
significantly reduced in CSCR patients compared with the control
group, suggesting that CSCR induces changes of choroidal capillary,
and may also cause the change of retinal capillaries, but the
mechanism needs further study.
The present study showed that the density of SMIR in
the annular C1 partition was significantly reduced in the CSCR
patients, and there was no statistically significance in the C2-C6
partitions, suggesting that the macular retinal vascular density
reduction in CSCR patients might be mainly concentrated near
central fovea region. However, there was no significant difference
in the changes of macular retinal blood vessel density in patients
with CSCR, indicating that there was no correlation between macular
area and vascular density in patients with CSCR. This might be
related to serous detachment caused by CSCR.
There are two main retinal blood supply systems. The
outer retina is mainly supplied through the choroidal blood
circulation, and retinal blood circulation provides supply and
nutrition for the inner retina. The blood choriocapillary layer
nourishes retinal neuroepithelial layer (layer of retinal neurons
to the outer plexiform layer) of the optic nerve, and usually is
the only source of nutrition for fovea (14). The retinal blood flow and choroidal
blood circulation changes will directly affect the vision of
patients. We found that the total macular retinal microvessel
density and visual acuity had significant correlation, and
reduction of the macular retinal microvascular density did not have
effects on visual acuity. That might be because of the difference
in disease stage, which needs further investigation with a larger
sample size.
OCTA plays an important role in the diagnosis of
CSCR, because it can not only be used to observe the retinal
neuroepithelial layer morphology and the changes of RPE, but also
can be used to track the changes of the disease, such as subretinal
fluid changes (15). CSCR induces
retinal serous detachment, and causes decreased visual acuity,
central scotoma, metamorphopsia, dyschromatopsia, decreased visual
size and visual contrast sensitivity (9,16).
And there is a positive correlation between the serous detachment
height and vision quality. As a result, OCT tracks disease
condition mainly based on observation of changes of retinal
thickness and we found that changes of the central macular
thickness had a significant influence on visual acuity, which is
consistent with previous studies. In this study, OCTA showed
accumulation of subretinal fluid, RPE layer defects, and a typical
dye leakage from the choroid into the subretinal space in CSCR
patients. In most cases, the prognosis was good, with spontaneous
regression and good visual acuity. However, some patients might
suffer from vision impairment, which might be due to a recurrence
of the disease or a secondary angiogenesis (17). Filho et al reported that
OCTA had higher sensitivity and specificity compared with FAA
detection of chronic CSCR in CNV (18).
The reduction of retinal vascular network in CSCR
patients is regarded as an indicator of the progression of macular
degeneration, which is usually manifested as retinal vascular
disease. Changes of visual acuity occur in the early onset of
macular degeneration. CSCR manifested in OCTA as granular
inhomogeneous high reflection with small pieces of dark area at the
choriocapillary level, and 70% of the patients with high reflection
region matched with the high permeability area as seen with ICGA.
RPE CNV could be visualized by OCTA in 21% patients with double
signs of CSCR. OCTA can be used in the follow-up of patients with
CSCR, and can better show the choroidal ischemia after PDT. The
development of OCTA can promote our understanding of the
pathogenesis of CSCR (19).
Carlo et al (20) used OCTA to observe the irregular
shedding of RPE in CSCR patients, and found that CSCR patients
showed increased possibility of angiogenesis in irregular vascular
RPE shedding, which is helpful for the treatment. Shozo et
al (21) found that
inflammation at the high level of choroid reflex in the acute phase
of CSCR was associated with edema. The high reflecting area outside
the choroid is the expansion of the vascular lumen of the great
vessels, regardless of the onset of CSCR. Feucht et al
(22) found no changes in the
blood flow parameters associated with acute CSCR leakage in the
OCTA images of the superficial and deep retinal nerve plexus, the
outer retina, and the choroidal capillaries. But the changes of
intraocular choroidal blood flow could be quantified. Studies
(23) have shown that retinal
abnormalities, especially lattice degeneration, are often seen in
patients with CSCR. Therefore, the authors suggested that CSCR
patients should be regularly examined for the retinal health,
including the assessment of the peripheral retina.
There are some inadequacies in the present study.
For example, we only observed the density of SMIR, SMAR and SC1
changes in the macular of patients with CSCR. Whether it is
consistent with choroidal microvascular changes, or happens before
or after the choroidal microvascular changes, and whether it can be
used as an early indicator of self-healing remain to be further
explored.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China [NSFC no. 81160118 (Yi Sh),
81460092 (Yi Sh) and 81660152 (Yi Sh)].
References
|
1
|
Kang S, Park YG, Kim JR, Seifert E,
Theisen-Kunde D, Brinkmann R and Roh YJ: Selective retina therapy
in patients with chronic central serous chorioretinopathy: A pilot
study. Medicine (Baltimore). 95:e25242016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guyer DR, Yannuzzi LA, Slakter JS,
Sorenson JA, Ho A and Orlock D: Digital indocyanine green
videoangiography of central serous chorioretinopathy. Arch
Ophthalmol. 112:1057–1062. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klatt C, Saeger M, Oppermann T, Pörksen E,
Treumer F, Hillenkamp J, Fritzer E, Brinkmann R, Birngruber R and
Roider J: Selective retina therapy for acute central serous
chorioretinopathy. Br J Ophthalmol. 95:83–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adhi M and Duker JS: Optical coherence
tomography-current and future applications. Curr Opin Ophthalmol.
24:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miwa Y, Murakami T, Suzuma K, Uji A,
Yoshitake S, Fujimoto M, Yoshitake T, Tamura Y and Yoshimura N:
Relationship between functional and structural changes in diabetic
vessels in optical coherence tomography angiography. Sci Rep.
6:290642016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spaide RF, Klancnik JM Jr and Cooney MJ:
Retinal vascular layers imaged by fluorescein angiography and
optical coherence tomography angiography. JAMA Ophthalmol.
133:45–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kraus MF, Potsaid B, Mayer MA, Bock R,
Baumann B, Liu JJ, Hornegger J and Fujimoto JG: Motion correction
in optical coherence tomography volumes on a per A-scan basis using
orthogonal scan patterns. Biomed Opt Expr. 3:1182–1199. 2012.
View Article : Google Scholar
|
|
8
|
Jia Y, Tan O, Tokayer J, Potsaid B, Wang
Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J and Huang
D: Split-spectrum amplitude decorrelation angiography with optical
coherence tomography. Opt Express. 20:4710–4725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang M, Munch IC, Hasler PW, Prünte C and
Larsen M: Central serous chorioretinopathy. Acta Ophthalmol.
86:126–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kishi S, Yoshida O, Matsuoka R and Kojima
Y: Serous retinal detachment in patients under systemic
corticosteroid treatment. Japanese J ophthalmol. 45:640–647. 2001.
View Article : Google Scholar
|
|
11
|
Prunte C and Flammer J: Choroidal
capillary and venous congestion in central serous
chorioretinopathy. Am J Ophthalmol. 121:26–34. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piccolino FC and Borgia L: Central serous
chorioretinopathy and indocyanine green angiography. Retina.
14:231–242. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caccavale A, Romanazzi F, Imparato M,
Negri A, Morano A and Ferentini F: Central serous
chorioretinopathy: A pathogenetic model. Clin Ophthalmol.
5:239–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cunha-Vaz J, Bernardes R and Lobo C:
Blood-retinal barrier. Eur J Ophthalmol. 21:S3–S9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montero JA and Ruiz-Moreno JM: Optical
coherence tomography characterisation of idiopathic central serous
chorioretinopathy. Br J Ophthalmol. 89:562–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daruich A, Matet A and Behar-Cohen F:
Central serous chorioretinopathy. Dev Ophthalmol. 58:27–38. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chalam KV and Sambhav K: Optical coherence
tomography angiography in retinal diseases. J Ophthalmic Vis Res.
11:84–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonini Filho MA, de Carlo TE, Ferrara D,
Adhi M, Baumal CR, Witkin AJ, Reichel E, Duker JS and Waheed NK:
Association of choroidal neovascularization and central serous
chorioretinopathy with optical coherence tomography angiography.
JAMA Ophthalmol. 133:899–906. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bousquet E, Bonnin S, Mrejen S, Krivosic
V, Tadayoni R and Gaudric A: Optical coherence tomography
angiography of flat irregular pigment epithelium detachment in
central serous chorioretinopathy. Retina. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Carlo TE, Rosenblatt A, Goldstein M,
Baumal CR, Loewenstein A and Duker JS: Vascularization of irregular
retinal pigment epithelial detachments in chronic central serous
chorioretinopathy evaluated with OCT angiography. Ophthalmic Surg
Lasers Imaging Retina. 47:128–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sonoda S, Sakamoto T, Kuroiwa N, Arimura
N, Kawano H, Yoshihara N, Yamashita T, Uchino E, Kinoshita T and
Mitamura Y: Structural changes of inner and outer choroid in
central serous chorioretinopathy determined by optical coherence
tomography. PLoS One. 11:e01571902016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feucht N, Maier M, Lohmann CP and Reznicek
L: OCT angiography findings in acute central serous
chorioretinopathy. Ophthalmic Surg Laser Imaging Retina.
47:322–327. 2016. View Article : Google Scholar
|
|
23
|
Oztas Z, Akkin C, Ismayilova N, Nalcaci S
and Afrashi F: The importance of the peripheral retina in patients
with central serous chorioretinopathy. Retina. 2017. View Article : Google Scholar : PubMed/NCBI
|