Introduction
Fungal rhinosinusitis (FRS) is an infectious and/or
allergic disease of the rhinosinuses caused by fungi. While
considered uncommon, there have been an increasing number of cases
reported over the last two decades (1). Invasive FRS (IFRS), a type of FRS, is
an aggressive, often destructive and rapidly progressive infection,
which is histopathologically characterized by the presence of
hyphal invasion within the sinus mucosa, submucosa, blood vessels
or bone and is classified as either acute or chronic (2). Acute invasive FRS (AIFRS), a subtype
of IFRS, has a high mortality rate (50–80%) in immunocompromised
patients (3). Aspergillus
species (spp) and Mucorales are the major pathogenic fungi
implicated in FRS. Different fungi have different pathogenic
mechanisms and susceptibility to different antifungal drugs, thus
cases of FRS that are caused by different types of fungi vary with
regard to therapeutic options and the prognosis of patients.
Mucorales infection exhibits a more rapid course of progression and
greater invasiveness and is more likely to invade the arterial
intimal layer, which can lead to thrombosis, hemorrhagic and
ischemic necrosis and is, therefore, associated with high mortality
rates (4,5). Currently, amphotericin B is the
primary drug used to treat patients infected with Mucorales and
intravenous voriconazole is the primary drug for Aspergillus
spp infection. Accurate and timely diagnosis of the fungal species
is of clinical importance; however, sensitive diagnostic assays
have yet to be developed.
At present, there are various laboratory tests that
are used to identify fungi types, including culture, serological
differentiation, molecular techniques and histopathological
analysis (6). Because of its
simplicity, the existence of established methods, reasonable cost
and relatively fast and accurate diagnostic performance,
histopathological analysis is one of the major methods applied to
identify causative fungi clinically. Histopathological analysis
includes the following staining techniques: Hematoxylin and eosin
(H&E), Periodic Acid-Schiff (PAS), Gomori methenamine silver
(GMS) and mucin 5B (MUC5B) immunohistochemical staining. Of the
staining methods mentioned, MUC5B immunohistochemical staining has
strong specificity and high sensitivity as it is based on an
antigen-antibody response. However, not all Aspergillus spp
cases are positively identified with MUC5B immunohistochemical
staining. Additional immunohistochemical staining methods with
strong specificity and high sensitivity are required to improve the
histopathological diagnosis of FRS.
The present study aimed to develop a novel
immunohistochemical staining assay to differentiate between
Aspergillus spp and Mucorales, the two major types of
pathogenic fungi implicated in FRS. Interferon-γ (IFN-γ) is a
protein dimer that has an essential role in the innate and adaptive
phases of an immune response (7,8).
Although protein is a major component of the fungal cell wall, the
presence of the IFN-γ antigen on the fungal cell wall has not yet
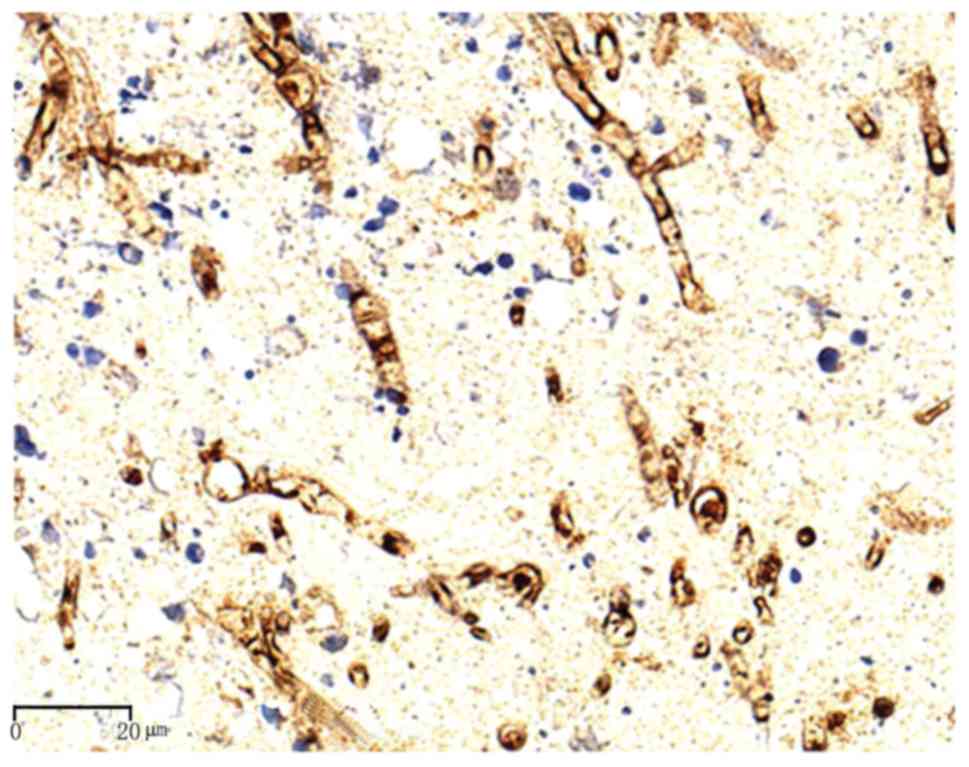
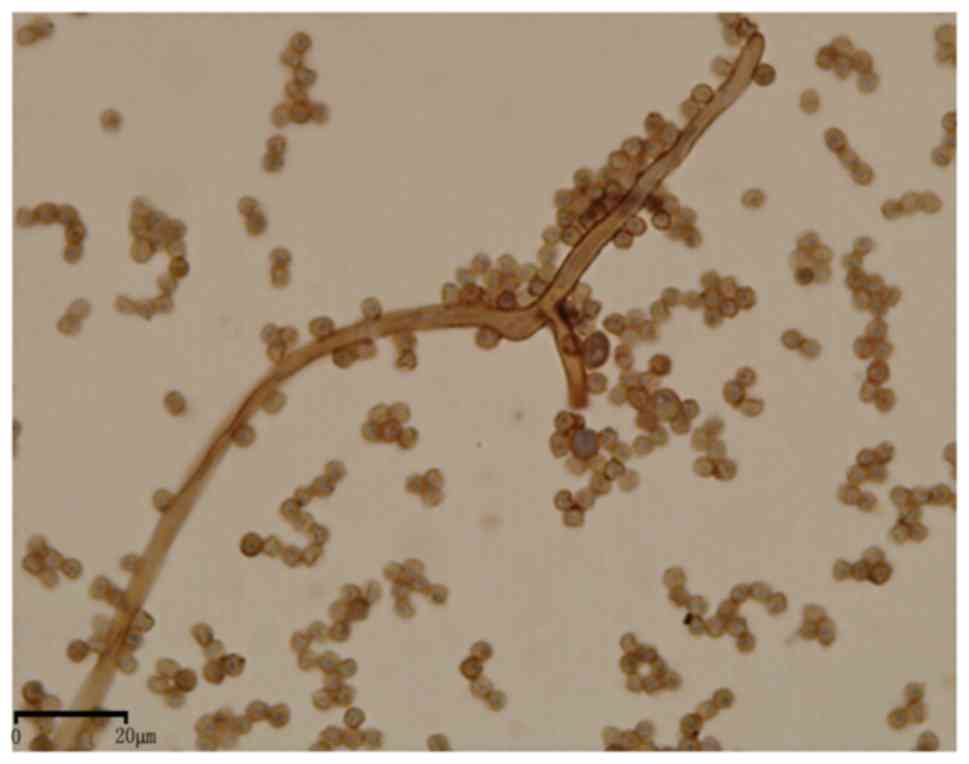
been demonstrated. AIFRS animal pre-experiments revealed that
Aspergillus fumigatus stained positive for IFN-γ. The hyphae
and conidia of A. fumigatus were stained diffuse brown on
the cell wall (Fig. 1). Therefore,
it was hypothesized that the IFN-γ antigen may be specific to
Aspergillus spp and that IFN-γ antibody may be used as a
diagnostic marker to differentiate between Aspergillus spp
and other major pathogenic fungi that cause FRS, including
Mucorales. Validation of these assumptions may provide a novel
method for identifying the causative fungal type in FRS with high
specificity and sensitivity, which may allow earlier diagnosis and
appropriate treatment of FRS. In the current study, an IFN-γ
antibody immunohistochemical assay was performed on formalin-fixed
paraffin-embedded nasal tissue specimens of FRS patients and
H&E, PAS and GMS staining was performed for comparison, to
observe the differences in the staining patterns of
Aspergillus spp and Mucorales. In addition, IFN-γ antibody
was used to stain cultures of Aspergillus spp and Mucorales,
derived from FRS patients, to validate the results of the
immunohistochemical assay on formalin-fixed paraffin-embedded
specimens of FRS.
Materials and methods
Samples
Formalin-fixed paraffin-embedded blocks of 51 nasal
tissue specimens of FRS patients (30 males and 21 females, mean age
32 years old; 27 infected by Aspergillus spp and 24 infected
by Mucorales (Rhizopus sp.) were obtained from the
Department of Pathology of Beijing Tongren Hospital. Samples were
randomly selected based on a diagnosis of FRS from pathological and
clinical data and fungal culture results between 2005–2012, which
was when the FRS diagnosis was determined. Samples that were
missing any of the aforementioned data were excluded. The cases in
the present study included IFRS, fungal balls and allergic FRS. The
types of causative fungi were blindly determined by two experienced
pathologists following assessment of pathological sections stained
with H&E, PAS and GMS, combined with clinical features and
cultures, before immunohistochemistry assays were performed. The
present study was approved by the ethics committee of the Beijing
Tongren Hospital (Beijing, China).
Formalin-fixed paraffin-embedded blocks of 10
esophageal cancer specimens isolated from patients were randomly
selected from the Department of Pathology of Beijing Tongren
Hospital and blindly determined as such by two experienced
pathologists. Cultures were provided by the bacterial laboratory of
Tongren Hospital in Beijing. Randomly selected Aspergillus
spp (A. fumigatus, n=10; A. flavus, n=8; and A.
terreus, n=8) and Mucorales cultures (n=8) were cultured for 5
days at 25°C on Vogel's glucose agar.
Groups
Group A was the experimental group and consisted of
formalin-fixed paraffin-embedded blocks of 51 FRS specimens (27
Aspergillus spp and 24 Mucorales) and 34 cultures of these
specimens (26 Aspergillus spp and 8 Mucorales). Group B
consisted of formalin-fixed paraffin-embedded blocks of 10
esophageal cancer specimens, which served as the positive control
group. Group C was the negative control group, in this group, IFN-γ
antibody was replaced by PBS when performing immunohistochemical
assay of Group A specimens.
Immunohistochemical assay of
formalin-fixed paraffin-embedded specimens
Sections of formalin-fixed paraffin-embedded
specimens (3 µm) were dried in an oven at 37°C for 30 min,
deparaffinized three times for 5 min in xylene and rehydrated with
graded ethanol solutions and distilled water. Endogenous peroxidase
in the sections was deactivated by incubating in 3%
H2O2 for 10 min. Following rinsing with
distilled water, antigen retrieval was performed using EDTA
solution at a high temperature (800W) in a microwave oven for 8
min. After cooling in distilled water for 5 min and rinsing with
PBS twice for 5 min, sections were subsequently incubated with
primary antibody (IFN-γ antibody; orb10878; 1:200; Biorbyt Ltd.,
Cambridge, UK) overnight at 4°C. The sections were subsequently
rinsed with distilled water and PBS, and EnVision+ horseradish
peroxidase rabbit antibody was added (K5007; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) for 30 min at room
temperature (25°C). Following washing with distilled water and PBS,
the sections were incubated in 3,3′-diaminobenzidine solution
(Dako; Agilent Technologies, Inc.) for 2–3 min. Chromogenic time
was determined by observation using a light microscope. The
sections were washed in distilled water, counterstained with
hematoxylin and dehydrated with xylene prior to mounting.
Esophageal cancer specimens were analyzed as positive controls
using the same method. In negative controls, primary antibody was
replaced by PBS.
Immunocytochemistry of cultures
Mucor cultures were added to slides, fixed in
95% ethanol for ~1 h at room temperature and incubated with the
aforementioned IFN-γ antibody (1:200; Biorbyt) overnight at 4°C.
The remaining procedures were the same as those described in the
previous section for the immunohistochemical assay of
formalin-fixed paraffin-embedded specimens. Esophageal cancer
specimens were used as positive controls. PBS was used instead of
primary antibody in the negative controls. The immunohistochemical
processes described were also repeated with another IFN-γ antibody
(PTG:15365-1-AP; 1:100; Proteintech Group, Inc., Chicago, IL, USA)
to verify the results.
Statistical analysis
Statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). The significance of
the results was evaluated by the χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
The majority of Aspergillus spp have
segmented forms and a shape that resemble antlers. Mucorales
species are thicker and the majority have right angled hyphae.
However, in certain clinical cases, Aspergillus spp become
thicker and deformed, perhaps affected by the clinical
characteristics of patients or the process of preparing sections,
making it difficult to distinguish them from other fungi based on
simple morphology. Thus, a highly specific immunohistochemical
method to identify fungal types is required. The standard for a
positive immunohistochemistry result in the present study was a
brown staining of the cell wall or cytoplasm of the fungus.
Immunohistochemistry of formalin-fixed
paraffin-embedded specimens
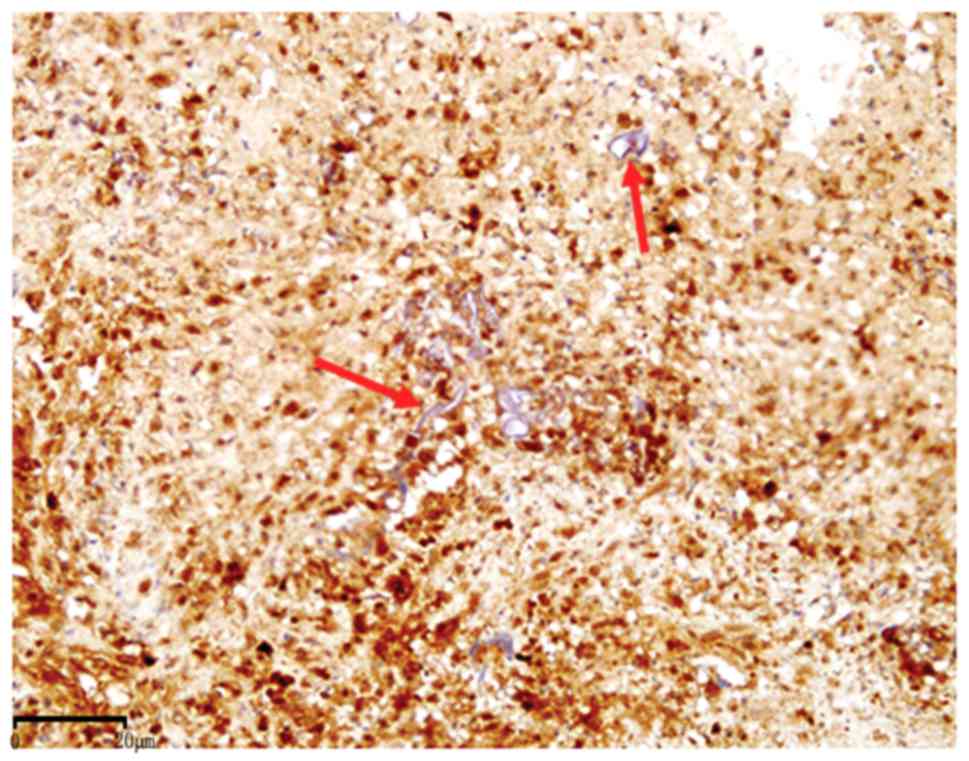
The results of immunohistochemical staining with
IFN-γ antibody in formalin-fixed paraffin-embedded FRS specimens
are presented in Table I and
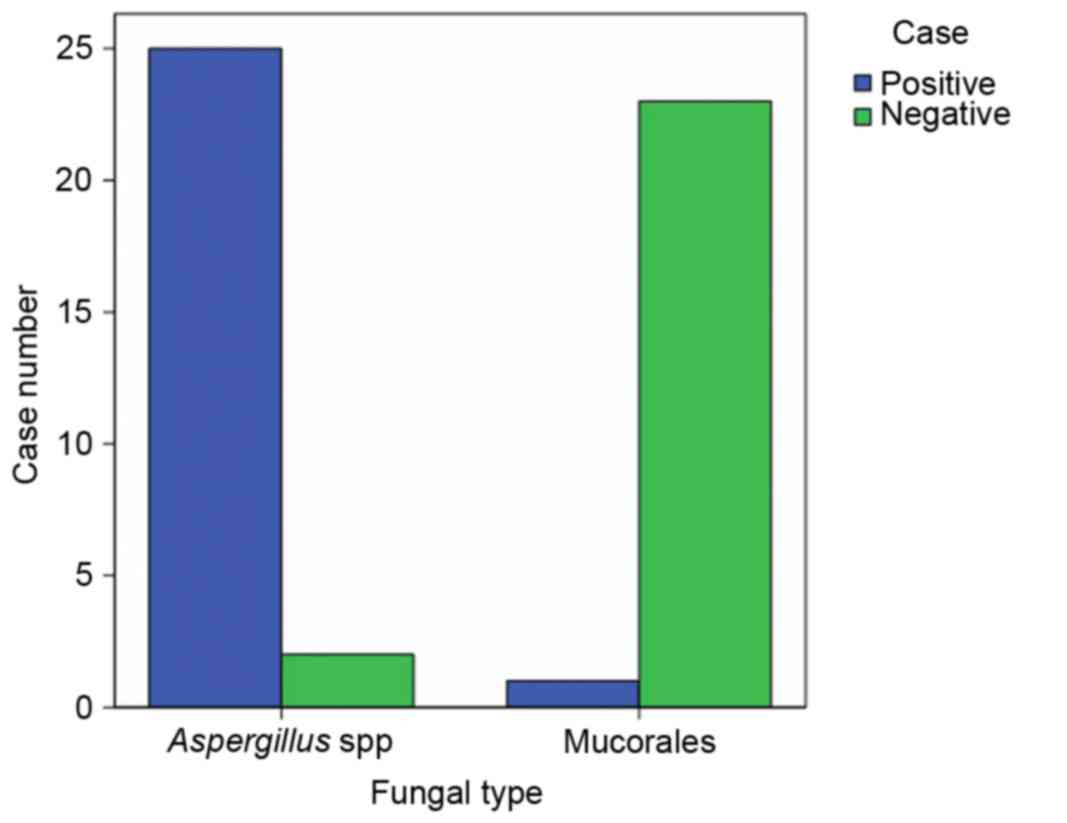
Fig. 2. IFN-γ expression was
positive in 25 and negative in 2 Aspergillus spp specimens.
Therefore, the IFN-γ-positive rate in Aspergillus spp was
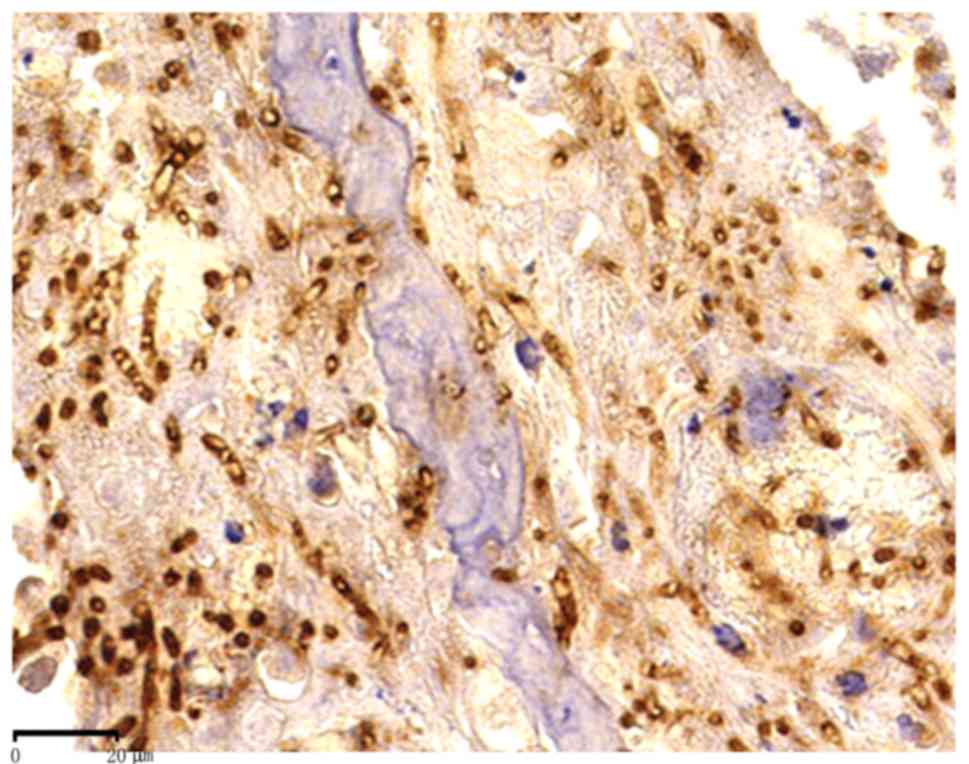
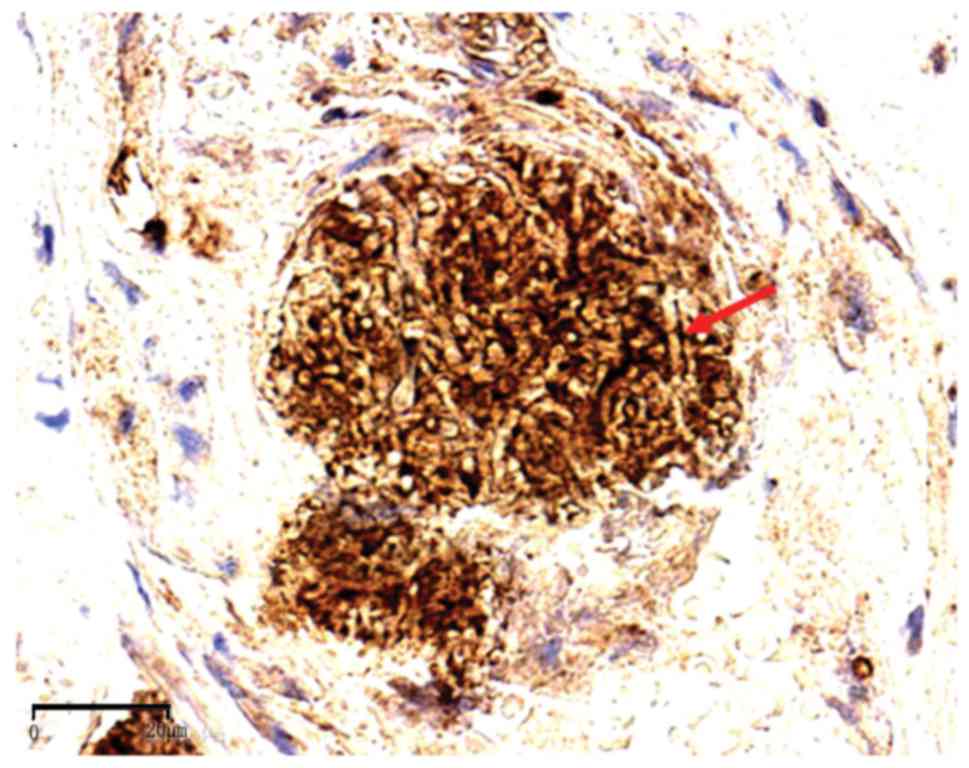
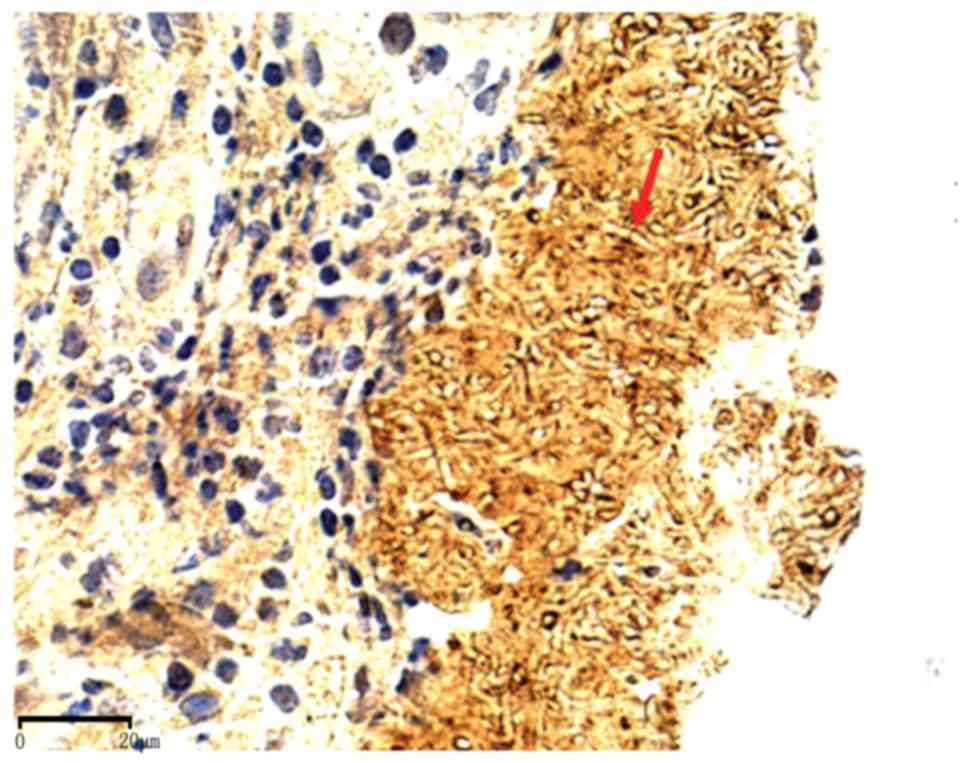
92.6% (25/27). The hyphae and conidia of Aspergillus spp
exhibited diffuse brown staining on the cell wall (Figs. 3, 4 and 5).
The two negative Aspergillus cases were fungal balls.
Aspergillus spp specimens from cases with allergic FRS and
IFRS were all stained positive. By contrast, IFN-γ expression was
negative in all Mucorales cell walls (Fig. 6), with the exception of one case,
therefore, IFN-γ staining was positive in only 4.2% of Mucorales
specimens; this was significantly lower than the percentage of
Aspergillus spp specimens that stained positive
(P<0.001).
 | Table I.IFN-γ expression in Aspergillus
spp and Mucorales formalin-fixed paraffin-embedded specimens. |
Table I.
IFN-γ expression in Aspergillus
spp and Mucorales formalin-fixed paraffin-embedded specimens.
|
| IFN-γ
expression |
|---|
|
|
|
|---|
| Fungal type | Positive cases
(%) | Negative cases
(%) |
|---|
| Aspergillus
sppa | 25 (92.5) | 2 (7.4) |
|
Mucoralesb | 1 (4.2) | 23 (95.8) |
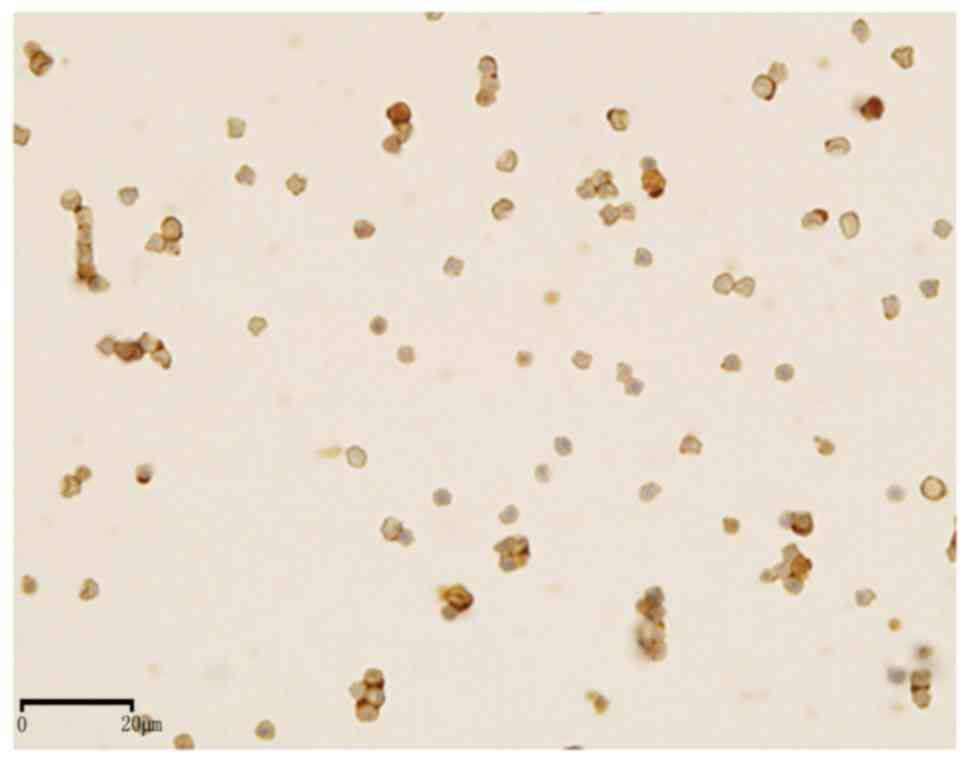
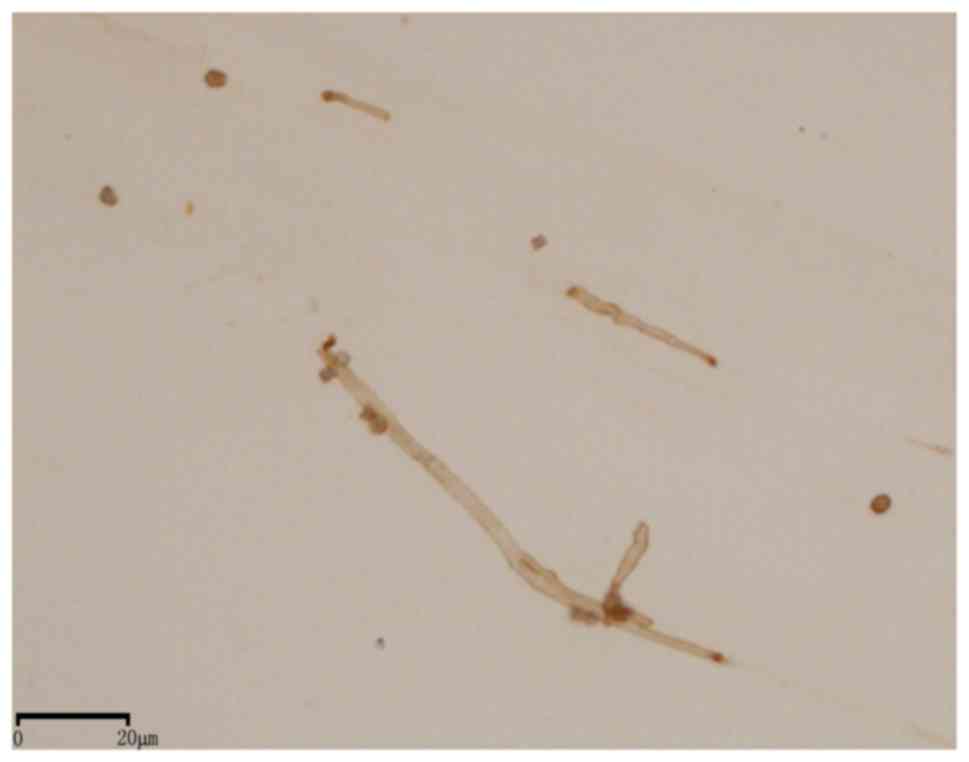
Immunocytochemistry of cultures
The results of immunocytochemical staining of
cultures for IFN-γ are summarized in Table II, which demonstrates that
positive immunoreactivity was observed in all A. fumigatus
(Fig. 7), A. flavus
(Fig. 8) and A. terreus
(Fig. 9) cultures. The positive
staining rate for Aspergillus spp was 100% (26/26), whereas
all Mucor spp (Fig. 10)
stained negative for IFN-γ. The cell walls of positive hyphae and
conidia exhibited diffuse brown staining. A significant difference
in IFN-γ expression was observed between Aspergillus spp and
Mucor spp (P<0.001). In the positive control group (Group
B), all cancer cell membranes exhibited positive brown staining,
while all specimens in the negative control group (Group C)
exhibited negative staining results. Identical immunohistochemical
staining results from the paraffin-embedded sections and the
cultures were obtained with the two IFN-γ antibodies from different
manufacturers.
 | Table II.IFN-γ expression in of
Aspergillus spp and Mucor spp cultures. |
Table II.
IFN-γ expression in of
Aspergillus spp and Mucor spp cultures.
|
| IFN-γ
expression |
|---|
|
|
|
|---|
| Fungal type | Positive cases
(%) | Negative cases
(%) |
|---|
| Aspergillus
sppa | 26 (100) | 0 (0) |
|
Mucorb | 0 (0) | 8 (100) |
Discussion
The present study performed immunohistochemical
staining for IFN-γ in samples of FRS and may have developed a novel
diagnostic immunohistochemical approach to distinguish between
types of fungi that are associated with FRS, which may increase the
potential for earlier and more accurate diagnosis and treatment of
FRS.
FRS was once considered to be a rare disorder;
however, it is currently reported with increasing frequency
worldwide (9–11). In the current classification
system, FRS is classified into invasive and noninvasive types
(12). Based on guidelines from
the International Society for Human and Mycology Group, IFRS may be
classified as acute, chronic or granulomatous, while the
non-invasive types of FRS are allergic FRS and fungus balls
(13). IFRS is a lethal disease
that has a fatality rate of 50–80% in AIFRS (3). Currently, surgery and antifungal
drugs are the major treatments for FRS. Early, accurate diagnosis
and systematic antifungal drug treatment is critical for the
management of FRS. Aspergillus spp and Mucorales are the
major types of pathogenic fungi that cause FRS. Aspergillus
spp account for 80% of FRS cases, while Mucorales are the most
aggressive and dangerous pathogens that have been implicated in
FRS, which means they are associated with a high mortality rate
(3,14,15).
The pathogenic mechanism of different fungi can vary and they may
also be susceptible to different antifungal drugs. Therefore, a
method that can rapidly and accurately identify the types of fungi
that are causative in different FRS cases is key for early
diagnosis and the appropriate treatment of FRS.
In recent years, various methods have been developed
to identify the types of fungi that are implicated in FRS cases.
Culture and histopathology-based tests have been supplemented with
molecular and proteomic techniques, and also antigen detection
methods (16–18). However, all methods possess
limitations. The culture method is insensitive, has potential for
laboratory contamination and often takes too long (usually 5 to 12
days) to obtain results. Although molecular assays, including
nucleic acid amplification tests and nucleic acid hybridization,
have potential as they exhibit high sensitivity and specificity,
the lack of test standardization and limited validation data for
many fungal nucleic acid tests have hindered their general
acceptance and broad application in clinical laboratories (16). The disadvantages of protein pattern
recognition, such as matrix-assisted laser desorption
ionization-time of flight mass spectrometry, include the
requirement of culture (they are not amenable to direct sample
testing), database supplementation (particularly for molds) and,
occasionally, manual spectral analysis (19). The application of the antigen
detection method in immunocompromised patients is limited. Although
histopathology-based testing also possesses limitations, it has
many advantages in clinical application, including the fact that
pathologists are familiar with it, it is easy to perform, reagents
are readily available, it provides rapid diagnosis and has
relatively high accuracy. Therefore, at present, histopathological
identification is the definitive method for diagnosing fungal
sinusitis in clinics (20).
IFN-γ is a protein dimer that has an essential role
in the innate and adaptive phases of an immune response (8,21,22).
It is required for optimal activation of phagocytes, it
collaborates in the generation of the protective antibody response
and favors the development of a T helper 1 cell protective response
(7,8). In addition to polysaccharides,
protein is also an important component of the fungal cell wall.
However, prior to the present study, it was not established whether
the fungal cell wall contained IFN-γ antigen. In a pre-experiment
on AIFRS animals, A. fumigatus in all infected cases
exhibited brown staining when IFN-γ antibody was applied.
Therefore, it was hypothesized that IFN-γ may be one of the
components of the Aspergillus cell wall and IFN-γ antibody
may be used to identify the causative fungal types in cases of
FRS.
The results of the present study demonstrated that
all Aspergillus spp and Mucorales specimens exhibited
positive staining with H&E, PAS and GMS. GMS exhibited the
strongest staining contrast between Aspergillus spp and
Mucorales (data not shown). However, none of the tests accurately
identified the fungal type, particularly for deformed and swollen
fungi. Based on the principle of the specific antigen-antibody
reaction, immunohistochemical staining with IFN-γ antibody was
markedly different between Aspergillus spp and Mucorales,
92.5% Aspergillus spp stained positive, with diffuse brown
staining on the cell wall, whereas Mucorales cell walls were all
negatively stained, with the exception of one case. These results
demonstrated that this may be a potentially useful, differential
diagnostic test to distinguish between Aspergillus spp and
Mucorales fungi.
In order to confirm that the IFN-γ antigen was of
fungal origin and not from the host tissue, the present study also
performed immunocytochemistry on cultures of Aspergillus spp
and Mucorales using IFN-γ antibody. Again, IFN-γ expression was
observed in the cell walls of all Aspergillus spp, including
A. fumigatus, A. flavus and A. terreus. By
contrast, all Mucorales cell walls were negative. These results
confirmed that IFN-γ antibody may be used as a new supplementary
test for the clinical diagnosis of FRS.
In order to verify that it was the IFN-γ antibody,
and not other substances present in the primary antibody, that had
positively stained the Aspergillus spp, the present study
applied a second IFN-γ antibody produced by a different
manufacturer to repeat the staining process and the observed
results were identical, which further confirmed the reliability of
this staining method for identifying fungal pathogens in FRS. In
conclusion, immunohistochemical staining with IFN-γ is a novel
method for distinguishing between Aspergillus spp and
Mucorales, and may be a beneficial supplementary test for current
immunohistochemical methods in the diagnosis of FRS. IFN-γ may be
one of the antigen components of the cell wall of
Aspergillus spp, however, this requires further studies.
Acknowledgements
The present study was supported in part by the
Department of Pathology, Beijing Tongren Hospital (Beijing, China)
and the National Natural Science Foundation of China (grant no.
81070769).
References
|
1
|
Chakrabarti A, Das A and Panda NK:
Overview of fungal rhinosinusitis. Indian J Otolaryngol Head Neck
Surg. 56:251–258. 2004.PubMed/NCBI
|
|
2
|
Seo J, Kim HJ, Chung SK, Kim E, Lee H,
Choi JW, Cha JH, Kim HJ and Kim ST: Cervicofacial tissue infarction
in patients with acute invasive fungal sinusitis: Prevalence and
characteristic MR imaging findings. Neuroradiology. 55:467–473.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donovan ST, Thompson JW, Sandlund JT,
Adderson EE, Pivnick EK and Harreld JH: Imaging of acute invasive
fungal rhinosinusitis in a patient with gorlin syndrome and acute
lymphocytic leukemia. Case Rep Otolaryngol.
2013:2723142013.PubMed/NCBI
|
|
4
|
Kobayashi K, Kami M, Murashige N, Kishi Y,
Fujisaki G and Mitamura T: Breakthrough zygomycosis during
voriconazole treatment for invasive aspergillosis. Haematologica.
89:ECR422004.PubMed/NCBI
|
|
5
|
Ma L, Xu R, Shi J, Zhou W, Xu G, Jiang G,
Li G and Chen Z: Identification of fungi in fungal ball sinusitis:
Comparison between MUC5B immunohistochemical and Grocott
methenamine silver staining. Acta Otolaryngol. 133:1181–1187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piao YS, Zhang Y, Yang X, He CY and Liu
HG: The use of MUC5B antibody in identifying the fungal type of
fungal sinusitis. Hum Pathol. 39:650–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kearney S, Delgado C and Lenz LL:
Differential effects of type I and II interferons on myeloid cells
and resistance to intracellular bacterial infections. Immunol Res.
55:187–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gozalbo D, Maneu V and Gil ML: Role of
IFN-gamma in immune responses to Candida albicans infections. Front
Biosci (Landmark Ed). 19:1279–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Challa S, Uppin SG, Hanumanthu S,
Panigrahi MK, Purohit AK, Sattaluri S, Borgohain R, Chava A, Vemu L
and Jagarlapudi MM: Fungal rhinosinusitis: A clinicopathological
study from South India. Eur Arch Otorhinolaryngol. 267:1239–1245.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Montone KT, Livolsi VA, Feldman MD, Palmer
J, Chiu AG, Lanza DC, Kennedy DW, Loevner LA and Nachamkin I:
Fungal rhinosinusitis: A retrospective microbiologic and pathologic
review of 400 patients at a single university medical center. Int J
Otolaryngol. 2012:6848352012.PubMed/NCBI
|
|
11
|
Callejas CA and Douglas RG: Fungal
rhinosinusitis: What every allergist should know. Clin Exp Allergy.
43:835–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rupa V and Thomas M: Different types of
fungal sinusitis occurring concurrently: Implications for therapy.
Eur Arch Otorhinolaryngol. 270:603–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chakrabarti A, Denning DW, Ferguson BJ,
Ponikau J, Buzina W, Kita H, Marple B, Panda N, Vlaminck S,
Kauffmann-Lacroix C, et al: Fungal rhinosinusitis: A categorization
and definitional schema addressing current controversies.
Laryngoscope. 119:1809–1818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aribandi M, McCoy VA and Bazan C III:
Imaging features of invasive and noninvasive fungal sinusitis: A
review. Radiographics. 27:1283–1296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thompson GR III and Patterson TF: Fungal
disease of the nose and paranasal sinuses. J Allergy Clin Immunol.
129:321–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wengenack NL and Binnicker MJ: Fungal
molecular diagnostics. Clin Chest Med. 30:391–408, viii. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ostrosky-Zeichner L: Invasive mycoses:
Diagnostic challenges. Am J Med. 125 1 Suppl:S14–S24. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Griffin AT and Hanson KE: Update on fungal
diagnostics. Curr Infect Dis Rep. 16:4152014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenvinge FS, Dzajic E, Knudsen E, Malig
S, Andersen LB, Løvig A, Arendrup MC, Jensen TG, Gahrn-Hansen B and
Kemp M: Performance of matrix-assisted laser desorption-time of
flight mass spectrometry for identification of clinical yeast
isolates. Mycoses. 56:229–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michael RC, Michael JS, Ashbee RH and
Mathews MS: Mycological profile of fungal sinusitis: An audit of
specimens over a 7-year period in a tertiary care hospital in Tamil
Nadu. Indian J Pathol Microbiol. 51:493–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schroder K, Hertzog PJ, Ravasi T and Hume
DA: Interferon-gamma: An overview of signals, mechanisms and
functions. J Leukoc Biol. 75:163–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toomer KH and Chen Z: Autoimmunity as a
double agent in tumor killing and cancer promotion. Front Immunol.
5:1162014. View Article : Google Scholar : PubMed/NCBI
|