Introduction
Optic nerve injury results from damage to the optic
nerve in craniocerebral trauma, with the majority of cases caused
through an indirect mechanism (1).
Optic nerve injury is commonly the result of ocular surgical
injury, which refers to the complete or partial loss of the optic
nerve due to the effect of external force or through direct contact
with tools (2). The progressive
death of retinal ganglion cells (RGCs) is a major cause of
irreversible visual impairment following optic nerve injury.
Clinically, there are no effective treatments for recovering visual
function at present. However, a previous study examined the
Wallerian degeneration of disconnected axonal fragments following
secondary axotomy 12 weeks following injury to an optic nerve; the
results revealed that a number of nerve fibers initiated Wallerian
degeneration within the days and weeks following the initial
mechanical injury to the optic nerve (3).
Growth-associated protein-43 (GAP-43) is a cell
membrane phosphorylation protein of the nerve terminal membrane,
which belongs to the family of calmodulin-binding proteins
(4). The protein consists of two
functional regions, including a membrane binding area and G protein
reaction zone (5). It is mainly
distributed in the brain, cerebellum, hippocampus, spinal cord and
autonomic nervous system. In general, the levels of GAP-43 in young
animals are 20 times higher than those of adult animals, whose
organs have developed fully (6).
GAP-43 is a neuronal-specific protein, which regulates multiple
aspects of neuronal development, plasticity and regeneration
(7). It is closely associated with
nerve growth and synapse formation, particularly in nerve
regeneration. It has been shown to promote bone marrow mesenchymal
stem cell differentiation in a rat model of traumatic optic
neuropathy (8), which may be
effective for the treatment of central nervous system disorders
(9). Investigation of the time
course of the expression of GAP-43 in the retina of goldfish, in
which axons are able to regrow for full restoration of visual
function following optic nerve transection, demonstrated that
GAP-43 was a useful biochemical marker for monitoring the entire
period of optic nerve regeneration in the fish (10). Existing evidence also shows that
GAP-43 can increase by 20–100 times following peripheral nerve
damage (11,12).
Nerve injury usually induces high levels of
inflammatory cytokines, and these inflammatory factors lead to
neuritis injury (13). The
toll-like receptor 4 (TLR4) signaling pathway acts via a myeloid
differentiation factor 88 (MyD88)-independent pathway, leading to
the subsequent activation of nuclear factor-κB (NF-κB) gene
binding, which induces a variety of cytokines (14). MyD88 is a key joint molecule of TLR
signaling, with an important role in transferring upstream
information. MyD88 is a member of the Toll/interleukin-1 receptor
(IL-1R) family and the family members of the death domain family
(15). Mechanistic experiments
have showed that dioscin significantly increases the levels of heat
shock protein 70, decreases the levels of TLR4, MyD88, tumor
necrosis factor receptor-associated factor 6, cyclooxygenase-2,
c-Jun N-terminal kinase, extracellular signal-regulated kinase and
p38 mitogen-activated protein kinase phosphorylation, suppresses
the nuclear translocation of NF-κB and high mobility group box 1
(HMGB1), and subsequently decreases the mRNA levels of IL-1β, IL-6,
tumor necrosis factor-α, intercellular adhesion molecule-1 and
interferon-γ (16). The
TLR4-mediated phosphatase and tensin homolog/phosphoinositide
3-kinase/AKT/NF-κB signaling pathway in rat hippocampal neurons is
associated with the activation of a neuroinflammatory response
(17). Another study showed that
TLR4 is critical in streptozotocin-induced diabetic retinopathy at
the level of inflammatory cytokine induction, via MyD88-dependent
and MyD88-independent pathways (18).
MicroRNAs (miRNAs) are non-coding, single-stranded
small RNA molecules, which can inhibit or upregulate the
transcription of a target mRNA, or induce its degradation through
complete or incomplete pairing with the 3′ untranslated region
(3′UTR) of the target mRNA, consequently regulating the expression
of genes (19–21). Studies have shown that miRNA-204
(miR-204) has multiple physiological functions, including that of a
master regulator in the regulation of lens morphogenesis in
vertebrates, and tumor-suppressor genes in the types of cancer
investigated (22); miR-204
regulates the differentiation process of mesenchymal stem cells
through decreasing the expression of Runt-related transcription
factor 2 in mesenchymal stem cells (23); therefore, downregulating the
expression of miR-204 may be a novel strategy for treating aplastic
anemia (24). In addition, this
miRNA is considered particularly important in regulating eye
development, affecting multiple ocular functions (22), as it is indispensable for retinal
pigmented epithelium (RPE) differentiation and development of the
adjacent neuroretina (25), and is
involved in corneal epithelial cell proliferation and migration
(26). Studies have indicated that
miRNAs are important in the regulation of several neural correlates
of the loop in diseases, including brain trauma and cerebral
ischemia, the pathogenesis of which may be contributed to by the
change of synaptic plasticity (21,27).
In addition, investigations have indicated that the expression of
miR-204 is high in nerve terminals; however, the mechanism involved
in the regulation of visual injury remains to be fully elucidated
(28).
The present study aimed to examine the function of
miR-204 through modulating the expression of GAP-43 in the repair
process following optic nerve injury. A model of optic nerve injury
was established in Sprague-Dawley (SD) rats, and the expression
levels of miR-204 and GAP-43 were detected in retinal blood vessels
of model and normal SD rats. Subsequently, miR-204 mimic or
inhibitor was injected into the model SD rats, and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis was performed to detect miR-204 and the mRNA levels of
GAP-43. The protein expression levels of GAP-43 TLR4, MyD88 and
NF-κB were detected using western blot analysis, and the apoptosis
of retinal cells was detected using the terminal deoxynucleotidyl
transferase mediated dUTP nick end labeling (TUNEL) method. The
results may provide a theoretical basis for the clinical treatment
of optic nerve injury.
Materials and methods
Reagents and equipment
The miR-204 mimic and inhibitor were from Shenggong
Biotech Co. (Shanghai, China), for injecting into the cavities of
the retina. Primary antibodies against GAP-43, TLR4, MyD88 and
NF-κB were from Abcam (Cambridge, UK); the ChemiDoc™ XRS gel
imaging system and fluorescence qPCR instrument were from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA); SYBR-Green qPCR SuperMix
was from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA); the TUNEL apoptosis kit was from Beyotime Institute of
Biotechnology (Haimen, China); the mini double vertical
electrophoresis and mini transfer electrophoresis apparatus were
from Beijing 61 Instrument Factory (Beijing, China); the inverted
fluorescence microscope was from Leica Microsystems, Inc. (Buffalo
Grove, IL, USA).
Animals and induction of optic nerve
crush injury
A total of 20 male SD rats (8–12 weeks; 150–200 g),
all of which conformed to the standard of first class animal, were
purchased from Hunan Slack King Experimental Animal Co. (Changsha,
China). The animals were reared in the isolators under the
conditions of a 10–15°C temperature, humidity of 55–75% and a 12 h
light:dark cycle, with free access to food and drinking water. The
animals had no external eye disease, and the direct and indirect
light reflexes of pupils in both eyes were normal. The 20 SD rats
were randomly divided into the following four groups, each group
containing 5 rats: Normal control group; model + miR-204 mimic
group; model + miR-204 inhibitor group; and model group. The
present study was approved by the Ethics Committee of the
Department of Ophthalmology, The Second Affiliated Hospital of
Nanchang University, Medical School of Nanchang University
(Jiangxi, China).
Animal model
The animal model was established using the following
methods: Pentobarbital sodium (30 mg/kg) was used for anesthesia by
intraperitoneal injection, followed by routine disinfection,
draping, and subcutaneous injection of lidocaine in the upper
eyelid for local anesthesia. A horizontal incision of 1 cm was made
in the region, 3 mm from the eyelid. The subcutaneous tissue was
separated bluntly, entering the eye socket closely along the
orbital bone. A section of orbital fat was removed, part of the
extraocular muscle was cut, and the optic nerve was carefully
exposed, holding the optic nerve for 10 sec, 2 mm away from the
posterior globe, avoiding damage to the ophthalmic artery. A fundus
microscope was used to observe the above process. Those rats in
which retinal blood flow was recovered within 5 min following
crushing injury were included in the experiment. Following
lamination oversewing, erythromycin eye ointment was administered
topcially onto the wound and an intramuscular injection of
penicillin sodium (1.2 µg daily) was administered postoperatively
for 3 days to promote healing.
RNA extraction and fluorescence
RT-qPCR analysis
The retinal vascular tissues of the SD rats were
used for RNA extraction, following which cDNA was synthesized
according to the manufacturer's instructions of the RT kit. This
cDNA was used as the template for fluorescence qPCR, using GAPDH as
an internal control. The relative expression levels of miR-204,
GAP-43, TLR4, MyD88 and NF-κB in each group were calculated.
RNA was extracted from the frozen retina biopsies
using the mirVana™ miRNA isolation kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.). RT-qPCR analysis of the miR-204
transcripts was performed using TaqMan® microRNA assays
(Applied Biosystems; Thermo Fisher Scientific, Inc.), using RNU48
levels to normalize the data. The RT-qPCR reactions were performed
in triplicate in 296-well plates and were run in a QuantStudio™ 12K
Flex Real-Time PCR system. The 2−∆∆Cq method (29) was used to determine the relative
quantitative levels of miR-204, TLR4, MyD88, NF-κB and GAPDH.
RT-qPCR analysis of gene expression was performed using SYBR Green
PCR master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and the expression of GAPDH was used as an endogenous control for
normalization purposes. PCR reaction was performed using the
following conditions: Pre-denaturation at 95°C for 5 min.
denaturalization at 95°C for 1 min, annealing at 60°C for 45 sec,
72°C for 45 sec, 35 cycles of amplification, and final extension at
72°C for 10 min. A total of 2 mg RNA was reverse transcribed to
cDNA. cDNA was amplified using the following primers: TLR4 forward,
5′-AAGTATGGCAGAGGTGAA-3′ and reverse, 5′-GGAATAAAGTCCCTGTAGTG-3′;
MyD88 forward, 5′-GCGATGTTTCCCACCCTT-3′ and reverse,
5′-CTTCTTCCGCACGCTCAC-3′; GAP-43 forward, 5′-AGGAGGAGGGCAGCAAAG-3′
and reverse, 5′-CGGCGAGTTATCAGTGGAAG; miR-204 forward,
5′-TGGCTACAGTCTTTCTTCA-3′ and reverse, 5′-CTCATGGGACAGTTATGG-3′;
NF-κB forward, 5′-CACCCTGACCTTGCCTAT-3′ and reverse,
5′-TGAAGCTGCCAGTGCTAT-3′; GAPDH forward, 5′-GTCTGCCACGATAACACC-3′
and reverse, 5′-CAATACAACAAGCCCACTC-3′.
Western blot analysis
The retinal vascular tissue of the SD rats was
homogenized in tissue lysis solution to disintegrate protein for 30
min, following which the homogenates were centrifuged at 8,880 × g,
for 10 min at 4°C. BCA method (Beyotime Institute of Biotechnology)
was used to determine the protein concentrations. A total of 30 µl
protein was loaded for 10% SDS-PAGE and transferred onto immobilon
P membranes (EMD Millipore, Billerica, MA, USA). The membranes were
incubated with blocking solution of 5% skim milk dissolved in
Tris-buffered saline for 1 h at room temperature, followed by an
overnight incubation with rabbit primary antibodies against GAP-43
(1:100,000, cat. no. ab75810), TLR4 (1:1,000, cat. no. ab22048),
MyD88 (1:1,000, cat. no. ab2068) and NF-κB (1:1,000, cat. no
ab32360) (all from Abcam) at 4°C. The membranes were next incubated
with secondary horseradish peroxidase-conjugated donkey anti-rabbit
IgG (1:1,000, cat. no. ab150077; Abcam) for ~1 h at room
temperature. Chemiluminescence was used to detect the relative
levels of the target proteins, with β-actin used as an internal
control. The blots were visualized by an enhanced chemiluminesence
system (Amersham; GE Healtcare, Chicago, IL, USA).
TUNEL staining
As mentioned above, 20 healthy SD rats were randomly
divided into four groups, each group containing 5 rats: Normal
control group; model + miR-204 mimic group; model + miR-204
inhibitor group; and model group. In order to evaluate apoptosis in
the retinas of the SD rats, a TUNEL assay was used for the
detection of apoptotic cells. TUNEL-positive cells represent
apoptotic cells (30). After
injection of PBS (100 ml) in the left ventricle of rats once every
month for 8 months, the right atrial blood outflow was blocked, and
tissues were fixed with 4% paraformaldehyde (100 ml). On removal of
the eye, it was completely stripped of the retina by trypsin
digestion, following which the glass body and inner limiting
membrane were peeled off, and retinal nerve tissue was removed. The
complete retinal capillary network was transferred onto a slide
with the retinal capillary network of the optic disc at the center,
divided into three regions. The slide was examined in a
blinded-manner under an optical microscope for counting cells and
acellular vasculature. The retinal vasculature was then stained
using the Dead end apoptosis detection system (Promega Corporation,
Madison, WI, USA). TUNEL-positive cells were labeled with
fluorescein-conjugated streptavidin and the numbers of
TUNEL-positive cells were determined per unit area
(mm2).
Statistical analysis
Significant differences between groups were analyzed
using one-way analysis of variance and Tukey's multiple comparison
test in Prism 5.0 software (GraphPad Software, Inc., San Diego, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Optic nerve injury upregulates miR-204
and inhibits the mRNA expression of GAP-43
The levels of miR-204 and GAP-43 in the retina of
the optic nerve injury model SD rats and the normal control SD rats
were detected using RT-qPCR analysis, the results of which are
shown in Fig. 1. The results
showed that miR-204 was effectively induced by optic nerve injury
(P<0.05), whereas the mRNA level of neuroprotective GAP-43 was
significantly inhibited by optic nerve injury (P<0.05), compared
with that in the normal SD rats (Fig.
1A). This suggested that optic nerve injury was a destructive
effect in the body.
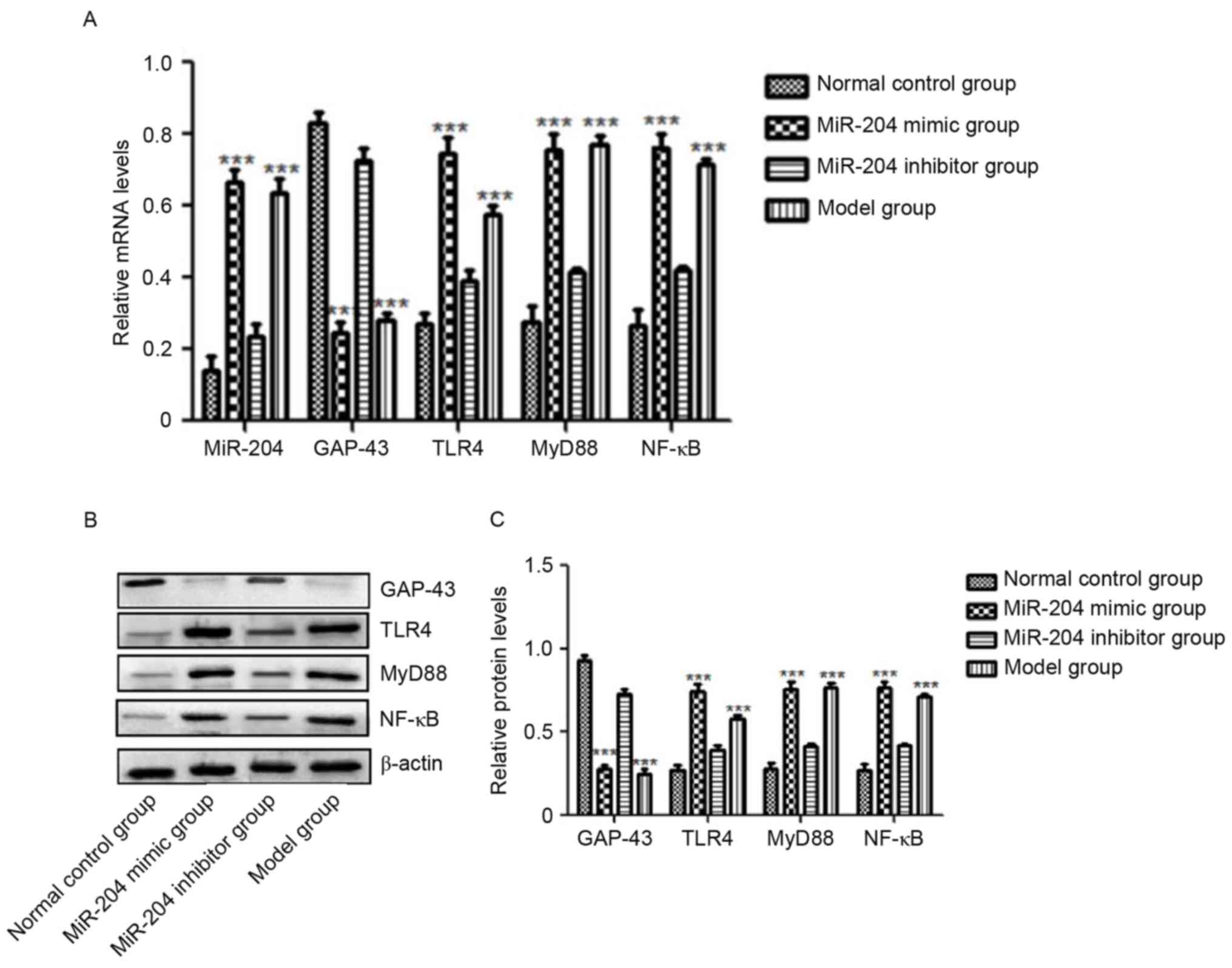 | Figure 1.Expression levels of miR-204, GAP-43,
TLR4, MyD88 and NF-κB. Relative levels of miR-204, GAP-43, TLR4,
MyD88 and NF-κB in the retina of each group demonstrated by (A)
reverse transcription-quantitative polymerase chain reaction and
(B) western blot analyses. (C) Quantification of protein levels.
Compared with the normal SD rats, the level of miR-204 in SD rats
with optic nerve injury was significantly increased (**P<0.05,
***P<0.01) and the mRNA level of GAP-43 was significantly
inhibited by optic nerve injury (**P<0.05, ***P<0.01).
miR-204 increased the gene levels of TLR4/MyD88/NF-κB, and
decreased the mRNA and protein expression of GAP-43. miR, microRNA;
GAP-43, growth-associated protein-43; TLR4, toll-like receptor 4;
MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor-κB;
SD, Sprague-Dawley. |
miR-204 decreases the level of GAP-43
and promotes the TLR4/MyD88/NF-κB pathway
The levels of miR-204, GAP-43, TLR4, MyD88 and NF-κB
in each group, following the overexpression and inhibition of
miR-204 by injection of miR-204 mimic or miR-204 inhibitor, were
detected using RT-qPCR analysis for miRNAs and mRNAs, and western
blot analysis for proteins (Fig.
1A-C). As shown in Fig. 1A,
which show the results of the RT-qPCR analysis of miR-204 and the
GAP-43, TLR4, MyD88 and NF-κB mRNAs in each group, miR-204 was
effectively increased by the miR-204 mimic, compared with that in
the normal control group (P<0.05), which was the similar to the
result in the model group (Fig.
1A). The results for GAP-43 were the opposite in these two
groups, being significantly inhibited in the miR-204 mimic and
model groups (P<0.05). Compared with the normal control group,
the levels of TLR4, MyD88 and NF-κB in the miR-204 mimic group and
model group were significantly increased, and the differences were
statistically significant (P<0.05). These three genes were
effectively inhibited by the miR-204 inhibitor, compared with model
group (Fig. 1A). They remained
marginally higher than those in the normal control group, however,
the difference was not statistically significant. The proteins
levels showed similar results to mRNAs for GAP-43, TLR4, MyD88 and
NF-κB (Fig. 1B and C).
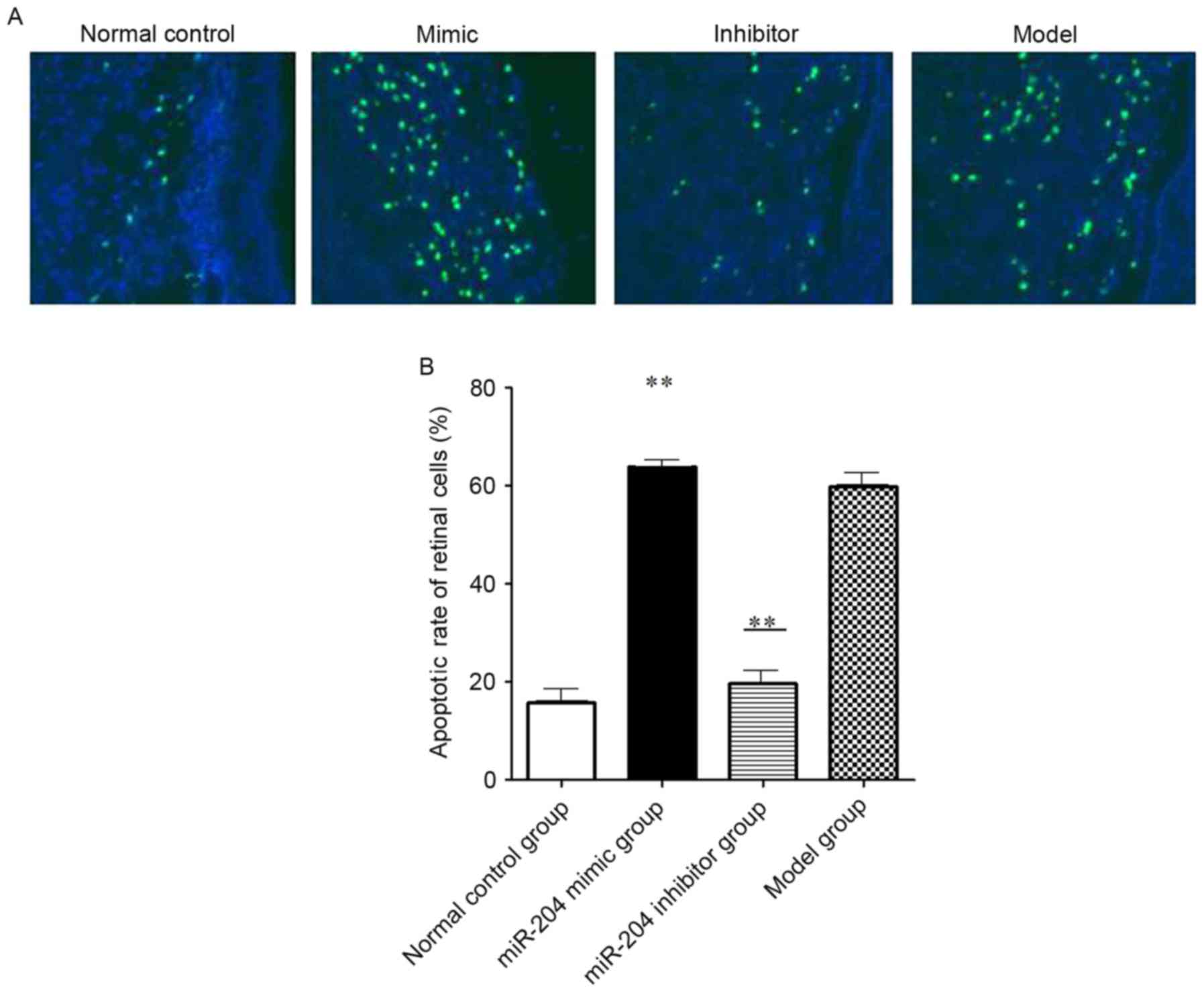
miR-204 increases the apoptosis of
retinal cells
In order to evaluate apoptosis in the retina of SD
rats, a TUNEL assay was used for the detection of apoptotic cells.
TUNEL-positive cells represent the apoptotic cells, as shown in
Fig. 2. The results showed that,
compared with the normal control group, the apoptotic rates in the
miR-204 mimic group and the model group were significantly
increased (Fig. 2A and B). The
numbers of TUNEL-positive cells in the retina of the miR-204 mimic
group and the model group were significantly higher, compared with
that of the control group (P<0.05). Compared with the normal
control group, the number of TUNEL-positive cells of the miR-204
inhibitor group was increased, however, this difference was not
statistically significant (P>0.05).
Discussion
The survival of RGCs is the most important
requirement for regeneration of the optic nerve following injury,
therefore, it is important to protect ganglion cell activity and
inhibit the apoptosis of ganglion cells (31). GAP-43 is a presynaptic protein,
which is key in the regulation of axonal growth and modulating
synapse formation (6). The
increase of GAP-43 can exert neuroprotective effects in the central
nervous system, including the production of progesterone (7–9). It
has been shown that the level of GAP-43 is enhanced in axon
regeneration (32). However, the
overexpression of GAP-43 was shown to aggravate RGC death in
experimental chronic intraocular pressure (IOP) elevation injury,
which indicates that the function of this same protein is complex
according to the different pathogenesis (33). The results of the present study
demonstrated that a low expression of GAP-43 (Fig. 1) was associated with the apoptotic
activity of the retinal cell (Fig.
2). Optic nerve injury inhibited the level of GAP-43 (Fig. 1), which possibly reflected the
destructive effect of optic nerve injury. Optic nerve injury
activated the TLR4/MyD88/NF-κB pathway (Fig. 1), which was consistent with the
result of a previous study, which showed that neuroinflammation was
involved in the death of RGCs following optic nerve injury
(31).
The expression patterns of miRNAs have been analyzed
in a variety of ocular tissues and multiple pathologies, and the
downregulation of miRNAs, including miR-181c, miR-497 and miR-204,
have been associated with chronically elevated IOP (34). In addition, the significant
elevation of miRNAs, including miR-223, miR142-5p and miR142-3p,
may be involved in autoimmune uveoretinitis (35). miR-204 is a widely distributed
miRNA, which has target genes belonging to different pathways, and
is key in eye development and neural differentiation processes,
including axon guidance (34). In
addition, a key role of miR-204 in the differentiation of the RPE
was verified in vivo through the RPE-specific conditional
mutagenesis of Dicer1 or Dgcr8 in mice (35). It has been found that miR-204 was
expressed at high levels in nerve cells and axons (36). In the present study, the results
showed that miR-204 was increased in the retinal injury model,
which reflected adjusting of function to retinal damage repair.
When given sufficient light to stimulate rats, which
have survived in the dark for 10 days, the expression level of
GAP-43 has been shown to increase slowly, eventually reaching a
maximum (37). This suggests that
GAP-43 may be involved in the repair and regeneration of retinal
light damage. A previous study found that, in the normal retina,
the immunoreactivity of GAP-43 is predominantly in the inner
plexiform layer, but in a rat model of retinal ischemia reperfusion
injury model induced by elevated intraocular pressure, GAP-43
immunoreactivity appeared in retinal ganglion cells; the expression
of GAP-43 peaked at 190% in 3 days and the rate reduced in 7 days
(38). Therefore, it was
hypothesized that optic ganglion cells have the potential for
partial regeneration as GAP-43 level increases.
The present study observed the effects on the GAP-43
protein induced by miR-204 through the injection of miR-204 mimic
and inhibitor in the established optic nerve injury model. GAP-43
was decreased in the miR-204 mimic group, whereas injection with
miR-204 inhibitor increased the level of GAP-43 (Fig. 1). This suggested that miR-204
downregulated the protein expression of GAP-43, which was the
similar to the result of a previous study, in which miR-204 was
expressed at high levels in the glia, but low levels in the retinal
neurons (23).
Studies have indicated that TLR4 is important in
cerebral hemorrhage, cerebral infarction, and cerebral hemorrhage
reperfusion injury (14–16). Another study indicated that TLR4
produces a response that causes brain damage (39). In the present study, retinal
tissues were stretched using the technique of stretched preparation
of the retina, following which the apoptosis of retinal cells was
detected using the TUNEL method. The results showed that the
apoptotic rates in the miR-204 mimic group and model group were
significantly increased, compared with that in the normal control
group (P<0.05). The apoptotic rate was effectively inhibited in
the miR-204 inhibitor group, compared with that in the model group
(P<0.05; Fig. 2). These results
suggested that miR-204 was involved in retinal injury repair
through modulating the expression of GAP-43.
The present study also examined the function of
miR-204 in the TLR4, MyD88 and NF-κB pathways following injection
of miR-204 mimic or inhibitor into the model rats using RT-qPCR and
western blot analyses. The results showed that, compared with the
normal control group, the levels of TLR4, MyD88 and NF-κB in the
miR-204 mimic group and model group were significantly increased,
and this difference was statistically significant (P<0.05). The
miR-204 inhibitor effectively inhibited the expression levels of
TLR4, MyD88 and NF-κB, compared with those in the model group. The
results showed that the levels of TLR4, MyD88 and NF-κB increased
significantly in the optic nerve injury group, and treatment with
miR-204 inhibitor following optic nerve injury decreased the levels
of TLR4, MyD88 and NF-κB, which was consistent with the result of a
previous study (13).
Taken together, the above results indicated that
miR-204 may have been the result of the stress response of retinal
injury, which downregulated the expression of neuroprotective
factor GAP-43, and upregulated the levels of TLR4, MyD88 and NF-κB.
However, further experiments are required to elucidate the definite
regulatory mechanism of miR-204 in retinal injury.
In conclusion, the results of the present study
indicated that miR-204 promoted the apoptosis of retinal cells
through inhibiting GAP-43, which provide theoretical guidance for
the function of GAP-43 in retinal injury.
Acknowledgements
This study was supported by the Key Project of
Jiangxi Science and Technology Agency: The Research and Application
of The Standardized Diagnosis and Treatment of Diabetic Retinopathy
in Province Model (grant no. 20161BBG70204).
References
|
1
|
Balla L, Ianovici N and Costin D:
Pathology of the optic nerve injury. Rev Med Chir Soc Med Nat Iasi.
116:1087–1090. 2012.PubMed/NCBI
|
|
2
|
Xue F, Wu K, Wang T, Cheng Y, Jiang M and
Ji J: Morphological and functional changes of the optic nerve
following traumatic optic nerve injuries in rabbits. Biomed Rep.
4:188–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maxwell WL, Bartlett E and Morgan H:
Wallerian degeneration in the optic nerve stretch-injury model of
traumatic brain injury: A stereological analysis. J Neurotrauma.
32:780–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grasselli G and Strata P: Structural
plasticity of climbing fibers and the growth-associated protein
GAP-43. Front Neural Circuits. 7:252013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu F, Liao F, Li W, Han Y and Liao D:
Progesterone alters Nogo-A, GFAP and GAP-43 expression in a rat
model of traumatic brain injury. Mol Med Rep. 9:1225–1231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao JC, Zhang LX, Zhang Y and Shen YF:
The differential regulation of Gap43 gene in the neuronal
differentiation of P19 cells. J Cell Physiol. 227:2645–2653. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Williams KR, McAninch DS, Stefanovic S,
Xing L, Allen M, Li W, Feng Y, Mihailescu MR and Bassell GJ:
hnRNP-Q1 represses nascent axon growth in cortical neurons by
inhibiting Gap-43 mRNA translation. Mol Biol Cell. 27:518–534.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Q, Liu Z, Wang C, Nie L, He Y, Zhang
Y, Liu X and Su G: Lentiviral-mediated growth-associated protein-43
modification of bone marrow mesenchymal stem cells improves
traumatic optic neuropathy in rats. Mol Med Rep. 12:5691–5700.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan H, Kang X, Lu S and Liu L: The
therapeutic effects of bone marrow mesenchymal stem cells after
optic nerve damage in the adult rat. Clin Interv Aging. 10:487–490.
2015.PubMed/NCBI
|
|
10
|
Kaneda M, Nagashima M, Mawatari K, Nunome
T, Muramoto K, Sugitani K and Kato S: Growth-associated protein43
(GAP43) is a biochemical marker for the whole period of fish optic
nerve regeneration. Adv Exp Med Biol. 664:97–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guarnieri S, Morabito C, Paolini C,
Boncompagni S, Pilla R, Fanò-Illic G and Mariggiò MA: Growth
associated protein 43 is expressed in skeletal muscle fibers and is
localized in proximity of mitochondria and calcium release units.
PloS One. 8:e532672013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takei H, Buckleair LW, Rivera A and Powell
SZ: Brain tissue microarrays in neurodegenerative diseases:
Validation of methodology and immunohistochemical study of
growth-associated protein-43 and calretinin. Pathol Int.
57:775–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lucas SM, Rothwell NJ and Gibson RM: The
role of inflammation in CNS injury and disease. Br J Pharmacol. 147
Suppl 1:S232–S240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeFrancesco-Lisowitz A, Lindborg JA, Niemi
JP and Zigmond RE: The neuroimmunology of degeneration and
regeneration in the peripheral nervous system. Neuroscience.
302:174–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin S, Liang Y, Zhang J, Bian C, Zhou H,
Guo Q, Xiong Y, Li S and Su B: Microglial TIR-domain-containing
adapter-inducing interferon-β (TRIF) deficiency promotes retinal
ganglion cell survival and axon regeneration via nuclear factor-κB.
J Neuroinflammation. 9:392012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi M, Zheng L, Qi Y, Han X, Xu Y, Xu L,
Yin L, Wang C, Zhao Y, Sun H, et al: Dioscin attenuates renal
ischemia/reperfusion injury by inhibiting the TLR4/MyD88 signaling
pathway via up-regulation of HSP70. Pharmacol Res. 100:341–352.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen M, Xiang Z and Cai J: The
anti-apoptotic and neuro-protective effects of human umbilical cord
blood mesenchymal stem cells (hUCB-MSCs) on acute optic nerve
injury is transient. Brain Res. 1532:63–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Latchney SE, Masiulis I, Zaccaria KJ,
Lagace DC, Powell CM, McCasland JS and Eisch AJ: Developmental and
adult GAP-43 deficiency in mice dynamically alters hippocampal
neurogenesis and mossy fiber volume. Dev Neurosci. 36:44–63. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adlakha YK and Neeru Saini: Brain
microRNAs and insights into biological functions and therapeutic
potential of brain enriched miRNA-128. Mol Cancer. 13:332014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ouyang YB, Stary CM, White RE and Giffard
RG: The use of microRNAs to modialate redox and immune response to
stroke. Antioxid Redox Signal. 22:187–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Pan H and Li R: The dual regulatory
role of miR-204 in cancer. Tumor Biol. 37:11667–11677. 2016.
View Article : Google Scholar
|
|
23
|
Huang J, Zhao L, Xing L and Chen D:
MicroRNA-204 regulates Runx2 protein expression and mesenchymal
progenitor cell differentiation. Stem Cells. 28:357–364.
2010.PubMed/NCBI
|
|
24
|
Zhao J, Wang C, Song Y and Fang B: Arsenic
trioxide and microRNA-204 display contrary effects on regulating
adipogenic and osteogenic differentiation of mesenchymal stem cells
in aplastic anemia. Acta Biochim Biophys Sin (Shanghai).
46:885–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banaei-Esfahani A, Moazzeni H, Naseri
Nosar P, Amin S, Arefian E, Soleimani M, Yazdani S and Elahi E:
MicroRNAs that target RGS5. Iran J Basic Med Sci. 18:108–114.
2015.PubMed/NCBI
|
|
26
|
Ohana R, Weiman-Kelman B, Raviv S, Tamm
ER, Pasmanik-Chor M, Rinon A, Netanely D, Shamir R, Solomon AS and
Ashery-Padan R: MicroRNAs are essential for differentiation of the
retinal pigmented epithelium and maturation of adjacent
photoreceptors. Development. 142:2487–2498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
An J, Chen X, Chen W, Liang R, Reinach PS,
Yan D and Tu L: MicroRNA expression profile and the role of miR-204
in corneal wound healing. Invest Ophthalmol Vis Sci. 56:3673–3683.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Natera-Naranjo O, Aschrafi A, Gioio AE and
Kaplan BB: Identification and quantitative analyses of microRNAs
located in the distal axons of sympathetic neurons. RNA.
16:1516–1529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim J, Jo K, Lee IS, Kim CS and Kim JS:
The extract of Aster koraiensis prevents retinal pericyte apoptosis
in diabetic rats and its active compound, chlorogenic acid inhibits
AGE formation and AGE/RAGE interaction. Nutrients. 8:E5852016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morishita S, Oku H, Horie T, Tonari M,
Kida T, Okubo A, Sugiyama T, Takai S, Hara H and Ikeda T: Systemic
simvastatin rescues retinal ganglion cells from optic nerve injury
possibly through suppression of astroglial NF-kB activation. PLoS
One. 9:e843872014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gupta SK, Mishra R, Kusum S, Spedding M,
Meiri KF, Gressens P and Mani S: GAP-43 is essential for the
neurotrophic effects of BDNF and positive AMPA receptor modulator
S18986. Cell Death Differ. 16:624–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang C, Cen LP, Liu L, Leaver SG, Harvey
AR, Cui Q, Pang CP and Zhang M: Adeno-associated virus-mediated
expression of growth-associated protein-43 aggravates retinal
ganglion cell death in experimental chronic glaucomatous injury.
Mol Vis. 19:1422–1432. 2013.PubMed/NCBI
|
|
34
|
Conte I, Merella S, Garcia-Manteiga JM,
Migliore C, Lazarevic D, Carrella S, Marco-Ferreres R, Avellino R,
Davidson NP and Emmett W: The combination of transcriptomics and
informatics identifies pathways targeted by miR-204 during
neurogenesis and axon guidance. Nucleic Acids Res. 42:7793–7806.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohana R, Weiman-Kelman B, Raviv S, Tamm
ER, Pasmanik-Chor M, Rinon A, Netanely D, Shamir R, Solomon AS and
Ashery-Padan R: MicroRNAs are essential for differentiation of the
retinal pigmented epithelium and maturation of adjacent
photoreceptors. Development. 142:2487–2498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Natera-Naranjo O, Aschrafi A, Gioio AE and
Kaplan BB: Identification and quantitative analyses of microRNAs
located in the distal axons of sympathetic neurons. RNA.
16:1516–1529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
López-Costa JJ, Goldstein J, Mangeaud M
and Saavedra JP: Expression of GAP-43 in the retina of rats
following protracted illumination. Brain Res. 900:332–336. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ju WK, Gwon JS, Park SJ, Kim KY, Moon JI,
Lee MY, Oh SJ and Chun MH: Growth-associated protein 43 is
up-regulated in the ganglion cells of the ischemic rat retina.
Neuroreport. 13:861–865. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alfonso-Loeches S, Pascual-Lucas M, Blanco
AM, Sanchez-Vera I and Guerri C: Pivotal role of TLR4 receptors in
alcohol-induced neuroinflammation and brain damage. J Neurosci.
30:8285–8295. 2010. View Article : Google Scholar : PubMed/NCBI
|