Introduction
Osteoarthritis (OA) is the most frequently diagnosed
musculoskeletal disorder. It is a multifactorial and slowly
progressing degenerative joint disease (1). The role of inflammation in OA
progression has been previously reported (2–5).
IL-1β is an important cytokine in OA progression by acting as a
catabolic factor to reduce the synthesis of two primary cartilage
components, the type II collagen (COL2A1) and aggrecan (ACAN)
(6). Moreover, the level of IL-1β
in the synovial fluid, synovial membrane, cartilage, and
subchondral bone layer is elevated in patients with OA (7). Previous reports demonstrated that
treatment with IL-1β decreases COL2A1 expression (6) and increases MMP-13 expression
(8). Therefore, IL-1β plays a
critical role during OA progression.
In mammals, the aquaporin (AQP) family consists of
thirteen subtypes (AQP0 to 12) and these proteins are expressed in
various tissues (9). AQP
expression has been previously reported in several animal models. A
previous study demonstrated the expression of AQP1 and AQP3 in
normal equine articular chondrocytes (10). Another study showed that AQP1,
AQP3, and AQP6 were expressed in rat mandibular condylar
cartilage, although only AQP3 mRNA was highly upregulated in
rat OA cartilage (11).
Additionally, an increase in AQP1 expression in meniscus
tissue was demonstrated in an experimentally-induced rat OA model
(12).
AQP1 is a membrane protein found in the red blood
cells (13). It is a 28-kDa water
channel formed by six transmembrane domains with N- and C-termini
at the inner cytosolic site of the cell membrane (14). AQP1 is highly expressed in the
anus, gallbladder, and liver, and it is moderately expressed in the
hippocampus and ependymal cells of the central nervous system
(15). The presence of AQP1 was
also confirmed in human endothelia and certain water-transporting
epithelia, mammary epithelium, articular chondrocytes,
synoviocytes, and synovial microvessels (9). In the central nervous system, the
expression of AQP1 is restricted to the choroid plexus region of
the brain under normal conditions (16), but it is expressed in the
microvascular endothelia and reactive astrocytes of brain tumors
where it is thought to play a role in the development of vasogenic
edema (17). Recently, enhanced
AQP1 expression was observed in several diseases (18–20).
AQP1 expression is increased in patients with autoimmune and
alcoholic pancreatitis (18),
Alzheimer's disease (19), and
rheumatoid and psoriatic arthritis (20).
Several studies have demonstrated the expression of
AQP1 and AQP3 in human articular cartilage (15,20–23).
Mobasheri et al (15)
showed moderate AQP1 expression in chondrocytes residing in the
deep zone of the articular cartilage, adjacent to the subchondral
bone in normal human femoral head articular cartilage. They also
reported AQP1 expression in the synovial microvessels and
synoviocytes in normal joints, but the expression was upregulated
in the inflamed synovium of patients with RA, suggesting that edema
formation and synovial fluid accumulation in RA joints may be a
consequence of elevated AQP1 expression in the synovium (20). However, functional analysis was not
performed in these previous studies (15,20–23).
We hypothesized that AQP1 is highly expressed in OA cartilages, and
AQP1 may increase the expression of catabolic factors. Thus, we
evaluated AQP1 functions in human OA chondrocytes.
Materials and methods
Human cartilage samples
OA cartilage tissues were obtained from the
cartilage of lateral femoral condyles of patients with end-stage
varus-type OA during total knee arthroplasty surgery (n=31). The
diagnosis of OA was based on clinical, laboratory, and radiographic
evaluations. These patients showed apparent macroscopic OA
progression. As a control, normal chondrocytes (as determined
macroscopically) were obtained from the hip cartilage of patients
that underwent surgery for femoral neck fracture with no recorded
tumor complication (n=12). All primary cartilage samples were
obtained in accordance with the World Medical Association
Declaration of Helsinki of ethical principles for medical research
involving human subjects. The present study was approved by the
ethical review board of Kobe University Graduate School of Medicine
(Kobe, Hyōgo, Japan) and all patients provided written informed
consent. The average age of OA patients in this study was 76.4
years, and the average age of patients with femoral neck fracture
was 85.1 years, but there was no significant difference in age
between the two groups.
Chondrocyte isolation and culture
Chondrocytes were isolated from the cartilage
tissues and cultured. Previously, we have used OA chondrocytes
isolated from the knee joint (24–26).
Briefly, tissue samples were minced and digested in Dulbecco's
modified Eagle's medium (DMEM, cat. no. D5746; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) containing 0.2% collagenase D (Roche,
Basel, Switzerland) at 37°C for 3 h under 5% CO2 in a
Petri dish. Dissociated cells were cultured in DMEM supplemented
with 10% fetal bovine serum (FBS) (BioWhittaker; Lonza Group,
Basel, Switzerland), 50 U/ml penicillin, and 0.05 mg/ml
streptomycin. After overnight culture, non-adherent cells were
removed, and adherent cells were further incubated in fresh medium.
Five to six days after incubation, chondrocytes reached confluence
and were re-plated into 6-well plates at a density of
2.0×105 cells/well in DMEM. All experiments were
conducted using first-passage cells. To confirm the chondrocyte
properties, the expression of type II collagen was assessed by
reverse transcription-polymerase chain reaction (RT-PCR). We
confirmed that type II collagen was expressed at a higher level in
normal human hip chondrocytes than in OA knee chondrocytes, while
type X collagen was expressed at a higher level in OA knee
chondrocytes than in normal hip chondrocytes (data not shown).
RNA isolation and RT-PCR
To evaluate the expression of different AQP
genes, OA chondrocytes (n=4) were cultured in 6-well plates with
DMEM for 12 h, and RNA was extracted using the QIA shredder and
RNeasy Mini kit (Qiagen, Hilden, Germany) according to the
manufacturer's instructions. For RT-PCR, 1 µg of total RNA was
reverse transcribed to first-strand complementary DNA (cDNA) using
1.25 µM oligo-dT primers in 40 µl PCR buffer II containing 2.5 mM
MgC12, 0.5 mM dNTP mix, 0.5 U RNase inhibitor, and 1.25
U MuLV reverse transcriptase (PerkinElmer, Inc., Foster City, CA,
USA) at 42°C for 60 min. The cDNA amplification was performed under
the following PCR conditions: 94°C for 5 min; followed by 35 cycles
of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec; and a
final extension at 72°C for 2 min using AmpliTaq Gold DNA
Polymerase (cat. no. N8080241; Thermo Fisher Scientific, Inc.,
Rockford, IL, USA) and specific primers (Table I). The amplicons, along with the
TrackIt 50 bp DNA ladder (cat. no. 10488-043; Thermo Fisher
Scientific, Inc.), were resolved using 3% polyacrylamide gel
electrophoresis, and the signals were visualized using the LAS-3000
mini system (FujiFilm, Tokyo, Japan).
 | Table I.Sequence of primers used in reverse
transcription- and reverse transcription-quantitative polymerase
chain reaction. |
Table I.
Sequence of primers used in reverse
transcription- and reverse transcription-quantitative polymerase
chain reaction.
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| GAPDH |
GTTCGACAGTCAGCCGCATC |
GGAATTTGCATGGGTGGA |
| AQP0 |
TGTACTGGGTAGGCCCAATC |
CCCCTCCACGTAAACTCAGA |
| AQP1 |
TGGACACCTCCTGGCTATTG |
GGGCCAGGATGAAGTCGTAG |
| AQP2 |
CACCCCTGCTCTCTCCATA |
GAAGACCCAGTGGTCATCAAAT |
| AQP3 |
GCTGTATTATGATGCAATCTGGC |
TAAGGGAGGCTGTGCCTATG |
| AQP4 |
GAAGGCATGAGTGACAGACC |
ATTCCGCTGTGACTGCTTTC |
| AQP5 |
GCCACCTTGTCGGAATCTAC |
TAAAGCATGGCAGCCAGGAC |
| AQP6 |
CACCTCATTGGGATCCACTT |
GTTGTAGATCAGTGAGGCCA |
| AQP7 |
ATCTCTGGAGCCCACATGAA |
GAAGGAGCCCAGGAACTG |
| AQP8 |
GTGCCTGTCGGTCATTGAG |
CAGGGTTGAAGTGTCCACC |
| AQP9 |
TCTCTGAGTTCTTGGGCACG |
GGTTGATGTGACCACCAGAG |
| AQP10 |
GATAGCCATCTACGTGGGTG |
CACAGAAAGCAGACAGCAAC |
| AQP11 |
TCCGAACCAAGCTTCGTATC |
TAGCGAAAGTGCCAAAGCTG |
| AQP12 |
ACTTGTTCTTCTGGCCGTAG |
CTTACTGGAGTACGTGCAGG |
| MMP-3 |
ATTCCATGGAGCCAGGCTTTC |
CATTTGGGTCAAACTCCAACTGTG |
| MMP-13 |
TGCTGCATTCTCCTTCAGGA |
ATGCATCCAGGGGTCCTGGC |
|
ADAMTS-4 |
GGCTAAAGCGCTACCTGCTA |
GAGTCACCACCAAGCTGACA |
|
ADAMTS-5 |
TATGACAAGTGCGGACTATG |
TTCAGGGCTAAATAGGCAGT |
| COL2A1 |
CCCAGAGGTGACAAAGGAGA |
CACCTTGGTCTCCAGAAGGA |
| ACAN |
GGCACTAGTCAACCCTTTGG |
CTGAACCCTGGTAACCCTGA |
RT-quantitative PCR (RT-qPCR)
The relative mRNA levels of human AQP1,
matrix metalloproteinase (MMP)-3, MMP-13, a
disintegrin and metalloprotease with thrombospondin motifs
(ADAMTS)-4, ADAMTS-5, COL2A1, and ACAN in OA
chondrocytes (n=19) and normal hip chondrocytes (n=4) were analyzed
by SYBR-Green real-time PCR using the ABI prism 7500
sequence-detection system (Applied Biosystems, Foster City, CA,
USA). The expression of the genes of interest was normalized
against that of the GAPDH housekeeping gene using the
comparative quantification cycle (Cq)-value method. The difference
between the mean Cq values of the gene of interest and those of the
housekeeping gene was denoted as ΔCq, and the difference between
the ΔCq of unknown samples and that of the calibrated sample was
denoted as ΔΔCq. The relative value of gene expression was
calculated as the 2−ΔΔCq (27). Sequences of the primers used for
detection are listed in Table
I.
Histological evaluation of cartilage
degeneration
The femoral condyles (n=5) and femoral head (n=5)
were fixed in 4% paraformaldehyde for 24 h, dehydrated in graded
alcohol solutions, decalcified with 14% EDTA for 7 days, and
embedded in paraffin wax. Histological sections were obtained at
10-µm intervals.
Immunohistochemistry
Deparaffinized sections were digested with
proteinase (Dako, Glostrup, Denmark) for 10 min and treated with 3%
hydrogen peroxide (Wako Pure Chemical, Ltd., Industries, Osaka,
Japan) to block endogenous peroxidase activity. The sections were
treated with a 1:50 dilution of anti-AQP1 antibody (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight, and
subsequently treated with peroxidase-labeled anti-mouse
immunoglobulin (Histofine Simple Stain MAX PO; Nichirei Bioscience,
Tokyo, Japan) at room temperature for 30 min. The signal was
developed as brown-reaction products using a peroxidase substrate
3,3′-diaminobenzidine (Histofine Simple Stain DAB solution;
Nichirei Bioscience), and the sections were examined under a
microscope. Hematoxylin stain was used as a counter stain. Numbers
of AQP1-positive cells were counted in five areas of
high-magnification fields at both the superficial and deep zones of
the cartilage tissue by triple-blinded observers. The average
percentage of AQP1-positive cells from the total cell count was
calculated. Positive cells superior of the tidemark were included
in the assessment.
Explant culture
Normal hip cartilages (n=3) were harvested under
sterile conditions and explant pieces were placed in a culture dish
containing DMEM with or without IL-1β. Forty-eight h after
stimulation, the explants were fixed in 4% paraformaldehyde for 24
h, decalcified with 14% EDTA for 7 days, dehydrated in graded
alcohol solutions, and embedded in paraffin wax. Histological
sections were obtained at 10-µm intervals.
IL-1β stimulation
Chondrocytes were cultured in 6-well plates with or
without stimulation with 10 ng/ml recombinant human IL-1β/IL-1F2
(cat. no. 201-LB; R&D Systems) for 12 h.
Small interfering RNA (siRNA)
transfection
OA chondrocytes (n=7) were placed onto 6-well plates
at a density of 2.0×105 cells/well in DMEM supplemented
with 10% FBS and 100 U/ml of penicillin-streptomycin. After
subculturing at 37°C for 24 h under 5% CO2, the medium
was replaced with fresh serum-free medium, and chondrocytes were
transfected with 0.5 nM of non-specific control siRNA (negative
control no. 2 siRNA; cat. no. AM4613; Thermo Fisher Scientific,
Inc.) or AQP1-specific siRNA (cat. no. 4390824, assay ID: s1515 or
s1516) using the Lipofectamine RNAiMAX transfection reagent (cat.
no. 13778150) (both from Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Twelve hours after transfection, the cells
were treated with IL-1β.
Western blot analysis
OA chondrocytes (n=3) were lysed in a buffer
containing 25 mM Tris, 1% Nonidet P-40, 150 mM NaCl, 1.5 mM EDTA,
and a protease/phosphatase inhibitor mix (Roche Diagnostics, Basel,
Switzerland). The lysates were centrifuged to remove cellular
debris. The supernatants were collected, mixed with 4X
electrophoresis sample buffer, electrophoresed on a 7.5–15%
polyacrylamide gradient gel (Biocraft, Tokyo, Japan), and
transferred onto a blotting membrane (GE Healthcare Life Sciences,
Little Chalfont, UK). Membranes were incubated with antibodies
against AQP1 or α-tubulin (cat. no. T9026; Sigma-Aldrich).
Horseradish peroxidase (HRP)-conjugated goat anti-mouse
immunoglobulin G antibody (IgG Ab) was used as a secondary antibody
and proteins were visualized using the ECL plus reagent (GE
Healthcare Life Sciences) in a Chemilumino Analyzer LAS-3000 mini
(Fujifilm). Protein expression was determined by
semi-quantification of digitally captured images using the NIH
ImageJ software (http://imagej.nih.gov/ij/). Three different samples
were analyzed.
Immunocytochemistry/immunofluorescence
(ICC/IF)
OA chondrocytes (n=3) were placed onto a glass-based
dish (Iwaki, Tokyo, Japan) at a density of 2.0×105
cells/well in DMEM. Chondrocytes were transfected with non-specific
control (0.5 nM) (Qiagen) or AQP1-specific siRNA-1 (0.5 nM) using
the Lipofectamine RNAiMAX transfection reagent. Twelve hours after
transfection, the cells were left untreated or stimulated with 10
ng/ml of recombinant human IL-1β/IL-1F2 (R&D Systems) for 12 h.
After stimulation, the chondrocytes were fixed with 4%
paraformaldehyde at room temperature for 30 min and permeabilized
with 0.25% Triton X-100 (Nacalai Tesque, Kyoto, Japan) for 30 min.
Fixed chondrocytes were then incubated with human anti-AQP1 mouse
monoclonal antibody (1:50 dilution; Santa Cruz Biotechnology, Inc.)
and mouse anti-ADAMTS-4 rabbit polyclonal antibody (1:500 dilution;
Thermo Fisher Scientific, Inc.) in Can Get Signal immunostain
Solution A (Toyobo, Osaka, Japan) overnight at 4°C. After the
primary antibody incubation, the chondrocytes were incubated with
goat anti-mouse immunoglobulin Alexa Fluor Plus 555 (1:200
dilution) and goat anti-rabbit immunoglobulin Alexa Fluor Plus 488
(1:200 dilution) (both from Thermo Fisher Scientific, Inc.) as the
secondary antibodies for 60 min at room temperature. The nuclei
were stained with DAPI (Nacalai Tesque), and images were viewed and
captured using a BZ-X700 microscope (Keyence, Osaka, Japan).
Numbers of AQP1-positive cells, ADAMTS-4-positive cells, and
DAPI-stained nuclei were counted in four areas of
high-magnification fields by triple-blinded observers. The average
percentage of AQP1-positive cells and ADAMTS-4-positive cells from
the total nuclei count was calculated.
Statistical analysis
The Mann-Whitney U test for comparisons between two
groups and one-way analysis of variance with Tukey-Kramer's post
hoc test were applied to analyze differences between time points or
between culture conditions. P-values <0.05 indicated
statistically significant differences. Results are presented as
mean values ± standard error (SE) with 95% confidence intervals
(CI). Data analysis was performed using the Bell Curve for Excel
software (Social Survey Research Information Co., Ltd., Tokyo,
Japan).
Results
AQP1 was highly expressed in human OA
chondrocytes
The expression of various AQPs was examined
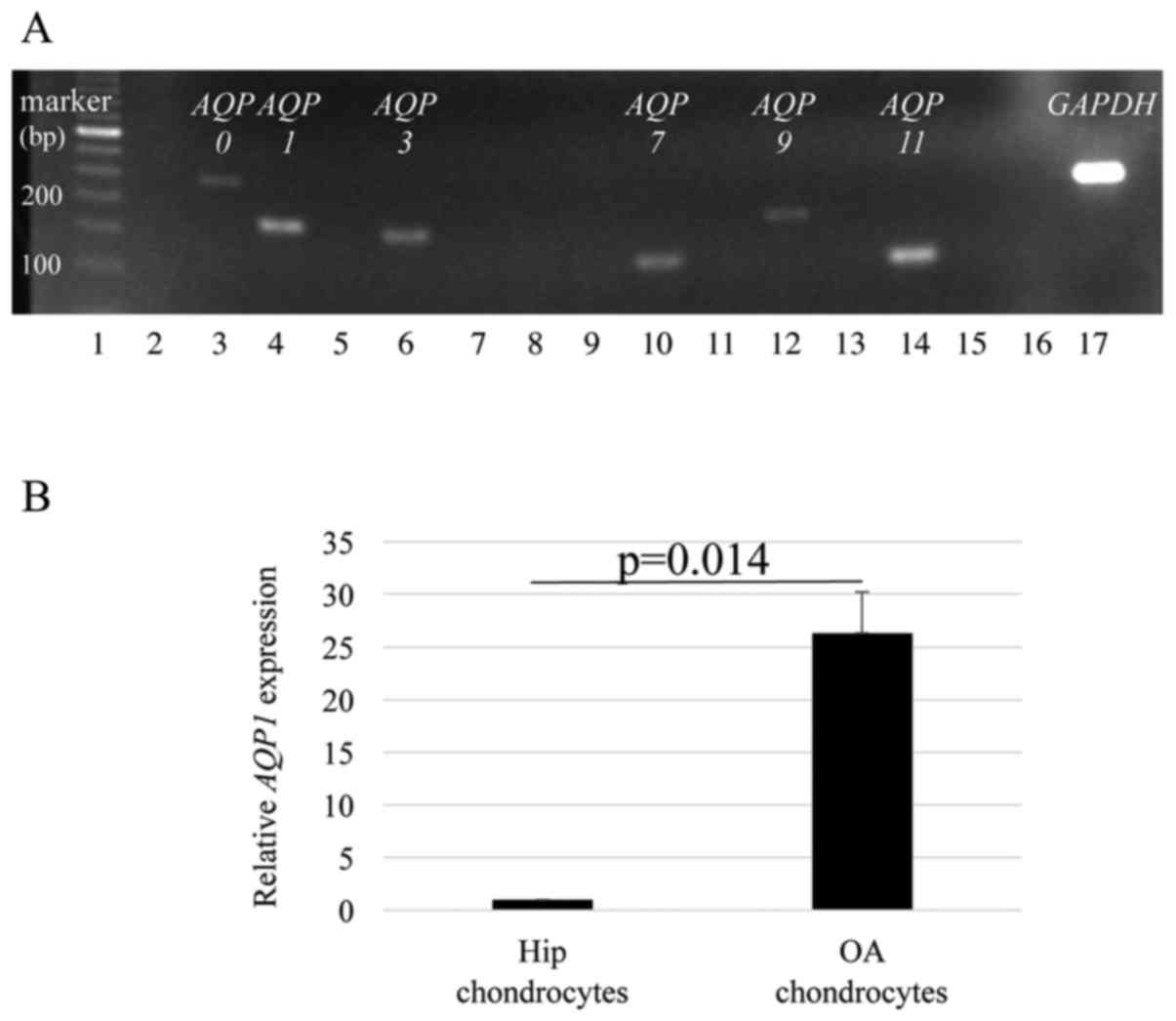
in OA chondrocytes from four patients with knee OA. Representative
electrophoresis results with PCR products showed that AQP0, 1,
3, 7, 9 and 11 were expressed in OA chondrocytes
(Fig. 1A). The average AQP1
mRNA expression in OA chondrocytes was significantly higher than
that in normal chondrocytes from hip cartilage of four individuals
(P=0.014) (Fig. 1B).
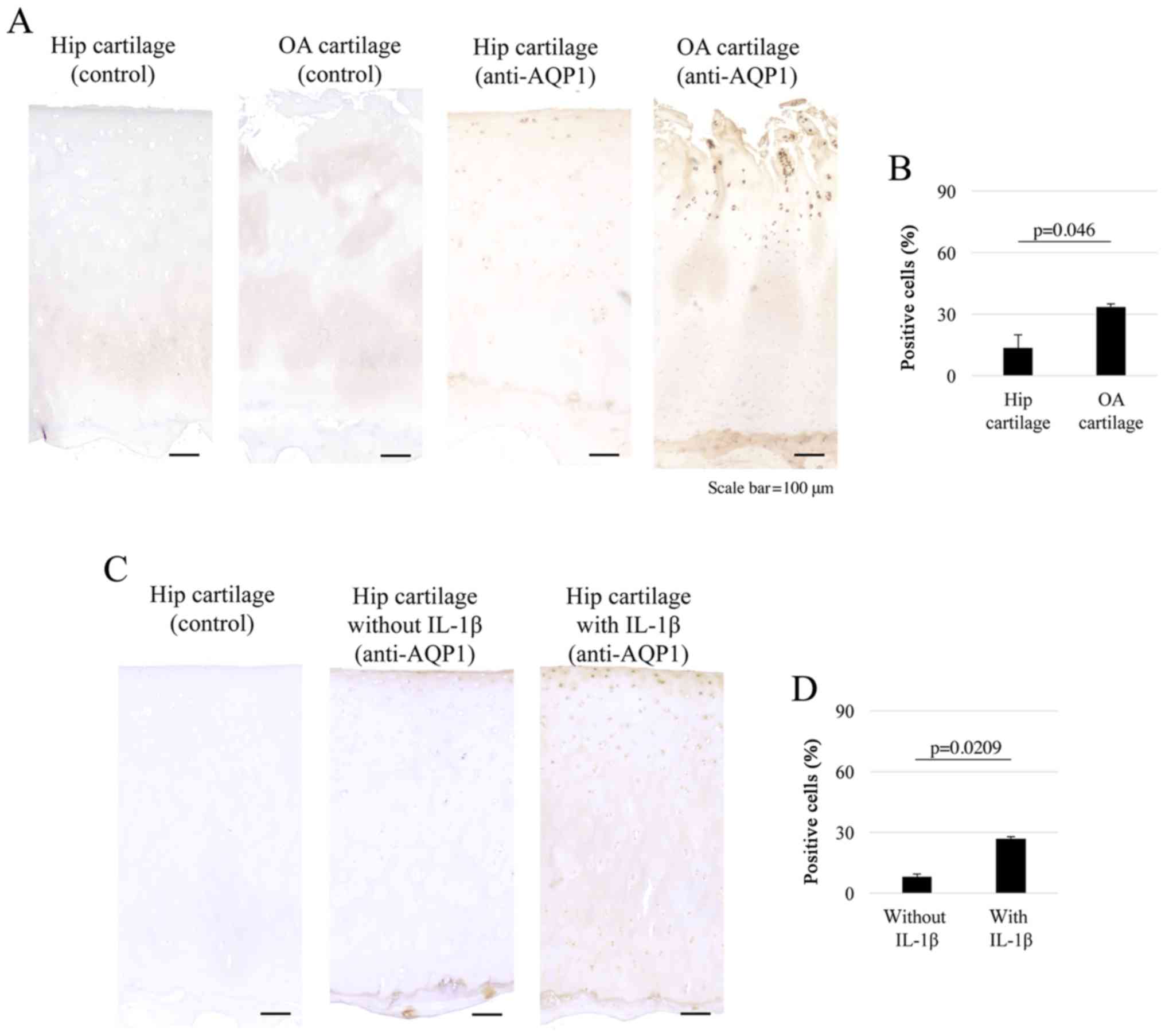
Immunohistochemistry results showed that surface layer of the
articular cartilage was clearly degenerated in the OA group
compared to that in the normal hip cartilage group. AQP1 was
expressed in the superficial to middle layers in both OA (n=5) and
hip (n=5) cartilage tissues (Fig.
2A), and the percentage of AQP1-positive cells was
significantly higher in OA cartilages than in normal hip cartilages
(AQP1-positive cell percentage: OA, 33.5%; normal hip, 13.4%;
P=0.046) (Fig. 2B). Furthermore,
AQP1 was expressed in the superficial layers in hip explant
cartilages with or without IL-1β treatment (Fig. 2C). The percentage of AQP1-positive
cells was significantly higher in explant cartilages treated with
IL-1β than in those without IL-1β (AQP1-positive cell percentage:
With IL-1β, 26.9%; without IL-1β, 8.2%; P=0.0209) (Fig. 2D). Together, these results
indicated that AQP1 was highly expressed in human OA chondrocytes,
and the expression levels were increased in response to IL-1β.
IL-1β increased the expression of AQP1
and catabolic factors in OA chondrocytes
RT-qPCR analysis showed that the level of
AQP1 mRNA was significantly higher in OA chondrocytes
treated with 10 ng/ml IL-1β than in untreated chondrocytes. At 4 h
after IL-1β stimulation, the level of AQP1 mRNA was 5-fold
higher than that of the control (0 h; not treated with IL-1β)
(Fig. 3A). The mRNA levels of
MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 were increased
significantly following treatment with 10 ng/ml of IL-1β (Fig. 3B-E). The mRNA levels of
MMP-3 and MMP-13 were increased in a time-dependent
manner until 24 h after stimulation (Fig. 3B and C). The level of
ADAMTS-4 mRNA was increased at 12 h after stimulation, but
it was decreased at 24 h after stimulation (Fig. 3D). The level of ADAMTS-5
mRNA was increased at 12 and 24 h after stimulation (Fig. 3E). In contrast, the levels of
COL2A1 and ACAN mRNAs were significantly decreased at
12 and 24 h after stimulation (Fig. 3F
and G). These results indicated that IL-1β stimulation
increased the expression of AQP1 and catabolic factors, but
decreased the expression of anabolic factors in OA chondrocytes. It
is important to note that although AQP1 mRNA expression
peaked 4 h after IL-1β treatment, the level was still significantly
higher than that of the control at 12 h after treatment. At 12 h
post-IL-1β treatment, we also observed the peak of ADAMTS-4
and ADAMTS-5 mRNA expression, while that of COL2A1
and ACAN decreased. Thus, we investigated changes in mRNA
expression at 12 h after stimulation in subsequent experiments.
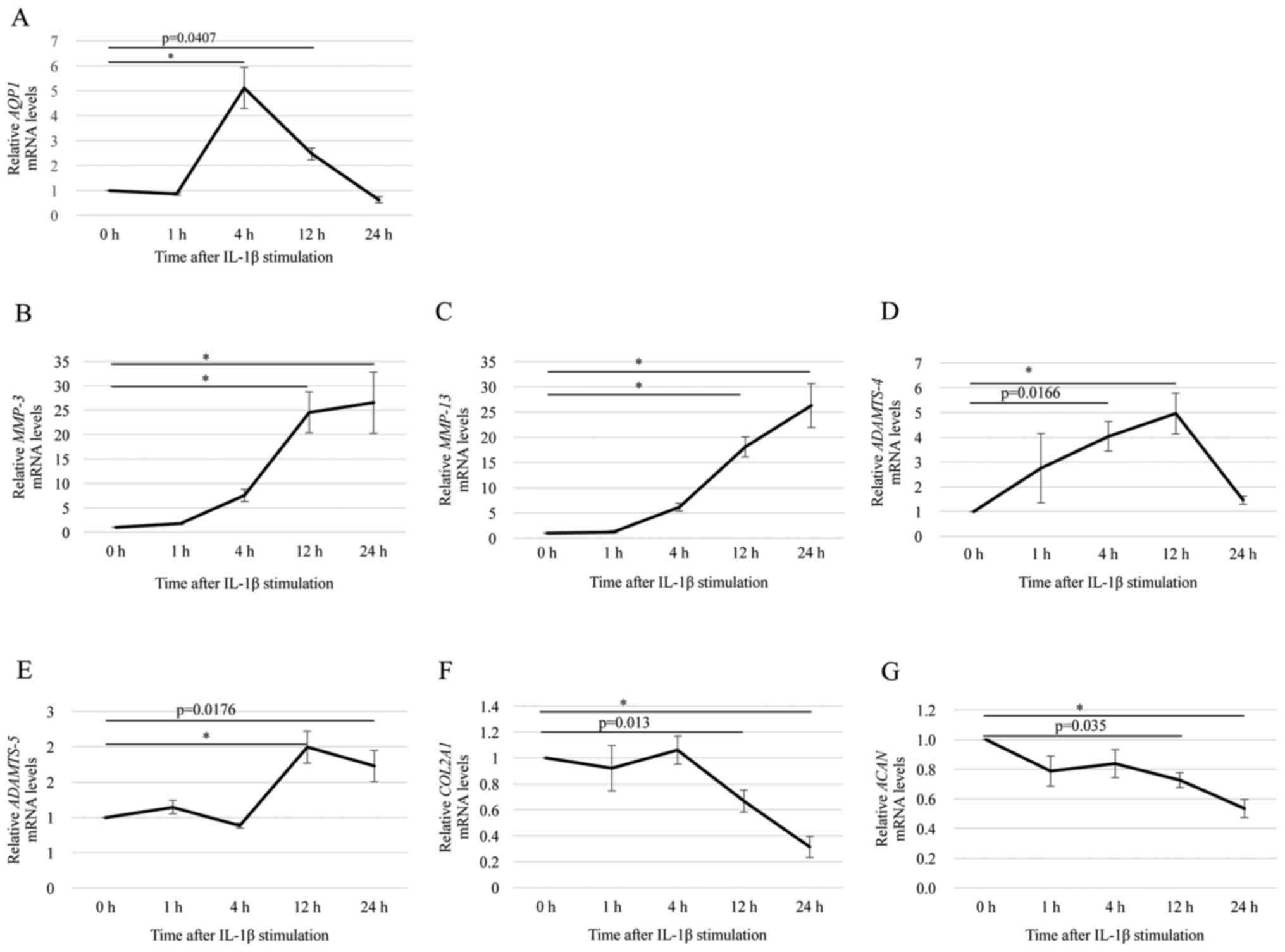 | Figure 3.Effects of IL-1β stimulation on the
expression of AQP1, catabolic factors and anabolic factors
in OA chondrocytes. Changes in the mRNA level of (A) AQP1,
(B) MMP-3, (C) MMP-13, (D) ADAMTS-4, (E)
ADAMTS-5, (F) COL2A1 and (G) ACAN in OA
chondrocytes stimulated with IL-1β for 1, 4, 12 or 24 h were
assessed by reverse transcription-quantitative polymerase chain
reaction. GAPDH was used as the endogenous control. The
value for each gene expression level without IL-1β stimulation (0
h) normalized to GAPDH was set as 1. Data are presented as
the mean ± standard error (n=8). *P<0.001, as indicated. IL,
interleukin; AQP1, aquaporin 1; OA, osteoarthritis; MMP, matrix
metalloproteinase; ADAMTS, a disintegrin and metalloprotease with
thrombospondin motifs; COL2A1, cartilage components type II
collagen; ACAN, aggrecan. |
Downregulation of AQP1 decreased
ADAMTS-4 expression in OA chondrocytes
To investigate the functions of AQP1 in
chondrocytes, two AQP1-specific siRNAs were used to transfect the
OA chondrocytes with or without IL-1β stimulation. IL-1β
stimulation significantly increased the level of AQP1 mRNA
in OA chondrocytes (Fig. 4A).
However, the level of AQP1 mRNA was significantly decreased
in chondrocytes transfected with AQP1-specific siRNAs (Fig. 4A). The knockdown efficiency of
AQP1 using AQP1 siRNA-1 (s1515) and −2 (s1516) with IL-1β
stimulation was 94.3 and 88.5%, respectively relative to the
expression in the non-specific siRNA group with IL-1β stimulation
(Fig. 4A). Western blot results
verified that AQP1 siRNA-1 and −2 significantly decreased AQP1
expression at the protein level in chondrocytes with or without
IL-1β stimulation when compared to the level in cells transfected
with non-specific siRNA (Fig. 4B and
C). These results indicated that the AQP1-specific siRNAs
downregulated AQP1 at both the mRNA and protein levels. The level
of ADAMTS-4 mRNA was also significantly decreased to 64.2
and 65.8% using AQP1 siRNA-1 and −2, respectively under IL-1β
stimulation (Fig. 4F). However,
the mRNA levels of MMP-3, MMP-13, ADAMTS-5, COL2A1, and
ACAN were not significantly changed by the AQP1-specific
siRNA transfection (Fig. 4D and E, and
G-I). These results indicated that AQP1 downregulation
decreased ADAMTS-4 expression in OA chondrocytes, but did
not affect the expression of other related genes.
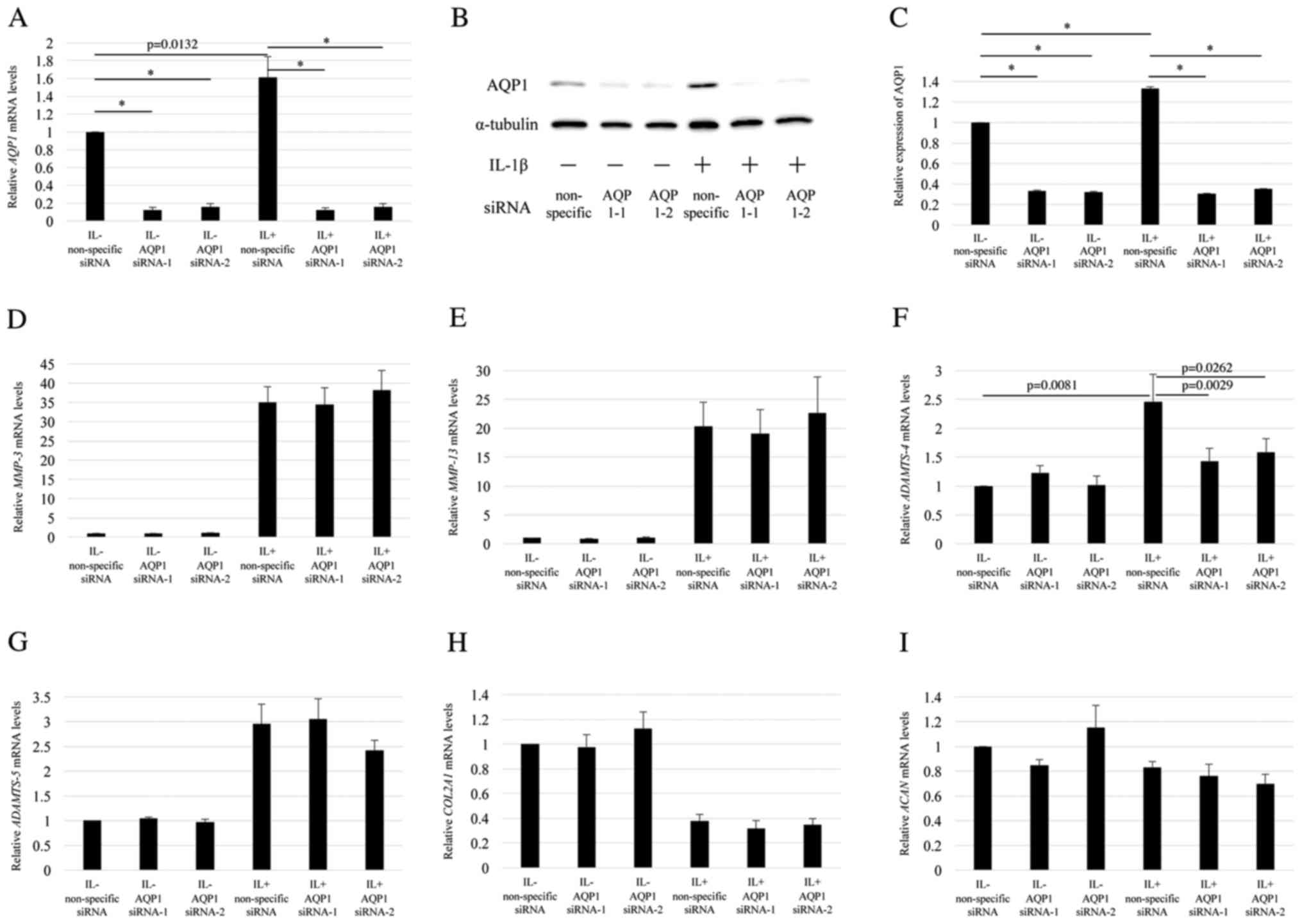 | Figure 4.Effects of AQP1-specific siRNA
transfection on IL-1β-induced expression of catabolic factors in OA
chondrocytes. OA chondrocytes were transfected with two different
AQP1-specific siRNAs for 12 h and then stimulated without (IL-) or
with IL-1β (IL+) for 12 h. (A) AQP1 knockdown efficiency was
assessed by RT-qPCR. AQP1 protein expression levels were (B)
assessed by western blotting and (C) the protein bands were
semi-quantified (n=3). The AQP1 expression level in cells
transfected with non-specific siRNA without IL-1β stimulation was
set as 1. Changes in the mRNA level of (D) MMP-3, (E)
MMP-13, (F) ADAMTS-4, (G) ADAMTS-5, (H)
COL2A1 and (I) ACAN were assessed by RT-qPCR.
GAPDH was used as the endogenous control. The value for each
gene expression level in cells treated with non-specific siRNA
without IL-1β stimulation normalized to GAPDH was set as 1.
Data are presented as the mean ± standard error (n=7). *P<0.001,
as indicated. AQP1, aquaporin 1; siRNA, small interfering RNA; IL,
interleukin; OA, osteoarthritis; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; MMP, matrix
metalloproteinase; ADAMTS, a disintegrin and metalloprotease with
thrombospondin motifs; COL2A1, cartilage components type II
collagen; ACAN, aggrecan. |
AQP1 and ADAMTS-4 were co-localized in
chondrocytes
To investigate the relationship between AQP1 and
ADAMTS-4, we assessed AQP1 and ADAMTS-4 expression with or without
IL-1β stimulation in human articular chondrocytes using
immunofluorescence. Representative single-color images showing
DAPI, AQP1, and ADAMTS-4 staining, as well as merged images are
shown (Fig. 5A). The results
showed that in the absence of IL-1β stimulation, AQP1 was
expressed, while ADAMTS-4 was not. Following IL-1β stimulation, the
expression of both AQP1 and ADAMTS-4 was increased and the two
molecules co-localized. However, AQP1 and ADAMTS-4 expression was
decreased with AQP1-specific siRNA transfection. The results showed
that AQP1-specific siRNA transfection reduced the percentage of
AQP1 and ADAMTS-4 co-localization and ADAMTS-4 expression in OA
chondrocytes (Fig. 5B and C).
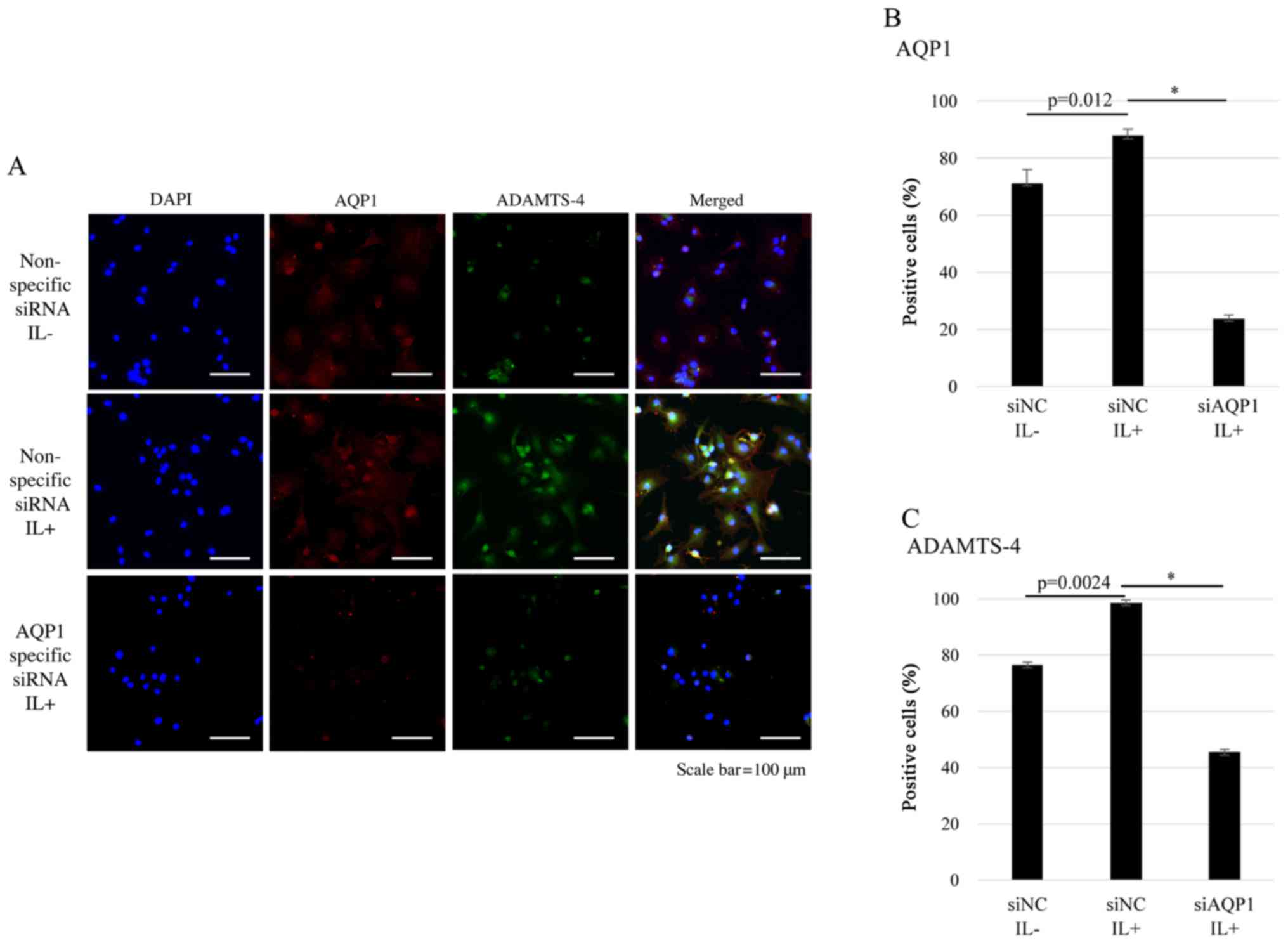 | Figure 5.Immunostaining of OA human articular
chondrocytes. DAPI, AQP1 and ADAMTS-4 staining are shown in blue,
red and green, respectively. (A) OA articular chondrocytes were
either transfected with non-specific control siRNA (siNC) without
(IL-) or with IL-1β stimulation (IL+) for 12 h, or transfected with
siAQP1 with IL-1β stimulation (IL+) for 12 h. The percentage of (B)
AQP1- and (C) ADAMTS-4 positive cells (percentage of positive cells
relative to DAPI-stained nuclei count with 95% confidence interval)
is shown. Scale bar, 100 µm. Data are presented as the mean ±
standard error (n=3). *P<0.001, as indicated. AQP1, aquaporin 1;
siRNA/NC, small interfering RNA/negative control; siAQP1,
AQP1-specific siRNA; IL, interleukin; OA, osteoarthritis; ADAMTS-4,
a disintegrin and metalloprotease with thrombospondin motifs 4;
DAPI, 4′,6-diamidino-2-phenylindole. |
Discussion
Geyer et al (21) showed that the increase in AQP1
expression in damaged tissues was restricted to chondrocytes in the
superficial areas, while non-lesional regions of human OA of the
knee displayed lack of AQP1 expression. Hagiwara et al
(23) suggested that AQP1 is
expressed in the articular cartilage of the knee in human, and the
expression is localized in chondrocytes in both intact and early
degenerative cartilage regions. In this study, we demonstrated that
AQP1 was highly expressed in the superficial to middle zones of OA
articular cartilages, and in the superficial zone of hip explant
cartilages treated with IL-1β. The regions where AQP1 is expressed
may be related to progression of OA. We also observed that OA
chondrocytes had significantly higher levels of AQP1 mRNA
compared to those in normal chondrocytes from hip cartilage.
Recent reports have also described various AQP1
functions other than its role in water-dependent homeostasis
(28–30). Meng et al (30) demonstrated that AQP1 enhances the
migration of bone marrow mesenchymal stem cells by regulating the
focal adhesion kinase and β-catenin. Another study found enhanced
MMP-2 and −9 expression upon AQP1 downregulation in LTEP-A2 and LLC
lung cancer cell lines (29). In
the present study, we demonstrated that the AQP1 downregulation
decreased ADAMTS-4 expression in OA chondrocytes, but did not
affect the expression of other catabolic genes. Further, we
observed that AQP1 and ADAMTS-4 co-localized and the expression of
ADAMTS-4 was decreased upon AQP1 downregulation in OA chondrocytes.
Based on these results, AQP1 may directly affect ADAMTS-4
expression in OA chondrocytes.
ACAN is the principal cartilage extracellular matrix
proteoglycan that gives cartilage its characteristic
compressibility, while ADAMTS is a family of proteases (31). A previous study showed that
downregulation of ADAMTS-5 expression protects mice from
arthritis-induced ACAN degradation (32). In vitro, downregulation of
ADAMTS-4 and ADAMTS-5 was associated with ACAN cleavage prior to
collagen degradation by MMP-13 (33,34).
ADAMTS-4 is predominantly expressed in OA cartilage, while ADAMTS-5
is constitutively expressed in both OA cartilage and normal hip
cartilage. Further, the active form of ADAMTS-4 is overexpressed in
OA chondrocytes, which directly correlates with the degradation of
cartilage tissue (34). MMP and
ADAMTS proteases cleave ACAN at different sites within the protein
core (35). These findings
supported our results that AQP1 knockdown suppressed IL-1β-induced
ADAMTS-4, but not ADAMTS-5, MMP-3, and MMP-13
expression.
Recently, Graziano et al (36) demonstrated a relationship between
AQP1 and cartilage differentiation. However, we showed that AQP1
silencing did not affect MMP-13 expression in OA
chondrocytes. Substantial phenotypic differences between
differentiated chondrocytes used by Graziano et al (36) and OA chondrocytes in our study may
explain the discrepancy. Cai et al (37) showed that AQP4 over-expression
exacerbated the severity of adjuvant-induced arthritis in rat
articular cartilages. This report supported our findings that AQP1
may control ADAMTS-4 expression in inflammatory chondrocytes.
Nevertheless, further studies are needed to clarify the mechanism
of IL-1β-induced ADAMTS-4 regulation by AQP1.
Our study had several limitations. First, synovial
fluid is produced in the synovium, and AQP is known to function in
water metabolism in tissues. However, AQP1 functions in the
synovial tissue were not assessed in the present study and should
be addressed in a future study. Second, a previous report showed
that AQP1 expression positively correlated with caspase-3
expression and activity, suggesting that AQP1 promoted caspase-3
activation and thereby contributed to chondrocyte apoptosis and the
development of OA (28). Thus,
AQP1 roles in chondrocyte apoptosis should also be evaluated in
future studies.
In conclusion, we demonstrated that AQP1 was highly
expressed in the superficial to middle zones of OA articular
cartilages, and the level of AQP1 mRNA was significantly
higher in OA than in normal chondrocytes from hip cartilages.
Further, AQP1 downregulation decreased ADAMTS-4
expression in OA chondrocytes, but did not change the expression of
other catabolic and anabolic genes evaluated. We also observed the
co-localization of AQP1 and ADAMTS-4 expression, and a decrease in
ADAMTS-4 expression upon AQP1 downregulation in OA chondrocytes.
Our results indicated that AQP1 may play a role in maintaining the
homeostasis of cartilage tissues; thus, catabolic factors may be
suppressed by regulating AQP1 expression during OA progression.
Acknowledgements
The authors thank Mr. Takeshi Ueha, Ms. Kyoko
Tanaka, Ms. Minako Nagata, and Ms. Maya Yasuda for their technical
assistance, and Dr. Kazunari Ishida (Kobe Kaisei Hpspital) and Dr.
Naoko Shima (Hyogo Prefectural Rehabilitation Central Hospital) for
kindly providing the cartilage tissues. We would like to thank
Editage (www.editage.jp) for English language
editing.
References
|
1
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee. Diagnostic and therapeutic criteria committee of the
American Rheumatism association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berenbaum F and van den Berg WB:
Inflammation in osteoarthritis: Changing views. Osteoarthritis
Cartilage. 23:1823–1824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldring MB: The role of cytokines as
inflammatory mediators in osteoarthritis: Lessons from animal
models. Connect Tissue Res. 40:1–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernandes JC, Martel-Pelletier J and
Pelletier JP: The role of cytokines in osteoarthritis
pathophysiology. Biorheology. 39:237–246. 2002.PubMed/NCBI
|
|
6
|
Goldring MB, Birkhead J, Sandell LJ,
Kimura T and Krane SM: Interleukin 1 suppresses expression of
cartilage-specific types II and IX collagens and increases types I
and III collagens in human chondrocytes. J Clin Invest.
82:2026–2037. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mengshol JA, Vincenti MP, Coon CI,
Barchowsky A and Brinckerhoff CE: Interleukin-1 induction of
collagenase 3 (matrix metalloproteinase 13) gene expression in
chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear
factor kappaB: Differential regulation of collagenase 1 and
collagenase 3. Arthritis Rheum. 43:801–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agre P, King LS, Yasui M, Guggino WB,
Ottersen OP, Fujiyoshi Y, Engel A and Nielsen S: Aquaporin water
channels-from atomic structure to clinical medicine. J Physiol.
542:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mobasheri A, Trujillo E, Bell S, Carter
SD, Clegg PD, Martín-Vasallo P and Marples D: Aquaporin water
channels AQP1 and AQP3, are expressed in equine articular
chondrocytes. Vet J. 168:143–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng J, Ma X, Ma D and Xu C: Microarray
analysis of differential gene expression in temporomandibular joint
condylar cartilage after experimentally induced osteoarthritis.
Osteoarthritis Cartilage. 13:1115–1125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Musumeci G, Leonardi R, Carnazza ML,
Cardile V, Pichler K, Weinberg AM and Loreto C: Aquaporin 1 (AQP1)
expression in experimentally induced osteoarthritic knee menisci:
An in vivo and in vitro study. Tissue Cell. 45:145–152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Preston GM, Carroll TP, Guggino WB and
Agre P: Appearance of water channels in Xenopus oocytes expressing
red cell CHIP28 protein. Science. 256:385–387. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borgnia M, Nielsen S, Engel A and Agre P:
Cellular and molecular biology of the aquaporin water channels.
Annu Rev Biochem. 68:425–458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mobasheri A and Marples D: Expression of
the AQP-1 water channel in normal human tissues: A semiquantitative
study using tissue microarray technology. Am J Physiol Cell
Physiol. 286:C529–C537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oshio K, Watanabe H, Song Y, Verkman AS
and Manley GT: Reduced cerebrospinal fluid production and
intracranial pressure in mice lacking choroid plexus water channel
Aquaporin-1. FASEB J. 19:76–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saadoun S, Papadopoulos MC, Davies DC,
Bell BA and Krishna S: Increased aquaporin 1 water channel
expression in human brain tumours. Br J Cancer. 87:621–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ko SB, Mizuno N, Yatabe Y, Yoshikawa T,
Ishiguro H, Yamamoto A, Azuma S, Naruse S, Yamao K, Muallem S and
Goto H: Aquaporin 1 water channel is overexpressed in the plasma
membranes of pancreatic ducts in patients with autoimmune
pancreatitis. J Med Invest. 56 Suppl:S318–S321. 2009. View Article : Google Scholar
|
|
19
|
Huysseune S, Kienlen-Campard P, Hébert S,
Tasiaux B, Leroy K, Devuyst O, Brion JP, De Strooper B and Octave
JN: Epigenetic control of aquaporin 1 expression by the amyloid
precursor protein. FASEB J. 23:4158–4167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mobasheri A, Moskaluk CA, Marples D and
Shakibaei M: Expression of aquaporin 1 (AQP1) in human synovitis.
Ann Anat. 192:116–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geyer M, Grässel S, Straub RH, Schett G,
Dinser R, Grifka J, Gay S, Neumann E and Müller-Ladner U:
Differential transcriptome analysis of intraarticular lesional vs
intact cartilage reveals new candidate genes in osteoarthritis
pathophysiology. Osteoarthritis Cartilage. 17:328–335. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trujillo E, González T, Marín R,
Martín-Vasallo P, Marples D and Mobasheri A: Human articular
chondrocytes, synoviocytes and synovial microvessels express
aquaporin water channels; upregulation of AQP1 in rheumatoid
arthritis. Histol Histopathol. 19:435–444. 2004.PubMed/NCBI
|
|
23
|
Hagiwara K, Shinozaki T, Matsuzaki T,
Takata K and Takagishi K: Immunolocalization of water channel
aquaporins in human knee articular cartilage with intact and early
degenerative regions. Med Mol Morphol. 46:104–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawakita K, Nishiyama T, Fujishiro T,
Hayashi S, Kanzaki N, Hashimoto S, Takebe K, Iwasa K, Sakata S,
Nishida K, et al: Akt phosphorylation in human chondrocytes is
regulated by p53R2 in response to mechanical stress. Osteoarthritis
Cartilage. 20:1603–1609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayashi S, Fujishiro T, Hashimoto S,
Kanzaki N, Chinzei N, Kihara S, Takayama K, Matsumoto T, Nishida K,
Kurosaka M and Kuroda R: p21 deficiency is susceptible to
osteoarthritis through STAT3 phosphorylation. Arthritis Res Ther.
17:3142015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwasa K, Hayashi S, Fujishiro T, Kanzaki
N, Hashimoto S, Sakata S, Chinzei N, Nishiyama T, Kuroda R and
Kurosaka M: PTEN regulates matrix synthesis in adult human
chondrocytes under oxidative stress. J Orthop Res. 32:231–237.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao H, Gui J, Wang L, Xu Y, Jiang Y, Xiong
M and Cui Y: Aquaporin 1 contributes to chondrocyte apoptosis in a
rat model of osteoarthritis. Int J Mol Med. 38:1752–1758. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei X and Dong J: Aquaporin 1 promotes the
proliferation and migration of lung cancer cell in vitro. Oncol
Rep. 34:1440–1448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng JH, Ma XC, Li ZM and Wu DC:
Aquaporin-1 and aquaporin-3 expressions in the temporo-mandibular
joint condylar cartilage after an experimentally induced
osteoarthritis. Chin Med J (Engl). 120:2191–2194. 2007.PubMed/NCBI
|
|
31
|
Porter S, Clark IM, Kevorkian L and
Edwards DR: The ADAMTS metalloproteinases. Biochem J. 386:15–27.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Glasson SS, Askew R, Sheppard B, Carito B,
Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al:
Deletion of active ADAMTS5 prevents cartilage degradation in a
murine model of osteoarthritis. Nature. 434:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song RH, Tortorella MD, Malfait AM, Alston
JT, Yang Z, Arner EC and Griggs DW: Aggrecan degradation in human
articular cartilage explants is mediated by both ADAMTS-4 and
ADAMTS-5. Arthritis Rheum. 56:575–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naito S, Shiomi T, Okada A, Kimura T,
Chijiiwa M, Fujita Y, Yatabe T, Komiya K, Enomoto H, Fujikawa K and
Okada Y: Expression of ADAMTS4 (aggrecanase-1) in human
osteoarthritic cartilage. Pathol Int. 57:703–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Struglics A, Larsson S, Pratta MA, Kumar
S, Lark MW and Lohmander LS: Human osteoarthritis synovial fluid
and joint cartilage contain both aggrecanase- and matrix
metalloproteinase-generated aggrecan fragments. Osteoarthritis
Cartilage. 14:101–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Graziano ACE, Avola R, Pannuzzo G and
Cardile V: Aquaporin1 and 3 modification as a result of
chondrogenic differentiation of human mesenchymal stem cell. J Cell
Physiol. 233:2279–2291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai L, Lei C, Li R, Chen WN, Hu CM, Chen
XY and Li CM: Overexpression of aquaporin 4 in articular
chondrocytes exacerbates the severity of adjuvant-induced arthritis
in rats: An in vivo and in vitro study. J Inflamm (Lond). 14:62017.
View Article : Google Scholar : PubMed/NCBI
|