Introduction
Prostate cancer (PCa) is one of the most prevalent
diagnosed malignancies and is the sixth leading cause of
cancer-associated death in males worldwide (1,2).
Globally, it is anticipated that the incidence of PCa may increase
to 1.7 million novel cases with 499,000 mortalities by 2030
(3). Furthermore, the 5-year
survival rate of patients with PCa is only 29% (4). Despite developments in the treatment
of PCa, the annual morbidity rate has increased by 14% since 1990
(5). Although numerous and
extensive studies have been performed to investigate PCa, the
pathogenesis of the tumorigenesis and progression of PCa has yet to
be completely elucidated. Therefore, further investigation of the
molecular mechanisms of PCa is required.
In recent decades, an increasing number of studies
have demonstrated that inflammation is associated with the
tumorigenesis and progression of cancer (6–8),
particularly prostaglandins (PGs). PGs are lipid mediators that are
derived from arachidonic acid via the cyclooxygenase (COX) pathway,
which is an important molecular target in cancer therapy (9–11).
In prostate tissue, PGE2 is the most abundant
proinflammatory mediator and excessive levels of PGE2
have been reported in PCa (12).
PGE2 exerts various effects, including promoting cancer
cell growth, proliferation, invasion and metastasis (13), upregulating antiapoptotic proteins
and regulating the immune system (14,15).
PGE2, which binds to four cognate E prostanoid receptors
(EP1, EP2, EP3 and EP4) (16,17),
stimulates PCa cell proliferation and modulates various kinase
pathways, including those regulated by phosphatidylinositol
3-kinase (PI3K)/Akt and protein kinase A (PKA) (18–22).
Of these receptors, EP4 is the most common prostanoid receptor and
is closely associated with inflammatory diseases and cancer
(23–26). The authors' previous studies
demonstrated that EP4 overexpression was associated with the
progression of PCa to a castration-resistant form, and the use of
an EP4 antagonist, ONO-AE3-208, in vivo inhibited the progression
of PCa to a castration-resistant form via regulation of androgen
receptor activation, which indicated that the expression of the EP4
receptor was positively associated with the metastatic malignant
phenotype of PCa (27–30). Although evidence indicates that
non-steroidal anti-inflammatory drugs that inhibit COX-2 are
effective, they are limited by their well-known side effects, which
include cardiovascular complications (31). Therefore, as a downstream factor of
PGE2 and COX-2, evidence has demonstrated that EP4 may
be closely associated with PCa and may be a potential novel target
for PCa treatment.
Furthermore, metastasis is a complex multistep
process that is regulated by various mechanisms. It is considered
that increases in migration and the potential for invasion are the
most crucial steps in tumor metastasis (32). The activation or overexpression of
matrix metalloproteinases (MMPs), receptor activator of nuclear
factor-κB ligand (RANKL) and runt-related transcription factor 2
(RUNX2) is involved in cell growth and bone metastasis via cyclic
(c)AMP-PKA and PI3K-Akt signaling pathways (21,33–35).
MMPs, which are calcium-dependent zinc-containing endopeptidases
(36), have essential functions in
tissue remodeling during numerous physiological or pathological
processes, which include morphogenesis, angiogenesis, tissue
repair, cirrhosis, arthritis and metastasis (37). Specifically, MMP-2 and MMP-9 are
considered to be implicated in metastasis. Studies have
demonstrated that the abnormal expression of MMPs enhances the
invasion and metastasis of tumor cells via cAMP-PKA and PI3K-Akt
signaling pathways (21,34). In humans, the RANKL protein is the
product of the TNFSF11 gene (38,39).
Certain reports have indicated that RANKL expression allows
favorable microenvironmental conditions for cancer cell migration,
and RANKL is also reported to be an important signal regulator in
cancer-induced bone loss (40,41).
RUNX2 is a transcription factor that is associated with osteoblast
differentiation (42). The
expression level of RUNX2 was reported to be significantly higher
in metastatic PCa and was positively associated with EP4 receptor
expression in PCa (19).
Therefore, the detailed molecular mechanisms of MMP, RANKL and
RUNX2 activation in PCa require further investigation.
Based on the literature and our previous results,
the present study was designed to investigate the hypothesis that
PGE2 may upregulate the protein and mRNA expression
levels of MMPs, RANKL and RUNX2 by binding to the EP4 receptor and
activating cAMP-PKA and PI3K-Akt signaling pathways, thus promoting
the cell proliferation and invasion of PCa cells.
Materials and methods
Materials and reagents
The PC-3 cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA). Roswell Park Memorial
Institute (RPMI)-1640 medium and fetal bovine serum (FBS) were from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
PGE2 and PGE1 alcohol were obtained from
Cayman Chemical Company (Ann Arbor, MI, USA). The EP4 receptor
antagonist ONO-AE3-208 was donated by the Department of Urology,
Kyoto University (Kyoto, Japan). Lipofectamine 2000 was purchased
from Thermo Fisher Scientific, Inc., SQ22536, forskolin, H89 and
LY294002 were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Cell Counting Kit-8 (CCK-8) was obtained from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). The High Pure RNA
isolation kit was from Roche Applied Science (Mannheim, Germany)
and the PrimeScript RT reagent kit was from Takara Biotechnology
Co., Ltd. (Dalian, China). Radioimmunoprecipitation assay (RIPA)
lysis buffer was from Beijing Solarbio Science & Technology
Co., Ltd., (Beijing, China). The Transwell unit (BD-BioCoat
Matrigel Invasion Chambers) were from BD Biosciences (San Jose, CA,
USA). TRIzol reagent was from Invitrogen (Thermo Fisher Scientific,
Inc.).
Cell treatments
To determine the effect on MMP-2, MMP-9, RANKL and
RUNX2 mRNA and protein expression varying doses and concentrations
of the EP4 receptor selective agonist PGE1 were used on
cells. PC-3 cells were treated with PGE1 alcohol (10 µM)
for 0, 0.5, 1, 1.5 and 2 h at 37°C or PGE1 alcohol at 0,
0.1, 0.3, 1, 3 and 10 µM for 2 h at 37°C. Then the effect of
PGE1 or PGE2 and ONO-AE3-208 on protein
expression in cells was investigated. PC-3 cells were treated with
PGE1 alcohol (10 µM) for 0, 0.5, 1, 1.5 and 2 h at 37°C
or PGE1 alcohol at 0, 0.1, 0.3, 1, 3 and 10 µM for 2 h.
PC-3 cells were pretreated for 1 h with the EP4 receptor selective
antagonist ONO-AE3-208 (10 µM) and then stimulated with
PGE2 (10 µM) or PGE1 alcohol (10 µM), and
protein expression of MMP-2, MMP-9, RANKL and RUNX2 were determined
by western blotting after 2 h. The effect of EP4 siRNA on protein
expression was analysed. PC-3 cells were transfected with EP4 siRNA
or negative control siRNA for 72 h at 37°C and then stimulated with
PGE2 (10 µM) or PGE1 alcohol (10 µM) in
serum-free medium for 2 h, and protein expression of MMP-2, MMP-9,
RANKL and RUNX2 were determined by western blotting. To test the
effect of adenyl cyclase activator and inhibitors, PC-3 cells were
treated with forskolin (10 µM), and protein expression of MMP-2,
MMP-9, RANKL and RUNX2 WERE determined aftr 2 h by western
blotting. PC-3 cells were pretreated for 1 h with SQ22536 (200 µM)
and then stimulated with PGE1 alcohol (10 µM), and
protein expression of MMP-2, MMP-9, RANKL and RUNX2 were determined
after 2 h by western blotting. The effect of a PKA inhibitor was
also investigated on the protein expression of MMP-2, MMP-9 RANKL
and RUNX2. PC-3 cells were pretreated for 1 h with the PKA specifc
inhibitor H89 (10 µM) and then stimulated with PGE1 alcohol (10
µM), and protein expression of MMP-2, MMP-9, RANKL and RUNX2 were
determined after 2 h by western blotting.
Cell lines and culture
PC-3 cells were cultured in RMPI-1640 medium
supplemented with 10% fetal bovine serum (FBS) at 37°C in a
humidified air atmosphere containing 5% CO2. The cells
were digested by trypsin once every 3 days. The experiments were
performed when cells reached 80% confluency and were conducted in
serum-free medium.
Small interfering (si)RNA
interference
The siRNA targeting human EP4 receptor (siRNA ID:
s11455) was purchased from Thermo Fisher Scientific, Inc., siRNA
specific to MMP-2, MMP-9, RUNX2 and RANKL were from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The siRNA specific to genes
were as follows: EP4 siRNA sense, 5′-TTCAGTTCCTTCCTCATCCTCGCC-3′
and antisense, 5′-CTGTCTTCCGCAGGAGGATGTATA-3′; MMP-2 siRNA sense,
5′-GUGGCCAACUACAACUUCUTT-3′ and antisense,
5′-AGAAGUUGUAGUUGGCCACTT-3′; MMP-9 siRNA sense,
5′-CUAUGGUCCUCGCCCUGAATT-3′ and antisense,
5′-UUCAGGGCGAGGACCAUAGAG-3′; RUNX2 siRNA sense,
5′-CAAGGACAGAGUCAGAUUAUU-3′ and antisense,
5′-UAAUCUGACUCUGUCCUUGUU-3′; RANKL siRNA sense,
5′-UCCCAUCGGGUUCCCAUAAdTdT-3′ and antisense,
5′-AUUCUGUAGAAGCAGCGCCdTdT-3′; and NC-siRNA sense,
5′-UGGUUUACAUGUUCCAAUAUU-3′ and antisense,
5′-UAUUGGAACAUGUAAACCAUU-3′ PC-3 cells (2×105) were
plated in 6-well plates for 24 h at 37°C, resulting in a 30–50%
confluent cell monolayer. The cells were subsequently transfected
with the 80 µM targeting siRNA or negative control (NC) siRNA
(Shanghai GenePharma Co., Ltd., Shanghai, China) using
Lipofectamine 2000 for 24 h. Following transfection with EP4 or NC
siRNA for 72 h, depletion of target protein was confirmed by
western blotting or reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) analysis, and the cells were subsequently
used for further experiments.
Cell proliferation assay
A CCK-8 assay was performed to assess the effect of
MMP-2, MMP-9, RANKL and RUNX2 siRNA transfection on cell
proliferation. PC-3 cells transfected with 80 nM NC or target siRNA
(5×103) were seeded in 96-well plates. Subsequently,
every 24 for 72 h, a batch of cells were stained with 10 µl CCK-8
regent at 37°C for 2 h. The reaction was quantified with an
automatic plate reader at 450 nm. Each experiment was triplicated
and performed three times independently.
Cell invasion assay
RMPI-1640 medium without FBS (100 µl) containing
3×104 cells were seeded in the upper chamber in the
presence or absence of 10 µM PGE2, and 750 µl RMPI-1640
culture medium with 1% FBS was added to the lower chamber. The cell
invasion assay was conducted following 24 h. The cell invasion
activity of PC-3 cells was assessed using BD BioCoat Matrigel
Invasion Chambers. The cells were washed with PBS and resuspended
in RMPI-1640 medium without FBS at a density of 3×104
cells/ml. Cell suspension (500 µl) was placed onto the upper
chamber coated with Matrigel and 750 µl RMPI-1640 culture medium
with 1% FBS was added to the lower chamber of the Transwell. After
24 h incubation at 37°C in a 5% CO2 incubator, the cells
on the upper surface of the filters were removed by wiping with a
cotton swab. The filters were fixed in 70% ethanol at 37°C and
stained with hematoxylin for 30 min. The stained cells were counted
under a light microscope in six randomly selected fields at
magnification, ×200. At least three chambers from three different
experiments were analyzed.
Western blot analysis
Prior to western blotting, 5×106 PC-3
cells were incubated with PGE2 at 0, 0.1, 0.3, 1, 3 and
10 µM for 2 h at 37°C to investigate protein expression of MMP-2,
MMP-9, RANKL and RUNX2 to varying concentrations of
PGE2. PC-3 cells were also treated with 10 µM
PGE2 for 0, 0.5, 1, 1.5 and 2 h at 37°C to investigate
the effect of different durations of incubation. Total cell lysates
were extracted from the cells using RIPA lysis buffer followed by
repetitive pipetting and lysis on the ice for 30 min. The contents
were collected into centrifuge tubes and centrifuged at at
1.6×104 × g for 30 min at 4°C. Loading buffer was
subsequently added to the supernatant and the samples were treated
at 100°C for 5 min to denature the proteins. Protein concentrations
of lysates were measured using Bio-Rad protein assay dye reagent
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Samples (10 µl)
were loaded into each well for 12% SDS-PAGE and proteins were
transferred to a polyvinylidene difluoride membrane. The membranes
were soaked with 8% non-fat milk for 1 h at room temperature and
incubated with the corresponding primary antibodies overnight at
4°C with gentle agitation. Then, the membranes were washed with
0.1% PBS-Tween-20 (PBST) three times (10 min each time). For
western blotting, the primary antibodies were MMP-2 (cat. no.
40994), MMP-9 (cat. no. 13667), RANKL (cat. no. 5312), RUNX2 (cat.
no. 12556) and GAPDH (cat. no. 5174), all at 1:10,000, the
membranes were washed with PBST three times. Subsequently,
membranes were incubated with secondary antibodies [the peroxidase
conjugated secondary anti-rabbit IgG (cat. no. 8885) or anti-mouse
IgG (cat. no. 8887) antibodies (all from Cell Signaling Technology,
Inc., Danvers, MA, USA), all at 1:5,000] for 2 h at room
temperature. The signals were detected using enhanced
chemiluminescent western blotting substrate (Pierce; Thermo Fisher
Scientific, Inc.) and analyzed using Image Lab 4.0 analysis
software (Bio-Rad Laboratories, Inc.). GAPDH was used as an
internal control.
RT-qPCR
PC-3 cells were incubated at 37°C with
PGE2 at 0, 0.1, 0.3, 1, 3 and 10 µM for 1 h and mRNA
expression of MMP-2, MMP-9, RANKL and RUNX2 was examined. Total RNA
was extracted from the human PCa tissues or cells using
TRIzol™ reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. cDNA was
synthesized from total RNA (1 µg) using a First-Strand cDNA
Synthesis kit (GE Healthcare, Chicago, IL, USA) at 65°C for 5 min
qPCR was performed using SYBR Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and monitored using a
GeneAmp 5700 Sequence Detection System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in triplicate. PCR conditions were:
Pre-incubation at 95°C for 10 min (1 cycle) followed by 40 cycles
of 95°C for 15 sec (30 cycles), 60°C for 30 sec (30 cycles), and
72°C for 30 sec (30 cycles). The mRNA expression was quantified
using the 2−ΔΔCq method (43), and expressed as the relative
quantity of target mRNA normalized to the GAPDH, respectively.
Relative expression was calculated using the comparative cross
threshold (Cq) method. The following primer pairs were employed in
the present study: MMP-2, 5′-AGACATACATCTTTGCTGGAGACA-3′ (forward)
and 5′-CTTGAAGAAGTAGCTGTGACCG-3′ (reverse); MMP-9,
5′-TTTGAGTCCGGTGGACGATG-3′ (forward) and 5′-TTGTCGGCGATAGGAAGGG-3′
(reverse); RANKL, 5′-AATAGAATATCAGAAGATGGCACTC-3′ (forward) and
5′-TAAGGAGGGGTTGGAGACCTCG-3′ (reverse); RUNX2,
5′-GTTTGTTCTCTGACCGCC-3′ (forward) and 5′-CCAGTTCTGAAGCACCTGA-3′
(reverse); EP4, 5′-CATCTTACTCATTGCCACCT-3′ (forward) and
5′-TACTGAGCACTGTCTTTCTC-3′ (reverse); and GAPDH,
5′-TTCCAGGAGCGAGATCCCT-3′ (forward) and 5′-CACCCATGACGAACATGGG-3′
(reverse). The qPCR reactions were performed in triplicate and
included no-template controls.
Statistical analysis
GraphPad Prism 5.0 statistical software (GraphPad
Software, Inc., La Jolla, CA, USA) was used to analyze experimental
data. Data are presented as the mean ± standard deviation. One-way
analysis of variance (ANOVA) test with post hoc tests performed
using Student-Newman-Keuls test. ANOVA or paired t-tests were
performed to analyze the intergroup differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
PGE2 induces the protein
and mRNA expression of MMP-2, MMP-9, RUNX2 and RANKL in PCa
cells
PC-3 cells were treated with various doses of
PGE2 for different durations in order to determine the
direct effect of PGE2 on the expression levels of MMP-2,
MMP-9, RUNX2 and RANKL in PCa cells. Subsequently, the protein and
mRNA expression levels of MMP-2, MMP-9, RUNX2 and RANKL were
analyzed by western blotting and RT-qPCR, respectively. The results
of western blotting demonstrated that the protein expression levels
were increased with increasing durations of PGE2
treatment (0–2 h; Fig. 1A). In
addition, the protein expression of all four proteins was
upregulated following treatment with 0.1–10 µM PGE2,
compared with the 0 µM group (Fig.
1B). Furthermore, the mRNA expression levels of MMP-2, MMP-9,
RUNX2 and RANKL were increased by PGE2 treatment, with
expression peaking at a 1 h duration of treatment with
PGE2 (Fig. 1C).
Furthermore, the mRNA levels were increased following treatment
with 0.1–10 µM PGE2 in the PC-3 cells, compared with the
0 µM group (Fig. 1D). These
results indicate that PGE2 upregulated the expression of
MMP-2, MMP-9, RUNX2 and RANKL at the protein and mRNA level in PC-3
cells.
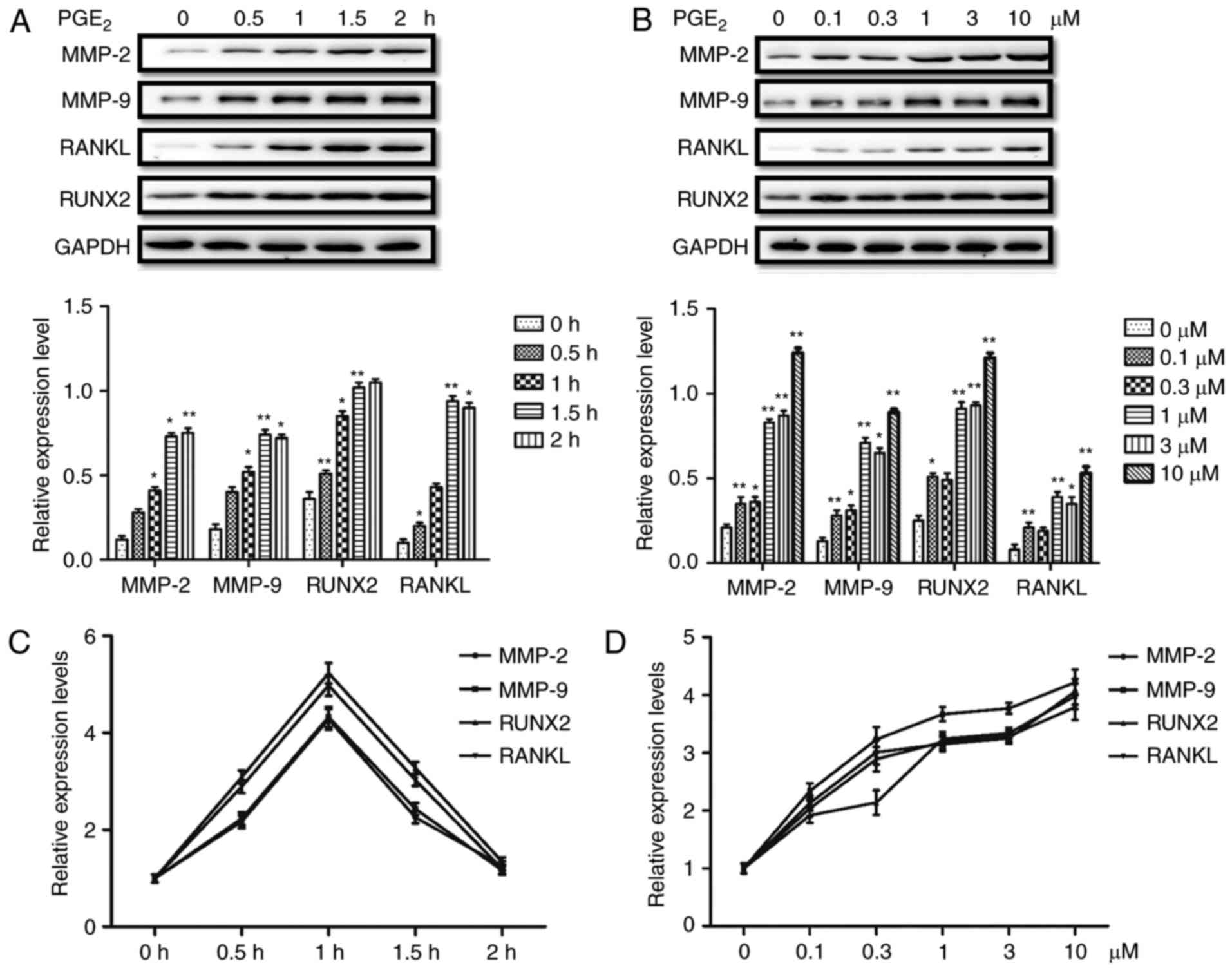 | Figure 1.PGE2 upregulates the
protein and mRNA expression levels of MMP-2, MMP-9, RANKL and RUNX2
in PC-3 prostate cancer cells. (A) Time course of the effects of
PGE2 on the protein expression levels of MMP-2, MMP-9, RANKL and
RUNX2 in PC-3 cells. (B) Effect of various PGE2
concentrations on the protein expression levels of MMP-2, MMP-9,
RANKL and RUNX2 in PC-3 cells. (C) Time course of the effects of
PGE2 on the mRNA expression levels of MMP-2, MMP-9,
RANKL and RUNX2 in PC-3 cells. (D) Effect of various PGE2
concentrations on the mRNA expression levels of MMP-2, MMP-9, RANKL
and RUNX2 in PC-3 cells. Results are presented as the mean ±
standard deviation of three independent experiments. *P<0.05 and
**P<0.01 vs. 0 h or 0 µM group. PGE2, prostaglandin E2; MMP,
matrix metalloproteinase; RANKL, receptor activator of nuclear
factor-κB ligand; RUNX2, runt-related transcription factor 2. |
Knockdown of MMP-2, MMP-9, RUNX2 and
RANKL inhibits the proliferation and invasion of PCa cells
Previous studies have demonstrated that
PGE2 accelerates the proliferation and invasion of PCa
cells (44,45), however, the underlying mechanism
remains unclear. The results of the present study demonstrated that
PGE2 directly upregulated the protein and mRNA
expression levels of MMP-2, MMP-9, RUNX2 and RANKL, which are key
transcription factors associated with cell proliferation and the
bone metastasis of PCa (33–42).
Therefore, further investigation of the role of MMP-2, MMP-9, RUNX2
and RANKL upregulation in PGE2-induced PC-3 cell
proliferation and invasion is required. As demonstrated in Fig. 2A, transfection of PC-3 cells with
MMP-2, MMP-9, RUNX2 and RANKL siRNAs significantly blocked
PGE2-induced proliferation. Furthermore, the results in
Fig. 2B demonstrate that MMP-2,
MMP-9, RUNX2 and RANKL siRNAs inhibited PGE2-induced
invasion in PC-3 cells. Western blot analysis confirmed that the
protein expression levels of MMP-2, MMP-9, RUNX2 and RANKL were
reduced by siRNA transfection in PC-3 cells (Fig. 2C). These results indicate that
MMP-2, MMP-9, RUNX2 and RANKL may have an important role in the
proliferation and invasion of PCa cells.
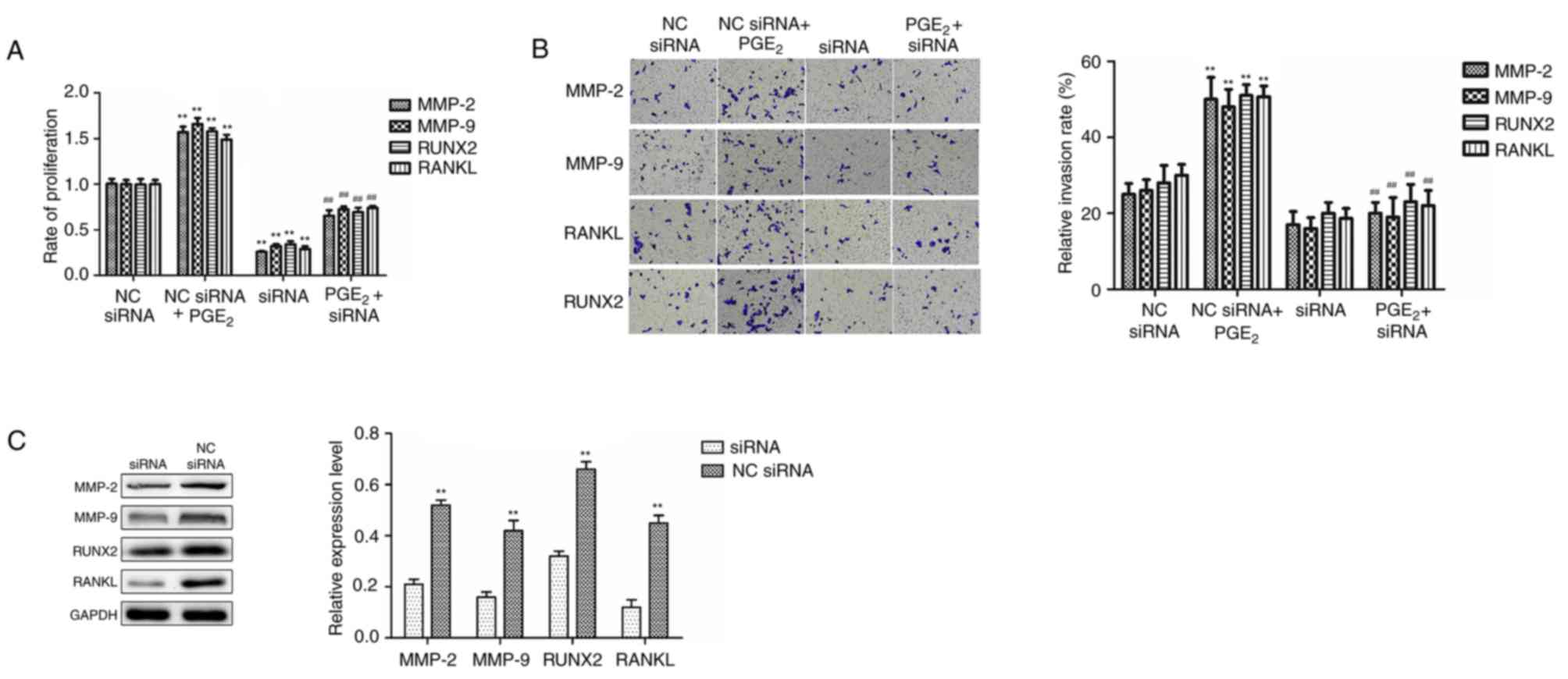 | Figure 2.Knockdown of MMP-2, MMP-9, RANKL and
RUNX2 using siRNA reduces PGE2-induced PC-3 PCa cell
proliferation and invasion. (A) Knockdown of MMP-2, MMP-9, RANKL
and RUNX2 using siRNA reduced PGE2-induced increases in
PCa cell proliferation. (B) Knockdown of MMP-2, MMP-9, RANKL and
RUNX2 using siRNA blocked PGE2-induced increases in PCa
cell invasion. The cell invasion assay was conducted after 24 h and
magnification was ×200. (C) The efficiency of MMP-2, MMP-9, RANKL
and RUNX2 siRNA transfection in PC-3 cells was confirmed by western
blotting. Results are presented as the mean ± standard deviation of
three independent experiments. **P<0.01 vs. NC siRNA group;
##P<0.01 vs. NC siRNA + PGE2 group. MMP,
matrix metalloproteinase; RANKL, receptor activator of nuclear
factor-κB ligand; RUNX2, runt-related transcription factor 2;
siRNA, small interfering RNA; PGE2, prostaglandin
E2; PCa, prostate cancer; N.C., negative control. |
The EP4 receptor is involved in the
PGE2-induced protein expression of MMP-2, MMP-9, RUNX2
and RANKL in PCa cells
Overexpression of the EP4 receptor has been reported
in various types of cancer, and increased EP4 receptor signaling
has been previously associated with the migration, invasion and
bone metastasis of various tumor types (19,24,46).
The authors' previous studies demonstrated that an EP4 receptor
antagonist significantly inhibited the proliferation, invasion and
metastatic abilities in PCa cells (27,28).
Therefore, the present study investigated whether the EP4 receptor
may participate in the PGE2-induced protein expression
of MMP-2, MMP-9, RUNX2 and RANKL in PC-3 cells. In PC-3 cells that
were treated with PGE1 alcohol, an EP4 receptor
selective agonist, the protein expression levels of MMP-2, MMP-9,
RUNX2 and RANKL were increased with increases in treatment
concentration and duration (Fig. 3A
and B). Furthermore, the mRNA expression levels were also
upregulated by PGE1 alcohol at different concentrations
and durations of treatment in PC-3 cells (Fig. 3C and D). These results indicated
that PGE1 alcohol upregulated the protein and mRNA
expression levels of MMP-2, MMP-9, RUNX2 and RANKL in PCa cells,
which was similar to the effects observed following treatment of
PC-3 cells with PGE2. Therefore, the EP4 receptor may
have an important role in the regulation of MMP-2, MMP-9, RUNX2 and
RANKL expression levels in PC-3 cells.
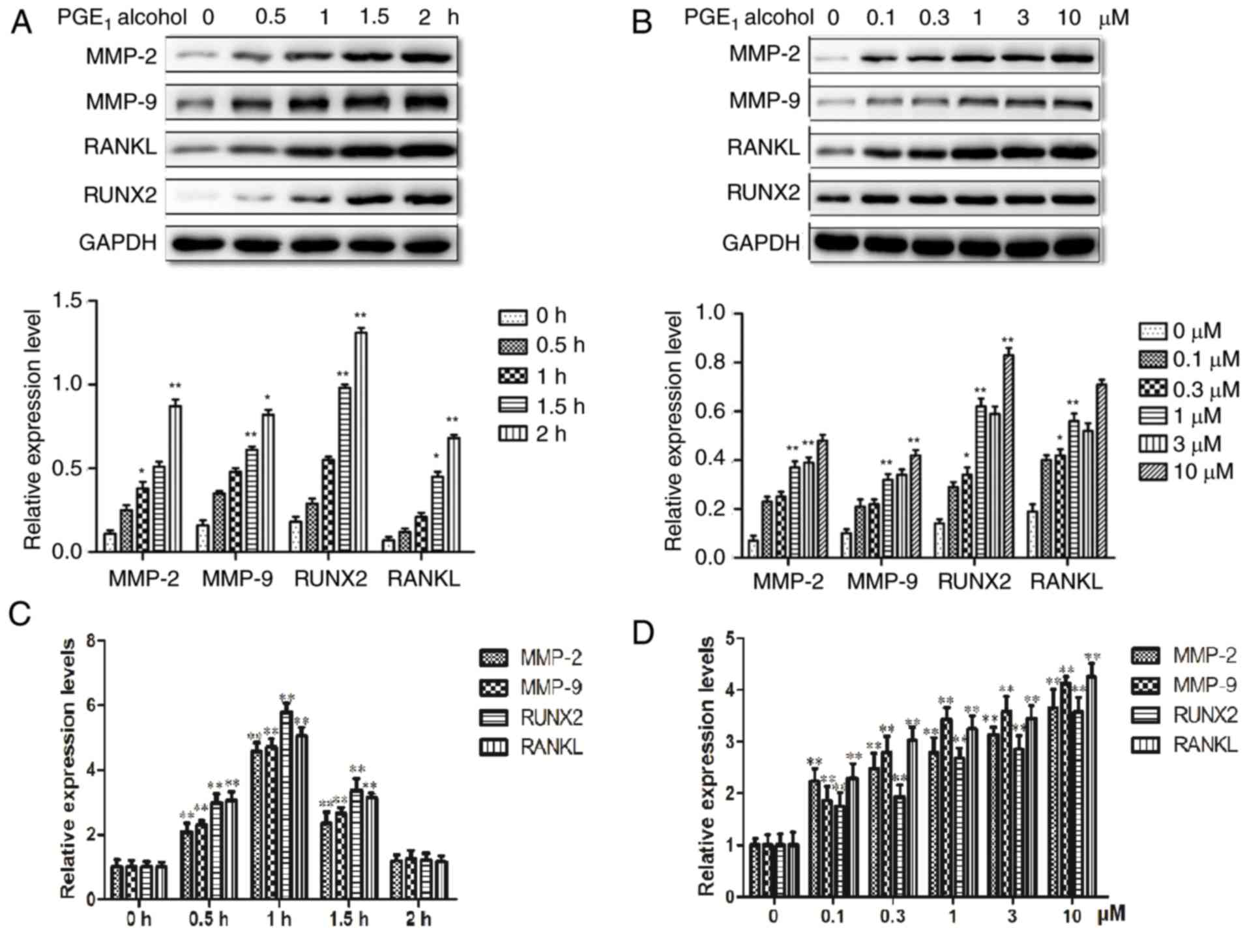 | Figure 3.The EP4 receptor selective agonist,
PGE1 alcohol, mimics the upregulatory effect of
PGE2 on the expression levels of MMP-2, MMP-9, RANKL and
RUNX2 in PC-3 prostate cancer cells. (A) Time course of the effects
of PGE1 alcohol on the protein expression levels of
MMP-2, MMP-9, RANKL and RUNX2 in PC-3 cells. (B) Effect of various
concentrations of PGE1 alcohol on the protein expression
levels of MMP-2, MMP-9, RANKL and RUNX2 in PC-3 cells. (C) Time
course of the effects of PGE1 alcohol on the mRNA expression levels
of MMP-2, MMP-9, RANKL and RUNX2 in PC-3 cells. (D) Effect of
various concentrations of PGE1 alcohol on the mRNA expression
levels of MMP-2, MMP-9, RANKL and RUNX2 in PC-3 cells. Results are
presented as the mean ± standard deviation of three independent
experiments. *P<0.05 and **P<0.01 vs. 0 h or 0 µM group. PGE,
prostaglandin E; EP4, PGE2 receptor EP4; MMP, matrix
metalloproteinase; RANKL, receptor activator of nuclear factor-κB
ligand; RUNX2, runt-related transcription factor 2. |
In order to further confirm the effects of the EP4
receptor on PGE2-induced protein and mRNA expression,
the PC-3 cells were pretreated with an EP4 receptor selective
antagonist or EP4 receptor siRNA. As demonstrated in Fig. 4A, pretreatment of PC-3 cells with
ONO-AE3-208, the EP4 receptor selective antagonist, markedly
reduced PGE2- and PGE1 alcohol-induced
protein expression levels of MMP-2, MMP-9, RUNX2 and RANKL.
Furthermore, transfection of siRNA targeting the EP4 receptor also
significantly blocked PGE2- and PGE1
alcohol-induced upregulation of the protein expression levels in
PC-3 cells (Fig. 4B). RT-qPCR was
performed to determine the efficiency of transfection with EP4
receptor siRNA, and the results demonstrated that EP4 siRNA
significantly lowered the protein expression of the EP4 receptor in
PC-3 cells, compared with cells transfected with NC siRNA (Fig. 4C). These results indicate that the
EP4 receptor may be involved in the regulation of the protein
expression levels of MMP-2, MMP-9, RUNX2 and RANKL in PCa
cells.
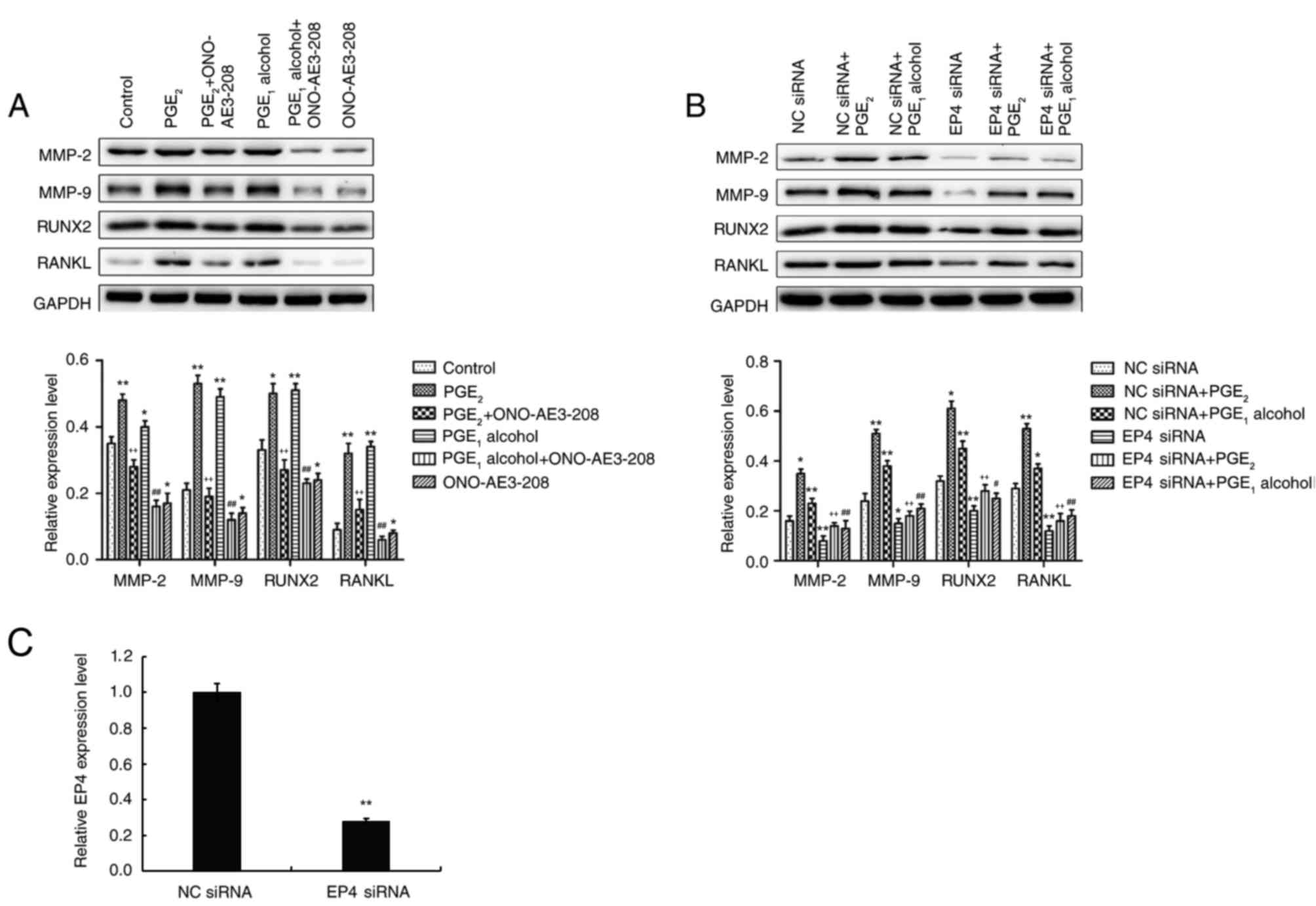 | Figure 4.The EP4 receptor antagonist,
ONO-AE3-208, or siRNA interference attenuates
PGE2-induced MMP-2, MMP-9, RANKL and RUNX2 expression in
PC-3 prostate cancer cells. (A) Effect of the EP4 receptor
selective antagonist, ONO-AE3-208, on PGE2- and
PGE1 alcohol-induced increases in the protein expression
levels of MMP-2, MMP-9, RANKL and RUNX2 in PC-3 cells. (B) Effect
of EP4 receptor siRNA on PGE2- and PGE1
alcohol-induced increases in the protein expression of MMP-2,
MMP-9, RANKL and RUNX2 in PC-3 cells. (C) RNAi effciency of EP4
siRNA in PC-3 cells. Following transfection of PC-3 cells with EP4
or NC siRNA for 72 h, the transfection efficiency was confirmed
using reverse transcription-quantitative polymerase chain reaction
to detect mRNA levels of EP4. Results are presented as the mean ±
standard deviation of three independent experiments. For parts A
and B: *P<0.05 and **P<0.01 vs. control group;
++P<0.01 vs. PGE2 group;
#P<0.05 and ##P<0.01 vs.
PGE1 alcohol group. For part C: **P<0.05 vs. NC siRNA
group. PGE, prostaglandin E; EP4, PGE2 receptor EP4;
siRNA, small interfering RNA; MMP, matrix metalloproteinase; RANKL,
receptor activator of nuclear factor-κB ligand; RUNX2, runt-related
transcription factor 2; NC, negative control. |
The cAMP-PKA signaling pathway is
involved in the upregulation of the protein expression levels of
MMP-2, MMP-9, RUNX2 and RANKL in PCa cells
As a G-protein-coupled receptor, the EP4 receptor
normally couples with Gsα protein to activate adenylate
cyclase (AC) and elevate intracellular cAMP levels, which has been
associated with the occurrence and development of tumors (16,17).
To confirm the role of the cAMP-PKA downstream signaling pathway in
the EP4 receptor-mediated upregulation of MMP-2, MMP-9, RUNX2 and
RANKL expression, an activator (forskolin) and inhibitor (SQ22536)
of AC were employed to treat the PC-3 cells. Following treatment,
the effects on the protein expression levels of MMP-2, MMP-9, RUNX2
and RANKL in PC-3 cells were determined by western blotting. The
results demonstrated that the protein expression levels in PC-3
cells pretreated with forskolin were significantly upregulated
(Fig. 5A), while levels were
markedly reduced when pretreated with SQ22536 (Fig. 5B), compared with the respective
control groups. In addition, increased cAMP levels lead PKA
activation, a key signaling protein, which can regulate gene
expression (47). To investigate
the involvement of PKA activation in EP4 receptor-mediated
upregulation of protein expression in PCa cells, PC-3 cells were
pretreated with the PKA specific inhibitor, H89. As demonstrated in
Fig. 5C, the PGE1
alcohol-induced upregulation of protein expression levels in PC-3
cells pretreated with H89 was significantly reduced. These results
indicated that PKA may have an important role in EP4
receptor-mediated protein upregulation in PC-3 cells. Therefore,
the cAMP-PKA signaling pathway may be involved in the upregulation
of the protein expression levels of MMP-2, MMP-9, RUNX2 and RANKL
in PCa cells.
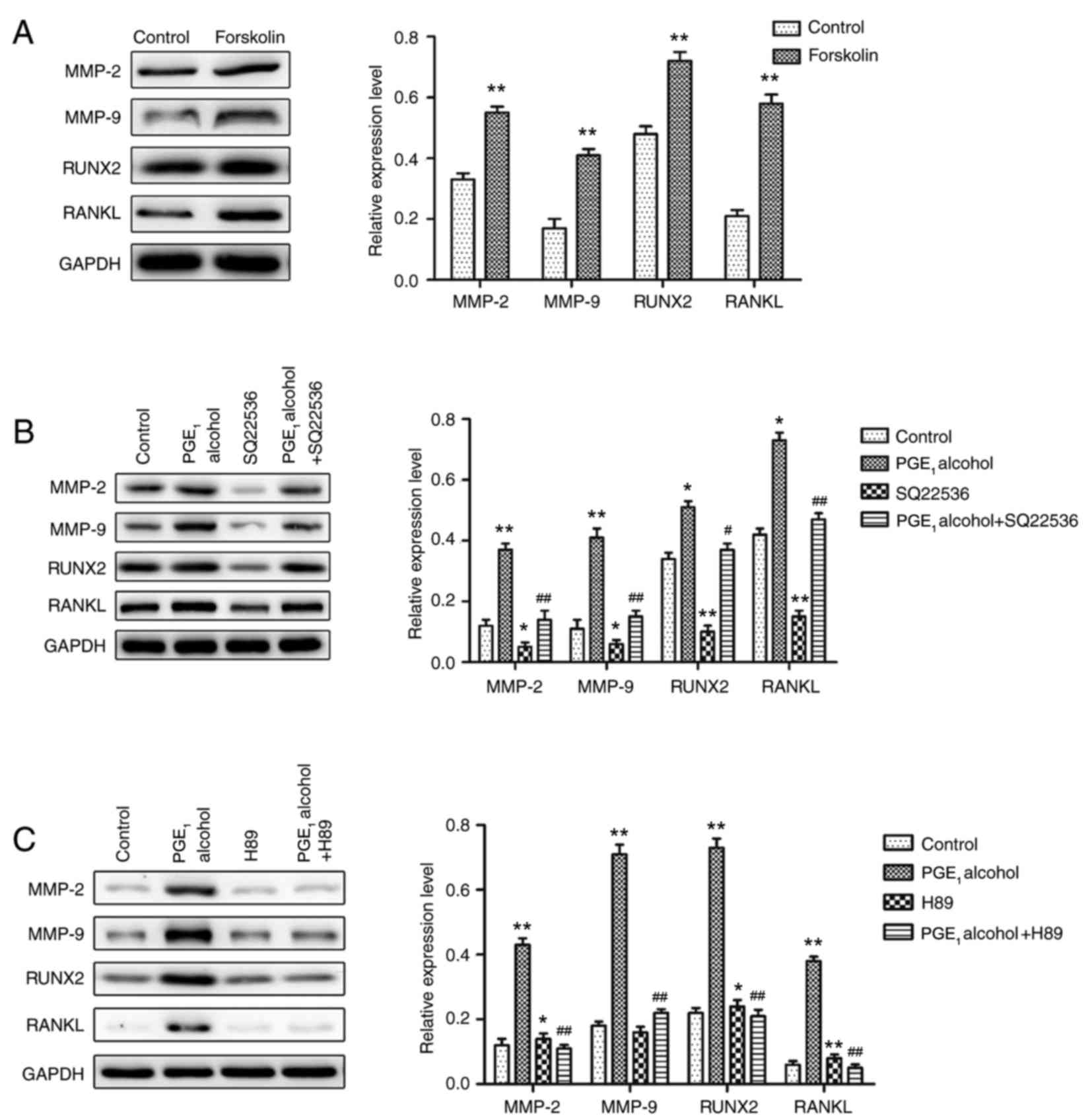 | Figure 5.Involvement of the cAMP-PKA signaling
pathway in EP4 receptor-mediated increases in the protein
expression levels of MMP-2, MMP-9, RANKL and RUNX2 in PC-3 prostate
cancer cells. (A) The specific AC activator, forskolin, induced
increases in the protein expression levels of MMP-2, MMP-9, RANKL
and RUNX2 in PC-3 cells. (B) The specific AC inhibitor, SQ22536,
reduced EP4 receptor-mediated increases in the protein expression
levels of MMP-2, MMP-9, RANKL and RUNX2 in PC-3 cells. (C) The
specific PKA inhibitor, H89, reduced EP4 receptor-mediated
increases in the protein expression levels of MMP-2, MMP-9, RANKL
and RUNX2 in PC-3 cells. Results are presented as the mean ±
standard deviation of three independent experiments. *P<0.05 and
**P<0.01 vs. control group; #P<0.05 and
##P<0.01 vs. PGE1 alcohol group. cAMP,
cyclic AMP; PKA, protein kinase A; PGE1, prostaglandin
E1; EP4, PGE2 receptor EP4; MMP, matrix
metalloproteinase; RANKL, receptor activator of nuclear factor-κB
ligand; RUNX2, runt-related transcription factor 2; AC, adenylate
cyclase. |
The PI3K-Akt signaling pathway is
involved in the upregulation of the protein expression levels of
MMP-2, MMP-9, RUNX2 and RANKL in PCa cells
As discussed in the previous paragraph, the results
of the current study demonstrated that the cAMP-PKA signaling
pathway may be involved in the upregulation of the protein
expression levels of MMP-2, MMP-9, RUNX2 and RANKL in PCa cells.
Furthermore, it has been previously reported that the EP4 receptor
activates the Akt signaling pathway to regulate cell proliferation
and gene expression (45,46). To determine the involvement of the
Akt signaling pathway in the upregulation of the protein expression
levels of MMP-2, MMP-9, RUNX2 and RANKL in PCa cells, PC-3 cells
were pretreated with a specific inhibitor PI3K, LY294002, and the
effects on the protein expression levels in PC-3 cells were
determined by western blotting. As demonstrated in Fig. 6, treatment of PC-3 cells with
LY294002, the specific inhibitor of PI3K, resulted in a
dose-dependent decrease in protein expression levels in
PGE1 alcohol-treated cells. Based on these results, the
PI3K-Akt signaling pathway may be involved in the upregulation of
MMP-2, MMP-9, RUNX2 and RANKL protein expression in PCa cells.
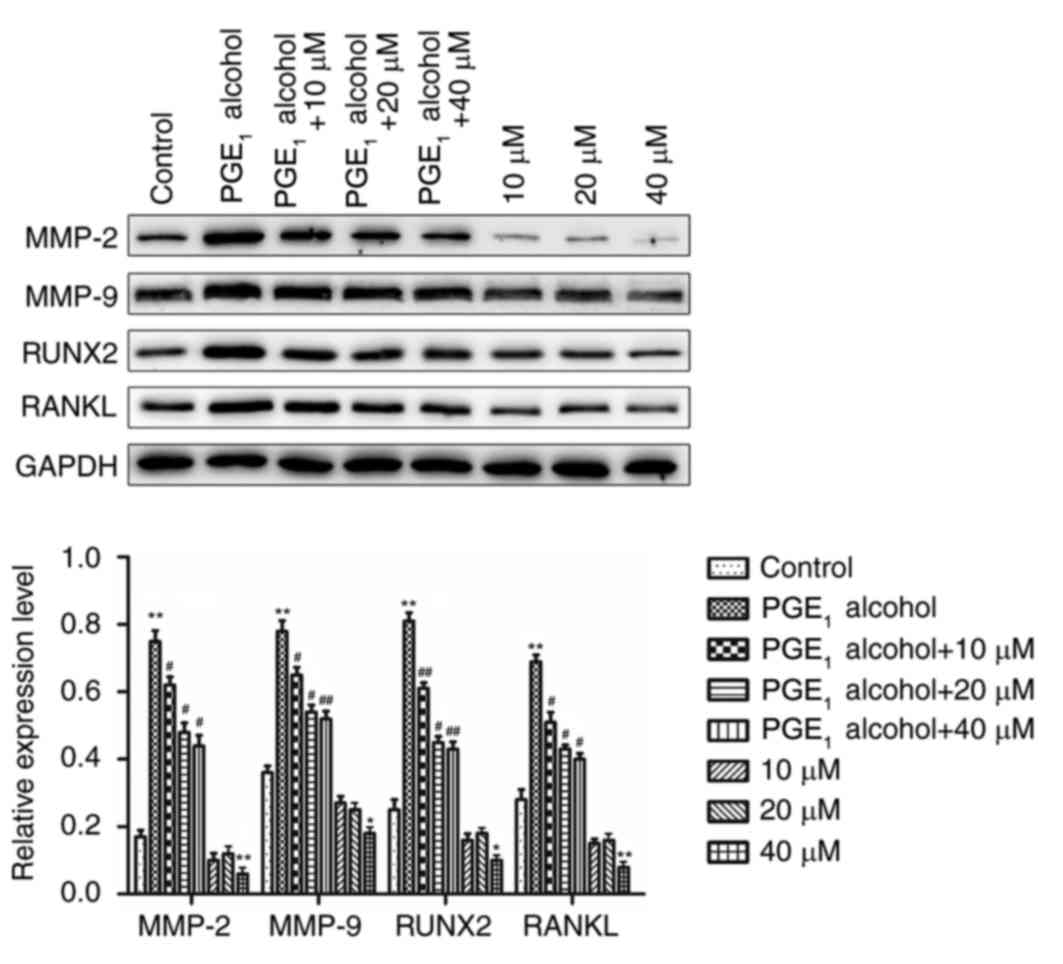 | Figure 6.Involvement of the PI3K-Akt signaling
pathway in EP4 receptor-mediated increases in the protein
expression levels of MMP-2, MMP-9, RANKL and RUNX2 in PC-3 prostate
cancer cells. The specific PI3K inhibitor, LY294002, reduced the
EP4 receptor-mediated increases in the protein expression levels of
MMP-2, MMP-9, RANKL and RUNX2 in PC-3 cells. Results are presented
as the mean ± standard deviation of three independent experiments.
*P<0.05 and **P<0.01 vs. control group; #P<0.05
and ##P<0.01 vs. PGE1 alcohol group. PI3K,
phosphatidylinositol 3-kinase; PGE1, prostaglandin E1; EP4, PGE2
receptor EP4; MMP, matrix metalloproteinase; RANKL, receptor
activator of nuclear factor-κB ligand; RUNX2, runt-related
transcription factor 2; 10 µM, 10 µM LY294002; 20 µM, 20 µM
LY294002; 40 µM, 40 µM LY294002. |
Based on the results presented in the current study,
we hypothesize that EP4 receptor expression increases the secretion
of PGE2 and the protein and mRNA expression of MMP-2,
MMP-9, RANKL and RUNX2 in PCa cells. PGE2, in turn, acting through
the EP4 receptor, activates cAMP-PKA and PI3K-Akt pathways, which
is required for the effects of PGE2 on the cell
migration and invasion of PCa cells. A summary of this potential
process is presented in Fig. 7.
Therefore, targeting of the downstream signaling components
regulated by PGE2 may be a potential therapeutic
approach for overcoming severe side effects and health risks
associated with the use of COX-2 inhibitors in patients with
PCa.
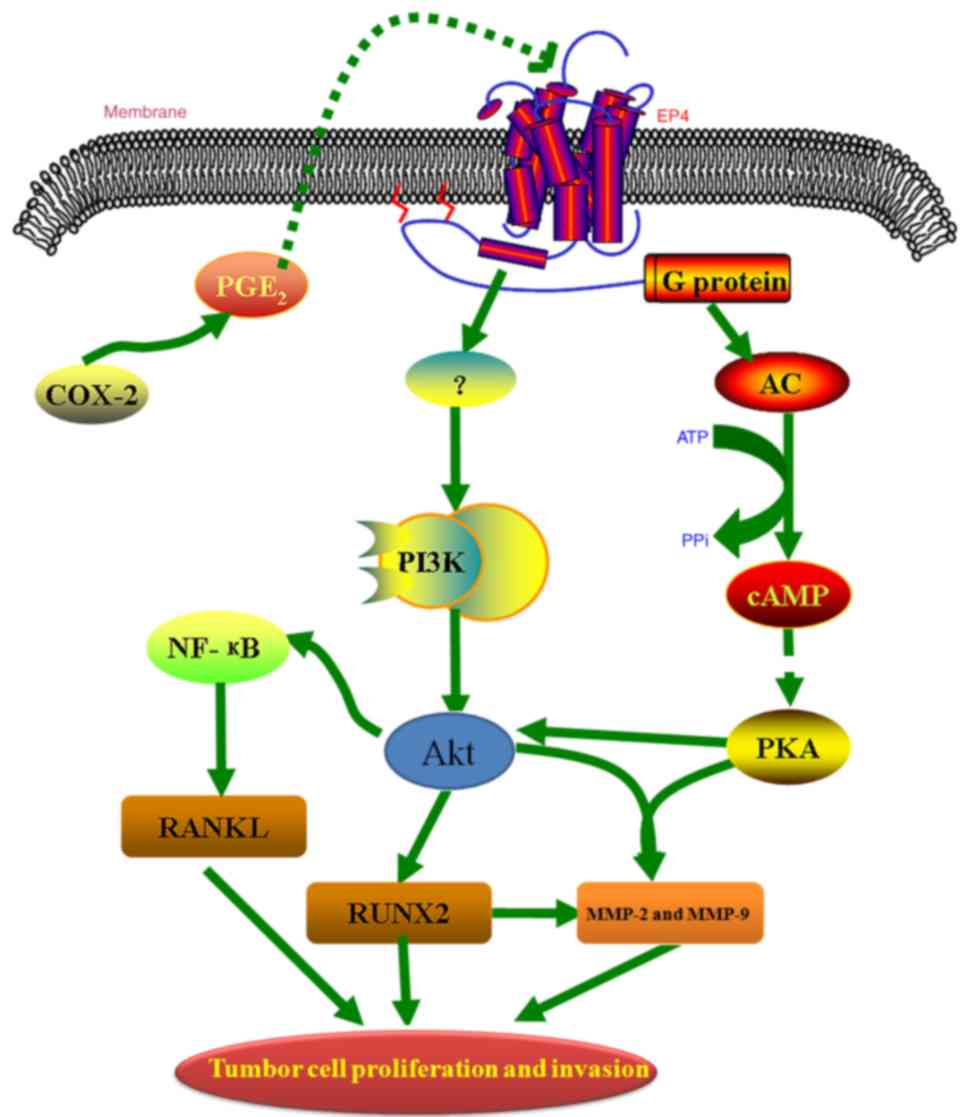 | Figure 7.A proposed model indicating that PGE2
may promote the proliferation and invasion of prostate cancer cells
by upregulating the expression of MMP-2, MMP-9, RANKL and RUNX2 via
the EP4 receptor and the cAMP-PKA/PI3K-Akt signaling pathways.
PGE2, prostaglandin E2; MMP, matrix
metalloproteinase; RANKL, receptor activator of nuclear factor-κB
ligand; RUNX2, runt-related transcription factor 2; EP4,
PGE2 receptor EP4; cAMP, cyclic AMP; PKA, protein kinase
A; PI3K, phosphatidylinositol 3-kinase; COX, cyclooxygenase; AC,
adenylate cyclase; NF-κB, nuclear factor-κB; PPi, inorganic
pyrophosphate. |
Discussion
Malignant tumors are a serious threat to human
health. Clinically, PCa is one of the most common types of
malignant tumor, which have become a major health problem globally.
However, at present, the detailed molecular mechanisms underlying
PCa remain unclear. Previous studies have demonstrated that
PGE2 is associated with proliferation, invasion and
metastasis in PCa cells. Although certain signaling pathways have
been identified, including transactivation of epidermal growth
factor receptor (EGFR) (47) and
phosphorylation of focal adhesion kinase (48), the detailed molecular mechanisms of
PGE2 in PCa require further investigation.
Numerous studies have demonstrated that
PGE2 is closely associated with various human diseases,
including malignant tumors (42,49,50).
PGE2 has been reported to significantly enhance cancer
cell proliferation, adhesion, invasion, metastasis and
angiogenesis, and has therefore been implicated in the
tumorigenesis and progression of various cancer types, including
prostate (51), breast (52) and liver cancer (53). At the cellular level,
PGE2, as a bioactive lipid, exerts various functions and
activates the signal transduction pathway by binding to specific
receptors on the cell surface membrane, which occurs in a paracrine
or autocrine manner. It has been confirmed that there are four
types of PGE2 receptor (EP1, EP2, EP3 and EP4), which
are encoded by different genes. Various studies have reported that
the EP4 receptor has an important role in various
PGE2-induced cancers, and the EP4 receptor is usually
coupled with Gsα protein to activate AC and elevate intracellular
cAMP levels (54,55).
Our previous studies demonstrated that the EP4
receptor and its antagonist significantly dominate cell invasion,
migration and bone metastasis in PCa, which provided a research
direction for the investigation of the underlying mechanisms. The
aim of the current study was to investigate the
PGE2-EP4-PKA/PI3K-Akt signaling pathways and the key
factors associated with the abnormal proliferation and metastasis
of osteoblasts. Numerous studies have reported that MMPs, RANKL and
RUNX2 may be involved in bone metastasis which is mediated via the
cAMP-PKA and PI3K-Akt signaling pathways, and have been reported to
be activated or overexpressed in various cancer types, including
PCa (56–59). MMPs exhibit key roles in tissue
remodeling, which is observed in various physiological and
pathological processes, including morphogenesis, angiogenesis,
tissue repair, cirrhosis, arthritis and metastasis (33). MMP-2 and MMP-9 are considered to be
associated with metastasis. RANKL is a protein that is encoded by
the TNFSF11 gene in humans. Certain reports have indicated RANKL
expression allows optimal microenvironmental conditions to be
established to influence cancer cell migration, and RANKL is
reported to be an important signal regulator in cancer-induced bone
loss (37,38). RUNX2 is a key transcription factor
that is associated with osteoblast differentiation. The expression
level of RUNX2 was reported to be significantly higher in
metastatic PCa and was positively correlated with EP4 receptor
expression in PCa (39).
Therefore, it is important to investigate the detailed molecular
mechanisms of MMPs, RANKL and RUNX2 activation in PCa. In the
present study, the results demonstrated that PGE2
induced the protein and mRNA expression of MMP-2, MMP-9, RANKL and
RUNX2, which was associated with increased proliferation and
invasion in PCa cells and occurred primarily through the EP4
receptor.
Within the signaling pathway, the EP4 receptor
normally couples with Gsα protein to activate AC and
elevate intracellular cAMP levels in cells. Subsequently, the
elevated cAMP levels lead to the activation of three major targets,
including PKA, exchange protein directly activated by cAMP and
cyclic nucleotide-gated ion channels (60). Of these targets, PKA has been
reported to regulate various cellular processes, including
metabolism, signal transduction and gene expression (61). The present study demonstrated that
the cAMP-PKA signaling pathway may also be implicated in the EP4
receptor-mediated upregulation of MMP-2, MMP-9, RANKL and RUNX2
protein expression in PCa cells, as determined using an agonist and
inhibitor of AC, and a specific inhibitor of PKA.
The PI3K-Akt pathway is associated with migration
and survival of cells. In addition to PKA signaling pathway, the
EP4 receptor also exerts effects through activation of Akt. Akt,
also termed protein kinase B, has been demonstrated to be involved
in the regulation of cell survival, proliferation and protein
synthesis, and associated with tumor development (55,62).
It has also been reported that the EP4 receptor may also function
by coupling with non-G-proteins, such as activation of EGFR on the
membrane and the intracellular Akt pathway. For example, in
intrahepatic cholangiocarcinoma, the EP1 receptor led to the
activation of Akt by binding to the Src kinase and EGFR. In a
cutaneous tag, the EP2 receptor can activate the Akt pathway
through β-arrestin 1-Src to signal transduction. Akt is inhibited
by the specific inhibition of PI3K, for example, by using LY294002.
Therefore, we hypothesized that the PI3K-Akt pathway may be
involved in the EP4 receptor-mediated upregulation of protein
expression in PCa cells. The results in present study confirmed the
hypothesis that the pretreatment of PCa cells with the specific
inhibitor of PI3K significantly inhibited the EP4 receptor-mediated
upregulation of protein expression.
The results of the current study confirmed that the
EP4 receptor activated the cAMP-PKA/PI3K-Akt signaling pathway in
PCa cells. Furthermore, it was demonstrated that this pathway may
be involved in the EP4 receptor-mediated upregulation of MMP-2,
MMP-9, RANKL and RUNX2 protein expression in PCa cells. In summary,
the present study demonstrated that PGE2 directly
upregulated the expression of MMP-2, MMP-9, RANKL and RUNX2,
potentially via the EP4 receptor and cAMP-PKA/PI3K-Akt signaling
pathways, and thus promoted the proliferation and invasion of PC-3
cells. Therefore, targeting of the EP4 receptor may have potential
as a novel therapeutic strategy to prevent and treat PCa; targeting
of the downstream signaling components regulated by PGE2
may be an improved therapeutic approach for overcoming severe side
effects and health risks associated with the use of COX-2
inhibitors in patients with PCa.
Acknowledgements
The present study was supported by the China
Postdoctoral Science Foundation (grant no. 2016M602981) and China
Jiangsu Planned Projects for Postdoctoral Research Funds (grant no.
1601160B). The abstract was presented at the 2017 Great Wall
International Translational Andrology and Urology Forum and Huaxia
Medical Forum-Genitourinary, Aug 11–13, 2017 in Shenyang, China,
and was published as abstract no. AB063 in Translational Andrology
and Urology 6 (Suppl 3): August 2017.
References
|
1
|
Bashir MN: Epidemiology of prostate
cancer. Asian Pac J Cancer Prev. 16:5137–5141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carroll PR: Early stage prostate cancer-do
we have a problem with over-detection, overtreatment or both? J
Urol. 173:1061–1062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistic, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berasain C, Castillo J, Perugorria MJ,
Latasa MU, Prieto J and Avila MA: Inflammation and liver cancer:
New molecular links. Ann N Y Acad Sci. 1155:206–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakagawa H and Maeda S: Inflammation- and
stress-related signaling pathways in hepatocarcinogenesis. World J
Gastroenterol. 18:4071–4081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramakrishna G, Rastogi A, Trehanpati N,
Sen B, Khosla R and Sarin SK: From cirrhosis to hepatocellular
carcinoma: New molecular insights on inflammation and cellular
senescence. Liver cancer. 2:367–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ono K, Akatsu T, Murakami T, Kitamura R,
Yamamoto M, Shinomiya N, Rokutanda M, Sasaki T, Amizuka N, Ozawa H,
et al: Involvement of cyclo-oxygenase-2 in osteoclast formation and
bone destruction in bone metastasis of mammary carcinoma cell
lines. J Bone Miner Res. 17:774–781. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rizzo MT: Cyclooxygenase-2 in oncogenesis.
Clin Chim Acta. 412:671–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hawk ET, Viner JL, Dannenberg A and DuBois
RN: COX-2 in cancer-a player that's defining the rules. J Natl
Cancer Inst. 94:545–546. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee KS, Lee HJ, Ahn KS and Kim SH, Nam D,
Kim DK, Choi DY, Ahn KS, Lu J and Kim SH:
Cyclooxygenase-2/prostaglandin E2 pathway mediates icariside II
induced apoptosis in human PC-3 prostate cancer cells. Cancer Lett.
280:93–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi T, Uehara H, Bando Y and Izumi
K: Soluble EP2 neutralizes prostaglandin E2-induced cell signaling
and inhibits osteolytic tumor growth. Mol Cancer Ther. 7:2807–2816.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Richie-Jannetta R, Nirodi CS, Crews BC,
Woodward DF, Wang JW, Duff PT and Marnett LJ: Structural
determinants for calcium mobilization by prostaglandin E2 and
prostaglandin F2alpha glyceryl esters in RAW 264.7 cells and H1819
cells. Prostaglandins Other Lipid Mediat. 92:19–24. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murakami M and Kudo I: Recent advances in
molecular biology and physiology of the prostaglandin
E2-biosynthetic pathway. Prog Lipid Res. 43:3–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hata AN and Breyer RM: Pharmacology and
signaling of prostaglandin receptors: Multiple roles in
inflammation and immune modulation. Pharmacol Ther. 103:147–166.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Negishi M, Sugimoto Y and Ichikawa A:
Prostaglandin E receptors. J Lipid Mediat Cell Signal. 12:379–391.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
George RJ, Sturmoski MA, Anant S and
Houchen CW: EP4 mediates PGE2 dependent cell survival through the
PI3 kinase/AKT pathway. Prostaglandins Other Lipid Mediat.
83:112–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang HF, Shu P, Murphy TF, Aisner S,
Fitzhugh VA and Jordan ML: Significance of divergent expression of
prostaglandin EP4 and EP3 receptors in human prostate cancer. Mol
Cancer Res. 11:427–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Madrigal-Martinez A, Cazana FJ and
Fernandez-Martinez yA: Role of intracellular prostaglandin E2 in
cancer-related phenotypes in PC3 cells. Int J Biochem Cell Biol.
59:52–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yen JH, Kocieda VP, Jing H and Ganea D:
Prostaglandin E2 induces matrix metalloproteinase 9 expression in
dendritic cells through two independent signaling pathways leading
to activator protein 1 (AP-1) Activation. J Biol Chem.
286:38913–38923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao L, Grebhardt S, Shi J, Peipe I, Zhang
J and Mayer D: Prostaglandin E2 stimulates S100A8 expression by
activating protein kinase A and CCAAT/enhancer-binding-protein-beta
in prostate cancer cells. Int J Biochem Cell Biol. 44:1919–1928.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Surhone LM, Tennoe MT and Henssonow SF:
Ep4 Receptor. Betascript Publishing; 2011
|
|
24
|
Kundu N, Ma X, Kochel T, Goloubeva O,
Staats P, Thompson K, Martin S, Reader J, Take Y, Collin P and
Fulton A: Prostaglandin E receptor EP4 is a therapeutic target in
breast cancer cells with stem-like properties. Breast Cancer Res
Treat. 143:19–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma X, Holt D, Kundu N, Reader J, Goloubeva
O, Take Y and Fulton AM: A prostaglandin E (PGE) receptor EP4
antagonist protects natural killer cells from PGE2-mediated
immunosuppression and inhibits breast cancer metastasis.
Oncoimmunology. 2:e226472013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia S, Ma J, Bai X, Zhang H, Cheng S,
Zhang M, Zhang L, Du M, Wang Y, Li H, et al: Prostaglandin E2
promotes the cell growth and invasive ability of hepatocellular
carcinoma cells by upregulating c-Myc expression via EP4 receptor
and the PKA signaling pathway. Oncol Rep. 32:1521–1530. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu S, Zhang Z, Ogawa O, Yoshikawa T,
Sakamoto H, Shibasaki N, Goto T, Wang L and Terada N: An EP4
antagonist ONO-AE3-208 suppresses cell invasion, migration and
metastasis of prostate cancer. Cell Biochem Biophys. 70:521–527.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu S, Goto T, Yoshikawa T, Zhang Z, Wang
L, Terada N and Ogawa O: Abstract 2813: EP4 antagonist suppresses
bone metastasis in prostate cancer. Cancer Res (AACR Annual Meeting
abstracts). 73:2813. 2014.
|
|
29
|
Xu S, Wang L, Ge LP, Zhou W and Zhang Z:
Effect of EP4 receptor antagonist on malignant phenotypes of
androgen-independent prostate cancer cells PC3. Jiangsu Med J.
40:R7372014.(In Chinese).
|
|
30
|
Xu S, Ge JP, Zhou WQ and Zhang ZY:
Inhibitory effect of ONO-AE3-208 on the formation of bone
metastasis of prostate cancer in mice. Zhonghua Nan Ke Xue.
20:684–689. 2014.(In Chinese). PubMed/NCBI
|
|
31
|
Harris RE: Cyclooxygenase-2 (cox-2)
blockade in the chemoprevention of cancers of the colon, breast,
prostate and lung. Inflammopharmacology. 17:55–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verma RP and Hansch C: Matrix
metalloproteinases (MMPs): Chemical-biological functions and
(Q)SARs. Bioorg Med Chem. 15:2223–2268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen S, Chen W, Zhang X, Lin S and Chen Z:
Overexpression of KiSS-1 reduces colorectal cancer cell invasion by
downregulating MMP-9 via blocking PI3K/Akt/NF-κB signal pathway.
Int J Oncol. 48:1391–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong BR, Rho J, Arron J, Robinson E,
Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS III,
Frankel WN, et al: TRANCE is a novel ligand of the tumor necrosis
factor receptor family that activates c-jun n-terminal kinase in T
cells. J Biol Chem. 272:251901997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anderson DM, Maraskovsky E, Billingsley
WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D
and Galibert L: A homologue of the TNF receptor and its ligand
enhance T-cell growth and dendritic-cell function. Nature.
390:175–179. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mayahara K, Yamaguchi A, Takenouchi H,
Kariya T, Taguchi H and Shimizu N: Osteoblasts stimulate
osteoclastogenesis via RANKL expression more strongly than
periodontal ligament cells do in response to PGE(2). Arch Oral
Biol. 57:1377–1384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmiedel BJ, Scheible CA, Nuebling T,
Kopp HG, Wirths S, Azuma M, Schneider P, Jung G, Grosse-Hovest L
and Salih HR: RANKL expression, function and therapeutic targeting
in multiple myeloma and chronic lymphocytic leukemia. Cancer Res.
73:683–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akech J, Wixted JJ, Bedard K, van der Deen
M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR,
Altieri DC, et al: Runx2 association with progression of prostate
cancer in patients: Mechanisms mediating bone osteolysis and
osteoblastic metastatic lesions. Oncogene. 29:811–821. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X and Klein RD: Prostaglandin E2
induces vascular endothelial growth factor secretion in prostate
cancer cells through EP2 receptor-mediated cAMP pathway. Mol
Carcinog. 46:912–923. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sha W, Olesch C, Hanaka H, Radmark O,
Weigert A and Brune B: Necrosis in DU145 prostate cancer spheroids
induces COX-2/mPGES-1-derived PGE2 to promote tumor growth and to
inhibit T cell activation. Int J Cancer. 133:1578–1588. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim JI, Lakshmikanthan V, Frilot N and
Daaka Y: Prostaglandin E2 promotes lung cancer cell migration via
EP4-betaArrestin1-c-Src signalsome. Mol Cancer Res. 8:569–577.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cooper DM: Regulation and organization of
adenylyl cyclases and cAMP. Biochem J. 375:517–529. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vo BT, Morton D Jr, Komaragiri S, Millena
AC, Leath C and Khan SA: TGF-β effects on prostate cancer cell
migration and invasion are mediated by PGE2 through activation of
PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kisslov L, Hadad N, Rosengraten M and Levy
R: HT-29 human colon cancer cell proliferation is regulated by
cytosolic phospholipase A(2)α dependent PGE(2)via both PKA and PKB
pathways. Biochim Biophys Acta. 1821:1224–1234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han C, Michalopoulos GK and Wu T:
Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor
tyrosine kinases and enhances invasiveness in human hepatocellular
carcinoma cells. J Cell Physiol. 207:261–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bai X, Wang J, Zhang L, Ma J, Zhang H, Xia
S, Zhang M, Ma X, Guo Y, Rong R, et al: Prostaglandin E2 receptor
EP1-mediated phosphorylation of focal adhesion kinase enhances cell
adhesion and migration in hepatocellular carcinoma cells. Int J
Oncol. 42:1833–1841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Peng Y, Shi J, Du X, Wang L, Klocker H, Mo
L, Mo Z and Zhang J: Prostaglandin E2 induces stromal cell-derived
factor-1 expression in prostate stromal cells by activating protein
kinase A and transcription factor Sp1. Int J Biochem Cell Biol.
45:521–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu J, Zhang Y, Frilot N, Kim JI, Kim WJ
and Daaka Y: Prostaglandin E2 regulates renal cell carcinoma
invasion through the EP4 receptor-Rap GTPase signal transduction
pathway. J Biol Chem. 286:33954–33962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jain S, Chakraborty G, Raja R, Kale S and
Kundu GC: Prostaglandin E2 regulates tumor angiogenesis in prostate
cancer. Cancer Res. 68:7750–7759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Reader J, Holt D and Fulton A:
Prostaglandin E2 EP receptors as therapeutic targets in breast
cancer. Cancer Metastasis Rev. 30:449–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bai XM, Zhang W, Liu NB, Jiang H, Lou KX,
Peng T, Ma J, Zhang L, Zhang H and Leng J: Focal adhesion kinase:
Important to prostaglandin E2-mediated adhesion, migration and
invasion in hepatocellular carcinoma cells. Oncol Rep. 21:129–136.
2009.PubMed/NCBI
|
|
54
|
Cherukuri DP, Goulet AC, Young RN,
Meuillet E, Regan WJ and Nelson MA: Prostagland in E2
(PGE2)-induced etracellular-regulated kinase
(ERK1/2)-phosphorylation is mediated by EP4 receptor in human colon
cancer cells. Cancer Res. 47:3872006.
|
|
55
|
Friis UG, Stubbe J, Uhrenhoil TR,
Svenningsen P, Nüsing RM, Skøtt O and Jensen BL: Prostaglandin E2
EP2 and EP4 receptor activation mediates cAMP-dependent
hyperpolarization and exocytosis of renin in juxtaglomerular cells.
Am J Physiol Renal Physiol. 289:F989–F997. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tauro M, Laghezza A, Tortorella P and
Lynch CC: Abstract 4858: A novel strategy for the selective and
tissue specific inhibition of MMPs in active breast cancer to bone
metastases. Cancer Res (AACR Annual Meeting abstracts). 74:pp.
48582014;
|
|
57
|
Jones DH, Nakashima T, Sanchez OH,
Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R,
Hojilla CV, et al: Regulation of cancer cell migration and bone
metastasis by RANKL. Nature. 440:692–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Baniwal SK, Khalid O, Gabet Y, Shah RR,
Purcell DJ, Mav D, Kohn-Gabet AE, Shi Y, Coetzee GA and Frenkel B:
Runx2 transcriptome of prostate cancer cells: Insights into
invasiveness and bone metastasis. Mol Cancer. 9:2582010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hussain A and Jayasekera O:
Characteristics of stage III and IV M0 prostate cancer patients in
Seer-medicare who develop bone Metastasis following diagnosis.
|
|
60
|
Sassone-Corsi P: The cyclic AMP pathway.
Cold Spring Harb Perspect Biol. 4:a0111482012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fimia GM and Sassone-Corsi P: Cyclic AMP
signalling. J Cell Sci. 114:1971–1972. 2001.PubMed/NCBI
|
|
62
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|





















