Introduction
Lung cancer is the most frequently diagnosed cancer
and the prominent reason for tumor-associated mortalities across
the globe (1). Chemotherapy alone
or combined with other therapies has an important role in the
treatment of lung cancer (2), an
example of this includes Cisplatin (DDP)-based chemotherapy
(3). Although remarkable advances
have been made in targeted therapy, the median survival time of
patients with advanced disease is typically less than a year
(4). As an initial treatment, the
cytotoxicity of DDP is effective; however, cancer cells frequently
develop resistance to DDP as a result of its frequent usage
(5). Therefore, the development of
novel therapeutic strategies or agents is important for improving
the survival rate of lung cancer patients.
Traditional Chinese medicine (TCM) has contributed
greatly to the prosperity of the Chinese population for several
thousand years and to the development of traditional medicine
worldwide (6). As an adjuvant
therapy, TCM reduces the toxicity and adverse reactions induced by
surgery, chemotherapy and radiotherapy, by acting synergistically
(7). TCM has been widely used in
the treatment of tumors, however is not the first-line therapeutic
and is second to modern western medicine in terms of mechanism
elucidation and evidence-based data. Further developments in the
area of TCM may enhance novel therapeutic strategies for cancer in
the future.
A novel Qiyusanlong (QYSL) decoction formula was
established by Professor Han Mingxiang (The First Affiliated
Hospital of Anhui University of Chinese Medicine, Hefei, China), a
doctor of TCM according to the folk prescription of ‘Fu Zheng Xiao
Ji’ (8). The QYSL decoction was
composed of 10 variants of Chinese medicine including astragalus
membranaceus (Huangqi), polygonatum odoratum (yuzu), scolopendra
(tianlong), pberetima (dilong), solanum nigrum (longkui),
herbahedyotis (baihushecao), semen coicis (yiyiren), euphorbia
helioscopia (zeqi), curcuma longa (eshu) and tendril-leaved
fritillary bulb (chuanbei). The QYSL decoction has been used in
clinical study for over 20 years in China, however, research
regarding the specific function of the QYSL decoction as a cancer
therapeutic, particularly in lung cancer, is limited. The author's
previously demonstrated that the QYSL decoction suppresses the
level of programmed cell death (PD)-1/PD-L1 in mice bearing Lewis
lung carcinoma (LLC) (8). To
further investigate the role of QYSL decoction on the progression
of LLC, the present study employed QYSL decoction to treat a
C57BL/6 mouse xenograft model using LLC cells. The present study
investigated the effect of QYSL decoction at three doses on lung
cancer in a mouse model, and its association with the Wnt/β-catenin
signaling pathway. The canonical Wnt/β-catenin pathway has an
essential role in different stages of tumor development, including
cancer cell proliferation, migration, invasion, tumorigenesis and
metastasis (9). Wnt/β-catenin
signaling is tightly regulated at multiple cellular levels
(10). Dysregulation of the
Wnt/β-catenin pathway may be an important factor contributing to
increased maintenance and proliferation signaling in various
cancers (11), including lung
cancer (12). Numerous regulatory
proteins are involved in Wnt/β-catenin pathway, including Axin2,
β-catenin, glycogen synthase kinase 3β (GSK3β), c-Myc, cyclin D1,
Dishevelled (Dvl), Secreted frizzled-related proteins 2 (SFRP 2)
and cluster of differentiation 44 variation 6 (CD44v6) (13). The present study demonstrated that
QYSL decoction and DDP regulated the expression of regulatory
proteins in Wnt/β-catenin, including Wnt1, Wnt2, Wnt5a, GSK3β, and
affected the signals of CD44v6 and Survivin in tumor tissues.
The results of the present study revealed that QYSL
decoction exhibited an inhibitory effect on lung cancer growth via
the Wnt/β-catenin pathway, which verified the direct anti-tumor
effect of QYSL decoction in cancer. These data demonstrated that
the combination of QYSL decoction and DDP may represent a novel
future approach in treatment for lung cancer.
Materials and methods
Reagents
TRIzol® buffer (catalog no. 87801),
RevertAidTM first strand cDNA Synthesis Kit (catalog no. 00174486)
and enhanced chemiluminescence (ECL) kit (catalog no. QE218149)
were purchased from Thermo Fisher Scientific, Inc., (Waltham, MA,
USA). Polyclonal anti-Wnt1 (catalog no. AA56134), anti-GSK3β
(catalog no. CN33151), anti-phosphorylated (p)-GSK3β (catalog no.
CN33151), anti-SFRP-2 (catalog no. CC22141) antibodies were
purchased from Bioworld Technology, Inc., (St Louis Park, MN, USA).
Monoclonal anti-Wnt2 (catalog no. GR203181-1), anti-Wnt5a (catalog
no. CC36131), anti-β-catenin (catalog no. GR177612-31),
anti-Survivin (catalog no. GR209492-1) and polyclonal anti-Dvl-1
antibodies (catalog no. 109145) were obtained from Abcam
(Cambridge, UK). Anti-CD44V6 antibody (catalog no. bs-20756R) was
purchased from BIOSS (Beijing, China). DAB kit (catalog no.
K137726A) was obtained fromOrigene Technologies, Inc., (Beijing,
China).
All crudes of QYSL decoction drugs and Cisplatin
(DDP; catalog no. 411016CE) were purchased from dispensary of TCM
and dispensary for western medicine in the First Affiliated
Hospital of Anhui University of Chinese Medicine (Hefei, China).
The decoction consisted of 30 g astragalus membranaceus (huangqi),
10 g polygonatum odoratum (yuzu), 6 g scolopendra subspinipes
mutilans (tianlong), 6 g pberetima (dilong), 20 g solanum nigrum
(longkui), 20 g herbahedyotis (baihushecao), 20 g semen coicis
(yiyiren), 6 g euphorbia helioscopia (zeqi), 10 g curcuma longa
(eshu) and 6 g tendril-leaved fritillary bulb (chuanbei). QYSL
decoction was stored at 4°C at a concentration of 4.024 g/ml.
Animals
A total of 60 male SPF grade C57BL/6 mice (6–8 weeks
old), weighing 20±2 g, were purchased from Cavens Laboratory Animal
Co., Ltd. (Changzhou, China; catalog no. SCXK 2011-0003). Mice were
housed in air-filtered laminar flow cabinets with a 12-h light
cycle and food and water ad libitum. All the animal care and
experimental procedures followed were approved by the Institutional
Animal Care and Use Committee of Anhui University of Chinese
Medicine. Lewis lung carcinoma LLCs were obtained from Institute of
Shanghai Academy of Life Sciences, Chinese Academy of Sciences
(Shanghai, China), and maintained in Dulbecco's modified Eagle's
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 U/ml streptomycin (Origene
Technologies, Inc.), and kept in an incubator at 37°C, in a
humidified atmosphere containing 5% CO2.
Tumor xenografts in nude mice
LLCs were suspended in serum-free medium and a total
of 1×105 (0.1 ml) LLCs per mouse were inoculated
subcutaneously into the left dorsal flanks of nude mice. When
tumors reached an average volume of approximately 3–5
mm3 (10 days following injection), the mice were
considered as established with xenografts. The next day, mouse
models were randomized into six groups (n=8 per group): i) Control
group (C): Mice received dosage of 0.2 ml/10 g physiological saline
via intragastric administration for 21 days, and 0.4 ml of
physiological saline by intraperitoneal injection once a week. ii)
Low dosage of QYSL decoction group (LQ): Mice underwent
intragastric administration of 20.12 g/kg QYSL decoction for 21
days, and 0.4 ml physiological saline via intraperitoneal injection
once a week. iii) Medium dosage of QYSL decoction group (MQ): Mice
underwent intragastric administration of 40.24 g/kg QYSL for 21
days, and 0.4 ml physiological saline via intraperitoneal injection
once a week. iv) High dosage of QYSL decoction group (HQ): Mice
received intragastric administration with doses of 80.48 g/kg QYSL
for 21 days, and 0.4 ml physiological saline via intraperitoneal
injection once a week. v) DDP group (DDP): Mice received
intragastric administration of 0.2 ml/10 g physiological saline for
30 days, and 0.4 ml DDP via intraperitoneal injection once a week.
vi) HQ+DDP group: Mice received intragastric administration of
80.48 g/kg QYSL for 21 days, and 0.4 ml DDP via intraperitoneal
injection once a week. Following a 22-day period, tumor xenografts
were isolated, and measured with the precision electronic balance
and the tumor inhibition ratio was calculated. The tumor inhibition
ratio=(the average quality of tumor in C group-the average quality
of tumor in experience group)/the average quality of tumor in C
group × 100%.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from xenograft tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
quality and quantity of RNA were determined by measuring the
absorbance at wavelengths of 260 and 280 nm using Nano Drop-2000
ultra microspectrophotometer (Thermo Fisher Scientific, Inc.). A
total of one µg of RNA was reverse transcribed into cDNA using
RevertAidTM first strand cDNA Synthesis kit according to the
manufacturer's protocol. C-myc, Cyclin D1 and Axin2 cDNAs were
amplified using SYBR Premix Ex Taq kit (Takara Bio, Inc.) with
LightCycler 480 PCR system (Roche Diagnostics, Indianopolis, IN,
USA). The thermocycling conditions for the PCR assay cycles were as
follows: 95°C for 5 min, 40 cycles of 95°C for 10 sec, 95°C for 10
sec and 60°C for 30 sec. The mRNA levels of the target gene were
normalized to the level of β-actin using the 2−ΔΔCq
method (14) and were represented
as fold induction. The primers used for RT-qPCR were as follows:
Forward, 5′-TGAGGAAACGACGAGAACAG-3′ and reverse,
5′-ACGAGAGATTCCAGCTCCTC-3′ for c-myc; forward,
5′-ATCTCCCTTGATTCAAACGC-3′ and reverse, 5′-GCCCAATGAAAGACCAATCT-3′
for cyclin D1; forward, 5′-CAGTGAGCTGGTTGTCACCT-3′ and reverse,
5′-CTGAGCTGCTCCTTGAAGTG-3′ for Axin2; forward,
5′-CCTCTATGCCAACACAGTGC-3′ and reverse, 5′-GTACTCCTGCTTGCTGATCC-3′
for β-actin.
Western blotting
Proteins were extracted from xenograft tissues using
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). The concentration of protein was measured
using a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China). A total of 50 µg protein was
subjected to 10% SDS-polyacrylamide gel and then transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). Subsequently, the membrane was blocked with 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) in Tris-buffered saline with
0.05% Tween-20 (TBST) for 1 h at room temperature and then
incubated overnight at 4°C with the primary antibodies against
β-actin (1:1,000), Wnt1 (1:500), Wnt2 (1:500), Wnt5a (1:1,000),
Dvl-1 (1:500), GSK3β (1:1,000), p-GSK3β (1:500) or SFRP-2
(1:1,000). Following washing with TBST three times, the membrane
was probed with peroxidase conjugated affinity purified goat
anti-mouse immunoglobulin G (Origene Technologies, Inc., Beijing,
China, 1:1,000 dilution, cat. no. TA100015) at 37°C for 2 h. The
level of β-actin was examined as an internal control. The membranes
were developed with an ECL kit (Pierce; Thermo Fisher Scientific
Inc.), and the gray value of the bands of interest was analyzed by
FlourChem V2.0 (ProteinSimple, San Jose, CA, USA).
Immunohistochemistry
Tumor xenografts were fixed immediately following
removal, in a 10% buffered formal in solution for a maximum of 48 h
at room temperature, prior to being dehydrated and
paraffin-embedded under vacuum conditions. Slides with paraffin
sections (3–5 µm) were deparaffinized. Endogenous peroxidase
activity was blocked by incubation in 3% H2O2
for 10 min at room temperature. The slides were incubated with the
primary anti-CD44v6 antibody (1:200 dilution) or anti-Survivin
antibody (1:500 dilution) overnight at 4°C. Sections were washed
with PBS and incubated with biotinylated anti-rabbit secondary
antibody for 2 h at 37°C. Diaminobenzidine
(DAB)-H2O2 was used as the chromogen to
reveal antibody binding sites. A total of five high-powered fields
were randomly chosen using an inverted microscope. Positive cells
were marked in brown or dark nankeen. The average optical density
was quantified with JEDR801D software (Jetta Technology, Nanjing,
Jiangsu, China). The staining results were evaluated and scored
independently by two pathologists.
Statistical analysis
All experiments were performed at least three times
using independent samples. All data are expressed as the mean ±
standard deviation. Statistical analysis was conducted using SPSS
software, version 12.0 software (SPSS, Inc., Chicago, IL, USA). The
Student's t-test was used to compare the difference between two
individual groups, and one-way analysis of variance followed by
Dunnett's post hoc testwas used to compare the difference among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
QYSL decoction inhibits tumor
growth
To explore the role of the QYSL decoction on lung
cancer, the present studyestablished a C57BL/6 mouse xenograft
model using LLC and administered different doses of QYSL decoction
to treat mice. In Table I, there
were no significant differences among C, LQ and MQ groups
(P>0.05). However, the tumor size in HQ, DDP or HQ+DDP groups
was decreased compared with the C group (P<0.05; P<0.01;
P<0.01). No significant difference was observed between DDP and
HQ+DDP groups, however the tumor masses in the DDP and HQ+DDP
groups were lower compared with HQ group (P<0.01). Following
this, the tumor inhibition ratio from different groups was
analyzed. Compared with C group, the tumor inhibition ratios of LQ,
MQ, HQ, DDP and HQ+DDP groups were increased (P<0.01). These
data demonstrated that high doses of QYSL decoction and DDP
significantly suppressed tumor growth, suggesting that QYSL
decoction may function as a lung cancer therapeutic.
 | Table I.Tumor mass and inhibition ratio of
different groups. |
Table I.
Tumor mass and inhibition ratio of
different groups.
| Groups | n | Mass (g) | Ratio (%) |
|---|
| C | 8 | 5.12±0.63 | 0 |
| LQ | 8 | 4.53±1.34 | 13.51 |
| MQ | 8 | 4.27±0.62 | 18.32 |
| HQ | 8 |
3.45±1.05a | 33.86 |
| DDP | 8 |
1.49±0.68b | 73.01 |
| HQ+DDP | 8 |
1.48±0.71b | 73.23 |
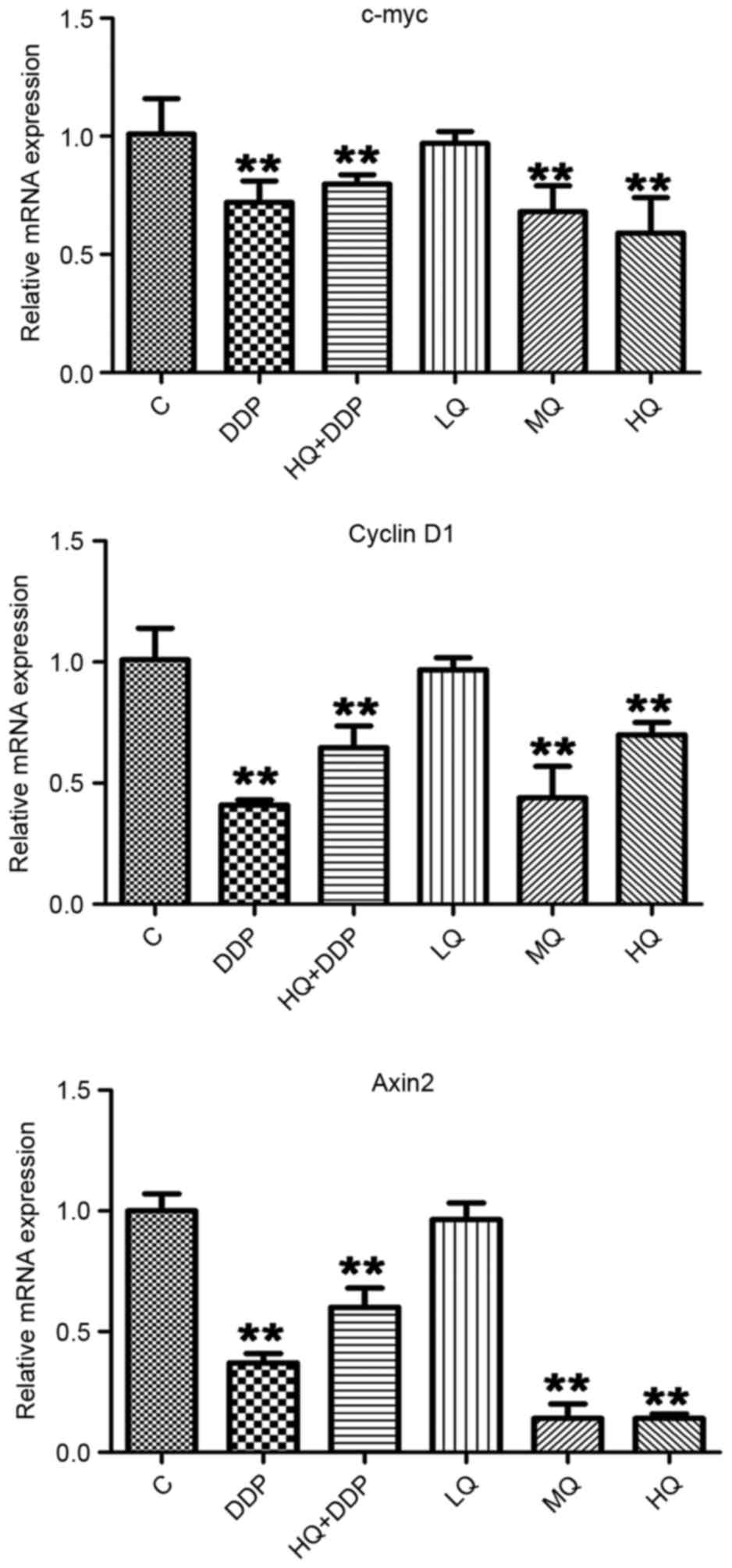
QYSL decoction inhibits mRNA levels of
c-myc, cyclin D1 and Axin 2, detected via RT-qPCR
To examine the mechanism by which QYSL decoction
exhibits its inhibitory effects in lung tumor development, the
present study first detected the mRNA levels of c-myc, cyclin D1
and Axin 2 following different treatments. In Fig. 1, there was no significant
difference between C and LQ groups in the mRNA levels of c-myc,
cyclin D1 and Axin2 (P>0.05). Compared with C group, the levels
of c-myc, cyclin D1 and Axin2 in MQ, HQ, DDP and HQ+DDP groups were
significantly repressed (P<0.01). No significant differences in
the expression of c-myc among HQ, DDP and HQ+DDP groups were
observed. However, compared with HQ group, the level of cyclin D1
in DDP group were suppressed, however the expression of Axin2 was
upregulated (P<0.01). These data demonstrated that the QYSL
decoction and DDP inhibited the expression of c-myc, cyclin D1 and
Axin2, however, the QYSL decoction and DDP demonstrated different
degrees of inhibition, and the application of QYSL decoction
affected the role of DDP to a certain degree.
QYSL decoction exhibits varied effects
on Wnt/β-catenin pathway-associated proteins, detected via western
blotting
To further determine the mechanism of QYSL decoction
functioning in tumor inhibition, the protein levels of regulatory
proteins in Wnt/β-catenin pathway were detected. In Fig. 2A, compared with C group, different
dosages of QYSL decoction and DDP displayed different degrees of
inhibition on the protein levels of Wnt1, Wnt2, Wnt5a, GSK3β,
p-GSK3β, β-catenin and Dvl-1. Compared with C group, the protein
levels of Wnt1, Wnt2, Wnt5a, GSK3β, p-GSK3β, β-catenin and Dvl-1 in
LQ, MQ, HQ, DDP and HQ+DDP groups were significantly suppressed
(Fig. 2B-H; P<0.01), and the
HQ+DDP group demonstrated the most significant inhibitory effect on
the expression of the aforementioned genes, except β-catenin.
However, compared with the above genes, different doses of QYSL
decoction and DDP resulted in different effect on the protein level
of SFRP-2. Compared with C group, the levels of SFRP-2 in LQ, MQ,
DDP and HQ+DDP groups were upregulated (P<0.01), and HQ markedly
enhanced the expression of SFRP-2 (Fig. 2I; P<0.01). Overall, the results
revealed that the QYSL decoction and DDP modulated the regulatory
protein expression levels in Wnt/β-catenin pathway.
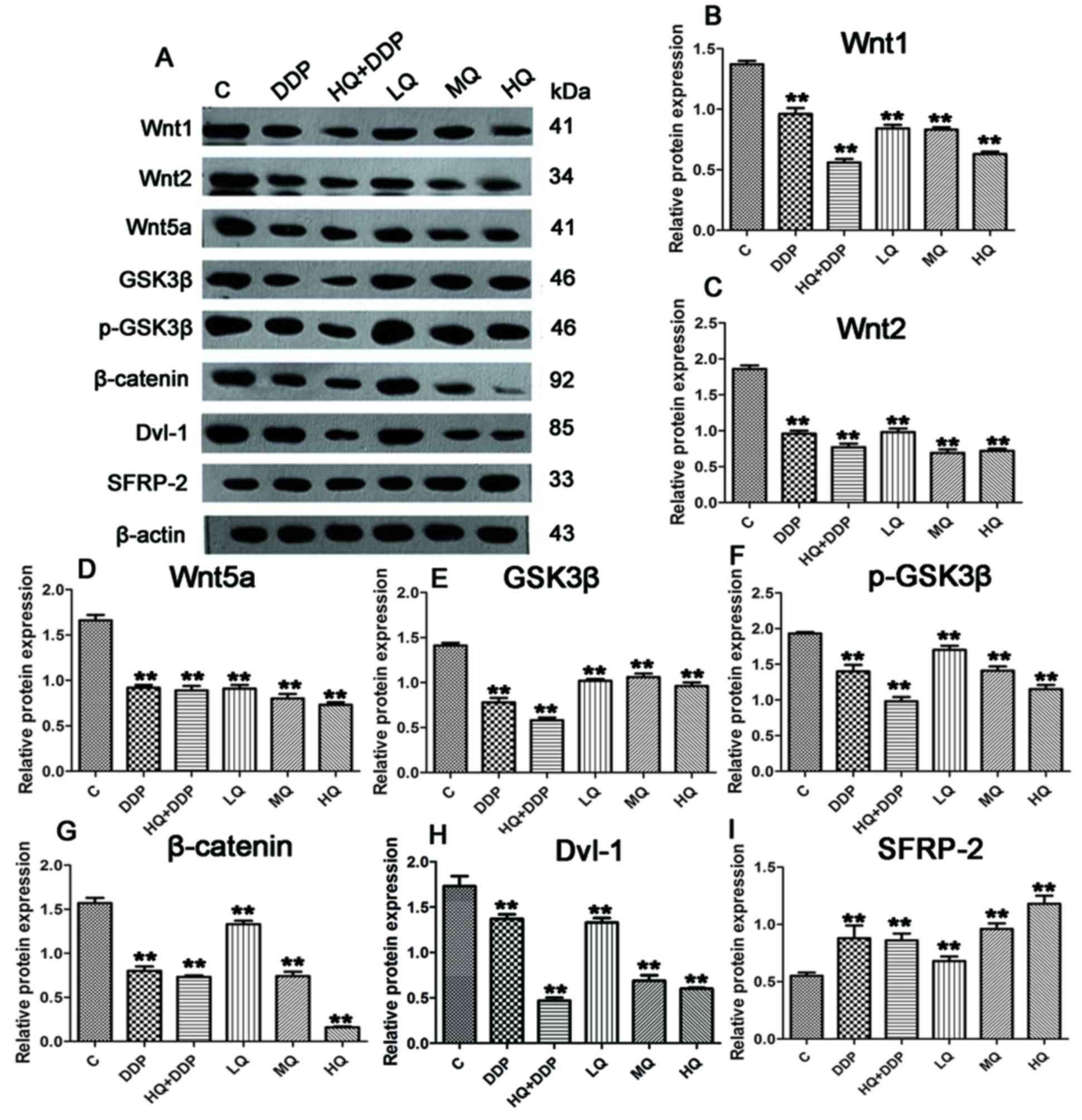 | Figure 2.QYSL decoction and DDP modulate the
expression levels of regulatory proteins in Wnt/β-catenin pathway.
The established growing xenograft lung cancer mice were randomized
into six groups (n=8 per group). i) control group (C): Mice
received dosage of 0.2 ml/10 g physiological saline via
intragastric administration for 21 days, and 0.4 ml of
physiological saline by intraperitoneal injection once a week. ii)
Low dosage of QYSL decoction group (LQ): Mice underwent
intragastric administration of 20.12 g/kg QYSL decoction for 21
days, and 0.4 ml physiological saline via intraperitoneal injection
once a week. iii) Medium dosage of QYSL decoction group (MQ): Mice
underwent intragastric administration of 40.24 g/kg QYSL for 21
days, and 0.4 ml physiological saline via intraperitoneal injection
once a week. iv) High dosage of QYSL decoction group (HQ): Mice
received intragastric administration with doses of 80.48 g/kg QYSL
for 21 days, and 0.4 ml physiological saline via intraperitoneal
injection once a week. v) DDP group (DDP): Mice received
intragastric administration of 0.2 ml/10 g physiological saline for
30 days, and 0.4 ml DDP via intraperitoneal injection once a week.
vi) HQ+DDP group: Mice received intragastric administration of
80.48 g/kg QYSL for 21 days, and 0.4 ml DDP via intraperitoneal
injection once a week. Tumor tissues proteins were extracted for
western blotting. (A) Representative image and quantification of
(B) Wnt1, (C) Wnt 2, (D) Wnt5a, (E) GSK3β, (F) p-GSK3β, (G)
β-catenin, (H) Dvl-1 and (I) SFRP-2 protein expression levels. The
gray value of the bands of interest was normalized to β-actin and
analyzed by FlourChem V2.0. **P<0.01 vs. (C). DDP, Cisplatin
(DDP)-based chemotherapy; QYSL, Qiyusanlong; p, phosphorylataed;
GSK3β, glycogen synthase kinase 3β; Dvl, Dishevelled; SFRP,
Secreted frizzled-related proteins. |
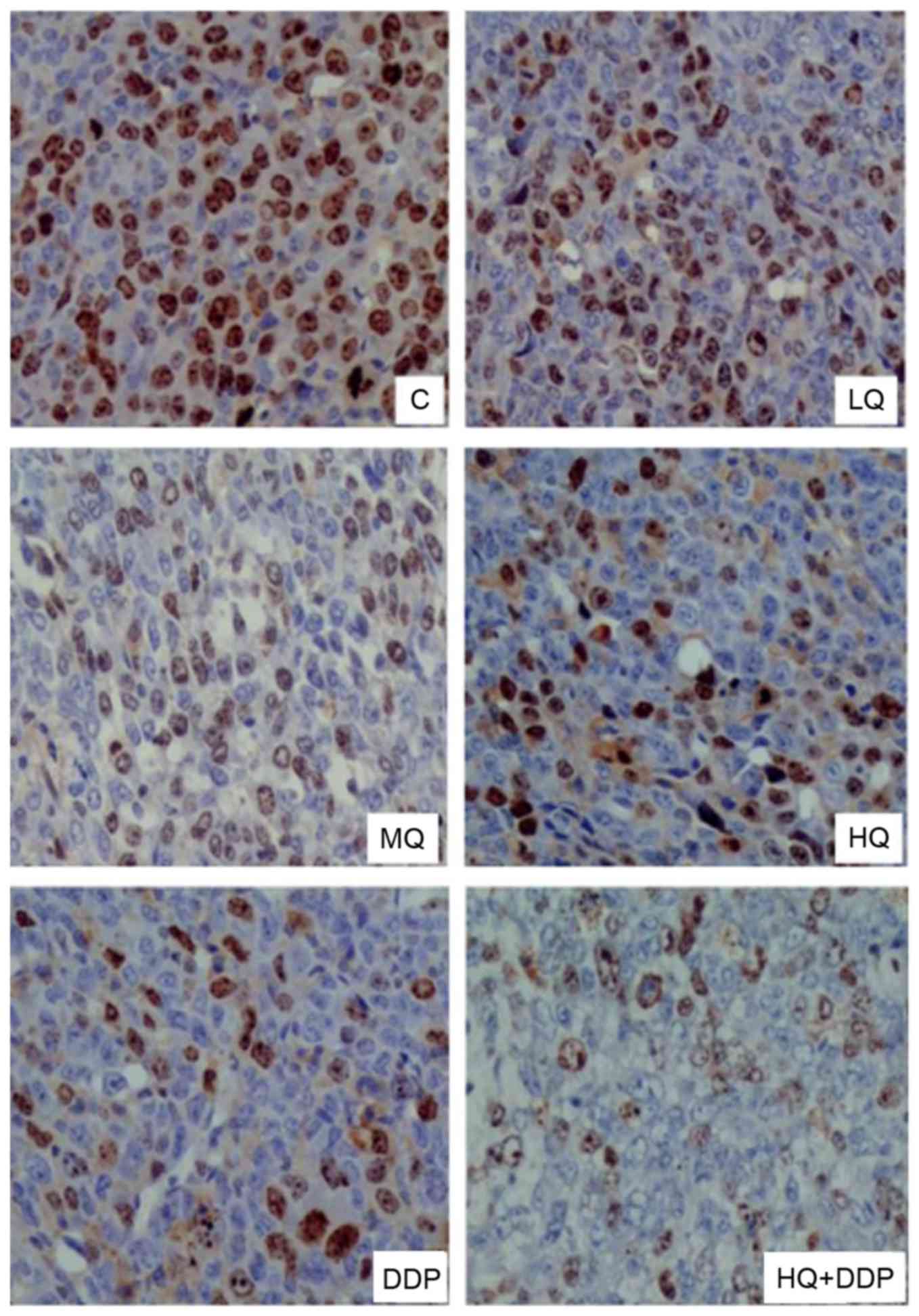
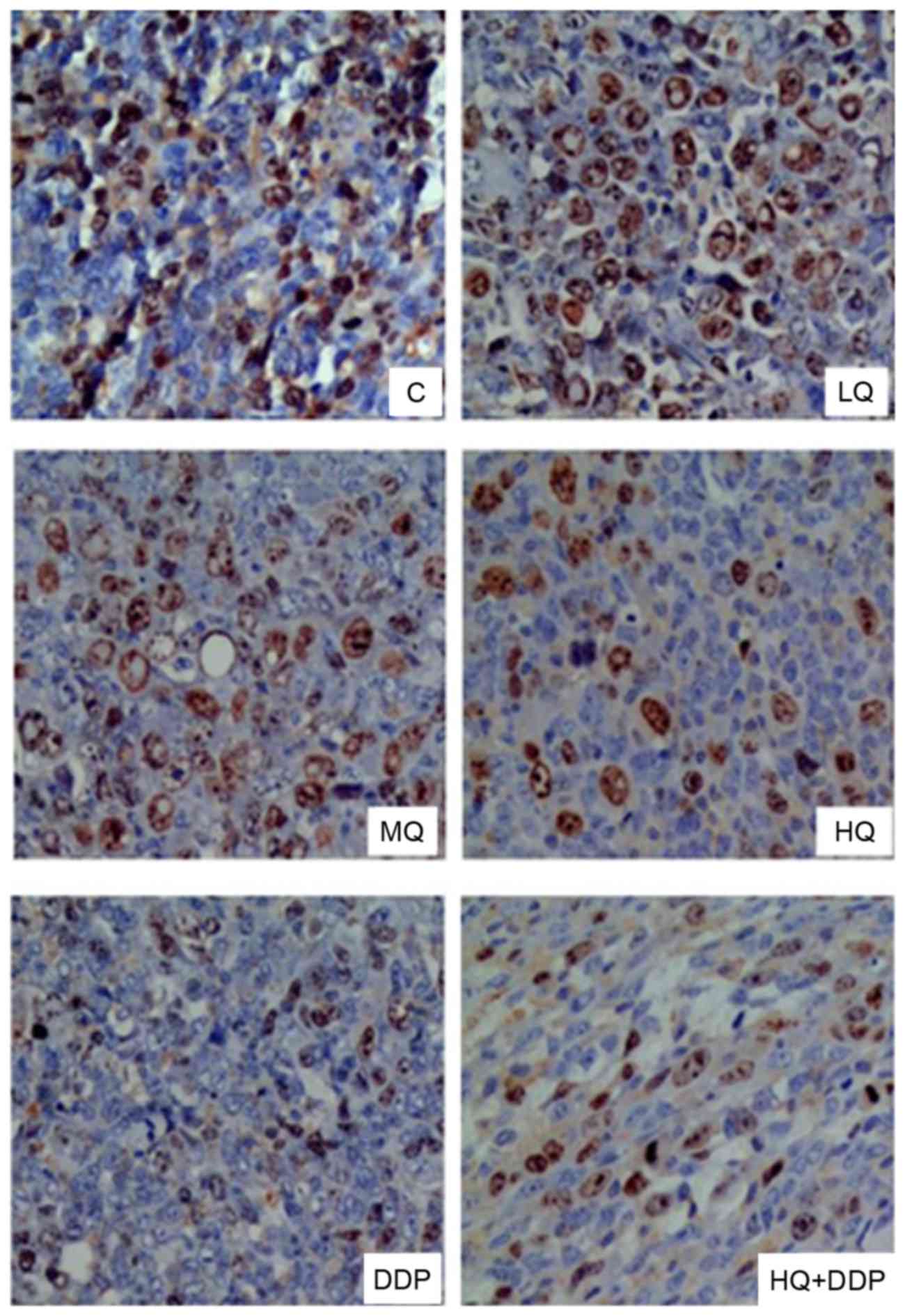
QYSL decoction inhibits protein
signals of CD44V6 and Survivin, detected via
immunohistochemistry
CD44 variant isoforms, particularly CD44v6, have
been identified as protein markers for metastatic behavior
innumerous malignant tumors (15).
Elevated expression of Survivin correlates with poor prognosis,
tumor recurrence and drug resistance in various human cancers,
including lung cancer (16). The
present study detected the signals of CD44v6 (Fig. 3) and Survivin (Fig. 4) via immunohistochemistry following
different treatments, presented in Table II. In Fig. 3, the signals of CD44v6 in MQ, HQ,
DDP and HQ+DDP groups decreased compared with C group (P<0.05),
and there were no significant differences among MQ, HQ and DDP
groups, however HQ+DDP group revealed the most significant
inhibitory effect. Similarly, there was no significant differences
observed in Survivin signals among C and LQ groups (P>0.05).
However, the signals of Survivin in MQ, HQ, DDP and HQ+DDP groups
were suppressed compared with the C group (Fig. 4; P<0.05), and there was no
significant difference among the MQ, HQ, DDP and HQ+DDP groups
(P>0.05). These data revealed that QYSL decoction and DDP
inhibited the protein levels of CD44v6 and Survivin.
 | Table II.Signals of CD44v6 and Survivin in
tumor tissues from different groups. |
Table II.
Signals of CD44v6 and Survivin in
tumor tissues from different groups.
| Groups | n | CD44v6 | Survivin |
|---|
| C | 6 | 0.85±0.12 | 0.83±0.10 |
| LQ | 6 | 0.81±0.07 | 0.83±0.11 |
| MQ | 6 |
0.75±0.06a |
0.76±0.05a |
| HQ | 6 |
0.74±0.08a |
0.71±0.07a |
| DDP | 6 |
0.74±0.08a |
0.72±0.07a |
| HQ+DDP | 6 |
0.65±0.11a |
0.71±0.11a |
Discussion
Lung cancer is a leading contributor to
cancer-associated mortalities worldwide (4). Chemotherapy has improved overall
survival, however remains limited at <12 months median overall
survival (17). Adverse effects,
including nausea and vomiting, sore mouth, diarrhea, hepatotoxicity
and immunosuppression, are commonly reportedin patients with
cancers treated with chemotherapy. A previous report suggested that
a variety of TCM may extensively be used for managing these adverse
effects (18). QYSL decoction,
established by Professor Han Mingxiang, an old doctor of TCM
according to the folk prescription of ‘Fu Zheng Xiao Ji’, was
composed from 10 kinds of Chinese medicine including astragalus
membranaceus (Huangqi), polygonatum odoratum (yuzu), scolopendra
(tianlong), pberetima (dilong), solanum nigrum (longkui),
herbahedyotis (baihushecao), semen coicis (yiyiren), euphorbia
helioscopia (zeqi), curcuma longa (eshu) and tendril-leaved
fritillary bulb (chuanbei). The function of QYSL decoction in
cancer therapy has not been fully elucidated. The present study
aimed to expand current treatments of lung cancer and provide a
novel candidate as alung cancer treatment. The present study
employed the QYSL decoction to treat lung cancer xenografts in nude
mice models to observe tumor growth following different treatments.
The results revealed that QYSL decoction regulated tumor growth via
modulating the regulatory proteins in the Wnt/β-catenin
pathway.
QYSL decoction was composed of 10 types of Chinese
medicine. These aforementioned herbs have previously been
categorized to exhibit anti-cancer properties. Astragalus
membranaceus (Huangqi) has a wide range of immunopotentiating
effects and is widely used as an adjuvant medicine during cancer
therapy (19). Polygonatum
odoratum (yuzu) extracts induce apoptosis of MDA-MB-231 breast
cancer cells (20) and A549 human
lung cancer cells (21). Extracts
of scolopendra (tianlong) subspinipes mutilans induce cell cycle
arrest and apoptosis in A375 human melanoma cells (22). Aqueous extract of solanum nigrum
(longkui) leaves suppress tumor growth and enhance cytotoxicity of
DDP (23). HerbaHedyotidis
(baihushecao) is the ‘assistant and attendant’ herb with ‘bitter
and cold’ properties, resulting in ‘heat-clearing and
detoxification’ and ‘pain-alleviating’ effects to reduce or control
tumor size (7). The components of
semen coicis(yiyiren) exert anti-cancer actions via inhibiting
proliferation and inducing apoptosis of cancer cells, in addition
to increasing the sensitivity of patients to chemotherapeutic
agents (24). Euphorbia
helioscopia (zeqi) extract inhibits hepatocellular carcinoma growth
in nude mice xenografts (25). A
previous study indicated that curcuma longa (eshu) exerts
anti-cancer effects in a variety of biological pathways involved in
mutagenesis, apoptosis, tumorigenesis, cell cycle regulation and
metastasis (26). These previous
reports indicate that the components of QYSL decoction exhibit
anti-tumor functions in various cancers. The present study
demonstrated that the application of QYSL decoction suppressed
tumor growth, which was in agreement with previous reports. These
data provided evidence that QYSL decoction maybe used as a novel
therapy for lung cancer treatment.
Wnt/β-catenin signaling is an evolutionarily
conserved and versatile pathway that is known to be involved in
embryonic development, tissue homeostasis and a wide variety of
human diseases. In the present study, the administration of QYSL
decoction and DDP affected the mRNA and protein levels of the
regulatory proteins in the Wnt/β-catenin pathway, suggesting that
QYSL decoction may interfere with lung cancer development via the
Wnt/β-catenin pathway. Stimulation of the canonical Wnt pathway
ultimately results in the activation of β-catenin, which promotes
the transcription of proteins involved in cell proliferation,
including c-Myc and Cyclin D1 (27). The present study demonstrated that
the application of a high dose of QYSL decoction significantly
reduced the expression levels of c-myc, Cyclin D1, Axin2, Wnt1,
Wnt2, Wnt5a, GSK3β, p-GSK3β and β-catenin, suggesting that QYSL
decoction interacts with the Wnt/β-catenin pathway. Dvl family
proteins located upstream of the Wnt pathway are overexpressed in
lung cancer (28). Dvl-1 and Dvl-3
affected the biological behavior of lung cancer cells via canonical
and non canonical Wnt pathway (28). In the present study, QYSL
decoction, mimicking chemotherapy DDP, suppressed the expression of
Dvl-1 and suppressed the cyclin D1, c-myc and axin 2 levels. These
results revealed that there may be a new mechanism by which Dvl-1
was associated with lung cancer development. Therefore, further
investigations need to be performed in our further work. SFRP2, an
identified member of the SFRPs family of molecules, is able to
inhibit the Wnt-induced increase of free β-catenin and influence
cell cycle progression and tumor cell proliferation (29). CD44v6, a downstream factor of Wnt
signaling, acts as a marker for predicting cancer metastasis
(30). The knockdown of CD44v6
expression may result in depression of tumor metastases and cell
invasion (31). Survivin, a member
of an apoptosis inhibitor family, was a unique target for tumor
therapy (32). DDP and QYSL
decoction repressed the signals of CD44v6 and survivin. Notably,
the effect of QYSL decoction was milder compared with DDP,
suggesting that TCM QYSL decoction may be more effectively
tolerated than DDP.
In conclusion, the results of the present study
demonstrated that different doses of QYSL decoction resulted in
various effects on tumor growth and the expression of regulatory
proteins in the Wnt/β-catenin pathway. The combination of DDP and a
high dose of QYSL decoction significantly enhanced the inhibitory
effect on lung cancer. Therefore, clinical trials may attempt to
administer high doses of QYSL decoction combined with DDP in cancer
therapy in the future. However, the effective doses of QYSL
decoction and DDP on cancer inhibition require further
investigation. QYSL decoction and DDP repressed lung cancer
development via monitoring the expression of regulatory proteins in
Wnt/β-catenin pathway. Furthermore, the effect of QYSL decoction
was milder, however when combined with the chemotherapy drug,
enhanced the overall inhibitory effect. Further investigations may
help provide novel strategies for lung cancer therapy by targeting
the Wnt signaling pathway in the future.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Anhui Province (grant no. 1708085MH197).
Glossary
Abbreviations
Abbreviations:
|
QYSL
|
qiyusanlong
|
|
TCM
|
traditional Chinese medicine
|
|
LLC
|
Lewis lung carcinoma cells
|
|
GSK3β
|
glycogen synthase kinase 3β
|
|
Dvl
|
Dishevelled
|
|
SFRP
|
Secreted frizzled-related proteins
|
|
CD44v6
|
cluster of differentiation 44
variation 6
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hwang KE and Kim HR: Response evaluation
of chemotherapy for lung cancer. Tuberc Respir Dis (Seoul).
80:136–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu N, Xiong Y and Wang C: Bu-Zhong-Yi-Qi
decoction, the water extract of chinese traditional herbal
medicine, enhances cisplatin cytotoxicity in A549/DDP cells through
induction of apoptosis and autophagy. Biomed Res Int.
2017:36927972017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Somasundaram A and Burns TF: The next
generation of immunotherapy: Keeping lung cancer in check. J
Hematol Oncol. 10:872017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ou Y, Zhai D, Wu N and Li X:
Downregulation of miR-363 increases drug resistance in
cisplatin-treated HepG2 by dysregulating Mcl-1. Gene. 572:116–122.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin HQ, Gong AG, Wang HY, Duan R, Dong TT,
Zhao KJ and Tsim KW: Danggui Buxue Tang (Astragali Radix and
Angelicae Sinensis Radix) for menopausal symptoms: A review. J
Ethnopharmacol. 199:205–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Z, Chen S, Cai J, Zhang E, Lan L,
Zheng J, Liao L, Yang X, Zhou C and Du J: Traditional Chinese
medicine syndrome-related herbal prescriptions in treatment of
malignant tumors. J Tradit Chin Med. 33:19–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Tong J and Li Z: Qiyusanlong
decoction inhibits the level of PD-1/PD-L1 in mice bearing Lewis
lung carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 32:770–774.
2016.(In Chinese). PubMed/NCBI
|
|
9
|
Jiang Q, He M, Guan S, Ma M, Wu H, Yu Z,
Jiang L, Wang Y, Zong X, Jin F and Wei M: MicroRNA-100 suppresses
the migration and invasion of breast cancer cells by targeting
FZD-8 and inhibiting Wnt/β-catenin signaling pathway. Tumour Biol.
37:5001–5011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan P, Zhang B, Xi GM, Wu Y, Liu HB, Liu
YF, Xu WJ, Zhu QQ, Cai F, Zhou ZJ, et al: PRC1 contributes to
tumorigenesis of lung adenocarcinoma in association with the
Wnt/β-catenin signaling pathway. Mol Cancer. 16:1082017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu T and Li C: Convergence between
Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 9:2362010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salahshor S, Naidoo R, Serra S, Shih W,
Tsao MS, Chetty R and Woodgett JR: Frequent accumulation of nuclear
E-cadherin and alterations in the Wnt signaling pathway in
esophageal squamous cell carcinomas. Mod Pathol. 21:271–281. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Zhu L, Zuo W, Zeng Z, Huang L, Lin
F, Lin R, Wang J, Lu J, Wang Q, et al: MicroRNA-mediated epigenetic
targeting of survivin significantly enhances the antitumor activity
of paclitaxel against non-small cell lung cancer. Oncotarget.
7:37693–37713. 2016.PubMed/NCBI
|
|
17
|
Zhuang B, Du L, Xu H, Xu X, Wang C, Fan Y,
Cong M, Yin J, Li H and Guan H: Self-assembled Micelle Loading
Cabazitaxel for therapy of Lung Cancer. Int J Pharm. 499:146–155.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taixiang W, Munro AJ and Guanjian L:
Chinese medical herbs for chemotherapy side effects in colorectal
cancer patients. Cochrane Database Syst Rev: CD004540. 2005.
|
|
19
|
Xiao WL, Motley TJ, Unachukwu UJ, Lau CB,
Jiang B, Hong F, Leung PC, Wang QF, Livingston PO, Cassileth BR and
Kennelly EJ: Chemical and genetic assessment of variability in
commercial Radix Astragali (Astragalus spp.) by ion trap LC-MS and
nuclear ribosomal DNA barcoding sequence analyses. J Agric Food
Chem. 59:1548–1556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tai Y, Sun YM, Zou X, Pan Q, Lan YD, Huo
Q, Zhu JW, Guo F, Zheng CQ, Wu CZ and Liu H: Effect of Polygonatum
odoratum extract on human breast cancer MDA-MB-231 cell
proliferation and apoptosis. Exp Ther Med. 12:2681–2687. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu L, Liu T, Xiao Y, Li X, Zhu Y, Zhao Y,
Bao J and Wu C: Polygonatum odoratum lectin induces apoptosis and
autophagy by regulation of microRNA-1290 and microRNA-15a-3p in
human lung adenocarcinoma A549 cells. Int J Biol Macromol.
85:217–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma W, Liu R, Qi J and Zhang Y: Extracts of
centipede Scolopendra subspinipes mutilans induce cell cycle arrest
and apoptosis in A375 human melanoma cells. Oncol Lett. 8:414–420.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang MY, Hung CH, Chang CH, Tseng TH and
Wang CJ: Solanum nigrum suppress angiogenesis-mediated tumor growth
through inhibition of the AKT/mTOR pathway. Am J Chin Med.
44:1273–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Xu F, Wang G, Diao X and Li Y:
Kanglaite injection plus chemotherapy versus chemotherapy alone for
non-small cell lung cancer patients: A systematic review and
meta-analysis. Curr Ther Res Clin Exp. 69:381–411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng J, Han W, Wang Z, Shao Y, Wang Y and
Zhang Y, Li Z, Xu X and Zhang Y: Hepatocellular carcinoma growth is
inhibited by euphorbia helioscopia L. Extract in nude mice
xenografts. Biomed Res Int. 2015:6010152015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kocaadam B and Şanlier N: Curcumin, an
active component of turmeric (Curcuma longa), and its effects on
health. Crit Rev Food Sci Nutr. 57:2889–2895. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Yang ZQ, Wang Y, Miao Y, Liu Y,
Dai SD, Han Y and Wang EH: Dishevelled-1 and dishevelled-3 affect
cell invasion mainly through canonical and noncanonical Wnt
pathway, respectively and associate with poor prognosis in nonsmall
cell lung cancer. Mol Carcinog. 49:760–770. 2010.PubMed/NCBI
|
|
29
|
Liu Y, Zhou Q, Zhou D, Huang C, Meng X and
Li J: Secreted frizzled-related protein 2-mediated cancer events:
Friend or foe? Pharmacol Rep. 69:403–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Xiao L, Luo CH, Zhou H, Zeng L,
Zhong J, Tang Y, Zhao XH, Zhao M and Zhang Y: CD44v6 promotes
β-catenin and TGF-β expression, inducing aggression in ovarian
cancer cells. Mol Med Rep. 11:3505–3510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun W and Chen G: Impact and mechanism of
non-steroidal anti-inflammatory drugs combined with
chemotherapeutic drugs on human lung cancer-nude mouse transplanted
tumors. Oncol Lett. 11:4193–4199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garg H, Suri P, Gupta JC, Talwar GP and
Dubey S: Survivin: A unique target for tumor therapy. Cancer Cell
Int. 16:492016. View Article : Google Scholar : PubMed/NCBI
|