Introduction
Breast cancer is one of the most commonly diagnosed
types of cancer in women worldwide, which usually develops from
breast tissues, including the milk ducts or lobules (1). Breast cancer is the uncontrolled
growth of malignant cells, and is associated with high incidence
and high mortality rates. It is estimated that breast cancer
comprises 11% of all types of cancer diagnosed worldwide on an
annual basis (2). A combination
treatment is used in the treatment of human breast cancer,
including surgery, radiotherapy, chemotherapy, hormone therapy and
biological therapy (3–5). Until now, surgery remains the most
common treatment strategy for breast cancer. Traditional prognostic
factors include age, menopausal status, status of axillary lymph
nodes, tumor size, histological features, and estrogen and
progesterone receptors (6), and
the prognosis of breast cancer is closely associated with the
invasion and metastasis early on. It is reported that peritoneal
metastases (7,8), lymph node metastasis (9,10)
and brain metastasis (11–13) in breast cancer are usually
associated with a poor prognosis following standard treatments. The
progression, invasion and metastasis of breast cancer involve
multiple genetic mutations, leading to the activation of oncogenes
and the inactivation of tumor suppressor genes (14,15).
Therefore, it is important to identify a novel and effective target
in cancer-associated signaling pathways, which may assist in the
treatment of breast cancer.
The Notch 1 signaling pathway is a highly conserved
pathway in the majority of multicellular organisms (16,17).
It can maintain the character of adult stem cells by affecting the
communication between adjacent cells. It is also involved in the
tumorigenesis of various types of tumor by affecting
differentiation, proliferation and apoptosis (18,19).
The activation of Notch has been reported to cause mammary
carcinoma. Xu et al found that Notch receptors were of
prognostic value in breast cancer, and that a high mRNA expression
levels of Notch 1 were correlated with poor overall survival (OS)
in patients with progesterone receptor-negative breast cancer
(20). In addition, abnormal Notch
1 signaling was found to be important in patients with colorectal
cancer (21). Pant et al
reported on a first-in-human phase I study of the oral Notch
inhibitor, LY900009, in patients with advanced cancer, and found
that LY900009 inhibited plasma levels of amyloid-β peptide in a
dose-dependent manner with 80–90% inhibition observed in the
30–60-mg cohorts (22). Notch
signaling has been reported as a novel therapeutic target to
prevent the recurrence of breast cancer and regulate long
non-coding (Lnc)RNAs at the downstream target of the Notch pathway.
In breast cancer, it has been found that Notch 1 promotes cell
proliferation by regulating the GAS5 LncRNA (19).
In the present study, the molecular mechanism
underlying the effects of Notch 1 on the proliferation and invasion
of human breast cancer cells was examined. In addition, the
association between the Notch 1 signaling pathway and Wnt/β-catenin
signaling pathway, and the variation in β-catenin location were
examined. The results may provide novel clues for determining
whether Notch 1 may be used as a novel target in the treatment of
breast cancer.
Materials and methods
Cell lines
The MDA-MB-231, MCF-7 and MCF-10A breast cancer cell
lines were purchased from Shanghai Bioleaf Biotech Co., Ltd.
(Shanghai, China). Human mammary epithelial cells (HMECs; cat no.
A10565) isolated from adult female breast tissue were obtained from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The cells were
cultured in Dulbecco's modified Eagles medium (DMEM), which was
purchased from GE Healthcare Life Sciences (Little Chalfont, UK)
supplemented with 10% serum at 37°C in an atmosphere of 5%
CO2. Lentiviral particles of Notch 1 short hairpain
(sh)RNA (cat no. sc-36095-V) and control (N.C.) shRNA lentiviral
particles (cat no. sc-108080) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). MRK003 (cat no. 205885)
was obtained from MedKoo Biosciences, Inc. (Chapel Hill, NC, USA).
MTT reagent was obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany).
MTT assay
The breast cancer cells (3×105
cells/well) were plated into 48-well plates and transfected with
Notch 1 shRNA or N.C. shRNA for 48, 72 and 96 h, respectively. The
cell proliferation was then evaluated using an MTT assay. In a
separate experiment, the breast cancer cells (3×105
cells/well) were treated with different concentrations of Notch 1
inhibitor, MRK003, for 24, 48 and 72 h, respectively at 37°C. The
concentrations of MRK003 were 0, 0.5, 1.0 and 5.0 µM. MRR reagent
(10 µl of 5 mg/ml; Sigma-Aldrich; Merck KGaA) was added into the
medium 4 h prior to assessment. Finally, 150 µl of DMSO was added
and the purple crystals were dissolved for 10 min. The plates were
read at a test wavelength of 490 nm and a reference wavelength of
570 nm.
Western blot analysis
Breast cancer cell (2×106 cells/well)
lysates were prepared and (15 µl) the proteins were separated by
10% SDS-PAGE. The polyvinylidene difluoride membrane was blocked
with 5% bovine serum albumin and incubated with primary antibodies
at 4°C overnight and secondary antibodies for 1 h at room
temperature, respectively. The antibodies used were as follows:
Anti-Notch 1, anti-GSK-3β, anti-MMP-2, anti-MMP-9, anti-β-catenin,
anti-β-actin and horseradish peroxidase (HRP)-conjugated goat
anti-mouse secondary antibody. The primary antibody against Notch 1
(mN1A; cat no. sc-32745; 1:1,000) was obtained from Santa Cruz
Biotechnology, Inc., which is a mouse monoclonal immunoglobulin
(Ig)G1 provided at 200 µg/ml. The GSK-3β antibody (H-76; cat no.
sc-9166; 1:1,000) was obtained from Santa Cruz Biotechnology, Inc.,
and is a rabbit polyclonal IgG provided at 200 µg/ml. The MMP2
antibody (cat no. 10373-2-AP; 1:1,000) and MMP-9 antibody (cat no.
10375-2-AP; 1:1,000) are rabbit polyclonal antibodies, and were
obtained from Wuhan Sanying Biotechnology (Wuhan, China). The
secondary goat anti-mouse IgG H&L HRP-conjugated (cat no.
ab6789; 1:5,000) and goat anti-rabbit IgG H&L HRP-conjugated
(cat no. ab6721; 1:5,000) were obtained from Abcam (Cambridge, UK).
β-catenin antibody (cat no. ab16051; 1:1,000) was obtained from
Abcam. β-actin antibody (cat no. HC201-01; 1:500) was purchased
from TransGen Biotechnology (Beijing, China). The bands were
visualized with an enhanced chemiluminescence detection system (GE
Healthcare, Chicago, IL, USA) and protein levels were quantified
using a gel imaging system Gel Doc™ XR system with Quantity One
software (version 4.4) (both from Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Transwell assay
The MCF-7 cells and MDA-MB-231 cells were cultured
in DMEM medium supplemented with 10% fetal bovine serum (FBS).
Transwell assays were performed using a 24-well format with 8-µm
pore size (cat no. ECM509; Chemicon International, Inc., Temecula,
CA, USA). Briefly, the lower compartment was filled with 2 ml of
DMEM with 0.5% FBS containing 40 µg/ml collagen I, and the
Transwell insert was placed into the medium in the lower
compartment. Subsequently, 5×103 MCF-7 cells or
MDA-MB-231 cells were plated in the upper compartment and incubated
at 37°C and 5% CO2 for 48 h. The breast cancer cells on
the lower side of the insert filter were then immediately fixed in
5% glutaraldehyde for 10 min, and stained with 1% crystal violet in
2% ethanol for 20 min. The cells were washed with ddH2O
and the numbers of cells on the lower side of the filter were
counted under a light microscope.
Statistical analysis
The data were analyzed using the SPSS software
version 19.0 (IBM Corp., Armonk, NY, USA). The expression levels of
proteins, including Notch 1, and the migration rates of cells were
analyzed with two sets of independent samples t-tests. The
comparison of multiple groups was performed with multiple
comparisons using a two-way analysis of variance and Dunnett's
test. The experiment was repeated twice and there were three
replicates for each well in the MTT assay. The data are presented
as the mean ± standard error of the mean.
Results
Expression levels of Notch 1 are
higher in MDA-MB-231 and MCF-7 cells
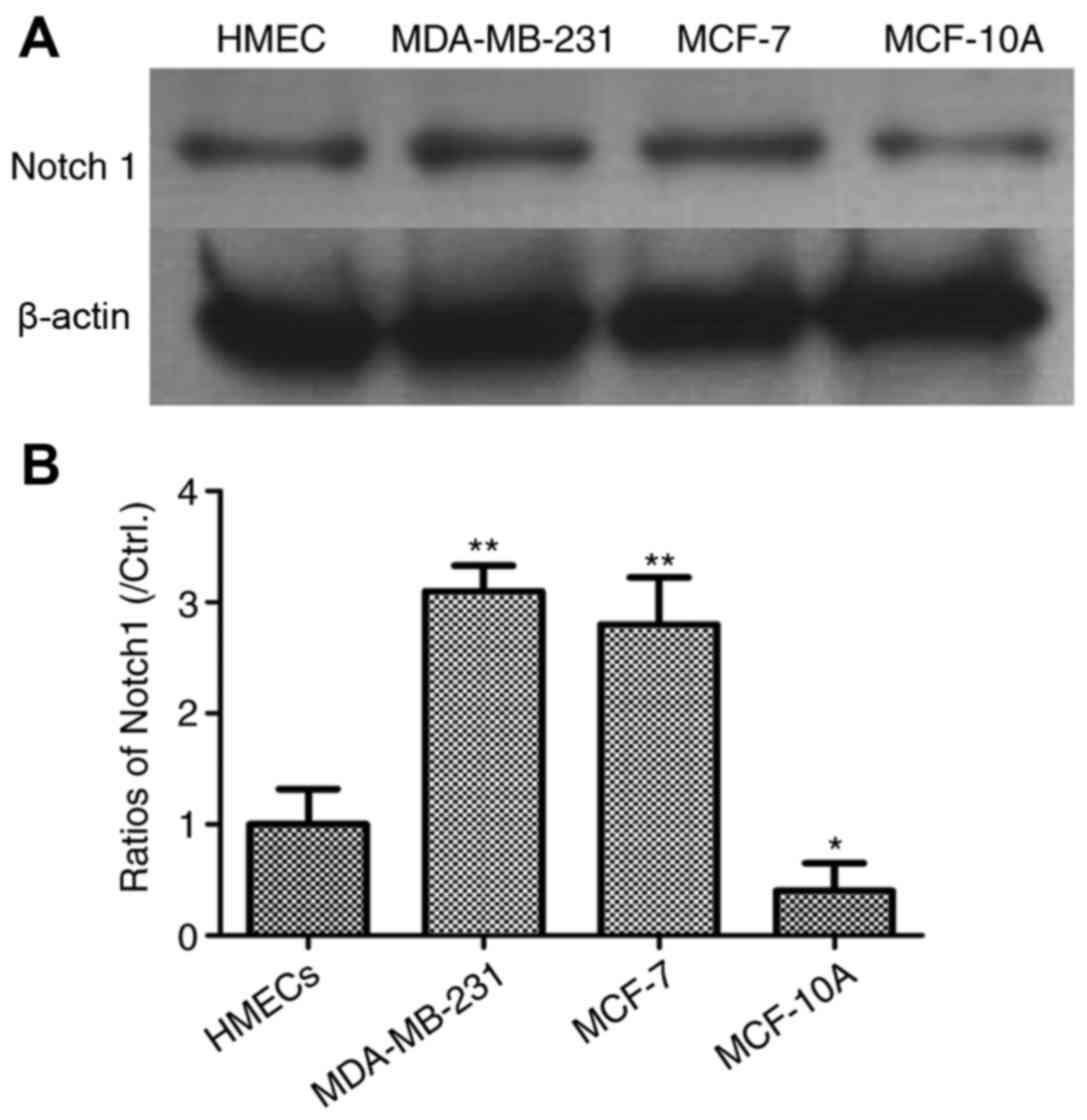
In order to examine the role of Notch 1 in the
progression of human breast cancer, three breast cancer cell lines
were used as a cell model, including MDA-MB-231, MCF-10A and MCF-7
cells. MDA-MB-231 cells have a high invasive ability, whereas
MCF10A cells are a non-invasive cell line. Usually, MCF-7 breast
cancer cells have a poor invasive phenotype. Normal HMECs were used
as negative controls. The expression levels of Notch 1 were
detected using western blot analysis. As shown in Fig. 1, the results demonstrated that the
levels of Notch 1 were significantly increased in the MDA-MB-231
and MCF-7 cells (P<0.01), compared with levels in the HMECs.
However, the level of Notch 1 was lower in the MCF-10A cells,
compared with the HMECs (P<0.05).
Knock down of the expression of Notch
1 significantly inhibits the proliferation of breast cancer
cells
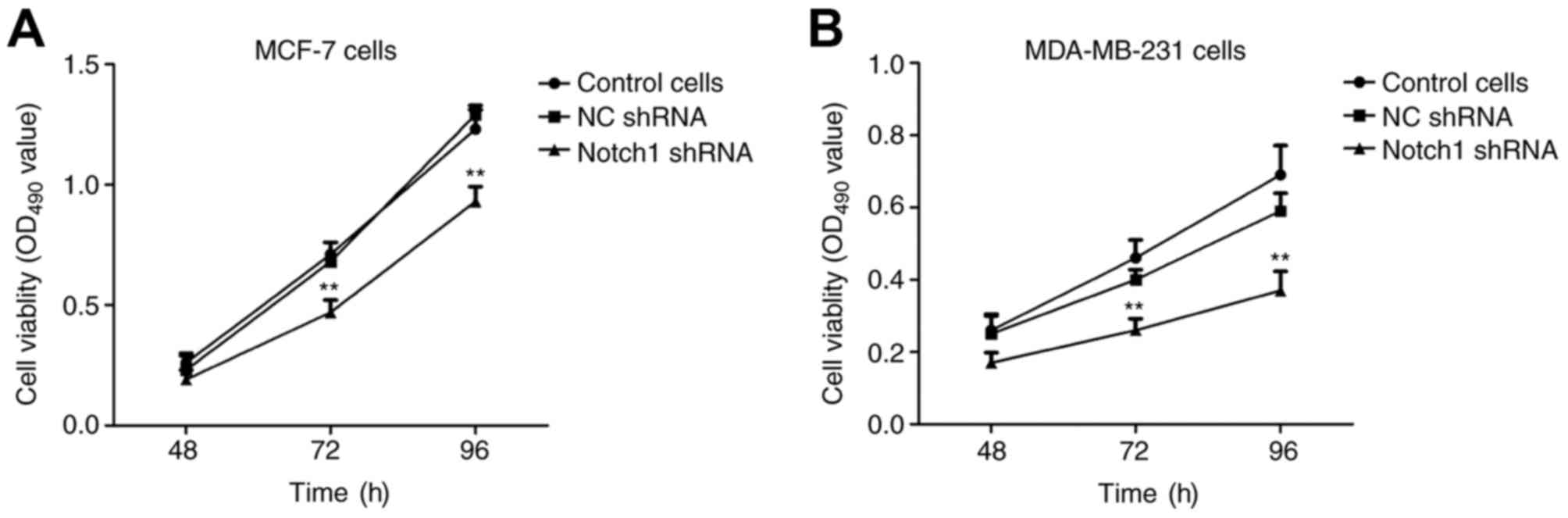
In order to determine the role of Notch 1 in the
proliferation of breast cancer cells, MCF-7 cells were transfected
with Notch 1 shRNA and N.C. shRNA for 48, 72 and 96 h,
respectively. The cell viability (OD 490 value) was significantly
lower in the Notch 1-transfected MCF-7 cells, compared with that in
the N.C. shRNA-transfected MCF-7 cells (P<0.01; Fig. 2A). This was consistent with the
results in MDA-MB-231 cells (Fig.
2B). Taken together, these results demonstrated that Notch 1
was an important in promoting the proliferation of breast cancer
cells.
MRK003 inhibits the proliferation of
MCF-7 and MDA-MB-231 cells in a time- and dose-dependent
manner
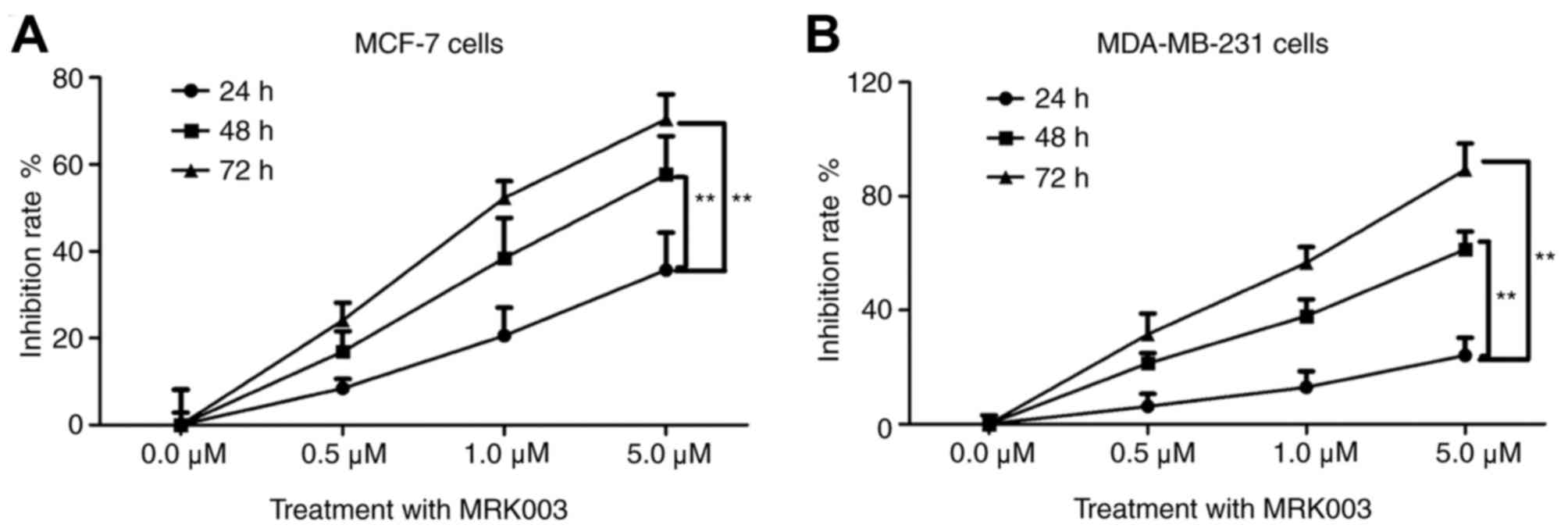
MRK003 is a potent and selective γ-secretase
inhibitor, which can lead to the downregulation of the nuclear
Notch 1 intracellular domain and inhibits the role of Notch 1
(23). In order to further confirm
the role of Notch 1 in breast cancer cells, the present study used
different concentrations of MRK003 (0.0, 0.5, 1.0 and 5.0 µM) for
24, 48 and 72 h, respectively. As shown in Fig. 3A, MRK003 effectively inhibited the
proliferation of MCF-7 cells as the concentration of the Notch 1
inhibitor increased. In addition, the inhibition rate was
significantly increased in a time-dependent manner (P<0.01).
This was consistent with the results in the invasive MDA-MB-231
cell line (Fig. 3B). These results
demonstrated that inhibiting Notch 1 by MRK003, the Notch 1
inhibitor, significantly suppressed the proliferation of breast
cancer cells in a dose- and time-dependent manner.
Notch 1 knockdown inhibits the
invasive ability of MCF-7 and MDA-MB-231 cells
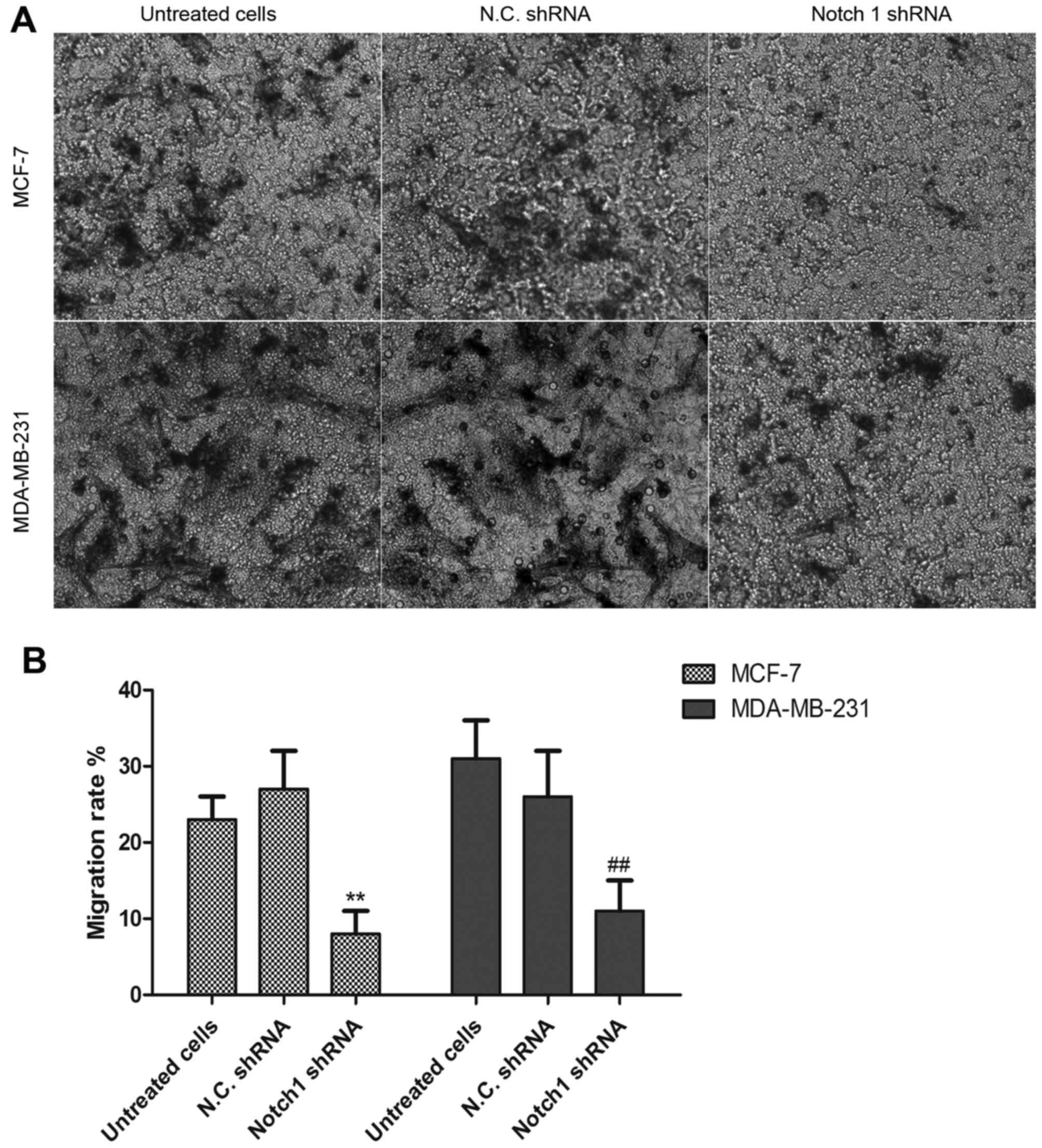
To further examine the effects of Notch 1 on the
invasive ability of the MCF-7 and MDA-MB-231 breast cancer cells, a
Transwell assay was performed to detect the effects of Notch 1 on
cell invasion. The MCF-7 cells or MDA-MB-231 cells were transfected
with Notch 1 shRNA or N.C. shRNA and cultured for 48 h, and the
invasive cells were observed, as shown in Fig. 4A. The results demonstrated that the
migration rate of the Notch 1 shRNA-transfected cells was
significantly decreased, compared with that of the N.C.
shRNA-transfected cells (Fig. 4B),
suggesting that the Notch 1 signaling pathway may contribute to the
invasion and migration of breast cancer cells.
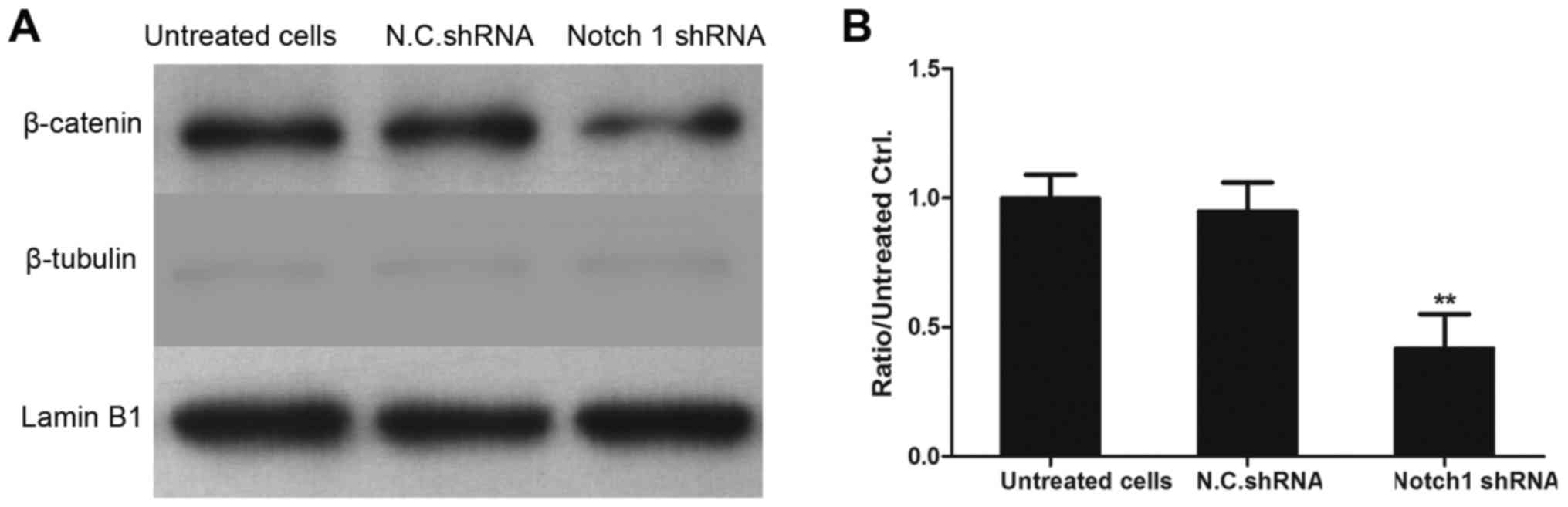
Interference with Notch 1 reduces the
expression of β-catenin, MMP-2 and MMP-9
The present study also investigated whether
knockdown of the expression of Notch 1 affected the β-catenin
signaling pathway. The MDA-MB-231 cells were transfected with Notch
1 shRNA and N.C. shRNA for 48 h, and the levels of GSK-3β and
β-catenin were determined using western blot analysis. As exhibited
in Fig. 5, the expression levels
of β-catenin, MMP-2 and MMP-9 were significantly decreased in the
Notch 1 shRNA-transfected MDA-MB-231 cells, compared with levels in
the N.C. shRNA-transfected cells. However, no significant change in
the expression level of GSK-3β was observed, compared with the N.C.
shRNA-transfected cells (P>0.05). These results demonstrated
that Notch 1, as an oncogene in breast cancer cells, promoted the
proliferation and invasion of breast cancer cells partly by
regulating MMP-2 and MMP-9 involving the β-catenin-related
signaling pathway.
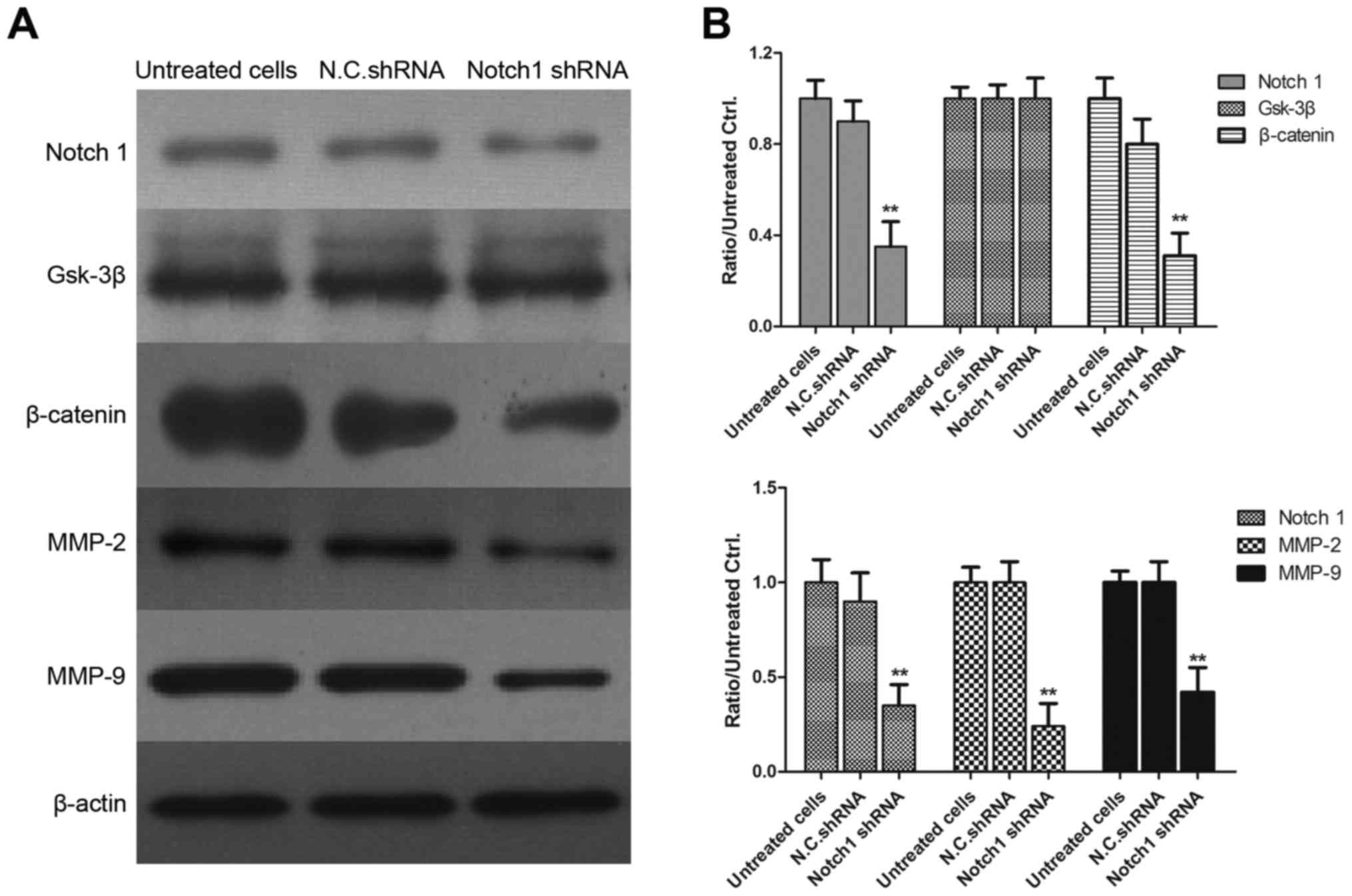 | Figure 5.Interference with Notch 1 reduces the
expression of β-catenin, MMP-2 and MMP-9. (A) MDA-MB-231 cells were
transfected with Notch 1 shRNA or N.C. shRNA for 48 h, and the
levels of Notch 1, GSK-3β, β-catenin, MMP-2 and MMP-9 were
determined using western blot analysis. (B) Gray values of Notch 1,
GSK-3β, β-catenin, MMP-2 and MMP-9 are shown. **P<0.01, compared
with N.C. shRNA-transfected cells. MMP, matrix metalloproteinase;
GSK-3β, glycogen synthase kinase 3β; shRNA, short hairpin RNA;
N.C., negative control. |
Interference with Notch 1 decreases
the levels of nuclear β-catenin in MDA-MB-231 cells
To examine whether the knockdown of Notch 1 affected
the translocation of β-catenin in MDA-MB-231 cells, the levels of
nuclear Notch 1 were determined using western blot analysis. As
exhibited in Fig. 6, the
expression of Notch 1 in the nucleus was significantly decreased in
the Notch 1 shRNA-transfected MDA-MB-231 cells, compared with
expression in the N.C. shRNA-transfected cells. Therefore, it was
hypothesized that downstream target genes, including cyclin D1,
c-Myc and other associated genes, may be downregulated in Notch 1
shRNA-interference of MDA-MB-231 cells.
Discussion
Breast cancer is the second most frequently
diagnosed cancer in women and is life threatening. The mechanism
underlying the tumorigenesis of breast cancer has been investigated
previously, involving the regulation of the p38/mitogen-activated
protein kinase (MAPK) signal transduction pathway (24), the c-Jun N-terminal
kinase/stress-activated protein kinase signaling pathway (25), MAPK/extracellular signal-regulated
signaling pathway (26) and human
epidermal growth factor receptor 2 signaling pathway (27). The Notch 1 signaling pathway is
also reported to be a highly conserved signaling pathway, which is
found to regulate the development and progression of cancer
(18,28). In the present study, it was found
that the Notch 1 signaling pathway was important in the
proliferation and invasion of breast cancer cells.
In the present study, three breast cancer cell lines
were cultured and the levels of Notch 1 were detected using western
blot analysis. The results showed that the levels of Notch1 were
higher in the invasive cell lines (MDA-MB-231 and MCF-7 cells),
compared with that in the non-invasive cell line (MCF10A). The
results also showed that Notch 1 was abnormally expressed in human
breast cancer cell lines, suggesting Notch 1 may be involved in the
proliferation and invasion of breast cancer cells. This was
confirmed by the results of a Transwell assay, suggesting that
Notch 1 had a higher potential to promote the invasion and
migration of breast cancer cells.
The molecular mechanisms and the role of Notch 1 in
breast cancer were also investigated. A number of studies have
demonstrated that the dysregulation and abnormality of conserved
Wnt/β-catenin signaling occurs in human breast cancer (29,30).
The present study further examined the interplay between the Notch
1 and Wnt/β-catenin signaling pathway. The results of the western
blot analysis revealed that the knockdown of endogenous Notch 1
significantly decreased the expression of β-catenin and
downregulated the levels of nuclear β-catenin. The expression
levels of MMP-2 and MMP-9 were also detected using western blot
analysis, and the results demonstrated that knockdown of the
expression of Notch 1 significantly decreased the expression of
MMP-2 and MMP-9, suggesting that the invasive ability was
suppressed in the Notch 1 shRNA-transfected cells. Consistent with
this, cell viability was significantly decreased in the Notch 1
shRNA-transfected MCF-7 and MDA-MB-231 cells, compared with that in
the N.C. shRNA-transfected cells.
Taken together, the present study found that the
knockdown of Notch 1 decreased the levels of MMP-2 and MMP-9 in
human breast cancer cells, which was consistent with previous
evidence that MMP-2 and MMP-9 correlate significantly with tumor
size (28), invasion and
metastasis in breast cancer (31).
In terms of the underlying molecular mechanism, it may be that the
inhibition of Notch 1 significantly suppressed the proliferation
and invasion of breast cancer cells by decreasing the β-catenin
signaling pathway. Therefore, it was concluded that Notch 1 is
important in the progression and invasion of breast cancer, and may
be used as a target in therapy for patients with breast cancer.
References
|
1
|
Wang Y, Yu J, Cui R, Lin J and Ding X:
Curcumin in treating breast cancer: A review. J Lab Autom.
21:723–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asif HM, Sultana S, Ahmed S, Akhtar N and
Tariq M: HER-2 positive breast cancer - a mini-review. Asian Pac J
Cancer Prev. 17:1609–1615. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu L, Li L, Li Y, Wang J and Wang Q:
Chinese herbal medicine as an adjunctive therapy for breast cancer:
A systematic review and meta-analysis. Evid Based Complement
Alternat Med. 2016:94692762016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
George BP and Abrahamse H: A review on
novel breast cancer therapies: Photodynamic therapy and plant
derived agent induced cell death mechanisms. Anticancer Agents Med
Chem. 16:793–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nicolini A, Carpi A, Ferrari P, Biava PM
and Rossi G: Immunotherapy and hormone-therapy in metastatic breast
cancer: A review and an update. Curr Drug Targets. 17:1127–1139.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Nijnatten TJ, Schipper RJ, Lobbes MB,
Nelemans PJ, Beets-Tan RG and Smidt ML: The diagnostic performance
of sentinel lymph node biopsy in pathologically confirmed node
positive breast cancer patients after neoadjuvant systemic therapy:
A systematic review and meta-analysis. Eur J Surg Oncol.
41:1278–1287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bertozzi S, Londero AP, Cedolini C, Uzzau
A, Seriau L, Bernardi S, Bacchetti S, Pasqual EM and Risaliti A:
Prevalence, risk factors, and prognosis of peritoneal metastasis
from breast cancer. Springerplus. 4:6882015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petekkaya I, Ayyildiz V, Kizilarslanoglu
MC, Sahin U, Gezgen G, Roach EC, Karcaaltincaba M and Altundag K:
Prognosis of breast cancer in patients with peritoneal metastasis.
Breast. 21:420–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuoka H, Tsujimoto M, Yoshidome K,
Nakahara M, Kodama R, Sanke T and Nakamura Y: Cytoplasmic CXCR4
expression in breast cancer: Induction by nitric oxide and
correlation with lymph node metastasis and poor prognosis. BMC
Cancer. 8:3402008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu MQ, Hu P, Gao J, Wei WD, Xiao XS, Tang
HL, Li X, Ge QD, Jia WH, Liu RB and Xie XM: Low expression of
tyrosine-protein phosphatase nonreceptor type 12 is associated with
lymph node metastasis and poor prognosis in operable
triple-negative breast cancer. Asian Pac J Cancer Prev. 14:287–292.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Venkitaraman R, Joseph T, Dhadda A,
Chaturvedi A and Upadhyay S: Prognosis of patients with
triple-negative breast cancer and brain metastasis. Clin Oncol (R
Coll Radiol). 21:729–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wikman H, Westphal L, Schmid F, Pollari S,
Kropidlowski J, Sielaff-Frimpong B, Glatzel M, Matschke J, Westphal
M, Iljin K, et al: Loss of CADM1 expression is associated with poor
prognosis and brain metastasis in breast cancer patients.
Oncotarget. 5:3076–3087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamura J, Masuda N, Yasojima H, Mizutani
M, Kuriyama K, Nakamori S, Sekimoto M, Mano M, Tanaka E and Nonaka
M: Clinicopathological factors related to the prognosis of
metastatic breast cancer patients after development of brain
metastasis. Breast Care (Basel). 10:387–392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Jin K, van Pelt GW, van Dam H, Yu X,
Mesker WE, Ten Dijke P, Zhou F and Zhang L: c-Myb enhances breast
cancer invasion and metastasis through the Wnt/β-Catenin/Axin2
pathway. Cancer Res. 76:3364–3375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi M, Cao M, Song J, Liu Q, Li H, Meng F,
Pan Z, Bai J and Zheng J: PinX1 inhibits the invasion and
metastasis of human breast cancer via suppressing NF-κB/MMP-9
signaling pathway. Mol Cancer. 14:662015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
D'Angelo RC, Ouzounova M, Davis A, Choi D,
Tchuenkam SM, Kim G, Luther T, Quraishi AA, Senbabaoglu Y, Conley
SJ, et al: Notch reporter activity in breast cancer cell lines
identifies a subset of cells with stem cell activity. Mol Cancer
Ther. 14:779–787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W and Grivennikov SI: Top Notch
cancer stem cells by paracrine NF-κB signaling in breast cancer.
Breast Cancer Res. 15:3162013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Yuan Y, Cui J, Xiao T and Jiang
D: Paeoniflorin inhibits proliferation and invasion of breast
cancer cells through suppressing Notch-1 signaling pathway. Biomed
Pharmacother. 78:197–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pei J and Wang B: Notch-1 promotes breast
cancer cells proliferation by regulating LncRNA GAS5. Int J Clin
Exp Med. 8:14464–14471. 2015.PubMed/NCBI
|
|
20
|
Xu J, Song F, Jin T, Qin J, Wu J, Wang M,
Wang Y and Liu J: Prognostic values of Notch receptors in breast
cancer. Tumour Biol. 37:1871–1877. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vinson KE, George DC, Fender AW, Bertrand
FE and Sigounas G: The Notch pathway in colorectal cancer. Int J
Cancer. 138:1835–1842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pant S, Jones SF, Kurkjian CD, Infante JR,
Moore KN, Burris HA, McMeekin DS, Benhadji KA, Patel BK, Frenzel
MJ, et al: A first-in-human phase I study of the oral Notch
inhibitor, LY900009, in patients with advanced cancer. Eur J
Cancer. 56:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizuma M, Rasheed ZA, Yabuuchi S, Omura N,
Campbell NR, de Wilde RF, De Oliveira E, Zhang Q, Puig O, Matsui W,
et al: The gamma secretase inhibitor MRK-003 attenuates pancreatic
cancer growth in preclinical models. Mol Cancer Ther. 11:1999–2009.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang X, Li T and Liu RH:
2α-Hydroxyursolic acid inhibited cell proliferation and induced
apoptosis in MDA-MB-231 human breast cancer cells through the
p38/MAPK signal transduction pathway. J Agric Food Chem.
64:1806–1816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang HT and Wang CQ: 27-O-(E)-p-coumaric
acyl ursolic acid via JNK/SAPK signal pathway regulates apoptosis
of human breast cancer MDA-MB-231 cell line. Zhongguo Zhong Yao Za
Zhi. 40:722–726. 2015.(In Chinese). PubMed/NCBI
|
|
26
|
Zhang Y, Song M, Cui ZS, Li CY, Xue XX, Yu
M, Lu Y, Zhang SY, Wang EH and Wen YY: Down-regulation of TSG101 by
small interfering RNA inhibits the proliferation of breast cancer
cells through the MAPK/ERK signal pathway. Histol Histopathol.
26:87–94. 2011.PubMed/NCBI
|
|
27
|
Lin YJ, Huang YH, Zhen YZ, Liu XJ and Zhen
YS: Rhein lysinate induces apoptosis in breast cancer SK-Br-3 cells
by inhibiting HER-2 signal pathway. Yao Xue Xue Bao. 43:1099–1105.
2008.(In Chinese). PubMed/NCBI
|
|
28
|
Zhou X, Teng L and Wang M: Distinct
prognostic values of four-Notch-receptor mRNA expression in ovarian
cancer. Tumour Biol. 37:6979–6985. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ranogajec I, Jakić-Razumović J, Puzović V
and Gabrilovac J: Prognostic value of matrix metalloproteinase-2
(MMP-2), matrix metalloproteinase-9 (MMP-9) and aminopeptidase
N/CD13 in breast cancer patients. Med Oncol. 29:561–569. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yi SJ, Li LL and Tu WB: MiR-214 negatively
regulates proliferation and WNT/β-catenin signaling in breast
cancer. Eur Rev Med Pharmacol Sci. 20:5148–5154. 2016.PubMed/NCBI
|
|
31
|
Huang Y, Zhao K, Hu Y, Zhou Y, Luo X, Li
X, Wei L, Li Z, You Q, Guo Q and Lu N: Wogonoside inhibits
angiogenesis in breast cancer via suppressing Wnt/β-catenin
pathway. Mol Carcinog. 55:1598–1612. 2016. View Article : Google Scholar : PubMed/NCBI
|