Introduction
Asthma is a chronic inflammatory disease of the
lower airway, and its prevalence continues to increase in the past
20 years (1). Asthma is
characterized by airflow obstruction, airway inflammation and
airway remodeling (2). Despite
many studies focusing on the pathogenesis of asthma, the underlying
mechanisms remain unclear. Airway smooth muscle is a major
component of the remodeled airway in patients with asthma (3). Previous studies demonstrated that
abnormal proliferation and migration of airway smooth muscle cells
(ASMCs) is important in the pathogenesis of asthma (4,5). In
addition, platelet-derived growth factor (PDGF)-BB has been
reported to induce ASMC proliferation and migration (6,7), and
elevated PDGF-BB expression is correlated with the severity of the
disease (8). Therefore, inhibiting
PDGF-BB-induced ASMC proliferation and migration may provide a
novel therapeutic method for asthma.
Paeoniflorin (PF) is one of the major active
ingredients of Paeonia lactiflora. There is substantial
evidence that PF exhibits anti-inflammatory, antitumor,
immunoregulatory, and nerve protective effects (9–12).
For example, PF treatment significantly suppresses proliferation
and invasion in human breast cancer cells (13). Recently, Sun et al (14) reported that PF oral administration
in asthmatic mice markedly reduced airway hyperresponsiveness to
aerosolized methacholine and decreased interleukin (IL)-5, IL-13,
IL-17 and eotaxin levels in bronchoalveolar lavage fluid. However,
the effects of PF on PDGF-BB-induced ASMC proliferation and
migration remain unknown. The present study was designed to
investigate the effects of PF treatment on human ASMCs and the
underlying mechanism.
Materials and methods
Cell culture
The human ASMC line was purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in
DMEM (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
medium containing 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin and
100 mg/ml streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) in a humidified atmosphere of 5% CO2 at 37°C.
ASMCs in passages 9–20 were used for the experiments presented in
this study.
Cell viability assay
Cell viability was measured using an MTT assay. In
brief, ASMCs at a density of 1×104 cells/well were
seeded in a 96-well plate and grown in DMEM medium containing 10%
FBS for 24 h. ASMCs were pretreated with PF (10, 20 and 40 nM;
Sigma-Aldrich; Merck KGaA) for 1 h prior to stimulation with
PDGF-BB (10 ng/ml; Sigma-Aldrich; Merck KGaA) for 24, 48 or 72 h.
Then, MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each
well and incubated for 4 h at 37°C in 5% CO2.
Subsequently, the supernatants were removed, and 150 µl of dimethyl
sulfoxide was added to dissolve the formazan crystals. The
absorbance at 450 nm was measured using a Spectra Max 190
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Cell migration assay
Cell migration was examined using transwell
chambers. In brief, following treatment as described above, ASMCs
at a density of 5×104 cells/well suspended in 0.1% FBS
medium were added into the upper chamber. DMEM medium (500 µl)
containing 10% FBS, with or without PDGF-BB, was added into the
lower chamber. Following incubation at 37°C for 24 h, cells
migrated to the lower surface of the transwell membrane were
stained with hematoxylin and eosin (Sigma-Aldrich; Merck KGaA), and
the mean number of cells per four fields of view was counted under
a light microscope. Migration was measured as the number of
cells/field.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from ASMCs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. cDNA was synthesized from the
extracted RNA (3 µg) using the SuperScript First-Strand cDNA
Synthesis SuperMix kit (Invitrogen; Thermo Fisher Scientific,
Inc.). qPCR analysis was performed using a 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) with
Fast Start Universal SYBR Green Master mix (Roche Diagnostics,
Basel, Switzerland). The specific primers were: α-smooth muscle
actin (α-SMA) sense, 5′-CTATTCCTTCGTGACTACT-3′ and antisense,
5′-ATGCTGTTATAGGTGGTGGTT-3′; β-actin sense,
5′-TTAGTTGCGTTACACCCTTTC-3′ and antisense,
5′-ACCTTCACCGTTCCAGTTT-3′. The PCR cycling program was 95°C for 3
min, then 35 cycles of 94°C for 20 sec, 59°C for 30 sec and 72°C
for 20 sec, and a final extension at 72°C for 5 min. β-actin was
used as a normalization control and the results were analyzed by
the method of 2−ΔΔcq (15).
Western blot analysis
ASMCs were washed with PBS and lysed using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Nantong, China). The cell lysate supernatants were
harvested by centrifugation at 10,000 × g for 10 min at 4°C. The
protein concentration was determined using a Bradford protein
assay. Equal amounts of protein (30 µg) were separated by 10%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(Sigma-Aldrich; Merck KGaA). Following blocking with 5% skim milk
in Tris-buffered saline (TBS) with 0.1% Tween-20 at room
temperature for 1 h, the membranes were incubated overnight at 4°C
with primary antibodies targeting α-SMA (1:2,500; cat. no.
sc-53142), phosphoinositide 3-kinase (PI3K, 1:2,000; cat. no.
sc-365290), phosphorylated (p)-PI3K (1:2,500; sc-293115), AKT
serine/threonine kinase 1 (Akt; (1:1,000; cat. no. sc-5298), p-Akt
(1:1,000; cat. no. sc-52940) and GAPDH (1:3,000, cat. no. sc-59540;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Then, the
membranes were washed with TBS containing 0.1% Tween for 10 min and
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:2,500, cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. Protein bands were evaluated by
enhanced chemiluminescence (Thermo Fisher Scientific, Inc.). The
optical densities of the bands were quantified using Gel-Pro
Analyzer v4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Data analysis
The results were analyzed using SPSS software
version 13.0 (SPSS, Inc., Chicago, IL, USA). All experiments were
performed at least in triplicate and results are expressed as mean
± standard deviation. Statistical significance was analyzed with
the one-way analysis of variance or the Student's two-tailed t-test
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PF treatment suppresses
PDGF-BB-induced ASMC growth
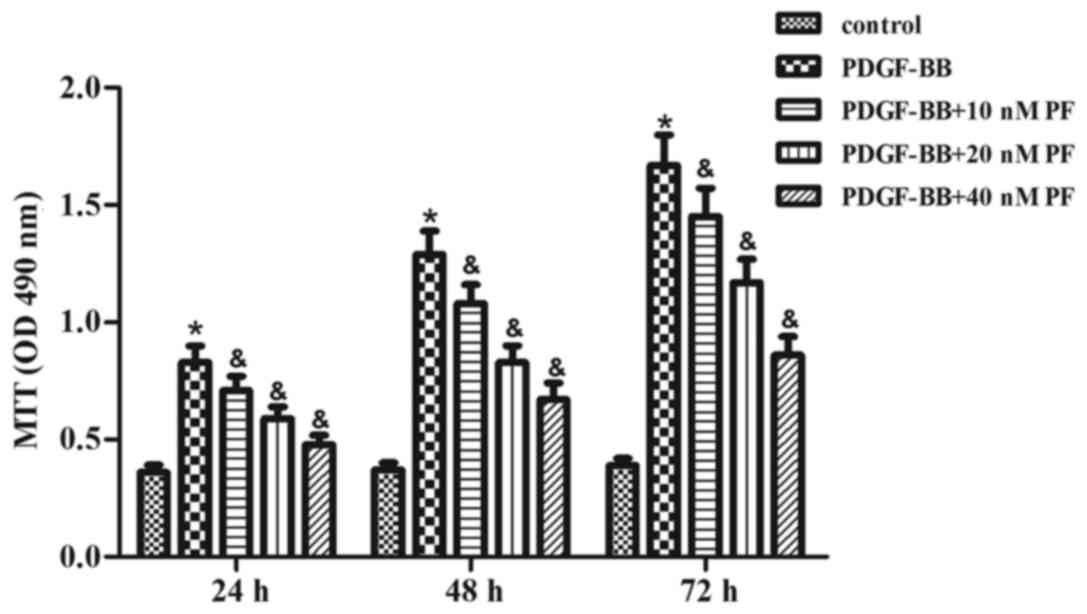
First, the effect of PF treatment on ASMC growth was
examined using the MTT assay. As indicated in Fig. 1, PDGF-BB stimulation significantly
induced growth in ASMCs over the course of 72 h, compared with the
control group. However, the PDGF-BB-induced effect on ASMC growth
was inhibited by PF pretreatment in a concentration-dependent
manner (Fig. 1).
PF treatment suppresses
PDGF-BB-induced ASMC migration
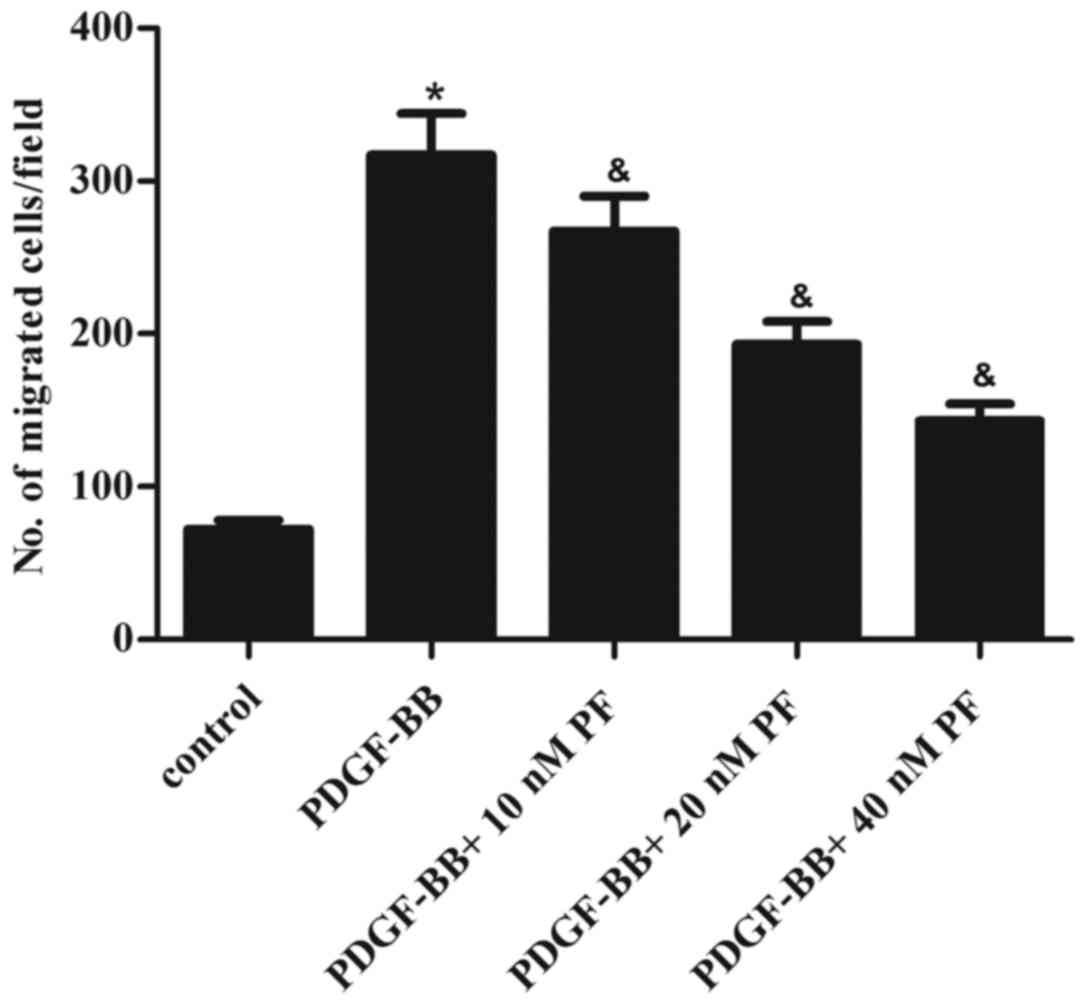
ASMC migration is hypothesized to be a key event in
the pathogenesis of asthma (16).
Thus, the effect of PF treatment on ASMC migration in response to
PDGF-BB stimulation was examined. The results of transwell
migration assays demonstrated that stimulation with PDGF-BB greatly
promoted the migration of ASMCs, compared with the control group
(Fig. 2). However, PF pretreatment
partially reversed this effect in a dose-dependent manner (Fig. 2).
PF treatment suppresses
PDGF-BB-induced α-SMA expression in ASMCs
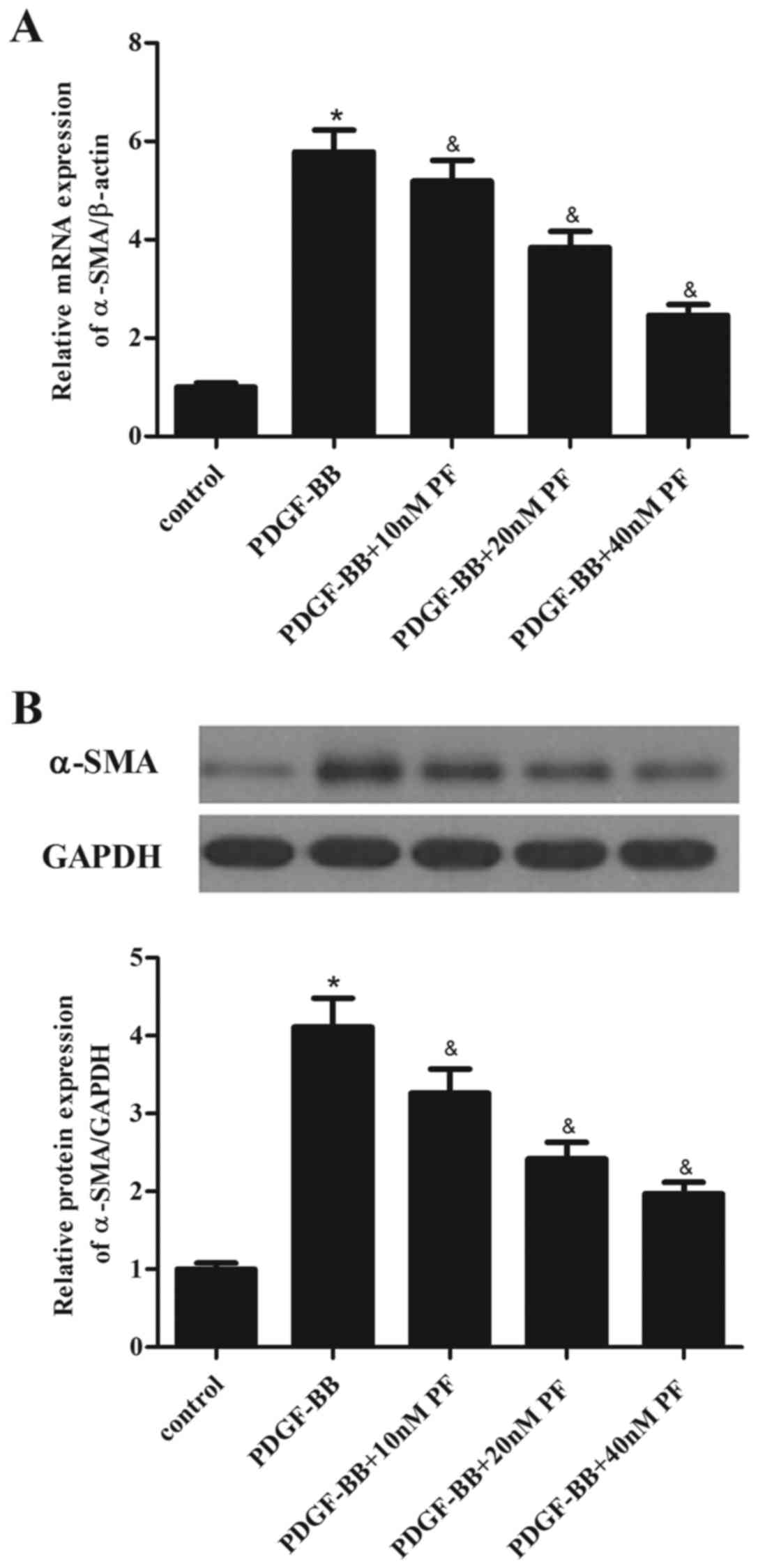
α-SMA is a major constituent of the contractile
apparatus of ASMCs, and its expression has been associated to the
progression of asthma (17).
Therefore, the effect of PF treatment on α-SMA expression in ASMCs
was investigated. As demonstrated in Fig. 3A, PDGF-BB stimulation in ASMCs
significantly increased the mRNA expression levels of α-SMA,
compared with the control group. However, PF pretreatment partially
inhibited the PDGF-BB-induced α-SMA mRNA overexpression (Fig. 3A). Similarly, the results of
western blot analysis demonstrated that PF pretreatment suppressed
PDGF-BB-induced α-SMA protein overexpression in ASMCs, compared
with cells treated with PDGF-BB alone (Fig. 3B).
PF treatment inhibits PDGF-BB-induced
phosphorylation of PI3K and Akt in ASMCs
To explore the signaling mechanisms involved in the
effects of PF on ASMC growth and migration, the phosphorylation
status of PI3K and Akt was investigated by western blotting. As
demonstrated in Fig. 4, PDGF-BB
stimulation significantly activated PI3K and Akt phosphorylation in
ASMCs compared with the control group. This activation was
partially reversed by PF pretreatment, with phosphorylation levels
for both PI3K and Akt significantly reduced compared with cells
treated with PDGF-BB alone (Fig.
4).
Discussion
Abnormal proliferation and migration of ASMCs is
involved in the progression of asthma (18). The present results demonstrated
that PF treatment significantly inhibited PDGF-BB-induced ASMC
growth and migration. PF pretreatment also suppressed
PDGF-BB-induced α-SMA expression in ASMCs. Finally, PF pretreatment
reduced PDGF-BB-induced phosphorylation of PI3K and Akt in
ASMCs.
ASMCs obtained from asthmatic patients proliferate
faster in culture than those obtained from non-asthmatic subjects
(19). It has been reported that
PF inhibits the proliferation of lung cancer A549 cells by blocking
cell cycle progression in the G0/G1 phase (20). PF has also been demonstrated to be
effective in the treatment of lung fibrosis in animal models
(21). In the present study, PF
treatment significantly inhibited PDGF-BB-induced growth of ASMCs,
suggesting that PF may attenuate asthma progression via suppressing
ASMC proliferation.
In addition, increased migration of ASMCs is thought
to participate in the progression of asthma. ASMCs from patients
with asthma exhibit more migratory capabilities compared with cells
from normal subjects (22). A
growing body of evidence indicates that PDGF-BB is a potent
chemoattractant that induces ASMC migration (23–25).
Thus, inhibition of ASMC migration represents a potentially
important therapeutic strategy for the treatment of asthma. In the
present study, PDGF-BB stimulation significantly induced ASMC
migration, while PF pretreatment significantly inhibited this
PDGF-BB-induced migration effect in ASMCs.
ASMC phenotypic switching (contractile to synthetic)
is a critical mediator of airway remodeling in asthma. The
phenotypic modulation of ASMCs contributes to the pathology of
asthma by altering proliferation, secretion of inflammatory
mediators and matrix deposition (26,27).
It has been reported that increased levels of α-SMA facilitate ASMC
proliferation and migration, which are important for asthma
progression (28). In vitro
PDGF-BB stimulation induces ASMC phenotypic switching (29,30).
PF treatment has been demonstrated to decrease the contents of
hydroxyproline, type I collagen and α-SMA in lung tissues of
bleomycin-induced pulmonary fibrosis (31). In the present study, PF
pretreatment suppressed PDGF-BB-induced α-SMA expression in ASMCs,
suggesting that PF may attenuate ASMC hyperplasia and
hypertrophy.
PDGF-BB has been reported to stimulate proliferation
and migration in ASMCs through PI3K signaling, which in turn
activates Akt signaling (32).
Inhibition of PI3K/Akt signaling significantly inhibits ASMC
proliferation and migration (33,34).
Furthermore, Zheng et al (35) reported that PF significantly
inhibits the expression of PI3K, Akt, p-Akt and p-signal transducer
and activator of transcription 3 in human gastric carcinoma MGC-803
cells, while a PI3K agonist (insulin-like growth factor 1) reverses
the effect of PF on MGC-803 cell proliferation. In the present
study, PDGF-BB treatment significantly activated PI3K and Akt
phosphorylation in ASMCs. The present results are consistent with
previous studies reporting that PI3K/Akt signaling mediates ASMC
proliferation and migration induced by PDGF-BB. However, in the
present study, PF pretreatment significantly suppressed
PDGF-BB-induced phosphorylation of PI3K and Akt. These data suggest
that PF inhibited PDGF-BB-induced human ASMC growth and migration
through suppressing the activation of the PI3K/Akt signaling
pathway.
In conclusion, the present study demonstrated for
the first time that PF inhibited ASMC growth and migration induced
by PDGF-BB. The inhibitory effect involved, at least partly,
inhibition of the PI3K/Akt signaling pathway. Therefore, the
present results provide evidence that PF may serve as a potential
therapeutic agent for the treatment of asthma in the future.
References
|
1
|
Barros R, Moreira A, Padrão P, Teixeira
VH, Carvalho P, Delgado L, Lopes C, Severo M and Moreira P: Dietary
patterns and asthma prevalence, incidence and control. Clin Exp
Allergy. 45:1673–1680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Halwani R, Al-Muhsen S and Hamid Q: Airway
remodeling in asthma. Curr Opin Pharmacol. 10:236–245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bara I, Ozier A, Tunon de Lara JM, Marthan
R and Berger P: Pathophysiology of bronchial smooth muscle
remodelling in asthma. Eur Respir J. 36:1174–1184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madison JM: Migration of airway smooth
muscle cells. Am J Respir Cell Mol Biol. 29:8–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirst SJ, Martin JG, Bonacci JV, Chan V,
Fixman ED, Hamid QA, Herszberg B, Lavoie JP, McVicker CG, Moir LM,
et al: Proliferative aspects of airway smooth muscle. J Allergy
Clin Immunol. 114 Suppl 2:S2–S17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spinelli AM, González-Cobos JC, Zhang X,
Motiani RK, Rowan S, Zhang W, Garrett J, Vincent PA, Matrougui K,
Singer HA and Trebak M: Airway smooth muscle STIM1 and Orai1 are
upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC
currents, proliferation, and migration. Pflugers Arch. 464:481–492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito I, Fixman ED, Asai K, Yoshida M,
Gounni AS, Martin JG and Hamid Q: Platelet-derived growth factor
and transforming growth factor-beta modulate the expression of
matrix metalloproteinases and migratory function of human airway
smooth muscle cells. Clin Exp Allergy. 39:1370–1380. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohno I, Nitta Y, Yamauchi K, Hoshi H,
Honma M, Woolley K, O'Byrne P, Dolovich J, Jordana M, Tamura G, et
al: Eosinophils as a potential source of platelet-derived growth
factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma. Am
J Respir Cell Mol Biol. 13:639–647. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim ID and Ha BJ: Paeoniflorin protects
RAW 264.7 macrophages from LPS-induced cytotoxicity and
genotoxicity. Toxicol In Vitro. 23:1014–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Zhou H, Wang CX, Li YS, Xie HY,
Luo JD and Zhou Y: Paeoniflorin inhibits growth of human colorectal
carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol.
50:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Yang Z, Yang S, Du J and Wang S:
Immunoregulatory effects of paeoniflorin exerts anti-asthmatic
effects via modulation of the Th1/Th2 equilibrium. Inflammation.
38:2017–2025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Xiong X and Liu Y: Protective effect
of paeoniflorin against optic nerve crush. J Huazhong Univ Sci
Technolog Med Sci. 27:650–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q, Yuan Y, Cui J, Xiao T and Jiang
D: Paeoniflorin inhibits proliferation and invasion of breast
cancer cells through suppressing Notch-1 signaling pathway. Biomed
Pharmacother. 78:197–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun J, Wu J, Xu C, Luo Q, Li B and Dong J:
Paeoniflorin attenuates allergic inflammation in asthmatic mice.
Int Immunopharmacol. 24:88–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joubert P, Lajoie-Kadoch S, Labonté I,
Gounni AS, Maghni K, Wellemans V, Chakir J, Laviolette M, Hamid Q
and Lamkhioued B: CCR3 expression and function in asthmatic airway
smooth muscle cells. J Immunol. 175:2702–2708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu C, Zhang J, Su M and Fan X: Nuclear
factor-ĸB mediates the phenotype switching of airway smooth muscle
cells in a murine asthma model. Int J Clin Exp Pathol.
8:12115–12128. 2015.PubMed/NCBI
|
|
18
|
Wei Y, Xu YD, Yin LM, Wang Y, Ran J, Liu
Q, Ma ZF, Liu YY and Yang YQ: Recombinant rat CC10 protein inhibits
PDGF-induced airway smooth muscle cells proliferation and
migration. Biomed Res Int. 2013:6909372013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michaeloudes C, Chang PJ, Petrou M and
Chung KF: Transforming growth factor-β and nuclear factor
E2-related factor 2 regulate antioxidant responses in airway smooth
muscle cells: Role in asthma. Am J Respir Crit Care Med.
184:894–903. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hung JY, Yang CJ, Tsai YM, Huang HW and
Huang MS: Antiproliferative activity of paeoniflorin is through
cell cycle arrest and the Fas/Fas ligand-mediated apoptotic pathway
in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol
Physiol. 35:141–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji Y, Wang T, Wei ZF, Lu GX, Jiang SD, Xia
YF and Dai Y: Paeoniflorin, the main active constituent of Paeonia
iactiflora roots, atteunates bleomycin-induced pulmonary fibrosis
in mice by suppressing the synthesis of type I collagen. J
Ethnopharmacol. 149:825–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parameswaran K, Radford K, Fanat A,
Stephen J, Bonnans C, Levy BD, Janssen LJ and Cox PG: Modulation of
human airway smooth muscle migration by lipid mediators and Th-2
cytokines. Am J Respir Cell Mol Biol. 37:240–247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ning Y, Huang H, Dong Y, Sun Q, Zhang W,
Xu W and Li Q: 5-Aza-2′-deoxycytidine inhibited PDGF-induced rat
airway smooth muscle cell phenotypic switching. Arch Toxicol.
87:871–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Kong H, Zeng X, Wang J, Wang Z, Yan
X, Wang Y, Xie W and Wang H: Iptakalim inhibits PDGF-BB-induced
human airway smooth muscle cells proliferation and migration. Exp
Cell Res. 336:204–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu YD, Wei Y, Wang Y, Yin LM, Park GH, Liu
YY and Yang YQ: Exogenous S100A8 protein inhibits PDGF-induced
migration of airway smooth muscle cells in a RAGE-dependent manner.
Biochem Biophys Res Commun. 472:243–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirst SJ, Walker TR and Chilvers ER:
Phenotypic diversity and molecular mechanisms of airway smooth
muscle proliferation in asthma. Eur Respir J. 16:159–177. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moir LM, Leung SY, Eynott PR, McVicker CG,
Ward JP, Chung KF and Hirst SJ: Repeated allergen inhalation
induces phenotypic modulation of smooth muscle in bronchioles of
sensitized rats. Am J Physiol Lung Cell Mol Physiol. 284:L148–L159.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu GN, Yang K, Xu ZP, Zhu L, Hou LN, Qi H,
Chen HZ and Cui YY: Protective effects of anisodamine on cigarette
smoke extract-induced airway smooth muscle cell proliferation and
tracheal contractility. Toxicol Appl Pharmacol. 262:70–79. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dekkers B, Prins A, Oldenbeuving G, Pool
K, Elzinga C, Meurs H, Schmidt M and Roscioni S: Epac and PKA
inhibit PDGF-induced airway smooth muscle phenotype modulation. Am
J Respir Crit Care Med. 181:A21422010.
|
|
30
|
Carlin SM, Roth M and Black JL: Urokinase
potentiates PDGF-induced chemotaxis of human airway smooth muscle
cells. Am J Physiol Lung Cell Mol Physiol. 284:L1020–L1026. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma J and Jemal A: Breast cancer
statisticsBreast Cancer Metastasis and Drug Resistance. Springer;
New York: pp. 1–18. 2013, View Article : Google Scholar
|
|
32
|
Chiou YL, Shieh JJ and Lin CY: Blocking of
Akt/NF-kappaB signaling by pentoxifylline inhibits platelet-derived
growth factor-stimulated proliferation in Brown Norway rat airway
smooth muscle cells. Pediatr Res. 60:657–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu D, Xie S, Sukkar MB, Lu X, Scully MF
and Chung KF: Inhibition of airway smooth muscle adhesion and
migration by the disintegrin domain of ADAM-15. Am J Respir Cell
Mol Biol. 37:494–500. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Walker TR, Moore SM, Lawson MF, Panettieri
RA Jr and Chilvers ER: Platelet-derived growth factor-BB and
thrombin activate phosphoinositide 3-kinase and protein kinase B:
Role in mediating airway smooth muscle proliferation. Mol
Pharmacol. 54:1007–1015. 1998.PubMed/NCBI
|
|
35
|
Zheng YB, Xiao GC, Tong SL, Ding Y, Wang
QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric carcinoma
cell proliferation through up-regulation of microRNA-124 and
suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol.
21:7197–7207. 2015. View Article : Google Scholar : PubMed/NCBI
|