Introduction
Diabetes mellitus is one of the major public health
problems around the world (1).
According to the compiled data of the International Diabetes
Federation, ~1 in 11 adults has diabetes mellitus worldwide, and
the number of diabetic patients may reach 642 million by the year
2040.
Diabetic cardiomyopathy (DCM) is defined as the
ventricular dysfunction in diabetic patients which do not rely on
some causes, coronary artery disease, hypertension and so on. DCM
is one of the main cardiovascular complications of diabetes which
has become a major cause of morbidity and mortality in diabetic
patients (2,3). Interstitial fibrosis is a
histological hallmark of DCM (4,5).
The normal structure of heart is composed of several
different cell types such as cardiomyocytes, cardiac fibroblasts,
endothelial cells, smooth muscle cells and extracellular matrix
(ECM) including collagens, larninin and fibrinogen (6,7).
Excessive deposition of ECM is one of the important pathological
alterations in diabetic hearts (8). Interstitial collagens such as
collagen-I and collagen-III are the major components of ECM in
heart, which play important roles in supporting and protecting
cardiomyocytes and maintaining the normal structure and function of
myocardial tissue ingredient. The excess production of collagen-I
and collagen-III proteins may be responsible for myocardial
interstitial collagen deposition, disorder, and even heart damage
(9). An abnormal amount of
collagen increases the hardness of the myocardium and reduces the
diastolic function of the heart. With the increase of myocardial
fibrosis, the myocardial systolic function is also damaged.
The isoflavone genistein (GEN;
4′,5,7-trihydroxyisoflavone), which is known to interact with
estrogen receptors and has weak estrogenic activity, is a
phytoestrogen found at high levels in soy products (10,11).
Studies have demonstrated that GEN plays a critical role in
anti-inflammatory, anti-oxidative and anti-proliferation effects
(12,13). Recent research has shown that GEN
can reduce cardiac inflammation and oxidative stress in DCM
(14). GEN can also protect the
liver from chronic injury in D-galactosamine-induced liver fibrosis
by inhibiting accumulation of the collagen matrix, suppressing the
expression of transforming growth factor-β (TGF-β) and activation
of the TGF-β/Smad signaling pathway (15). However, it is not clear whether the
TGF-β/Smad pathway is involved in the cardioprotective effect of
GEN on myocardial fibrosis in diabetic rats. The purpose of the
present study was to investigate the effects of GEN on myocardial
fibrosis in type 1 diabetic rats and to elucidate the underlying
mechanisms. This study may shed some light on GEN as a novel
effective medicine in treating DCM.
Materials and methods
Animals
Male Sprague-Dawley rats (6–7 weeks of age, 160–200
g) were obtained from of Bengbu Medical College Animal
Administration Center. The rats were given free access to normal
diet and water, and housed in cages at room temperature (22±1°C)
with a fixed 12 h light/dark cycle. All animal experiments were
approved by the Animal Ethics Committee of Bengbu Medical College
and conducted in accordance with ethical standards.
Chemicals and reagents
Streptozotocin and GEN were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Carboxymethylcellulose sodium
(CMC-Na) was purchased from Shanghai Sangon Biological Engineering
Co., Ltd., (Shanghai, China). Hydroxyproline (Hyp), creatine kinase
MB isozyme (CK-MB), lactate dehydrogenase (LDH), tumor necrosis
factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6)
assay kits were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, Jiangsu, China). Bradford and bicinchoninic
acid (BCA) protein assay kits were purchased from Beyotime
Biotechnology (Shanghai, China). Rabbit TGF-β1, Smad3,
phospho-Smad3 (p-Smad3) and Smad4 antibodies were purchased from
Abcam (Cambridge, MA, USA). Rabbit collagen-I and collagen-III
antibodies were purchased from Boster Biological Technology (Wuhan,
Hubei, China). Rabbit β-actin antibody was purchased from
Proteintech Group (Wuhan, Hubei, China). Goat anti-rabbit secondary
antibody was purchased from Biosharp Biotechnology (Hefei, Anhui,
China).
Induction of diabetes and experimental
protocol
All rats were divided randomly into 4 groups: Normal
control group (N), diabetic control group (D), low-dose GEN
treatment group (L) and high-dose GEN treatment group (H)
(n=8). Type 1 diabetes mellitus was induced in overnight
fasted rats by administering a single intraperitoneal injection of
55 mg/kg streptozotocin freshly dissolved in 0.1 mol/l sodium
citrate buffer (pH 4.5). The rats in the N group were received an
intraperitoneal injection with the same volume of sodium citrate
buffer. The rats with plasma fasting blood glucose (FBG) level
>16.7 mmol/l (72 h after injection) were considered as type 1
diabetic rats. The FBG level was monitored once a week during the
experimental period. After successful building models, from the
fifth week, the rats in the L and H groups were daily gavaged with
5 and 25 mg/kg GEN solution (freshly dissolved in CMC-Na) for 4
weeks, respectively. The doses of GEN were chosen according to a
method reported (16). The rats in
the N and D groups were daily gavaged with the same volume of
CMC-Na for 4 weeks.
Ventricular hemodynamic
measurements
Rats were anaesthetized by intraperitoneal injection
with chloral hydrate (100 mg/kg) and performed trachea cannula.
Right carotid artery was separated and intubated to the left
ventricle. Left ventricular systolic pressure (LVSP), left
ventricular end-diastolic pressure (LVEDP) and maximal rise/fall
rate of left ventricular pressure (± dp/dtmax)
were recorded by the Med-Lab Biological Recording system (Medease
Company, Nanjing, China).
Detection of FBG level, body-weight
(BW), heart-weight (HW) and HW/BW
After ventricular hemodynamic measurement, the blood
was collected and FBG level was measured. Rats were sacrificed and
their hearts were excised rapidly to place in ice-cold normal
saline, and HW/BW was calculated.
Determination of myocardial Hyp
content
Heart tissue (0.1 g) was homogenized in 0.9 ml
ice-cold normal saline. The supernatant was collected after
centrifugation for 20 min (3,000 rpm/min). The protein
concentration was measured by the Bradford protein assay kit. Hyp
content was measured according to the instruction manual.
Determination of serum CK-MB, LDH,
TNF-α, IL-1β and IL-6 levels
The blood was placed in centrifuge tubes and
centrifuged at 3,000 rpm/min for 20 min. The supernatant was
collected. CK-MB, LDH, TNF-α, IL-1β and IL-6 levels were measured
using the corresponding assay kits according to kit
instructions.
Histomorphological observation by
hematoxylin and eosin (H&E) staining and Masson's trichrome
staining
Fresh myocardial tissue was fixed in 4%
paraformaldehyde, embedded in paraffin, and cut into 5 µm thick
serial sections. After de-waxed, the sections were stained with
H&E and Masson's trichrome, respectively.
Ultrastructure observation under
transmission electron microscope
Fresh myocardial tissues were cut into the size of
1×1×1 mm, fixed in ice-cold 2.5% glutaraldehyde for 2 h, and
post-fixed in 1% osmium tetroxide for 1 h. Ultrathin sections
stained with uranyl acetate and lead citrate were observed with
JEM-1230 transmission electron microscope (JEOL, Tokyo, Japan).
Detection of TGF-β1, Smad3, p-Smad3,
Smad4, collagen-I and collagen-III protein by western blot
analysis
Myocardial tissue (0.1 g) was collected and
homogenized in 1 ml protein extraction buffer (990 µl lysis buffer
+ 10 µl Phenylmethanesulfonyl fluoride). The supernatant was
collected and protein concentration was measured using the BCA
protein assay kit. Proteins (50 µg) were separated using sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto polyvinylidene difluoride membranes. Membrane with p-Smad3 was
blocked in Tris buffered saline Tween-20 (TBST) containing 5%
bovine serum albumin, and membranes with TGF-β1, Smad3, Smad4,
collagen-I, collagen-III and β-actin were blocked in TBST
containing 5% nonfat milk at 37°C for 2 h, and then they were
incubated overnight at 4°C with the following primary antibodies:
Anti-TGF-β1 (1:1,000), anti-Smad3 (1:1,000), anti-p-Smad3
(1:1,000), anti-Smad4 (1:1,000), anti-collagen-I (1:400),
anti-collagen-III (1:400) and anti-β-actin (1:2,000). Membranes
were then washed with TBST and incubated with a horseradish
peroxidase conjugated goat anti-rabbit IgG secondary antibody for 1
h at room temperature. Autoradiographs were scanned with ChemiDoc
XRS system (Bio-Rad, Berkeley, CA, USA) and the band density was
determined with Quantity One software.
Statistical analysis
Data were expressed as mean ± SD. Statistical
comparisons were performed by one-way analysis of variance and the
Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes of ventricular hemodynamic
parameters
Compared with the N group, LVSP and
±dp/dtmax were decreased (P<0.01)
significantly, while LVEDP was increased (P<0.01) significantly
in the D group; Compared with the D group, there were no
statistical differences in ventricular hemodynamic parameters in
the L group; LVSP and ± dp/dtmax were increased
(P<0.01) significantly, LVEDP was decreased (P<0.01) in the H
group. Compared with the L group, LVSP and
±dp/dtmax were increased (P<0.05, P<0.01),
while LVEDP was decreased (P<0.05) in the H group (Fig. 1).
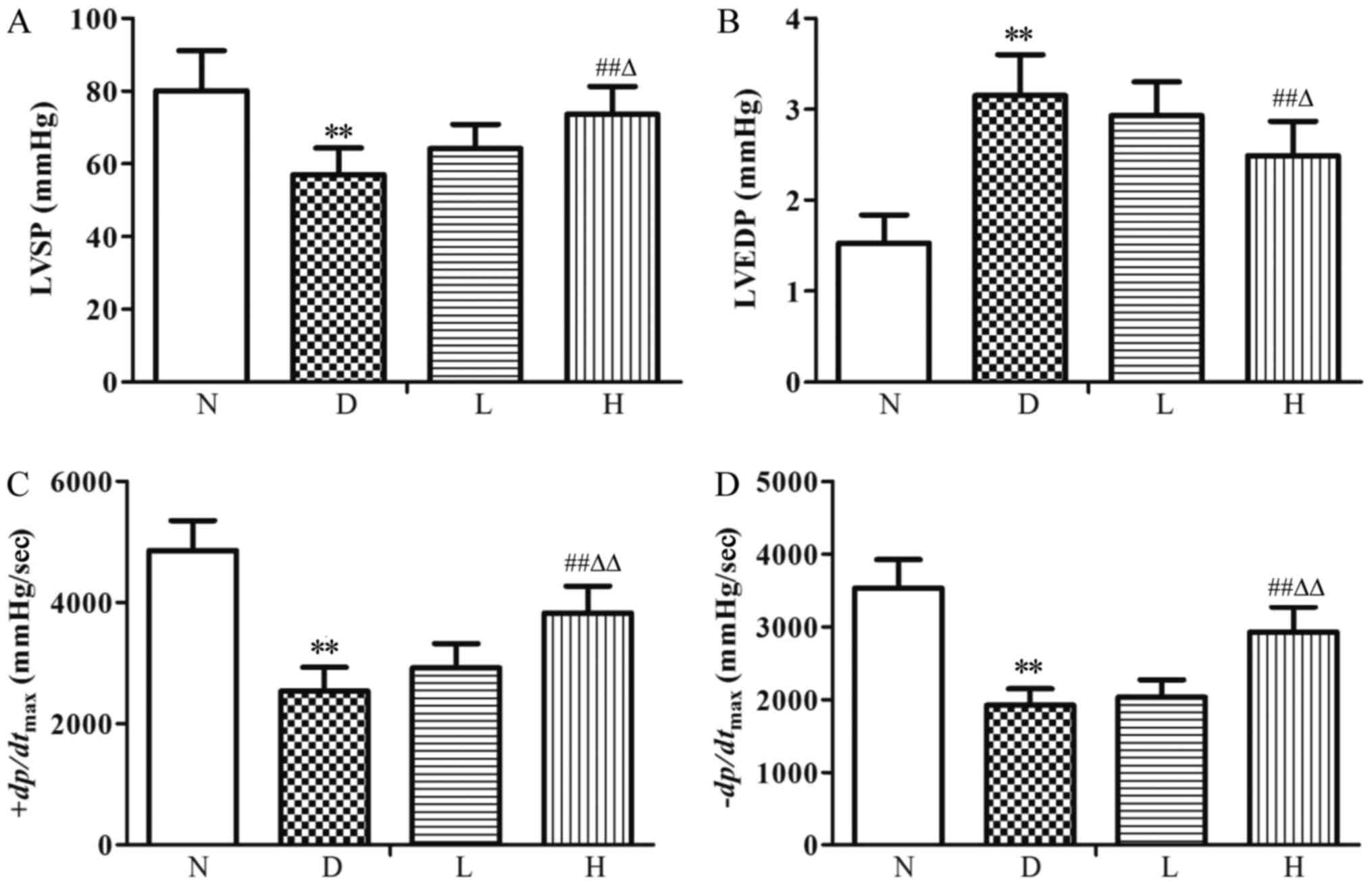 | Figure 1.Ventricular hemodynamic parameters in
the different groups. (A) LVSP, (B) LVEDP, (C)
+dp/dtmax and (D)-dp/dtmax.
Data were expressed as the mean ± standard deviation (n=8).
**P<0.01 vs. N group; ##P<0.01 vs. D group;
∆P<0.05 and ∆∆P<0.01 vs. L group. N,
normal control group; D, diabetic control group; L, low-dose
genistein treatment group; H, high-dose genistein treatment group;
LVSP, left ventricular systolic pressure; LVEDP, left ventricular
end diastolic pressure; +dp/dtmax, maximal rise
rate of left ventricular pressure; -dp/dtmax,
maximal fall rate of left ventricular pressure. |
Changes of FBG, BW, HW, HW/BW and Hyp
content
Compared with the N group, BW and HW were decreased
(P<0.01), while FBG, HW/BW and Hyp content were significantly
increased (P<0.01) in the D group. Compared with the D group,
there were no statistical differences in FBG and HW in the L group,
BW was increased (P<0.05), while HW/BW and Hyp content were
decreased (P<0.05); in the H group, there was no statistical
difference in FBG, while BW and HW were increased (P<0.01),
HW/BW and Hyp content were significantly decreased (P<0.01).
Compared with the L group, BW and HW were increased (P<0.01),
while HW/BW and Hyp content were decreased (P<0.05, P<0.01)
in the H group (Table I).
 | Table I.Fasting blood glucose, body and heart
weight and the ratio, and hydroxyproline content in the different
groups. |
Table I.
Fasting blood glucose, body and heart
weight and the ratio, and hydroxyproline content in the different
groups.
| Group | FBG (mmol/l) | BW (g) | HW (mg) | HW/BW (mg/g) | Hyp (µg/mg) |
|---|
| N |
5.54±0.87 |
443.90±31.23 |
1,261.27±136.97 |
2.89±0.24 |
3.15±0.42 |
| D |
24.91±3.23a |
230.50±26.55a |
811.45±61.60a |
3.64±0.29a |
6.83±0.87a |
| L |
24.67±3.40 |
263.21±28.17b |
852.02±63.25 |
3.35±0.27b |
5.97±0.70b |
| H |
21.86±3.02 |
359.28±29.38c,e |
1,078.64±119.86c,e |
3.02±0.25c,d |
4.52±0.66c,e |
Changes of serum CK-MB, LDH, TNF-α,
IL-1β and IL-6 levels
Compared with the N group, serum CK-MB, LDH, TNF-α,
IL-1β and IL-6 levels were increased (P<0.01) in the D group;
Compared with the D group, CK-MB, LDH, TNF-α, IL-1β and IL-6 levels
were decreased (P<0.05, P<0.01) in the L group; CK-MB, LDH,
TNF-α, IL-1β and IL-6 levels were decreased (P<0.01) in the H
group. Compared with the L group, CK-MB, LDH, TNF-α, IL-1β and IL-6
levels were decreased (P<0.01) in the H group (Fig. 2).
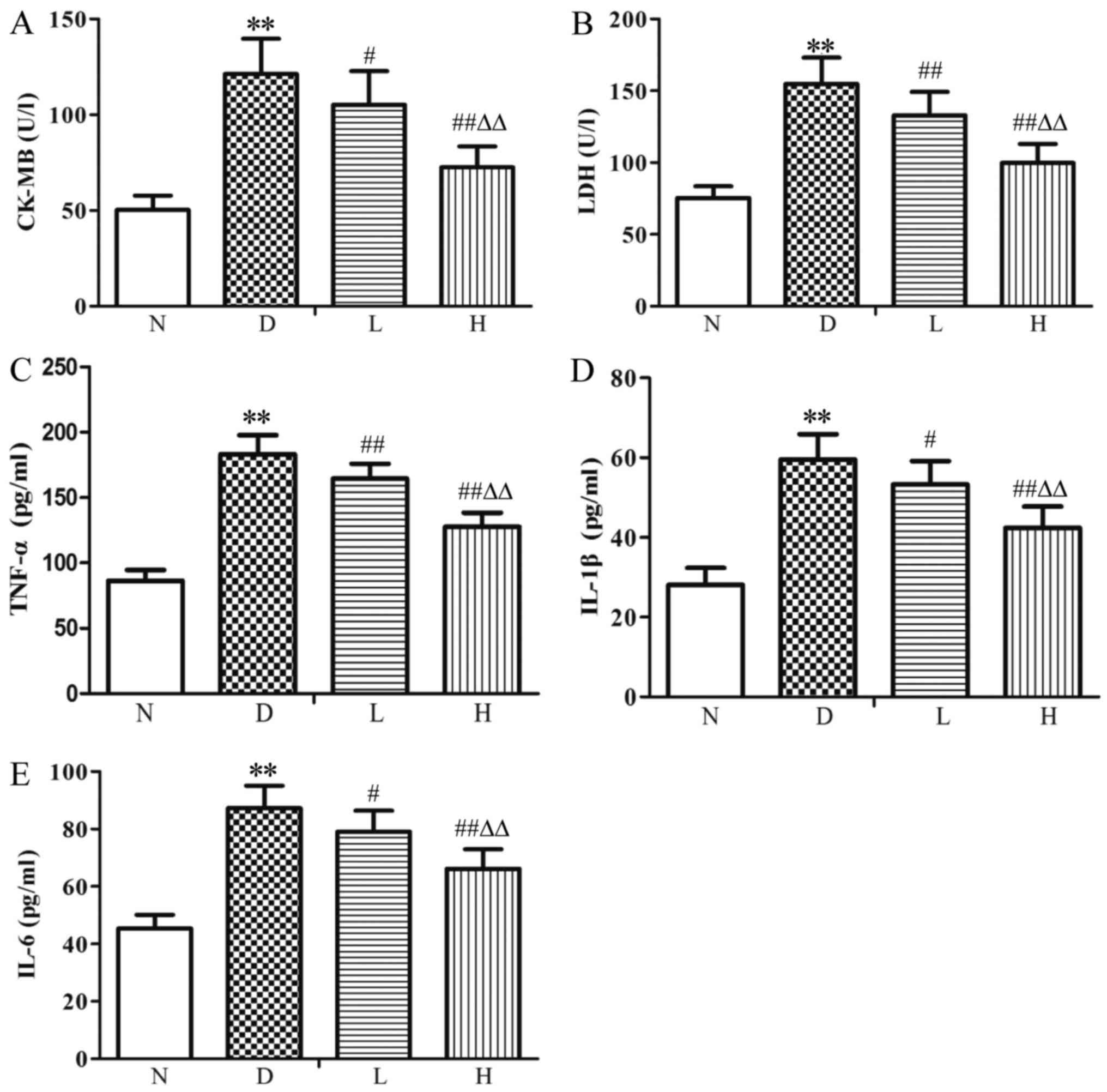 | Figure 2.Levels of (A) CK-MB, (B) LDH, (C)
TNF-α, (D) IL-1β and (E) IL-6 in the serum of the different groups.
Data were expressed as the mean ± standard deviation (n=8).
**P<0.01 vs. N group; #P<0.05 and
##P<0.01 vs. D group; ∆∆P<0.01 vs. L
group. N, normal control group; D, diabetic control group; L,
low-dose genistein treatment group; H, high-dose genistein
treatment group; CK-MB, creatine kinase MB isozyme; LDH, lactate
dehydrogenase; TNF-α, tumor necrosis factor-α; IL-1β,
interleukin-1β; IL-6, interleukin-6. |
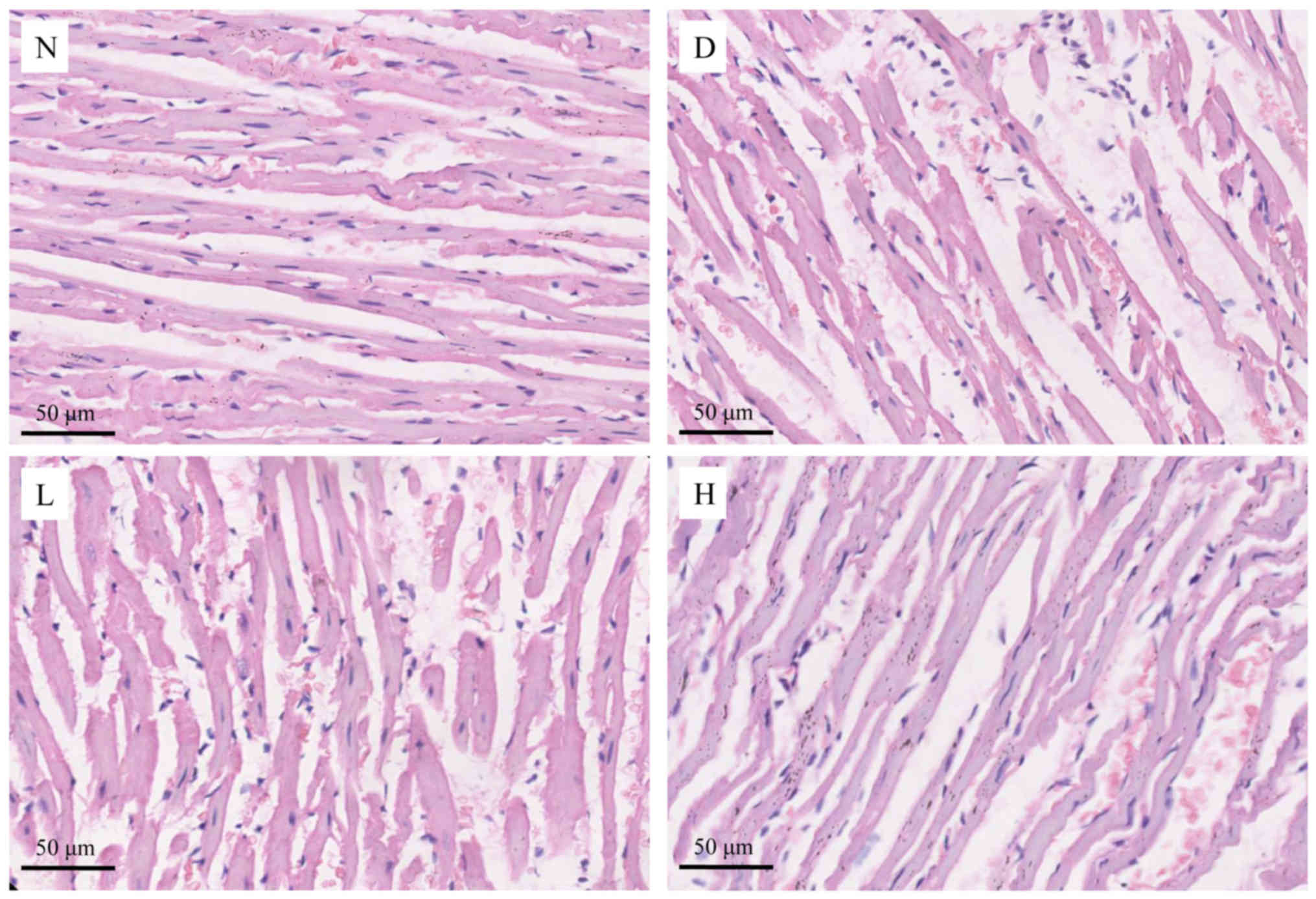
Histological changes in myocardial
tissue by H&E staining
In the N group, the myocardial fibers were arranged
in neat rows, the myocardial nuclei were clear, the myocardial gap
was normal and inflammatory cells soak was rare. In the D group,
the myocardial fibers were disordered, the cardiomyocytes were
swollen significantly and myocardial gap was widened. Inflammatory
cells were infiltrated significantly and the distribution of
cytoplasm was uneven in the D group. Compared with the D group, the
myocardial damage in the L group was relatively ameliorated,
however, the arrangement of myocardial fibers was disordered and
inflammatory cell infiltration was observed. The myocardial damage
in the H group was obviously ameliorated, most of the myocardial
cells were arranged neatly and the infiltration of inflammatory
cells was significantly reduced (Fig.
3).
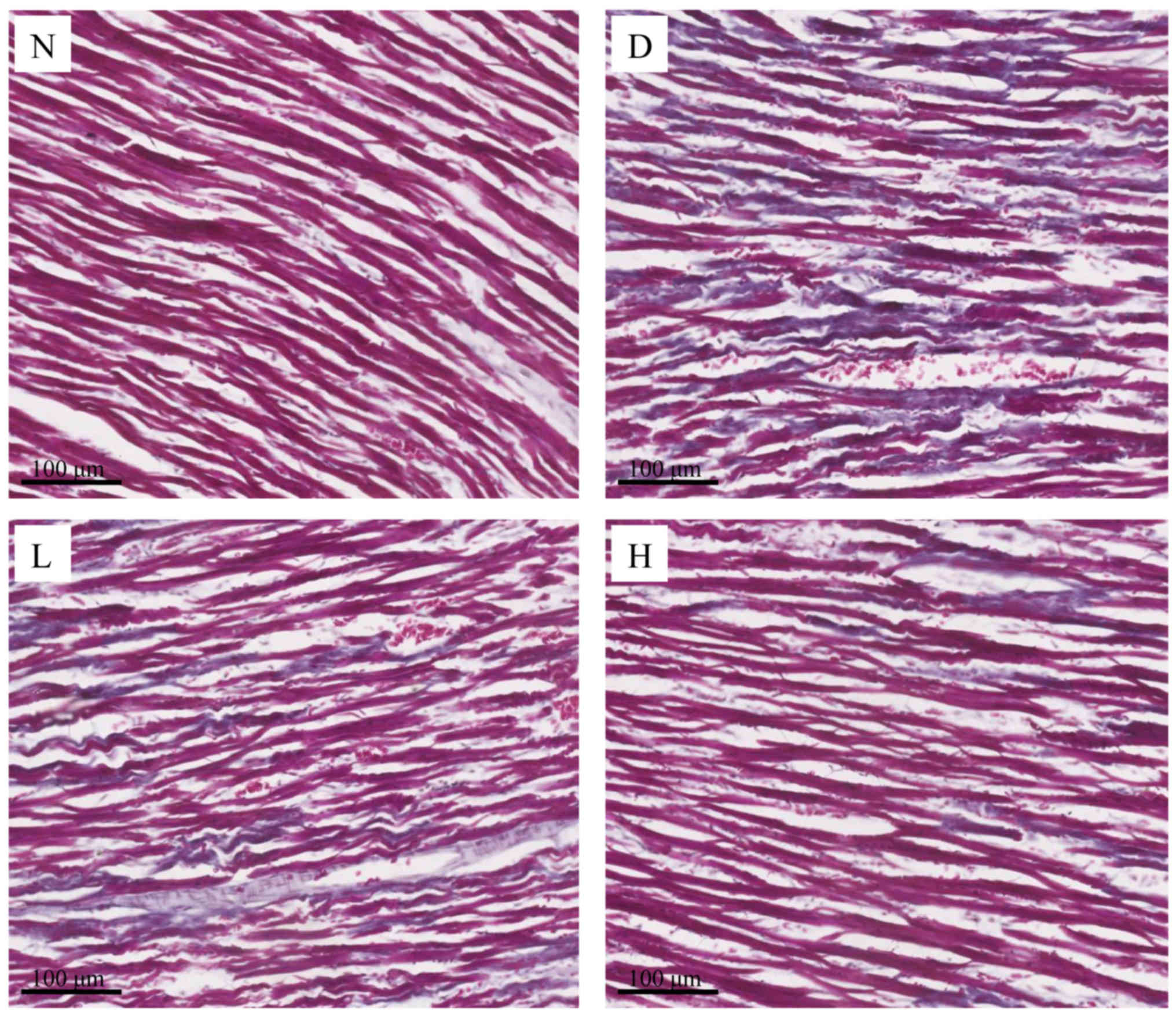
The morphology of collagen detection
by Masson's trichrome staining
In Masson's trichrome staining, the collagen fibers
were stained blue and cardiomyocytes were stained red. In the N
group, the myocardial fibers were arranged in neat row and the
myocardial gap was normal. The interstitial collagen fibers were
rare. In the D group, the cardiomyocytes were in a disordered
arrangement, the myocardial gap was widened and the interstitial
collagen fibers were increased obviously. Treated with GEN, the
cardiomyocytes were disrupted in some areas and collagen fibers
were decreased slightly in the L group; in the H group, the
cardiomyocytes were approximately arranged neatly, collagen fibers
were sparsely distributed, and interstitial collagen was dyed a
little blue (Fig. 4).
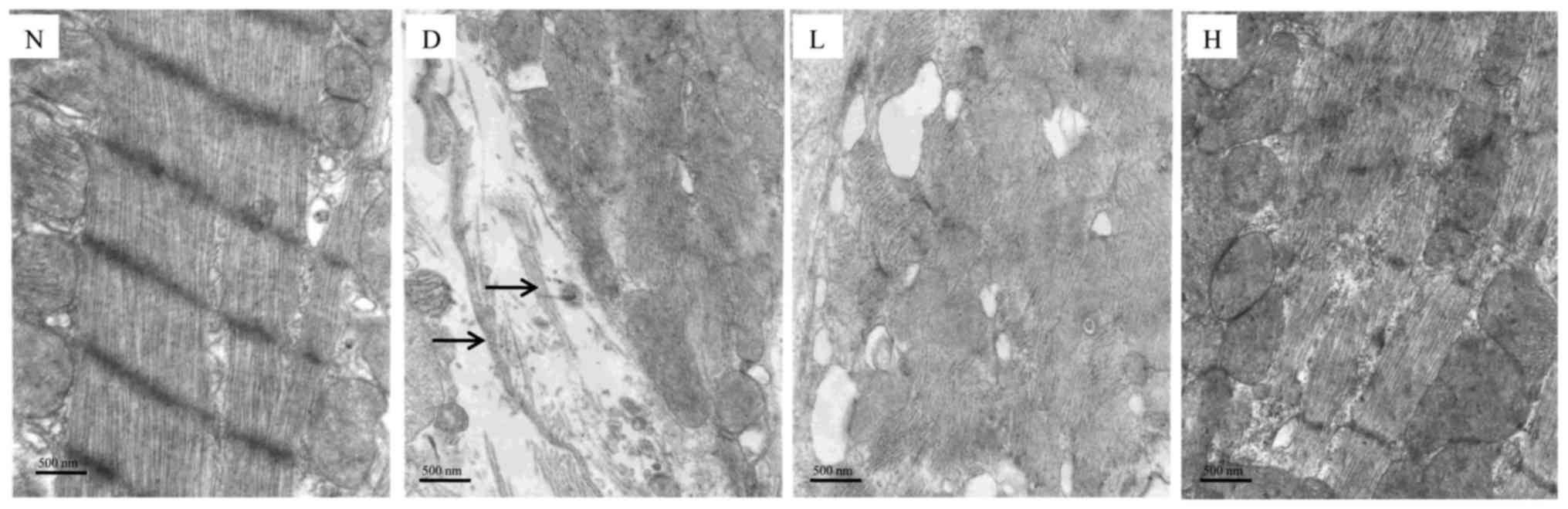
Changes in the myocardial
ultrastructure
In the N group, the sarcomeres of the myocardium
were arranged orderly and maintained homogenous length;
intermyofibrillar mitochondria were arranged in an ordered pattern
in myocardium. In the D group, the integrity of the cardiomyocyte
was destroyed and mitochondrial matrix was severely damaged. In
addition, the collagen fibers were found (shown with black arrows),
which indicated the severity of myocardial fibrosis. After
treatment with GEN, compared with the D group, myocardial injury
was alleviated in the L group; these injuries were significantly
ameliorated in the H group (Fig.
5).
Changes in myocardial TGF-β1, Smad3,
p-Smad3, Smad4, collagen-I and collagen-III at protein levels
Compared with the N group, levels of myocardial
TGF-β1, Smad3, p-Smad3, p-Smad3/Smad3, Smad4, collagen-I and
collagen-III were significantly increased (P<0.01) in the D
group. In contrast to D group, there was no statistical difference
in p-Smad3 expression, the levels of myocardial TGF-β1, Smad3,
p-Smad3/Smad3, Smad4, collagen-I and collagen-III were decreased
(P<0.05) in the L group; the levels of myocardial TGF-β1, Smad3,
p-Smad3, p-Smad3/Smad3, Smad4, collagen-I and collagen-III were
significantly decreased (P<0.01) in the H group. Compared with
the L group, the aforementioned indices were decreased (P<0.05,
P<0.01) in the H group (Fig.
6).
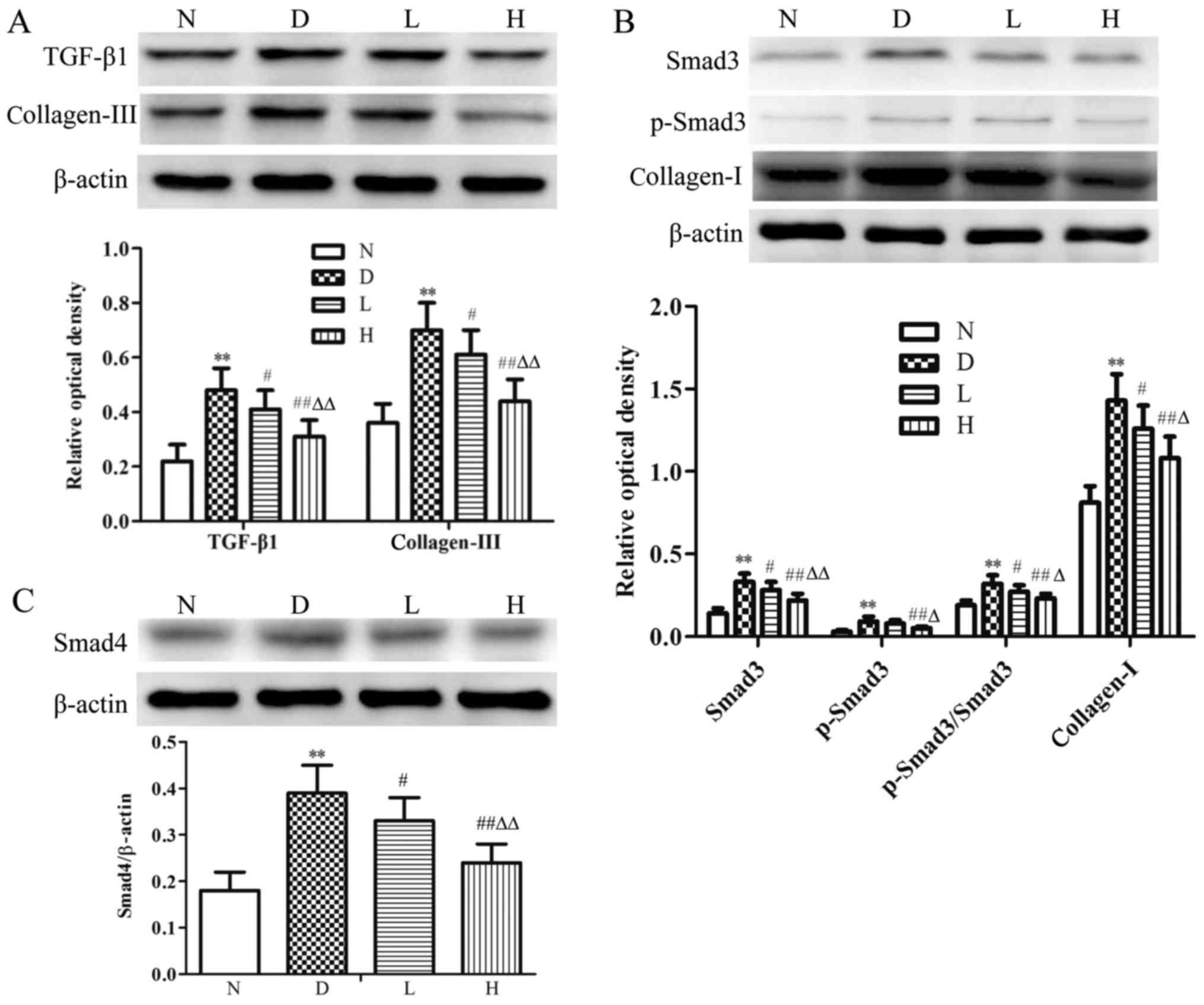 | Figure 6.Protein expression of TGF-β1, Smad3,
p-Smad3, Smad4, collagen-I and collagen-III in myocardial tissue in
the different groups. Representative western blotting and
quantitative analysis of the (A) TGF-β1, collagen-III, (B) Smad3,
p-Smad3, collagen-I and (C) Smad4 protein expression ratio in the
myocardial tissue of different groups. β-actin was used as a
loading control. Data were expressed as the mean ± standard
deviation (n=8). **P<0.01 vs. N group; #P<0.05 and
##P<0.01 vs. D group; ∆P<0.05 and
∆∆P<0.01 vs. L group. N, normal control group; D,
diabetic control group; L, low-dose genistein treatment group; H,
high-dose genistein treatment group; TGF-β1, transforming growth
factor-β1; Smad, mothers against decapentaplegic homolog; p-,
phosphorylated. |
Discussion
DCM is one of the most common complications of
diabetes, which major pathological characteristics are hypertrophy
or hyperplasia in cardiomyocytes. Excessive deposition of
myocardial interstitial collagen and myocardial fibrosis often
leads to cardiac hypertrophy and heart dysfunction, which plays an
important role in the occurrence and development of DCM (17).
The results in the present study showed that
compared with the N group, FBG, LVEDP and HW/BW were increased
significantly in the D group, while LVSP and
±dp/dtmax were decreased significantly, which
indicated the heart dysfunction and cardiomyocyte hypertrophy in
diabetic rats. In addition, there were histomorphological changes
and ultrastructure damage in the D group. The serum CK-MB, LDH,
TNF-α, IL-1β and IL-6 levels, Hyp content and the protein
expression of collagen-I and collagen-III in the D group were
significantly increased compared with the N group, which indicated
that myocardial injury, inflammatory reaction and myocardial
fibrosis were significantly aggravated in diabetic rats.
GEN is a type of plant estrogens, which exhibits
antioxidant, anti-inflammatory and anti-apoptotic effects. GEN can
prevent isoproterenol-induced cardiac hypertrophy in rats (18) and ameliorate adverse cardiac
effects induced by arsenic trioxide through preventing
cardiomyocytes apoptosis (19). In
the present study, we investigated the protective effects of low-
and high-dose GEN on myocardial tissue in diabetic rats. Compared
with the D group, the data showed that, although there was no
statistical difference in cardiac function in the L group, the
cardiac diastolic and systolic functions were improved in the H
group. In addition, HW/BW and the levels of TNF-α, IL-1β and IL-6
were decreased in the L and H groups. It is suggested that GEN can
alleviate myocardial hypertrophic changes and inflammatory reaction
to improve cardiac function, which indicated that GEN has a
protective effect in DCM.
In myocardial injury, CK-MB and LDH are two specific
indicators. CK-MB is a key enzyme in the energy metabolism of
cardiomyocytes, and LDH is an important dehydrogenase in the
process of anaerobic glycolysis (20). CK-MB and LDH activities were low in
the serum under normal physiological conditions. When the
myocardium injured and the cell membrane permeability changing,
leakage of intracellular proteins such as CK-MB and LDH could
occur. CK-MB and LDH activities were significantly increased in the
serum, which indicated that the degree of myocardial injury was
aggravated. Schmidt et al (21) reported that GEN can reduce LDH
release and inhibit proliferation of malignant glioma cells by
inhibiting protein tyrosine kinase activity. This study showed that
compared with N group, serum CK-MB and LDH activities were
significantly increased in the D group. After treating diabetic
rats with GEN, serum CK-MB and LDH activities were decreased in the
L and H groups. The results suggested that GEN can alleviate
myocardial injury through reducing CK-MB and LDH leakage in
diabetic rats.
In addition, some studies have reported that GEN,
which has anti-proliferative activity in many cell types and
inhibits tyrosine kinases, may benefit the cardiovascular system.
GEN can inhibit aortic smooth muscle cell proliferation in rat
(22) and abolish nucleoside
uptake by cardiac fibroblasts in vitro (23). Therefore, this raises the question
as to the exact mechanism by which GEN exerts its cardioprotective
effect in diabetic rat and whether the mechanism is associated with
regulation of myocardial collagen expression.
Evidence from human and experimental models of DCM
has implicated cardiac fibrosis as a strong pathological
contributor (24,25). Cardiac fibrosis can increase left
ventricle stiffness and decrease ventricular wall compliance, and
these results in systolic and in particular diastolic dysfunction
(26). Excess ECM, which is caused
by an imbalance between synthesis and degradation, plays an
important role in cardiac fibrosis. Therefore, the content of ECM
can be used to assess the degree of myocardial fibrosis. In heart,
the major components of ECM are interstitial collagens such as
collagen-I and collagen-III. Collagen-I accounts for 80% of total
collagen in the heart and the fiber is thick with good toughness
and strong anti-stretch function; collagen-III accounts for 11% of
total collagen and the fiber is thin with good stretch to maintain
the heart elasticity. Therefore, cardiac dysfunction would happen
when excessive deposition of these two collagens disrupts the
structure of the heart. Compared with the N group, data showed that
collagen-I and collagen-III protein expressions were significantly
increased in diabetic rats, which verified that myocardial collagen
content increased could aggravate the degree of fibrosis. In
contrast to D group, the protein expression of collagen-I and
collagen-III were significantly decreased in the L and H groups. We
suggest that GEN exhibits cardioprotective effect through
inhibiting the excessive production of myocardial collagen and
alleviating myocardial fibrosis in diabetic rats.
TGF-β1 is a key signaling molecule to induce cardiac
fibrosis by activating the proliferation and collagen production of
cardiac fibroblasts, and plays a significant role in the
development and progression of ECM metabolism (27). On the one hand, TGF-β1 can directly
induce the activation of ECM gene transcription and high
expression; on the other hand, TGF-β1 can suppress the degradation
of ECM by inhibiting matrix metalloproteinases and inducing tissue
inhibitor of metalloproteinase, which may lead to a deterioration
in myocardial injury and the occurrence and development of
myocardial fibrosis (28).
Smads are pivotal intracellular nuclear effectors of
TGF-β1 family members (29).
Ligand-induced activation of TGF-β family receptors with intrinsic
serine/threonine kinase activity triggers phosphorylation of
receptor-regulated Smads. Of these, Smad3 is phosphorylated by
TGF-β1, followed by binding with Smad4 to form a complex that
translocates to the nucleus. This signaling cascade finally
produces fibrotic mediators such as collagens (30,31).
Many studies have found that the TGF-β1/Smad3 signaling pathway
mediates a number of fibrotic diseases, such as hepatic fibrosis
(32) and pulmonary fibrosis
(33). Our data also showed that
compared with the N group, the protein expression of TGF-β1, Smad3,
p-Smad3 and Smad4 were significantly increased in the D group, this
confirmed that the TGF-β1/Smad3 pathway was involved in myocardial
fibrosis in diabetic rats. After treating diabetic rats with GEN,
the results showed that the expression levels of TGF-β1, Smad3,
p-Smad3/Smad3 and Smad4 were decreased in the L and H groups, and
this decrease was positively correlated with the dose of GEN. Thus,
we suggest that GEN can inhibit the TGF-β1/Smad3 signaling pathway
to regulate the collagens expression in DCM.
In conclusion, GEN can attenuate myocardial fibrosis
in type 1 diabetic rats, and the underlying mechanism may be
associated with the reduction of CK-MB and LDH leakage, inhibition
of inflammatory reaction, and suppression of the TGF-β1/Smad3
signaling pathway to regulate the collagens expression.
Acknowledgements
The present study was supported by the Natural
Science Research Project of the Education Commission of Anhui
Province (KJ2017A216), the Natural Science Research Project of
Bengbu Medical College (BYKY1621ZD), and the National Undergraduate
Innovation and Entrepreneurship Program (201510367009), China.
References
|
1
|
Mohan V, Seedat YK and Pradeepa R: The
rising burden of diabetes and hypertension in southeast Asian and
African regions: Need for effective strategies for prevention and
control in primary health care settings. Int J Hypertens.
2013:4090832013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ward ML and Crossman DJ: Mechanisms
underlying the impaired contractility of diabetic cardiomyopathy.
World J Cardiol. 6:577–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aneja A, Tang WH, Bansilal S, Garcia MJ
and Farkouh ME: Diabetic cardiomyopathy: Insights into
pathogenesis, diagnostic challenges and therapeutic options. Am J
Med. 121:748–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miki T, Yuda S, Kouzu H and Miura T:
Diabetic cardiomyopathy: Pathophysiology and clinical features.
Heart Fail Rev. 18:149–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu X, Bai T, Xu Z, Liu Q, Zheng Y and Cai
L: Pathophysiological fundamentals of diabetic cardiomyopathy.
Compr Physiol. 7:693–711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujiu K, Wang J and Nagai R:
Cardioprotective function of cardiac macrophages. Cardiovasc Res.
102:232–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cunnington RH, Nazari M and Dixon IM:
c-Ski, Smurf2 and Arkadia as regulators of TGF-beta signaling: New
targets for managing myofibroblast function and cardiac fibrosis.
Can J Physiol Pharmacol. 87:764–772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Liang E, Song X, Du Z, Zhang Y and
Zhao Y: Inhibition of Pin1 alleviates myocardial fibrosis and
dysfunction in STZ-induced diabetic mice. Biochem Biophys Res
Commun. 479:109–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Zhang L, Zhang Y, Xu JJ, Sun LL
and Li SZ: The protective role of liquiritin in high
fructose-induced myocardial fibrosis via inhibiting NF-κB and MAPK
signaling pathway. Biomed Pharmacother. 84:1337–1349. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patisaul HB and Jefferson W: The pros and
cons of phytoestrogens. Front Neuroendocrinol. 31:400–419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo TL, Germolec DR, Zheng JF, Kooistra L,
Auttachoat W, Smith MJ, White KL and Elmore SA: Genistein protects
female nonobese diabetic mice from developing type 1 diabetes when
fed a soy- and alfalfa-free diet. Toxicol Pathol. 43:435–448. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elmarakby AA, Ibrahim AS, Faulkner J,
Mozaffari MS, Liou GI and Abdelsayed R: Tyrosine kinase inhibitor,
genistein, reduces renal inflammation and injury in
streptozotocin-induced diabetic mice. Vascul Pharmacol. 55:149–156.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MJ and Lim Y: Protective effect of
short-term genistein supplementation on the early stage in
diabetes-induced renal damage. Mediators inflamm. 2013:5102122013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta SK, Dongare S, Mathur R, Mohanty IR,
Srivastava S, Mathur S and Nag TC: Genistein ameliorates cardiac
inflammation and oxidative stress in streptozotocin-induced
diabetic cardiomyopathy in rats. Mol Cell Biochem. 408:63–72. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ganai AA and Husain M: Genistein
attenuates D-GalN induced liver fibrosis/chronic liver damage in
rats by blocking the TGF-β/Smad signaling pathways. Chem Biol
Interact. 261:80–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sung MJ, Kim DH, Jung YJ, Kang KP, Lee AS,
Lee S, Kim W, Davaatseren M, Hwang JT, Kim HJ, et al: Genistein
protects the kidney from cisplatin-induced injury. Kidney Int.
74:1538–1547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kayama Y, Raaz U, Jagger A, Adam M,
Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM and Tsao
PS: Diabetic cardiovascular disease induced by oxidative stress.
Int J Mol Sci. 16:25234–25263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maulik SK, Prabhakar P, Dinda AK and Seth
S: Genistein prevents isoproterenol-induced cardiac hypertrophy in
rats. Can J Physiol Pharmacol. 90:1117–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan Y, Wang C, Zhang Y, Hang P, Liu Y, Pan
Z, Wang N and Du Z: Genistein ameliorates adverse cardiac effects
induced by arsenic trioxide through preventing cardiomyocytes
apoptosis. Cell Physiol Biochem. 31:80–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Y, Jia XJ, Zong QF, Zhang GJ, Ye HW, Hu
J, Gao Q and Guan SD: Remote ischemic postconditioning protects the
heart by upregulating ALDH2 expression levels through the PI3K/Akt
signaling pathway. Mol Med Rep. 10:536–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmidt F, Knobbe CB, Frank B, Wolburg H
and Weller M: The topoisomerase II inhibitor, GEN, induces G2/M
arrest and apoptosis in human malignant glioma cell lines. Oncol
Rep. 19:1061–1066. 2008.PubMed/NCBI
|
|
22
|
Yu JY, Lee JJ, Lim Y, Kim TJ, Jin YR,
Sheen YY and Yun YP: Genistein inhibits rat aortic smooth muscle
cell proliferation through the induction of p27kip1. J Pharmacol
Sci. 107:90–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pillai MS and Shivakumar K: Genistein
abolishes nucleoside uptake by cardiac fibroblasts. Mol Cell
Biochem. 332:121–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kain V, Kumar S and Sitasawad SL:
Azelnidipine prevents cardiac dysfunction in
streptozotocin-diabetic rats by reducing intracellular calcium
accumulation, oxidative stress and apoptosis. Cardiovasc Diabetol.
10:972011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li CJ, Lv L, Li H and Yu DM: Cardiac
fibrosis and dysfunction in experimental diabetic cardiomyopathy
are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asbun J and Villarreal FJ: The
pathogenesis of myocardial fibrosis in the setting of diabetic
cardiomyopathy. J Am Coll Cardiol. 47:693–700. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan Z, Zhao W, Zhang X, Wang B, Wang J,
Sun X, Liu X, Feng S, Yang B and Lu Y: Scutellarin alleviates
interstitial fibrosis and cardiac dysfunction of infarct rats by
inhibiting TGF-β1 expression and activation of p38-MAPK and ERK1/2.
Br J Pharmacol. 162:688–700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cutroneo KR: TGF-beta-induced fibrosis and
SMAD signaling: Oligo decoys as natural therapeutics for inhibition
of tissue fibrosis and scarring. Wound Repair Regen. 15 Suppl
1:S54–S60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Itoh S, Itoh F, Goumans MJ and Ten Dijke
P: Signaling of transforming growth factor-beta family members
through Smad protein. Eur J Biochem. 267:6954–6967. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hata A and Chen YG: TGF-β signaling from
receptors to smads. Cold Spring Harb Perspect Biol. 8:a0022612016.
View Article : Google Scholar
|
|
31
|
Ko JW, Shin NR, Park SH, Kim JS, Cho YK,
Kim JC, Shin IS and Shin DH: Pine bark extract
(Pycnogenol®) suppresses cigarette smoke-induced
fibrotic response via transforming growth factor-β1/Smad family
member 2/3 signaling. Lab Anim Res. 33:76–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu F, Liu C, Zhou D and Zhang L:
TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J
Histochem Cytochem. 64:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeon WY, Shin IS, Shin HK, Jin SE and Lee
MY: Aqueous extract of gumiganghwaltang, a traditional herbal
medicine, reduces pulmonary fibrosis by transforming growth
factor-β1/Smad signaling pathway in murine model of chronic asthma.
PLoS One. 11:e01648332016. View Article : Google Scholar : PubMed/NCBI
|