Introduction
Colorectal cancer (CRC) is one of the most common
types of tumor worldwide with one of the highest mortality rates
(1). Its occurrence is a complex
process and may be caused by genetic or epigenetic alterations and
different environmental factors (2). In recent years, with the development
of early diagnosis and advanced systemic treatments, the overall
prognosis has been ameliorated to a certain extent. However, for
those with advanced and metastatic diseases, the prognosis remains
poor (3,4).
Angiogenesis, the formation of new blood vessels,
may serve central roles in tumor occurrence and progression, and
even prognosis (5). The
newly-formed vessels faciliate nutrients and waste exchange for
growing tumors, in addition to providing entry points for
metastatic tumor cells (6).
Finding effective targets which may significantly inhibit
angiogenesis is likely to contribute to improved treatment outcomes
for patients with cancer.
As a member of the serine protease family, an
initial study reported that fibroblast activation protein-α (FAP-α)
was expressed in >90% of epithelial tumor stromal cells and was
able to serve important roles in proliferation, angiogenesis,
immune escape, invasion and metastasis, and tissue remolding
(7). Previous studies have
demonstrated that FAP-α expression is not confined to stromal cells
and is additionally expressed in certain malignant epithelial cells
(8–10). Huang et al (8) reported that the human breast cancer
cell line MDA-MB-231 expressing FAP-α grew more rapidly compared
with a control group. Cheng et al (9) reported that HEK293 cells
overexpressing FAP-α were 2–4 times more likely to develop tumors
in mice compared with HEK293 control cells, in addition to a 10- to
40-fold shift in tumor growth (9).
In 2003, Iwasa et al (10)
confirmed FAP-α expression in CRC cells using immunohistochemistry
(IHC) staining and reported that high FAP-α expression was
positively correlated with lymph node metastasis. However, the
study lacked further cellular experiments and the specific function
of FAP-α in CRC cells remains unknown. The present study focused on
the proangiogenic roles of FAP-α derived from CRC cells, not
mesenchymal cells, and further explored the potential mechanisms of
angiogenesis regulated by FAP-α.
Materials and methods
Cell lines and culture
Colorectal cancer cell lines SW1116 and HT29 and
human umbilical vein endothelial cells (HUVECs) were all purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). SW1116 and HT29 cells were cultured in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum (FBS; both
from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
HUVECs were cultured in endothelial cell medium (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 5% FBS and 1%
endothelial cell growth supplement (Invitrogen; Thermo Fisher
Scientific, Inc.). All cells were cultured at 37°C in 5%
CO2.
Tissue samples
With the approval from the Review Board and Ethics
Committee of the People's Hospital of Xintai City, 80 primary CRC
specimens were selected from patients who had undergone surgery
between January 2006 and December 2014 in the Xintai People's
Hospital (Shandong, China). No patients had received chemotherapy,
radiotherapy or immunomodulatory therapy prior to surgery.
IHC
Sections from CRC samples were cut to 4-µm
thickness, deparaffinized in xylene and rehydrated with a graded
ethanol series. The slides were boiled in 10 mmol/l citrate buffer
(pH 6.0) for 2.5 min at 100°C for antigen unmasking. The sections
were immersed in 3% H2O2 for 10 min to block
the endogenous peroxidase and in goat serum blocking solution (cat.
no. CW0130; CWbio Co., Ltd., Beijing, China) for 20 min to block
non-specific antigens. The slides were incubated at room
temperature for 2 h with primary antibodies for FAP-α (cat. no.
ab53066, rabbit anti-human, 1:300; Abcam, Cambridge, UK), VEGF-A
(cat. no. RAB-0157) and cluster of differentiation (CD)34
(kit-0004) (both from Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
China). The sections were washed with PBS and incubated in
horseradish peroxidase-conjugated goat anti-rabbit/mouse
immunoglobulin (Ig)G polymer (undiluted, cat. no. 9902; Fuzhou
Maixin Biotech Co., Ltd.) at room temperature for 30 min. Finally,
slides were stained with 3,3′-diaminobenzidine and counterstained
with 0.5% hematoxylin at room temperature. The assessment was
performed by two independent pathologists who were blinded to
clinical parameters and the clinical outcomes of the patients. The
proportion score represented the estimated fraction of positively
stained tumor cells: 0≤25%, 26≤1≤50%; 51≤2≤75%; 3>75%. The
intensity score represented the estimated average staining
intensity of positive tumor cells: 0, Negative; 1, weak; 2,
moderate; and 3, strong. The expression level of FAP-α and VEGF-A
was evaluated using the product of proportion score and intensity
score in 5 fields (×400, magnification) using a CX31 microscope
(Olympus Corporation, Tokyo, Japan) and mean value was obtained
(<4 as low expression, ≥4 as high expression). Microvessel
density (MVD) was determined by CD34 immunoreactivity and
quantified as described previously (11).
Transfection
Plasmid (0.5 µg) PcDNA3.1/FAP-vector (Shanghai
GenePharma Co., Ltd., Shanghai, China) was transfected into SW1116
cells at 4×104 cells/well in a 6-well plate using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) to
upregulate FAP-α expression, and p-GPU6/FAP-short hairpin (sh)RNA
(Shanghai GenePharma Co., Ltd.) was transfected into HT29 cells to
silence FAP-α expression. Empty plasmids were used as the control.
After 48 h, the supernatant was collected as conditioned medium
(CM) and protein was extracted for further research.
Western blot analysis
Protein were extracted from cells using
radioimmunoprecipitation lysis buffer containing 1%
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology, Haimen, China) and total proteins were measured
using a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. A total of 350
µg total protein was loaded in each well of a 5% acrylamide gel and
separated by a 10% separating gel prior to transfer onto a
polyvinylidene difluoride membrane. After being blocked in 5%
non-fat milk (cat. no. 232100; BD Biosciences, Franklin Lakes, NJ,
USA) at room temperature for 1 h, the membrane was incubated with
the primary antibodies at 4°C overnight and with peroxidase-linked
goat anti-rabbit-IgG (cat. no. SA00001-2, 1:5,000; Proteintech
Group Inc., Chicago, IL, USA) at room temperature for 1 h. Signals
were detected using enhanced chemiluminescence reagents (Pierce;
Thermo Fisher Scientific, Inc.) and analyzed using Image-Pro Plus
software (version 5.1; Media Cybernetics, Inc., Rockville, MD,
USA). The primary antibodies were as follows: FAP-α (cat. no.
ab53066, rabbit anti-human, 1:1,000; Epitomics, Burlingame, CA,
USA); VEGF-A (cat. no. 19003-1-AP, 1:1,000; Proteintech Group,
Inc.); VEGF-R2 (1:1,000; cat. no. ab2349; Abcam, Cambridge, MA,
USA); phosphorylated-extracellular signal-regulated kinase
(p-ERK)1/2 (cat. no. ab176660, 1:1,000; Epitomics; Abcam); ERK1/2
(cat. no. 9102, 1:1,000); phosphorylated-RAC-α
serine/threonine-protein kinase (cat. no. 13038, 1:1,000); and Akt
(cat. no. 4685, 1:1,000) (all from Cell Signaling Technology, Inc.)
and GAPDH (cat. no. 8727, 1:5,000; Wuhan Sanying Biotechnology,
Wuhan, China).
ELISA analysis
VEGF-A in the supernatant was detected using a
VEGF-A ELISA kit (cat. no. CSB-EL025833SH; Cusabio Biotech Co.,
Ltd., Wuhan, China), according to the manufacturer's instructions.
The experiment was repeated at least three times.
MTT assay of HUVECs
Cells were counted and plated in 96-well plate in
triplicate at 4×103 cells/well in 100 µl medium. An MTT
assay was performed dimethyl sulfoxide was used to dissolve
formazan at 6 and 48 h, according to the manufacturer's protocol
(Beijing Solarbio Science and Technology Co., Ltd., Beijing,
China). Optical density (OD) was measured at 490 nm. The
proliferation rate was calculated as the OD value at 48 h/the OD
value at 6 h. The experiment was repeated three times.
Statistical analysis
SPSS software version 13.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. All data are expressed as
the mean ± standard deviation, and the differences between groups
were analyzed using a one-way analysis of variance with Dunnett's
post hoc test. Survival curves were drawn using the Kaplan-Meier
method and compared using the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
IHC staining of FAP-α, VEGF-A and CD34
expression in CRC tissues
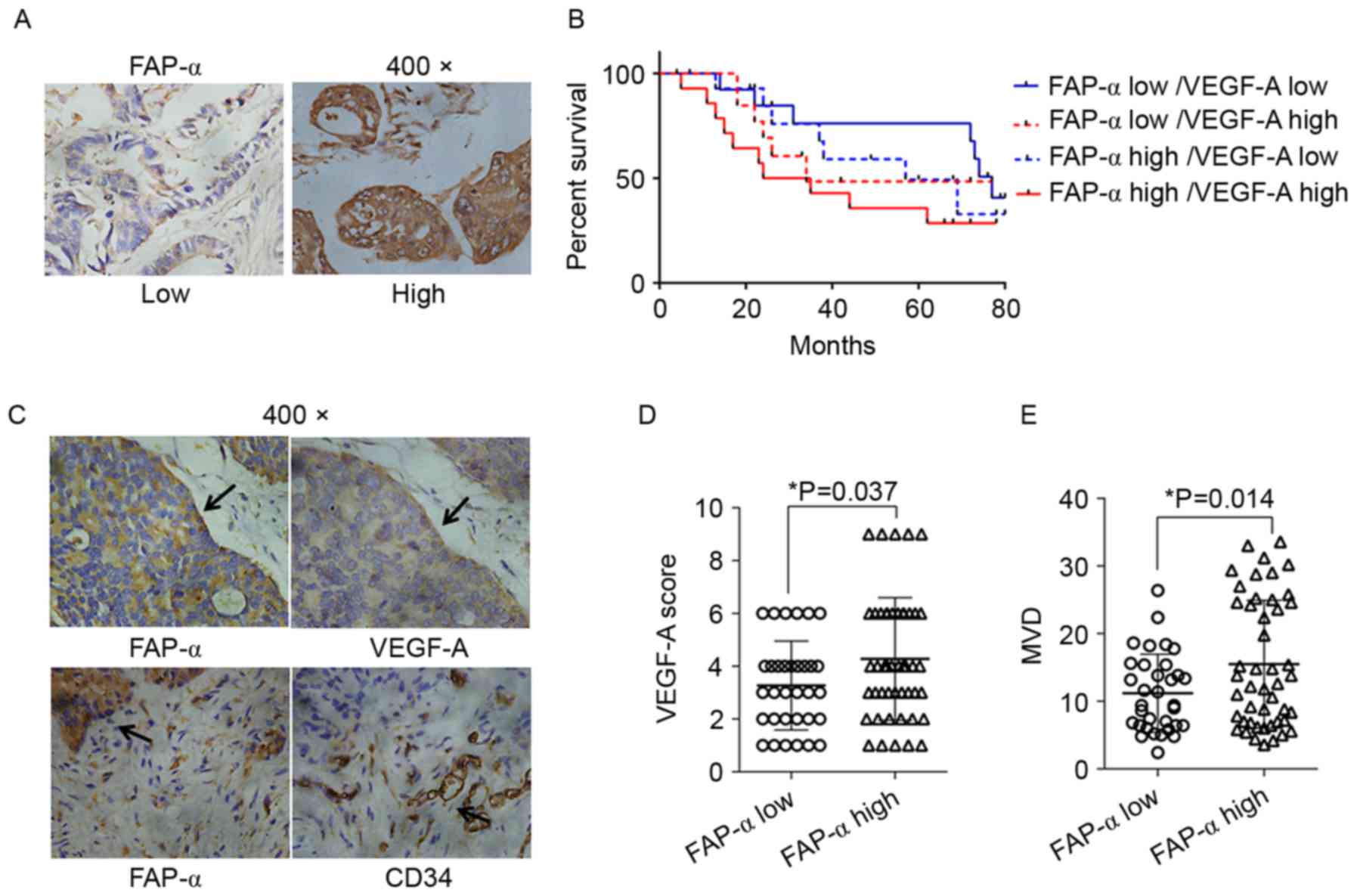
As illustrated in Fig.
1A, FAP-α expression was stained by IHC in the cytoplasm of CRC
cells, and sporadic staining was additionally identified in stromal
cells. As presented in Fig. 1B,
IHC staining of serial slides identified strong VEGF-A staining and
more microvessels in FAP-α high expression tissues. Statistical
analysis demonstrated that the general VEGF-A score (Fig. 1C) and MVD (Fig. 1D) in FAP-α high expression tissues
was increased compared with FAP-α low expression tissues, with a
statistically significant difference (P<0.05). Survival analysis
demonstrated that patients whose tissue samples possessed high
levels of FAP-α and VEGF-A demonstrated markedly worse outcomes in
OS compared with those whose samples possessed low FAP-α or low
VEGF-A expression levels, although the difference was only
statistically significant when compared with samples of the low
FAP-α and low VEGF-A expression group (P=0.049; Fig. 1E; Table I). All the results confirmed the
association between FAP-α and angiogenesis at the tissue level and
implied its important effects on the prognosis of patients with
CRC.
 | Table I.Overall survival differences between
different groups. |
Table I.
Overall survival differences between
different groups.
| Comparisons between
groups | P-value |
|---|
| FAP-α low/VEGF-A high
vs. FAP-α low/VEGF-A low | 0.457 |
| FAP-α high/VEGF-A low
vs. FAP-α high/VEGF-A high | 0.242 |
| FAP-α low/VEGF-A high
vs. FAP-α high/VEGF-A low | 0.833 |
| FAP-α low/VEGF-A low
vs. FAP-α high/VEGF-A high | 0.049a |
| FAP-α low/VEGF-A high
vs. FAP-α high/VEGF-A high | 0.308 |
| FAP-α low/VEGF-A low
vs. FAP-α high/VEGF-A low | 0.388 |
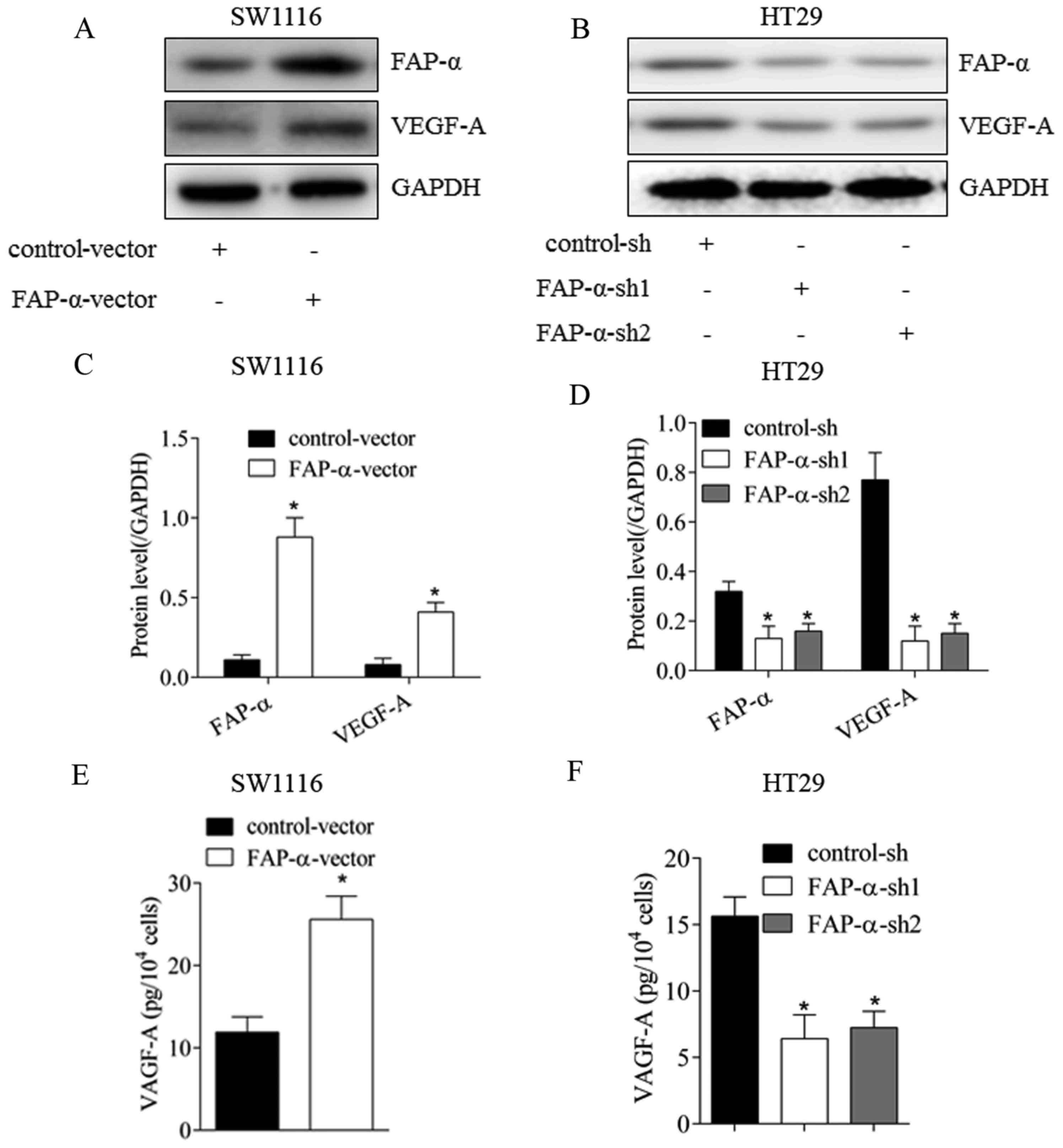
FAP-α may regulate VEGF-A expression
in CRC cells
To further confirm the proangiogenic roles of FAP-α
at the cell level, FAP-α expression was altered via transfection in
CRC cells (Fig. 2). As illustrated
in Fig. 2A and C, following
upregulation of FAP-α in SW1116 cells, VEGF-A expression was
significantly increased (P<0.05). The level of secreted VEGF-A
in the supernatant was additionally elevated (Fig. 2E; P<0.05). Following silencing
of FAP-α expression in HT29 cells, VEGF-A expression in the
cytoplasm (Fig. 2B and D;
P<0.05) and supernatant (Fig.
2F; P<0.05) declined significantly. These results verified
that FAP-α may effectively promote the expression of the important
proangiogenic factor VEGF-A in CRC cells.
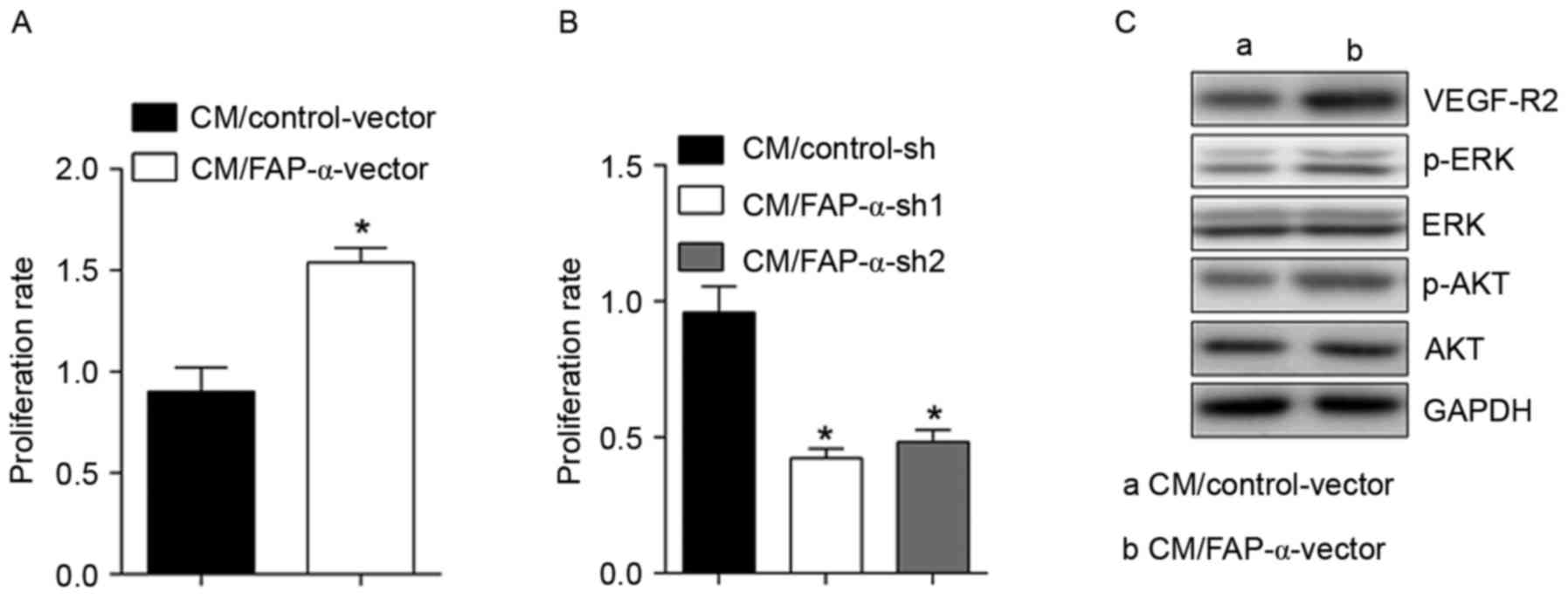
FAP-α in CRC cells may activate
HUVECs
To observe the effects of FAP-α shift on HUVECs,
HUVECs were exposed to CM derived from SW1116 cells with
overexpressed FAP-α (Fig. 3), and
it was observed that the proliferation rate of HUVECs increased
significantly from 0.9±0.11 to 1.54±0.07 (Fig. 3A). VEGF-R2, p-ERK and p-Akt were
significantly upregulated (Fig.
3C). Conversely, following treatment using CM derived from HT29
cells with silenced FAP-α, the proliferation rate of HUVECs
declined significantly from 0.96±0.09 to 0.42±0.04 or 0.48±0.04
(Fig. 3B). These results
demonstrated the activating roles of FAP-α in CRC cells on
HUVECs.
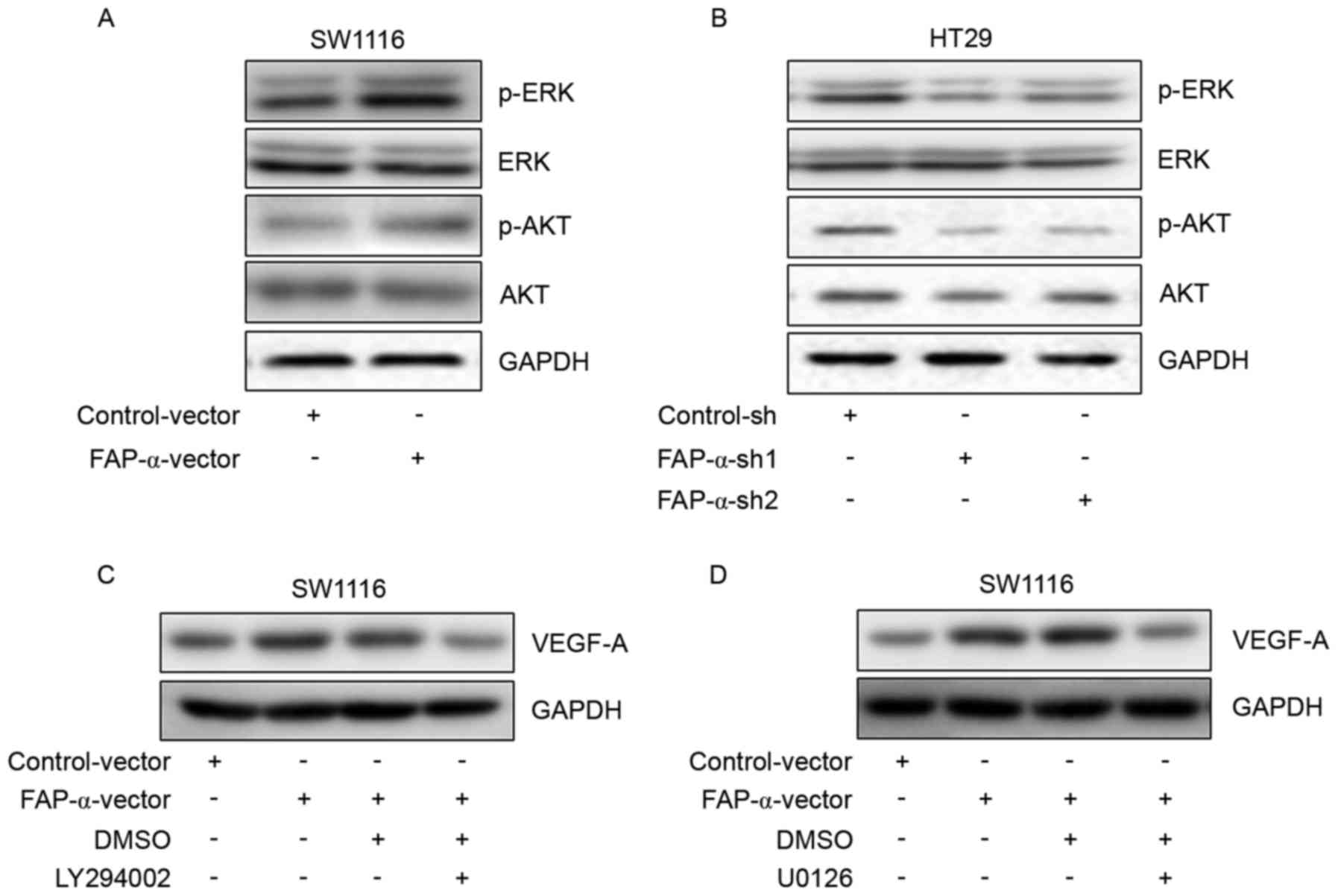
FAP-α regulates VEGF-A expression via
Akt and ERK signaling pathways
To further study the potential molecular mechanism
responsible for the proangiogenic effects of FAP-α, p-ERK and p-Akt
were selected as candidate signal targets. Western blot analysis
identified that FAP-α overexpression in SW1116 cells was able to
activate the phosphorylation of ERK and Akt (Fig. 4A). Phosphorylation of ERK and Akt
was additionally inhibited following FAP-α silencing in HT29 cells
(Fig. 4B). To further confirm
whether p-ERK and p-Akt are involved in the regulation of VEGF-A
expression, western blot analysis was performed and it was
identified that the Akt inhibitor LY294002 (Fig. 4C) and ERK inhibitor U0126 (Fig. 4D) significantly abrogated VEGF-A
upregulation induced by FAP-α. These results demonstrated that Akt
and ERK were involved in the proangiogenic effects of FAP-α.
Discussion
FAP-α is an integral membrane serine peptidase and
tumor stromal FAP-α has been considered to be an important
participant in tumor onset and progression, and even prognosis,
through enzymatic and non-enzymatic functions (12,13).
Wikberg et al (14)
reported that stromal FAP-α in tumor boundaries was not associated
with patient prognosis, while high stromal FAP-α expression in the
center of a tumor was positively correlated with poor prognosis. In
ovarian cancer, stromal FAP-α was positively correlated with lymph
node and omental metastasis, in addition to elevated lymphatic
density (15). Based on the
important roles of stromal FAP-α in tumor progression, FAP-α in
tumor cells has attracted increasing interest and its expression
has been detected in a number of types of epithelial tumor cells,
including breast cancer (16),
pancreatic adenocarcinoma (17),
gastric cancer (18), oral
squamous cell carcinoma (19),
ovarian cancer (20), cervical
cancer (21) and CRC (10). Jia et al (22) reported that FAP-α was significantly
associated with poor outcome in patients with breast cancer, and
promoted the proliferation and inhibited the migration of breast
cancer cells. Lai et al (23) identified that silencing of FAP-α in
the ovarian cancer cell line SKOV3 inhibited tumor growth in
vivo. Shi et al (17)
reported that increased FAP-α expression in pancreatic
adenocarcinoma cells was associated with tumor size, fibrotic
focus, perineural invasion and a worse clinical outcome. The
present study detected FAP-α expression at the tissue level via IHC
and at the cell level by western blotting, and further verified its
presence in CRC cells. IHC staining identified that FAP-α in CRC
cells was positively associated with MVD and VEGF-A expression,
implying its proangiogenic effects for the first time, to the best
of the authors' knowledge. VEGF-A is the most important regulatory
factor in tumor angiogenesis, and altered expression of VEGF-A has
been reported to be associated with a poor prognosis in various
types of human cancer (24,25).
The positive association of FAP-α and VEGF-A suggested that FAP-α
may function in angiogenesis by regulating VEGF-A expression. To
test this hypothesis, FAP-α expression was altered by transfection
and it was identified that VEGF-A expression exhibited the same
alterations in the cytoplasm and supernatant. Patients with double
high expression of FAP-α and VEGF-A exhibited the worst prognosis,
compared with the high FAP-α only or high VEGF-A only groups,
implying that FAP-α alone or VEGF-A alone may not exert significant
effects on prognosis, although their coexpression posessed notable
clinical significance. This was not consistent with a previous
study (24), possibly due to
different samples or the limited number of cases in the present
study.
VEGFR-2 is an essential mediator of VEGF-initiated
angiogenesis and serves crucial roles in regulating multiple
signaling pathways in endothelial cells which may regulate core
angiogenic responses, including proliferation, migration and tube
formation abilities (26,27). The Akt and ERK signaling pathways
are crucial participants in maintaining the different biological
behaviors of different cells including proliferation, migration,
differentiation, drug resistance, apoptosis and phenotype
maintenance (28–30). To detect the direct effects of
FAP-α alterations on HUVECs, HUVECs were treated using CM from
SW1116 cells with FAP-α overexpression, and it was identified that
the expression of VEGF-R2, p-Akt and p-ERK all increased
significantly, as did proliferation ability. CM from HT29 cells
with silenced FAP-α expression exerted the opposite effect on the
proliferation ability of HUVECs. This further confirmed the
proangiogenic function of FAP-α in CRC cells. p-Akt and p-ERK in
SW1116 cells and HT29 cells were significantly affected by FAP-α
expression. The p-Akt inhibitor LY294002 and the p-ERK inhibitor
U0126 were able to inhibit VEGF-A upregulation induced by FAP-α
overexpression. This implied that endogenous FAP-α in CRC cells may
regulate the VEGF-A expression of CRC cells and HUVEC activation
via the Akt and ERK signaling pathways.
In conclusion, the present study provided further
evidence of the presence of FAP-α in CRC cells, and additionally
demonstrated that FAP-α in CRC cells was able to promote
angiogenesis via the Akt and ERK signaling pathways. This provided
novel knowledge about the functions of endogenous FAP-α in tumor
cells and supplied further evidence for treating FAP-α as a target
in therapy.
References
|
1
|
Hubbard JM: Management of colorectal
cancer in older adults. Clin Geriatr Med. 32:97–111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arcaroli JJ, Tai WM, McWilliams R, Bagby
S, Blatchford PJ, Varella-Garcia M, Purkey A, Quackenbush KS, Song
EK, Pitts TM, et al: A NOTCH1 gene copy number gain is a prognostic
indicator of worse survival and a predictive biomarker to a Notch1
targeting antibody in colorectal cancer. Int J Cancer. 138:195–205.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dickinson KJ and Blackmon SH: Results of
pulmonary resection: Colorectal carcinoma. Thorac Surg Clin.
26:41–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuijer A, Furnée EJ and Smakman N:
Combined surgery for primary colorectal cancer and synchronous
pulmonary metastasis: A pilot experience in two patients. Eur J
Gastroenterol Hepatol. 28:15–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Zhang Y, Zhao Q, Wang J and He X:
MicroRNA-10a influences osteoblast differentiation and angiogenesis
by regulating β-catenin expression. Cell Physiol Biochem.
37:2194–2208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salem A and O'Connor JP: Assessment of
tumor angiogenesis: Dynamic contrast-enhanced MR imaging and
beyond. Magn Reson Imaging Clin N Am. 24:45–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mhawech-Fauceglia P, Yan L, Sharifian M,
Ren X, Liu S, Kim G, Gayther SA, Pejovic T and Lawrenson K: Stromal
expression of fibroblast activation protein alpha (FAP) predicts
platinum resistance and shorter recurrence in patients with
epithelial ovarian cancer. Cancer Microenviron. 8:23–31. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Y, Wang S and Kelly T: Seprase
promotes rapid tumor growth and increased microvessel density in a
mouse model of human breast cancer. Cancer Res. 64:2712–2716. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng JD, Dunbrack RL Jr, Valianou M,
Rogatko A, Alpaugh RK and Weiner LM: Promotion of tumor growth by
murine fibroblast activation protein, a serine protease, in an
animal model. Cancer Res. 62:4767–4772. 2002.PubMed/NCBI
|
|
10
|
Iwasa S, Jin X, Okada K, Mitsumata M and
Ooi A: Increased expression of seprase, a membrane-type serine
protease, is associated with lymph node metastasis in human
colorectal cancer. Cancer Lett. 199:91–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vermeulen PB, Gasparini G, Fox SB,
Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E,
Magnani E, et al: Second international consensus on the methodology
and criteria of evaluation of angiogenesis quantification in solid
human tumours. Eur J Cancer. 38:1564–1579. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly T1, Huang Y, Simms AE and Mazur A:
Fibroblast activation protein-α: A key modulator of the
microenvironment in multiple pathologies. Int Rev Cell Mol Biol.
297:83–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Simms AE, Mazur A, Wang S, León
NR, Jones B, Aziz N and Kelly T: Fibroblast activation protein-α
promotes tumor growth and invasion of breast cancer cells through
non-enzymatic functions. Clin Exp Metastasis. 28:567–579. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wikberg ML, Edin S, Lundberg IV, Van
Guelpen B, Dahlin AM, Rutegård J, Stenling R, Oberg A and Palmqvist
R: High intratumoral expression of fibroblast activation protein
(FAP) in colon cancer is associated with poorer patient prognosis.
Tumour Biol. 34:1013–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schauer IG, Sood AK, Mok S and Liu J:
Cancer-associated fibroblasts and their putative role in
potentiating the initiation and development of epithelial ovarian
cancer. Neoplasia. 13:393–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelly T, Kechelava S, Rozypal TL, West KW
and Korourian S: Seprase, a membrane-bound protease, is
overexpressed by invasive ductal carcinoma cells of human breast
cancers. Mod Pathol. 11:855–863. 1998.PubMed/NCBI
|
|
17
|
Shi M, Yu DH, Chen Y, Zhao CY, Zhang J,
Liu QH, Ni CR and Zhu MH: Expression of fibroblast activation
protein in human pancreatic adenocarcinoma and its
clinicopathological significance. World J Gastroenterol.
18:840–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mori Y, Kono K, Matsumoto Y, Fujii H,
Yamane T, Mitsumata M and Chen WT: The expression of a type II
transmembrane serine protease (Seprase) in human gastric carcinoma.
Oncology. 67:411–419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y,
Zhang Y, Hua S, Fu Q, Zhao M, et al: Downregulation of FAP
suppresses cell proliferation and metastasis through PTEN/PI3K/AKT
and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death
Dis. 5:e11552014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kennedy A, Dong H, Chen D and Chen WT:
Elevation of seprase expression and promotion of an invasive
phenotype by collagenous matrices in ovarian tumor cells. Int J
Cancer. 124:27–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin X, Iwasa S, Okada K, Mitsumata M and
Ooi A: Expression patterns of seprase, a membrane serine protease,
in cervical carcinoma and cervical intraepithelial neoplasm.
Anticancer Res. 23:3195–3198. 2003.PubMed/NCBI
|
|
22
|
Jia J, Martin TA and Jiang WG: FAP-α
(fibroblast activation protein-α) is involved in the control of
human breast cancer cell line growth and motility via the FAK
pathway. BMC Cell Biol. 15:162014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lai D, Ma L and Wang F: Fibroblast
activation protein regulates tumor-associated fibroblasts and
epithelial ovarian cancer cells. Int J Oncol. 41:541–550. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin J, Yun D, Tao Li, Yalei L, Yudong W,
Yan Z, Xinliang Z and Wei L: Differential expression of vascular
endothelial growth factor-A, -C and -D for the diagnosis and
prognosis of cancer patients with malignant effusions. Oncol Lett.
10:667–674. 2015.PubMed/NCBI
|
|
25
|
Zhao H, Wu Y, Chen Y and Liu H: Clinical
significance of hypoxia-inducible factor 1 and VEGF-A in
osteosarcoma. Int J Clin Oncol. 20:1233–1243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gheorghescu AK, Tywoniuk B, Duess J,
Buchete NV and Thompson J: Exposure of chick embryos to cadmium
changes the extra-embryonic vascular branching pattern and alters
expression of VEGF-A and VEGF-R2. Toxicol Appl Pharmacol.
289:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adeoye OO, Bouthors V, Hubbell MC,
Williams JM and Pearce WJ: VEGF receptors mediate hypoxic
remodeling of adult ovine carotid arteries. J Appl Physiol (1985).
117:777–787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Amico MA, Ghinassi B, Izzicupo P, Di
Ruscio A and Di Baldassarre A: IL-6 activates PI3K and PKCζ
signaling and determines cardiac differentiation in rat embryonic
H9c2 cells. J Cell Physiol. 231:576–586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ge C, Cawthorn WP, Li Y, Zhao G,
Macdougald OA and Franceschi RT: Reciprocal control of osteogenic
and adipogenic differentiation by ERK/MAP kinase phosphorylation of
Runx2 and PPARγ transcription factors. J Cell Physiol. 231:587–596.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishii M, Nakahara T, Ikeuchi S and
Nishimura M: β-amyrin induces angiogenesis in vascular endothelial
cells through the Akt/endothelial nitric oxide synthase signaling
pathway. Biochem Biophys Res Commun. 467:676–682. 2015. View Article : Google Scholar : PubMed/NCBI
|