Introduction
Nanotechnology, the branch of technology that
develops novel materials at size of <100 nm, has become one of
the most promising areas of engineering research (1). Manufactured metal oxide nanomaterials
are among the most widely used types of engineered nanomaterials
(2). Nickel oxide nanoparticles
(nano-NiO) are drawing significant research interest due to their
multiple applications, including as sensors, battery electrodes,
catalysts and cosmetics (3,4).
Because of the extensive potential of nano-NiO particles and their
emerging applications, it is essential to understand their
implications in human safety (5).
In recent years, toxicity and risk assessments of
nano-NiO have caught much attention. Single intratracheal
instillation of nano-NiO in rats induces oxidative stress,
inflammatory response, collagen deposition, and apoptosis in lung
tissues (6–8). In our previous studies, nano-NiO was
demonstrated to result in lung lesions following intratracheal
instillation for 6 weeks in rats and to enhance the nitrative
stress and inflammatory response (9), an effect that was associated with
nuclear factor-κB activation and T helper (Th)1/Th2 cell imbalance
(10). Other reports have also
demonstrated that nano-NiO induces toxicity in various cell lines,
including human bronchial epithelial cells, type II human alveolar
epithelial cells, human monocytes and mouse embryonic stem cells
(11–14). Suspensions of nano-NiO cause
oxidative stress in nauplii at concentrations 0.2–50 mg/l for 24
and 96 h (15).
The liver is the main organ where exogenous
materials are metabolized and eventually excreted (16). Therefore, liver cells exposed to
exogenous materials could result in liver dysfunction, cell injury,
and even organ failure. Pari et al (17) reported that nickel induces necrotic
and inflammatory response in rat livers by modulating the reactive
radicals due to oxidative stress. Nickel results in oxidative
stress and inflammatory response in mouse livers, which may be
mediated by the toll-like receptor 4/p38/cAMP response element
binding protein pathway (18).
Nickel sulfate triggers oxidative stress and apoptosis via the
c-Jun N-terminal kinase signaling pathway in the liver of
Carassius auratus (19).
Permenter et al (20)
reported that the mechanism of oxidative stress induced by nickel
sulfate in a rat liver-derived cell line may be the activation of
hypoxia inducible factor-1α transcription factor. Ahmad et
al (21) reported that nickel
chloride induces oxidative stress and apoptosis in human liver
cells through the mitochondrial pathway. Nickel sulfate has also
been reported to cause microvesicular steatosis and necrotic
hepatocytes in rat livers (22).
Sidhu et al (23) reported
that the activities of alkaline phosphatase and alanine
aminotransferase increased following treatment with nickel sulfate
in rat liver tissues.
While nickel and its compounds have been
demonstrated by these previous studies to lead to liver lesions,
few studies to date have addressed the potential liver toxicity of
nano-NiO in animal models. Ahamed et al (24) have demonstrated that nano-NiO
results in cytotoxicity via reactive oxygen species (ROS) and
enhances apoptosis in human liver cells (HepG2), which may be
mediated by the BCL2 associated X/BCL2 pathway (24). Another study has also demonstrated
that nickel nanoparticles induce cytotoxicity and oxidative stress
in HepG2 cells (25). Magaye et
al (26) reported that nickel
nanoparticles cause inflammation in the liver of rats, evident by
an increase of the liver coefficient in affected animals.
Nanoparticles of nickel ferrite induce cytotoxicity and oxidative
stress in human liver hepatocellular carcinoma cells in a
dose-dependent manner (27).
Katsnelson et al (28)
reported that nano-NiO administration in rats via intraperitoneal
injection leads to nickel deposition, increased numbers of Kupffer
cells, and akaryotic and binucleated hepatocytes in the liver
tissues.
Further research is required in order to evaluate
the safety of nano-NiO in the liver. In addition, the role of
oxidative stress in animal liver toxicity induced by nano-NiO
exposure remains unclear. In the present study, a rat model was
generated to observe the effects of nano-NiO administration by
examining various indicators of liver toxicity, including liver
weight, liver coefficient to body weight and histopathological
examination. Several biomarkers of nitrative and oxidative stress,
as well as the mRNA expression of heme oxygenase (HO)-1 and
metallothionein (MT)-1 were evaluated in order to explore the
potential mechanism of liver toxicity induced by nano-NiO.
Materials and methods
NiO particles characterization
NiO particles with diameters of 20 nm and 1 µm were
obtained from ST-nano Science and Technology Co., Ltd. (Shanghai,
China). The purities of both particles were detected with
inductively coupled plasma mass spectrometry (7500i; Agilent
Technologies, Inc., Santa Clara, CA USA). The specific surface area
of NiO particles was measured by the Brunauer Emmett Teller method
(3H-2000PS2; Beishide Instrument Technology Co., Ltd, Beijing,
China). The crystal structure of NiO particles were characterized
using an X-ray diffractometer (XRF-1800; Shimadzu Corporation,
Kyoto, Japan).
NiO sample preparation
Nano-NiO particles were dispread into physiological
saline at gradient concentrations of 0.015, 0.06, and 0.24 mg/ml,
following sterilization using a high pressure steam autoclave
(MLS-3751L; Panasonic Corporation, Osaka, Japan) at 120°C for 30
min, as well as concentration of micro-NiO particles at 0.24 mg/ml.
Avoiding the agglomeration of NiO particles, the suspensions were
dispersed using an ultrasonic homogenizer (CP750; Cole-Parmer
Instrument Co., Ltd., Vernon Hills, IL, USA) at 750 W for 30 min,
then mechanical vibration for 5 min.
Endotoxin assay of NiO samples
The Limulus Amebocyte Lysate (LAL) assay was
performed to identify whether NiO particles were contaminated by
endotoxins using a ToxinSensor Gel Clot endotoxin assay kit
(GenScript, Piscataway, NJ, USA). Twenty endotoxin-free vials were
divided into five groups (negative control, positive control,
saline, micro and nano-NiO groups), 0.1 ml LAL solution was added
into each tube, and then 0.1 ml endotoxin-free water, Escherichia
coli endotoxin standard, saline, 0.24 mg/ml micro or nano-NiO
suspensions were transferred into the respective vials. Each vial
was slowly turned upside down following incubation at 37°C for 1 h.
The gel remained intact when inverting the vial, and a positive
reaction meant that the endotoxin levels were >0.25 EU/ml. By
contrast, negative results showed that turbidity or viscosity
increased.
Animals and treatment
Forty adult male Wistar rats (age, 6–8 weeks;
weight, 200±20 g) were purchased from the Laboratory Animal Center
of Lanzhou University (Lanzhou, China), housed in stainless steel
cages in the laboratory with specific conditions (60% relative
humidity, 20±2°C, 12-h light/dark cycle). The animals were supplied
with commercial diet and water ad libitum. All procedures on
experimental animals complied with the ethics committee criteria of
Lanzhou University, and were approved by the Care and Use Committee
of Laboratory Animals of Lanzhou University (Lanzhou, Gansu,
China).
Following acclimatization for one week, the 40 rats
were randomized into five groups of 8 rats each: control group
(administered with only physiological saline), 0.015 mg/kg body
weight (b.w.) nano-NiO group, 0.06 mg/kg b.w. nano-NiO, 0.24 mg/kg
b.w. nano-NiO, and 0.24 mg/kg b.w. micro-NiO group. These dosages
of micro and nano-NiO were selected based on our previous studies
(9,10). Following anesthesia with inhaled
diethyl ether, rats received either physiological saline, or micro
or nano-NiO by intratracheal instillation twice a week for 6
consecutive weeks.
During the experimental period, body weight was
measured prior to each intratracheal instillation. At the end of 6
the weeks of exposure, animals were sacrificed and the liver tissue
was immediately excised and rinsed with ice-cold physiological
saline. Then, the liver tissue was weighted in order to calculate
the liver coefficient to body weight (wet weight in mg/100 g of
total body weight).
Histological examination
To explore if the liver was injured by the NiO
particle administration in rats, liver tissues were fixed with 4%
paraformaldehyde at room temperature for 48 h, embedded in
paraffin, cut into 5 µm sections, then transferred onto slides.
After removing the paraffin with xylene and dehydrating in graded
ethanol, the sections were rehydrated and stained with hematoxylin
and eosin. The slides were dehydrated with 95% and absolute
ethanol, cleared in xylene and sealed by neutral gum. Five staining
fields per sample were examined and photographed under a light
microscope (BX53; Olympus Corporation, Tokyo, Japan).
Preparation of tissue homogenate
The right lobe of each liver tissue was chopped,
diluted in physiological saline, and then grinded and centrifuged
at 1,100 × g for 10 min at 4°C. The supernatant was collected,
transferred into vials, and stored at −80°C for further analysis.
The protein concentration of the supernatant was measured with a
bicinchoninic acid total protein quantification kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China).
Nitrative stress assay
The enzyme activities of total nitric oxide synthase
(TNOS) and inducible nitric oxide synthase (iNOS) were assayed in
10% tissue homogenate by spectrophotometry at 530 nm wavelength,
and the nitric oxide (NO) content was determined in 10% tissue
homogenate at 550 nm wavelength, according to the instructions of
the respective detection kits (Nanjing Jiancheng Bioengineering
Institute).
Oxidative stress assay
The contents of hydroxyl radical (·OH), lipid
peroxidation (LPO), catalase (CAT), glutathione peroxidase
(GSH-Px), total superoxide dismutase (T-SOD) and total
antioxidative capacity (T-AOC) were tested in 0.5, 10, 0.5, 0.25, 1
and 10% tissue homogenate respectively using commercial kits
(Nanjing Jiancheng Bioengineering Institute) as per the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Approximately 95 mg of tissue from the liver was
resected from the experimental rats for RT-qPCR analysis of the
mRNA expression levels of HO-1 and MT-1. According to the
manufacturer's instructions, 1 ml TRIzol reagent (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract total RNA
from the liver tissue. Total RNA was assessed using a NanoDrop
2000C spectrophotometer (Thermo Fisher Scientific, Inc.), and only
samples of high quality, with A260/A280 ratios between 1.8 and 2.2,
were selected for reverse transcription. cDNA synthesis was
performed with the FastQuant RT kit (Tiangen Biotech Co., Ltd.,
Beijing, China). Then, qPCR was performed on an iQ5 real-time PCR
detection system (Bio-Rad Laboratories, Inc. Hercules, CA, USA)
using the qPCR SYBR-Green Mix (Tiangen Biotech Co., Ltd.). The
specific operating conditions were as follows: 95°C for 15 min for
initial denaturation, followed by 40 cycles of 95°C for 10 sec,
60°C for 20 sec, and 72°C for 32 sec. Primer sequences are listed
in Table I. The relative mRNA
expression levels were calculated using the Pfaffl method (29).
 | Table I.Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer | Sequence
(5′-3′) | Product size
(bp) |
|---|
| β-actin | Forward |
CCAACCGTGAAAAGATGA | 102 |
|
| Reverse |
TACGACCAGAGGCATACAG |
|
| HO-1 | Forward |
GAAGAGGAGATAGAGCGAAAC | 155 |
|
| Reverse |
CTGGTGTGTAAGGGATGG |
|
| MT-1 | Forward |
ACCTCCTGCAAGAAGAGC | 184 |
|
| Reverse |
GCACCTTTGCAGACACAG |
|
Statistical analysis
Results were expressed as mean ± standard deviation.
Significance of differences between groups were analyzed with
one-way analysis of variance followed by the least significant
difference test, using the SPSS 21.0 software (IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Particle characterization
To avoid the NiO particles agglomeration caused by
co-existing ions, van der Waals forces and static electricity,
ultrasound and vortex oscillation were used to retain the particles
in a scattered state (30). The
physicochemical characteristics of NiO particles were presented in
our previous study (9). The
endotoxin levels were <0.25 EU/ml in the micro and nano-NiO
suspensions, which is regarded as a negative result.
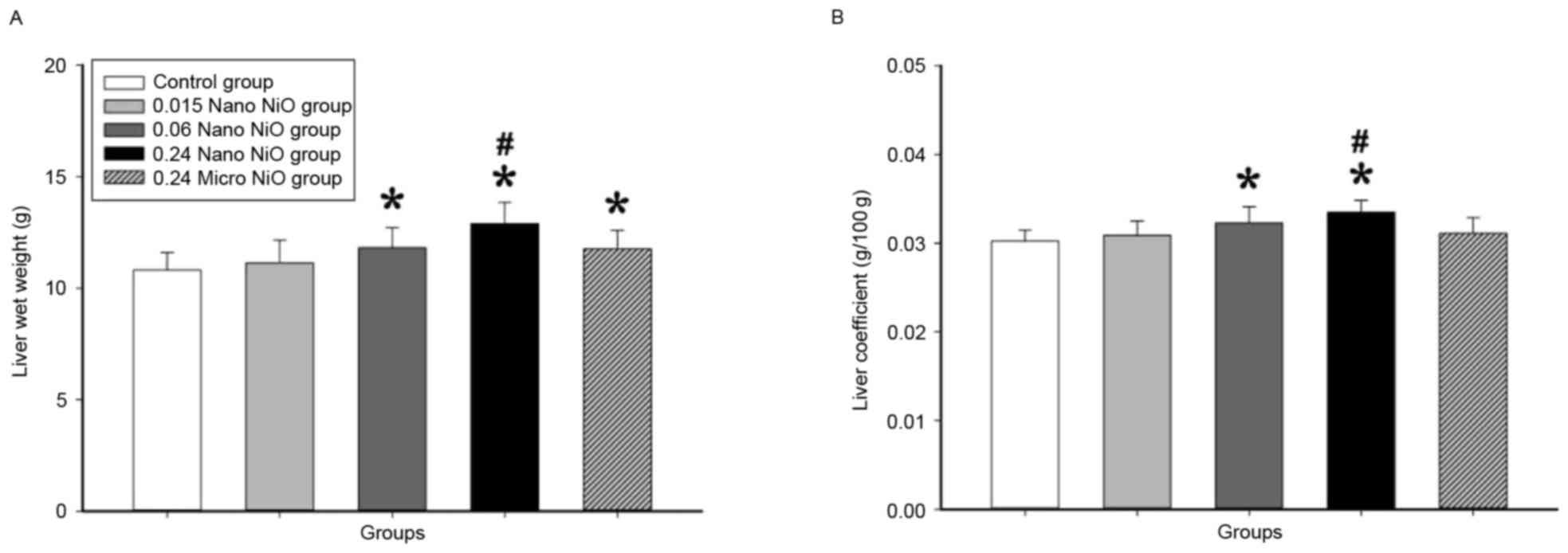
Liver coefficient to body weight
Liver coefficient reflected the degree of liver
injury caused by the NiO particles. As illustrated in Fig. 1, the liver wet weight and
coefficient to body weight significantly increased following
intratracheal instillation of 0.24 mg/kg nano-NiO, compared to the
control and the 0.24 mg/kg micro-NiO groups (P<0.05).
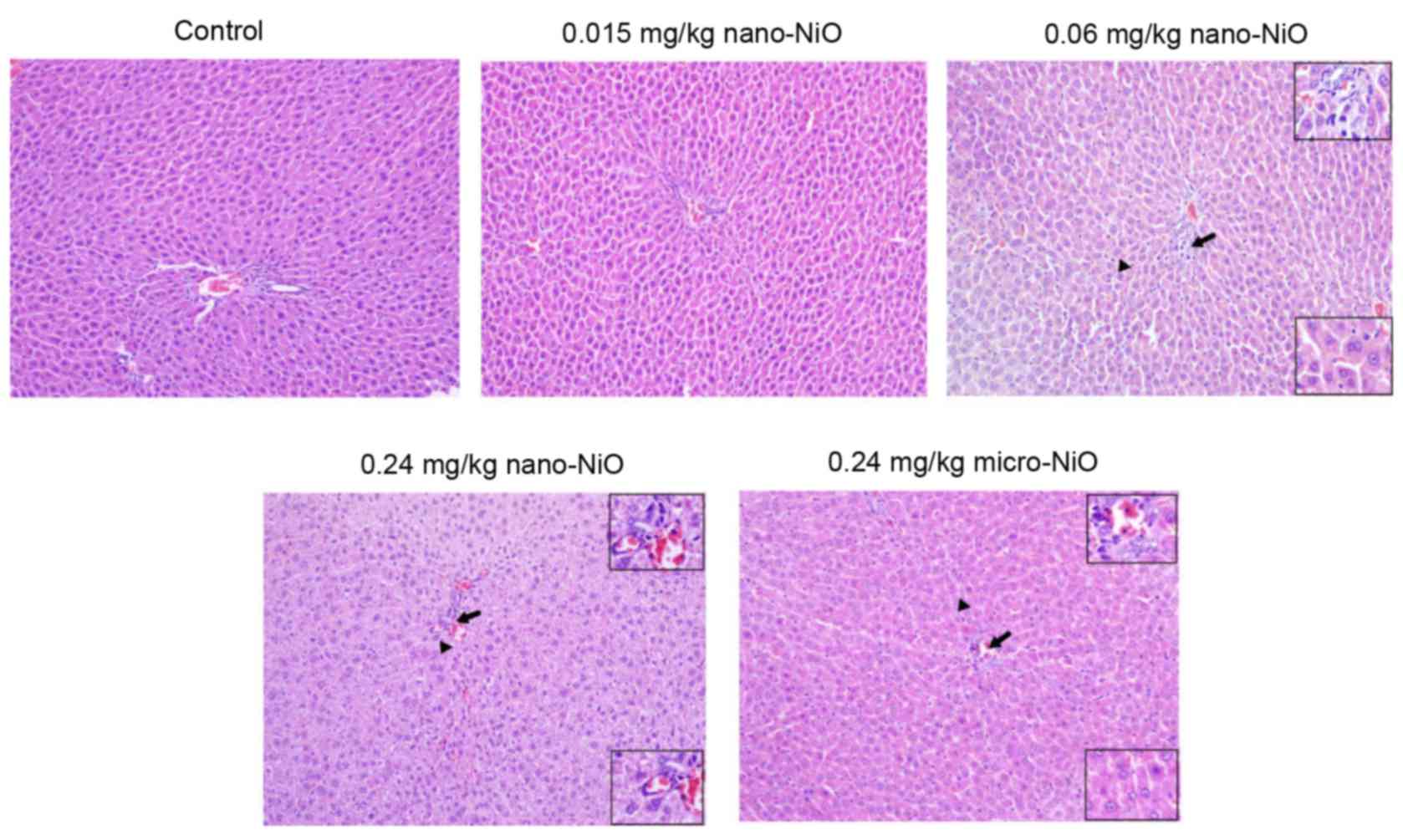
Histopathology
The subchorionic liver toxicity induced by nano-NiO
administration was evaluated in the experimental rats by observing
liver tissue sections that were stained by hematoxylin and eosin
(Fig. 2). The liver tissues of the
control group exhibited a normal liver structure, with consecutive
structure and clear outline. The distribution of hepatocytes was
scattered and dissociated in the 0.015 mg/kg nano-NiO group. In the
0.06 mg/kg nano-NiO group, the loosely arranged hepatocytes
appeared swollen, binucleated and infiltrated by lymphocytes.
Cellular edema, hepatic sinus disappearance and binucleated
hepatocytes, as well as cells mixed together with volume
enlargement, were observed in the 0.24 mg/kg nano-NiO group.
Finally, the 0.24 mg/kg micro-NiO group presented cellular edema,
neutrophil and lymphocyte infiltration, binucleated hepatocytes,
and sporadic spotty necrosis.
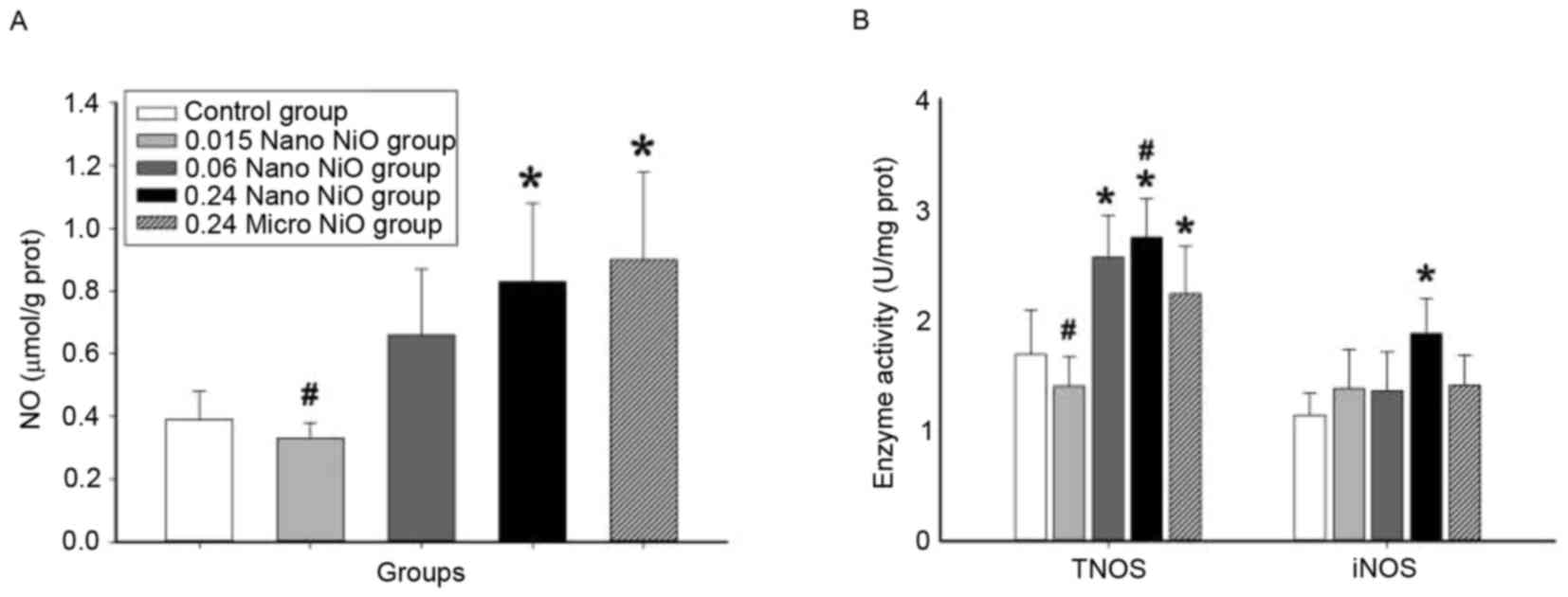
Nitrative stress assay
The indicators of nitrative stress (NO, TNOS and
iNOS) were measured in order to evaluate the degree of liver
toxicity induced by nano-NiO in rats. The changes of NO content,
and the activities of TNOS and iNOS in the different experimental
groups are depicted in Fig. 3. NO
content and TNOS and iNOS activities were significantly increased
in the 0.24 mg/kg nano-NiO group compared with the control group
(P<0.05). In addition, significant differences in the NO content
and TNOS activity were observed also in the micro-NiO and the 0.015
mg/kg nano-NiO groups compared with the control group
(P<0.05).
Oxidative stress assay
Oxidative stress indicators were examined in the
livers of the experimental groups in order to explore the mechanism
of liver injury induced by nano-NiO. As demonstrated in Fig. 4, the contents of ·OH and LPO
increased in the 0.24 mg/kg nano-NiO group compared with the
control group (P<0.05). In addition, the activities of the
antioxidant enzymes CAT, GSH-Px, T-SOD and T-AOC were significantly
decreased in the liver tissues of the 0.24 mg/kg nano-NiO group
compared with the control group (P<0.05).
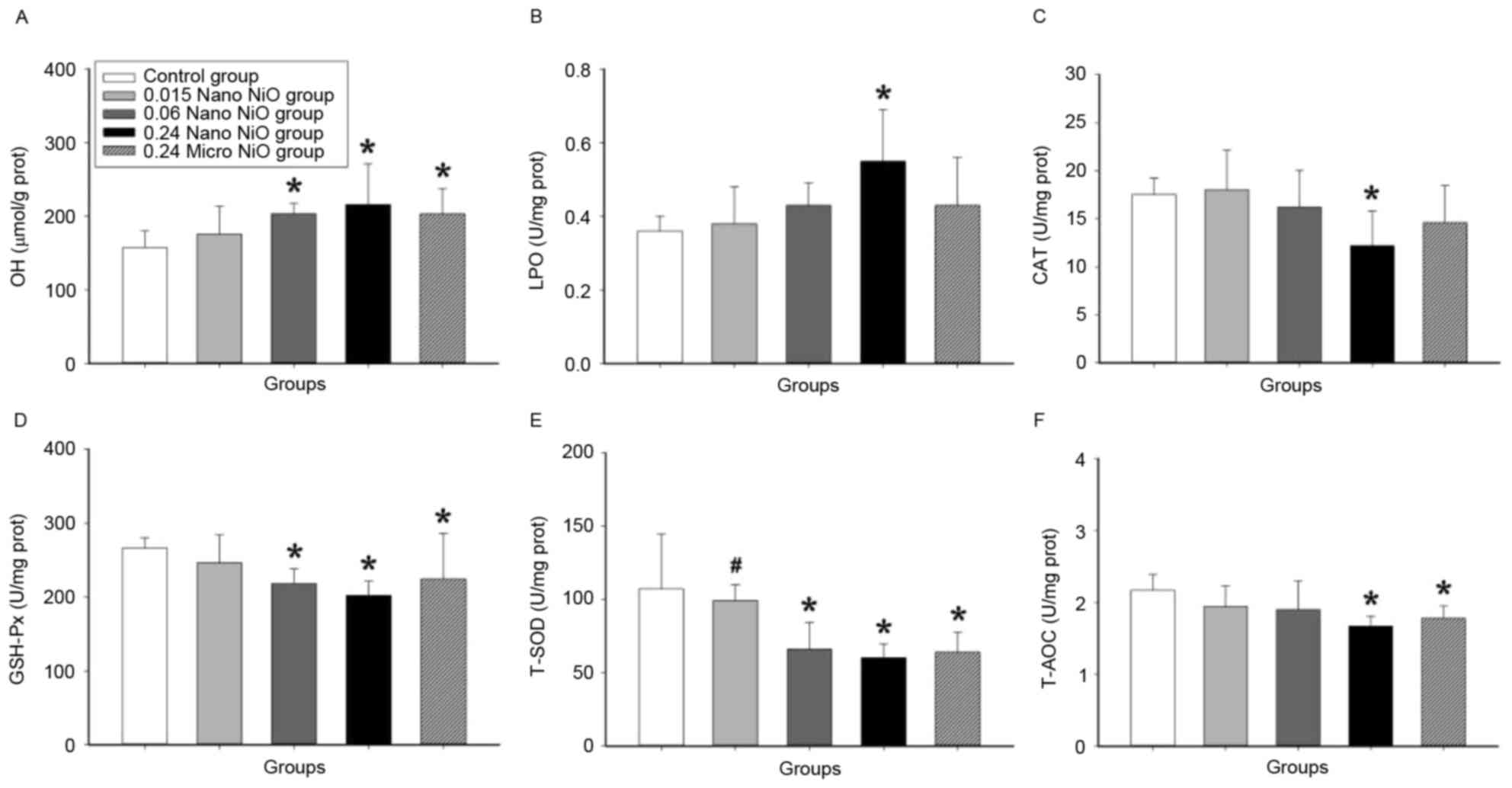 | Figure 4.Indicators of oxidative stress in rat
liver tissues following administration of NiO particles. Levels of
(A) ·OH, (B) LPO, (C) CAT, (D) GSH-Px, (E) T-SOD and (F) T-AOC were
measured in the control (saline) and NiO particle-administered
groups. Data are expressed as mean ± standard deviation (n=8).
*P<0.05 vs. control; #P<0.05 vs. 0.24 mg/kg
micro-NiO group. NiO, nickel oxide; ·OH, hydroxyl radical; LPO,
lipid peroxidation; CAT, catalase; GSH-Px, glutathione peroxidase;
T-SOD, total superoxide dismutas; T-AOC, total antioxidative
capacity; Nano, nanoparticles; Micro, microparticles. |
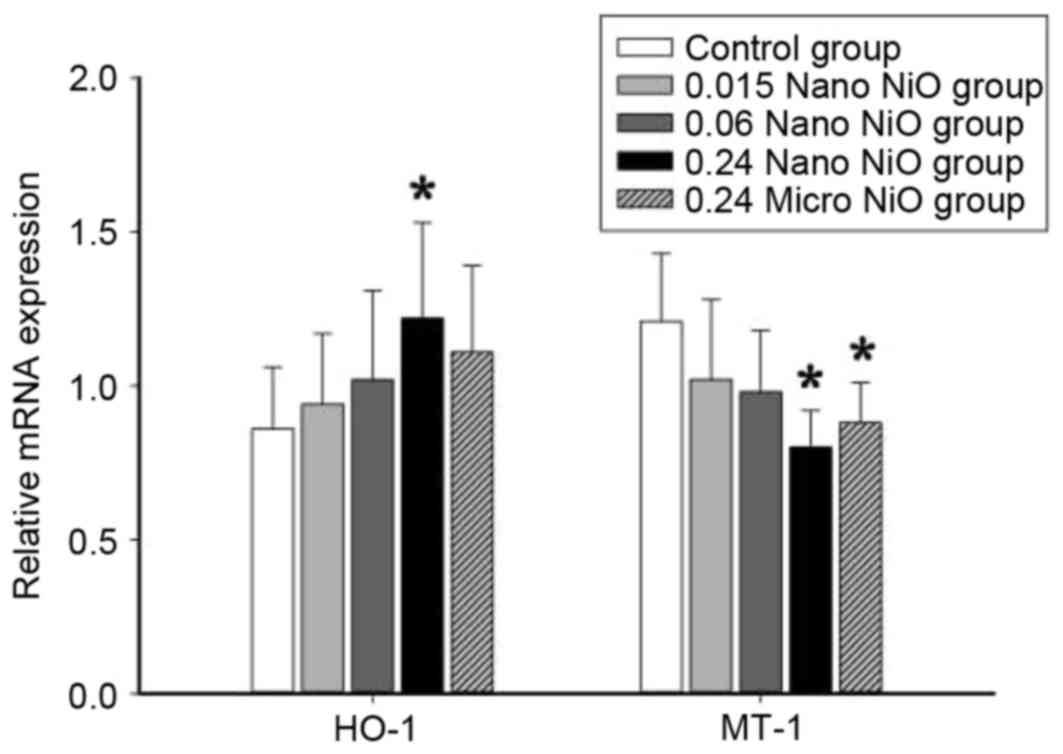
Analysis of HO-1 and MT-1 mRNA
expression
The relative mRNA expression levels of HO-1 and MT-1
were detected in the liver tissues in order to examine the effect
of nano-NiO administration in oxidative stress induction. As
demonstrated in Fig. 5, the
relative HO-1 mRNA levels were significantly elevated in the 0.24
mg/kg nano-NiO group compared with the control group (P<0.05).
By contrast, the relative MT-1 mRNA levels were reduced in the 0.24
mg/kg nano-NiO group compared with the control group (P<0.05;
Fig. 5).
Discussion
Nano-NiO particles are widely used in production
industries, however, important aspects of its toxicology, such as
the organism-level toxicokinetic and dose-response relationships,
can only be addressed through experiments on a whole mammalian
organism (28). The liver is the
main organ responsible for metabolism of both endogenous and
exogenous compounds, therefore it is one of the main target organs
for the toxic action of xenobiotics or their reactive metabolites
(31). In addition, the
respiratory system is the most common route of exposure to
particulate matters, especially nanoparticles (32), which enter the circulatory system
via the gastrointestinal tract and then get distributed in the
liver. To date, few studies on liver toxicity induced by nano-NiO
exist and the mechanism in animal models remains unclear. For all
these reasons, the present study generated a rat liver injury model
induced by nano-NiO administration via intratracheal instillation
and explored its potential mechanisms.
In the present study, an increase in liver wet
weight and coefficient to body weight were observed following
nano-NiO administration, suggesting liver damage induced by
nano-NiO. Observation of liver pathological changes revealed
cellular edema, hepatic sinus disappearance, binucleated
hepatocytes, and cells mixed together with volume enlargement. The
results suggested that the present animal model of liver injury was
successfully generated by intratracheal instillation of nano-NiO
twice a week for 6 consecutive weeks in rats.
The production of radical species, such as reactive
nitrogen species, has been proposed as an early event and indicator
of hepatotoxicity (33). Nitrative
stress leads to the activation of iNOS and oxidative metabolism of
NO (34). NO is a diffusible
molecule that mediates a variety of cellular physiological and
pathophysiological responses. Excessive production of NO produces
the formation of reactive nitrogen species, such as peroxynitrite
and nitrogen dioxide (35).
Exposure to toxic levels of ozone causes the upregulation of iNOS
in lung tissue (36). The
increased nitrite and nitrate levels in fish livers may be due to
the enhanced activity of TNOS and hence overproduction of NO
(37). In the present study, an
increase of NO, TNOS and iNOS were observed in liver tissue, which
implied that nano-NiO-induced nitrative stress could be involved in
liver toxicity.
Reactive nitrogen species as free radicals lead to
oxidative stress (38). At a
preliminary level, it is hypothesized that oxidative stress induced
by nanoparticles may be a major mechanism of their toxicity and is
often a focal point of studies (39). Oxidative stress could disturb
antioxidant defense mechanisms of the liver by affecting
antioxidant-related enzyme activities (40). When the body antioxidant capacity
can no longer protect the cell from oxidative damage, free radicals
accumulate and exert detrimental effects, including LPO and protein
oxidation (41). LPO, a process
contributing to the development of oxygen radical-related damages,
is one of the major causes of cell membranes damage (42). ·OH is the most reactive of all
reduced forms of dioxygen studied in recent years, along with lipid
peroxidation (43). The present
study demonstrated that levels of ·OH and LPO were increased
following nano-NiO particle administration, implying raised
oxidation levels.
Antioxidant enzymes, including SOD, CAT and GSH-Px,
prevent the oxidation-induced damages (43). SOD promotes the production of
O2 and H2O2 from O2-,
which in turn are converted to water by CAT, thus avoiding the
formation of ·OH. CAT is one of the crucial antioxidant enzymes
that protects the body from oxidative stress. T-AOC serves a
crucial role in the antioxidant defense system. Nickel sulfate
leads to decreasing activities of enzymatic antioxidants, including
SOD, CAT and GSH-Px, in rat livers (17). Sidhu et al (44) reported that sulfate administration
causes a significant inhibition in GSH content and SOD activity in
liver tissue (44). The present
study demonstrated that the activities of CAT, GSH-Px, SOD and
T-AOC were decreased in liver tissues following nano-NiO particle
administration, indicating that the antioxidant system failed to
maintain cellular redox equilibrium.
HO-1 and MT-1 genes are rapidly upregulated by
proinflammatory cytokines or oxidative stress, as a protection
mechanism against cellular stress in the liver (45). HO-1 is widely used as a sensitive
stress response marker in oxidative damage induced by xenobiotics
(46). MT-1 ameliorates the
effects of oxidative stress by scavenging free radicals or
preventing their formation (47).
Horie et al (48) reported
that the relative HO-1 gene levels increased following
intratracheal instillation of nano-NiO and nickel chloride in rat
bronchoalveolar lavage fluid (48). Increased mRNA expression levels of
MT-1 were observed in the lung and liver following nickel sulfate
exposure via a single intratracheal instillation (49). The present study demonstrated that
decreased MT-1 and increased HO-1 gene expression in liver tissue,
suggesting a deficient antioxidant and detoxification capacity
induced by nano-NiO administration in rats.
It has been hypothesized that smaller particle size
is associated with higher specific surface area and reactivity,
thus nanoparticles may release more ions for intracellular uptake
and mobilization, resulting in higher bioavailability than
microparticles (50). In order to
explore the size impact of the particles, liver toxicity was
examined following administration of both nano and micro-NiO, and
the present results indicated that nano-NiO produced a stronger
liver toxicity than micro-NiO particles.
In summary, nano-NiO administration via
intratracheal instillation led to the imbalance of oxidation and
antioxidants, as well as the occurrence of nitrative stress in
rats. The present study indicated that the liver toxicity may be
associated with nitrative stress, oxidative stress and abnormal
expression of HO-1 and MT-1 mRNA induced by nano-NiO. However, the
exact mechanism of liver toxicity induced by nano-NiO remains
unclear and further studies in vitro and in vivo will
be required. The current findings may provide helpful evidence for
the safety assessment of nano-NiO administration.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for the Central Universities of China (grant no.
lzujbky-2016-205) and the National Undergraduate Training Programs
for Innovation and Entrepreneurship (grant no. 201610730162).
References
|
1
|
Gajewicz A, Rasulev B, Dinadayalane TC,
Urbaszek P, Puzyn T, Leszczynska D and Leszczynski J: Advancing
risk assessment of engineered nanomaterials: Application of
computational approaches. Adv Drug Deliv Rev. 64:1663–1693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He X, Aker WG, Fu PP and Hwang HM:
Toxicity of engineered metal oxide nanomaterials mediated by
nano-bio-eco-interactions: A review and perspective. Environ Sci:
Nano. 2:564–582. 2015.
|
|
3
|
Andersen NI, Serov A and Atanassov P:
Metal oxides/CNT nano-composite catalysts for oxygen
reduction/oxygen evolution in alkaline media. Appl Catal B:
Environ. 163:623–627. 2015. View Article : Google Scholar
|
|
4
|
Liu RC, Liang F, Zhou W, Yang Y and Zhu Z:
Calcium-doped lanthanum nickelate layered perovskite and nickel
oxide nano-hybrid for highly efficient water oxidation. Nano
Energy. 12:115–122. 2015. View Article : Google Scholar
|
|
5
|
Zanni E, Palma SD, Chandraiahgari CR,
Bellis GD, Cialfi S, Talora C, Palleschi C, Sarto MS, Uccelletti D
and Mancini P: In vitro toxicity studies of zinc oxide nano- and
microrods on mammalian cells: A comparative analysis. Mater Lett.
179:90–94. 2016. View Article : Google Scholar
|
|
6
|
Ogami A, Morimoto Y, Myojo T, Oyabu T,
Murakami M, Todoroki M, Nishi K, Kadoya C, Yamamoto M and Tanaka I:
Pathological features of different sizes of nickel oxide following
intratracheal instillation in rats. Inhal Toxicol. 21:812–818.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horie M, Fukui H, Nishio K, Endoh S, Kato
H, Fujita K, Miyauchi A, Nakamura A, Shichiri M, Ishida N, et al:
Evaluation of acute oxidative stress induced by NiO nanoparticles
in vivo and in vitro. J Occup Health. 53:64–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duan WX, He MD, Mao L, Qian FH, Li YM, Pi
HF, Liu C, Chen CH, Lu YH, Cao ZW, et al: NiO nanoparticles induce
apoptosis through repressing SIRT1 in human bronchial epithelial
cells. Toxicol Appl Pharmacol. 286:80–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu A, Chang X, Sun Y, Zou L, Li S, Sun Y,
Li S, Sun Y, Zhou H and Li J: Role of oxidative stress and
inflammatory response in subchronic pulmonary toxicity induced by
nano nickel oxide in rats. J Nanosci Nanotechno. 17:1753–1761.
2017. View Article : Google Scholar
|
|
10
|
Chang X, Zhu A, Liu F, Zou L, Su L, Li S
and Sun Y: Role of NF-κB activation and Th1/Th2 imbalance in
pulmonary toxicity induced by nano-NiO. Environ Toxicol.
32:1354–1362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Veranth JM, Kaser EG, Veranth MM, Koch M
and Yost GS: Cytokine responses of human lung cells (BEAS-2B)
treated with micron-sized and nanoparticles of metal oxides
compared to soil dusts. Part Fibre Toxicol. 4:22007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karlsson HL, Gliga AR, Calleja FM,
Goncalves CS, Wallinder IO, Vrieling H, Fadeel B and Hendriks G:
Mechanism-based genotoxicity screening of metal oxide nanoparticles
using the ToxTracker panel of reporter cell lines. Part Fibre
Toxicol. 11:412014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horie M, Nishio K, Kato H, Endoh S, Fujita
K, Nakamura A, Miyauchi A, Kinugasa S, Hagihara Y, Yoshida Y and
Iwahashi H: The expression of inflammatory cytokine and heme
oxygenase-1 genes in THP-1 cells exposed to metal oxide
nanoparticles. J Nano Res. 30:116–127. 2015. View Article : Google Scholar
|
|
14
|
Lu S, Zhang W, Zhang R, Liu P and Wang Q,
Shang Y, Wu M, Donaldson K and Wang Q: Comparison of cellular
toxicity caused by ambient ultrafine particles and engineered metal
oxide nanoparticles. Part Fibre Toxicol. 12:52015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ates M, Demir V, Arslan Z, Camas M and
Celik F: Toxicity of engineered nickel oxide and cobalt oxide
nanoparticles to artemia salina in seawater. Water Air Soil Pollut.
227:pii: 702016. View Article : Google Scholar
|
|
16
|
Stine JG and Lewis JH: Current and future
directions in the treatment and prevention of drug-induced liver
injury: A systematic review. Expert Rev Gastroenterol Hepatol.
10:517–536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pari L and Prasath A: Efficacy of caffeic
acid in preventing nickel induced oxidative damage in liver of
rats. Chem Biol Interact. 173:77–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CM, Ma JQ, Liu SS, Feng ZJ and Wang
AM: Puerarin protects mouse liver against nickel-induced oxidative
stress and inflammation associated with the TLR4/p38/CREB pathway.
Chem Biol Interact. 243:29–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng GH, Liu CM, Sun JM, Feng ZJ and
Cheng C: Nickel-induced oxidative stress and apoptosis in carassius
auratus liver by JNK pathway. Aquat Toxicol. 147:105–111. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Permenter MG, Lewis JA and Jackson DA:
Exposure to nickel, chromium, or cadmium causes distinct changes in
the gene expression patterns of a rat liver derived cell line. PLoS
One. 6:e277302011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmad J, Alhadlaq HA, Siddiqui MA, Saquib
Q, Al-Khedhairy AA, Musarrat J and Ahamed M:
Concentration-dependent induction of reactive oxygen species, cell
cycle arrest and apoptosis in human liver cells after nickel
nanoparticles exposure. Environ Toxicol. 30:137–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knight JA, Plowman MR, Hopfer SM and
Sunderman FW Jr: Pathological reactions in lung, liver, thymus, and
spleen of rats after subacute parenteral administration of nickel
sulfate. Ann Clin Lab Sci. 21:275–283. 1991.PubMed/NCBI
|
|
23
|
Sidhu P, Garg ML, Morgenstern P, Vogt J,
Butz T and Dhawan DK: Role of zinc in regulating the levels of
hepatic elements following nickel toxicity in rats. Biol Trace Elem
Res. 102:161–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahamed M, Ali D, Alhadlaq HA and Akhtar
MJ: Nickel oxide nanoparticles exert cytotoxicity via oxidative
stress and induce apoptotic response in human liver cells (HepG2).
Chemosphere. 93:2514–2522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahmad J, Alhadlaq HA, Siddiqui MA, Saquib
Q, Al-Khedhairy AA, Musarrat J and Ahamed M:
Concentration-dependent induction of reactive oxygen species, cell
cycle arrest and apoptosis in human liver cells after nickel
nanoparticles exposure. Environ Toxicol. 30:137–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Magaye RR, Yue X, Zou B, Shi H, Yu H, Liu
K, Lin X, Xu J, Yang C, Wu A and Zhao J: Acute toxicity of nickel
nanoparticles in rats after intravenous injection. Int J
Nanomedicine. 9:1393–1402. 2014.PubMed/NCBI
|
|
27
|
Ahamed M, Akhtar MJ, Alhadlaq HA, Khan MA
and Alrokayan SA: Comparative cytotoxic response of nickel ferrite
nanoparticles in human liver HepG2 and breast MFC-7 cancer cells.
Chemosphere. 135:278–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katsnelson BA, Minigaliyeva IA, Panov VG,
Privalova LI, Varaksin AN, Gurvich VB, Sutunkova MP, Shur VY,
Shishkina EV, Valamina IE and Makeyev OH: Some patterns of metallic
nanoparticles' combined subchronic toxicity as exemplified by a
combination of nickel and manganese oxide nanoparticles. Food Chem
Toxicol. 86:351–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morimoto Y, Ogami A, Todoroki M, Yamamoto
M, Murakami M, Hirohashi M, Oyabu T, Myojo T, Nishi K, Kadoya C, et
al: Expression of inflammation-related cytokines following
intratracheal instillation of nickel oxide nanoparticles.
Nanotoxicology. 4:161–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao L, Du J, Ding W, Jia R, Liu Y, Xu P,
Teraoka H and Yin G: Hepatoprotective and antioxidant effects of
dietary Angelica sinensis extract against carbon
tetrachloride-induced hepatic injury in Jian Carp (Cyprinus carpio
var. Jian). Aquac Res. 47:1852–1863. 2016. View Article : Google Scholar
|
|
32
|
Magaye R, Gu Y, Wang Y, Su H, Zhou Q, Mao
G, Shi H, Yue X, Zou B, Xu J and Zhao J: In vitro and in vivo
evaluation of the toxicities induced by metallic nickel nano and
fine particles. J Mol Histol. 47:273–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shuhendler AJ, Pu K, Cui L, Uetrecht JP
and Rao J: Real-time imaging of oxidative and nitrosative stress in
the liver of live animals for drug-toxicity testing. Nat
Biotechnol. 32:373–380. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Li H, Lu N, Feng Y, Huang Y and Gao
Z: Iron increases liver injury through oxidative/nitrative stress
in diabetic rats: Involvement of nitrotyrosination of glucokinase.
Biochimie. 94:2620–2627. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sugiura H and Ichinose M: Oxidative and
nitrative stress in bronchial asthma. Antioxid Redox Signal.
10:785–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roberts RA, Laskin DL, Smith CV, Robertson
FM, Allen EM, Doorn JA and Slikker W: Nitrative and oxidative
stress in toxicology and disease. Toxicol Sci. 112:4–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Padmini E, Vijaya Geetha B and Usha Rani
M: Pollution induced nitrative stress and heat shock protein 70
overexpression in fish liver mitochondria. Sci Total Environ.
407:1307–1317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bian K and Murad F: Diversity of
endotoxin-induced nitrotyrosine formation in
macrophage-endothelium-rich organs. Free Radic Biol Med.
31:421–429. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Muñoz A and Costa M: Elucidating the
mechanisms of nickel compound uptake: A review of particulate and
nano-nickel endocytosis and toxicity. Toxicol Appl Pharmacol.
260:1–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao W, Xiao L, Liu G, Fang T, Wu X, Jia G,
Zhao H, Chen X, Wu C, Cai J and Wang J: Dietary arginine and
N-carbamylglutamate supplementation enhances the antioxidant
statuses of the liver and plasma against oxidative stress in rats.
Food Funct. 7:2303–2311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou BH, Zhao J, Liu J, Zhang JL, Li J and
Wang HW: Fluoride-induced oxidative stress is involved in the
morphological damage and dysfunction of liver in female mice.
Chemosphere. 139:504–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koc M, Taysi S, Buyukokuroglu ME and Bakan
N: Melatonin protects rat liver against irradiation-induced
oxidative injury. J Radiat Res. 44:211–215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bai K, Xu W, Zhang J, Kou T, Niu Y, Wan X,
Zhang L, Wang C and Wang T: Assessment of free radical scavenging
activity of dimethylglycine sodium salt and its role in providing
protection against lipopolysaccharide-induced oxidative stress in
mice. PLoS One. 11:e01553932016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sidhu P, Garg ML and Dhawan DK: Protective
role of zinc in nickel induced hepatotoxicity in rats. Chem Biol
Interact. 150:199–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abrahám S, Hermesz E, Szabó A, Ferencz A,
Jancsó Z, Duda E, Abrahám M, Lázár G and Lázár G Jr: Effects of
Kupffer cell blockade on the hepatic expression of metallothionein
and heme oxygenase genes in endotoxemic rats with obstructive
jaundice. Life Sci. 90:140–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gutierrez ER, Kamens RM, Tolocka M, Sexton
K and Jaspers I: A comparison of three dispersion media on the
physicochemical and toxicological behavior of TiO2 and NiO
nanoparticles. Chem Biol Interact. 236:74–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takano H, Inoue K, Yanagisawa R, Sato M,
Shimada A, Morita T, Sawada M, Nakamura K, Sanbongi C and Yoshikawa
T: Protective role of metallothionein in acute lung injury induced
by bacterial endotoxin. Thorax. 59:1057–1062. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Horie M, Fukui H, Endoh S, Maru J,
Miyauchi A, Shichiri M, Fujita K, Niki E, Hagihara Y, Yoshida Y, et
al: Comparison of acute oxidative stress on rat lung induced by
nano and fine-scale, soluble and insoluble metal oxide particles:
NiO and TiO2. Inhal Toxicol. 24:391–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wallenborn JG, Schladweiler MJ, Richards
JH and Kodavanti UP: Differential pulmonary and cardiac effects of
pulmonary exposure to a panel of particulate matter-associated
metals. Toxicol Appl Pharmacol. 241:71–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pietroiusti A: Health implication of
engineered nanomaterials. Nanoscale. 4:1231–1247. 2012. View Article : Google Scholar : PubMed/NCBI
|