Introduction
Septins belong to a class of cytoskeletal proteins
with GTPase activity, which can form intracellular filamentous
scaffolds. Previous studies have demonstrated that septins are
involved in numerous biological processes, such as cell mitosis,
polarity determination, vesicle trafficking and apoptosis (1–4). In
addition, a previous study revealed that septin-7 (SEPT7), a member
of septin family, can suppress cell cycle progression in yeast via
its highly homologous cDNA sequence to cell division control
protein 10 (5). Furthermore, a
previous study revealed that SEPT7 exerts a suppressive effect on
glioma cell proliferation and invasion and induces apoptosis, and
it has been suggested that SEPT7 exerted suppressive effects on the
growth and invasion of glioma cells, inducing cell apoptosis, which
suggested that SEPT7 is a glioma suppressor gene (6). Zhang et al (7) demonstrated that the silencing of
SEPT7 inhibits cell proliferation and apoptosis in human breast
cancer cells. In addition, it has been demonstrated that miR-127
can inhibit cell proliferation via suppression of SEPT7 expression
in hepatocellular carcinoma cells (HCC), which suggests that SEPT7
promotes cell proliferation and inhibits apoptosis (8). Therefore, it may be suggested that in
tumor-associated cells, SEPT7 may exhibit an inhibitory and
promotive effect on cell apoptosis; thus the exact molecular
mechanism of SEPT7 has not yet been determined. Our previous study
demonstrated that melatonin can induce cell apoptosis in the human
fetal osteoblastic cell line hFOB 1.19 by activating the
phosphorylated extracellular signal regulated kinase
(p-ERK)-p-eukaryotic translation initiation factor 2α
(eIF2α)-activating transcription factor 2 signal transduction
pathway (9), and ERS may mediate
this process. In the present study, in vitro experiments
were performed to further investigate whether SEPT7 inhibits
melatonin-induced apoptosis or whether it acts as a potential
target for melatonin in the hFOB 1.19 cell line. In the present
study, in vitro experiments were performed following the
construction and transfection of a SEPT7 overexpression plasmid
into hFOB 1.19 cells, and apoptosis/ERS associated proteins
including (p-eIF2α), 78 kDa glucose-regulated protein (GRP78),
C/EBP-homologous protein (CHOP), pro-caspase-3 and cleaved
caspase-3 were investigated to reveal whether SEPT7 has an
inhibitory or stimulative effect in melatonin-induced apoptosis, or
whether SPET7 acts as a potential target of melatonin in hFOB 1.19
cells.
Materials and methods
Cell culture and reagents
The hFOB 1.19 cell line, provided by the Department
of Biochemistry and Molecular Biology, the Mayo Clinic (Rochester,
MN, USA) (10) was maintained in a
1:1 mixture of Dulbecco's modified Eagle's medium (DMEM) and F12
medium without phenol red (HyClone, Laboratories; GE Healthcare
Life Sciences, Logan, UT, USA). Cells were supplemented with 10%
fetal bovine serum (FBS; Clark Bioscience, Richmond, VA, USA) and
maintained in an atmosphere of 5% CO2 and at a
temperature of 37°C. Medium was replaced with fresh medium every
two days. Cells were utilized in passages 8–11. Melatonin dissolved
in 0.2% dimethyl sulfoxide (DMSO) or vehicle treatment with 0.2%
DMSO (in culture medium, DMEM and F12) was performed 37°C with 10%
FBS. Melatonin was obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Primary monoclonal antibodies against GRP78
(cat. no. ab21685), caspase-3 (cat. no. ab90437), p-eIF2α (cat. no.
ab4837), SEPT7 (cat. no. ab186021), CHOP (cat. no. 194533) and goat
anti-rabbit secondary antibodies (cat. no. ab6721) were all
purchased from Abcam (Cambridge, MA, USA).
Overexpression plasmid
construction
The SEPT7 fragment was cloned from normal human cDNA
from hFOB 1.19 cells into GV230 plasmids (200 ng; Shanghai GeneChem
Co., Ltd., Shanghai, China) between XhoI/KpnI sites
to overexpress SEPT7 in hFOB 1.19 cells. Full-length SEPT7 gene
(4,377 bp; GenBank NM_001011553) was amplified by polymerase chain
reaction (PCR). The PCR primers used were as follows: Forward,
5′-CTGCTCACAATAGTTGATACCCC-3′ and reverse,
5′-TGTTCACTCGTGATTCTGCATT-3′. The PrimeSTAR HS DNA polymerase, was
also obtained from Shanghai GeneChem Co., Ltd., and cycled for 30
cycles following initial denaturation (98°C for 5 min) with the
following parameters: 72°C for 8 min. Following enzyme digestion
(Exnase™ II; 1 µl) using ClonExpress II One Step Cloning
kit (Vazyme Biotech Co., Ltd., Nanjing, China) and sequencing, the
PCR product was cloned into the XhoI/KpnI sites of
the GV230 expression vector. The recombinant GV230-SEPT7 plasmid
was confirmed via endonuclease digestion and DNA sequencing
(Shanghai GeneChem Co., Ltd.) prior to transfection into hFOB 1.19
cells using Lipofectamine 2000® (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). In addition, the GV230
vector (used as a negative control) was also transfected into cells
using Lipofectamine 2000®.
SEPT7 small interfering (si)RNA
transfection
Cells were cultured in DMEM and F12 medium
supplemented with 10% FBS (Clark Bioscience) in a humidified
incubator at 37°C and 5% CO2. At 70–80% confluence,
cells were transfected with SEPT7 small interfering (si)RNA (60 nM;
sense, 5′-CGACUACAUUGAUAGUAAAUU-3′ and antisense,
5′-UUUACUAUCAAUGUAGUCGAU-3′; (Shanghai Genechem Co., Ltd.) using
Lipofectamine® 2000 according to the manufacturer's
protocol (Invitrogen; Thermo Fisher Scientific, Inc.). Overall,
there were three control groups: Blank control (medium only),
transfection reagent control (to eliminate the toxic influence of
Lipofectamine 2000, and the influence of Lipofectamine 2000 on the
expression of the target gene) and scrambled siRNA control
(5′-GAAATTTATAACGATCAGTCT-3′).
Western blotting
Following treatment, proteins were extracted from
hFOB 1.19 cells via incubation with radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China)
for 30 min at 4°C. The supernatant containing total protein was
harvested and proteins were quantified using the bicinchoninic acid
method. Aliquots containing 50 µg protein per lane were separated
on a 12% gel using SDS-PAGE and then transferred to polyvinylidene
fluoride membranes at 60 V for 2 h at 4°C. Subsequently, membranes
were soaked in 5% blocking buffer, containing 25 mg bovine serum
albumin (Beyotime Institute of Biotechnology) in Tris-buffered
saline (TBS) buffer to final volume of 0.5 liters, at 4°C for 2 h.
Proteins were then incubated with primary antibodies against to
GRP78 (cat. no. ab21685), caspase-3 (cat. no. ab90437), p-eIF2α
(cat. no. ab4837), SEPT7 (cat. no. ab186021), CHOP (cat. no.
194533) and β-actin (cat. no. ab8226) were purchased from Abcam
(Cambridge, MA, USA), diluted at 1: 5,000 and incubated overnight
at 4°C, followed by incubation with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (1:10,000; cat. no.
ab6721) for 2 h at room temperature. The DNR imaging system (DNR
Bio-Imaging Systems, Ltd., Jerusalem, Israel) was used to visualize
specific bands using the BeyoECL Plus (Beyotime Institute of
Biotechnology) protocol, and the optical density of each band was
determined using ImageJ software (version 1.51; National Institute
of Health, Bethesda, MD, USA). The ratio between target proteins
and β-actin was determined and graphically presented.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from hFOB 1.19 cells using
E.Z.N.A.® Total RNA Midi kit (Omega Bio-Tek, Inc.,
Norcross, GA, USA) according to the manufacturer's protocol.
Samples were quantified spectrophotometrically at 260 nm, with
260/280 nm ratios of 1.8–2.0 considered to be acceptable. RNA
quality was confirmed using 1% agarose gel electrophoresis stained
with 1 µg/ml ethidium bromide. qPCR was performed using a
LightCycler® 480 High-Resolution Melting Master (Roche
Diagnostics, Basel, Switzerland) using SYBR Premix Ex
Taq™ II (Takara Biotechnology Co., Ltd., Dalian, China).
Specific primers for SEPT7 (forward, 5′-ACGGGTTAGGCTCTTGG-3′ and
reverse primer, 5′-CAGTGCGTGTCGTGGAGT-3′) were obtained from
Shanghai GeneChem Co., Ltd. Amplifications were performed in a
total volume of 20 µl and cycled 40 times following initial
denaturation (95°C for 30 sec) with the following parameters: 95°C
for 5 sec and 60°C for 30 sec. β-actin (forward,
5′-TCCTCCCTGGAGAAGAGCTA-3′ and reverse,
5′-TCAGGAGGAGCAATGATCTTG-3′) was used as an internal control.
Analysis of the melting curve was performed to further verify the
results of RT-qPCR. RT-qPCR data were quantified using the
2−ΔΔCq method (11).
Statistical analysis
Data are presented as mean ± standard error of the
mean and a minimum of three independent repeats were performed for
each experiment. SPSS software (version 20.0; IBM Corp., Armonk,
NY, USA) was used to perform data analysis. Independent t-tests or
one-way analysis of variance followed by the Student-Newman-Keuls
test were performed to investigate differences between multiple
groups. N-fold values of ≤0.5 and >2 gene expression compared
with control genes were considered to be significant. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of SEPT7 increased with the
increasing concentration of melatonin
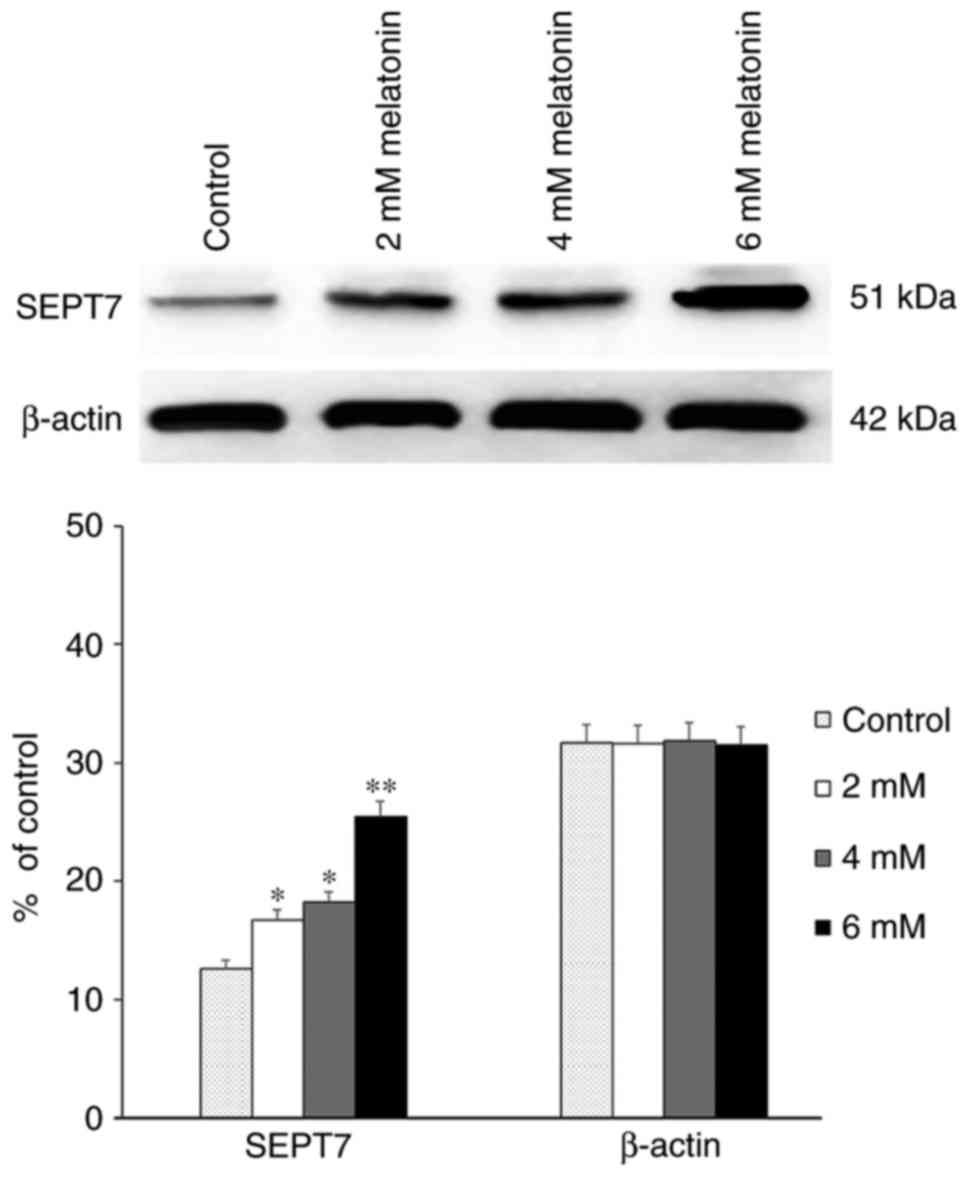
First, western blot analysis revealed that in hFOB
1.19 cells, the expression level of SEPT7 was significantly
upregulated in a dose-dependent manner following treatment with
differing concentrations of melatonin compared with the control
groups which did not receive any treatments (P<0.05) (Fig. 1).
The influence of SEPT7 overexpression
and inhibition on melatonin-induced apoptosis and ERS in human hFOB
1.19 cells
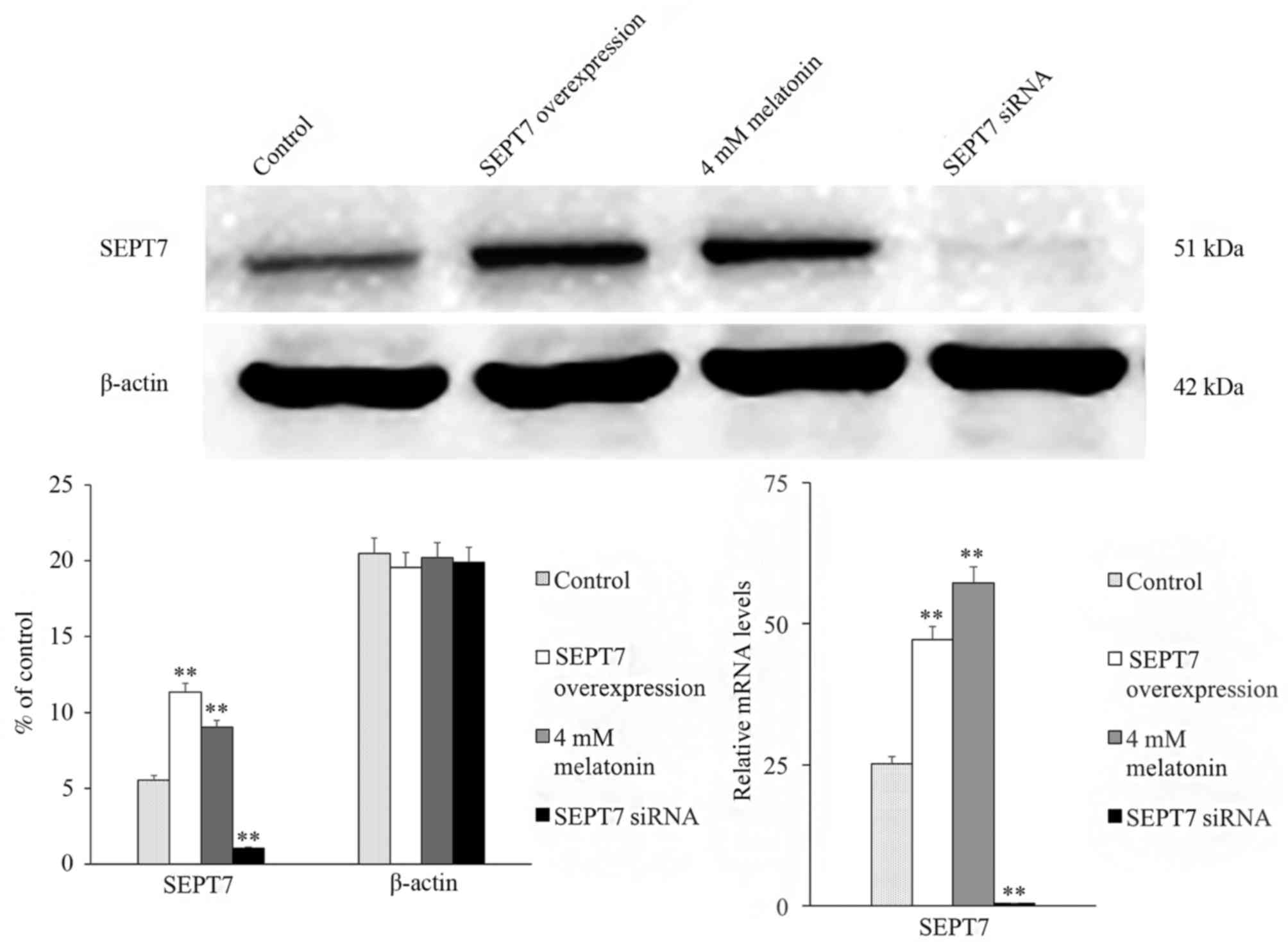
In addition, to assess whether SEPT7 affects
melatonin-induced apoptosis, the SEPT7 overexpression plasmid and
SEPT7 siRNA was constructed and transfected into hFOB 1.19 cells.
The expression of SEPT7 in the GV230-SEPT7 transfected group
demonstrated a marked increase in expression, and the expression
level of SEPT7 in the SEPT7 siRNA transfected group exhibited an
obvious decrease compared with the control group (the GV230 vector
transfected group; Fig. 2).
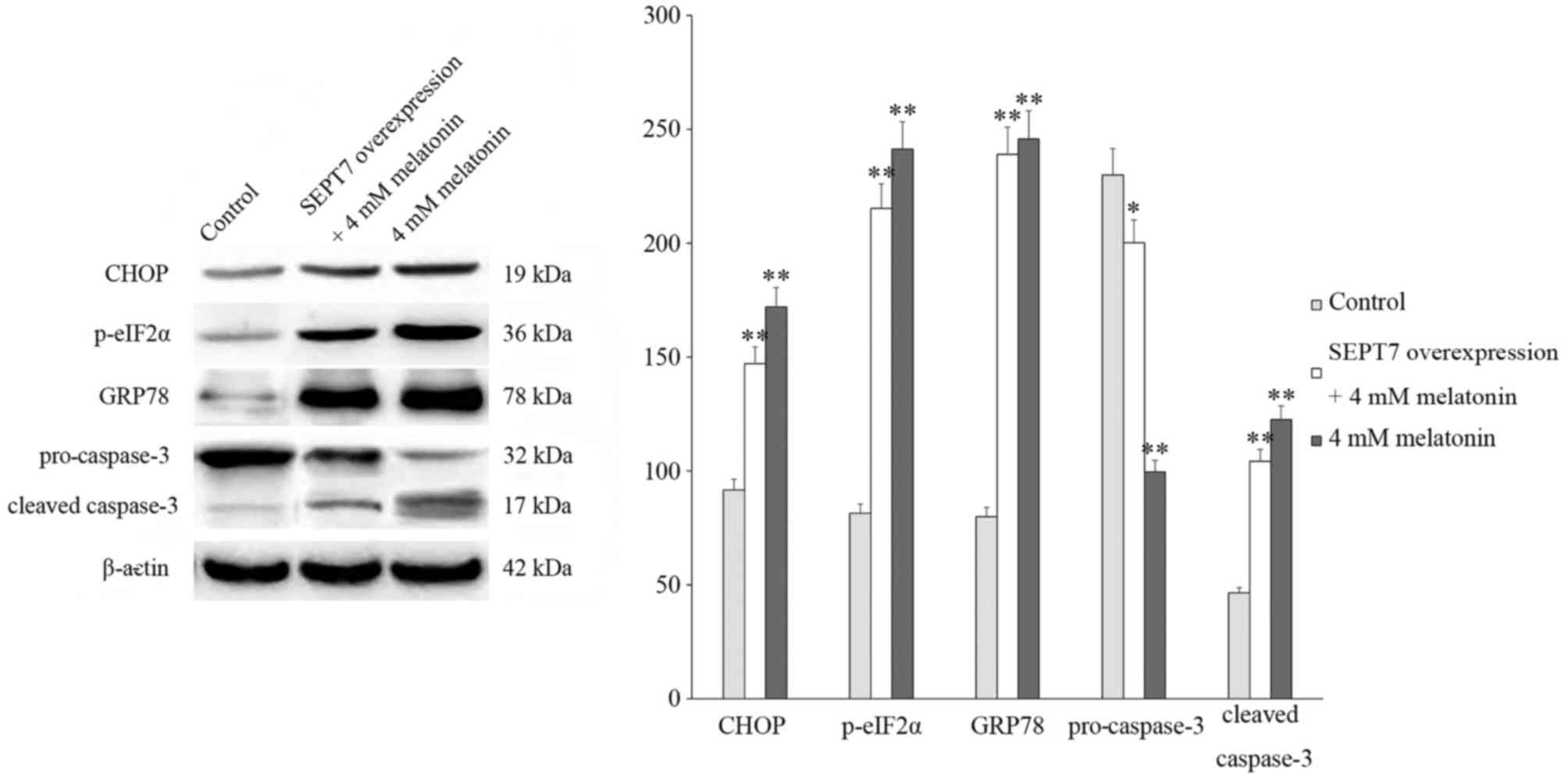
Following this, cells were treated with 4 mM
melatonin and the expression of proteins associated with cell
apoptosis and endoplasmic reticulum stress (ERS) were investigated
via western blotting. The results of these analyses revealed that
the levels of cleaved caspase-3, C/EBP homologous protein (CHOP),
78 kDa glucose-regulated protein (GRP78) and
phosphorylated-eukaryotic translation initiation factor 2α
(p-eIF2α) significantly increased and the expression of
pro-caspase-3 decreased following treatment with melatonin compared
with the control group which did not receive any treatments, thus
suggesting that the rate of cell apoptosis increased. In addition,
in the SEPT7 overexpression + 4 mM melatonin group, the levels of
cleaved caspase-3, CHOP, GRP78 and p-eIF2α were significantly
increased compared with the control group, and markedly suppressed
compared with the 4 mM melatonin group (Fig. 3).
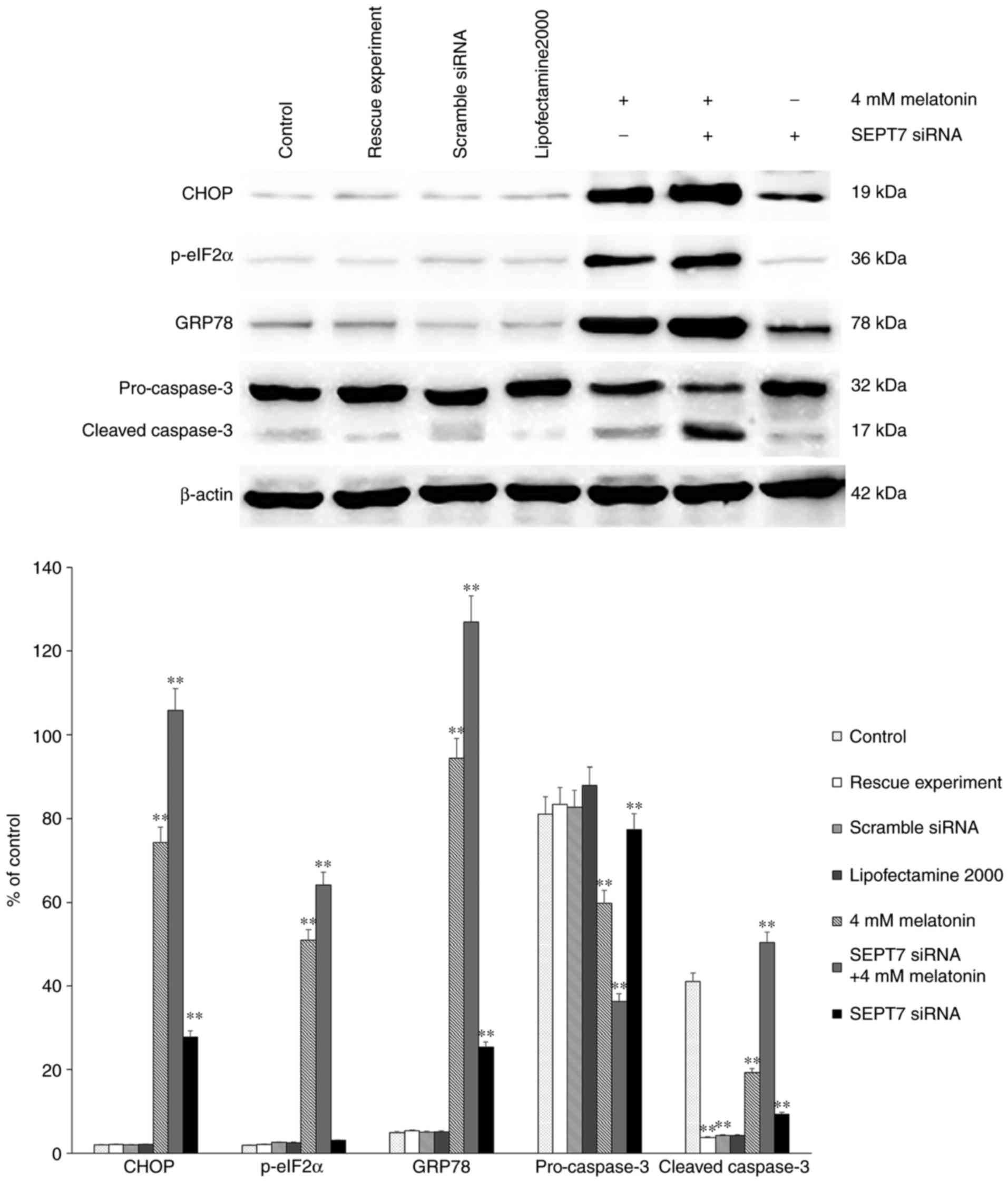
Finally, either SEPT7 siRNA or control siRNA were
transfected into osteoblasts using Lipofectamine® 2000
according to the manufacturer's protocol. There were three control
groups: Blank control, transfection reagent control and scramble
siRNA control. A rescue experiment was also performed via
transfection with the SEPT7 overexpression plasmid (Shanghai
Genechem Co., Ltd.). Western blot analysis was then performed to
assess the protein levels of CHOP, ATF4, p-eIF2α, pro-caspase-3 and
cleaved caspase-3. The results demonstrated that the levels of
CHOP, ATF4, p-eIF2α and cleaved caspase-3 in the group treated with
melatonin in combination with SEPT7 siRNA were increased
significantly, compared with those in the control groups (Fig. 4). Following transfection with SEPT7
siRNA, the expression level of SEPT7 decreased significantly
(Fig. 2). The SEPT7 overexpression
plasmid was then transfected into cells in order to perform the
rescue experiment. The results demonstrated that following
transfection with the SEPT7 overexpression plasmid the expression
level of SEPT7 was increased to a level similar to the 4 mM
melatonin-treated group, compared with the control group (Fig. 2). Therefore, the results suggest
that silencing of SEPT7 may enhance the intensity of
melatonin-induced apoptosis (by affecting the level of
apoptosis-related proteins) and ERS in human osteoblasts cell line
hFOB 1.19. Therefore concluding that the existence of SEPT7 may be
a protective factor in melatonin-induced apoptosis in human
osteoblasts cell line hFOB 1.19.
Discussion
It has previously been demonstrated that melatonin
is associated with aging, reproduction, tumor growth and multiple
other biological progresses in human cells (12–14).
Our previous study revealed that melatonin can regulate cell
apoptosis by inducing ERS in hFOB 1.19 cells (9). During melatonin-induced apoptosis,
proteins associated with well-established apoptotic pathways, such
as CHOP, pro-caspase-3, cleaved caspase-3 and p-eIF2α; were
upregulated, and thus may represent indicators for the occurrence
and rate of melatonin induced apoptosis.
Septins are a class of super protein families with a
molecular weight of 30–65 kDa and have conserved structures.
Previous studies have demonstrated that septin proteins are one of
the four cytoskeletal components, the other three being tubulin,
microfilament and intermediate fibrin (15,16),
and have important roles in the transportation of intracellular
substances (17,18), the regulation of cell division and
the cell cycle (19,20) as well as other physiological
processes, such as apoptosis. A previous study demonstrated that
under certain conditions, such as in the presence of apoptotic
factors, septin 4 (SEPT4) may become detached from the mitochondria
and be released into the cytoplasm following binding with an
inhibitor of cysteine and aspartic acid proteases, it is then
relieved of the inhibitory effect of X-linked inhibitor of
apoptosis protein and enhanced the activity of cysteine and
aspartic acid proteases afterwards, then finally promotes cell
apoptosis (21). However, it has
been demonstrated that septin 9 (SEPT9) is overexpressed in
numerous types of tumors and also promotes cell proliferation in
prostate cancer (22,23). Therefore, the effects of SEPT4 and
SEPT9 are opposite and thus the functions of the septin superfamily
associated with cell proliferation and apoptosis are unclear.
Considering that septin proteins have been identified in all
eukaryotes (15) and that the
association between SEPT7 and hFOB 1.19 cells has not been
extensively investigated, the present study aimed to investigate
whether SEPT7 has a role in melatonin-induced cell apoptosis in
hFOB 1.19 cells. The results of western blot analysis revealed that
the expression of SEPT7 was upregulated in melatonin-treated groups
in a dose-dependent manner, which suggests that SEPT7 may be a
protective factor in melatonin-induced apoptosis in hFOB 1.19
cells. To further investigate this, a SEPT7 overexpression plasmid
was constructed and transfected into hFOB 1.19 cells, and
apoptosis-associated proteins CHOP and caspase-3 were investigated
via western blot analysis. Results demonstrated the above-mentioned
proteins decreased in the SEPT7 overexpression group compared with
the 4 mM melatonin treatment group, which can demonstrate that the
existence of SEPT7 served a protective role in melatonin-induced
apoptosis in human osteoblasts cell line hFOB 1.19. The results
therefore suggested that SEPT7 attenuates apoptosis in hFOB 1.19
cells. Transfection with SEPT7-siRNA also demonstrated that SEPT7
has a protective, not a stimulative, role in melatonin-induced cell
apoptosis in hFOB 1.19 cells. Previous studies have revealed that
SEPT7 inhibits apoptosis via a caspase-independent pathway
(24), which is consistent with
our conclusions. However overexpression of SEPT7 inhibits cell
proliferation and induces G0/G1 phase arrest
in human glioma cells, both in vitro and in vivo
(25) which is in contrast with
our conclusions, the anti-apoptotic effect in normal cells
indicates that SEPT7 may serve a protective role in all kinds of
cells.
Our previous study (9) have demonstrated that
melatonin-induced cell apoptosis in hFOB 1.19 cells is associated
with the protein kinase RNA-like endoplasmic reticulum kinase
pathway, and the occurrence of ERS was demonstrated via
investigation into the expression levels of GRP78 and GRP94.
Previous study has suggested that the expression levels of GRP78
and GRP94 are significantly enhanced when ERS occurs (26). Therefore, the present study
investigated ERS-associated proteins GRP78 and p-eIF2α; the results
revealed that following transfection of the melatonin-treated cells
with the SEPT7 siRNA, the expression levels of GRP78 were
significantly increased compared with those in SEPT7 siRNA
non-transfected melatonin-treated cells. Furthermore, the levels of
GRP78 and p-eIF2α were significantly decreased in the SEPT7 siRNA
transfected group compared with the SEPT7 siRNA non-transfected and
melatonin-treated cells, which suggests that the intensity of the
ERS was decreased compared with the 4 mM melatonin group (Fig. 4). Taken together, it can be
suggested that SEPT7 inhibits melatonin-induced cell apoptosis via
suppression of ERS.
However, the underlying mechanism associated with
the effects of SEPT7 remains unclear. An ongoing study by the
authors suggests that this mechanism may be associated with certain
miRNAs (MiR-590-3p; Meng et al, unpublished data), and we
will investigate this further in later studies.
Acknowledgements
We would like to thank Dr Subramaniam M (Department
of Biochemistry and Molecular Biology, Mayo Clinic) for providing
the human fetal osteoblastic cell line hFOB 1.19. In addition, we
would like to thank the National Natural Science Foundation of
China (grant. no. 81472044) for their financial support and the
Shenyang Science and Technology Program-Population and Health
Special (17-230-9-04) of Shenyang supported by the Science and
Technology Bureau of Shenyang City, Liaoning Province.
References
|
1
|
Beites CL, Xie H, Bowser R and Trimble WS:
The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat
Neurosci. 2:434–439. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Field CM and Kellogg D: Septins:
Cytoskeletal polymers or signalling GTPases? Trends Cell Biol.
9:387–394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Larisch S, Yi Y, Lotan R, Kerner H, Eimerl
S, Tony Parks W, Gottfried Y, Birkey Reffey S, de Caestecker MP,
Danielpour D, et al: A novel mitochondrial septin-like protein,
ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell
Biol. 2:915–921. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kartmann B and Roth D: Novel roles for
mammalian septins: From vesicle trafficking to oncogenesis. J Cell
Sci. 114:839–844. 2001.PubMed/NCBI
|
|
5
|
Hou MS, Liu XB, Cao J and Chen B: SEPT7
overexpression inhibits glioma cell migration by targeting the
actin cytoskeleton pathway. Oncol Rep. 35:2003–2010. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia ZF, Pu PY, Kang CS, Wang GX, Zhang ZY,
Qiu MZ and Huang Q: Influence of SEPT7 on biological characters of
glioma cell line TJ905. Zhonghua Wai Ke Za Zhi. 45:1420–1423.
2007.(In Chinese). PubMed/NCBI
|
|
7
|
Zhang NZ, Liu L, Fan N, Zhang Q, Wang W,
Zheng M, Ma L, Li Y and Shi L: The requirement of SEPT2 and SEPT7
for migration and invasion in human breast cancer via MEK/ERK
activation. Oncotarget. 7:61587–61600. 2016.PubMed/NCBI
|
|
8
|
Zhou J, Lu S, Yang S, Chen H, Shi H, Miao
M and Jiao B: MicroRNA-127 post-transcriptionally downregulates
Sept7 and suppresses cell growth in hepatocellular carcinoma cells.
Cell Physiol Biochem. 33:1537–1546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng X, Zhu Y, Tao L, Zhao S and Qiu S:
Periostin has a protective role in melatonin-induced cell apoptosis
by inhibiting the eIF2α-ATF4 pathway in human osteoblasts. Int J
Mol Med. 41:1003–1012. 2018.PubMed/NCBI
|
|
10
|
Subramaniam M, Jalal SM, Rickard DJ,
Harris SA, Bolander ME and Spelsberg TC: Further characterization
of human fetal osteoblastic hFOB 1.19 and hFOB/ER alpha cells: Bone
formation in vivo and karyotype analysis using multicolor
fluorescent in situ hybridization. J Cell Biochem. 87:9–15. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative P C R
and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reiter RJ, Tan DX and Fuentes-Broto L:
Melatonin: A multi-tasking molecule. Prog Brain Res. 181:127–151.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akbarzadeh M, Rahbarghazi R, Nabat E,
Movassaghpour AA, Shanehbandi D, Faramarzian Azimi Maragheh B,
Matluobi D, Barazvan B, Kazemi M, Samadi N and Nouri M: The impact
of different extracellular matrices on melatonin effect in
proliferation and stemness properties of ovarian cancer cells.
Biomed Pharmacother. 87:288–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bavithra S, Selvakumar K, Sundareswaran L
and Arunakaran J: Neuroprotective effect of melatonin against PCBs
induced behavioural, molecular and histological changes in cerebral
cortex of adult male wistar rats. Neurochem Res. 42:428–438. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mostowy S and Cossart P: Septins: The
forth component of the cytoskeleton. Nat Rev Mol Cell Biol.
13:183–194. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weirich CS, Erzberger JP and Barral Y: The
septin family of GTPases: Architecture and dynamics. Nat Rev Mol
Cell Biol. 9:478–489. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kremer BE, Haystead T and Macara IG:
Mammalian septins regulate microtubule stability through
interaction with the micro tubule-binding protein MAP4. Mol Biol
Cell. 16:4648–4659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagata K and Inagaki M: Cytoskeletal
modification of Rho guanine nucleotide exchange factor activity:
Identification of a Rho guanine nucleotide exchange factor as a
binding partner for Sept9b, a mammalian septin. Oncogene. 24:65–76.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wloka C, Nishihama R, Onishi M, Oh Y,
Hanna J, Pringle JR, Krauss M and Bi E: Evidence that a septin
diffusion barrier is dispensable for cytokinesis in budding yeast.
Biol Chem. 392:813–829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MS, Froese CD, Xie H and Trimble WS:
Uncovering priciples that control septin-septin interactions. J
Biol Chem. 287:30406–30413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shehadeh L, Mitsi G, Adi N, Bishopric N
and Papapetropoulos S: Expression of lewy body protein septin 4 in
postmortem brain of Parkinson's disease and contro subjects. Mov
Disord. 24:204–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scott M, Hyland P, McGregor G, Hillan KJ,
Russell SE and Hall PA: Multimodality expression profiling shows
SEPT9 to be overexpressed in a wide range of human tumours.
Oncogene. 24:4688–4700. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amir S, Wang R, Matzkin H, Simons JW and
Mabjeesh NJ: MSF-A interacts with hypoxia-inducible factor-1alpha
and augments hypoxia-inducible factor transcriptional activation to
affect tumorigenicity and angiogenesis. Cancer Res. 66:856–866.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horowitz A, Lapointe JF, Eid R, Sheibani
S, Gharib N, Jones NK, Vali H, Mandato CA and Greenwood MT: The
human septin7 and the yeast CDC10 septin prevent Bax and copper
mediated cell death in yeast. Biochim Biophys Acta. 1833:3186–3194.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jia ZF, Huang Q, Kang CS, Yang WD, Wang
GX, Yu SZ, Jiang H and Pu PY: Overexpression of septin 7 suppresses
glioma cell growth. J Neurooncol. 98:329–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pizarro JG, Yeste-Velasco M, Esparza JL,
Verdaguer E, Pallàs M, Camins A and Folch J: The antiproliferative
activity of melatonin in B65 rat dopaminergic neuroblastoma cells
is related to the downregulation of cell cycle-related genes. J
Pineal Res. 45:8–16. 2008. View Article : Google Scholar : PubMed/NCBI
|