Introduction
Atrial fibrillation (AF) is the most common cardiac
arrhythmia, which is characterized by rapid, irregular electrical
and mechanical activation of the atria, leading to uncoordinated
contraction and atrial thrombi formation (1). AF has become an increasing
health-care burden; there is an increased risk of mortality and
morbidity in patients with AF, primarily due to stroke and heart
failure, resulting in disability and large healthcare costs
(2). The incidence of AF increases
with age (3,4). In addition, several factors have been
demonstrated to be associated with the development of AF, including
the presence of ischemic heart disease, hypertension, diabetes,
cardiopulmonary diseases and heavy alcohol consumption (5,6).
Furthermore, AF is a common postoperative complication,
particularly in patents following cardiothoracic surgery (7).
AF pathogenesis is very complex and multifactorial,
and the detailed molecular mechanism underlying AF development is
still unidentified. Beneficial efficacy has been reported for
current drugs used to treat AF, which primarily act by blocking
β-adrenergic receptors or ion channels; however, limited long-term
efficacy has been identified (8,9).
Thus, better understanding of the underlying molecular mechanism is
essential for developing novel therapeutic strategies for AF.
Previous studies indicated that atrial electrical remodeling and
structural remodeling are the pathological basis for AF generation
and maintenance (10,11). In addition, it is well accepted
that inflammation and oxidative stress serve an important role in
the development of AF (10,11).
Oxidase stress refers to the imbalance of
pro-oxidants and antioxidants in vivo, shifting the balance
towards pro-oxidants. An excess of reactive oxygen species (ROS)
can directly cause myocardial apoptosis and fibrosis (10). In addition, the increased level of
ROS, including superoxide and H2O2, has been
observed to be associated with AF development (12–14).
Increased levels of ROS also induce proteins, lipids and DNA
damage, and promotes inflammation by stimulating inflammatory
factor secretion from activated inflammatory cells (15,16).
Furthermore, ROS has been reported to be involved in cardiac
electrical and structural remodeling (17,18).
The promoting effects of ROS on inflammation and cardiac remodeling
increase susceptibility to AF. Antioxidants that are capable of
reducing peroxidation can reverse cardiac structural remodeling and
fibrosis (19). However, the
molecular mechanism of increased ROS production in AF and the
ROS-mediated downstream events have not been defined. NAD(P)H
oxidases (NOXs) are a major initiating source for increased ROS
production in cardiovascular diseases. The present study will
discuss its role in AF development.
Additionally, diabetes is an independent risk factor
for AF (20,21), and the mechanism underlying AF
development induced by diabetes may be associated with oxidase
stress (excess ROS) and inflammation (22,23).
Therefore, the present study was performed to explore the molecular
mechanism of increased ROS production and ROS-mediated downstream
events in AF induced by diabetes.
Materials and methods
Isolation and cultivation of atrial
fibroblasts
The present study obtained experimental animal use
approval from the Experimental Animal Administration Committee of
Tianjin Medical University and Tianjin Municipal Commission for
Experimental Animal Control (Tianjin, China), following the
guidelines established by the U.S. National Institutes of Health
(https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf).
Male New Zealand rabbits at 3–4 months old (n=60;
weight, 1.5–2.0 kg) provided by Tianjin Institute of Cardiology
(Tianjin, China) were used in the present study. Rabbits were
housed in a specific pathogen-free animal room maintained at 25°C,
60% humidity and on a 12-h light/dark cycle with free access to
food and water prior to experiments. Primarily, the rabbits were
anesthetized with an intravenous injection of ketamine (75 mg/kg)
and xylazine (0.75 mg/kg) and then sacrificed with a lethal dose of
thiopental (35 mg/kg). Hearts were removed via median thoracotomy
and immediately immersed in precooled phosphate-buffered saline
(PBS; Wuhan Boster Biological Technology, Ltd., Wuhan, China)
containing penicillin (100 U/ml) and streptomycin (100 µg/ml).
Subsequently, the epicardium and adipose tissue were cut using eye
scissors and the tissues of the left atrium were cut into 1
mm3 patches. These patches were rinsed with PBS until
the red blood cells were removed. Then atrial tissue samples were
subjected to enzymatic digestion (0.25% trypsin) at 37°C under
agitation (60 rpm) for 10 min and dispersed by gentle trituration
with a pipette. The supernatant was collected and mixed with 2 ml
Dulbecco's modified Eagle's medium (DMEM; Cellgro; Corning Inc.,
Corning, NY, USA) containing 4 mM glucose. The mixture was
centrifuged at 600 × g for 4 min at room temperature to precipitate
cells. Then cells were suspended with PBS and perfused with 0.1%
collagenase II for 10 min. DMEM containing 10% fetal bovine serum
(FBS; Junyao Biotechnology Co., Ltd., Beijing, China) was added to
the mixture to terminate digestion and centrifugation was performed
at 500 × g for 5 min at room temperature to pellet the cells. The
harvested cells were collected in DMEM containing 10% FBS and
incubated in an incubator with 37°C and 5% CO2 for 60–90
min. Following this, the adherent cells were harvested and
incubated in DMEM containing 20% FBS and 4 mM glucose at 37°C and
5% CO2. Cells were passaged three times.
Identification of atrial fibroblasts
by Masson trichrome staining
The cultured cells were placed on slides and fixed
in 4% formaldehyde at room temperature for 15 min. Following three
washes with DMEM, cells were stained in hematoxylin solution at
room temperature for 5 min. Excess dye was removed with
hydrochloric acid in ethanol and then cells were stained with
Ponceau-acid fuchsin at room temperature for 10 min. Cells were
immersed in 2% glacial acetic acid solution, followed by 1%
phosphomolybdic acid solution (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Without washing, cells were directly stained
with aniline blue at room temperature for 5 min. Excess dye was
removed with phosphomolybdic acid solution (1%) and glacial acetic
acid solution (1%). Finally, cells were dehydrated by an alcohol
gradient, washed with xylene and then sealed with neutral gum.
Identification of vimentin by
immunofluorescence
The cultured slides of atrial fibroblasts
(1×106/ml) were transferred to 6-well plates and washed
with cold PBS solution for 5 min. Then slides were treated with 4%
formaldehyde for fixation at 4°C for 15 min and permeated with 2%
Triton X-100 in PBS solution at 4°C for 20 min. Following washing
with PBS solution containing 0.25% Triton X-100, slides were
blocked with PBS containing 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) and 0.5% sheep serum albumin solution (BD Biosciences,
Franklin Lakes, NJ, CA, USA) for 4 h. Subsequently, slides were
incubated with mouse anti-vimentin antibodies (ab28028; Abcam,
Cambridge, MA, USA) diluted to 1:500 at 4°C overnight, followed by
horseradish peroxidase (HRP)-conjugated goat anti mouse IgG
(ab205719; Abcam) diluted to 1:1,000 for 1 h at room temperature.
Slides were counterstained with 4′6-diamidino-2-phenylindole for 10
min at room temperature and 10 random fields were viewed using a
confocal laser scanning microscope.
Treatments in atrial fibroblasts
The cultured atrial fibroblasts were randomly
divided into seven groups and treated as follows: i) NC group,
cells were cultured in DMEM medium containing 4 mM glucose without
any treatment; ii) Glu group, cells were cultured in DMEM medium
containing 25 mM glucose without other treatment; iii) Glu+Apo
group, cells were cultured in DMEM medium containing 25 mM glucose
and 100 µg/ml apocynin (Sigma-Aldrich; Merck KGaA); iv)
H2O2 group, cells were cultured in DMEM
medium containing 4 mM glucose and treated with 100 nmol/l
H2O2; v) H2O2+Apo
group, cells were cultured in DMEM medium containing 4 mM glucose
and 100 µg/ml apocynin, and treated with 100 nmol/l
H2O2; vi) Glu+H2O2
group, cells were cultured in DMEM medium containing 25 mM glucose
and treated with 100 nmol/l H2O2; vii)
Glu+H2O2+Apo group, cells were cultured in
DMEM medium containing 25 mM glucose and 100 µg/ml apocynin, and
treated with 100 nmol/l H2O2.
Detection of cell proliferation by MTS
assay
The cultured atrial fibroblasts (3,000/well)
following the different treatments were placed in 96-well plates
and detected for cell proliferation following incubation for 1, 3
and 5 days by MTS assay (Promega Corp., Madison, WI, USA). Briefly,
100 µl DMEM containing 20% MTS solution was added to each well of
the 96-well plates, which were then incubated in an incubator with
37°C and 5% CO2 for 2 h. The optical density (OD) was
then read at 490 nm with a microplate spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA). Cell proliferation was
evaluated according to the standard curve of OD value, which was
plotted based on different cell densities (0, 1×103,
3×103, 5×103, 1×104 and
2×104/well).
Western blot analysis
Following 72 h incubation, atrial fibroblasts were
washed with precooled PBS and lysed in radioimmunoprecipitation
assay buffer (150 mM NaCl, 1% Nonidet P40, 0.5% deoxysodium
cholate, 0.1% SDS and 50 mM Tris-HCl) solution containing 10 µl
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) for 20 min
on ice. The lysates were separated by centrifugation at 4°C and 600
× g for 20 min and the supernatant was collected. Total protein
concentration was evaluated using a bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Following this, protein samples (30 µg) were separated by 8–14%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). Then membranes were blocked in
5% non-fat milk in TBS with 0.05% Tween-20 (TBST) at room
temperature for 2 h. Following washing with TBST three times,
membranes were incubated with mouse anti-extracellular
signal-regulated kinase 1 (Erk1; ab32537; 1:1,000; Abcam),
phospho-Erk1 (ab131438; 1:1,000), Ras-related C3 botulinum toxin
substrate 1 precursor (Rac1; ab33186; 1:1,000), phospho-p38
(ab4822; 1:1,000), phospho-c-Jun N-terminal kinase 1 (Jnk1;
ab47337; 1:1,000), p22phox (ab75941; 1:1,000), matrix
metalloproteinase 2 (MMP2; ab2462; 1:1,000), MMP9 (ab58803; 1:500),
gp91phox (ab80508; 1:1,000), Jnk1 (ab213521; 1:1,000), p38
(ab-31828; 1:1,000) and β-actin (ab8226; 1:10,000) antibodies at
4°C overnight, followed by incubation with HRP-labeled secondary
antibodies (ab205719; 1:10,000; all from Abcam) at room temperature
for 2 h. Finally, membranes were washed with TBST and visualized by
enhanced chemiluminescence reagents and the Chemi-Doc imaging
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
expression of specific protein was quantified by the grey value
normalized to β-actin using TotalLab software version 1.11
(Ultra-Lum Inc., Claremont, CA, USA).
Statistical analysis
In the present study, data were presented as the
mean ± standard deviation and subjected to a one-way analysis of
variance followed by Duncan's multiple range test. P<0.05 was
considered to indicate a statistically significantly difference.
All statistical analysis was performed using SPSS software (version
21.0; IBM SPSS, Armonk, NY, USA).
Results
Cell morphology of atrial
fibroblasts
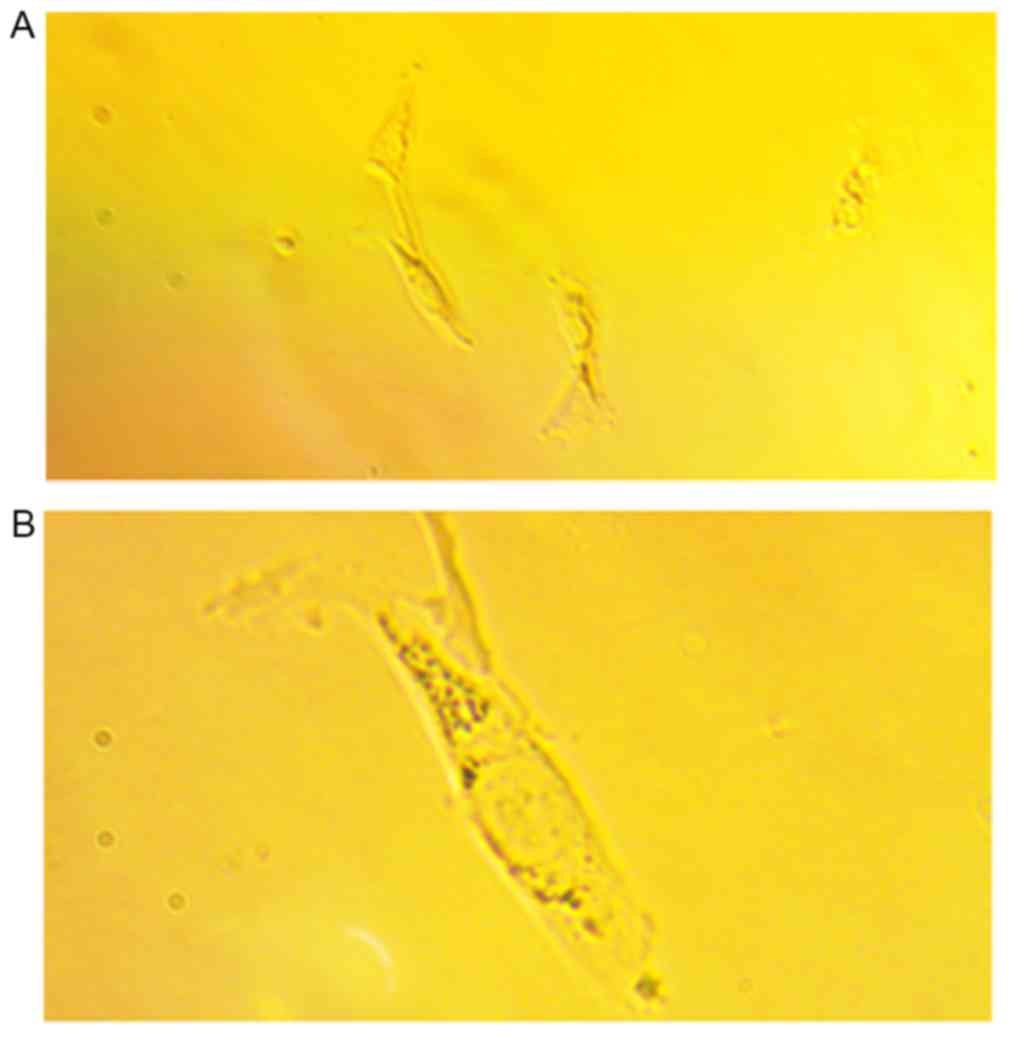
At 3 days following incubation, cell clumps crowded
with spindle cells were observed at ×100 magnification (Fig. 1A). At ×250 magnification, the
atrial fibroblasts were spindle-shaped with one or more
protuberances. Cell nuclei were large with finely dispersed
chromatin and 2–5 nucleoli (Fig.
1B). In addition, following Masson trichrome staining,
cytoplasmic granules such as ribosomes were stained in red and cell
nuclei were in pale blue. Granular or cord-like blue components
were observed, which represented the distribution of collagen in
atrial fibroblasts (data not shown).
Identification of vimentin in atrial
fibroblast
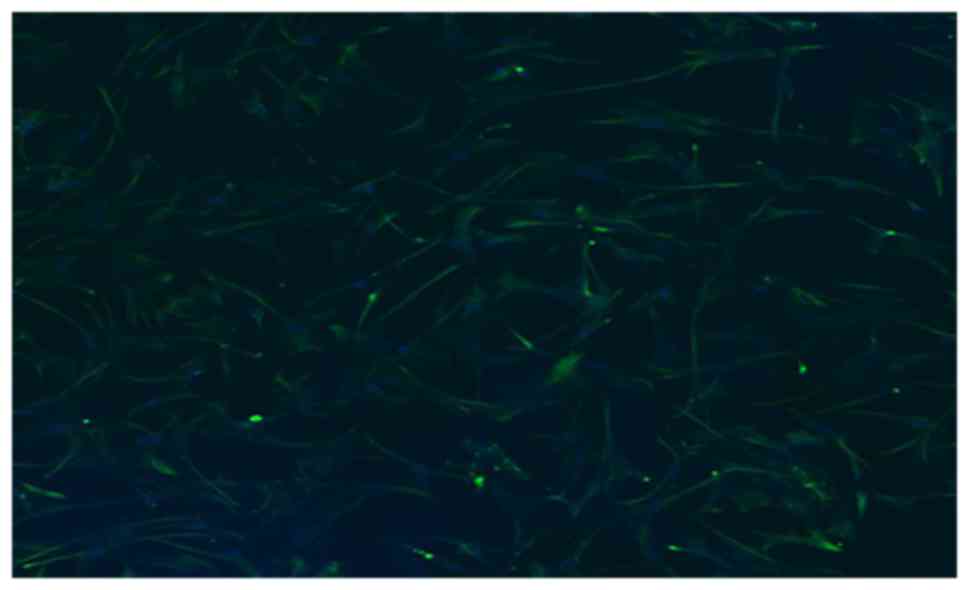
The expression of vimentin in atrial fibroblasts was
detected by immunofluorescence assay. As demonstrated in Fig. 2, a large number of
vimentin-positive cells (stained in green) were observed under
confocal laser scanning microscopy. This result indicated that the
atrial fibroblasts were successfully prepared.
Detection of cell proliferation
following the different treatments
In the present study, cell proliferation was
detected by MTS assay. To further identify the differences in cell
proliferation, the growth curves of atrial fibroblasts following
the different treatments based on cell counts on days 1, 3 and 5
were plotted and the slopes of the curves were calculated. The
average slopes in the different groups were presented in Fig. 3. The slope values in the Glu,
H2O2 and Glu+H2O2
groups were higher than those of the NC group, and there was a
significant increase in the slope value in the
H2O2 and Glu+H2O2
groups when compared with the NC group (P<0.05). The addition of
apocynin inhibited cell proliferation, as demonstrated by the
decrease in slope value in the Glu+Apo,
H2O2+Apo and
Glu+H2O2+Apo groups compared with the Glu,
H2O2 and Glu+H2O2
groups, respectively (Glu+H2O2+Apo vs.
Glu+H2O2, P<0.05). In addition, no significant
difference in slope value was observed when comparing the Glu+Apo,
H2O2+Apo and
Glu+H2O2+Apo groups with the NC group
(P>0.05).
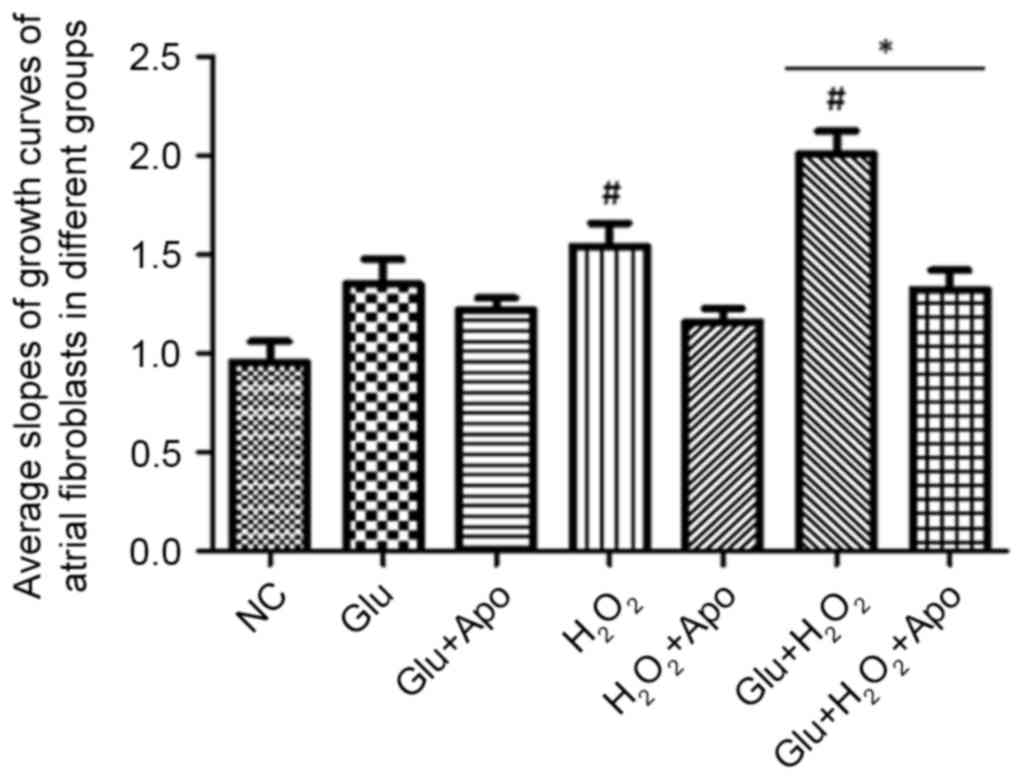 | Figure 3.Effects of high glucose,
H2O2 and apocynin on the slopes of growth
curves of atrial fibroblasts. #P<0.05 vs. NC group;
*P<0.05 as indicated. NC, normal control; Apo, apocynin; Glu
group, cells were treated with 25 mM glucose; Glu+Apo group, cells
were treated with 25 mM glucose and 100 µg/ml apocynin;
H2O2 group, cells were treated with 100
nmol/l H2O2; H2O2+Apo
group, cells were treated with 100 µg/ml apocynin and 100 nmol/l
H2O2; Glu+H2O2 group,
cells were treated with 25 mM glucose and 100 nmol/l
H2O2; Glu+H2O2+Apo
group, cells were treated with 25 mM glucose, 100 µg/ml apocynin
and 100 nmol/l H2O2. |
Detection of NADPH oxidase expression. Western
blotting was performed to detect the expression of the NADPH
oxidase subunits including Rac1 (regulatory subunit), p22phox and
gp91phox (structural subunits), and the results were presented in
Fig. 4. High glucose,
H2O2 stimulation and a combination of high
glucose and H2O2 treatment significantly
increased the expression of Rac1 compared to the NC group
(P<0.05) (Fig. 4A). However,
addition of apocynin suppressed the elevated expression of Rac1
induced by high glucose, H2O2 stimulation and
their combination, and a marked reduction was observed between
Glu+Apo and Glu groups, and Glu+H2O2+Apo and
Glu+H2O2 groups (P<0.05).
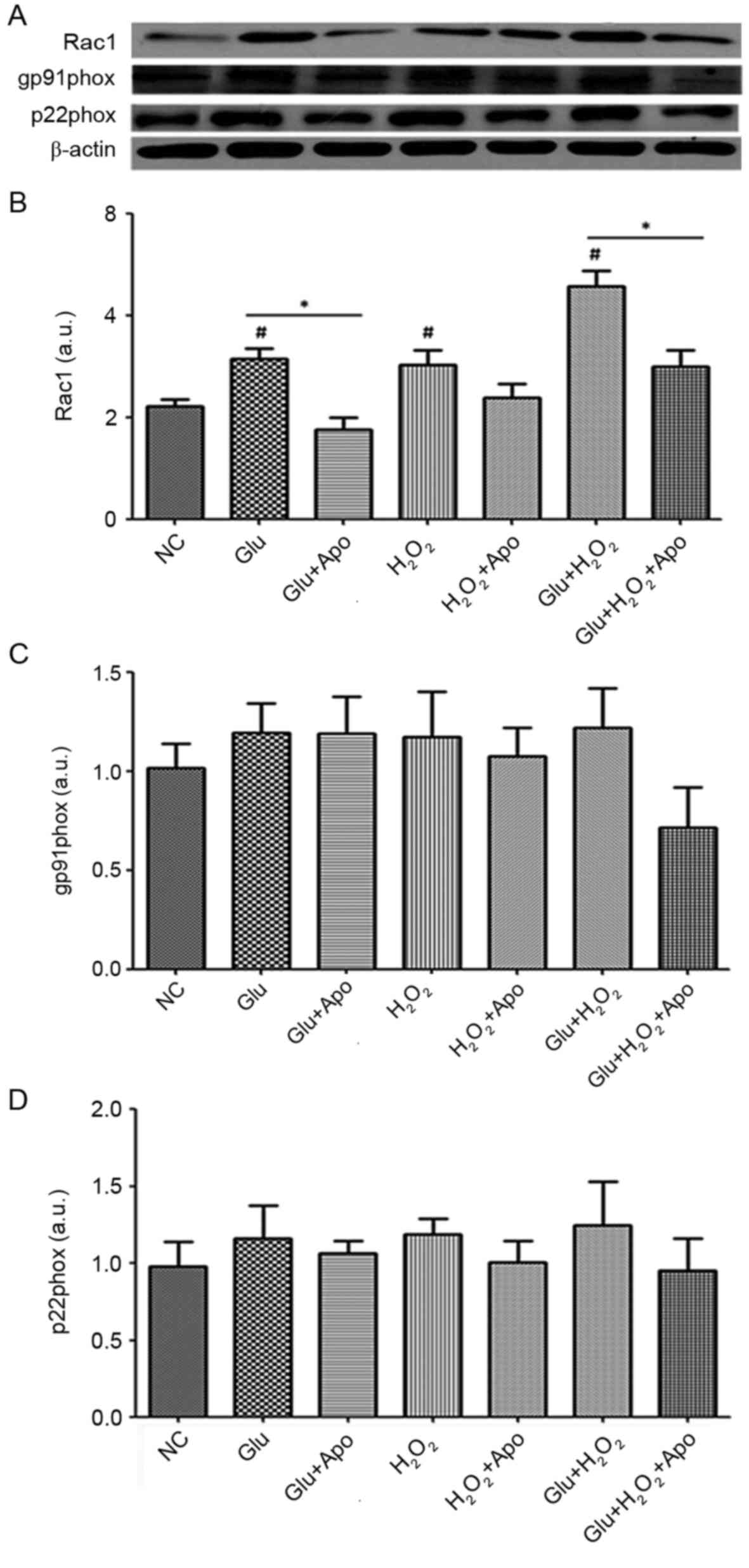 | Figure 4.Effects of high glucose,
H2O2 and apocynin on the expression of
NAD(P)H oxidative subunits. (A) Representative Western blotting
bands of Rac1, gp91phox and p22phox. (B) Quantitative expression
levels of Rac1 protein. (C) Quantitative expression levels of
gp91phox protein. (D) Quantitative expression levels of p22phox
protein. #P<0.05 vs. NC group; *P<0.05 as
indicated. NC, normal control; Apo, apocynin; Glu group, cells were
treated with 25 mM glucose; Glu+Apo group, cells were treated with
25 mM glucose and 100 µg/ml apocynin; H2O2
group, cells were treated with 100 nmol/l
H2O2; H2O2+Apo group,
cells were treated with 100 µg/ml apocynin and 100 nmol/l
H2O2; Glu+H2O2 group,
cells were treated with 25 mM glucose and 100 nmol/l
H2O2; Glu+H2O2+Apo
group, cells were treated with 25 mM glucose, 100 µg/ml apocynin
and 100 nmol/l H2O2; Rac1, Ras-related C3
botulinum toxin substrate 1 precursor; a.u., arbitrary units. |
Additionally, high glucose and
H2O2 stimulation did not affect the
expression of the structural subunits (p22phox and gp91phox), and
no significant differences in p22phox and gp91phox expression were
observed when comparing Glu, H2O2 and
Glu+H2O2 groups with the NC group (Fig. 4B and C). The addition of NADPH
oxidase inhibitor apocynin did not significantly alter the
expression of p22phox and gp91phox.
Detection of the expression and
activity of key factors involved in mitogen-activated protein
kinase (MAPK) signaling pathways
To reveal the ROS-mediated downstream events, the
present study also detected the expression of several key factors
involved in MAPK signaling pathways and the results were shown in
Fig. 5. When compared with the NC
group, no significant changes were observed in Erk1 and
phospho-Erk1 expression following treatment with high glucose,
H2O2 stimulation and their combination. The
addition of apocynin did not significantly change the expression of
Erk1 and phospho-Erk1 expression when compared with the
corresponding treatment. Although there was no significant change
in Jnk1 expression following high glucose and
H2O2 treatment, the phospho-Jnk1 expression
was significantly increased following H2O2
stimulation and the combination of high glucose and
H2O2 stimulation (P<0.05). Additionally,
the expression of phospho-Jnk1 was significantly reduced following
the addition of apocynin when compared with the
Glu+H2O2 and H2O2
groups (P<0.05). In addition, high glucose and
H2O2 treatment induced an increase in p38 and
phospho-p38 expression, and a significant difference was observed
following H2O2 treatment and combined
treatments for p38 expression, and for all treatments when
analyzing phospho-p38 expression (P<0.05). The increased
expression of p38 and phospho-p38 expression was significantly
alleviated by the addition of apocynin (P<0.05).
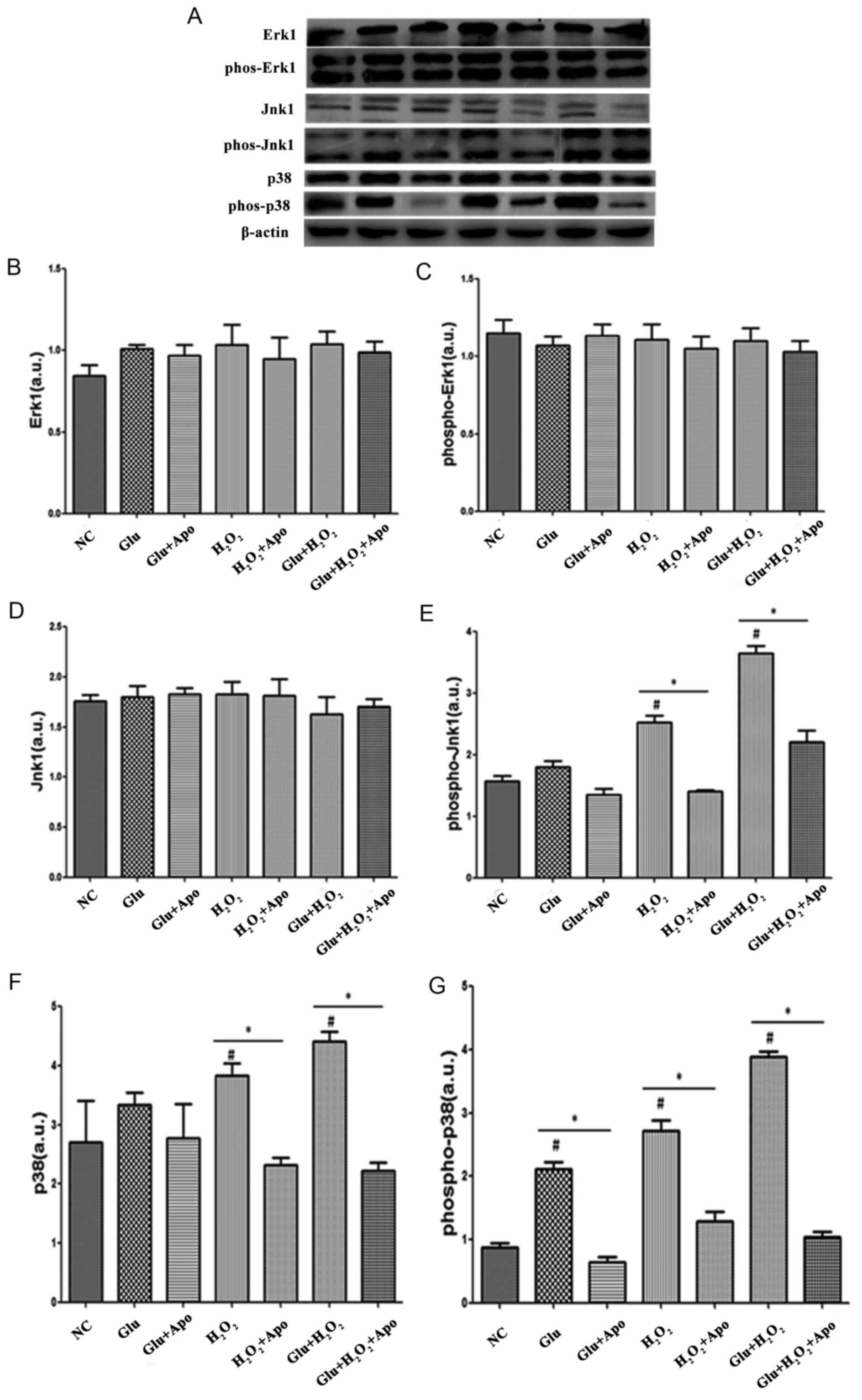 | Figure 5.Effects of high glucose,
H2O2 and apocynin on the expression of key
factors involved in mitogen-activated protein kinase signaling
pathways. (A) Representative western blotting bands of Erk1,
phospho-Erk1, Jnk1, phospho-Jnk1, p38 and phospho-p38. (B)
Quantitative expression levels of Erk1 protein. (C) Quantitative
expression levels of phospho-Erk1 protein. (D) Quantitative
expression levels of Jnk1 protein. (E) Quantitative expression
levels of phospho-Jnk1 protein. (F) Quantitative expression levels
of p38 protein. (G) Quantitative expression levels of phospho-p38
protein. #P<0.05 vs. NC group; *P<0.05, as
indicated. Erk1, extracellular signal-regulated kinase 1;
phospho/phos-, phosphorylated; Jnk1, c-Jun N-terminal kinase 1; NC,
normal control; Apo, apocynin; Glu group, cells were treated with
25 mM glucose; Glu+Apo group, cells were treated with 25 mM glucose
and 100 µg/ml apocynin; H2O2 group, cells
were treated with 100 nmol/l H2O2;
H2O2+Apo group, cells were treated with 100
µg/ml apocynin and 100 nmol/l H2O2;
Glu+H2O2 group, cells were treated with 25 mM
glucose and 100 nmol/l H2O2;
Glu+H2O2+Apo group, cells were treated with
25 mM glucose, 100 µg/ml apocynin and 100 nmol/l
H2O2; a.u., arbitrary units. |
Detection of the expression of
MMPs
Western blotting was also performed to explore the
expression of MMP2 and MMP9. As displayed in Fig. 6, the expression of MMP2 was
undetectable in NC and Glu groups, and H2O2
stimulation induced a low expression of MMP2. The combined
treatment of glucose and H2O2 induced an
increased expression of MMP2, while the addition of apocynin did
not significantly alter this increased expression of MMP2 induced
by glucose and H2O2 stimulation. In addition,
glucose, H2O2 stimulation and the combination
treatment significantly increased the expression of MMP9 when
compared with the NC group (P<0.05). Furthermore, the addition
of apocynin markedly reduced the expression of MMP9 when compared
with the corresponding group (Glu vs. Glu+Apo,
H2O2 vs. H2O2+Apo,
Glu+H2O2 vs.
Glu+H2O2+Apo; P<0.05).
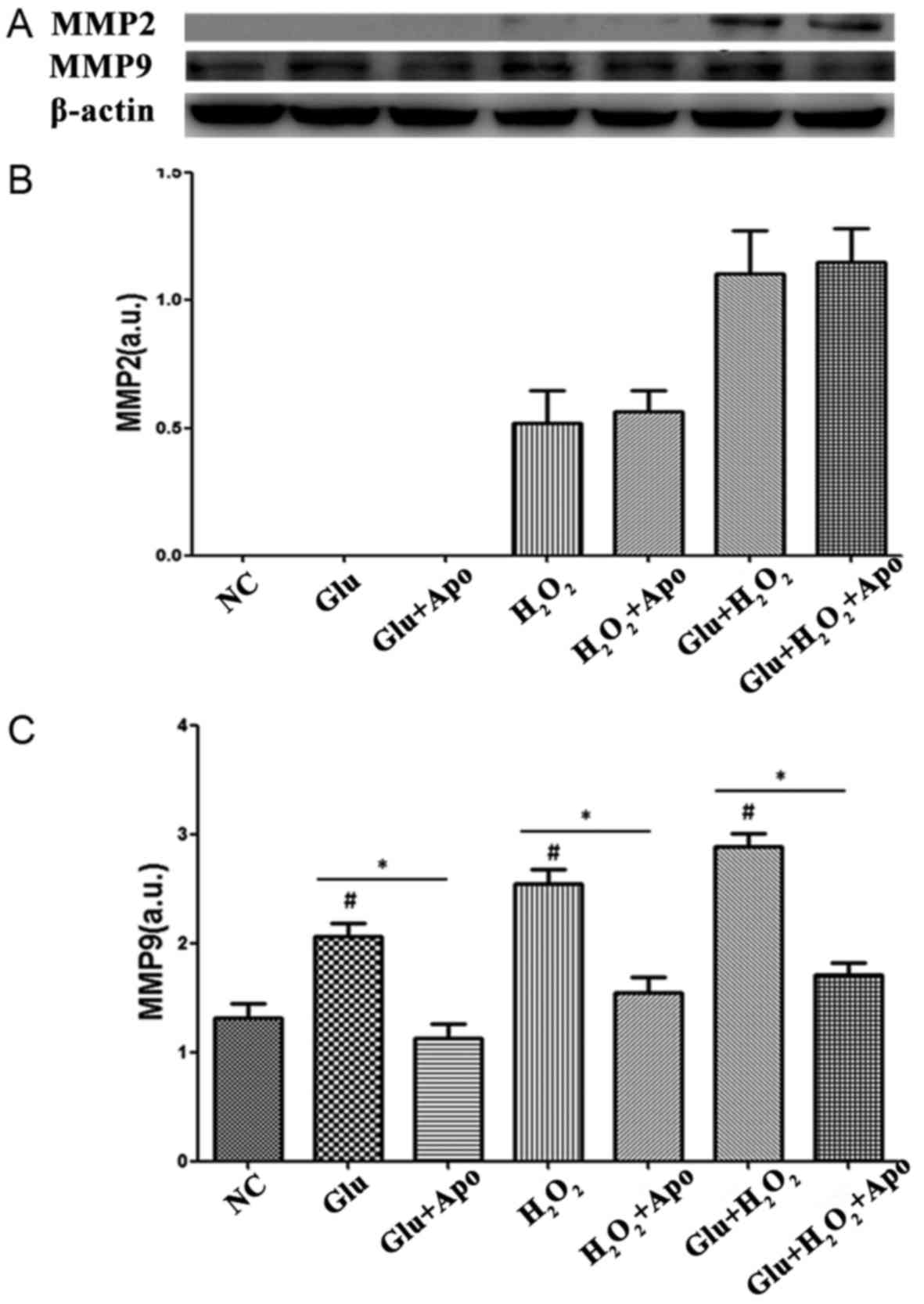 | Figure 6.Effects of high glucose,
H2O2 and apocynin on the expression of MMPs.
(A) Representative Western blotting bands of MMP2 and MMP9. (B)
Quantitative expression levels of MMP2 protein. (C) Quantitative
expression levels of MMP9 protein. #P<0.05 vs. NC
group; *P<0.05 as indicated. MMP, matrix metalloproteinase; NC,
normal control; Apo, apocynin; Glu group, cells were treated with
25 mM glucose; Glu+Apo group, cells were treated with 25 mM glucose
and 100 µg/ml apocynin; H2O2 group, cells
were treated with 100 nmol/l H2O2;
H2O2+Apo group, cells were treated with 100
µg/ml apocynin and 100 nmol/l H2O2;
Glu+H2O2 group, cells were treated with 25 mM
glucose and 100 nmol/l H2O2;
Glu+H2O2+Apo group, cells were treated with
25 mM glucose, 100 µg/ml apocynin, and 100 nmol/l
H2O2; a.u., arbitrary units. |
Discussion
Diabetes is a significant AF risk factor accounting
for 10–25% of AF cases (24),
while the underlying mechanisms associated with diabetes and AF
remain under speculation. Increasing evidence has indicated that
oxidative stress serves an important role in the pathogenesis of
AF, and an increased level of ROS has been reported to be
associated with AF development and maintenance (13,25).
In addition, ROS production has been suggested to be involved in
the AF pathophysiology in a diabetic rabbit model (26,27).
The present study was performed to investigate the molecular
mechanisms of increased ROS production in AF induced by diabetes as
well as ROS-mediated downstream signaling pathways. The primary
findings included: i) The atrial fibroblasts were successfully
prepared from the atrium of the rabbits using enzyme digestion and
differential adhesion; ii) high glucose and
H2O2 stimulation promoted cell proliferation
and the addition of apocynin inhibited this increase in atrial
fibroblast proliferation induced by high glucose and
H2O2 stimulation; iii) high glucose and
H2O2 stimulation induced an increase in the
expression of MMP9, NOXs subunits and key factors involved in the
MAPK signaling pathways; iv) and the promoting effects of high
glucose and H2O2 stimulation were attenuated
by apocynin.
In the present study, atrial tissue samples were
collected from the left atrium of rabbits and were digested by 2
enzymes (trypsin and collagenase II). Then, the atrial fibroblasts
were obtained from differential adhesion. Following cell
collection, the cultured cells were identified by cell morphology
under a microscope and vimentin expression by immunofluorescence
assay. The collected cells were spindle-shaped with one or more
protuberances. Cell nuclei were large with finely dispersed
chromatin and 2–5 nucleoli. Additionally, collagen was widely
distributed in collected cells following Masson trichrome staining
(data not shown). Vimentin, a type of intermediate filament
protein, is a specific marker of fibroblasts and is widely used for
identifying fibroblasts (28,29).
Following the results in the present study, a large number of
vimentin-positive cells were observed under confocal laser scanning
microscope. All of these results indicated that atrial fibroblasts
were successfully prepared.
Fibroblasts account for 75% of the total number of
cardiac cells (30). As these
cells are very small, fibroblasts only account for 10–15% of the
total volume of the heart. The fibroblasts combined with the
extracellular matrix constitute the cytoskeleton of heart (31). However, abnormal proliferation and
differentiation of fibroblasts could induce fibrosis of cardiac
tissues and dysfunction of cardiac function including AF. A
previous study indicated that oxidative stress is one of the
activators for abnormal proliferation and differentiation of
fibroblasts (32). In agreement
with this, H2O2 stimulation induced an
increase in the proliferation of atrial fibroblasts in the present
study. In addition, hyperglycemia can enhance the glycation of
proteins and lipids, leading to an increased generation of ROS
(33,34). Thus, the imbalance between ROS
production and scavenging through antioxidant mechanisms in
diabetes results in increased oxidative stress. As presented in
this study, a high glucose concentration also induced an increased
proliferation of atrial fibroblasts, and the combination of high
glucose and H2O2 stimulation presented
markedly promotional effects on atrial fibroblast
proliferation.
To reveal the underlying mechanism of increased ROS
production and ROS-mediated downstream events, the present study
detected the expression of several molecules including NOXs
subunits, key molecules involved in MAPK signaling pathways as well
as MMPs. NOXs are one of the primary sources for ROS and have been
shown to be involved in various cardiovascular disorders through
participating in redox signaling (35,36).
Following the results in the present study, the expression of NOXs
regulatory subunit Rac1 was significantly increased following high
glucose and H2O2 stimulation, which was
markedly attenuated by the addition of apocynin (the NOX
inhibitor). Recently, apocynin has been demonstrated to prevent AF
development and attenuate atrial remodeling in alloxan-induced
diabetic rabbits (37). The
present results indicated that ROS is excessively produced by NOXs
in diabetes, contributing to the development of AF. A previous
study indicated that diabetes enhanced oxidative stress,
inflammatory responses in coronary cells as well the expression of
MAPK signaling pathways (38).
Several factors involved in the MAPK pathway are known to regulate
the cellular response to stress, apoptosis and growth signals
(36). As demonstrated in previous
studies, phospho-p38 and JNK are activated by
H2O2 in perfused rat hearts (39), and there is an increased expression
of phospho-p38, phospho-JNK, ERK and phospho-ERK in diabetic
rabbits (37). In the present
study, it was demonstrated that a high level of glucose and
H2O2 stimulation increased the expression of
phospho-Jnk1, p38 and phospho-p38. These results indicated that the
diabetes induced increase in ROS production in AF may be involved
in MAPK signaling pathways, primarily in the signaling of
phospho-JNK, p38 and phospho-p38. In addition, the expression of
MMP2 and MMP9 were detected following different treatments. MMP9
expression was significantly increased following high glucose and
H2O2 treatment, and the addition of the NOX
inhibitor markedly attenuated the promotional effects on MMP9
expression induced by high glucose and H2O2
expression. However, MM2 expression did not markedly change
following the different treatments. These results of the present
study were consistent with a previous description, which reported
that the expression of MMP9 increased during fibrillation of atrial
tissue (40). These results
indicated that the oxidative stress primarily present or induced by
hyperglycemia would lead to the increased expression of MMP9, which
may have contributed to the atrial structural remodeling during AF
development.
In conclusion, the atrial fibroblasts were
successfully prepared using enzyme digestion and differential
adhesion in the present study. Hyperglycemia and oxidative stress
induced an increase in atrial fibroblasts proliferation, leading to
fibrosis of cardiac tissues and dysfunction of cardiac function,
including AF. In addition, during AF development, excessive
production of ROS was mainly derived from NOX activity and
activation of ROS induced the expression of the MAPK signaling
pathway, primarily including phospho-JNK, p38 and phospho-p38 and
MMP9. These results provide the theoretical basis for the
anti-oxidative therapy of atrial fibrillation caused by
diabetes.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 30900618,
81270245 and 81570298), the National Natural Science Foundation of
China Youth Science Foundation Project (grant no. 81700304), the
Tianjin Natural Science Foundation (grant no. 16JCZDJC34900), the
China Postdoctoral Science Foundation funded project (grant no.
2015M571272), the Scientific Research Fund Project of Key
Laboratory of Second Hospital of Tianjin Medical University (grant
no. 2017ZDSYS02), and the Youth Research Fund Project of Central
Laboratory of Second Hospital of Tianjin Medical University (grant
no. 2016ydey03).
References
|
1
|
Čihák R, Haman L and Heinc P: Summary of
the 2012 focused update of the ESC Guidelines for the management of
atrial fibrillation: Prepared by the Czech Society of Cardiology.
Cor Et Vasa. 54:e341–e351. 2012. View Article : Google Scholar
|
|
2
|
Lip GY and Tse HF: Management of atrial
fibrillation. Lancet. 370:604–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolf PA, Abbott RD and Kannel WB: Atrial
fibrillation as an independent risk factor for stroke: The
Framingham study. Stroke. 22:983–988. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalus JS, White CM, Caron MF, Coleman CI,
Takata H and Kluger J: Indicators of atrial fibrillation risk in
cardiac surgery patients on prophylactic amiodarone. Ann Thorac
Surg. 77:1288–1292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukahara K, Kotoh K, Doi T, Misaki T and
Sumi S: Impact of preoperative atrial fibrillation on the late
outcome of off-pump coronary artery bypass surgery. Eur J
Cardiothorac Surg. 38:366–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ad N, Henry L and Hunt S: The impact of
surgical ablation in patients with low ejection fraction, heart
failure, and atrial fibrillation. Eur J Cardiothorac Surg.
40:70–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanagaratnam P, Cherian A, Stanbridge RD,
Glenville B, Severs NJ and Peters NS: Relationship between
connexins and atrial activation during human atrial fibrillation. J
Cardiovasc Electrophysiol. 15:206–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel C, Salahuddin M, Jones A, Patel A,
Yan GX and Kowey PR: Atrial fibrillation: Pharmacological therapy.
Curr Probl Cardiol. 36:87–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanguinetti MC and Bennett PB:
Antiarrhythmic drug target choices and screening. Circ Res.
93:491–499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dudley SC Jr, Hoch NE, McCann LA,
Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI and
Langberg J: Atrial fibrillation increases production of superoxide
by the left atrium and left atrial appendage: Role of the NADPH and
xanthine oxidases. Circulation. 112:1266–1273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neuman RB, Bloom HL, Shukrullah I, Darrow
LA, Kleinbaum D, Jones DP and Dudley SC Jr: Oxidative stress
markers are associated with persistent atrial fibrillation. Clin
Chem. 53:1652–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang JP, Chen MC, Liu WH, Yang CH, Chen
CJ, Chen YL, Pan KL, Tsai TH and Chang HW: Atrial myocardial nox2
containing NADPH oxidase activity contribution to oxidative stress
in mitral regurgitation: Potential mechanism for atrial remodeling.
Cardiovasc Pathol. 20:99–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YM, Guzik TJ, Zhang YH, Zhang MH,
Kattach H, Ratnatunga C, Pillai R, Channon KM and Casadei B: A
myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative
stress in human atrial fibrillation. Circ Res. 97:629–636. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Youn JY, Kim AY, Ramirez RJ, Gao
L, Ngo D, Chen P, Scovotti J, Mahajan A and Cai H: NOX4-Dependent
hydrogen peroxide overproduction in human atrial fibrillation and
HL-1 atrial cells: Relationship to hypertension. Front Physiol.
3:1402012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Babusíková E, Kaplán P, Lehotský J,
Jesenák M and Dobrota D: Oxidative modification of rat cardiac
mitochondrial membranes and myofibrils by hydroxyl radicals. Gen
Physiol Biophys. 23:327–335. 2004.PubMed/NCBI
|
|
16
|
Lai LP, Su MJ, Lin JL, Lin FY, Tsai CH,
Chen YS, Huang SK, Tseng YZ and Lien WP: Down-regulation of L-type
calcium channel and sarcoplasmic reticular Ca(2+)-ATPase mRNA in
human atrial fibrillation without significant change in the mRNA of
ryanodine receptor, calsequestrin and phospholamban: An insight
into the mechanism of atrial electrical remodeling. J Am Coll
Cardiol. 33:1231–1237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li GR and Nattel S: Properties of human
atrial ICa at physiological temperatures and relevance to action
potential. Am J Physiol. 272:H227–H235. 1997.PubMed/NCBI
|
|
18
|
Carnes CA, Chung MK, Nakayama T, Nakayama
H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM,
et al: Ascorbate attenuates atrial pacing-induced peroxynitrite
formation and electrical remodeling and decreases the incidence of
postoperative atrial fibrillation. Circ Res. 89:E32–E38. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson EJ, Kypson AP, Rodriguez E,
Anderson CA, Lehr EJ and Neufer PD: Substrate-specific derangements
in mitochondrial metabolism and redox balance in the atrium of the
type 2 diabetic human heart. J Am Coll Cardiol. 54:1891–1898. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nichols GA, Reinier K and Chugh SS:
Independent contribution of diabetes to increased prevalence and
incidence of atrial fibrillation. Diabetes Care. 32:1851–1856.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goudis CA, Korantzopoulos P, Ntalas IV,
Kallergis EM, Liu T and Ketikoglou DG: Diabetes mellitus and atrial
fibrillation: Pathophysiological mechanisms and potential upstream
therapies. Int J Cardiol. 184:617–622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Heerebeek L, Hamdani N, Handoko ML,
Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ,
Schalkwijk CG, Bronzwaer JG, Diamant M, et al: Diastolic stiffness
of the failing diabetic heart: Importance of fibrosis, advanced
glycation end products, and myocyte resting tension. Circulation.
117:43–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Q, Liu T, Ng CY and Li G: diabetes
mellitus and atrial remodeling: Mechanisms and potential upstream
therapies. Cardiovasc Ther. 32:233–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohammad MR, Hashemzadeh M and Jamal MM:
Diabetes mellitus is a strong, independent risk for atrial
fibrillation and flutter in addition to other cardiovascular
disease. Int J Cardiol. 105:315–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korantzopoulos P, Kolettis TM, Galaris D
and Goudevenos JA: The role of oxidative stress in the pathogenesis
and perpetuation of atrial fibrillation. Int J Cardiol.
115:135–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu T, Zhao H, Li J, Korantzopoulos P and
Li G: Rosiglitazone attenuates atrial structural remodeling and
atrial fibrillation promotion in alloxan-induced diabetic rabbits.
Cardiovasc Ther. 32:178–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu H, Li G, Liu C, Li J, Wang X, Cheng L
and Liu T: Probucol prevents atrial remodeling by inhibiting
oxidative stress and TNF-α/NF-κB/TGF-β signal transduction pathway
in alloxan-induced diabetic rabbits. J Cardiovasc Electrophysiol.
26:211–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ieda M, Fu JD, Delgado-Olguin P, Vedantham
V, Hayashi Y, Bruneau BG and Srivastava D: Direct reprogramming of
fibroblasts into functional cardiomyocytes by defined factors.
Cell. 142:375–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shiraishi I, Takamatsu T, Minamikawa T,
Onouchi Z and Fujita S: Quantitative histological analysis of the
human sinoatrial node during growth and aging. Circulation.
85:2176–2184. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shiraishi I, Takamatsu T, Minamikawa T and
Fujita S: 3-D observation of actin filaments during cardiac
myofibrinogenesis in chick embryo using a confocal laser scanning
microscope. Anat Embryol (Berl). 185:401–408. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burstein B and Nattel S: Atrial fibrosis:
Mechanisms and clinical relevance in atrial fibrillation. J Am Coll
Cardiol. 51:802–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sayed AA, Khalifa M and Abd el-Latif FF:
Fenugreek attenuation of diabetic nephropathy in alloxan-diabetic
rats: Attenuation of diabetic nephropathy in rats. J Physiol
Biochem. 68:263–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aljofan M and Ding H: High glucose
increases expression of cyclooxygenase-2, increases oxidative
stress and decreases the generation of nitric oxide in mouse
microvessel endothelial cells. J Cell Physiol. 222:669–675.
2010.PubMed/NCBI
|
|
35
|
Li JM, Gall NP, Grieve DJ, Chen M and Shah
AM: Activation of NADPH oxidase during progression of cardiac
hypertrophy to failure. Hypertension. 40:477–484. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Griendling KK, Sorescu D and Ushio-Fukai
M: NAD(P)H oxidase: Role in cardiovascular biology and disease.
Circ Res. 86:494–501. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiu J, Zhao J, Li J, Liang X, Yang Y,
Zhang Z, Zhang X, Fu H, Korantzopoulos P, Liu T and Li G: NADPH
oxidase inhibitor apocynin prevents atrial remodeling in
alloxan-induced diabetic rabbits. Int J Cardiol. 221:812–819. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Zalewski A, Liu Y, Mazurek T,
Cowan S, Martin JL, Hofmann SM, Vlassara H and Shi Y:
Diabetes-induced oxidative stress and low-grade inflammation in
porcine coronary arteries. Circulation. 108:472–478. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Clerk A, Fuller SJ, Michael A and Sugden
PH: Stimulation of ‘stress-regulated’ mitogen-activated protein
kinases (stress-activated protein kinases/c-Jun N-terminal kinases
and p38-mitogen-activated protein kinases) in perfused rat hearts
by oxidative and other stresses. J Biol Chem. 273:7228–7234. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakano Y, Niida S, Dote K, Takenaka S,
Hirao H, Miura F, Ishida M, Shingu T, Sueda T, Yoshizumi M and
Chayama K: Matrix metalloproteinase-9 contributes to human atrial
remodeling during atrial fibrillation. J Am Coll Cardiol.
43:818–825. 2004. View Article : Google Scholar : PubMed/NCBI
|