Introduction
Endometrial carcinoma is one of the three malignant
tumors of the female genital tract, accounting for 20 to 30% of the
total number of female genital-tract malignant tumors (1). In 2015, 54,870 new cases of
endometrial carcinoma were diagnosed worldwide, and the number of
mortalities was ~10,170 (2). Early
endometrial cancer has a good prognosis; however, ~7% of cases
recur and the 3-year survival rate is relatively poor (3). Therefore, the underlying molecular
mechanism of endometrial cancer must be studied to prevent further
cancer development and to improve prognosis.
A previous study has suggested that the underlying
mechanisms of occurrence of endometrial cancer include an estrogen
and progesterone imbalance and tumor microenvironment factors
(4). Autotaxin (ATX) is a key
factor that regulates the tumor microenvironment. It is a primary
secreted enzyme with lysophospholipase D activity to catalyze
lysophosphatidylcholine (LPC) to lysophosphatidic acid (LPA). LPA,
a simple-structured glycerophospholipid, is an intercellular lipid
signaling molecule. LPA may bind to LPA receptors (LPA1-7), which
are G-protein coupled receptors, enabling LPA to exert multiple
biological functions. ATX has diverse roles in various different
types of cancers via the LPA and LPA receptors; these two represent
the ATX-LPA axis (5).
ATX promotes tumor cell proliferation, invasion,
metastasis and angiogenesis, which are closely associated with LPA.
In breast cancer, the ATX-LPA axis promotes the proliferation of
mammary epithelial cell lines (6).
The effect of LPC on cell migration was observed to be reduced by
inhibiting the secretion of ATX in human breast cancer and melanoma
cells. LPC promotes cell migration mediated by LPA catalyzed by ATX
(7). A positive feedback loop was
identified between ATX and vascular endothelial growth factor
(VEGF) in ovarian cancer cell lines. Exogenous VEGF may promote the
expression and secretion of ATX, and increase extracellular LPA
production. Accordingly, LPA induced VEGF receptor expression may
regulate the cellular response to VEGF (8). A previous study demonstrated that
LPA2 promoted the invasion of Hec-1A cells in endometrial cancer.
LPA2 increased the secretion of matrix metalloproteinase 7, which
suggested that LPA2 is an important factor in accelerating the
progression of endometrial carcinoma (9).
However, it appears that the role of ATX in
endometrial cancer has not yet been explored. The present study
investigated the effect of the ATX-LPA axis on endometrial cancer
cell proliferation. As the majority of endometrial cancers are
estrogen-dependent, the present study aimed to identify the
interaction between the ATX-LPA axis and estrogen in endometrial
cancer progression. Finally, this study preliminary clarified the
underlying molecular mechanism of the ATX-LPA axis in endometrial
cancer.
Materials and methods
Reagents and antibodies
Recombinant human ATX (also known as Ectonucleotide
Pyrophosphatase/Phosphodiesterase-2) was purchased from R&D
Systems, Inc., Minneapolis, MN, USA. 1-Oleoyl lysophosphatidic acid
sodium salt was from Tocris Bioscience (Bristol, UK). 17-β
estradiol (estrogen) was from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). PD98059 [the phosphorylated extracellular
signal-regulated kinase (p-ERK) inhibitor] was from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). ATX antibody
for western blotting was from Abcam (Cambridge, UK; ab77104) and
for immunohistochemistry from Santa Cruz Biotechnology (Texas, USA;
sc-374222). Antibodies against LPA receptors (LPA1, LPA2, LPA3)
were from Abcam (ab166903, ab38322 and ab219267, respectively).
Total/phosphorylated extracellular signal-regulated kinases
(t/p-ERK) were from Cell Signaling Technology, Inc. Danvers, MA,
USA (4695s and 4370s). Antibodies against GAPDH were from Santa
Cruz Biotechnology (sc-51907). Crystal violet staining solution was
from Tiangen Biotech Co., Ltd. (Beijing, China). Cell Counting kit
(CCK)-8 was from Beyotime Institute of Biotechnology, Haimen, China
(C0037). Transwell culture plates were from Corning Incorporated
(Corning, NY, USA). Lipofectamine 2000™ transfection reagent was
from Invitrogen, Thermo Fisher Scientific, Inc.
Cell culture, siRNA and
transfection
Ishikawa and Hec-1A cells are endometrial
adenocarcinoma cell lines. Ishikawa cells were kindly provided by
Dr Jonathan Braun (David Geffen School of Medicine, University of
California, CA, USA) and kept and subcultivated in our laboratory;
they are positive for estrogen receptor (ER). Ishikawa cells were
cultured in F12-Dulbecco's modified Eagle's medium (M&C Gene
Technology, Ltd., Beijing, China) supplemented with 10% fetal
bovine serum (FBS; HyClone; GE Healthcare, Chicago, IL, USA) at
37°C with 5% CO2 in a humidified atmosphere. The Hec-1A
cell line was from American Type Culture Collection (ATCC;
Manassas, VA, USA). Hec-1A cells with low ER expression were
cultured in McCoy's 5A medium (M&C Gene Technology, Ltd.), as
recommended by ATCC. Non-specific short interfering (si)RNA (si-NC)
and two siRNA sequences specific to ATX were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China; negative control:
Sense: 5′-UUCUCCGAACGUGUCACGUTT-3′, Antisense:
5′-ACGUGACACGUUCGGAGAATT-3′; ATX-siRNA1: Sense:
5′-AUCGACAAAAUUGUGGGGCTT-3′, Antisense:
5′-GCCCCACAAUUUUGUCGAUTT-3′; ATX-siRNA2: Sense:
5′-CGUCAUCUUUGUCGGAGACTT-3′, Antisense:
5′-GUCUCCGACAAAGAUGACGTT-3′). Ishikawa and Hec-1A cells were grown
until 60–70% confluent, and transfected with ATX siRNAs or siRNA-NC
as a negative control at 100 nm by using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The serum-free
medium (DMEM-F12 without FBS) with transfected siRNA was replaced
with culture medium (DMEM-F12 with 10% FBS) following 6 h
incubation. The transfected cells continued to be cultured until 48
h at 37°C in a 5% CO2 incubator.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the transfected cells
with ATX siRNAs or siRNA-NC by using TRIzol (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Reverse
transcription and cDNA synthesis involved the use of oligo-dT
primers with 2 µg total RNA and Superscript III reverse
transcriptase according to the First Strand cDNA Synthesis kit (cat
no. KR108; Tiangen Biotech Co., Ltd.). PCR involved use of 5% total
cDNA and was amplified with primers (ATX forward primer:
5′-CTTTCGGCCCTGAGGAGAGTA-3′, ATX reverse primer:
AGCAACTGGTCTTTCCTGTCT; LPA1 forward primer:
5′-AATCGGGATACCATGATGAGTCTT-3′, LPA1 reverse primer:
5′-CCAGGAGTCCAGCAGATGATAAA-3′; LPA2 forward primer:
5′-CGCTCAGCCTGGTCAAGACT-3′, LPA2 reverse primer:
5′-TTGCAGGACTCACAGCCTAAAC-3′; LPA3 forward primer:
5′-AGGACACCCATGAAGCTAATGAA-3′, LPA3 reverse primer:
5′-GCCGTCGAGGAGCAGAAC-3′; GAPDH forward primer:
CCTCCGGGAAACTGTGGCGTGATGG; GAPDH reverse primer:
AGACGGCAGGTCAGGTCCACCACTG), using the FastFire qPCR PreMix (SYBR
Green; cat no. FP207; Tiangen Biotech Co., Ltd.) with the
thermocycling conditions of 95°C for 5 sec, 56°C for 10 sec and
72°C for 15 sec for 40 cycles with the iCycleriQ real-time PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Melting curve analysis was performed for each sample to ensure that
a single product was produced in each reaction. The
2−∆∆Cq method was used for quantification (10).
Immunohistochemistry
The tissues of 10 endometrial carcinoma patients
were obtained previously from the Pathology Department of Peking
University People's Hospital (Beijing, China). The pathological
sections were already processed and paraffin embedded routinely
following surgery.
The present study was approved by the ethics
committee of Peking University People's Hospital (approval no.
2016PHB054-01). Slides of cancer samples continuous with
surrounding endometrial tissue were determined by a pathologist in
Pathology Department of Peking University People's Hospital. First,
slides were dewaxed in xylene twice. After that, different
concentrations of alcohol (95, 85 and 75%) were used to continue
dewaxing and slides were then placed in water. Subsequently,
peroxide enzyme was removed by adding 30%
H2O2 to the slides, which were then washed
three times with 1XPBS. Sections were then incubated with sealing
fluid (ZLI-9022; OriGene Technologies, Inc., Beijing, China) for 30
mins, and then the following primary antibodies: Mouse anti-ATX
(sc-374222; Santa Cruz Biotechnology), rabbit anti-LPA1, 2 and
3(ab166903, ab38322 and ab219267; Abcam) (dilution 1:400) and 1×
PBS (negative control) at 4°C for 12 h. Sections were then probed
with the mouse and rabbit secondary antibodies (PV-6001 and
PV-6002; OriGene Technologies, Inc.) for 1 h at room temperature,
followed by 3,3′diaminobenzidine color solution (ZLI-9018; OriGene
Technologies, Inc.) for 20 sec each section at room temperature.
The immunohistochemical results were analyzed using Image-Pro Plus
software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
From 5 randomly selected fields of each section (×400) images were
captured using a light microscope (Eclipse 501; Nikon Corporation,
Tokyo, Japan), the mean density (IOD SUM/area) and integrated
optical density (IOD) value of positive stained areas were
calculated and thence each sample's mean density. ATX, LPA1, LPA2
and LPA3 protein expression of 10 endometrial cancers and 10
non-carcinoma tissues samples were compared by t-test analysis.
Western blot analysis
The cells (2×106 from each well in
6-wells plates) were harvested, washed three times with 1XPBS, and
lysed in lysis buffer (R0020; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) for 30 min on ice. The
lysates were centrifuged (15 min at 12,000 × g, 4°C), and the
protein concentration was determined by a bicinchoninic acid
protein assay kit (23225; Thermo Fisher Scientific, Inc.). A total
of 20–60 µg protein in each sample was separated by 10% SDS-PAGE
and transferred onto nitrocellulose filter membranes (Merck KGaA).
Membranes for target protein (ATX, LPA1, LPA2, LPA3 and GAPDH) were
blocked with 5% skimmed milk for 1 h at room temperature. Membranes
for interest protein (T-ERK and P-ERK) were blocked with 5% bovine
serum albumin (BSA; B2064-50G; Sigma-Aldrich; Merck KGaA) for 1 h
at room temperature. These membranes were separately incubated with
primary antibodies ATX (ab77104; 1:1,000; Abcam), LPA1 (ab166903;
1:1,000, Abcam), LPA2 (ab38322; 1:1,000; Abcam), LPA3 (ab219267;
1:1,000; Abcam), T-ERK (4695s; 1:1,000; Cell Signaling Technology,
Inc.), P-ERK (4370s; 1:1,000; Cell Signaling Technology, Inc.) and
GAPDH (sc-51907; 1:5,000; Santa Cruz Biotechnology), with the 5%
skimmed milk or BSA dilutions recommended by the manufacturer, at
4°C overnight. Membranes were then washed using 1XTBST with 0.1%
Tween-20 and incubated with 1:3,000 secondary goat anti-rabbit IgG
(ZB-2301; OriGene Technologies, Inc.) or goat anti-mouse IgG
antibodies (ZB-2305; OriGene Technologies, Inc.). The membrane was
incubated with luminol reagent and enhanced chemiluminescence (Lot
No. 1614602, EMD Millipore, Billerica, MA, USA) for 30–120 sec at
room temperature. The optical density of bands was quantified by
using ImageJ software (v2.1.4.7; National Institutes of Health,
Bethesda, MD, USA).
CCK8 assay
Ishikawa cells grown until 60–70% confluent were
transfected with siRNA-NC or ATX siRNA and cultured for 24 h at
37°C with 5% CO2 in a humidified atmosphere, then cells
were seeded in 96-well culture plates at 2,000 cells per well. The
absorbance values of almost immediately adherent cells 4 h after
seeding were measured as the control group. Cells were incubated
with CCK8 (C0037; 1:100; Beyotime Institute of Biotechnology) for 2
h at 37°C. Absorbance was measured at a wavelength of 450 nm using
a microplate reader. Next, cell absorbance values were detected
using CCK8 methods at days 1, 2, 3, and 4, respectively.
Alternatively, cells were transfected with ATX siRNA and cultured
for 24 h at 37°C, then seeded in 96-well culture plates at 2,000
cells per well and treated with 10 nM 17β-estradiol or 40 µM LPA
for 48 h at 37°C. Cells were collected, incubated with CCK8 and the
absorbance was measured as described above. In addition, cells were
treated with 100 µM PD98059 (the p-ERK inhibitor) for 24 h at 37°C,
then seeded in 96-well culture plates at 2,000 cells per well and
treated with 10 nM 17β-estradiol or 40 µM LPA/0.5 nM ATX for 48 h
at 37°C. Cells were then collected, incubated with CCK8 and the
absorbance was measured as described above.
Colony formation assay
Ishikawa cells grown until 60–70% confluent were
transfected with siRNA and cultured for 24 h at 37°C, rinsed with
PBS twice, then digested with 0.25% trypsin solution for 2 min at
37°C. The neutralization reaction was induced by adding DMEM-F12
with 10% FBS and cells were centrifuged for 5 min at 300 × g at
room temperature, liquid-decanted and 1 ml fresh culture medium was
added. Cells were dispersed by pipetting repeatedly to create a
single-cell suspension with culture medium. Subsequently, cells
were counted using a cell counting chamber. A total of 500 cells
were added to each well of 6-well culture plates for ~6 days and
the medium was replaced every 3 days until cell clones were
observed with the naked eye. Cells were washed with PBS twice and
fixed in 4% paraformaldehyde for 15 min at room temperature. Cells
were then stained with crystal violet for 5 min at room
temperature, dried, images captured using an inverted microscope
and counted using ImageJ software (version 2.1.4.7; National
Institutes of Health).
Statistical analysis
Data are expressed as the mean ± standard error. All
experiments were repeated 3 times. Quantitative results were
compared using GraphPad Prism version 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). A two-tailed Student's paired
t-test was used to test for significance between two groups.
Multiple groups were compared by a one-way or two-way analysis of
variance with Tukey's post-test correction. P<0.05 was
considered to indicate a statistically significant difference.
Results
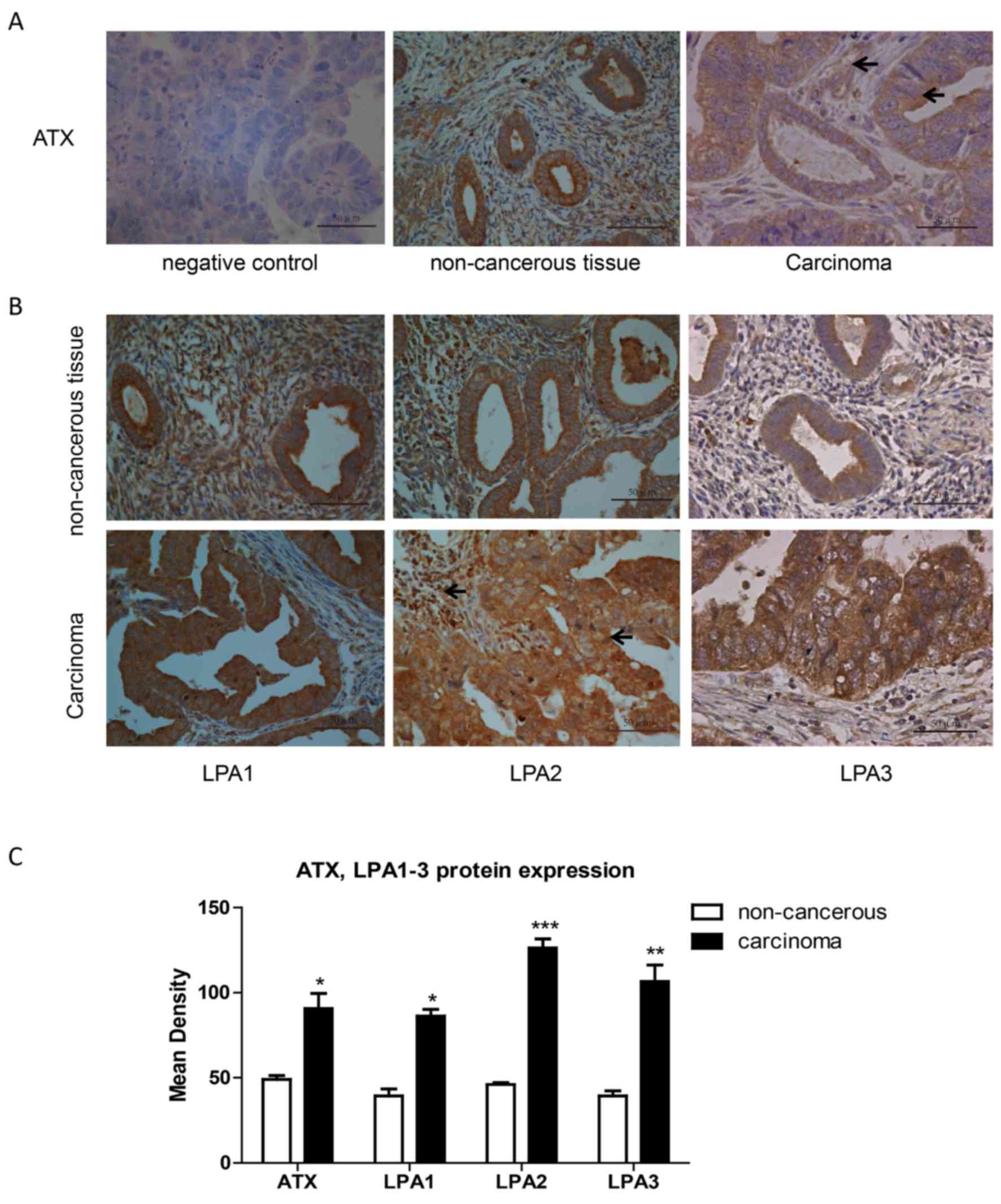
Expression of ATX and LPA1, 2 and 3 in
endometrial carcinoma tissues
Pathological sections of normal adjacent tissues and
carcinoma tissues were obtained from 10 endometrial adenocarcinoma
patients. Following immunohistochemistry, 5 randomly selected
fields of stained pathological sections were photographed under a
light microscope at ×400 magnification. The absorbance value of
positive regions was analyzed using the Image-Pro Plus software
version 6.0 (Media Cybernetics, Inc.). ATX expression was stronger
in endometrial carcinoma than in adjacent non-cancerous tissue
(Fig. 1A and C). Also, ATX was
expressed in the cytoplasm of cancer cells and stromal cells. LPA1,
2, 3 expression levels were greater in endometrial cancer tissues
than in adjacent non-cancerous tissue (Fig. 1B and C).
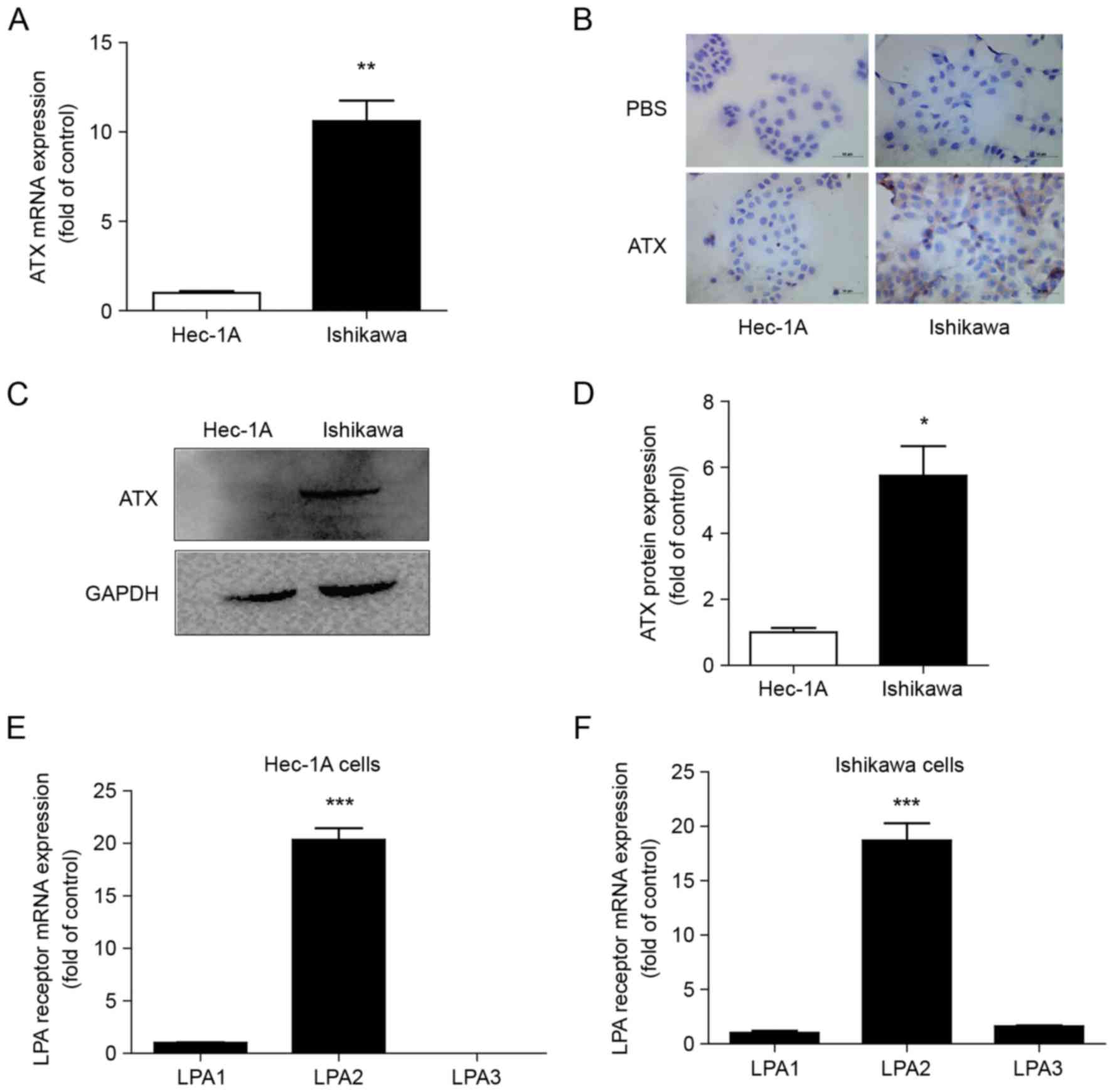
Differential expression of ATX and
LPA1, 2 and 3 in endometrial cancer cells
Ishikawa cells positive for ER and Hec-1A cells with
low ER expression exhibited ATX expression (Fig. 2A). However, ATX mRNA expression was
higher in Ishikawa cells than in Hec-1A cells (Fig. 2A). ATX was primarily expressed in
the cytoplasm of the cells by immunocytochemistry (Fig. 2B). ATX protein expression was also
greater in Ishikawa cells than in Hec-1A cells (Fig. 2C and D). The mRNA expression levels
of LPA1, 2 and 3 were detected in both cell types, and LPA2
expression was the greatest (Fig. 2E
and F).
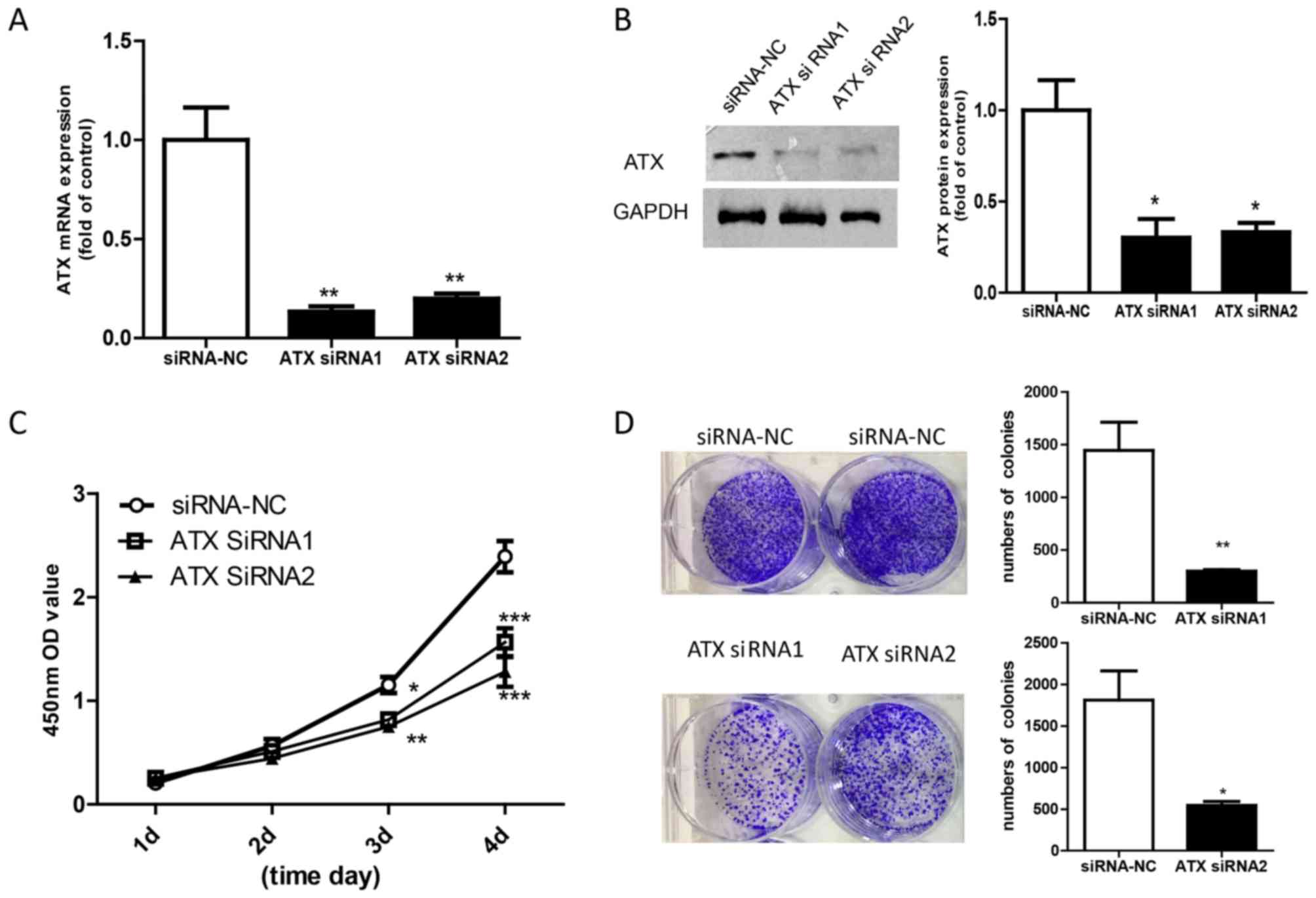
siRNA knockdown of ATX inhibits the
proliferation of Ishikawa cells
To understand the role of ATX in endometrial
adenocarcinoma, 2 siRNA sequences were used to obtain loss of
function of ATX in Ishikawa cells. The mRNA and protein expression
levels of ATX were significantly decreased following siRNA
knockdown of ATX compared with Ishikawa cells transfected with
siRNA-NC (Fig. 3A and B). The CCK8
assay revealed that the rate of cell growth was slower in cells
transfected with ATX siRNA than in Ishikawa cells transfected with
siRNA-NC (Fig. 3C). In addition,
Ishikawa cells exhibited lower colony numbers following ATX
knockdown (Fig. 3D).
Reduced ATX levels decrease
proliferation of Ishikawa cells induced by estrogen and LPA
ATX expression was significantly increased in
Ishikawa cells positive for ER. It was then determined whether
estrogen induced the expression of ATX by examining the mRNA
expression of ATX in Ishikawa cells treated with 17β-estradiol for
24 h. The mRNA expression of ATX was significantly induced by
estrogen compared with the control (Fig. 4A). It was subsequently determined
whether ATX was involved in estrogen- and LPA-induced cell
proliferation. Inhibition of ATX with siRNA decreased the cell
proliferation induced by estrogen, LPA or estrogen in combination
with LPA (Fig. 4B-D).
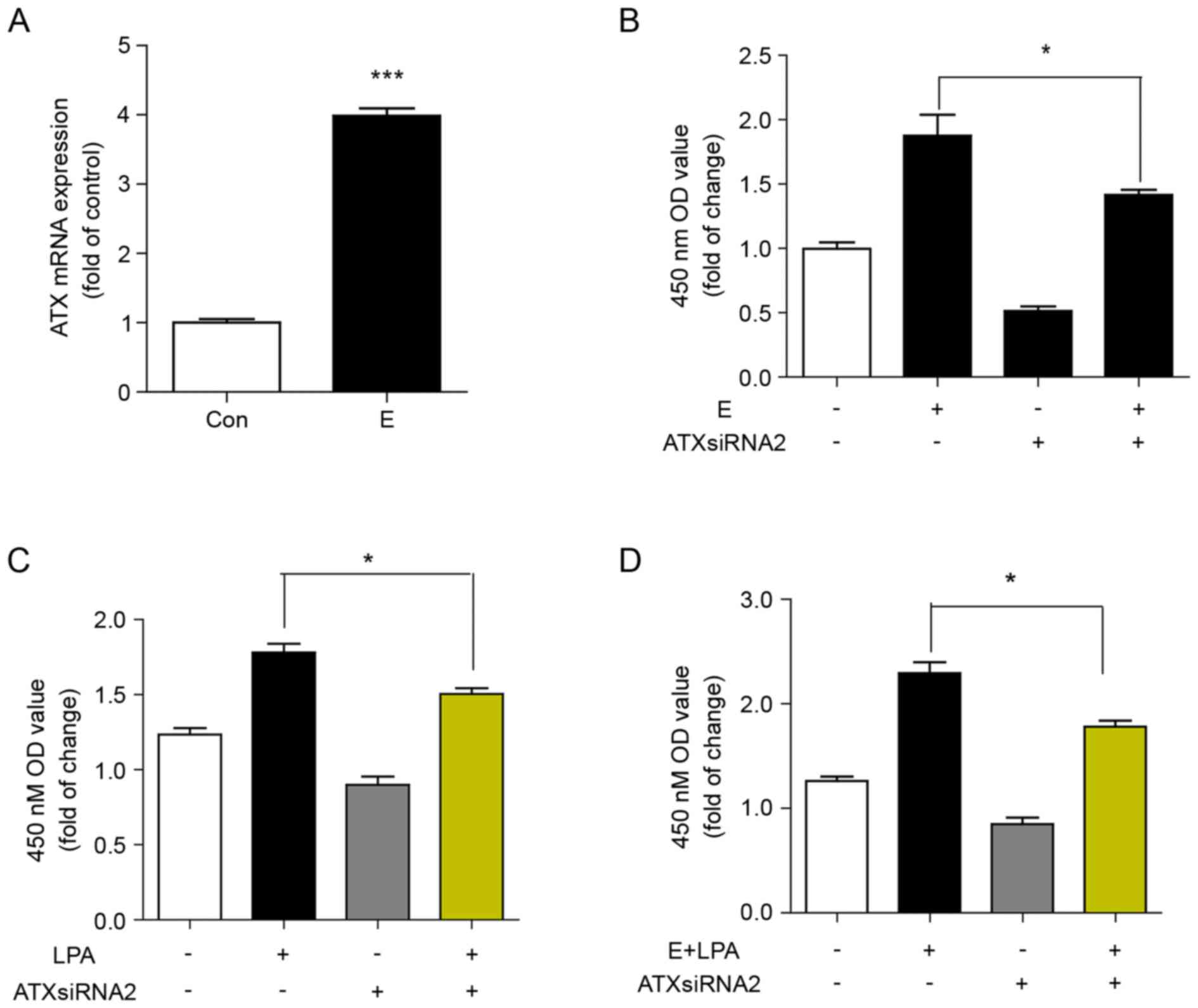 | Figure 4.Reduced ATX levels protect against
proliferation of Ishikawa cells induced by estrogen and LPA. (A)
Reverse transcription-quantitative polymerase chain reaction of the
mRNA expression of ATX in Ishikawa cells stimulated with
17β-estradiol (10 nM) for 24 h. ***P<0.001 vs. Con. (B) CCK8
assay of cell proliferation of Ishikawa cells transfected with ATX
siRNA or siRNA-NC for 24 h, then 2,000 cells/pore were stimulated
with or without 17β-estradiol for 48 h. *P<0.05, as indicated.
(C) CCK8 assay of cell proliferation of Ishikawa cells transfected
with ATX siRNA or siRNA-NC for 24 h, then stimulated with or
without LPA for 48 h. *P<0.05, as indicated. (D) CCK8 assay of
cell proliferation of Ishikawa cells transfected with ATX siRNA or
siRNA-NC for 24 h, then stimulated with or without 17β-estradiol
and LPA for 48 h. Data are shown as the mean ± standard deviation
(n=3). *P<0.05, as indicated. E, 17β-estradiol; con, control;
LPA, lysophosphatidic acid; ATX siRNA, autotaxin short interfering
RNA; siRNA-NC, nonsense negative control short interfering RNA; OD,
optical density; CCK, Cell Counting kit-8. |
ATX-LPA is involved in
estrogen-induced cell proliferation via ERK signaling
To confirm the effect of ATX on LPA receptors,
Ishikawa cells were transfected with ATX siRNA, and then the mRNA
expression levels of LPA1, 2 and 3 were determined. To varying
degrees, the expression of LPA 1, 2 and 3 receptors was reduced
following ATX siRNA treatment when compared with the siRNA-NC
group, and LPA2 expression was the most significantly reduced
(Fig. 5A). Ishikawa cells were
subsequently treated with different concentrations of LPA. LPA at
80, 40 and 20 µM significantly increased the phosphorylation of ERK
compared with 0 µM (Fig. 5B).
p-ERK expression levels were downregulated following siRNA
knockdown of ATX compared with the siRNA-NC group (Fig. 5C). Transfection with ATX siRNA
reduced LPA or estrogen-induced ERK phosphorylation (Fig. 5D and E). In addition, inhibition of
the ERK signaling pathway by PD98059 with estrogen and/or ATX and
LPA treatment decreased cell proliferation (Fig. 5F and G).
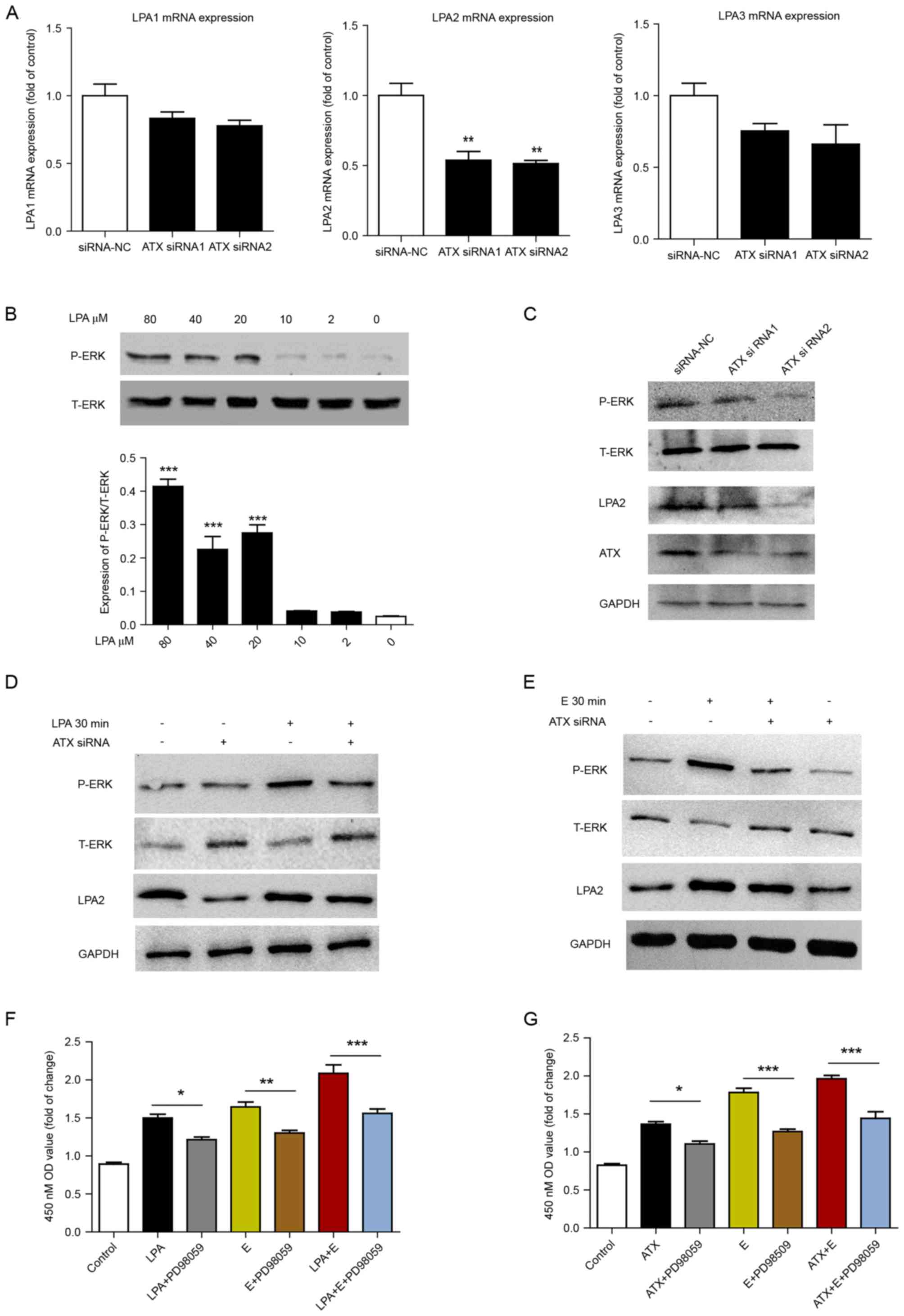 | Figure 5.ATX-LPA axis is involved in
estrogen-induced proliferation via ERK signaling. (A) Reverse
transcription-quantitative polymerase chain reaction of the mRNA
expression levels of LPA1, 2 and 3 in Ishikawa cells following
siRNA knockdown of ATX for 24 h. **P<0.01 vs. siRNA-NC. (B)
T-ERK and P-ERK levels following treatment with doses of LPA for 30
min in Ishikawa cells. ***P<0.001 vs. 0 µM LPA. Western blot
analysis of the protein expression of ATX, LPA2, P-ERK and T-ERK in
Ishikawa cells following (C) siRNA knockdown of ATX for 48 h, (D)
combined treatment with or without 40 µM LPA for 30 min, or (E)
17β-estradiol (10 nM) for 30 min. Cell Counting kit-8 assay of cell
proliferation following treatment with (F) LPA and/or
17β-estradiol, plus 100 µM PD98059 (ERK inhibitor) for 24 h and (G)
0.5 nM human recombinant ATX and/or 17β-estradiol plus 100 µM
PD98059 for 24 h. Data are shown as the mean ± standard deviation
(n=3). *P<0.05, **P<0.01 and ***P<0.001, as indicated.
ERK, extracellular signal-regulated kinase; T-ERK, total ERK;
P-ERK, phosphorylated ERK; LPA, lysophosphatidic acid; ATX,
autotaxin; OD, optical density; E, 17β-estradiol. |
Discussion
Endometrial adenocarcinoma represents 87 to 90% of
all diagnosed endometrial carcinomas. In the present study, ATX and
LPA receptors were highly expressed in endometrial adenocarcinoma
compared with surrounding non-cancerous endometrial tissue. The
expression levels of ATX and LPA receptors were positive in the
cytoplasm of endometrial cancer cells. Hence, the ATX-LPA axis may
serve an important role in the progression of endometrial
adenocarcinoma.
Wasniewski et al (11) investigated ATX and LPA receptor
expression in 37 endometrial cancers and 10 normal endometrial
samples, and demonstrated that ATX and LPA receptors were
overexpressed in endometrial carcinoma. High expression of LPA1 and
2 was positively associated with the depth of myoinvasion,
International Federation of Gynecology and Obstetrics stage and
body mass index of examined patients (11). However, the function of ATX was not
investigated in preliminary studies.
An epidemiological study reported that endometrial
carcinoma is frequently an estrogen-dependent tumor (12). The present study detected ATX
expression in endometrial cancer cell lines. Ishikawa and Hec-1A
endometrial cancer cell lines express high and low levels of ER,
respectively. The mRNA and protein expression levels of ATX were
higher in Ishikawa cells positive for ER and lower in Hec-1A cells
with low ER expression. ATX expression was strongly positive in
Ishikawa cells, with almost no expression in Hec-1A cells following
immunohistochemistry staining. Hence, estrogen may participate in
regulating ATX generation and secretion. The expression of ATX is
regulated by a number of tumor microenvironment factors. Kehlen
et al (13,14) demonstrated that epidermal growth
factor and basic fibroblast growth factor promote ATX mRNA
expression in thyroid cancer cells. The present study confirmed
that ATX mRNA levels were upregulated by estrogen. LPA receptor
expression in Ishikawa and Hec-A cells was examined, and the
expression of LPA1, 2 and 3 was greater in the two cell types. This
data suggested that the ATX-LPA axis may serve a role in the
development of endometrial carcinoma.
The results of cell proliferation in the present
study demonstrated that with siRNA knockdown of ATX, cell colony
number and cell proliferation rate decreased significantly. Sawada
et al (15) revealed that
concentrations of 1–15 µmol/l LPA may stimulate the growth of
ovarian cancer cells. Fishman et al (16) reported that LPA improved the
expression of cell surface adhesion molecule-1 integrin in ovarian
cancer cells and enhanced the ability of cell adhesion mediated by
collagen I. Meng et al (17) demonstrated that LPA inhibited
apoptosis induced by Fas and induced Fas translocation from the
cell membrane to the cytoplasm. Therefore, LPA, as a biologically
active substance with signal transduction, is closely associated
with the growth, adhesion and metastasis of cancer cells (17). In the present study, it was
revealed that ATX was involved in estrogen- and LPA-induced cell
proliferation.
The results of the present study also showed that
the mRNA expression levels of LPA2 decreased in Ishikawa cells
transfected with ATX siRNA. ERK inhibitors may prevent the
protective effect of LPA on cell apoptosis, which suggests that the
Ras/Raf1/mitogen-activated protein kinase kinase/ERK signaling
pathway may be involved in the protective effect of LPA on
apoptosis (18). Therefore, in the
current study, Ishikawa cells were treated with different
concentrations of LPA to observe ERK phosphorylation. LPA induced
ERK phosphorylation at high concentrations. In addition, ATX siRNA
transfection reduced the estrogen- and LPA-induced ERK
phosphorylation. The ERK inhibitor reduced the cell proliferation
induced by estrogen, ATX and LPA. The results suggested that the
mitogen-activated protein kinase (MAPK)/ERK signaling pathway may
be involved in the estrogen-ATX-LPA axis, inducing the
proliferation of endometrial cancer cells. The ATX-LPA axis may
facilitate estrogen-induced proliferation of endometrial cancer via
the MAPK/ERK signaling pathway.
The role of the ATX-LPA axis was preliminarily
revealed in endometrial cancer. A recent study indicated that ATX
may promote the recurrence and metastasis of breast cancer by
regulating the tumor inflammation microenvironment (19). Whether the ATX-LPA axis is involved
in regulating the imbalanced inflammatory microenvironment in
endometrial carcinoma is unknown. Further investigation is required
to study the role of the ATX-LPA axis in the progression of
endometrial carcinoma. The present study will provide ideas and
experimental basis for clinicians to identify novel molecular
targeted drugs for the treatment of endometrial cancer.
Acknowledgements
The present study was funded by National Key
Technology Research and Development Program of the Ministry of
Science and Technology of China (grant no. 2015BAI13B06) and
Research and Development Fund of Peking University People's
Hospital (grant no. RD2014-06).
References
|
1
|
Globocan. 2012, http://globocan.iarc.fr/Pages/online.aspx4–July.
2016
|
|
2
|
National Cancer Institute, .
PDQ® screening and prevention editorial board. PDQ
endometrial cancer prevention. Bethesda, MD: National Cancer
Institute; 2016, http://www.cancer.gov/types/uterine/hp/endometrial-prevention-pdq11–January.
2015[PMID: 26389477].
|
|
3
|
Jeppesen MM, Mogensen O, Hansen DG,
Iachina M, Korsholm M and Jensen PT: Detection of recurrence in
early stage endometrial cancer-the role of symptoms and routine
follow-up. Acta Oncol. 56:262–269. 2017. View Article : Google Scholar
|
|
4
|
Busch EL, Crous-Bou M, Prescott J, Chen
MM, Downing MJ, Rosner B, Mutter GL and De Vivo I: Endometrial
cancer risk factors, hormone receptors, and mortality prediction.
Cancer Epidemiol Biomarkers Prev. 26:727–735. 2017. View Article : Google Scholar
|
|
5
|
Benesch MG, Tang X, Venkatraman G, Bekele
RT and Brindley DN: Recent advances in targeting the
autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in
vivo. J Biomed Res. 30:272–284. 2016.
|
|
6
|
Volden PA, Skor MN, Johnson MB, Singh P,
Patel FN, McClintock MK, Brady MJ and Conzen SD: Mammary adipose
tissue-derived lysophospholipids promote estrogen receptor-negative
mammary epithelial cell proliferation. Cancer Prev Res (Phila).
9:367–378. 2016. View Article : Google Scholar :
|
|
7
|
Gaetano CG, Samadi N, Tomsig JL, Macdonald
TL, Lynch KR and Brindley DN: Inhibition of autotaxin production or
activity blocks lysophosphatidylcholine-induced migration of human
breast cancer and melanoma cells. Mol Carcinog. 48:801–809. 2009.
View Article : Google Scholar :
|
|
8
|
Ptaszynska MM, Pendrak ML, Bandle RW,
Stracke ML and Roberts DD: Positive feedback between vascular
endothelial growth factor-A and autotaxin in ovarian cancer cells.
Mol Cancer Res. 6:352–363. 2008. View Article : Google Scholar :
|
|
9
|
Hope JM, Wang FQ, Whyte JS, Ariztia EV,
Abdalla W, Long K and Fishman DA: LPA receptor 2 mediates
LPA-induced endometrial cancer invasion. Gynecol Oncol.
112:215–223. 2009. View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
Wasniewski T, Woclawek-Potocka I,
Boruszewska D, Kowalczyk-Zieba I, Sinderewicz E and Grycmacher K:
The significance of the altered expression of lysophosphatidic acid
receptors, utotaxin and phospholipase A2 as the potential
biomarkers in type 1 endometrial cancer biology. Oncol Rep.
34:2760–2767. 2015. View Article : Google Scholar
|
|
12
|
Droog M, Nevedomskaya E, Dackus GM, Fles
R, Kim Y, Hollema H, Mourits M, Nederlof PM, van Boven HH, Linn SC,
et al: Estrogen receptor α wields treatment-specific enhancers
between morphologically similar endometrial tumors. Proc Natl Acad
Sci USA. 114:pp. E1316–E1325. 2017; View Article : Google Scholar :
|
|
13
|
Kehlen A, Englert N, Seifert A, Klonisch
T, Dralle H, Langner J and Hoang-Vu C: Expression, regulation and
function of autotaxin in thyroid carcinomas. Int J Cancer.
109:833–838. 2004. View Article : Google Scholar
|
|
14
|
Kehlen A, Lauterbach R, Santos AN, Thiele
K, Kabisch U, Weber E, Riemann D and Langner J: IL-1 beta- and
IL-4-induced down-regulation of autotaxin mRNA and PC-1 in
fibroblast-like synoviocytes of patients with rheumatoid
arthritis(RA). Clin Exp Immunol. 123:147–154. 2001. View Article : Google Scholar :
|
|
15
|
Sawada K, Morishige Ki, Tahara M, Ikebuchi
Y, Kawagishi R, Tasaka K and Murata Y: Lysophosphatidic acid
induces focal adhesion assembly through Rho/Rho-associated kinase
pathway in human ovarian cancer cells. Gynecol Oncol. 87:252–259.
2002. View Article : Google Scholar
|
|
16
|
Fishman DA, Liu Y, Ellerbroek SM and Stack
MS: Lysophosphatidic acid promotes matrix metalloproteinase (MMP)
activation and MMP-dependent invasion in ovarian cancer cells.
Cancer Res. 61:3194–3199. 2001.
|
|
17
|
Meng Y, Kang S and Fishman DA:
Lysophosphatidic acid inhibits anti-Fas-mediated apoptosis enhanced
by actin depolymerization in epithelial ovarian cancer. FEBS Lett.
579:1311–1319. 2005. View Article : Google Scholar
|
|
18
|
Sun H, Zhu Q, Ren J, Wu L, Kong FZ, Li G
and Hu HF: Influence of lysophosphatidic acid on proliferation,
adhesion, migration and apoptosis of cervical cancer HeLa cells. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 25:702–705. 2009.(In
Chinese).
|
|
19
|
Benesch MG, Tang X, Dewald J, Dong WF,
Mackey JR, Hemmings DG, McMullen TP and Brindley DN: Tumor-induced
inflammation in mammary adipose tissue stimulates a vicious cycle
of autotaxin expression and breast cancer progression. FASEB J.
29:3990–4000. 2015. View Article : Google Scholar
|