Introduction
Skin wounds are the commonest body damage in
clinics, and their consequences are largely determined by the
quality of wound healing (1).
Wound healing is the process by which the damaged tissue is
repaired after trauma through an orchestrated cascade of stepwise
biochemical events including blood clotting with inflammation,
tissue growth (proliferation) and tissue remodeling (maturation)
(2,3). Cell turnover happens in those steps,
including the removal of the dysfunctional cells from the wound bed
in the forms of necrosis (4)
and/or apoptosis (5,6), the activation of cell proliferation
machinery to repaire the lost tissue (7,8) and
the remodeling of the regenerated tissue by inducing apoptosis of
unneeded cells (9). Apparently, a
reagent that well regulates cell death and proliferation would be
of practical values in better management of skin wounds.
Multiple molecular factors involve in skin wound
healing, of which the signal transduction pathways mediated by
Smad/TGFβ (10,11) and STAT3 (11,12)
play active roles through initiating or upregulating the expression
of a series of growth-promoting genes including anti-apoptotic
genes such as Survivin and Bcl-2 (13). It has been recognized that the
biological effects of Bcl-2 can be enhanced via interaction with
BAG3 (BCL2-associated athanogene 3) (14–16).
For instance, combined inhibition of Bcl-2 and BAG3 overcomes
apoptotic resistance in glioblastoma in vitro and in
vivo (15) and significant
decrease of BAG3 expression leads cardiac cells to apoptosis
(17). On the other hand, BAG3
promotes autophagy through interacting with autophagy-associated
protein Beclin 1 (18) because an
increased level of BAG3 results in stimulation of autophagy in
glioblastoma cells (19). Although
extensive cell turnover including apoptosis and autophagy occurs in
the skin wound tissues, the involvement and potential role(s) of
BAG3 in stepwise wound healing remains unknown.
It has long been recognized that maggot can be used
to accelerate skin wound healing in the manners of eating unhealthy
tissues and secreting/eliminating the bioactive products (20,21).
Our recent results demonstrate that the extract isolated from
maggots without secretion and elimination efficiently shortens the
wound closure time by enhancing Smad2/TGFβ and STAT3 signaling
activities, respectively (22).
Because Bcl-2 is one of the downstream effector of STAT3 signaling
(23) and its function is enhanced
by associating with BAG3 co-chaperone protein (24,25),
BAG3 expression pattern in wound tissue may be altered by maggot
extract and may functionally influence the apoptotic pathway and
autophagy-promoting activity of Beclin 1 in the post-trauma
regenerating tissues. To address these issues, BAG3, BCL-2, Beclin
1 and LC3 levels in rat skin wound tissues without and with maggot
extract treatment are analyzed and their relevance with the rates
of wound healing and cell proliferation are evaluated.
Materials and methods
Maggot extract preparation
The maintenance of Lcuprina blowflies and
their larvae, the ways of maggot collection and treatment
and the method for maggot extract preparation were conducted in the
manners described elsewhere (22).
We found that the full maggot extract (tissue lysate +
excretion/secretion) exerted the best repair promoting effects on
wound tissues in comparison with that of the mixture of
excretion/secretion (ES) and the tissue lysate prepared from the
maggots after excretion and secretion and the optimal working
concentration was 150 µg/ml (22).
Therefore, the vaseline-diluted full maggot extracts in the
concentration of 150 µg/ml was adopted to dress the wound beds.
Statement of assurance of proper
animal experiments
The protocol of animal study was designed in
compliance with the National Research Council's criteria for humane
care as outlined in ‘Guide for the Care and Use of Laboratory
Animals’ prepared by the Institute of Laboratory Animal Resources
and published by the National Institutes of Health (NIH Publication
No. 86–23, Revised 1985). Before conducting the experiments, the
contents of the present study were reviewed and approved by the
ethical and animal warfare committee of Dalian Medical University.
During the experiments, all animals received humane care. When the
experiments were finished, they were returned to the institutional
animal center without sacrificing.
Rat wound model
After getting the permission to conduct the animal
experiment from Institutional Ethics Committee and the Committee on
Research Animal Care of Dalian Medical University, sixteen 10-week
old male Sprague Dawley rats were provided by the Experimental
Animal Center of Dalian Medical University and reared under
specific pathogen-free/SPF condition. The rats were anaesthetized
with 12 mg/kg xylazine via intraperitoneal injection. A pair of 2
cm diameter round open wounds down to the muscle fascia was made on
the left for the purposes of sequential biopsy and on the right
flanks for wound area measurement, respectively (26). The animals were randomly divided
into two experimental groups of 8 animals/group as Group 1 (G1):
Dressed with vaseline only as untreated control; G2: Treated with a
mixture of vaseline and 150 µg/ml full maggot extract (without
excretion and secretion). The treatments lasted for 16 days by
daily dressing the reagents. The margins of individual wounds were
outlined in regular changed red and black colors at day 1, 3, 4, 6,
8, 10, 12, 14 and 16 by directly placing a transparency model sheet
on the wounds on the right flanks (12). The areas enclosed by the traced
wound margins of the two experimental groups were calculated by
Digital-transparency wound area measurement (12). The animal experiments were
restrictively followed the guidelines of the Association for
Assessment and Accreditation of Laboratory Animal Care,
International and repeated for three times for establishing
statistical significance.
Wound tissue biopsy
The tissues in the size of 0.3×0.3×0.2 cm were
biopsied from the margins and beds of the wounds on the left flanks
at the post-trauma times of day 1, day 4, day 7, day 12 and, if
available, day 16 or day 3, day 5, day 9 and, if available, day 14.
The biopsy was sequentially conducted at the 3, 6, 9 and 12 o'clock
positions of the round wounds. The collected tissues were snap
frozen in liquid nitrogen and stored at −80°C until use. The frozen
tissues were vertically sectioned in 5 mm thickness into 80–100
pieces which were immediately put into 40 µl cell lysate buffer for
protein isolation (27). The
remaining parts of the sample tissues were sectioned in 7 µm
thickness for histological and immunohistochemical staining.
TUNEL apoptosis assay
Terminal deoxynucleotide transferase (TdT)-mediated
dUTP-biotin nick-end labeling (TUNEL) assay was employed to detect
apoptotic cells according to producer's instructions (Promega
Corp., Madison, WI, USA). Briefly, the tissue samples were
pre-incubated with 3% bovine serum albumin (BSA) in PBS for 30 min
at RT to prevent nonspecific labeling and then incubated for 1 h at
37°C in a humid chamber with the TUNEL mixture containing 0.13 5
Uipl calf thymus TdT. After three 5 min washes with PBS at room
temperature, 100 µl anti-FITC-AP conj. was applied on each sample
for 30 min at 37°C. After resaturation in blocking reagent, the
tissues were treated for 1 hr at room temperature with a 1:120
diluted peroxidase-labeled anti-digoxigenin sheep Fab fragment,
followed by 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB)
color reaction.
Immunohistochemical staining
The statuses of BAG3, Bcl-2, Beclin 1 and LC3
expression in the wound tissues were analyzed immunohistochemical
staining by the method described elsewhere (28). The antibodies against those target
proteins were purchased from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA. Color reaction was developed using 3,
3′-diaminobenzidine tetrahydrochloride (DAB). The samples without
first antibody incubation were cited as negative control.
Western blot analysis
To validate the immunohistochemical results, total
cellular proteins were prepared from the wound tissues without and
with maggot extract treatment by the method described elsewhere
(12). The sample proteins (50
µg/lane) were separated in 10% sodium dodecylsulfate-polyacrylamide
gel electrophoresis and transferred to polyvinylidene difluoride
membrane (Amersham, Buckinghamshire, UK). The membrane was blocked
with 5% skimmed milk in TBS-T (10 mM Tris-HCl, pH 8.0, 150 mM NaCl
and 0.5% Tween 20) at 4°C, rinsed 10 min for three times with
TBS-T, followed by 3 h incubation at room temperature with the
first antibody and then 1 h incubation with HRP-conjugated
anti-mouse or anti-rabbit IgG (Zymed Lab, Inc., San Francisco, CA,
USA). The bound antibody was detected using the enhanced
chemiluminescence system (Roche GmbH, Mannheim, Germany). After
removing the labeling signal by incubation with stripping buffer,
the membrane was reprobed with other antibodies one by one until
all of the parameters were examined. The parameters checked are in
parallel with that of immunohistochemical staining. The results of
western blotting were quantified by densitometry analysis, using
Quantity One software according to producer's instruction (Bio-Rad
Lab., Berkeley, CA, USA). β-actin bands of individual samples were
cited as internal quantitative control.
Statistical analysis
The wound healing statuses of individual
experimental groups at different tracing times and MTT data were
evaluated by the independent-samples t-test and one-way ANOVA
methods with SPSS 11.5 software (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Maggot extract promoted wound
healing
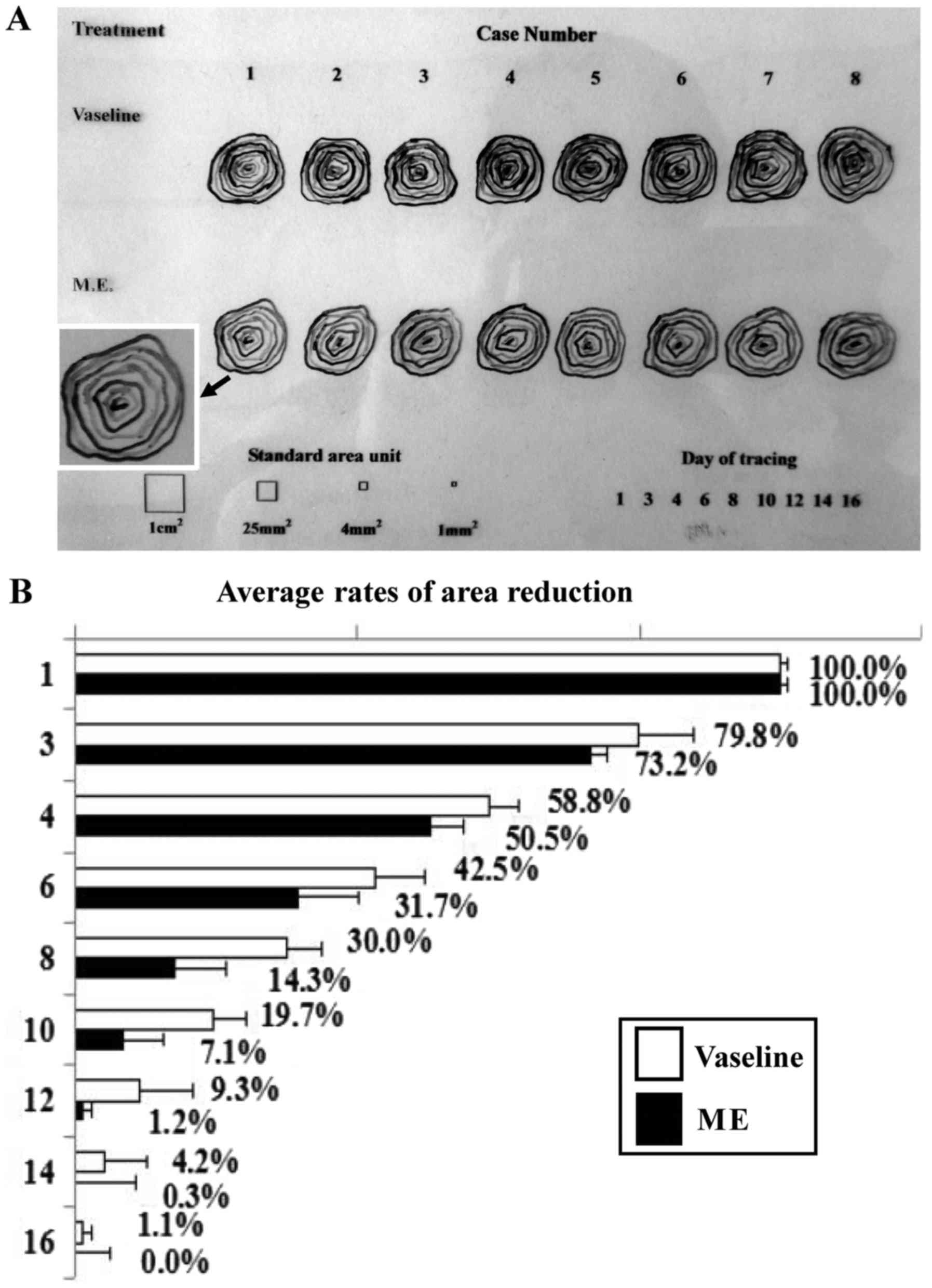
All together, 72 pieces of tracing message were
collected from 8 open skin wounds at 9 time points in the control
and 58 pieces from the experimental group respectively, which were
totally documented in one transparency model sheet (Fig. 1A). The repairing rates of the skin
wounds without and with maggot extract treatments were sequentially
evaluated by transparency tracing-digital calculation method
(12). As shown in Fig. 1B, the average unrepaired areas of
group 1 dressed only with vaseline were larger than that of group 2
treated by maggot extract at any observation time points
(P<0.05; t-test). Two of eight wounds in group 2 completely
closed at day 12, four at day 14 and the last two at day 16; in
contrast, all eight wounds in group 1 remained open at day 16
(Fig. 1A).
No effect of maggot extract on
TUNEL-negative cell death
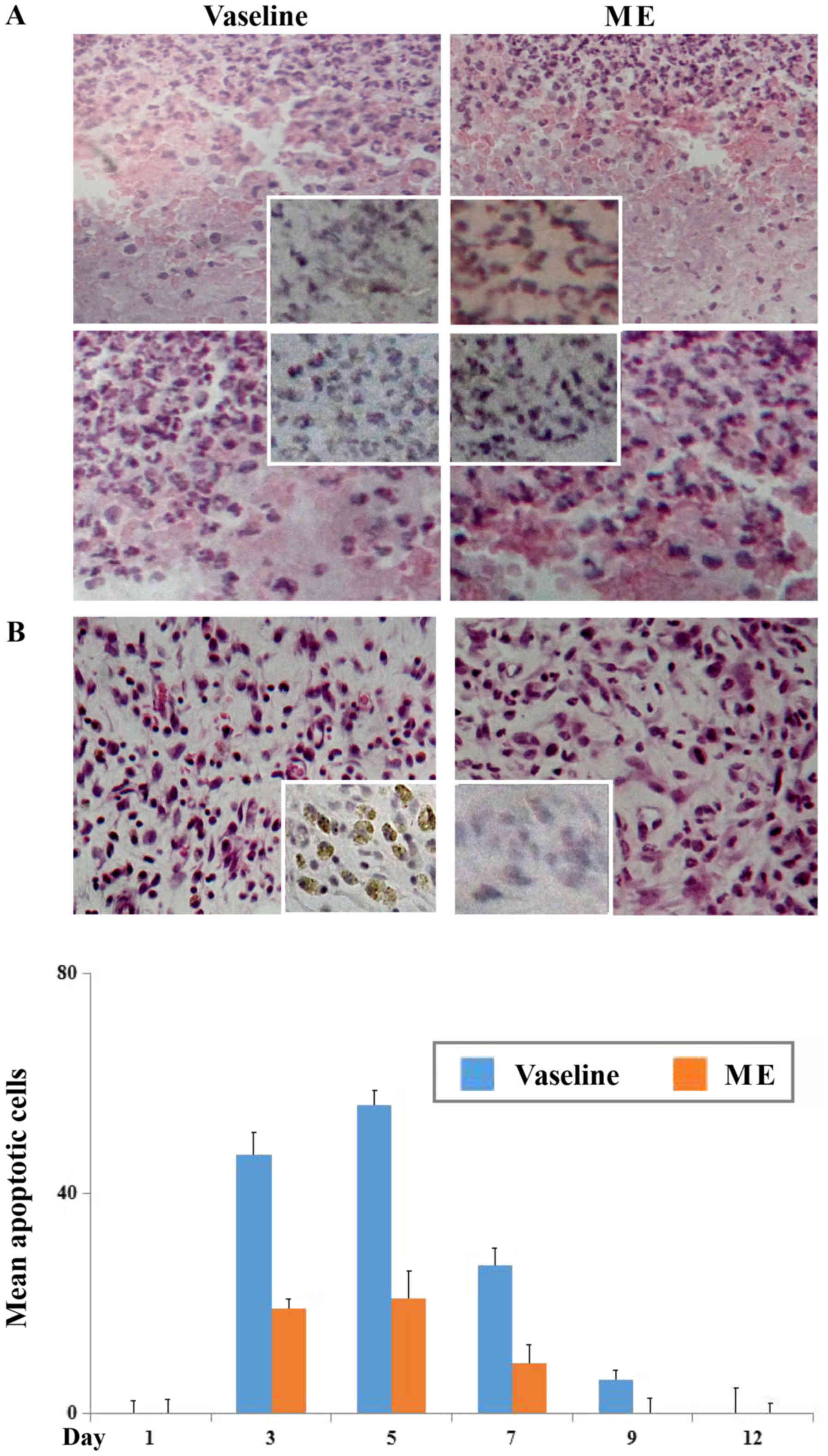
Distinct cell death was observed in the wound
tissues biopsied at the next two days (day 1 and day 2), which
became attenuate at day 3 after the trauma irrespective to maggot
extract treatment (Fig. 2A). To
scrutinize the feature of the cell death, TUNEL assay was performed
on those tissues, which revealed the rarity of TUNEL-positive cells
in the regions with extensive cell death at the early wound stage
(Fig. 2A).
Maggot extract reduced apoptosis in
wound tissues
As shown in Fig.
2B, the dead cells in small sizes were common in the wound
beds. TUNEL-positive cells (insets in Fig. 2B) were more frequently observed in
the control group from day 3 in the average apoptosis rate of
47/vision field (X 40), became most remarkable at day 6 (56/vision
field) and subsided thereafter in the time related fashion
(Fig. 2C). The incidence of
apoptotic cells were lesser common in the wound tissues treated by
maggot extract at the corresponding time points (P<0.05) and
became uncommon after day 9 of the trauma.
Maggot extract-enhanced BAG3 and Bcl-2
expression
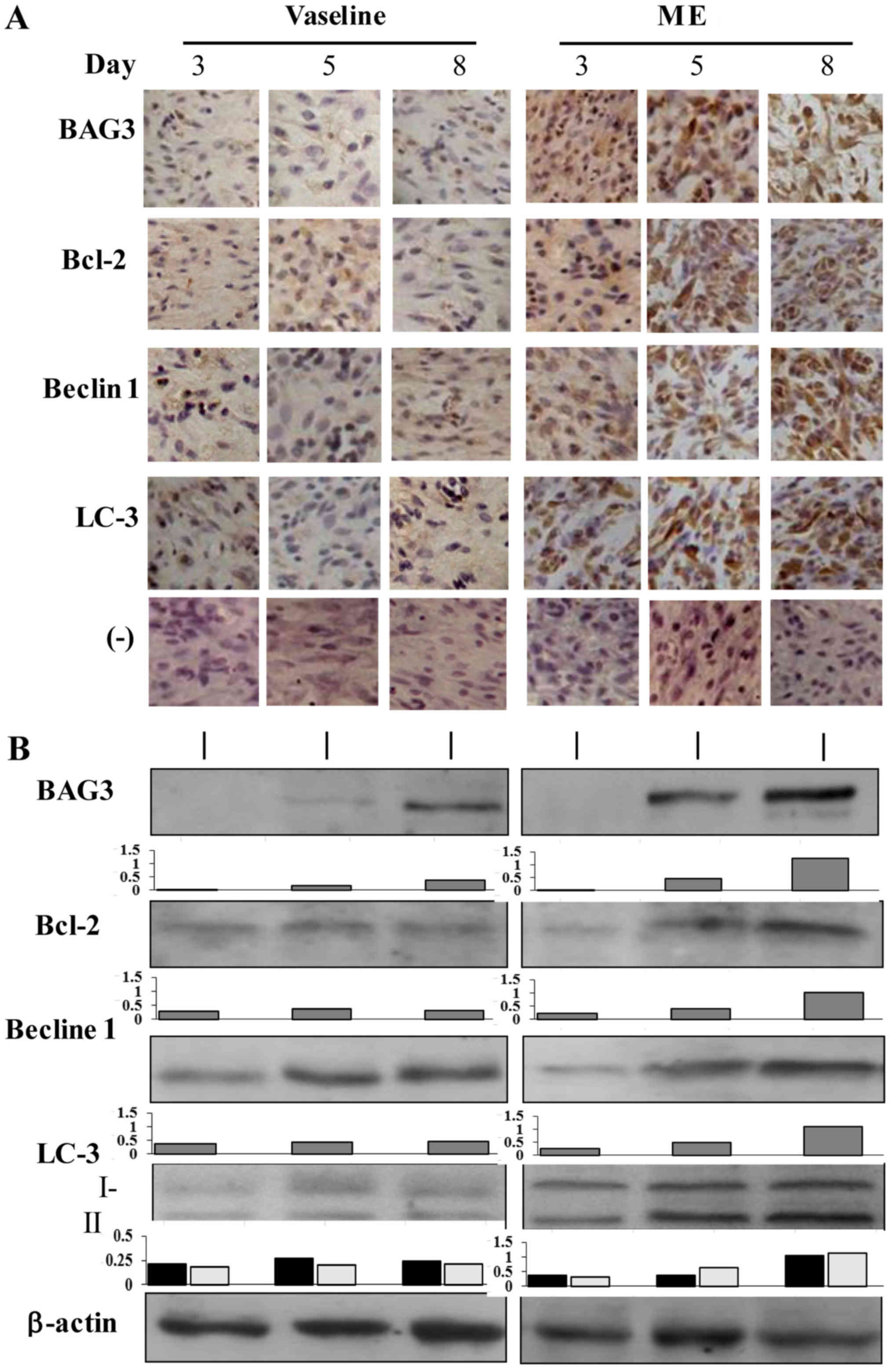
The results of immunohistochemical staining
(Fig. 3A) demonstrated that the
level of BAG3 was expressed in low levels in the wound tissues of
the control group, which was increased in maggot extract treated
tissues. The immunohistochemical staining pattern of Bcl-2 was
similar with that of BAG3 in terms of its elevated level after
maggot extract treatment, especially at day 5. The results of
western blotting for BAG3 and Bcl-2 were in accordance with that of
immunohistochemical staining, showing increased production of BAG3
and Bcl-2 proteins in maggot extract treated tissues (Fig. 3B).
Beclin 1 and LC3 upregulation in
extract-treated wound tissues
As shown in Fig. 3A and
B, Beclin 1 was weakly expressed in the wound tissues and was
upregulated following maggot extract treatment especially in the
first week after the trauma. LC3 as another autophagy-associated
factor was upregulated as well in the extract-treated wound tissues
in the pattern as similar as Beclin 1. Western blotting
demonstrated two LC3 bands in the molecular weights about 18 kDa
and 16 kDa, indicating the presence of the original (type-I) and
enzymaticaly cleaved active form (type-II) of LC3 protein. The
fraction of LC3 II was increased in the maggot extract-treated
samples, especially those collected at day 3, day 5 and day 8 time
points (Fig. 3B).
Discussion
Acute and chronic skin wounds are the common
injuries in clinics and the aim of their treatments is to reduce
patients' suffering through promoting the reconstruction of damaged
tissues (29). Wound healing is
stepwise processes with closely orchestrated biochemical
events/cascades (30) in which the
damaged or unhealthy cells are removed in the forms of necrosis
and/or apoptosis, accompanied with the repair of lost tissues via
active proliferation of the intact cell components. Apparently, a
well balanced cell loss and gain is essential for normal wound
healing. Because the full maggot extract efficiently shortens the
tissue closure time of rat skin wounds, we speculate that this
natural bioactive mixture may also exert certain impact(s) on the
cell death and maintenance in the wound tissues, although the
effective components in the extract remain to be identified.
Addressing these issues would provide further evidence for the
practical use of maggot extract in the skin wound managements.
In the acute wound tissues, two types of cell death
can be observed, they are, as demonstrated in the present study,
necrosis due to cell damage and apoptosis induced by death
signal-activated intrinsic suicide program (31). Although maggot extract shortens the
wound closing time by promoting cell growth (12), it fails to rescue the cells from
necrosis at the early days of skin damage, suggesting that this
type of cell death is caused by direct physical damage or severe
ischemia status (32) and is
therefore unavoidable and irreversible. On the other hand, maggot
extract effectively decrease the incidences of TUNEL-positive cells
especially at the proliferation phase, indicating the reduced
apoptotic pressure and enhanced anti-apoptosis activities in its
treated wound tissues. Alternatively, the expression of
anti-apoptosis factors may be upregulated by maggot extract, which
prevent the cells from apoptosis through improving cell maintenance
environment in the wound tissues and/or blocking the intracellular
cascade of death signal transduction.
Many factors in the wound tissues can trigger the
programmed cell death, of which cytochrome C and ROS released from
mitochondrium are important death signals (33). Bax and Bak are pro-apoptosis
proteins, which cause the increased permeability of mitochondrial
membrane (34). Therefore, an
inhibitor that suppresses Bax and Bak actions can stabilize the
permeability of mitochondrial membrane and prevent the initiation
of suicide program in the stressed cells. Bcl-2 is such inhibitor,
because it is localized to the outer membrane of mitochondria,
where it inhibits the apoptosis-promoting effects of Bak and Bax
and sequesters the procaspases 8 and 9 (35,36).
BAG3 is another apoptosis-preventive protein and its knockdown
leads to apoptosis-associated lower leg venous ulcers (25). Interestingly, BAG3 synergizes the
anti-apoptotic effect of Bcl-2 (37). However, the statuses of Bcl-2 and
BAG3, their impacts on apoptotic activity in wound tissues and
their relevance with maggot extract promoted wound healing remain
largely unknown. Our results demonstrate the upregulated BAG3 and
Bcl-2 expression accompanied with reduction of apoptotic incidence
in maggot extract treated tissues, suggesting the involvement of
BAG3 and Bcl-2 in maggot extract inhibited apoptosis. It would also
be possible that, in additional to improving cell proliferation
environment, maggot extract inhibits apoptosis during wound healing
through upregulating BAG3 and Bcl 2 expression. These findings
further prove the efficacy of maggot extract in the treatment of
skin wounds from the aspect of apoptosis regulation.
Autophagy is an adaptive response to environmental
stresses including nutrient starvation due to tissue destruction or
blood shortage (38,39). Autophagic activity is closely
associated with the levels of Beclin 1 expression and LC3 II
generated by enzymatic cleavage (40). Increasing evidence reveals that
BAG3 promotes autophagy via association with Beclin 1 (24,41).
However, no report has been so far available concerning the status
of autophagy in acute skin wounds and its relevance to maggot
extract promoted wound healing. We find that Beclin 1 and LC3 II
production is increased in maggot extract treated tissues.
Importantly, the cells with enhanced Beclin 1 and LC3 co-labeling
are abundant in the tissues with reduced apoptotic incidence and
BAG3 upregulation. These phenomena provide a cue to elucidate the
potential cell protective effects of autophagy in the wound tissues
to overcome nutrient shortage before the nutritional system has not
been re-established in the regenerating tissues. The reduction of
Beclin 1 and LC3 expression in the later stage of wound healing
would support this notion. In this context, maggot extract
regulated autophagic activity in the wound healing processes may be
an attempt to prevent the intact cells from apoptosis rather than
an additional cause of death.
Taken together, the results of the present study
show remarkable apoptosis and low autophagy activity in the early
stage of acute skin wound healing. Maggot extract, though failing
to inhibit necrosis, efficiently facilitates wound closure and
reduces the extent of apoptosis presumably through upregulating
Bacl-2 expression. The elevated Beclin 1 expression and LC3 II
fraction in maggot extract treated wound tissues indicate the
enhanced autophagic activity. BAG3 is up-regulated by maggot
extract, which may exert anti-apoptotic and autophagic effects in
the wound tissues by interaction with Bcl-2 and Beclin 1,
respectively. The above findings thus provide further cellular and
molecular evidence for the effectiveness of maggot extract in local
care of skin wounds. The above conclusion would be further
strengthened by an experiment in which larvae are applied to the
animal wound healing model mimicking a clinical treatment, followed
by assessment of the parameters checked in the present study.
Acknowledgements
The present study was supported by the grants from
National Natural Science Foundation of China (No. 81272786) and the
special research fund for outstanding scholar of Dalian Medical
University to Dr. Jia Liu. We thank the staffs in the Animal Center
of Dalian Medical University for their humane care of the rats used
this study during and after the experiments.
References
|
1
|
Eming SA, Martin P and Tomic-Canic M:
Wound repair and regeneration: Mechanisms, signaling, and
translation. Sci Transl Med. 26:265sr62014. View Article : Google Scholar
|
|
2
|
Leavitt T, Hu MS, Marshall CD, Barnes LA,
Lorenz HP and Longaker MT: Scarless wound healing: Finding the
right cells and signals. Cell Tissue Res. 365:483–493. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pereira RF and Bártolo PJ: Traditional
therapies for skin wound healing. Adv Wound Care (New Rochelle).
5:208–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottrup F, Jørgensen B and Karlsmark T:
News in wound healing and management. Curr Opin Support Palliat
Care. 3:300–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reinke JM and Sorg H: Wound repair and
regeneration. Eur Surg Res. 49:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson A and DiPietro LA: Apoptosis and
angiogenesis: An evolving mechanism for fibrosis. FASEB J.
27:3893–3901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Landén NX, Li D and Ståhle M: Transition
from inflammation to proliferation: A critical step during wound
healing. Cell Mol Life Sci. 73:3861–3885. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ali N, Hosseini M, Vainio S, Taïeb A,
Cario-André M and Rezvani HR: Skin equivalents: Skin from
reconstructions as models to study skin development and diseases.
Br J Dermatol. 173:391–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cappuzzello C, Doni A, Dander E,
Pasqualini F, Nebuloni M, Bottazzi B, Mantovani A, Biondi A,
Garlanda C and D'Amico G: Mesenchymal stromal cell-derived PTX3
promotes wound healing via fibrin remodeling. J Invest Dermatol.
136:293–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walraven M, Beelen RH and Ulrich MM:
Transforming growth factor-β (TGF-β) signaling in healthy human
fetal skin: A descriptive study. J Dermatol Sci. 78:117–124. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Honma M, Minami-Hori M, Takahashi H and
Iizuka H: Podoplanin expression in wound and hyperproliferative
psoriatic epidermis: Regulation by TGF-β and STAT-3 activating
cytokines, IFN-γ, IL-6, and IL-22. J Dermatol Sci. 65:134–140.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li PN, Li H, Wu ML, Wang SY, Kong QY,
Zhang Z, Sun Y, Liu J and Lv DC: A cost-effective
transparency-based digital imaging for efficient and accurate wound
area measurement. PLoS One. 7:e380692012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sano S, Chan KS and DiGiovanni J: Impact
of Stat3 activation upon skin biology: A dichotomy of its role
between homeostasis and diseases. J Dermatol Sci. 50:1–14. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jacobs AT and Marnett LJ: HSF1-mediated
BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated
colon cancer cells via stabilization of anti-apoptotic Bcl-2
proteins. J Biol Chem. 284:9176–9183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karpel-Massler G, Shu C, Chau L, Banu M,
Halatsch ME, Westhoff MA, Ramirez Y, Ross AH, Bruce JN, Canoll P
and Siegelin MD: Combined inhibition of Bcl-2/Bcl-xL and Usp9X/Bag3
overcomes apoptotic resistance in glioblastoma in vitro and in
vivo. Oncotarget. 6:14507–14521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Behl C: BAG3 and friends: Co-chaperones in
selective autophagy during aging and disease. Autophagy. 7:795–798.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arimura T, Ishikawa T, Nunoda S, Kawai S
and Kimura A: Dilated cardiomyopathy-associated BAG3 mutations
impair Z-disc assembly and enhance sensitivity to apoptosis in
cardiomyocytes. Hum Mutat. 32:1481–1491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Behl C: Breaking BAG: The Co-chaperone
BAG3 in health and disease. Trends Pharmacol Sci. 37:672–688. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Merabova N, Sariyer IK, Saribas AS,
Knezevic T, Gordon J, Turco MC, Rosati A, Weaver M, Landry J and
Khalili K: WW domain of BAG3 is required for the induction of
autophagy in glioma cells. J Cell Physiol. 230:831–841. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Menon J: Maggot therapy: A literature
review of methods and patient experience. Br J Nurs. 21:S38–S42.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sherman RA: Mechanisms of maggot-induced
wound healing: What do we know, and where do we go from here? Evid
Based Complement Alternat Med 2014. 5924192014.
|
|
22
|
Li PN, Li H, Zhong LX, Sun Y, Yu LJ, Wu
ML, Zhang LL, Kong QY, Wang SY and Lv DC: Molecular events
underlying maggot extract promoted rat in vivo and human in vitro
skin wound healing. Wound Repair Regen. 23:65–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dixon BJ, Chen D, Zhang Y, Flores J,
Malaguit J, Nowrangi D, Zhang JH and Tang J: Intranasal
administration of interferon beta attenuates neuronal apoptosis via
the JAK1/STAT3/BCL-2 pathway in a rat model of neonatal
hypoxic-ischemic encephalopathy. ASN neuro. 8:pii:
17590914166704922016. View Article : Google Scholar
|
|
24
|
Rosati A, Graziano V, De Laurenzi V,
Pascale M and Turco MC: BAG3: A multifaceted protien that regulates
major cell pathways. Cell Death Dis. 2:e1412011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campitiello N, Faenza M, Pagliara D, Baldi
C, Zeppa P, Rosati A and Rubino C: Expression of the anti-apoptotic
BAG3 protein in leg venous ulcerative tissues. Cell Death Discov.
2:150682016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lundberg C and Gerdin B: The role of
histamine and serotonin in the inflammatory reaction in an
experimental model of open wounds in the rat. Scand J Plast
Reconstr Surg. 18:175–180. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Chen XY, Kong QY and Liu J:
Cytopathological evaluations combined RNA and protein analyses on
defined cell regions using single frozen tissue block. Cell Res.
12:117–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong LX, Zhang Y, Wu ML, Liu YN, Zhang P,
Chen XY, Kong QY, Liu J and Li H: Resveratrol and STAT inhibitor
enhance autophagy in ovarian cancer cells. Cell Death Discov.
2:150712016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Greaves NS, Iqbal SA, Hodgkinson T, Morris
J, Benatar B, Alonso-Rasgado T, Baguneid M and Bayat A: Skin
substitute-assisted repair shows reduced dermal fibrosis in acute
human wounds validated simultaneously by histology and optical
coherence tomography. Wound Repair Regen. 23:483–494. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gurtner GC and Chapman MA: Regenerative
medicine: Charting a new course in wound healing. Adv Wound Care
(New Rochelle). 5:314–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosińczuk J, Taradaj J, Dymarek R and
Sopel M: Mechanoregulation of wound healing and skin homeostasis.
Biomed Res Int. 2016:39434812016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Johnson A and DiPietro LA: Apoptosis and
angiogenesis: An evolving mechanism for fibrosis. FASEB J.
27:3893–3901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moseley R, Hilton JR, Waddington RJ,
Harding KG, Stephens P and Thomas DW: Comparison of oxidative
stress biomarker profiles between acute and chronic wound
environments. Wound Repair Regen. 12:419–429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luna-Vargas MP and Chipuk JE:
Physiological and pharmacological control of BAK, BAX, and beyond.
Trends Cell Biol. 26:906–917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O'Neill KL, Huang K, Zhang J, Chen Y and
Luo X: Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak
through the outer mitochondrial membrane. Genes Dev. 30:973–988.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luna-Vargas MP and Chipuk JE: The deadly
landscape of pro-apoptotic BCL-2 proteins in the outer
mitochondrial membrane. FEBS J. 283:2676–2689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tahrir FG, Knezevic T, Gupta MK, Gordon J,
Cheung JY, Feldman AM and Khalili K: Evidence for the role of BAG3
in mitochondrial quality control in cardiomyocytes. J Cell Physiol.
232:797–805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takagi A, Kume S, Maegawa H and Uzu T:
Emerging role of mammalian autophagy in ketogenesis to overcome
starvation. Autophagy. 12:709–710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaushal GP and Shah SV: Autophagy in acute
kidney injury. Kidney Int. 89:779–791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanida I: Autophagosome formation and
molecular mechanism of autophagy. Antioxid Redox Signal.
14:2201–2214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gamerdinger M, Carra S and Behl C:
Emerging roles of molecular chaperones and co-chaperones in
selective autophagy: Focus on BAG proteins. J Mol Med (Berl).
89:1175–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|