Introduction
Osteoarthritis (OA) is a degenerative joint disease
characterized by the erosion of articular cartilage and subchondral
bone and inflammation of the synovial membrane (1,2). The
etiology of OA is affected by factors including aging, injury,
obesity and heredity. OA currently affects ~25% of the population
aged >18 years and is expected to be the greatest cause of
disability among patients aged >40 years (3,4).
Recent studies have increasingly recognized the
importance of the synovial membrane in the progression of OA
(5). The synovial membrane is
significantly altered prior to the occurrence of visible cartilage
degeneration (6). Prieto-Potin
et al reported that the histological pattern of the synovial
membrane in OA patients was characterized by hyperplasia of
synovial lining and stromal vascularization (7). Inflammatory cells (including
macrophages, as well as B- and T-lymphocytes) and cytokines
[including interleukin (IL)-6, IL-1β and tumor necrosis factor-α]
have been detected in the synovial membranes of OA patients
(8–10). It has been demonstrated that the
synovium is associated with pain and poor functioning of the knee
(11). However, the detailed
pathogenic mechanisms in the synovia in the progression of OA have
remained poorly understood and there is currently no available
intervention strategy to slow down disease progression in patients
with OA.
Microarray technology has markedly expanded the
capacity of investigators to examine the pathogenic processes of
various diseases (12,13). This technology is an important tool
for current functional genomic research. Numerous studies have used
microarray technology to identify certain OA-specific proteins,
including activating transcription factor 4, G protein-coupled
receptor 18, S100 calcium-binding protein A9/A8, prostaglandin D2
synthase and stromal cell-derived factor 1 (14,15).
However, the sex of a patient may have a key role in the
development of OA: Epidemiological studies have revealed a higher
incidence of OA in females than in males (16,17).
Furthermore, the use of certain medicines, including non-steroidal
anti-inflammatory drugs or methotrexate, may also affect gene
expression in synovial membranes affected by OA. In the present
study, to exclude the effects of sex and medication use on the
synovial membrane, genetic expression data of samples from female
patients with OA who did not take any medicines and from healthy
females were extracted. A bioinformatics analysis was performed to
explore key genes whose functions are important in the synovial
membranes of female patients with OA. Subsequently, Gene Ontology
(GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment analyses were performed, with the aim of gaining a
better understanding of the molecular mechanisms of the synovia in
female patients with OA.
Materials and methods
Microarray data
The Gene Expression Ominibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) stores
original submitter-supplied records as well as curated DataSets.
Two gene expression profiles (GSE55457 and GSE55584) were obtained
from the GEO database. To eliminate the potential effect of sex and
medication, only data of synovial membranes from healthy females
and female patients with OA who did not take any medicines were
used. Data of two normal synovial membranes (GSM1337306 and
GSM1337310) and two synovial membranes affected by OA (GSM1337327
and GSM1337330) were obtained from the GSE55457 dataset, while the
data of three synovial membranes affected by OA (GSM1339628,
GSM1339629 and GSM1339632) were obtained from the GSE55584
dataset.
Identification of differentially
expressed genes (DEGs)
Analysis was performed by using the matrix
visualization and analysis platform Morpheus (https://software.broadinstitute.org/morpheus/). The
expression of mRNAs with a signal-to-noise ratio of >2 or <-2
were defined as DEGs (18).
Protein-protein interaction (PPI)
network analysis
To understand the functional interactions between
these DEGs, a PPI network was constructed by using the web-based
tool STRING (http://www.string-db.org).
Subsequently, the PPI network was visualized by using Cytoscape
software (http://www.cytoscape.org/). The top
ten nodes ranked by the degree of interactions in the PPI network
were selected. Subsequently, the plug-in Molecular Complex
Detection (MCODE) was used to screen modules of the PPI network in
Cytoscape. The selection criteria were set as follows: MCODE scores
≥3 and number of nodes ≥3.
GO and pathway enrichment
analysis
In order to analyse the functions and pathways that
the DEGs in the top module from the PPI network are involved in, GO
and KEGG pathway enrichment analyses were performed using ClueGo
(19). A cut-off of 0.4 was set
for kappa score and terms including at least 3 genes were
retrieved.
Results
Identification of DEGs
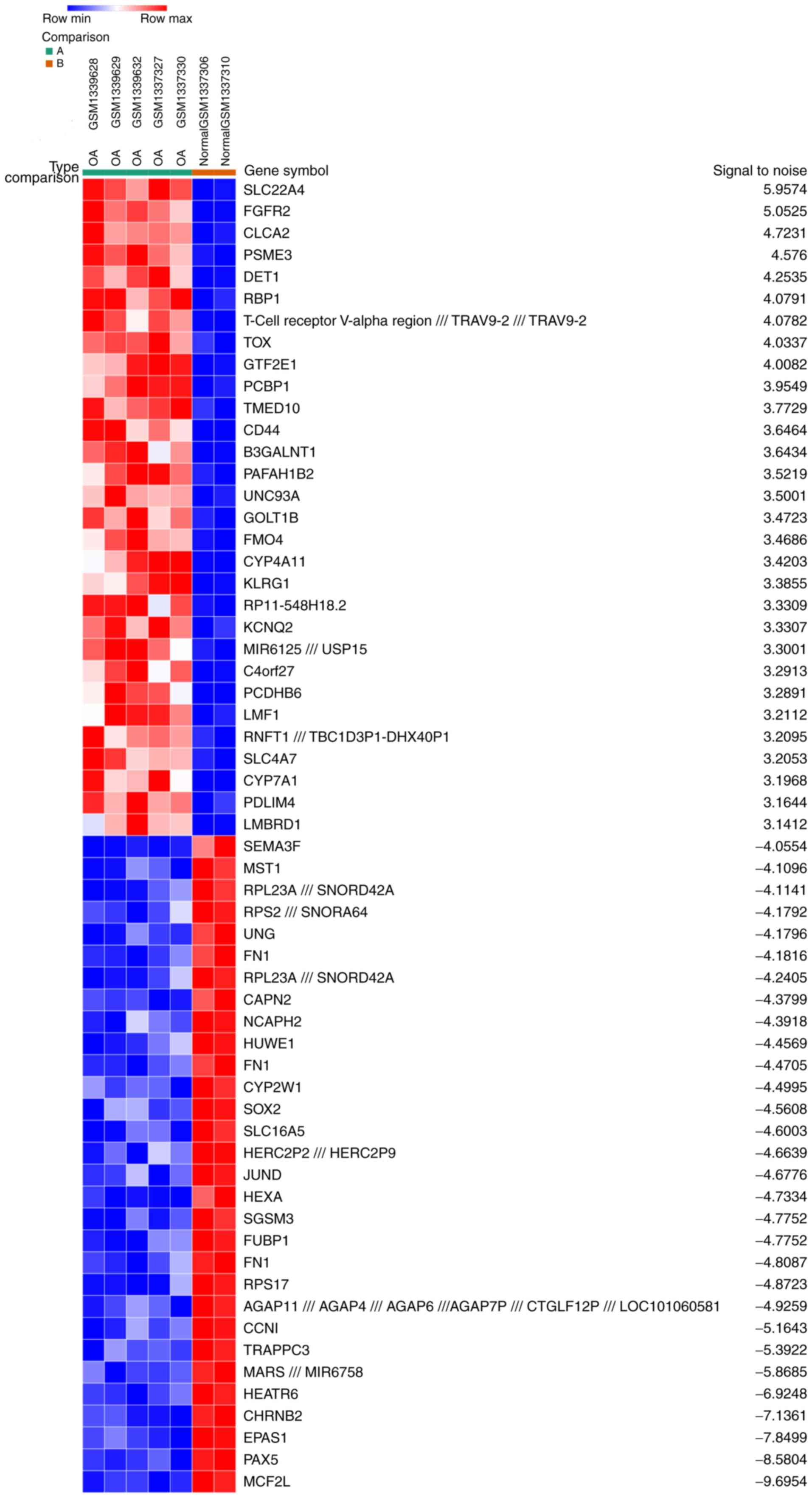
A total of 5 OA samples and 2 normal synovial
samples were analyzed from the GSE55457 and GSE55584 datasets. The
gene expression profiles were analyzed with Morpheus software. This
analysis identified 164 up- and 213 downregulated DEGs in the OA
samples compared with the normal samples. An expression heat map of
the top 30 up- and downregulated DEGs is presented in Fig. 1.
PPI network construction and module
analysis
Based on the information in the STRING and Cytoscape
databases, the top 10 hub nodes with the highest degree of
interaction were screened. These hub genes were ubiquitin (UB) C,
ribosomal protein (RP) L23A, mammalian target of rapamycin (mTOR),
heat shock protein 90 α family class A member 1 (HSP90AA1), RPS28,
RPL37A, RPS24, RPS4X, RPS18 and UBB (Table I). Among these genes, UBC had the
highest degree of interaction with 97 nodes. Six modules from the
PPI network satisfied the criteria of MCODE scores ≥3 and number of
nodes ≥3 (Table II). The results
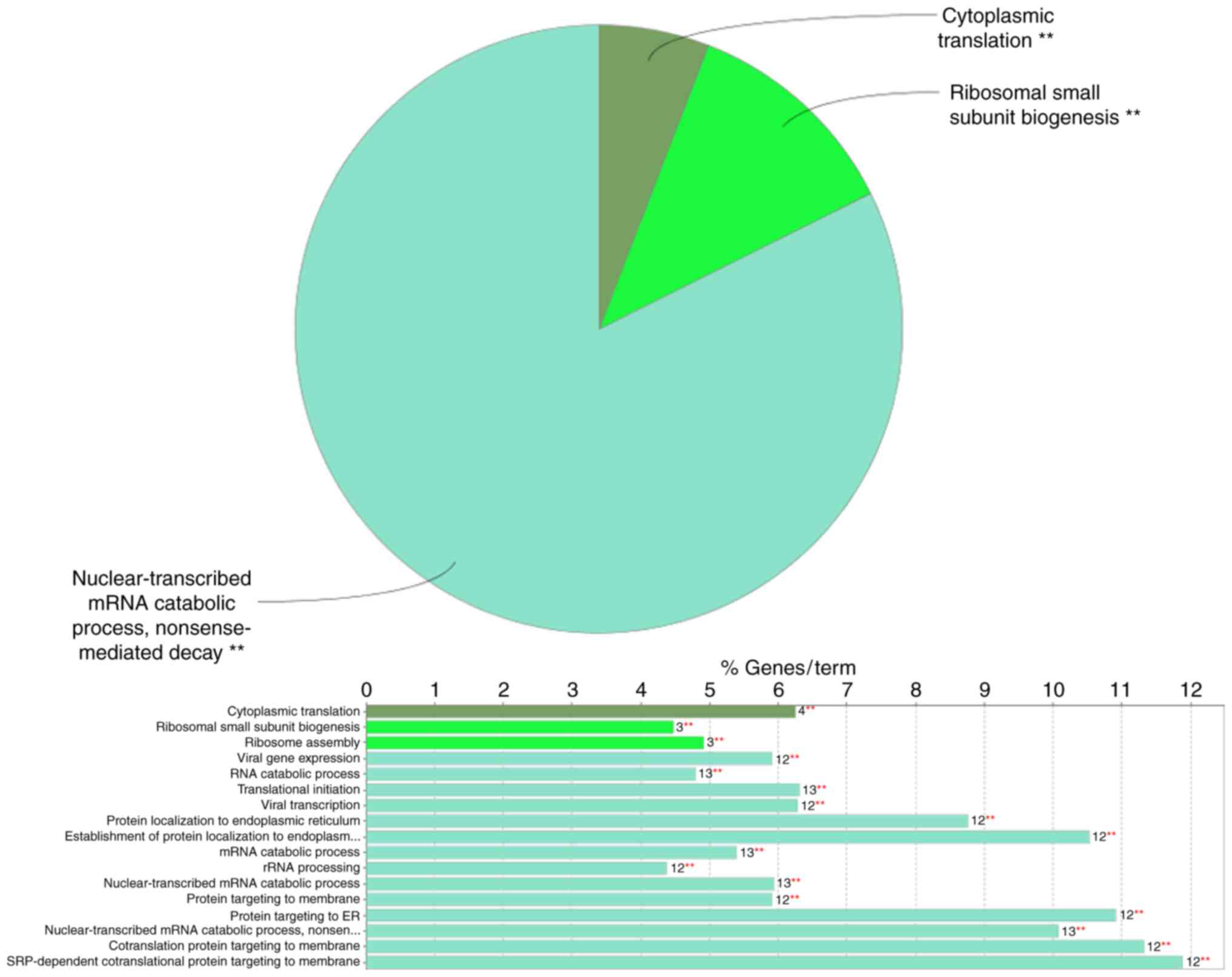
of the GO analysis indicated that in the category ‘biological
process’ (BP), the DEGs included in the top module (Fig. 2) were significantly enriched in the
GO terms ‘nuclear-transcribed mRNA catabolic process’, ‘nonsense
mediated decay’ (GO:000184), ‘cytoplasmic translation’ (GO:0002181)
and ‘ribosomal small subunit biogenesis’ (GO:0042274) (Fig. 3). In the GO category ‘molecular
function’ (MF), the DEGs included in the top module were
significantly enriched in ‘ribosomal (r)RNA binding’ (GO:0019843)
(Table III). The KEGG pathway
enrichment analysis of the DEGs included in the top module revealed
that these genes were mainly associated with the ribosome pathway
(KEGG:03010) (Table III).
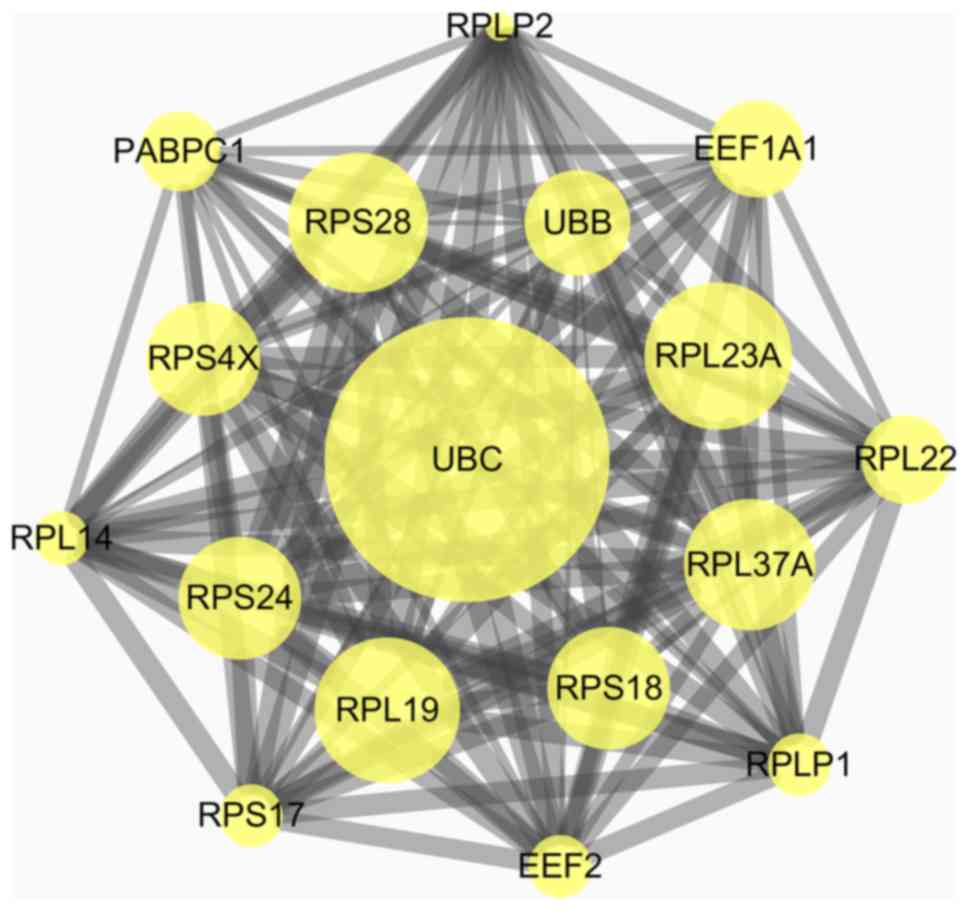 | Figure 2.Top module from the protein-protein
interaction network. UBC, ubiquitin C; UBB, ubiquitin B; RPLP1,
ribosomal protein lateral stalk subunit P1; RPLP2, ribosomal
protein lateral stalk subunit P2; PABPC1, polyadenylate-binding
protein; RPS28, ribosomal protein 28; RPS4X, ribosomal protein S4,
X-Linked; RPS24, ribosomal protein S24; RPS17, ribosomal protein
S17; RPS18, ribosomal protein S18; RPL23A, ribosomal protein L23a;
RPL14, ribosomal protein L14; RPL19, ribosomal protein L19; RPL37A,
ribosomal protein L37a; RPL22, ribosomal protein L22; EEF1A1,
elongation factor 1-α 1; EEF2, eukaryotic translation elongation
factor 2. |
 | Table I.Top ten genes with the highest degree
of interaction in the protein-protein interaction network. |
Table I.
Top ten genes with the highest degree
of interaction in the protein-protein interaction network.
| Gene | Degree of
interaction |
|---|
| UBC | 97 |
| RPL23A | 20 |
| MTOR | 20 |
| HSP90AA1 | 20 |
| RPS28 | 17 |
| RPL37A | 16 |
| RPS24 | 15 |
| RPS4X | 15 |
| RPS18 | 14 |
| UBB | 13 |
 | Table II.Six modules from the protein-protein
interaction network. |
Table II.
Six modules from the protein-protein
interaction network.
| Cluster no. | Score | Nodes (n) | Edges (n) | Node IDs |
|---|
| 1 | 16.5 | 17 | 132 | RPL19, RPS17, RPL23A,
EEF1A1, RPS24, UBC, RPLP1, RPL37A, RPL22, RPLP2, RPL14, RPS4X,
RPS28, RPS18, EEF2, PABPC1, UBB |
| 2 | 4 | 10 | 18 | ATG14, CSNK1A1,
CHEK1, BECN1, MTOR, CSNK1E, CFLAR, GAPDH, YWHAB, UVRAG |
| 3 | 3 | 3 | 3 | PCBP1, SNRPD1,
HNRNPUL1 |
| 4 | 3 | 3 | 3 | BUB3, CLASP2,
KIF18A |
| 5 | 3 | 3 | 3 | SEC31B, COPB1,
ARF4 |
| 6 | 3 | 3 | 3 | FPR1, PTGER3,
GRM3 |
 | Table III.Analysis of GO terms in the category
MF and KEGG pathways in the top module in the protein-protein
interaction network. |
Table III.
Analysis of GO terms in the category
MF and KEGG pathways in the top module in the protein-protein
interaction network.
| Term | GO ID | Function | Associated
genes |
|---|
| MF | GO:0019843 | rRNA binding | EEF2, RPL19,
RPL23A, RPS18, RPS4X |
| KEGG pathway | GO:0003010 | Ribosome | RPL14, RPL19,
RPL22, RPL23A, RPL37A, RPLP1, RPLP2, RPS17, RPS18, RPS24, RPS28,
RPS4X |
Discussion
In the present study, the DEGs and associated PPIs
in the synovial membranes of female patients with OA compared with
those in healthy females were investigated. These results may
contribute to a better understanding of the etiology of the
synovial membrane in OA in female patients. In addition, the
identified hub genes may act as potential candidate biomarkers for
the diagnosis of OA. As presented in Table I, the top 10 hub genes in the PPI
network included UBC, RPL23A, mTOR, HSP90AA1, RPS28, RPL37A, RPS24,
RPS4X, RPS18 and UBB. All hub genes were downregulated in the
synovia of female patients with OA.
UBC and UBB are genes encoding ubiquitin in the
mammalian genome. UB is a small protein that is involved in the
maintenance of chromatin structure and the regulation of gene
expression. Based on previous studies reporting that UB is
essential for the growth of cancer cells (20,21),
the present study hypothesized that the upregulation of UBB and UBC
may be a therapeutic strategy for maintaining the normal function
of cells in the synovium.
mTOR is a major repressor of autophagy and it was
previously demonstrated that mTOR is overexpressed in cartilage
affected by OA (22). By
inhibiting mTOR, authophagy is activated, which reduces cartilage
damage and synovial inflammation in an experimental model of OA
(22,23). The present bioinformatics analysis
indicated that mTOR was downregulated in the synovium of OA
patients, suggesting that autophagy was activated in the early
stage of OA.
RPs are the major constituents of the ribosome
complex and their functions are essential for cell growth,
proliferation and homeostasis. RPL23A has been detected in the
cytoplasm of synovial cells from patients with rheumatoid arthritis
(RA) or OA as well as in healthy individuals, and that in RA
patients, RPL23A was attached to T cells (24). Downregulation of RPL23A may also
cause abnormal functioning of the synovium in OA patients. Tang and
Wade (25) reported that, in zebra
finches, the total number of cells expressing RPL37 was lower in
females than in males, and declined with increasing age in females.
These results may explain why OA often occurs in older females.
RPS24 insufficiency inhibits the proliferation of cancer cells
(26) and causes distinct cell
cycle defects in diamond-blackfan anemia (27). The biological functions of RPS28,
RPS18 and RPS4X in OA remain elusive at present. Anthony and
Liebman (28) reported that
alterations in RPS-18 may affect the translational accuracy in
Saccharomyces cerevisiae. Furthermore, studies suggested
that RPS28, RPS18 and RPS4X are associated with Diamond-Blackfan
anemia (29–31). Based on these previous studies, it
may be speculated that decreased levels of RPS28, RPS18 and RPS4X
in synovial membranes may affect the functions of key proteins in
rRNA processing, which may result in the degeneration of the
synovium.
The HSP90AA1 gene encodes the Hsp90α protein. At
present, the relevance of its expression to the progression of OA
remains largely elusive. In a previous study, the upregulated
expression of HSP90AA1 was demonstrated to be significantly
associated with tumors and was critical for the stability of
proteins that are vital for tumor progression (32). This result indicated that the level
of HSP90AA1 downregulation may have an effect on cell viability in
the synovium.
The ClueGo analysis provided the GO terms in the
categories BP and MF, as well as KEGG pathway terms associated with
the DEGs included in the top module. With regard to BP terms, the
DEGs included in the top module of the PPI network were mainly
enriched in ‘nuclear-transcribed mRNA catabolic process’, ‘nonsense
mediated decay’, ‘cytoplasmic translation’ and ‘ribosomal small
subunit biogenesis’, which may otherwise lead to the synthesis of
dysfunctional proteins (33). The
other two enriched BP terms, ‘cytoplasmic translation’ and
‘ribosomal small subunit biogenesis’, have important roles in
protein formation. In the GO category MF, the DEGs from the top
module of the PPI network were enriched in the term ‘rRNA binding’.
KEGG pathway enrichment analysis indicated a significant role of
‘ribosome’ pathways in the degeneration of the synovial
membrane.
As the association of most of the genes that were
identified to be associated with OA in the present study were not
previously reported, a further study will be performed to verify
the gene expression levels of the screened genes in synovial
samples from female patients with OA and healthy patients.
Furthermore, cells extracted from those samples will be cultured to
identify the molecular mechanisms of OA associated with these
genes. In addition, the progression of synovial membrane
degradation will be assessed in gene knockout rat models to further
verify the functions of these genes.
The limitation of the present study is that only two
normal synovial membrane samples and five OA synovial membrane
samples were included in the analysis, and inclusion of other
samples may change the present results. It is therefore necessary
to collect more synovial membrane samples from female patients with
OA to detect the expression levels of significantly DEGs.
In conclusion, the present study describes the
molecular mechanism that may be involved in the degeneration of
synovial membranes in female patients with OA. These mechanisms
include ‘nuclear-transcribed mRNA catabolic process’, ‘cytoplasmic
translation’ and ‘ribosomal small subunit biogenesis’. In addition,
the present study reported the major hub genes that may be involved
in the molecular mechanisms associated with the synovium in female
patients with OA. Sex disparities should be taken into
consideration when further molecular biological experiments on OA
are performed.
Acknowledgements
The authors would like to thank Mr. Jintong Ji
(Department of Orthopaedics, Union Hospital, Tongji Medical
College, Huazhong University of Science and Technology, Wuhan,
China) for his continuous support and language editing of this
article. This research was supported by the Natural Science
Foundation of Hubei Province (grant no. WJ2017Q025) and the Science
and Technology Department of Hubei Province (grant no.
2016CFB303).
Glossary
Abbreviations
Abbreviations:
|
OA
|
osteoarthritis
|
|
GEO
|
Gene Expression Omnibus
|
|
IL-6
|
interleukin 6
|
|
GPR18
|
G protein-coupled receptor 18
|
|
DEGs
|
differentially expressed genes
|
|
PPI
|
protein-protein interaction
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Liu W, He J, Lin R, Liang J and Luo Q:
Differential proteomics of the synovial membrane between bilateral
and unilateral knee osteoarthritis in surgeryinduced rabbit models.
Mol Med Rep. 14:2243–2249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen S, Fang XQ, Zhang JF, Ma Y, Tang XZ,
Zhou ZJ, Wang JY, Qin A and Fan SW: Lycorine protects cartilage
through suppressing the expression of matrix metalloprotenases in
rat chondrocytes and in a mouse osteoarthritis model. Mol Med Rep.
14:3389–3396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Helmick CG, Felson DT, Lawrence RC,
Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD,
Merkel PA, et al: Estimates of the prevalence of arthritis and
other rheumatic conditions in the United States. Part I. Arthritis
Rheum. 58:15–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas E, Peat G and Croft P: Defining and
mapping the person with osteoarthritis for population studies and
public health. Rheumatology (Oxford). 53:338–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kubosch EJ, Lang GM, Fürst D, Kubosch DC,
Izadpanah K, Rolauffs B, Südkamp NP and Schmal H: The potential for
synovium-derived stem cells in cartilage repair. Curr Stem Cell Res
Ther. Oct 2–2017.(Epub ahead of print). PubMed/NCBI
|
|
6
|
Mathiessen A and Conaghan PG: Synovitis in
osteoarthritis: Current understanding with therapeutic
implications. Arthritis Res Ther. 19:182017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prieto-Potin I, Largo R, Roman-Blas JA,
Herrero-Beaumont G and Walsh DA: Characterization of multinucleated
giant cells in synovium and subchondral bone in knee osteoarthritis
and rheumatoid arthritis. BMC Musculoskelet Disord. 16:2262015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deligne C, Casulli S, Pigenet A, Bougault
C, Campillo-Gimenez L, Nourissat G, Berenbaum F, Elbim C and Houard
X: Differential expression of interleukin-17 and interleukin-22 in
inflamed and non-inflamed synovium from osteoarthritis patients.
Osteoarthritis Cartilage. 23:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuca-Warnawin EH, Kurowska WJ, Radzikowska
A, Massalska MA, Burakowski T, Kontny E, Słowińska I, Gasik R and
Maśliński W: Different expression of chemokines in rheumatoid
arthritis and osteoarthritis bone marrow. Reumatologia. 54:51–53.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takano S, Uchida K, Miyagi M, Inoue G,
Fujimaki H, Aikawa J, Iwase D, Minatani A, Iwabuchi K and Takaso M:
Nerve growth factor regulation by TNF-α and IL-1β in synovial
macrophages and fibroblasts in osteoarthritic mice. J Immunol Res.
2016:57063592016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salaffi F, Ciapetti A and Carotti M: The
sources of pain in osteoarthritis: A pathophysiological review.
Reumatismo. 66:57–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das DK, Ali T, Krampis K and Ogunwobi OO:
Fibronectin and androgen receptor expression data in prostate
cancer obtained from a RNA-sequencing bioinformatics analysis. Data
Brief. 11:131–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong C, Sun S, Liu B, Wang J and Chen X:
Identification of potential therapeutic target genes, key miRNAs
and mechanisms in oral lichen planus by bioinformatics analysis.
Arch Oral Biol. 78:122–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramos YF, Bos SD, Lakenberg N, Böhringer
S, den Hollander WJ, Kloppenburg M, Slagboom PE and Meulenbelt I:
Genes expressed in blood link osteoarthritis with apoptotic
pathways. Ann Rheum Dis. 73:1844–1853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ungethuem U, Haeupl T, Witt H, Koczan D,
Krenn V, Huber H, von Helversen TM, Drungowski M, Seyfert C, Zacher
J, et al: Molecular signatures and new candidates to target the
pathogenesis of rheumatoid arthritis. Physiol Genomics. 42A:1–282.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blagojevic M, Jinks C, Jeffery A and
Jordan KP: Risk factors for onset of osteoarthritis of the knee in
older adults: A systematic review and meta-analysis. Osteoarthritis
Cartilage. 18:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kee CC: Osteoarthritis: Manageable scourge
of aging. Nurs Clin North Am. 35:199–208. 2000.PubMed/NCBI
|
|
18
|
Venet D, Detours V and Bersini H: A
measure of the signal-to-noise ratio of microarray samples and
studies using gene correlations. PLoS One. 7:e510132012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh C, Park S, Lee EK and Yoo YJ:
Downregulation of ubiquitin level via knockdown of polyubiquitin
gene Ubb as potential cancer therapeutic intervention. Sci Rep.
3:26232013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian Y, Ding W, Wang Y, Ji T, Sun S, Mo Q,
Chen P, Fang Y, Liu J, Wang B, et al: Correction: Ubiquitin B in
cervical cancer: Critical for the maintenance of cancer stem-like
cell characters. PLoS One. 11:e01528132016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Vasheghani F, Li YH, Blati M,
Simeone K, Fahmi H, Lussier B, Roughley P, Lagares D, Pelletier JP,
et al: Cartilage-specific deletion of mTOR upregulates autophagy
and protects mice from osteoarthritis. Ann Rheum Dis. 74:1432–1440.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ribeiro M, López de Figueroa P,
Nogueira-Recalde U, Centeno A, Mendes AF, Blanco FJ and Caramés B:
Diabetes-accelerated experimental osteoarthritis is prevented by
autophagy activation. Osteoarthritis Cartilage. 24:2116–2125. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ito Y, Hashimoto M, Hirota K, Ohkura N,
Morikawa H, Nishikawa H, Tanaka A, Furu M, Ito H, Fujii T, et al:
Detection of T cell responses to a ubiquitous cellular protein in
autoimmune disease. Science. 346:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang YP and Wade J: Sex- and age-related
differences in ribosomal proteins L17 and L37, as well as androgen
receptor protein, in the song control system of zebra finches.
Neuroscience. 171:1131–1140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Sui J, Li X, Cao F, He J, Yang B,
Zhu X, Sun Y and Pu YD: RPS24 knockdown inhibits colorectal cancer
cell migration and proliferation in vitro. Gene. 571:286–291. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Badhai J, Fröjmark ASJ, Davey E, Schuster
J and Dahl N: Ribosomal protein S19 and S24 insufficiency cause
distinct cell cycle defects in Diamond-Blackfan anemia. Biochim
Biophys Acta. 1792:1036–1042. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anthony RA and Liebman SW: Alterations in
ribosomal protein RPS28 can diversely affect translational accuracy
in Saccharomyces cerevisiae. Genetics. 140:1247–1258.
1995.PubMed/NCBI
|
|
29
|
Doherty L, Sheen MR, Vlachos A, Choesmel
V, O'Donohue MF, Clinton C, Schneider HE, Sieff CA, Newburger PE,
Ball SE, et al: Ribosomal protein genes RPS10 and RPS26 are
commonly mutated in Diamond-Blackfan anemia. Am J Hum Genet.
86:222–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gazda HT, Preti M, Sheen MR, O'Donohue MF,
Vlachos A, Davies SM, Kattamis A, Doherty L, Landowski M, Buros C,
et al: Frameshift mutation in p53 regulator RPL26 is associated
with multiple physical abnormalities and a specific pre-ribosomal
RNA processing defect in diamond-blackfan anemia. Hum Mutat.
33:1037–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gripp KW, Curry C, Olney AH, Sandoval C,
Fisher J, Chong JX; UW Center for Mendelian Genomics, ; Pilchman L,
Sahraoui R, Stabley DL and Sol-Church K: Diamond-Blackfan anemia
with mandibulofacial dystostosis is heterogeneous, including the
novel DBA genes TSR2 and RPS28. Am J Med Genet A. 164A:1–2249.
2014.PubMed/NCBI
|
|
32
|
Shen H, Zhu H, Song M, Tian Y, Huang Y,
Zheng H, Cao R, Lin J, Bi Z and Zhong W: A selenosemicarbazone
complex with copper efficiently down-regulates the 90-kDa heat
shock protein HSP90AA1 and its client proteins in cancer cells. BMC
Cancer. 14:6292014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hentze MW and Kulozik AE: A perfect
message: RNA surveillance and nonsense-mediated decay. Cell.
96:307–310. 1999. View Article : Google Scholar : PubMed/NCBI
|