Introduction
Osteosarcoma (OS), the most common primary malignant
tumor of bone, has two age-specific peaks in incidence in children,
adolescents (0–24 years old) and the elderly (≥60 years old)
(1–3). The incidence rate of OS is ~5–10
million/year worldwide (4). With
the combination of surgery and neoadjuvant chemotherapy treatment
from 1980, patients with osteosarcoma have an improved survival
time. However, the metastases or recurrence of the 5-year overall
survival rate for OS patients is 20%, which contributes to the
majority of mortalities, while 65% of patients have localized
disease (5). Additionally, the
reduced effectiveness of cytotoxic drugs due to acquired
chemoresistance is growing (6).
Therefore, finding novel therapeutic agents is important to improve
the prognosis.
Traditional Chinese medicines (7) have been used as a promising agent to
treat osteosarcoma in patients with metastasis, chemoresistance and
recurrence. Paeoniflorin (PF) is isolated from the peony root.
Previous research have demonstrated the proliferation inhibition
and apoptosis induction by PF in many different tumor cells
including hepatoma (8–10), cervical (11,12),
gastric (13–15), colorectal (16) and lung (17) cancers.
However, it remains to be determined whether PF has
a treatment effect on osteosarcoma. Therefore, the present study
investigated the anti-tumor effect of PF and the specific mechanism
on human osteosarcoma cells. Since PF could inhibit proliferation
and induce apoptosis in multiple neoplasias (18–20),
it is highly possible that PF has the capacity to suppress
proliferation and mediate apoptosis in human osteosarcoma cells.
Additionally, cell cycle proteins, caspase protein and the B-cell
lymphoma 2 (Bcl-2) family were investigated in relation to the
effect of PF on osteosarcoma.
Materials and methods
Reagents and antibodies
PF was from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany), with a purity >98%. The molecular formula of PF is
C23H28O11, and its molecular
weight is 480.46. PF was dissolved in PBS to produce a stock
solution. The PF stock solution was diluted in a cell culture
medium prior to its use in each experiment. Trypsin (0.25%), PBS,
fetal bovine serum (FBS), Eagle's Minimum Essential medium (EMEM)
and Mcroy' 5A medium were purchased from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Hoechst 33258 and an MTS kit
were from Promega Corporation (Madison, WI, USA). Antibodies
against caspase-3 (cat. no. 9662), cleaved-caspase-3 (cat. no.
9661), poly (ADPribose) polymerase (PARP; cat. no. 9532),
cleaved-PARP (cat. no. 5625), BH3 interacting domain death agonist
(Bid; cat. no. 2002), Bcl-2 (cat. no. 4223), Bcl-2 X-associated
protein (Bax; cat. no. 5023), Bcl-extra large (XL; cat. no. 2764),
cyclin B1 (cat. no. 4135), p21 (cat. no. 2947) and β-actin (cat.
no. 3700) were from Cell Signaling Technology, Inc., (Beverly, MA,
USA). Antibodies against cyclin-dependent kinase (CDK)1 (cat. no.
ab133327) and phosphorylated (p)-CDK1 (p-Y15; cat. no. ab133463)
were purchased from Abcam (Cambridge, UK).
Cells and cell culture
The HOS [CRL-1547TM, American Type Culture
Collection, Manassas, VA, USA (ATCC)] and Saos-2 (HTB-85TM; ATCC)
human osteosarcoma cell lines were from the Cell Bank of Shanghai
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). HOS cells were cultured in EMEM and
Saos-2 cells were cultured in Mcroy' 5A medium containing 10% FBS,
penicillin (100 U/ml) and streptomycin (100 µg/ml; Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany). All cell lines were cultured at
37°C in a 5% (v/v) CO2 humidified incubator. The present
study was approved by the Ethics Committee of the Second Affiliated
Hospital of Zhejiang University Medical School (Zhejiang,
China).
Cell viability assay
The viability of osteosarcoma cells treated with PF
was measured by an MTS assay. In brief, cells were seeded into
96-well plates (5,000–6,000 cells/well) overnight, then they were
treated with PF at concentrations ranging from 200 to 500 µM for
0–48 h. The MTS kit was added and cells were incubated at 37°C in a
humidified incubator for 1–3 h following the manufacturer's
protocol. Absorbance was read on a MR7000 microplate reader
(Dynatech Nevada, Inc., Carson City, NV, USA) at 490 nm and the
probit model was used to obtain IC50 values. Data were averaged in
six replicates. Each assay was tested in triplicate.
Hoechst staining
To assess the characteristic morphological changes
of osteosarcoma cell apoptosis, Hoechst 33258 staining followed by
fluorescent microscopy was performed. Cells were treated with PF
for 24 h. Subsequently, cells were stained with Hoechst 33258 at
room temperature for 10 min. Subsequently, cells were washed twice
with PBS and observed under a fluorescence microscope (Olympus
Corporation, Tokyo, Japan) to identify chromatin condensation and
nuclei fragmentation.
Morphological apoptosis
The morphological ultra-structure changes of treated
and control cells were observed using transmission electron
microscopy (TEM). Cells were fixed with 2.5% glutaraldehyde at 4°C
for 4 h and post-fixed in 1% osmium tetroxide at 4°C for 1 h. The
cell pellets were embedded in epoxy resin following dehydration in
different concentrations of alcohol. Sections (0.5-µm) were treated
with uranyl acetate and lead citrate, then observed at a
magnification of ×8,300 under a transmission electron
microscope.
Apoptosis proportion analysis
Apoptosis caused by PF was detected by Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining
(Biouniquer Technology, Nanjing, China). In brief, cells were
incubated in six-well plates at a density of 2×105
cells/well and treated with varying concentrations of PF (0–500 µM)
for 24 h. The cells were stained using Annexin V-FITC (5 µl) and PI
(5 µl) for 15 min in the dark. A flow cytometer (FACSCalibur; BD
Biosciences, San Jose, CA, USA) and ModFit LT (version 3.3; Verity
Software House, Topshame, ME, USA) was used to analyze the
samples.
Cell cycle analysis
PI/RNase staining buffer (BD Biosciences) was used
to determine cell cycle with flow cytometry. In brief, after
incubation with different concentrations of PF (0, 200, 300 and 500
µM) for 24 h, ~5×105 cells were fixed with 70% ethanol
at −20°C for 24 h. Following this, the cells were incubated with
PI/RNase and tested with a flow cytometer (FACSCalibur).
Western blot analysis
Cells (5×105) were incubated in 60-mm
dishes and treated with PF for 24 h. Protein lysates were obtained
by lysing cells with radioimmunoprecipitation assay lysis buffer
(Sigma-Aldrich; Merck KGaA) containing a mixture of protease
inhibitors (Sigma-Aldrich; Merck KGaA) for 0.5 h at 4°C to extract
total proteins. Next, a bicinchoninic acid total protein
quantitation kit was used to identify the protein content. Total
proteins (60 µg) were separated by electrophoresis on a 5% stacking
gel and an 8–12% separating gel. Proteins were transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA) and blocked with 5% bovine serum albumin at 20°C for 1 h.
Membranes were incubated with primary antibodies (1:1,000) at 4°C
for 12 h. After that, the membranes were washed five times with TBS
with 0.1% Tween 20 and then incubated with goat anti-rabbit and
anti-mouse immunoglobulin G horseradish peroxide conjugated
secondary antibodies (Pierce; Thermo Fisher Scientific, Inc.;
1:5,000) at 37°C for 1 h. Following this, an Enhanced
Chemiluminescence kit was used to visualize proteins with exposure
to X-ray film (Kodak, Rochester, NY, USA). BandScan 5.0 software
(Glyko, Inc., Novato, CA, USA) were used to analyze the density of
strips. The relative expression amount of the target protein is
expressed as: (Objective protein optical density value)/(β-actin
optical density value) ×10.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The experiments were triplicated to obtain the mean
values. The statistical differences were calculated by unpaired
Student's t-test or one-way analysis of variance followed by
Dunnett's test using SPSS software (version 17.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
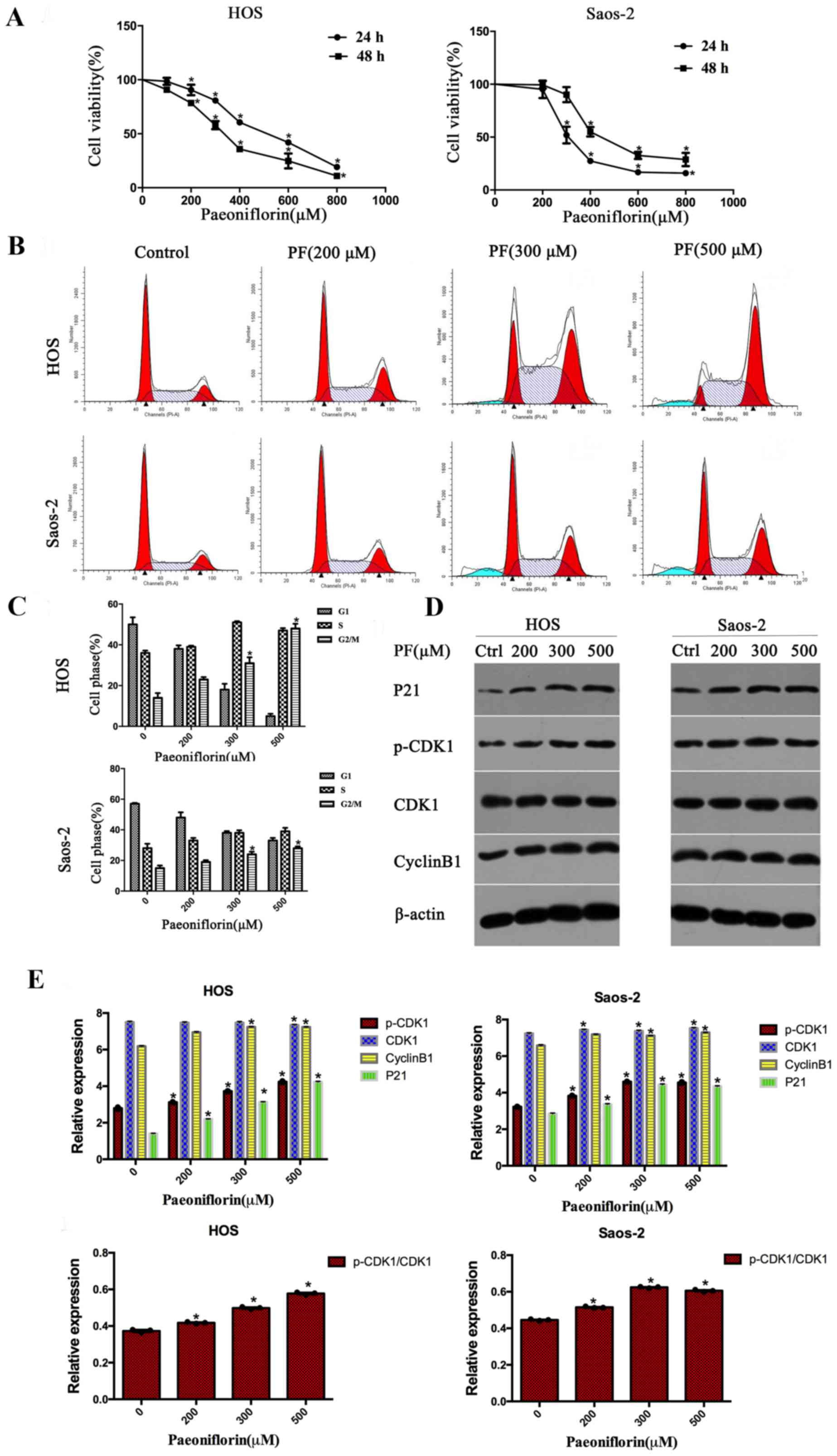
PF inhibits cell viability in
osteosarcoma cells in a dose- and time-dependent manner
To study the effects of PF on the growth of
osteosarcoma cells, an MTS assay was used to measure the cell
viability of HOS and Saos-2 cells. As the concentration of PF
increased, cell viability decreased; following incubation with PF
for 48 h, the IC50 values of PF were 363.29 µM for HOS and 351.24
µM for Saos-2 (Fig. 1A). The
results indicated that PF has the capability to inhibit cell
viability in a dose- and time-dependent manner.
PF induces cell cycle arrest at the
G2/M phase and regulates cell cycle protein expression levels in
osteosarcoma
In order to demonstrate whether PF inhibits cell
proliferation through mediating cell cycle arrest, flow cytometry
analyses were used to examine the distribution of the cell cycle.
As presented in Fig. 1B and C,
after incubating with PF for 48 h, HOS and Saos-2 cells exhibited a
significant increase in G2/M phase arrest, with a corresponding
decline in the G0/G1 and S phases in a dose-dependent manner. To
determine the underlying mechanism, the protein expression levels
of cyclin B1, CDK1, p-CDK1 and p21, which have been demonstrated to
be cell cycle-regulating proteins, were assessed. The protein
expression levels of cyclin B1, p-CDK1, CDK1 and p21 increased
after treating with PF in HOS and Saos-2 cells in a dose-dependent
manner (Fig. 1D and E).
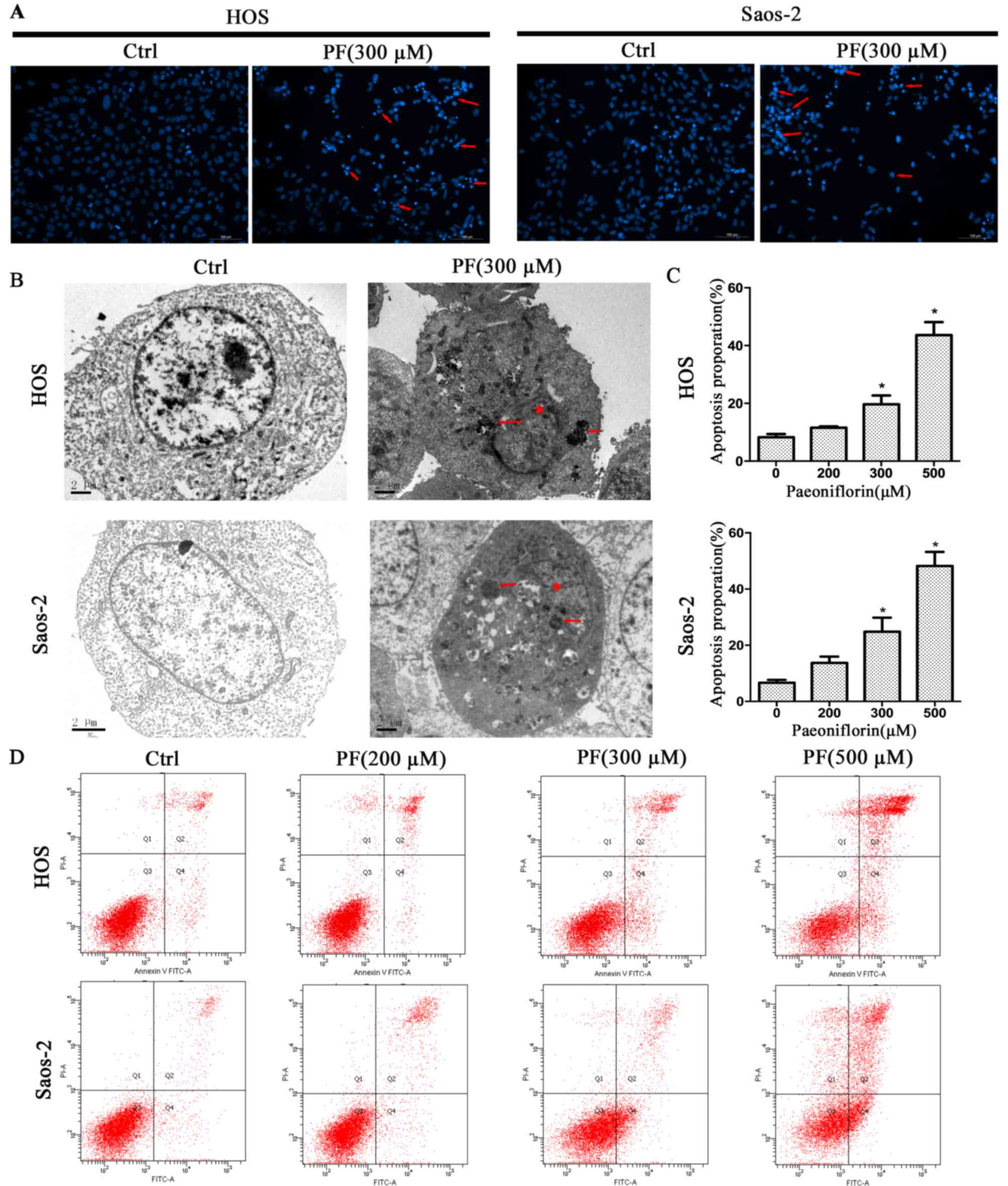
PF induces apoptosis in human
osteosarcoma cell lines
To investigate the inhibition mechanism of PF in the
proliferation of osteosarcoma cells, Hoechst 33258, TEM and flow
cytometry analyses were used in the present study. The Hoechst
33258 staining revealed morphological changes when the cells were
treated with PF, such as karyopyknosis and nuclear fragmentation
(Fig. 2A). Furthermore, TEM
revealed more detailed apoptotic morphological features in cells
treated with PF compared with control cells, including cell
shrinkage, a denser cytoplasm, cytoplasmic vacuoles, karyopyknosis
and the presence of apoptotic bodies (Fig. 2B). Next, flow cytometry analysis
was used to quantify PF-induced apoptosis in human osteosarcoma
cells. PF led to apoptosis in osteosarcoma cell lines in a
dose-dependent manner, consistent with the results of the MTS
assay. Following treatment with PF, Saos-2 cells exhibited
increased rates of apoptosis from 6.3% at 0 µM to 48.7% at 500 µM,
and HOS cells from 8.8% at 0 µM to 43.8% at 500 µM (Fig. 2C and D). The result was consistent
with the finding demonstrated by the MTS assay.
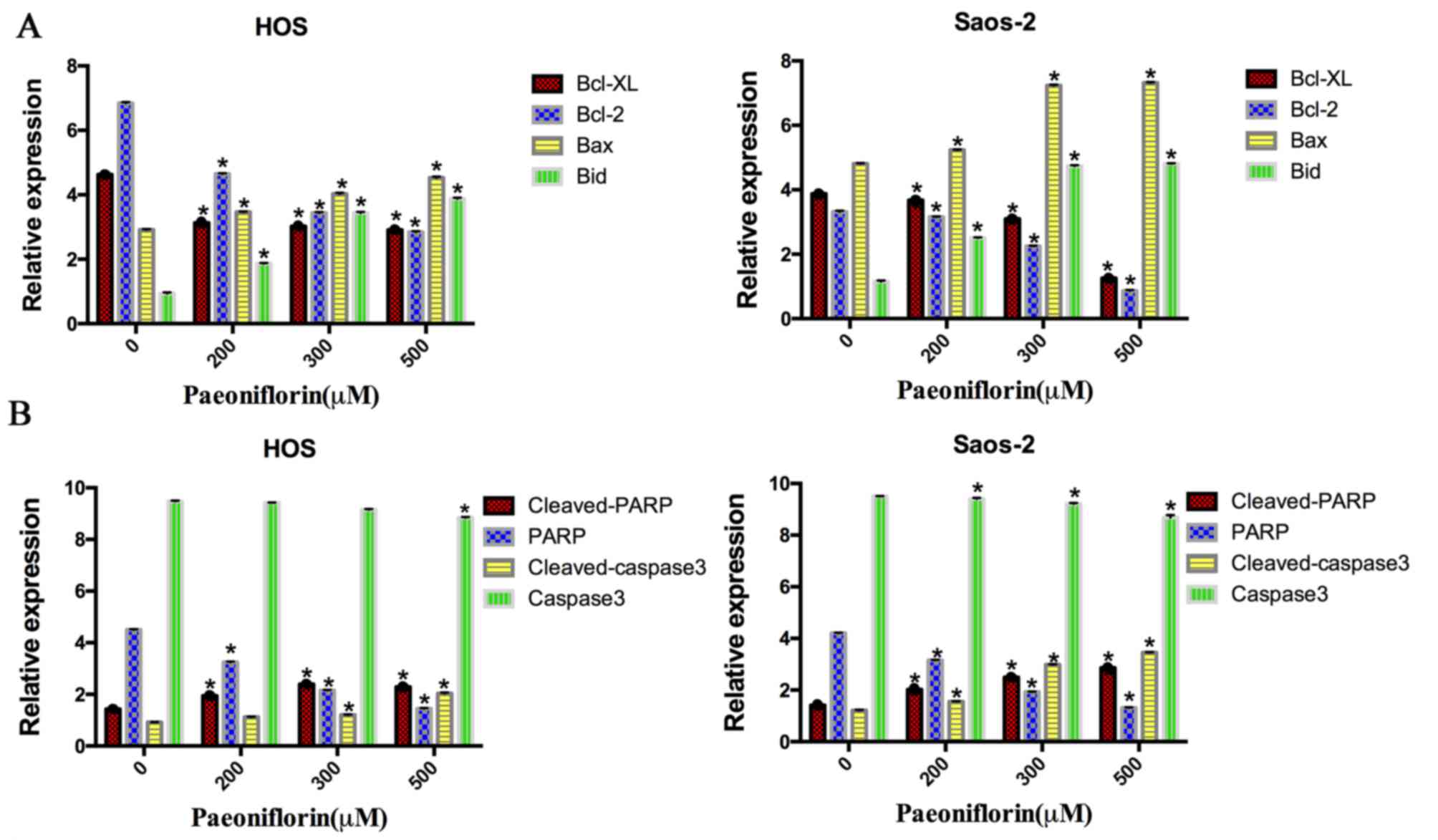
PF induces the apoptosis in the human
osteosarcoma cells by regulating the expression of Bcl-2, Bcl-XL,
Bax, Bid, PARP and caspase-3 in osteosarcoma cells
To detect the potential cell signaling pathways in
apoptosis induced by PF, the protein expression levels of the
caspase-3, PARP and Bcl-2 family were examined (Fig. 3). The expression levels of Bcl-2
and Bcl-XL protein were downregulated following PF treatment in a
dose-dependent manner, in both cell lines. However, the levels of
Bax and Bid protein were upregulated after incubation with PF for
48 h (P<0.05). Additionally, the levels of cleaved caspase-3 and
cleaved PARP protein were significantly upregulated in a
dose-dependent manner (P<0.05). Therefore, these results
demonstrated that apoptosis induced by PF occurred via
downregulation of the anti-apoptotic proteins Bcl-XL and Bcl-2 and
upregulation of the pro-apoptotic proteins Bax, Bid, PARP and
caspase-3 (Fig. 4).
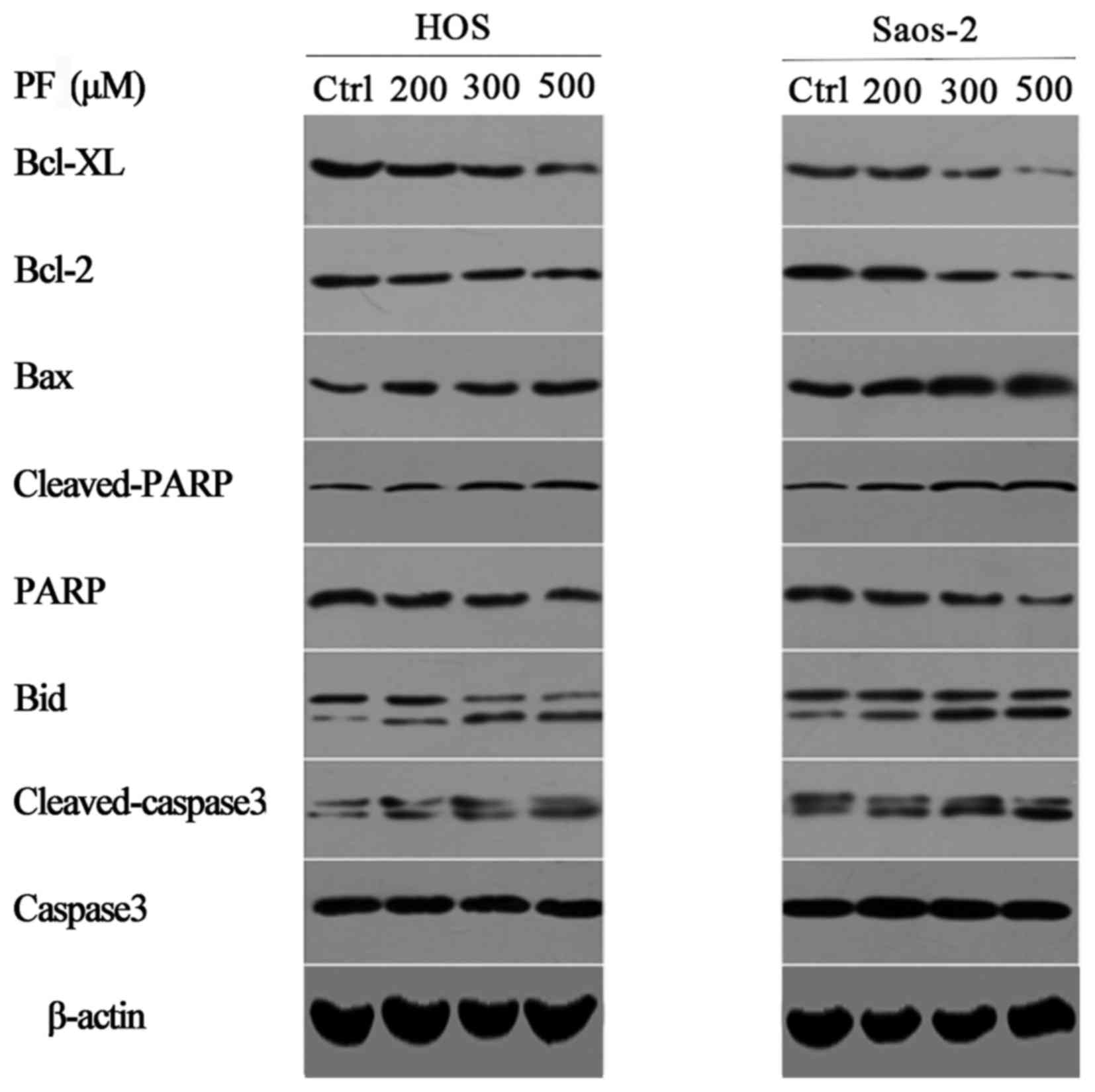 | Figure 3.Representative western blot images of
cleaved caspase-3, caspase-3, cleaved PARP, PARP and the Bcl-2
family of proteins, including Bid, Bax, Bcl-2 and Bcl-XL in human
osteosarcoma cells treated with PF for 24 h. β-actin served as a
loading control. PF, paeoniflorin; Bcl-2, B-cell lymphoma 2; XL,
extra large; Bax, Bcl-2 X-associated protein; PARP, poly
(ADPribose) polymerase; Bid, BH3 interacting domain death agonist;
Ctrl, control. |
Discussion
There is increasing evidence indicating the
anti-tumor properties of PF in multiple cancer cells, but the
impact of PF on human osteosarcoma cells remains to be determined.
In this study, it was indicated that PF inhibited the proliferation
of osteosarcoma cells in a dose- and time-dependent manner.
To elucidate the mechanisms underlying the
anticancer properties of PF, further studies were further
conducted. Annexin V-FITC/PI double staining illustrated that PF
causes G2/M phase cell cycle arrest and apoptosis in a
dose-dependent manner within a 0–500 µM range. Fluorescence and
electron microscopy revealed morphological changes following PF
treatment, such as cell shrinkage, a denser cytoplasm, cytoplasmic
vacuoles, karyopyknosis and the presence apoptotic bodies. The
present study illustrated in vitro that PF has a therapeutic
effect on osteosarcoma through G2/M phase cell cycle arrest and
inducing apoptosis.
Consistent with previous reports, the present study
demonstrated that the cell cycle G2 checkpoint (21) is also a target of PF. The cyclin
B1/CDK1 complex is an important regulators of G2/M cell arrest
(22–24). p21 was demonstrated to serve a role
in regulating G2 phase cell cycle progression through the
p53-dependent or p53-independent signaling pathways (25,26).
As HOS and Saos-2 cells are p53-mutant, p53-independent factors
serve a central role in the upregulation of p21.
Caspases, a cysteine protease family, act as an
important regulator of apoptosis. Once the cell is in the process
of apoptosis, the stimuli will trigger a caspase signaling cascade
(27). Caspase-3 marks the end of
the downstream effect of caspases (28,29),
the primary executioner of programmed cell death. PARP is the
substrate of caspase-3 (30), a
key regulator of apoptosis. In the present study, the dose of PF
upregulated the cleavage of caspase-3 and cleaved-PARP and
simultaneously inhibited the protein expression levels of
procaspase-3 and PARP, while increasing the rate of apoptosis.
These findings illustrated that PF inhibits osteosarcoma cells by
activating apoptotic pathways.
To further illuminate the specific molecular
mechanisms, related upstream apoptotic proteins were investigated.
Llambi et al (31) reported
that mitochondria-mediated apoptosis is mainly associated with the
Bcl-2 family. The Bcl-2 family can be separated into two
categories: One involving anti-apoptotic proteins including Bcl-2
and Bcl-XL, the other involving is pro-apoptotic proteins, mainly
including Bax and Bid (32). Bid
is a proapoptotic BH3-only member of the Bcl-2 family, and when
cleaved into the truncated Bid, it will translocate to the
mitochondria to cause apoptosis (33,34),
which is also called mitochondrial outer membrane permeabilization
(MOMP), the final common pathway of apoptosis (35,36).
Bax forms the MOMP pore, while Bcl-2 and Bcl-XL, the anti-apoptotic
proteins, locate in the outer wall of mitochondria and inhibit its
formation (37,38). Therefore, in the pathway of
apoptosis, the ratio of Bax/Bcl-2 and Bax/Bcl-XL serve an important
role, in addition to the expression levels of Bcl-2 family members
(39). The results indicated that
PF suppresses HOS and Saos-2 cells proliferation in vitro
through the mitochondrial signaling pathway.
In conclusion, although the specific mechanism of PF
in osteosarcoma has not been totally identified, these data
indicated that PF may induce apoptosis of osteosarcoma cells
directly or indirectly through the mediation of apoptosis,
resulting in G2/M cell cycle arrest, indicating a potential
therapeutic agent for osteosarcoma. However, further in vivo
studies are required to further elucidate these mechanisms.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472504), the
National Natural Science Foundation of China (grant no. 81401822)
and the Science and Technology Planning Project of Zhejiang
Province (grant no. 2013C33231).
References
|
1
|
Knops RR, van Dalen EC, Mulder RL,
Leclercq E, Knijnenburg SL, Kaspers GJ, Pieters R, Caron HN and
Kremer LC: The volume effect in paediatric oncology: A systematic
review. Ann Oncol. 24:1749–1753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rainusso N, Wang LL and Yustein JT: The
adolescent and young adult with cancer: State of the art-bone
tumors. Curr Oncol Rep. 15:296–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuijjer ML, Hogendoorn PC and
Cleton-Jansen AM: Genome-wide analyses on high-grade osteosarcoma:
Making sense of a genomically most unstable tumor. Int J Cancer.
133:2512–2521. 2013.PubMed/NCBI
|
|
5
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen C, Wang W, Tao L, Liu B, Yang Z and
Tao H: Chloroquine blocks the autophagic process in
cisplatin-resistant osteosarcoma cells by regulating the expression
of p62/SQSTM1. Int J Mol Med. 32:448–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang CZ, Zhang X, Li H, Tao YQ, Tao LJ,
Yang ZR, Zhou XP, Shi ZL and Tao HM: Gallic acid induces the
apoptosis of human osteosarcoma cells in vitro and in vivo via the
regulation of mitogen-activated protein kinase pathways. Cancer
Biother Radiopharm. 27:701–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gong WG, Lin JL, Niu QX, Wang HM, Zhou YC,
Chen SY and Liang GW: Paeoniflorin diminishes ConA-induced IL-8
production in primary human hepatic sinusoidal endothelial cells in
the involvement of ERK1/2 and Akt phosphorylation. Int J Biochem
Cell Biol. 62:93–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu S, Sun W, Wei W, Wang D, Jin J, Wu J,
Chen J, Wu H and Wang Q: Involvement of the prostaglandin E
receptor EP2 in paeoniflorin-induced human hepatoma cell apoptosis.
Anticancer Drugs. 24:140–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu JT, He W, Song SS and Wei W:
Paeoniflorin inhibited the tumor invasion and metastasis in human
hepatocellular carcinoma cells. Bratisl Lek Listy. 115:427–433.
2014.PubMed/NCBI
|
|
11
|
Zhang L and Zhang S: Modulating Bcl-2
family proteins and caspase-3 in induction of apoptosis by
paeoniflorin in human cervical cancer cells. Phytother Res.
25:1551–1557. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang LL, Zhang SL and Wang SZ: Relevant
study on apoptosis of cervical cancer HeLa cells induced by
paeoniflorin. Zhonghua Yi Xue Za Zhi. 90:3371–3375. 2010.(In
Chinese). PubMed/NCBI
|
|
13
|
Fang S, Zhu W, Zhang Y, Shu Y and Liu P:
Paeoniflorin modulates multidrug resistance of a human gastric
cancer cell line via the inhibition of NF-κB activation. Mol Med
Rep. 5:351–356. 2012.PubMed/NCBI
|
|
14
|
Zheng YB, Xiao GC, Tong SL, Ding Y, Wang
QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric carcinoma
cell proliferation through up-regulation of microRNA-124 and
suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol.
21:7197–7207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu H, Li W, Wang T, Shu Y and Liu P:
Paeoniflorin suppress NF-kappaB activation through modulation of I
kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human
gastric carcinoma cells. Biomed Pharmacother. 62:659–666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Zhou H, Wang CX, Li YS, Xie HY,
Luo JD and Zhou Y: Paeoniflorin inhibits growth of human colorectal
carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol.
50:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hung JY, Yang CJ, Tsai YM, Huang HW and
Huang MS: Antiproliferative activity of paeoniflorin is through
cell cycle arrest and the Fas/Fas ligand-mediated apoptotic pathway
in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol
Physiol. 35:141–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S and Liu W: Paeoniflorin inhibits
proliferation and promotes apoptosis of multiple myeloma cells via
its effects on microRNA29b and matrix metalloproteinase2. Mol Med
Rep. 14:2143–2149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Q, Chen GL, Li YJ, Chen Y and Lin FZ:
Paeoniflorin inhibits macrophage-mediated lung cancer metastasis.
Chin J Nat Med. 13:925–932. 2015.PubMed/NCBI
|
|
20
|
Nie XH, Ou-yang J, Xing Y, Li DY, Dong XY,
Liu RE and Xu RX: Paeoniflorin inhibits human glioma cells via
STAT3 degradation by the ubiquitin-proteasome pathway. Drug Des
Devel Ther. 9:5611–5622. 2015.PubMed/NCBI
|
|
21
|
Vairapandi M, Balliet AG, Hoffman B and
Liebermann DA: GADD45b and GADD45g are cdc2/cyclinB1 kinase
inhibitors with a role in S and G2/M cell cycle checkpoints induced
by genotoxic stress. J Cell Physiol. 192:327–338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Stefansson B, Wang W, Schaefer EM
and Brautigan DL: Phosphorylation of the Pro-X-Thr-Pro site in
phosphatase inhibitor-2 by cyclin-dependent protein kinase during
M-phase of the cell cycle. Cell Signal. 18:1318–1326. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sabour Alaoui S, Dessirier V, de Araujo E,
Alexaki VI, Pelekanou V, Lkhider M, Stathopoulos EN, Castanas E,
Bagot M, Bensussan A and Tsapis A: TWEAK affects keratinocyte G2/M
growth arrest and induces apoptosis through the translocation of
the AIF protein to the nucleus. PloS One. 7:e336092012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Khanal P, Savage P, She YM, Cyr TD
and Yang X: YAP-induced resistance of cancer cells to antitubulin
drugs is modulated by a Hippo-independent pathway. Cancer Res.
74:4493–4503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dalvai M, Mondesert O, Bourdon JC,
Ducommun B and Dozier C: Cdc25B is negatively regulated by p53
through Sp1 and NF-Y transcription factors. Oncogene. 30:2282–2288.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang W, Liu Y, Zhao N, Chen H, Qiao L,
Zhao W and Chen JJ: Role of Cdk1 in the p53-independent abrogation
of the postmitotic checkpoint by human papillomavirus E6. J Virol.
89:2553–2562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang CZ, Zhang JK, Shi ZL, Liu B, Shen CQ
and Tao HM: Matrine induces caspase-dependent apoptosis in human
osteosarcoma cells in vitro and in vivo through the upregulation of
Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother
Pharmacol. 69:317–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao LJ, Zhou XD, Shen CC, Liang CZ, Liu B,
Tao Y and Tao HM: Tetrandrine induces apoptosis and triggers a
caspase cascade in U2-OS and MG-63 cells through the intrinsic and
extrinsic pathways. Mol Med Rep. 9:345–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang SJ, Lee SA, Park MG, Kim JS, Yu SK,
Kim CS, Kim JS, Kim SG, Oh JS, Kim HJ, et al: Induction of
apoptosis by diphenyldifluoroketone in osteogenic sarcoma cells is
associated with activation of caspases. Oncol Rep. 31:2286–2292.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sairanen T, Szepesi R,
Karjalainen-Lindsberg ML, Saksi J, Paetau A and Lindsberg PJ:
Neuronal caspase-3 and PARP-1 correlate differentially with
apoptosis and necrosis in ischemic human stroke. Acta Neuropathol.
118:541–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Llambi F, Moldoveanu T, Tait SW,
Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP and Green DR:
A unified model of mammalian BCL-2 protein family interactions at
the mitochondria. Mol Cell. 44:517–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kvansakul M and Hinds MG: The Bcl-2
family: Structures, interactions and targets for drug discovery.
Apoptosis. 20:136–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grohm J, Plesnila N and Culmsee C: Bid
mediates fission, membrane permeabilization and peri-nuclear
accumulation of mitochondria as a prerequisite for oxidative
neuronal cell death. Brain Behav Immun. 24:831–838. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan XJ and Whang YE: PTEN sensitizes
prostate cancer cells to death receptor-mediated and drug-induced
apoptosis through a FADD-dependent pathway. Oncogene. 21:319–327.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suh DH, Kim MK, Kim HS, Chung HH and Song
YS: Mitochondrial permeability transition pore as a selective
target for anti-cancer therapy. Front Oncol. 3:412013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jonas EA, Porter GA Jr, Beutner G,
Mnatsakanyan N and Alavian KN: Cell death disguised: The
mitochondrial permeability transition pore as the c-subunit of the
F(1)F(O) ATP synthase. Pharmacol Res. 99:382–392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pang X, Moussa SH, Targy NM, Bose JL,
George NM, Gries C, Lopez H, Zhang L, Bayles KW, Young R and Luo X:
Active Bax and Bak are functional holins. Genes Dev. 25:2278–2290.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lindenboim L, Ferrando-May E, Borner C and
Stein R: Non-canonical function of Bax in stress-induced nuclear
protein redistribution. Cell Mol Life Sci. 70:3013–3027. 2013.
View Article : Google Scholar : PubMed/NCBI
|