Introduction
Cerebrovascular disease has been identified as the
third leading cause of mortality (1), and cerebrovascular accidents are
among the main causes of mortality and disability worldwide with
great clinical and socioeconomic impact. Ischemic stroke accounts
for ~80% of all cerebrovascular accidents worldwide (2), while carotid artery stenosis or
occlusion accounts for 15–20% of ischemic stroke (3), as it can result in brain ischemia and
infarction. Carotid endarterectomy is the standard operation for
the prevention of cerebral infarction, whereas vascular restenosis
is one of the major complications following vascular anastomosis,
often causing failure of the surgery (4). The 3-year rate of restenosis of ≥50%
of anastomotic sites following carotid endarterectomy has been
reported as 5% (5). Therefore, the
prevention of restenosis following vascular anastomosis is of great
significance for patients at risk of cerebrovascular accidents.
Postoperative vascular restenosis is a complex
pathophysiological process. Excessive proliferation and migration
of vascular smooth muscle cells (VSMCs) are critical for the
development of restenosis, and inflammatory responses are involved
in the molecular mechanisms underlying these events (6). Following surgically-induced vascular
anastomosis, the injured endangium and the exposed subendothelial
tissues have been demonstrated to induce the expression of numerous
biologically active factors, including chemokines, cytokines and
cell adhesion molecules, which can trigger inflammatory responses,
leading to leukocyte adhesion and platelet activation, and can
enhance VSMC proliferation and migration (7). Numerous alterations have been
reported to occur in VSMCs following vascular injury, including
their transition from a contractile phenotype to an active
phenotype with secretory functions; secretory VSMCs can produce
paracrine mediators, including tumor necrosis factor (TNF)-α and
interleukin (IL)-6 (8). This
phenotypic alteration further enhances the synthesis of cytokines
and growth factors, which promote the proliferation and migration
of VSMCs and maintain their active state in the inflammatory
environment. This vicious cycle of events causes the sustained
production of cytokines and growth factors that ultimately lead to
the development of intimal hyperplasia. Therefore, it may be
hypothesized that the inhibition of the inflammatory response
following vascular injury is a promising strategy to suppress VSMC
proliferation, and thus vascular hyperplasia (9).
TNF-α and IL-6 are critical mediators during
inflammatory responses. TNF-α and IL-6 have been reported to be
involved in nuclear factor (NF)-κB-mediated pathways (10), which can stimulate DNA synthesis
and intercellular adhesion molecule-1 expression. These molecular
pathways have been revealed to be involved in VSMC proliferation
and migration, as well as fibroblast proliferation, thus leading to
intimal hyperplasia (11,12). NF-κB-mediated signaling pathways
have been reported to regulate the transcription of several target
genes (13). In the absence of
appropriate stimuli, NF-κB and its inhibitory factor κB (IκB)
combine to form an inactive complex in the cytoplasm. Various
stimuli can activate the IκB kinase in the cell membrane to promote
IκB phosphorylation and degradation, leading to the release of
active NF-κB. NF-κB can then translocate to the nucleus, where it
can modulate the transcription of several proinflammatory genes,
including TNF-α and IL-6. These proinflammatory factors have been
reported to mediate chronic inflammatory responses in the vascular
endothelium and promote VSMC proliferation and migration to the
tunica intima (14). Proliferating
cell nuclear antigen (PCNA) has been identified as an accessory
protein of DNA polymerase δ, which is involved in DNA replication,
and is thus critical for the initiation of cellular proliferation.
The expression levels of PCNA have been demonstrated to reliably
reflect the rate of cellular proliferation (15). Therefore, the detection of PCNA
expression levels in vascular tissue allows the evaluation of VSMC
proliferation. A previous study reported that synthetic selectin
can inhibit cerebral ischemia-reperfusion injury in rats (16). Thus, in the present study synthetic
E-selectin was selected for further evaluation.
In the present study, rats underwent vascular
anastomosis and were treated with synthetic E-selectin in order to
investigate the putative inhibitory effects of E-selectin on VSMC
proliferation and thus arterial restenosis. Synthetic E-selectin
was demonstrated to suppress the expression of inflammatory
factors, thus suggesting that it may be able to attenuate
inflammatory responses and inhibit VSMC proliferation.
Materials and methods
Animals
A total of 90 adult male Sprague-Dawley rats (age,
8–10 weeks; weight, 250–300 g) were purchased from the Animal
Center of the Chinese Academy of Sciences (Shanghai, China). The
rats were kept in standard cages (5 rats/cage) and housed in
temperature- (~25°C) and humidity- (50–60%) controlled animal
quarters, under 12/12 h light/dark cycles with free access to food
and water. Care was taken to avoid unnecessary stress and
discomfort to the rats throughout the experimental period.
The animal use and care protocols, including all
operation procedures, were approved by the Institutional Animal
Care Committee of Soochow University (Jiangsu, China) and conformed
to the Guide for the Care and Use of Laboratory Animals by the
National Institutes of Health (17).
Carotid artery anastomosis model
Rats were anesthetized with 4% chloral hydrate (400
mg/kg) via intraperitoneal injection. The body was placed in the
supine position, and the limbs and head were fixed with rubber
bands on the operating table. Body temperature was maintained at
37.5±0.5°C with an automatic heating pad (Letica Scientific
Instruments, Barcelona, Spain). The anterior part of the neck was
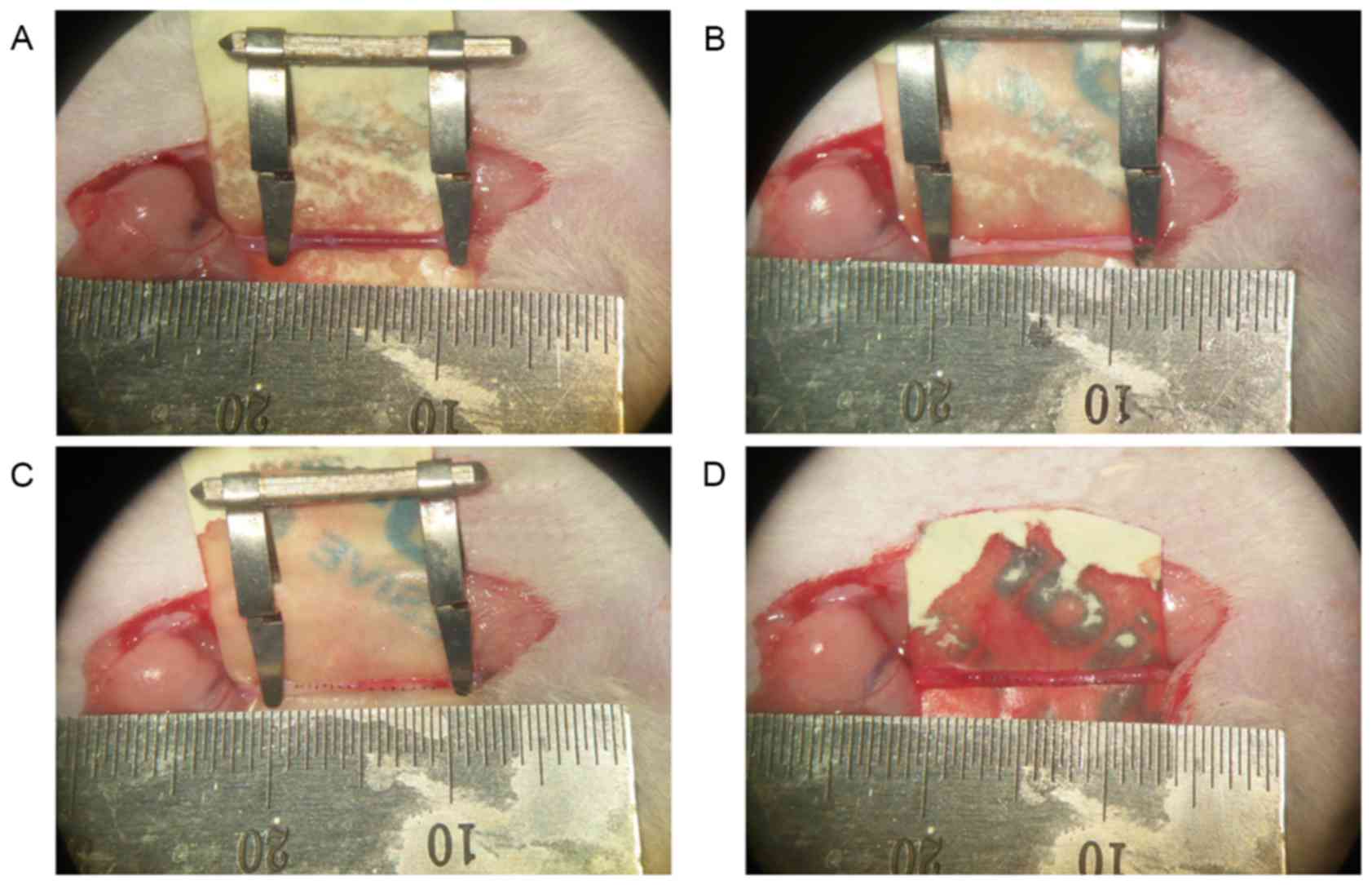
shaved and the exposed skin was sterilized. A ~1.5 cm incision was
made along the upper neck midline, and the muscle layer was
dissected under a surgical microscope to isolate the right common
carotid artery. Two vascular occlusion clips were placed on the
artery to temporarily block blood flow; the distance between the
clips was ~1 cm. A longitudinal incision was made in the blood
vessel wall between the clips and the incision was closed using
interrupted sutures (Fig. 1).
Following suturing, a Microvascular Doppler (Nanjing Kejin
Industrial Co., Ltd, Nanjing, China) was used to monitor blood flow
in the proximal and distal end of the anastomosis, to determine
whether the anastomosis remained unblocked. A total of ~15 min
elapsed from the initiation of blood vessel blockage to the
completion of vascular anastomosis. Then 25 U Heparin (Yan Sheng
Co., Shanghai, China) was injected intravenously to prevent the
formation of blood clots. The skin was continuously sutured and
disinfected. The rats were allowed to recover for 45 min following
surgery. Subsequently, they were returned to their cages and the
room temperature was maintained at 25±1°C. To prevent dehydration,
20 ml 0.9% NaCl solution was injected subcutaneously, immediately
following the operation.
Experimental design
Among the total 90 rats, 72 rats survived the
carotid endarterectomy and were randomly assigned to the following
three groups (n=24 rats/group): Control, operation and treatment
groups. Rats in the treatment group were injected with 10 mg/kg
synthetic E-selectin (Prospec-Tany TechnoGene, Ltd., East
Brunswick, NJ, USA) into the femoral vein immediately following
surgery; rats in the operation group received an equal volume of
saline. The dose of E-selectin was chosen according to Morikawa
et al (16). Rats in the
control group underwent a temporary ~20 min blockage of the right
carotid artery without vascular suturing, and received no further
interventions.
Sample collection
Serum samples were collected as follows: Rats from
the three groups (6 rats/group/day) were anaesthetized with 4%
chloral hydrate (400 mg/kg) via intraperitoneal injection on
postoperative day 1, 3, 7 and 14, and blood samples (4 ml) were
collected from the heart. Blood samples were centrifuged at room
temperature for 16 min at 8,000 × g. The supernatants were
cryopreserved at −20°C, and serum samples from all postoperative
days were collectively tested. Serum levels of TNF-α and IL-6 were
evaluated using enzyme-linked immunosorbent assay (ELISA).
All the rats were euthanized by cervical dislocation
following anaesthesia with 4% chloral hydrate (400 mg/kg) via
intraperitoneal injection. Following euthanasia, vascular tissue
samples of rats were collected as follows: On postoperative day 1,
3, 7 and 14, the skin was cut along the original incision and the
right common carotid artery was separated. The ends of the
anastomosis were clipped and the vascular lumen was flushed with
heparinized saline. Vascular tissue samples were collected, fixed
and stored in 10% paraformaldehyde solution at 4°C for 24 h. The
mRNA and protein expression levels of NF-κB p65 in vascular tissue
samples were assessed using western blot analysis and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
respectively. In addition, NF-κB binding activity was assessed
using electrophoretic mobility shift assay (EMSA), and the
percentage of PCNA-positive cells in vascular tissue was detected
using immunohistochemistry.
ELISA
The serum levels of inflammatory mediators were
quantified using specific rat ELISA kits, according to the
manufacturers' protocols. The TNF-α ELISA kit (cat. no. RA20035;
Bio-Swamp Life Science, Shanghai, China) and the IL-6 kit (cat. no.
RA20607; Bio-Swamp Life Science) were used. Briefly, 100 µl
standard or serum sample was added to each well and the plate was
covered with a plate sealer. Plates were incubated for 2 h at 37°C,
aspirated and 100 µl Detection Reagent A working solution was added
to each well. The plates were covered with the plate sealer and
incubated for 1 h at 37°C. Subsequently, the plates were aspirated,
washed three times and 100 µl Detection Reagent B working solution
was added to each well. The plates were covered with the plate
sealer and incubated for 30 min at 37°C. Plates were then
aspirated, washed five times, 90 µl Substrate Solution was added to
each well and plates were covered with a new plate sealer and
incubated for 15–25 min at 37°C in the dark. Finally, 50 µl Stop
Solution was added to each well. Samples were immediately measured
at 450 nm using a microplate reader. Duplicate readings for each
standard, control and sample were averaged and the average zero
standard optical density (OD) was subtracted. The standard OD curve
was plotted using regression analysis to estimate the best fit. The
standard curve was then used to estimate the sample concentrations,
which were multiplied by the dilution ratio, thus yielding the
actual protein concentration in each serum sample.
Immunohistochemistry
Immunohistochemistry was used to evaluate the
immunoreactivity of PCNA in paraformaldehyde-fixed
paraffin-embedded vascular tissue sections. Briefly, the sutures in
the blood vessel walls were removed, the tissue was fixed in 4%
paraformaldehyde at 4°C for 24 h and then embedded in paraffin.
Tissue samples were sliced coronally into 4 µm sections, which were
then deparaffinized and rehydrated in graded concentrations of
ethanol in distilled water. Endogenous peroxidase activity was
blocked with 3% H2O2 for 5 min at room
temperature, followed by a brief rinse in distilled water and a
15-min wash in PBS. Sections were placed in 10 mM citrate buffer
(pH 6.0) and heated in a microwave oven at 95°C for 30 min for
antigen retrieval. Sections were cooled at room temperature for 20
min and rinsed in PBS. Non-specific binding was blocked by a 40-min
incubation in Immunol staining blocking buffer (cat. no. P0102;
Beyotime Institute of Biotechnology, Haimen, China) at 37°C.
Sections were then incubated with an anti-PCNA mouse monoclonal
antibody (cat. no. ab29; 1:200; Abcam, Cambridge, MA, USA) for 1 h
at room temperature, followed by a 15-min wash in PBS. Following
incubation with biotinylated goat anti-mouse secondary antibody
(cat. no. PAB10760; 1:500; Abnova, Taipei, Taiwan) and
streptavidin-biotin complex (Wuhan Boster Biological Technology,
Ltd., Wuhan, China) for 30 min at room temperature,
3,3′-diaminobenzidine was used for the chromogenic detection of
immunoreactive complexes, and counterstaining was performed with
hematoxylin. Subsequently, the sections were dehydrated using an
ethanol gradient of 80, 95 and 100%, and then covered with
coverslips. The sections were examined by a light microscope (CX23;
Olympus Corporation, Tokyo, Japan) and the cells counted; cells
with intense or moderate brown staining were classified as
PCNA-positive, whereas cells with weak or no staining were
classified as PCNA-negative. The number of PCNA-positive cells in
each section was determined in 10 randomly selected microscope
fields throughout similar regions of the studied vessel (×400
magnification), and the mean PCNA-positive cell number per visual
field was calculated.
Extraction of nuclear proteins
Nuclear proteins were extracted and quantified as
previously described (18).
Briefly, frozen vascular tissue samples were homogenized with 1 ml
lysis buffer, composed of 10 mM HEPES (pH 7.9), 2 mM
MgCl2, 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol (DTT)
and 0.5 mM phenylmethylsulfonyl fluoride (PMSF), purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The homogenates
were centrifuged at 1,000 × g for 30 sec at 4°C to discard tissue
fragments. The supernatants were then incubated on ice for 20 min
and centrifuged at 5,000 × g for 10 min at 4°C. The crude nuclear
pellets were suspended in 200 µl ice-cold buffer, containing 20 mM
HEPES (pH 7.9), 25% glycerol, 1.5 mM MgCl2, 20 mM KCl,
0.1 mM EDTA, 0.5 mM PMSF and 1 mM DTT, and incubated on ice for 30
min with frequent mixing. The samples were then centrifuged at
12,000 × g for 15 min at 4°C. The supernatants were collected as
nuclear extracts and stored at −80°C for further analysis. Protein
concentration was determined using a bicinchoninic acid assay kit
with bovine serum albumin as the standard (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
EMSA
NF-κB DNA-binding activity was detected using a Gel
Shift Assay system (Promega Corporation, Madison, WI, USA). The
NF-κB consensus oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was
5′-end-labeled with [γ-32P]-adenosine triphosphate
(PerkinElmer, Inc., Waltham, MA, USA). Nuclear proteins (40 µg)
were preincubated in a total volume of 9 µl binding buffer, which
consisted of 10 mM Tris-HCl (pH 7.5), 4% glycerol, 1 mM
MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl and 0.05
mg/ml poly(deoxyinosinic-deoxycytidylic) acid for 10 min at room
temperature. Following the addition of the 32P-labeled
oligonucleotide probe, incubation was continued for 20 min at room
temperature. The DNA-protein binding specificity was determined
using a competition experiment as a control, with the addition of a
100-fold molar excess of unlabeled NF-κB oligonucleotide (specific
competitor) or unlabeled activating protein 2 oligonucleotide
(non-specific competitor) to the binding reaction 10 min prior to
the addition of the 32P-labeled probe in the nuclear
extract. The reaction was terminated following the addition of 1 µl
gel loading buffer, and the mixture was subjected to non-denaturing
4% polyacrylamide gel electrophoresis in Tris-borate-EDTA buffer.
The gel was vacuum-dried and exposed to X-ray film (Fuji Hyperfilm,
Tokyo, Japan) at −70°C using an intensifying screen (Medical
Devices Co., Ltd., Zhejiang, China). The relative intensity of
radiolabeled bands was analyzed by ImageJ version 1.47 (National
Institutes of Health, Bethesda, MD, USA).
RT-qPCR
mRNA expression levels of NF-κB p65 were determined
in vascular tissue samples using RT-qPCR. Total RNA was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
RNA quality was assessed by gel visualization and
spectrophotometric analysis using the
OD260/OD280 ratio. The quantity of RNA was
measured with a spectrophotometer using OD260. According
to the manufacturers' protocols, total RNA was reverse transcribed
into cDNA using M-MLV Reverse Transcriptase (cat. no. M530B;
Promega Corporation) and oligo dT primers (Shanghai ShineGene
Molecular Biotech, Inc., Shanghai, China). cDNA was used for
RT-qPCR with the Rotor-Gene 3000 real-time PCR cycler (Qiagen China
Co., Ltd., Shanghai, China) using SYBR Green as the fluorescent
probe. The following primers were used: NF-κB, sense
5′-TTTGATAACCGTGCCCCCAA-3′, antisense 5′-GCCAGGTCCCGTGAAATACA-3′;
and GAPDH, sense 5′-TCTCTGCTCCTCCCTGTTCT-3′ and antisense
5′-TACGGCCAAATCCGTTCACA-3′. The reaction mixtures contained diluted
cDNA, SYBR Green I Nucleic Acid Gel Stain (Invitrogen; Thermo
Fisher Scientific, Inc.), 20 µM of each primer and nuclease-free
water, to a final volume of 25 µl. Thermocycling conditions were as
follows: Initial denaturation at 95°C for 5 min, followed by 40
cycles of amplification (denaturation at 94°C for 30 sec, annealing
at 55°C for 30 sec and extension at 72°C for 5 min). GAPDH was used
as an internal loading control. The 2∆∆Cq method was
used for normalization (19). All
samples were analyzed in triplicate.
Western blot analysis
Western blot analysis was used to determine NF-κB
p65 protein expression levels. Briefly, frozen vascular tissue
samples were lysed in radioimmunoprecipitation buffer (cat. no.
P0013; Beyotime Institute of Biotechnology) and centrifuged at
12,000 × g for 20 min at 4°C. Protein concentration was determined
using the Bradford assay (Nanjing Jiancheng protein assay kit;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Equal
amounts of extracted protein samples (60 µg) were separated by 10%
SDS-PAGE and transferred onto a polyvinylidene difluoride membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was
blocked with 5% skim milk for 2 h at room temperature and incubated
overnight at 4°C with anti-NF-κB p65 (1:150; cat. no. SAB4502610;
Sigma-Aldrich, Merck KGaA) and anti-GAPDH (1:6,000; cat. no. G9545;
Sigma-Aldrich, Merck KGaA) primary antibodies in PBS containing
0.1% Tween-20 (PBST). Following 6 washes in PBST, the membrane was
incubated at room temperature with horseradish
peroxidase-conjugated secondary antibody (1:400; cat. no. ab6721;
Abcam) for 2 h. The protein bands were visualized using Amersham
Enhanced Chemiluminescence Western Blotting Detection Reagent (GE
Healthcare Life Sciences, Chalfont, UK) and were exposed to X-ray
film. Developed films were digitized using an Epson Perfection 2480
scanner (Epson America, Inc., Long Beach, CA, USA). Blots were
semi-quantified by densitometric analysis using ImageJ2× software
(National Institutes of Health, Bethesda, MD, USA), and protein
expression levels were normalized to GAPDH.
Statistical analysis
The statistical significance of the differences
between groups was assessed using one-way analysis of variance,
followed by a post hoc Fisher's least significant difference test
for multiple comparisons. Data are expressed as the mean ± standard
deviation of at least three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using SPSS software version 12.0
(SPSS, Inc., Chicago, IL, USA).
Results
General observations
No significant alterations were detected in body
weight, temperature, mean arterial blood pressure and injected
arterial blood gas data among experimental groups (data not shown).
All rats survived for the duration of the experiments, from the
induction to the unblocking of experimental anastomoses.
Morphological alterations following
arterial anastomosis
Among rats in the operation group, no marked
thickening in blood vessels was observed 3 days following
anastomosis, and suture adhesion was not obvious. However,
peripheral tissue edema developed, which disappeared 7 days
post-anastomosis. On day 7, the anastomosis tissue appeared thicker
compared with on day 3, whereas some of the sutures had adhered to
the surrounding tissue. On day 14, granulation tissue development
was obvious surrounding the anastomosis, and sutures had thickened,
suggesting that intimal hyperplasia had developed. Among rats in
the treatment group, thickening and adhesion of vascular
anastomoses was less pronounced compared with in the operation
group at the corresponding time points (data not shown).
Synthetic E-selectin suppresses NF-κB
binding activity
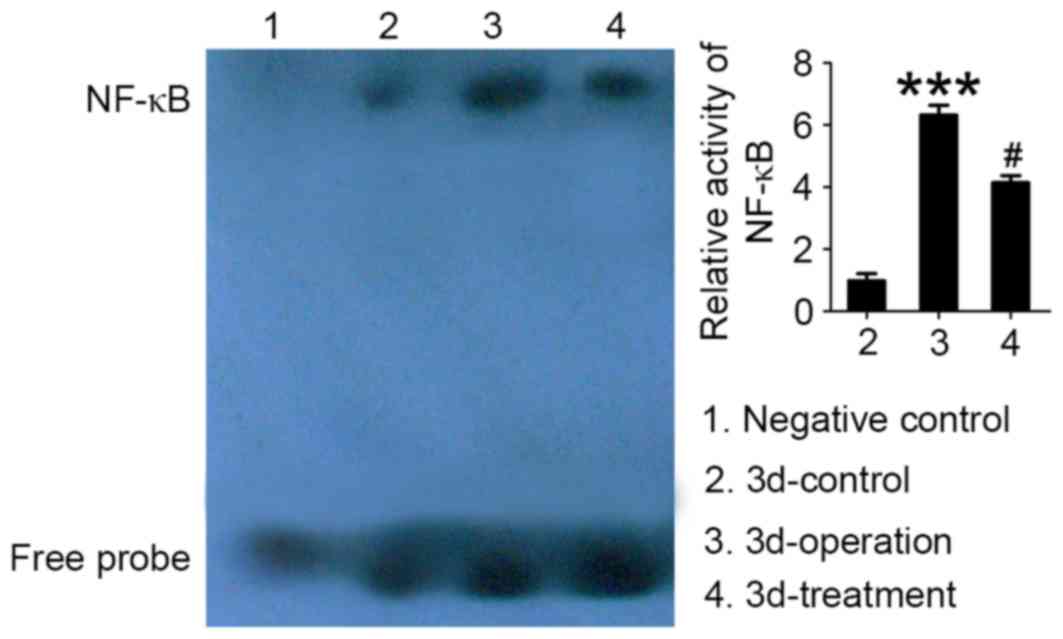
Autoradiography results, following EMSA of NF-κB
DNA-binding activity in tissue samples isolated from vascular
anastomoses, are presented in Fig.
2. Low NF-κB binding activity, corresponding to a weak
autoradiography signal, was detected in rats in the control group.
Conversely, NF-κB binding activity in anastomosis tissue samples
was significantly increased in rats in the operation group compared
with in the control group (P<0.01; Fig. 2). Following treatment with
synthetic E-selectin, NF-κB binding activity appeared to be
significantly downregulated in the vascular tissue surrounding the
anastomotic site compared with the operation group (P<0.05).
These results suggested that synthetic E-selectin may prevent NF-κB
activation and its subsequent nuclear translocation.
Synthetic E-selectin downregulates
NF-κB p65 protein expression
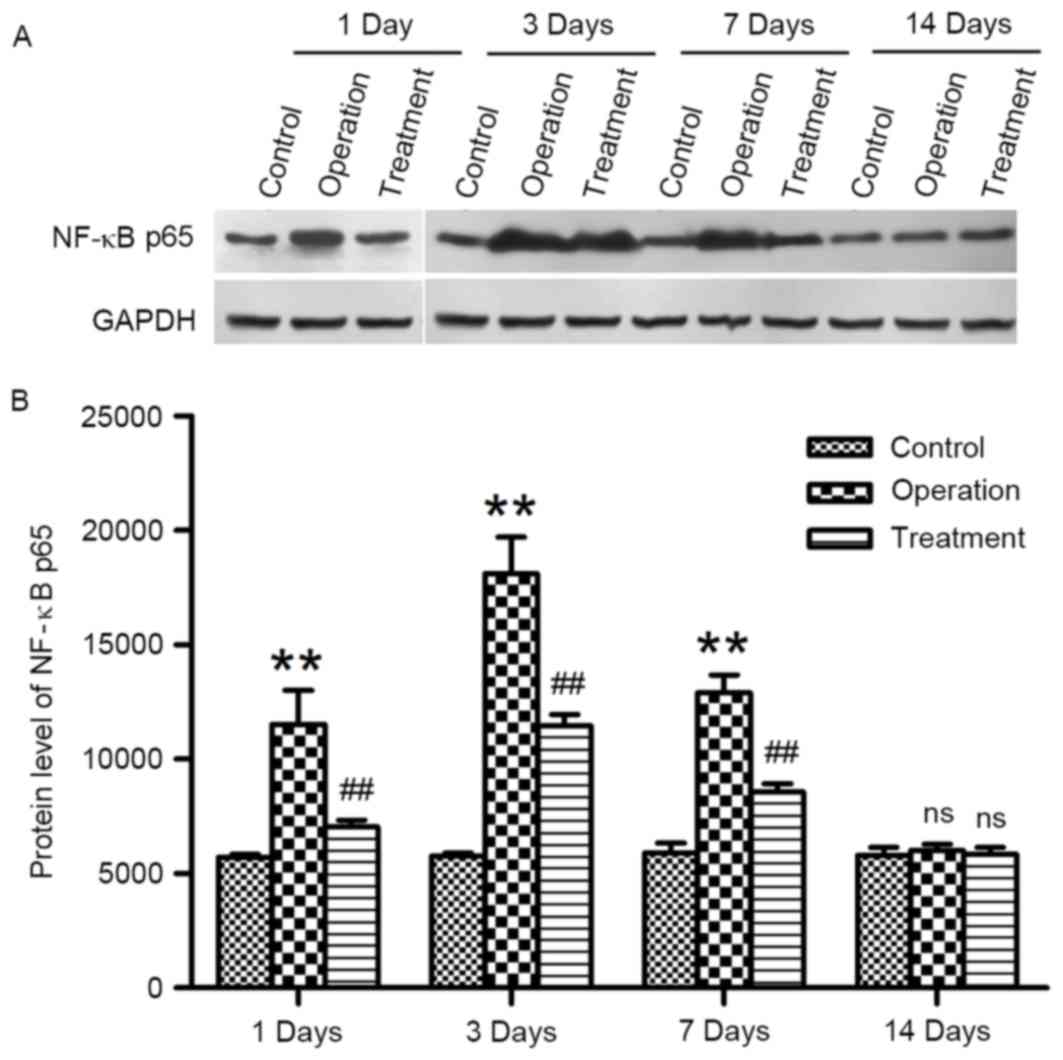
Western blot analysis was used to evaluate the
alterations in NF-κB p65 protein expression levels following
arterial anastomosis. NF-κB p65 protein expression levels remained
consistently low in rats in the control group at the various time
points (Fig. 3). Conversely, NF-κB
p65 levels were significantly upregulated in vascular tissue
surrounding the anastomotic site isolated from rats in the
operation group, suggesting the activation of the NF-κB pathway.
NF-κB p65 protein expression levels in rats treated with synthetic
E-selectin were significantly lower at the corresponding time
points following anastomosis compared with in rats from the
operation group (P<0.01; Fig.
3). These results suggested that synthetic E-selectin may
inhibit the upregulation in NF-κB expression following carotid
artery anastomosis.
Synthetic E-selectin downregulates
NF-κB p65 mRNA expression
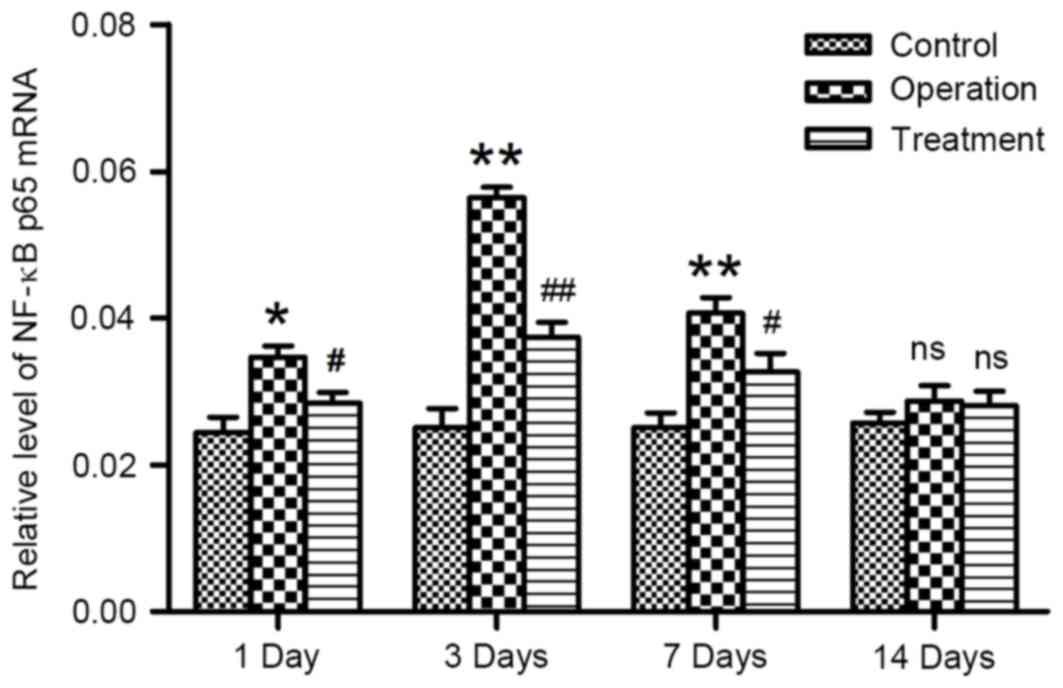
RT-qPCR was used to investigate alterations in the
gene expression of NF-κB p65 following arterial anastomosis. NF-κB
p65 mRNA expression levels remained consistently low in rats in the
control group throughout the duration of the study. Conversely,
following carotid artery anastomosis, NF-κB p65 mRNA expression
appeared to be significantly upregulated (P<0.05; Fig. 4). Treatment with synthetic
E-selectin was demonstrated to significantly downregulate NF-κB p65
mRNA expression levels compared with the operation group at the
corresponding time points post-anastomosis (P<0.05; Fig. 4). These results suggested that
synthetic E-selectin may suppress NF-κB expression at the mRNA
level.
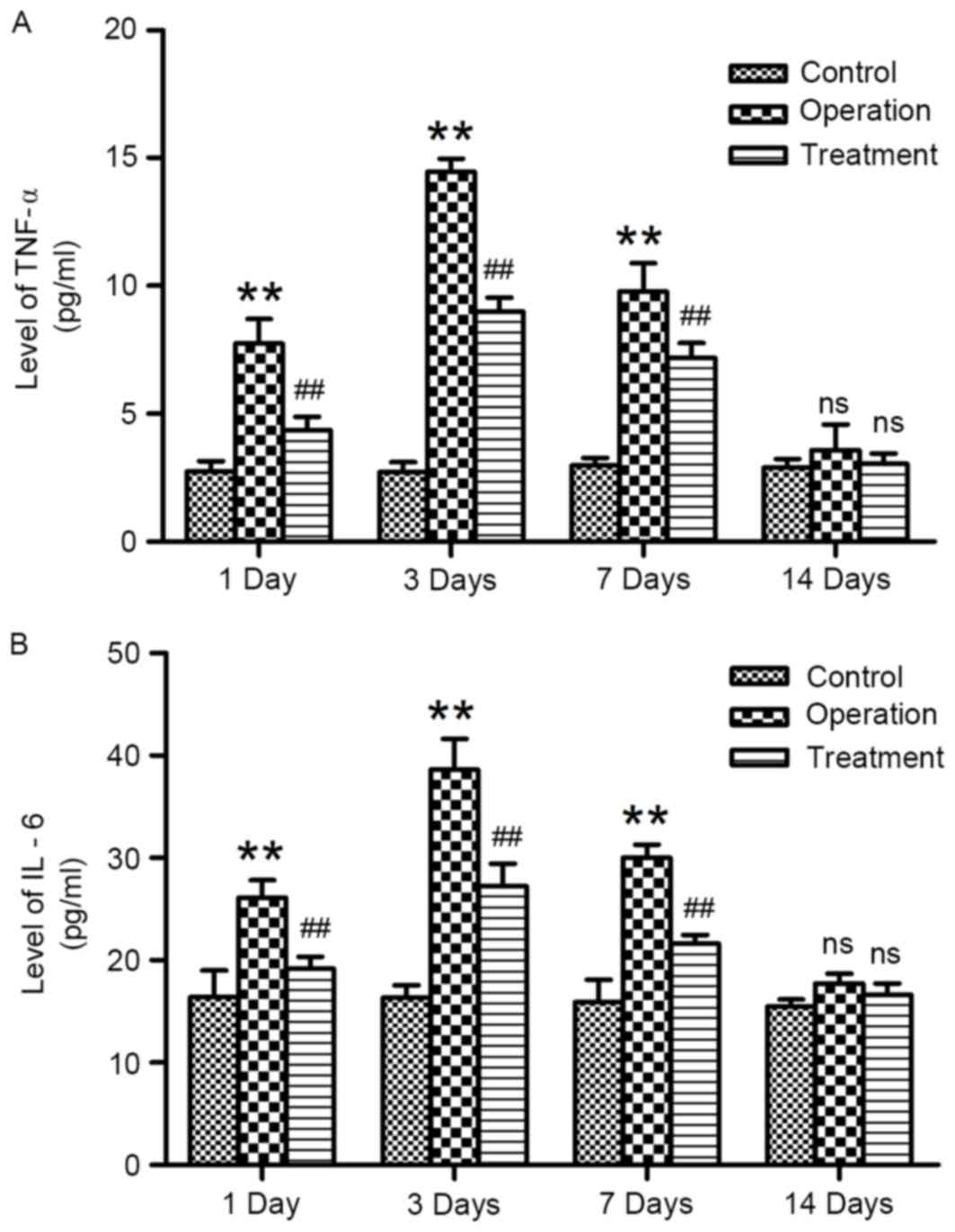
Synthetic E-selectin reduces serum
concentrations of TNF-α and IL-6
The concentrations of TNF-α and IL-6 in rat serum
samples were determined using ELISA. TNF-α and IL-6 concentrations
in rats from the control group remained low throughout the duration
of the experiments. Following anastomosis, the serum concentrations
of TNF-α and IL-6 were significantly increased on days 1, 3 and 7
post-surgery compared with the control group (Fig. 5); serum concentrations peaked on
day 3. Serum samples isolated from E-selectin-treated rats
following anastomosis exhibited significantly reduced TNF-α and
IL-6 concentrations compared with samples from the operation group
(P<0.01; Fig. 5). However, no
statistically significant differences were detected among the
control, operation and treatment groups on day 14 post-surgery.
These results indicated that treatment with synthetic E-selectin
may prevent the increase in serum TNF-α and IL-6 levels following
arterial anastomosis.
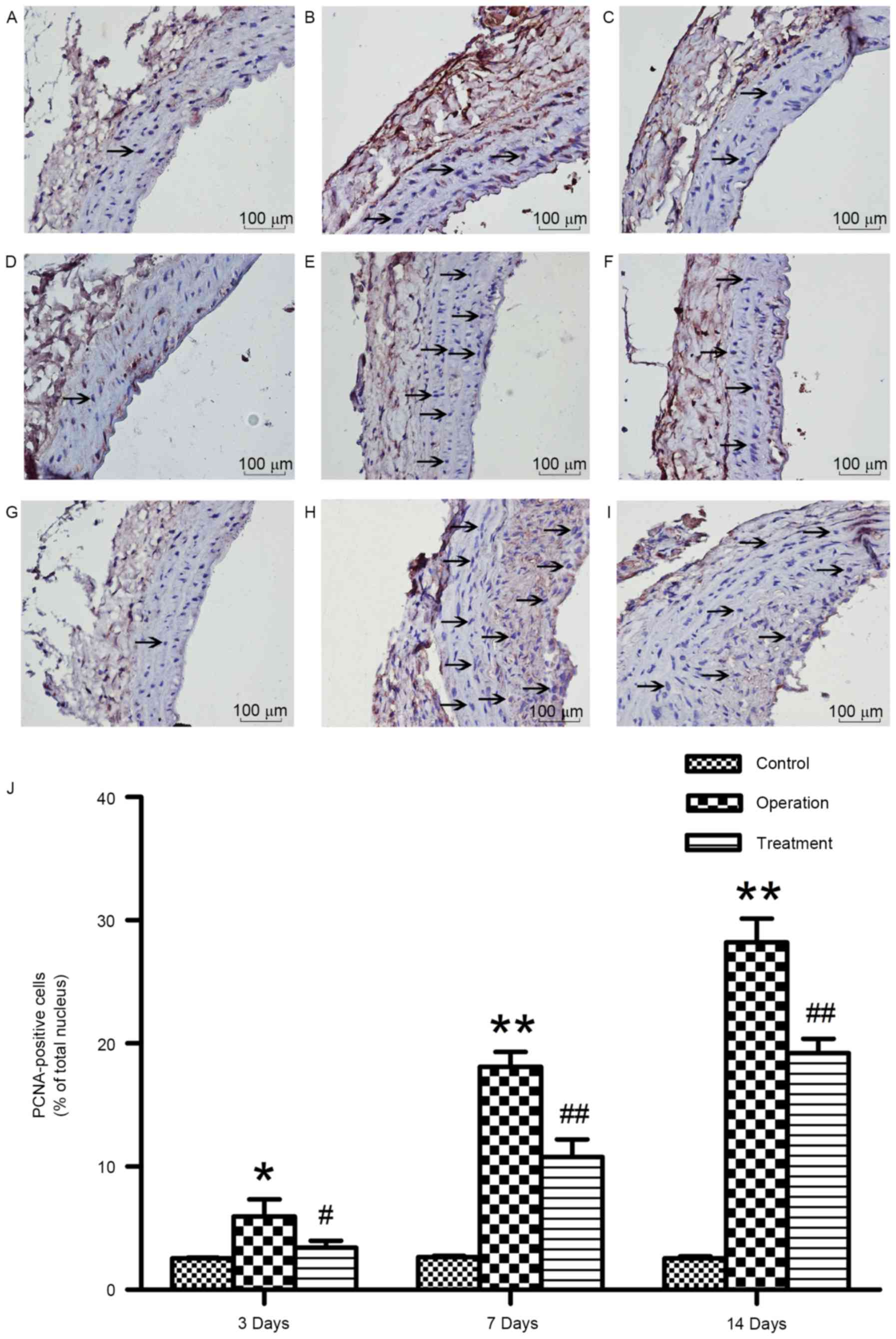
Synthetic E-selectin suppresses VSMC
proliferation
PCNA levels have been reported to reflect the degree
of cellular proliferation (15).
Therefore, in the present study, PCNA immunoreactivity was
investigated in vascular tissue surrounding the anastomotic site in
order to assess VSMC proliferation. Rats in the control group
exhibited negligible numbers of PCNA-positive cells at the various
time points (Fig. 6).
PCNA-positive cell numbers were significantly increased in rats in
the operation group as early as 3 days post-anastomosis; PCNA
immunoreactivity remained high throughout the course of the study
(Fig. 6). Notably, rats treated
with synthetic E-selectin demonstrated significantly reduced
PCNA-positive cell numbers following anastomosis compared with rats
in the operation group (P<0.01; Fig. 6). These results suggested that
synthetic E-selectin administration post-anastomosis may suppress
the proliferative capabilities of VSMCs.
Discussion
The present study demonstrated that intimal
hyperplasia was induced following carotid artery anastomosis,
whereas it could be effectively suppressed by the administration of
synthetic E-selectin. The molecular mechanisms underlying the
effects of synthetic E-selectin on neointimal formation were also
investigated. A significant increase in the production of the
proinflammatory cytokines TNF-α and IL-6, which has been reported
to promote VSMC proliferation (8),
was detected following vascular injury. In addition, NF-κB mRNA and
protein expression levels were also revealed to be upregulated
post-anastomosis. Notably, TNF-α and IL-6 serum levels were
significantly decreased following treatment with synthetic
E-selectin, which also appeared to suppress NF-κB-mediated
signaling. In addition, the results demonstrated that synthetic
E-selectin-induced suppression of the NF-κB pathway was primarily
in the early stage after anastomosis. Furthermore, synthetic
E-selectin administration was revealed to inhibit VSMC
proliferation, thus suggesting that it may be able to reduce
postoperative vascular restenosis.
Neointimal hyperplasia has been identified as a
critical mechanism during the development of restenosis that often
follows vascular anastomosis (20). VSMC proliferation has been
implicated in intimal hyperplasia; following vascular injury, VSMCs
have been reported to undergo phenotypic alteration from a
contractile to a secretory type (8). Since their proliferative and
migratory capabilities are thus potentiated, VSMCs can migrate from
the basement membrane to the tunica intima, resulting in vascular
wall thickening and luminal stenosis (21). However, the molecular mechanisms
involved in the phenotypic transformation of VSMCs have yet to be
elucidated.
The pathophysiological mechanisms underlying
postoperative arterial restenosis have been reported to include
aberrant inflammation that follows vascular injury (22,23).
The proinflammatory cytokines TNF-α and IL-6 are critical in the
development of inflammation following vascular injury, as
inflammatory cells release large amounts of TNF-α and IL-6, which
have been reported to participate in the pathological processes
leading to arterial wall damage (24). Selzman et al (12) demonstrated that TNF-α induced VSMC
proliferation via activating the NF-κB pathway. In addition,
following NF-κB activation in VSMCs, IL-6 mRNA expression has been
revealed to be enhanced, leading to the induction of inflammatory
responses in the vascular wall (25). The present study demonstrated that
TNF-α and IL-6 levels began to increase on day 1 post-anastomosis
and peaked on day 3; by day 7 they had begun to decrease, returning
to physiological levels by day 14 post-surgery. Furthermore, a
corresponding increase was detected in the number of PCNA-positive
cells. Notably, following treatment with synthetic E-selectin, a
decrease in TNF-α and IL-6 serum levels was apparent, which was
accompanied by a marked decrease in PCNA-positive cell numbers in
vascular tissue surrounding the site of anastomosis. These results
suggested that IL-6 and TNF-α may be involved in VSMC proliferation
underlying the development of stenosis during the early
postoperative stages. Therefore, it may be hypothesized that TNF-α
and IL-6 may be associated with the proliferation and migration of
VSMCs.
In the present study, NF-κB activation in VSMCs
appeared to accompany the upregulation in TNF-α and IL-6 serum
levels. Activated NF-κB has been reported to enhance the
transcription of proinflammatory genes, thus promoting the release
of several factors that mediate inflammation and promote VSMC
proliferation (25). Sekiguchi
et al (26) demonstrated
that angiotensin II promoted the expression of proinflammatory
cytokines, via inducing NF-κB activation, whereas the inhibition of
NF-κB activity was revealed to suppress the expression of
inflammatory factors. The involvement of NF-κB in cell cycle
regulation has also been reported. NF-κB has been demonstrated to
bind the promoter of the cell cycle-associated protein cyclin D1
and activate its transcription, thus enhancing cell cycle
progression from G1 to S phase and promoting cellular
proliferation (27).
NF-κB-mediated pathways have also been reported to underlie the
pathophysiological processes during VSMC proliferation (28). NF-κB inhibitors and decoy
oligonucleotides have been revealed to reduce macrophage
recruitment and VSMC accumulation in experimental vein grafts
(29–31). The present study demonstrated that
treatment with synthetic E-selectin effectively suppressed the
production and activation of NF-κB, which was accompanied by the
corresponding production of inflammatory cytokine levels and VSMC
proliferation.
The present study evaluated the effects of synthetic
E-selectin on NF-κB-mediated pathways following arterial
anastomosis. Serum levels of the proinflammatory factors TNF-α and
IL-6 were revealed to be significantly upregulated post-injury,
thus suggesting that these factors may act directly on VSMCs to
promote their proliferation and migration during the progression of
restenosis. The present results suggested that following its
activation and nuclear translocation, NF-κB may enhance the
transcription of inflammatory mediators and promote cellular
proliferation, which may in turn trigger the release of more
inflammatory mediators and further promote VSMC proliferation and
migration. Furthermore, NF-κB levels appeared to peak 3 days
post-anastomosis. At this time point, inflammatory factor levels
were also at their highest, and PCNA-positive cell numbers appeared
to be increased, indicating the initiation of VSMC proliferation.
Synthetic E-selectin was revealed to significantly inhibit NF-κB
activation, downregulate the levels of inflammatory mediators, and
reduce the number of PCNA-positive cells, thus suggesting that it
may be able to inhibit VSMC proliferation and attenuate neointimal
hyperplasia. Therefore, it may be hypothesized that the molecular
mechanisms underlying the inhibitory effects of synthetic
E-selectin on VSMC proliferation may involve the inhibition of
NF-κB-mediated pathways. Notably, previous studies have reported
that ethanol extracts of Prunella vulgaris and Akebia
quinata exerted their anti-inflammatory effects via inhibiting
the p38 mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK)/NF-κB signaling pathway in
TNF-α-stimulated human aortic smooth muscle cells (26,27).
However, whether E-selectin may be involved in p38 MAPK/ERK/NF-κB
signaling pathways during inflammatory responses in VSMCs following
carotid artery anastomosis remains to be elucidated.
In conclusion, the present study suggested that
following treatment with synthetic E-selectin, the proliferative
and migratory capabilities of VSMCs were significantly decreased.
Furthermore, synthetic E-selectin was demonstrated to suppress the
production and activation of NF-κB, the production of the
proinflammatory cytokines TNF-α and IL-6, as well as downregulate
PCNA-positive cells in the perianastomotic tissue. Therefore, it
may be hypothesized that synthetic E-selectin is able to inhibit
VSMC proliferation and migration, and the molecular mechanisms
underlying its actions may involve the suppression of NF-κB
pathways to inhibit the release of inflammatory cytokines, and thus
VSMC proliferation, during the early stages post-anastomosis.
However, arterial restenosis is a complex pathological process
associated with numerous factors; therefore, the interaction
between synthetic E-selectin and the NF-κB pathway in restenosis
requires further study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371280), Suzhou
Basic Research on Science and Technology (grant no. SYS201535) and
the Graduate Innovation Program of Jiangsu Province (grant no.
CXZZ13_0836).
References
|
1
|
Jia Q, Liu LP and Wang YJ: Stroke in
China. Clin Exp Pharmacol Physiol. 37:259–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HJ, Kim SK, Park HJ, Chung JH, Chun J,
Yun DH and Kim YO: Polymorphisms of IGFI contribute to the
development of ischemic stroke. Exp Ther Med. 3:93–98. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaturvedi S, Bruno A, Feasby T, Holloway
R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, et
al: Carotid endarterectomy-an evidence-based review: Report of the
therepeutics and technology assessment subcommittee of the american
academy of neurology. Neurology. 65:794–801. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matter CM, Ma L, von Lukowicz T, Meier P,
Lohmann C, Zhang D, Kilic U, Hofmann E, Ha SW, Hersberger M, et al:
Increased balloon-induced inflammation, proliferation, and
neointima formation in apolipoprotein E (ApoE) knockout mice.
Stroke. 37:2625–2632. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arguizan C, Tringuart L, Touboul PJ, Long
A, Feasson S, Terriat B, Gobin-Metteil MP, Guidolin B, Cohen S and
Mas JL: EVA-3S Investigators: Restenosis is more frequent after
carotid stenting than after endarterectomy: The EVA-3S study.
Stroke. 42:1015–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matter CM, Chadjichristos CE, Meier P, von
Lukowicz T, Lohmann C, Schuler PK, Zhang D, Odermatt B, Hofmann E,
Brunner T, et al: Role of endogenous Fas (CD95/Apo-1) ligand in
balloon-induced apoptosis, inflammation, and neointima formation.
Circulation. 113:1879–1887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaturvedi S, Bruno A, Feasby T, Holloway
R, Benavente O, Cohen SN, Cote R, Hess D, Saver J, Spence JD, et
al: Carotid endarterectomy-an evidence-based review: Report of the
therepeutics and technology assessment subcommittee of the american
academy of neurology. Neurology. 65:794–801. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hay C, Micko C, Prescott MF, Liau G,
Robinson K and De Leon H: Differential cell cycle progression
patterns of infiltrating leukocytes and resident cells after
balloon injury of the rat carotid artery. Arterioscler Thromb Vasc
Biol. 21:1948–1954. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li YT, Swales KE, Thomas GJ, Warner TD and
Bishop-Bailey D: Farnesoid X receptor ligands inhibit vascular
smooth muscle cell inflammation and migration. Arterioscler Thromb
Vasc Biol. 27:2606–2611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-κB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Couffinhal T, Duplàa C, Labat L, Lamaziere
JM, Moreau C, Printseva O and Bonnet J: Tumor necrosis factor-alpha
stimulates ICAM-1 expression in human vascular smooth muscle cells.
Arterioscler Thromb. 13:407–414. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Selzman CH, Shames BD, Reznikov LL, Miller
SA, Meng X, Barton HA, Werman A, Harken AH, Dinarello CA and
Banerjee A: Liposomal delivery of purified inhibitory-kappBalpha
inhibits tumor necrosis factor-alpha-induced human vascular smooth
muscle proliferation. Circ Res. 84:867–875. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nurmi A, Lindsberg PJ, Koistinaho M, Zhang
W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N,
Schwaninger M and Koistinaho J: Nuclear factor-kappaB contributes
to infarction after permanent focal ischemia. Stroke. 35:987–991.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams AJ, Hale SL, Moffett JR, Dave JR,
Elliott PJ, Adams J and Tortella FC: Delayed treatment with MLN519
reduces infarction and associated neurologic deficit caused by
focal ischemic brain injury in rats via antiinflammatory mechanisms
involving nuclear factor-kappaB activation, gliosis, and leukocyte
infiltration. J Cereb Blood Flow Metab. 23:75–87. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ross G, Jiang Y, Landberg G, Nielsen NH,
Zhang P and Lee MY: Determination of the epitope of an inhibitory
antibody to proliferating cell nuclear antigen. Exp Cell Res.
226:208–213. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morikawa E, Zhang SM, Seko Y, Toyoda T and
Kirino T: Treatment of focal cerebral ischemia with synthetic
oligopeptide corresponding to lectin domain of selectin. Stroke.
27:951–955. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th.
Washington (DC): National Academies Press (US); 2011
|
|
18
|
Chen G, Shi JX, Hang CH, Xie W, Liu J and
Liu X: Inhibitory effect on cerebral inflammatory agents that
accompany traumatic brain injury in a rat model: A potential
neuroprotective mechanism of recombinant human erythropoietin
(rhEPO). Neurosci Lett. 425:177–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bassiouny HS, White S, Glagov S, Choi E,
Giddens DP and Zarins CK: Anastomotic intimal hyperplasia:
Mechanical injury or flow induced. J Vasc Surg. 15:708–716. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Austin GE, Ratliff NB, Hollman J, Tabei S
and Phillips DF: Intimal proliferation of smooth muscle cells as an
explanation for recurrent coronary artery stenosis after
percutaneous transluminal coronary angioplasty. J Am Coll Cardiol.
6:369–375. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong YJ, Jeong MH, Song SJ, Sim DS, Kim
JH, Lim KS, Hachinohe D, Ahmed K, Hwang SH, Lee MG, et al: Effects
of ramiprilat-coated stents on neointimal hyperplasia,
inflammation, and arterial healing in a porcine coronary restenosis
model. Korean Circ J. 41:535–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou XC, Huang RC, Zhang B, Yin D, Liang
B, Wang SP, Guan QG, Sun XZ, Miao ZL, He XZ, et al: Inflammation
inhibitory effects of sirolimus and paclitaxel-eluting stents on
interleukin-1β-induced coronary artery in-stent restenosis in pigs.
Chin Med J (Engl). 123:2405–2409. 2010.PubMed/NCBI
|
|
24
|
Warner SJ and Libby P: Human vascular
smooth muscle cells. Target for and source of tumor necrosis
factor. J Immunol. 142:100–109. 1989.PubMed/NCBI
|
|
25
|
Kranzhöfer R, Schmidt J, Pfeiffer CA, Hagl
S, Libby P and Kübler W: Angiotensin induces inflammatory
activation of human vascular smooth muscle cells. Arterioscler
Thromb Vasc Biol. 19:1623–1629. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sekiguchi K, Li X, Coker M, Flesch M,
Barger PM, Sivasubramanian N and Mann DL: Cross-regulation between
the renin-angiotensin system and inflammatory mediators in cardiac
hypertrophy and failure. Cardiovasc Res. 63:433–442. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hinz M, Krappmann D, Eichten A, Heder A,
Scheidereit C and Strauss M: NF-kappaB function in growth control:
Regulation of cyclin D1 expression and G0/G1-to-S-phase transition.
Mol Cell Biol. 19:2690–2698. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramana KV, Chandra D, Srivastava S,
Bhatnagar A, Aggarwal BB and Srivastava SK: Aldose reductase
mediates mitogenic signaling in vascular smooth muscle cells. J
Biol Chem. 277:32063–32070. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimizu N, Azuma N, Nishikawa T, Hirata S,
Morishita R, Kaneda Y and Sasajima T: Effect on vein graft intimal
hyperplasia of nuclear factor-kB decoy transfection using the
second generation of HVJ vector. J Cardiovasc Surg (Torino).
48:463–470. 2007.PubMed/NCBI
|
|
30
|
Miyake T, Aoki M, Shiraya S, Tanemoto K,
Ogihara T, Kaneda Y and Morishita R: Inhibitory effects of NFkappaB
decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit
vein graft model. J Mol Cell Cardiol. 41:431–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gareus R, Kotsaki E, Xanthoulea S, van der
Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de
Winther MP and Pasparakis M: Endothelial cell-specific NF-kappaB
inhibition protects mice from atherosclerosis. Cell Metab.
8:372–383. 2008. View Article : Google Scholar : PubMed/NCBI
|