Introduction
Aging is accompanied by cognitive decline that is
associated with morphological and functional alterations in
hippocampal neurons, which have an important role in learning and
memory (1–3). Glial cells additionally influence the
function of hippocampal neurons during aging; decreased long-term
potentiation (LTP) is linked to age-associated microglial
activation in perforant path-granule cells in the hippocampus
(4). Exogenous astrocyte-derived
glial cell derived neurotrophic factor and D-serine in the
hippocampal CA1 area are able to reverse age-induced cognitive
deficits by increasing neurotransmitter synthesis, and enhancing
N-methyl-D-aspartic acid receptor-dependent LTP, respectively
(5,6). Furthermore, epigenetic memory stored
in the chromatin of mature oligodendrocytes is reduced in the
corpus callosum, due to alterations in gene expression (7).
Oligodendrocytes form myelin, which is involved in
the fast saltatory conduction of nerve impulses in the central
nervous system (CNS) (8); 20–30%
of the proteins of which myelin is composed are specific to myelin
and oligodendrocytes (9). Myelin
basic protein and proteolipid protein account for ~80% of the total
myelin protein, and oligodendrocyte-specific protein (OSP) is the
3rd most abundant protein, accounting for ~7% of the total myelin
protein (8,10). OSP is primarily expressed in
oligodendrocytes, and it exhibits channel functions and
oligodendrocyte growth regulation in the CNS (10).
Age-associated alterations in oligodendrocyte
protein expression have been studied in various brain regions,
including the cortex and corpus callosum of monkeys (11,12),
the corpus callosum of rats (13)
and the hippocampus of mice (14,15).
Additionally, increased numbers of newly-generated oligodendrocytes
in the hippocampus (14) and
spinal cord (16) during the aging
process have been reported. However, to date, limited studies have
reported the distribution of OSP in the hippocampus at various
ages. Therefore, the objective of the present study was to
determine the age-dependent alterations in OSP expression, an
oligodendrocyte marker, in the gerbil hippocampus as a good model
of aging (17,18).
Materials and methods
Experimental animals
Male gerbils (Meriones unguiculatus; n=84)
were supplied by the Experimental Animal Center, Kangwon National
University (Chuncheon, South Korea) and used at post-natal month
(PM) 1 (young), PM 2, PM 3, PM 4, PM 6 (adult) and PM 24 (aged).
The gerbils were housed at 23±3°C and 55±5% relative humidity in a
12-h light/dark cycle and were allowed free access to food and
water. Gerbils (n=14 in each group) were handled following the
National Institutes of Health (Bethesda, MD, USA) Guide for the
Care and Use of Laboratory Animals. The experimental protocol of
the present study was approved (approval no. KW-160802-2) by the
Institutional Animal Care and Use Committee of Kangwon National
University (Chuncheon, South Korea).
Western blot analysis
Animals (n=7 in each group) were used to examine
alterations in OSP expression levels. Western blotting was
performed according to a previously published method (19). Briefly, following sacrifice of the
animals, the hippocampus was removed and the hippocampal tissues
were homogenized and centrifuged, and the supernatants were
subjected to western blot analysis. The membranes were incubated
with Rabbit anti-OSP (cat. no. ab53041; 1:1,000; Abcam, Cambridge,
MA, USA) and mouse anti-β actin (cat. no. A5441; 1:5,000;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) overnight at 4°C.
Following washing 3 times with PBST (each for 10 min;
Sigma-Aldrich; Merck KGaA), the membrane was incubated with
peroxidase-conjugated donkey anti-rabbit IgG or goat anti-mouse IgG
(cat. no. sc-2305 or cat. no. sc-2031; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature,
and an ECL kit (Pierce; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The results of the western blot analysis were scanned,
and densitometric analysis was applied for quantification of the
bands as relative optical density (ROD) using ImageJ software
(version 1.46; National Institutes of Health). The ROD ratio was
calibrated as the percentage expression compared with PM 1 gerbils,
which was designated as 100% following normalization to each
β-actin band.
Immunohistochemistry
To examine the age-dependent alterations in OSP
immunoreactivity in the hippocampus during normal aging,
immunohistochemical staining and subsequent quantitative analysis
was performed, according to a previously published protocol
(20). Gerbils (n=7 in each group)
were intraperitoneally anesthetized with pentobarbital sodium (40
mg/kg) and transcardially perfused with 4% paraformaldehyde. Brain
tissues were cut into 25-µm thick sections at −20°C. Rabbit
anti-OSP (1:500; Abcam) was used as the primary antibody and
incubated overnight at 4°C. Following washing three times for with
PBS (each for 10 min; Sigma-Aldrich), the brain tissues were
incubated with biotinylated goat anti-rabbit (cat. no. BA-1000;
1:200; Vector Laboratories Inc., Burlingame, CA, USA) for 2 h at
room temperature and streptavidin peroxidase complex (cat. no.
SA-5004; 1:200; Vector Laboratories Inc.) for 45 min at room
temperature. A negative control test was performed using pre-immune
serum instead of the primary antibody to establish specificity of
the immunostaining. The negative control resulted in no
immunoreactivity in the stained sections.
To quantitatively analyze OSP immunoreactivity,
digital images of hippocampal sections were captured from six
sections per gerbil using a AxioM1 light microscope at 20×
magnification (Zeiss AG, Oberkochen, Germany) equipped with a
digital camera (Axiocam; Zeiss AG). OSP immunoreactive neurons were
counted in a 400×400-µm square area at the center of the CA1 area,
CA2/3 areas and the dentate gyrus using Optimas image analysis
software (version 6.5; CyberMetrics Corporation, Pheonix, AZ, USA).
Cell numbers were determined by calculating the mean total cell
number obtained from the 7 sections per gerbil.
Statistical analysis
The experiments were repeats three times. Data are
expressed as the mean ± standard error of the mean. Statistical
analysis was performed using one-way analysis of variance with a
post hoc Tukey's test for multiple comparisons by GraphPad Instat
version 3.05 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
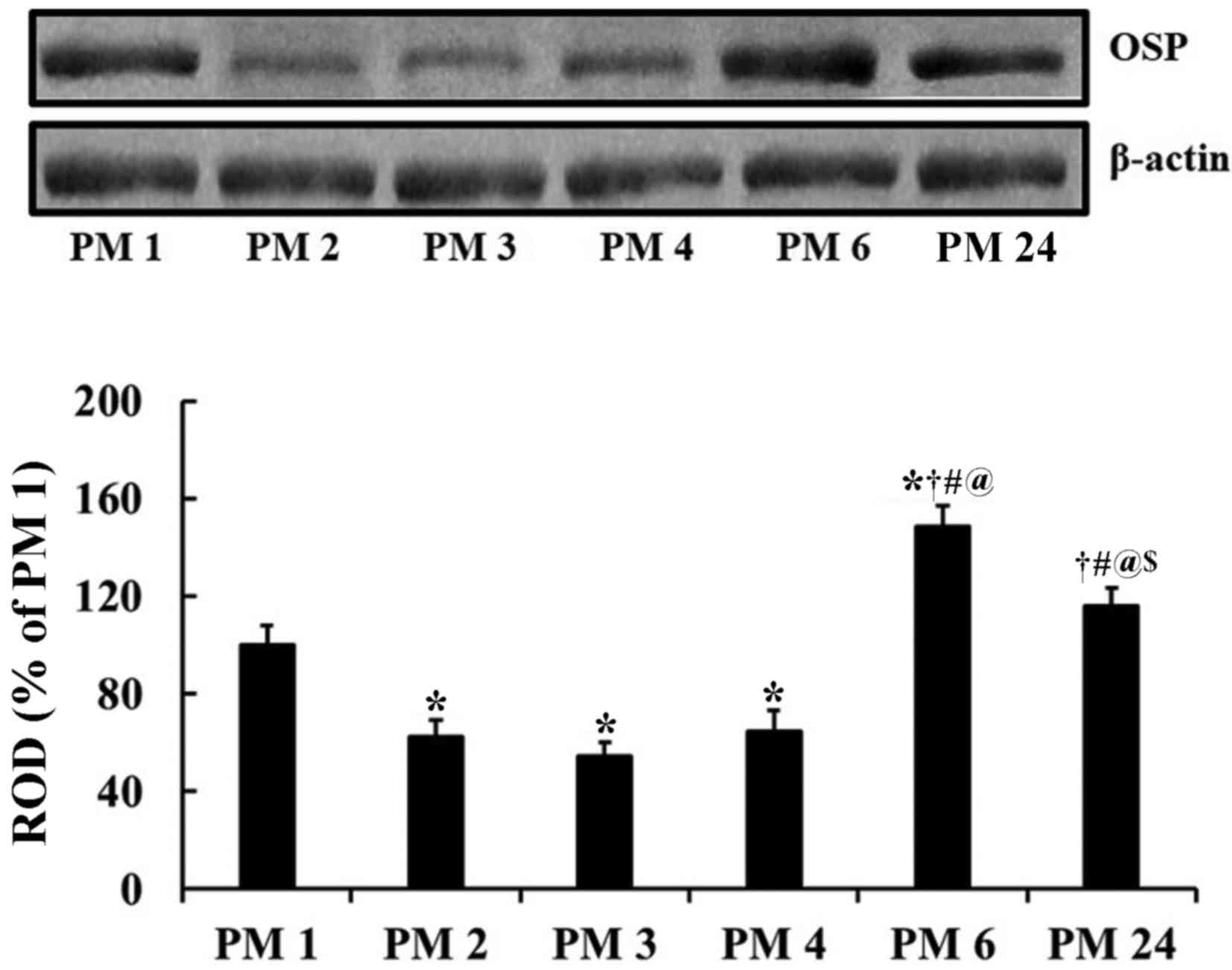
OSP protein levels
Significant alterations in OSP protein expression
were detected between groups via western blot analysis
[F(5,36)=23.493; P<0.0001]. OSP protein levels in the gerbil
hippocampus were significantly altered during normal aging. The
lowest OSP expression was detected in the PM 3 group and the
highest in the PM 6 group (Fig.
1). OSP levels in the PM 24 group were significantly decreased
compared with the PM 6 group, although they were higher compared
with those detected in the PM 1 group (Fig. 1).
OSP immunoreactivity
Patterns of OSP immunoreactivity were different
according to gerbil age and hippocampal subregions during normal
aging (Figs. 2–4). OSP immunoreactivity was detected in
cells in the PM 1–4 groups and in fibers in the PM 6 and PM 24
groups.
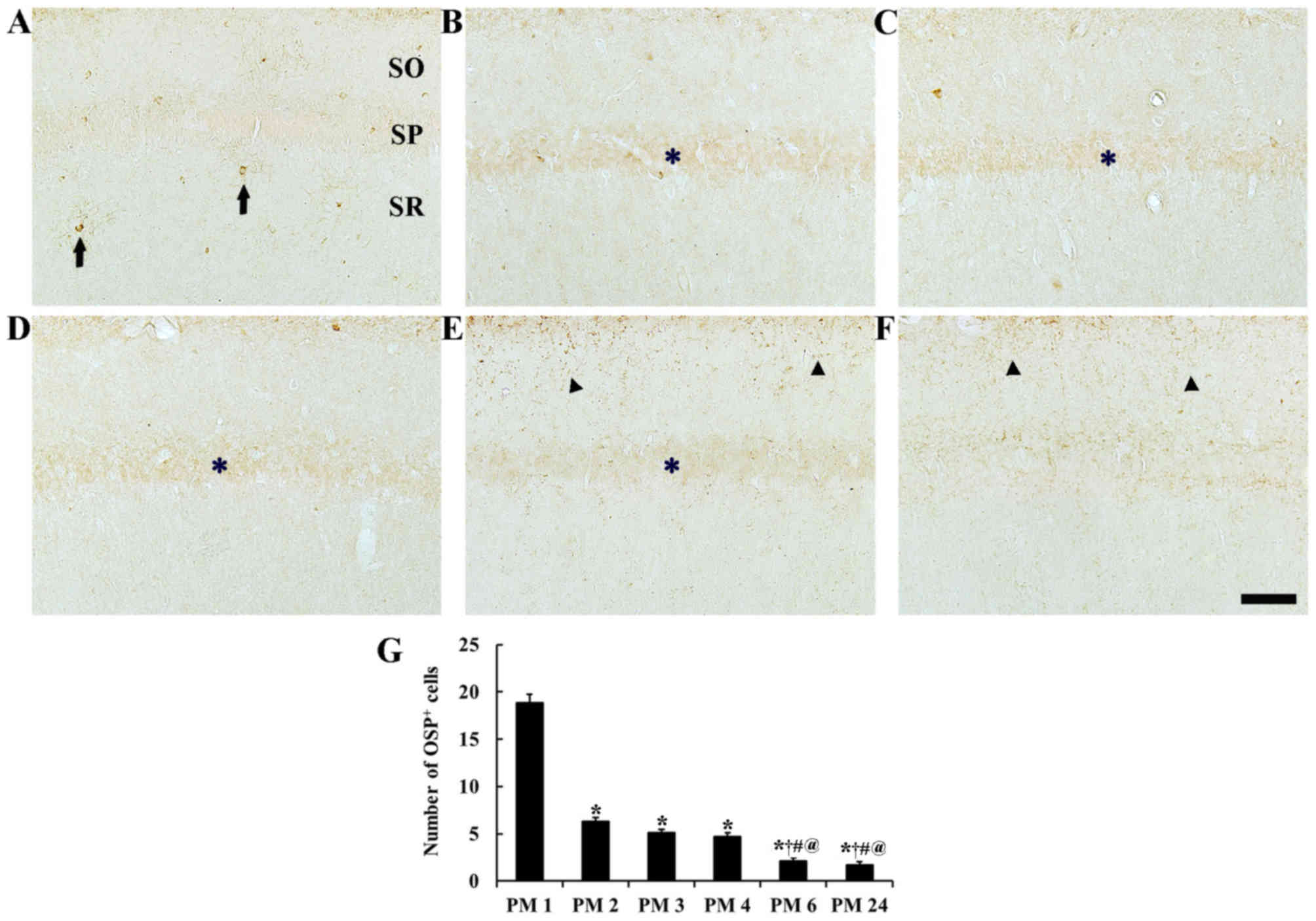 | Figure 2.OSP immunohistochemistry in the
hippocampal CA1 area of the (A) PM 1, (B) PM 2, (C) PM 3, (D) PM 4,
(E) PM 6 and (F) PM 24 groups. OSP immunoreactive cell bodies are
indicated by the arrows and were mainly detected in the SR at PM 1,
with numbers gradually decreasing with age. OSP immunoreactive
fibers are indicated by arrowheads and were markedly increased in
the SO at PM 6 and 24. OSP immunoreactivity was detected in the SP
at PM 2–6 and is indicated by the asterisk. Scale bar, 100 µm. (G)
Mean number of OSP immunoreactive cell bodies in the CA1 area. n=7
per group. *P<0.05 vs. PM 1, †P<0.05 vs. PM 2,
#P<0.05 vs. PM 3, @P<0.05 vs. PM 4.
Bars indicate the mean ± standard error of the mean. OSP,
oligodendrocyte-specific protein; PM, post-natal month; SR, stratum
radiatum; SO, stratum oriens; SP, stratum pyramidale. |
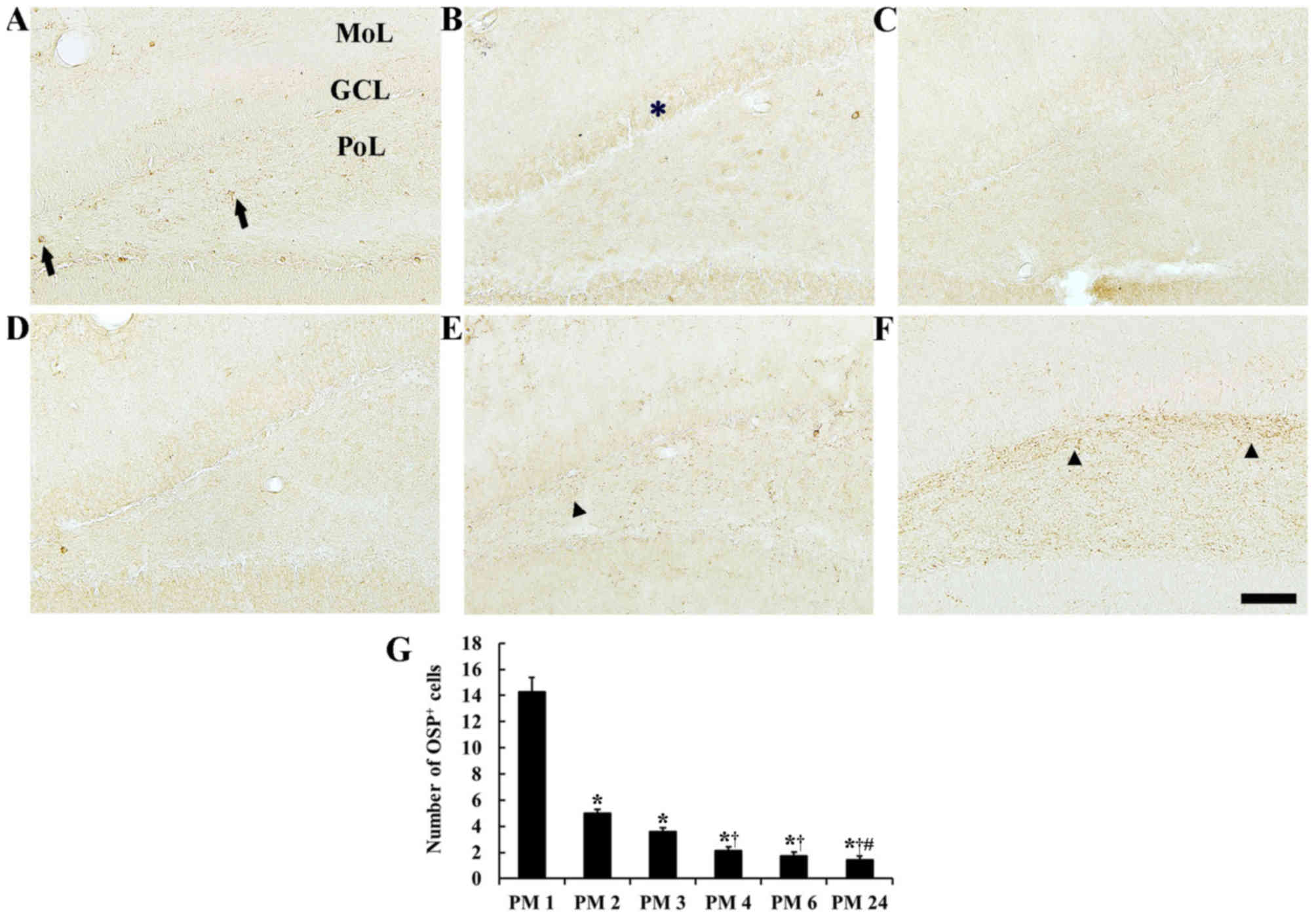 | Figure 4.OSP immunohistochemistry in the
dentate gyrus of the (A) PM 1, (B) PM 2, (C) PM 3, (D) PM 4, (E) PM
6 and (F) PM 24 groups. OSP immunoreactive cell bodies are
indicated by the arrows. Many cell bodies were detected in the PoL
until PM 2, where numbers decreased. OSP immunoreactive fibers are
indicated by arrowheads and detection markedly increased in the PoL
at PM 6 and PM 24. OSP immunoreactivity was observed in the GCL at
PM 2, as indicated by the asterisk. Scale bar, 100 µm. (G) Mean
number of OSP immunoreactive cell bodies in the dentate gyrus. n=7
per group. *P<0.05 vs. PM 1, †P<0.05 vs. PM 2,
#P<0.05 vs. PM 3 group. Bars indicate the mean ±
standard error of the mean. OSP, oligodendrocyte-specific protein;
PM, post-natal month; PoL, polymorphic layer; GCL, granule cell
layer; MoL, molecular cell layer. |
CA1 area
Significant alterations in OSP-positive cell number
were detected [F(5,36)=176.98; P<0.0001]. In the PM 1 group,
several OSP immunoreactive cell bodies were observed in the stratum
oriens and radiatum. Negligible numbers of OSP immunoreactive
fibers were detected in the CA1 area (Fig. 2A). In the PM 2 group, a significant
decrease in the number of OSP immunoreactive cell bodies was
observed in the CA1 area (Fig.
2G), and OSP immunoreactive fibers were uncommon (Fig. 2B). However, pyramidal cells of the
stratum pyramidale, or pyramidal neurons, had weak OSP
immunoreactivity (Fig. 2B). In the
PM 3 and PM 4 groups, OSP immunoreactive cell body numbers
(Fig. 2G), OSP immunoreactive
fiber distribution and OSP immunoreactivity in pyramidal neurons
were similar to those in the PM2 group (Fig. 2C, D). In the PM 6 group, OSP
immunoreactive cell bodies had shrunk in size and significantly
reduced in numbers compared with the PM 4 group (Fig. 2G); however, OSP immunoreactive
fibers were increased in the stratum oriens of the CA1 area
(Fig. 2E). In the PM 24 group, OSP
immunoreactive cell body numbers were not significantly different
compared with the PM 6 group (Fig.
2G), and OSP immunoreactive fibers were increased in the
stratum oriens and pyramidale of the CA1 area (Fig. 2F). Additionally, OSP
immunoreactivity in pyramidal neuron was negligible (Fig. 2F).
CA2/3 region
Significant alterations in the number of
OSP-positive cells were detected [F(5,36)=542.50; P<0.0001]. In
the PM 1 group, numerous OSP immunoreactive cell bodies were found
in all layers, although OSP immunoreactive fibers were rarely
detected (Fig. 3A). In the PM 2
group, the number of OSP immunoreactive cell bodies significantly
decreased (Fig. 3G) compared to
that in the PM1 group and few OSP immunoreactive fibers were
observed (Fig. 3B). However,
pyramidal neurons of the stratum pyramidale exhibited OSP
immunoreactivity (Fig. 3B). In the
PM 3 and 4 groups, numbers of OSP immunoreactive cell bodies had
decreased compared with the PM 2 group (Fig. 3G) and OSP immunoreactivity in
pyramidal neuron gradually decreased with age (Fig. 3C, D). In the PM 6 group, a few
small OSP immunoreactive cell bodies were observed (Fig. 3G); however, OSP immunoreactive
fibers had significantly increased in all layers, particularly in
the stratum radiatum (Fig. 3E). In
the PM 24 group, the number of OSP immunoreactive cell bodies was
similar to the PM 6 group (Fig.
3G); however, OSP immunoreactive fibers were evenly distributed
in the stratum oriens and lucidum (Fig. 3F). OSP immunoreactivity in
pyramidal neuron in the CA2/3 region was negligible (Fig. 3F).
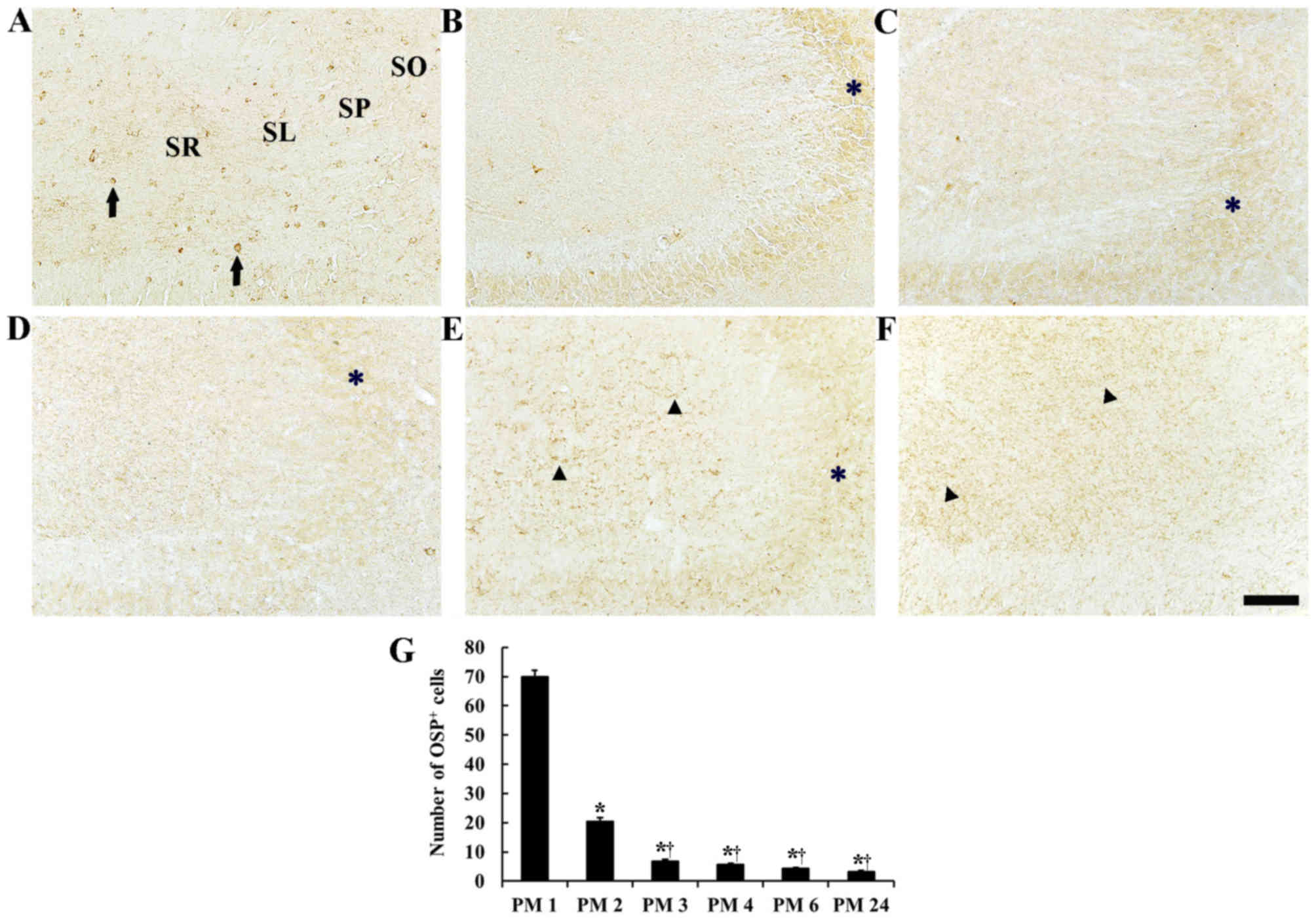 | Figure 3.OSP immunohistochemistry in the
hippocampal CA2/3 area of the (A) PM 1, (B) PM 2, (C) PM 3, (D) PM
4, (E) PM 6 and (F) PM 24 groups. OSP immunoreactive cell bodies
are indicated by the arrows and were detected abundantly throughout
all layers. OSP+ cell body numbers significantly reduced from PM 2.
The numerous OSP immunoreactive fibers are indicated by arrowheads
and were abundantly detected in the SR at PM 6 and 24. OSP
immunoreactivity is indicated by the asterisk and was observed in
the SP at PM 2–6. Scale bar, 100 µm. (G) Mean number of OSP
immunoreactive cell bodies in the CA2/3 area. n=7 per group.
*P<0.05 vs. PM 1, †P<0.05 vs. PM 2. Bars indicate
the mean ± standard error of the mean. OSP,
oligodendrocyte-specific protein; PM, post-natal month; SR, stratum
radiatum; SO, stratum oriens; SL, stratum lucidum; SP, stratum
pyramidale. |
Dentate gyrus
Significant alterations in the number of OSP
positive cells were detected in the dentate gyrus [F(5,36)=87.037;
P<0.0001]. In the PM 1 group, OSP immunoreactive cell bodies
were primarily detected in the polymorphic layer; the detection of
OSP immunoreactive fibers in the dentate gyrus was uncommon
(Fig. 4A). In the PM 2 group, the
number of OSP immunoreactive cell bodies significantly decreased in
the polymorphic layer (Fig. 4G)
and few OSP immunoreactive fibers were detected (Fig. 4B). OSP immunoreactivity was also
detected in cells of the granule cell layer, which are neurons
(Fig. 4B). In the PM 3 and 4
groups, OSP immunoreactive cell bodies gradually decreased with age
(Fig. 4G) and OSP immunoreactivity
in granule cells was very weak (Fig.
4C and D). In the PM 6 group, the number of OSP immunoreactive
cell bodies had decreased (Fig.
4G) and OSP immunoreactive fibers had marginally increased in
the polymorphic layer (Fig. 4E).
OSP immunoreactivity in granule cells was not observed (Fig. 4F). In the PM 24 group, the number
of OSP immunoreactive cell bodies was similar to the PM 6 group
(Fig. 4G); however, OSP
immunoreactive fibers had marginally increased in the polymorphic
layer (Fig. 4F).
Discussion
Myelin serves an important role in the function of
nervous tissue, and alterations in myelin-specific proteins causes
a several neurological disorders (21,22).
However, little is known about how OSP expression is affected by
aging in the hippocampus. In the present study, alterations in OSP
levels and immunoreactive structures were investigated in gerbil
hippocampi at 1, 2, 3, 4, 6 and 24 months with western blot
analysis and immunohistochemistry. It was demonstrated that OSP
levels and immunoreactive structures were significantly altered
with age.
In the present study, OSP immunoreactive cell bodies
were observed in the gerbil hippocampus at 1–4 months. At these
ages, the detection of OSP immunoreactive fibers was uncommon.
However, abundant OSP immunoreactive fibers were detected from 6
months. It was additionally revealed that the distribution of OSP
immunoreactive cell bodies and fibers was markedly different
according to the layers of the hippocampal subregions.
Distributions of other oligodendrocyte proteins in rodent brains
has been studied previously using various antibodies. For example,
Yamada and Jinno (14) reported
that the immunoreactivity of oligodendrocyte transcription factor
(Olig2), a basic helix-loop-helix transcription factor encoded by
Olig2 gene, is detected in cell bodies in the hippocampus of the
C57BL/6J mouse between 2 and 12 months of age. Additionally,
2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), a
myelin-associated enzyme that makes up 4% of the total CNS myelin
protein, is detected in the fibers in the hippocampus of ICR mice
between 2 and 59 weeks of age (23). Xie et al (24) reported that the levels of myelin
oligodendrocyte glycoprotein in the rat brain significantly
decrease from 5 months of age and are progressively downregulated
until 26 months. Similarly, the present study demonstrated that
levels of OSP protein in the gerbil hippocampus were highest in the
PM 6 group, and decreased in the PM 24 group. Based on the results
of previous research and the current study, expression patterns of
proteins in the myelin- or oligodendrocyte may be different
according to the kind of antibodies used.
In the present study, the number of OSP
immunoreactive cell bodies in the gerbil hippocampus abruptly
decreased at 2 months. Subsequently, the numbers gradually
decreased with increasing age. A small number of OSP immunoreactive
cell bodies were observed at 6 and 24 months; however, no
significant difference in numbers was detected between the groups.
In the C57BL/6J mouse, it has been reported that Olig2
immunoreactive cells are distributed in all hippocampal subregions
between 2 and 10 months of age, with no significant difference in
the numbers of Olig2 immunoreactive cells between mouse hippocampal
subregions (14). It has
additionally been demonstrated that the age-associated decrease in
remyelination efficiency is due to the impairment of
oligodendrocyte progenitor recruitment and differentiation
(25). Based on previous research
and the current study, OSP and Olig2 immunoreactive cell numbers
may be significantly decreased from an early age, which may be
associated with dysfunction of myelination.
OSP immunoreactive fiber density was significantly
increased in the CA1-3 areas and the dentate gyrus at 6 months in
the present study. The density was increased in the polymorphic
layer of the dentate gyrus at 24 months. Regarding age-dependent
alterations in myelin- or oligodendrocyte-associated proteins in
the CNS, it has been reported that CNPase immunoreactive fiber
density significantly decreases in the hippocampal CA1 region from
10 months in normal (23) and
senescence-accelerated mice (15).
Therefore, the expression of myelin-associated proteins in fibers
may be differentially altered in various brain regions during
normal aging.
In conclusion, the present study demonstrated that
the expression pattern of OSP immunoreactivity in the gerbil
hippocampus was significantly different according to hippocampal
subregion and the layers in the subregions. OSP was detected in
cell bodies prior to adult age, and in fibers from adult gerbils.
The present results suggested that OSP expression alterations may
be part of the normal aging process.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
NRF-2017R1D1A1B03030161), by the Bio & Medical Technology
Development Program of the NRF funded by the Korean government,
MSIP (grant no. NRF-2015M3A9B6066835), and by a Priority Research
Centers Program grant (grant no. NRF-2009-0093812) through the
National Research Foundation of Korea funded by the Ministry of
Science, ICT and Future Planning.
References
|
1
|
Rosenzweig ES and Barnes CA: Impact of
aging on hippocampal function: Plasticity, network dynamics and
cognition. Progress Neurobiol. 69:143–179. 2003. View Article : Google Scholar
|
|
2
|
Neves G, Cooke SF and Bliss TV: Synaptic
plasticity, memory and the hippocampus: A neural network approach
to causality. Nat Rev Neurosci. 9:65–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen L, Xu J, Zhan T, Wang H, Huang X, Liu
W, Yang X and Zhan R: The occurrence of diffuse axonal injury in
the brain: Associated with the accumulation and clearance of myelin
debris. Neural Regen Res. 9:1902–1906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lynch MA: Age-related neuroinflammatory
changes negatively impact on neuronal function. Front Aging
Neurosci. 1:62009.
|
|
5
|
Pertusa M, Garcia-Matas S, Mammeri H,
Adell A, Rodrigo T, Mallet J, Cristòfol R, Sarkis C and Sanfeliu C:
Expression of GDNF transgene in astrocytes improves cognitive
deficits in aged rats. Neurobiol Aging. 29:1366–1379. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mothet J, Rouaud E, Sinet PM, Potier B,
Jouvenceau A, Dutar P, Videau C, Epelbaum J and Billard JM: A
critical role for the glial-derived neuromodulator d-serine in the
age-related deficits of cellular mechanisms of learning and memory.
Aging cell. 5:267–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen S, Liu A, Li J, Wolubah C and
Casaccia-Bonnefil P: Epigenetic memory loss in aging
oligodendrocytes in the corpus callosum. Neurobiol Aging.
29:452–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baumann N and Pham-Dinh D: Biology of
oligodendrocyte and myelin in the mammalian central nervous system.
Physiol Rev. 81:871–927. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Campagnoni AT and Macklin WB: Cellular and
molecular aspects of myelin protein gene expression. Mol Neurobiol.
2:41–89. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bronstein JM, Micevych PE and Chen K:
Oligodendrocyte-specific protein (OSP) is a major component of CNS
myelin. J Neurosci Res. 50:713–720. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peters A and Sethares C: Oligodendrocytes,
their progenitors and other neuroglial cells in the aging primate
cerebral cortex. Cereb Cortex. 14:995–1007. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sloane JA, Hinman JD, Lubonia M, Hollander
W and Abraham CR: Age-dependent myelin degeneration and proteolysis
of oligodendrocyte proteins is associated with the activation of
calpain-1 in the rhesus monkey. J Neurochem. 84:157–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L, Lu W, Yang Z, Yang S, Li C, Shi X
and Tang Y: Age-related changes of the oligodendrocytes in rat
subcortical white matter. Anat Rec (Hpboken). 294:487–493. 2011.
View Article : Google Scholar
|
|
14
|
Yamada J and Jinno S: Age-related
differences in oligodendrogenesis across the dorsal-ventral axis of
the mouse hippocampus. Hippocampus. 24:1017–1029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka J, Okuma Y, Tomobe K and Nomura Y:
The age-related degeneration of oligodendrocytes in the hippocampus
of the senescence-accelerated mouse (SAM) P8: A quantitative
immunohistochemical study. Biol Pharm Bull. 28:615–618. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lasiene J, Matsui A, Sawa Y, Wong F and
Horner PJ: Age-related myelin dynamics revealed by increased
oligodendrogenesis and short internodes. Aging Cell. 8:201–213.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Selaković V, Rauš Balind S, Radenović L,
Prolić Z and Janać B: Age-dependent effects of ELF-MF on oxidative
stress in the brain of Mongolian gerbils. Cell Biochem Biophys.
66:513–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bae EJ, Chen BH, Shin BN, Cho JH, Kim IH,
Park JH, Lee JC, Tae HJ, Choi SY, Kim JD, et al: Comparison of
immunoreactivities of calbindin-D28k, calretinin and parvalbumin in
the striatum between young, adult and aged mice, rats and gerbils.
Neurochem Res. 40:864–872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahn JH, Chen BH, Shin BN, Cho JH, Kim IH,
Park JH, Lee JC, Tae HJ, Lee YL, Lee J, et al: Intravenously
Infused F3. Olig2 Improves memory deficits via restoring
myelination in the aged hippocampus following experimental ischemic
stroke. Cell Transplant. 25:2129–2144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn JH, Choi JH, Park JH, Kim IH, Cho JH,
Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH, et al: Long-term
exercise improves memory deficits via restoration of myelin and
microvessel damage and enhancement of neurogenesis in the aged
gerbil hippocampus after ischemic stroke. Neurorehabil Neural
Repair. 30:894–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lazzarini RA, Griffin JW, Lassman H, Nave
KA, Miller R and Trapp BD: Myelin biology and disorders. Elsevier
Inc; pp. 11822003
|
|
22
|
Agosta F, Dalla Libera D, Spinelli EG,
Finardi A, Canu E, Bergami A, Bocchio Chiavetto L, Baronio M, Comi
G, Martino G, et al: Myeloid microvesicles in cerebrospinal fluid
are associated with myelin damage and neuronal loss in mild
cognitive impairment and Alzheimer disease. Annals of neurology.
76:813–825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayakawa N, Kato H and Araki T:
Age-related changes of astorocytes, oligodendrocytes and microglia
in the mouse hippocampal CA1 sector. Mech Ageing Dev. 128:311–316.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie F, Zhang JC, Fu H and Chen J:
Age-related decline of myelin proteins is highly correlated with
activation of astrocytes and microglia in the rat CNS. Int J Mol
Med. 32:1021–1028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sim FJ, Zhao C, Penderis J and Franklin
RJ: The age-related decrease in CNS remyelination efficiency is
attributable to an impairment of both oligodendrocyte progenitor
recruitment and differentiation. J Neurosci. 22:2451–2459.
2002.PubMed/NCBI
|