Introduction
Nasal polyps are a heterogeneous disease
characterized by predominant infiltration of inflammatory cells,
structural fibrosis, edematous stromal tissue, and thickening of
the basement membrane (1). The
prevalence is about 4% (2). Much
of the nasal polyp stroma is dense with fibroblasts, which produce
many kinds of cytokines for polymorphonuclear leukocytes. The
fibroblasts in nasal polyp tissue can be transformed into
myofibroblasts, which are the main source of extracellular matrix
in nasal polyps (3,4). This suggests the importance of
controlling fibroblast functions, such as proliferation, collagen,
and cytokine production, in the management and treatment of nasal
polyps (5,6).
Bleomycin A5 is a cytotoxic antibiotic derived from
Streptomyces verticellus. It has antineoplastic,
antibacterial, and antiviral properties. Intralesional bleomycin A5
has recently come to be used in the treatment of keloid and
hypertrophic scars (7,8). Several studies have besen performed
to explain the exact mechanism by which bleomycin A5 resolves
keloids and hypertrophic scars. Some researchers have shown that
cultured human dermal fibroblasts treated with bleomycin A5 can
diminish collagen synthesis (9).
Similarly, administration of bleomycin A5 to cultured fibroblasts
causes a reduction in lysyl oxidase levels (10). It has also been reported that
bleomycin A5 induces apoptosis in epithelial cells and fibroblasts
in the lungs through production of reactive oxygen species (ROS),
and activation of the Fas-FasL associated caspase cascade-mediated
pathway (11,12).
Recently, intralesional bleomycin A5 injections have
been used to treat nasal polyps in China. It has also been reported
that bleomycin A5 induces apoptosis among eosinophils in nasal
polyp tissue (13). Based on
previous studies, it is here hypothesized that bleomycin A5 may
also have a pro-apoptotic effect on NPDFs. For this reason, this
study was designed to ascertain whether the apoptotic signaling
pathway of bleomycin A5 becomes activated in NPDFs.
Materials and methods
Isolation, culture, and identification
of fibroblasts
Here, 10 patients (6 females and 4 males, 36.3±4.1
years) who were non-smokers and had either not been treated with
glucocorticoids (systemic or topical), antihistamines,
non-steroidal anti-inflammatory drugs, or macrolide antibiotics for
at least 1 month or who had ceased using the drug at least 1 month
due to lack of alleviation or even aggravation of symptoms, were
recruited from the Department of Otorhinolaryngology at Sun Yat-sen
Memorial Hospital. All participants provided written informed
consent in advance, and nasal polyp tissues were obtained during
surgery. The study was approved by the Ethics Committee of Sun
Yat-sen Memorial Hospital.
NPDFs were isolated from nasal polyp tissue from
patients who had chronic rhinosinusitis with nasal polyps using a
previously described method (14).
Briefly, nasal polyp tissue was cut into pieces, re-suspended in
DMEM/F12 containing 1 mg/ml collagenase I in a centrifuge tube, and
then placed in a 5% CO2 humidified atmosphere at 37°C.
After 12 h, the tissues were re-suspended in DMEM/F12 with 10% FBS
and cultured in a 5% CO2-humidified atmosphere at 37°C,
and the medium was changed every 48 h. Trypsin enzymatic digestion
and differential attachment were used to isolate fibroblast
cells.
The isolated fibroblasts were identified
immunocytochemistry using vimentin as a positive marker and CK
(pan) as a negative marker. After 3 rounds of washing with PBS, the
cells were incubated for 30 min with 5% BSA to block non-specific
sites. The coverslips were then incubated with primary antibody
(vimentin or CK) (1:200 dilution) in 1% BSA in a humidified chamber
overnight at 4°C. Then the coverslips were incubated with HRP
conjugated anti-rabbit secondary antibody for 30 min, and washed 3
times for 5 min each with PBS. DAB substrate was used to detect HRP
activity. Another group of NPDFs were grown in 6-well plates using
DMEM/F12 containing 10% FBS with or without 100, 200, or 400 µM
bleomycin A5 for 48 h in a 5% CO2 humidified atmosphere
at 37°C to assess the influence of bleomycin A5 on NPDFs.
CCK-8 array of NPDFs
Cultured NPDFs (1×104) were seeded in
each well of a 96-well plate in triplicate. After attachment, the
cells were treated with bleomycin A5 at various concentrations
(from 0 to 400 µM at 50 µM intervals) for 24, 48, or 72 h. Then
cells were incubated for 3 h with CCK-8 reagent (100 µl/ml medium)
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Absorbance
was determined at 490 nm using a microplate reader (Thermo Fisher
Scientific Inc., Cramlington, UK).
Flow cytometric Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide assay of fibroblast
cells
An Annexin V/PI Apoptosis kit (Thermo Fisher
Scientific Inc.) was used to assess the rate of apoptosis of
fibroblast cells after bleomycin A5 treatment in accordance with
the manufacturer's instructions. Briefly, after incubation in
6-well plates with DMEM/F12 with 10% FBS medium in the presence of
various concentrations of BLE (0, 100, 200 or 400 µM) for 48 h, the
cultured fibroblast cells were gently suspended in binding buffer
and incubated for 15 min at room temperature in the dark with 5 µl
Annexin V-FITC and 10 µl PI. The Annexin V-FITC- and PI-labelled
cells were analyzed using a flow cytometer (BD Biosciences,
Burlington, MA, USA). Using flow cytometry, dot plots of PI on the
y-axis, against Annexin V-FITC on the x-axis were used to
distinguish viable cells, which are negative for PI and Annexin
V-FITC, cells in the early stages of apoptosis (Annexin
V-positive/PI-negative), and cells in late apoptosis or full
necrosis (Annexin V-FITC-positive/PI-positive staining).
Reverse transcription-quantitative
polymerase chain reaction
After bleomycin A5 treatment at various
concentrations (including 0, 100, 200, and 400 µM), fibroblasts
were homogenized in 1 ml TRIzol reagent (Invitrogen, Carlsbad, CA,
USA). Total RNA was obtained, and 2 µg RNA/cell sample was reverse
transcribed into complementary DNA (cDNA) using the reverse
transcription system (Toyobo Co., Ltd., Osaka, Japan). Quantitative
polymerase chain reaction was performed using Roche Light cycler 96
system (Roche Diagnostics, Basel, Switzerland) with a 10 µl volume
mixture containing 2 µl cDNA, 0.2 µl of each primer, and 5 µl
SYBR-Green (Toyobo Co., Ltd.). The relative mRNA expression levels
of apoptosis-related genes were compared using the
2−ΔΔCq method. The sequences of the primers used for PCR
were ascertained from the primer bank and are listed as follows:
GADPH forward, 5′-CAGTGCCAGCCTCTGCTCAT-3′ and reverse,
5′-ATACTCAGCACCAGCACAT-3′; Bcl-2 forward,
5′-CTGGGATGCCTTTGTGGAAC-3′ and reverse, 5′-GGCAGGCATGTTGACTTCAC-3′;
Bax forward, 5′-CCAAGAAGCTGAGCGAGTGT-3′ and reverse,
5′-CAGCCCATGATGGTTCTGAT-3′; caspase-3 forward,
5′-ATGCAGCAAACCTCAGGGAA-3′ and reverse,
5′-GTCGGCCTCCACTGGTATTT-3′.
Western blot analysis
NPDFs treated with bleomycin A5 were homogenized in
RIPA buffer (Sigma-Aldrich;. Merck KGaA, Darmstadt, Germany) on ice
for 30 min. Using a BCA protein assay kit (Beyotime Institute of
Biotechnology, Beijing, China), equal amounts of proteins were
separated using 10% sodium dodecyl sulfate-polyacrymide (SDS) gel
electrophoresis and transferred onto polyvinylidene fluoride
membranes (Milipore, Billerica, MA, USA). They were then blocked
using 5% non-fat milk in TBS-0.1% Tween-20 for 1 h at room
temperature. The membranes were then incubated overnight at 4°C
with primary antibodies for caspase-3 (1:500 dilution), cleaved
caspase-3 (1:1,000 dilution), poly(ADP-ribose) polymerase (PARP)
(1:1,000 dilution), active PARP (1:1,000 dilution), Bax (1:2,000
dilution), Bcl-2 (1:1,000 dilution), or GADPH (1:10,000 dilution)
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After
washing in TBST, the membranes were incubated with
horseradish-peroxidase-conjugated anti-rabbit secondary antibodies
for 1 h at room temperature and then visualized using an enhanced
chemiluminescence kit (Millipore, Billerica, MA, USA). Images of
the bands were captured using a Bio-Rad Gel Doc XR documentation
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The levels
of relative protein expression were determined by densitometry and
standardized to the GAPDH levels using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
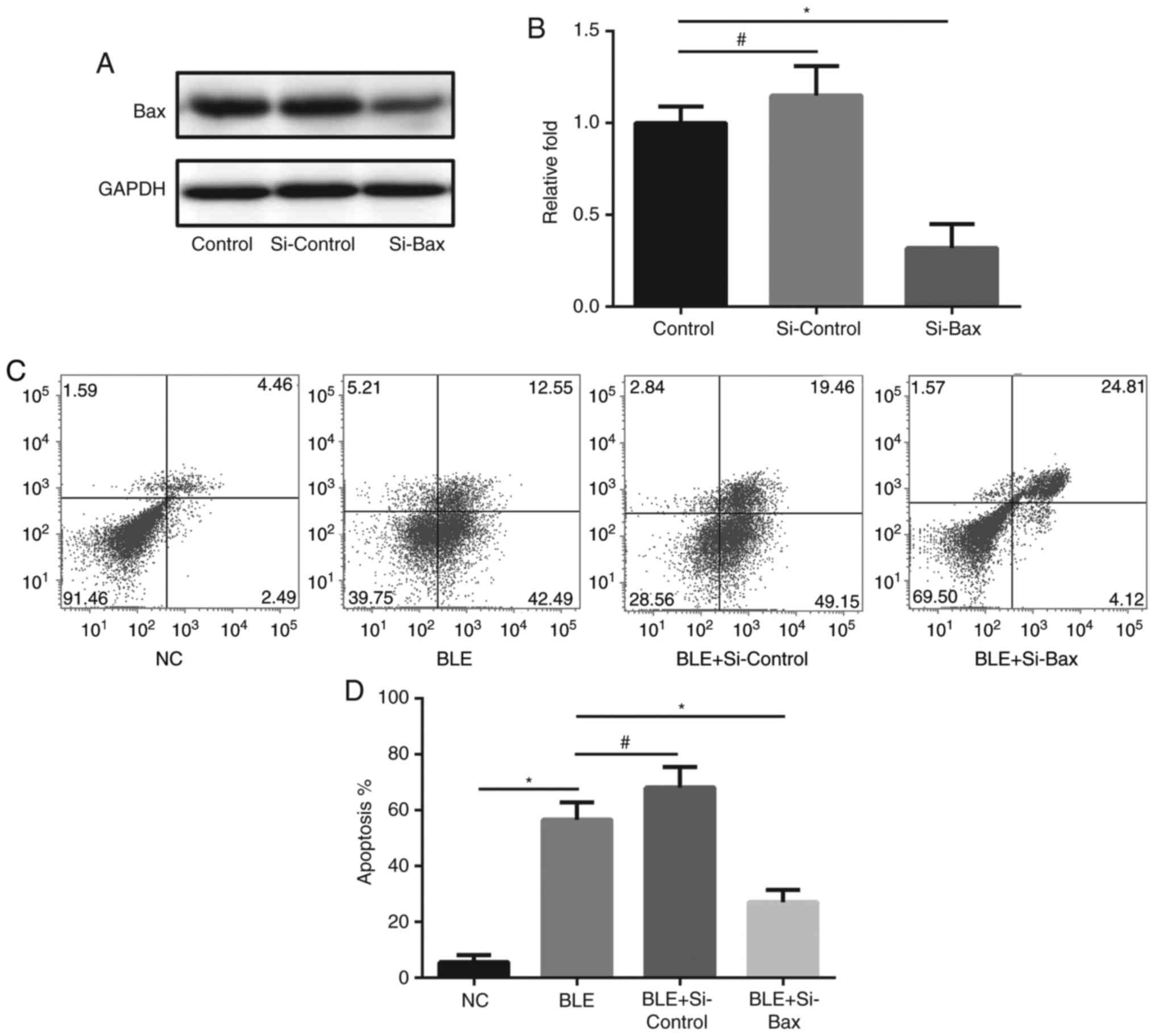
Bax gene silencing by siRNA
Mitochondria-mediated caspase-dependent
pro-apoptotic protein Bax was inhibited using small interfering RNA
(siRNA). Cells (2.5×105/well) were plated in 6-well
plates and transfected with 20 nM Bax-targeting siRNA or control
siRNA (Santa Cruz Biotechnology, Inc.) using Lipofectamine 3000 kit
(Life Technologies, Japan) according to the manufacturer's
instructions and cultured for 72 h at 37°C in a 5% CO2
incubator. The cells were then analyzed for Bax protein levels by
western blot analysis using an anti-Bax antibody. The membranes
were re-probed with an anti-GAPDH antibody to verify equal loading.
Then, transfected NPDFs were treated with 200 µM BLE for 48 h and
analyzed for apoptosis by Annexin V/PI staining in a flow
cytometer. Annexin V+ cells were considered to be
apoptotic cells.
Statistical analysis
All experiments were performed 3 times to provide
sufficient data. The differences between the experimental
conditions and the controls were analyzed through one-way analysis
of variance and Tukey's test (GraphPad Prism, version 5; GraphPad
Software, San Diego, CA, USA). Statistical significance was
determined at the 95% confidence level. P-values <0.05 were
considered to indicate a statistically significant difference.
Results
Identification of NPDFs
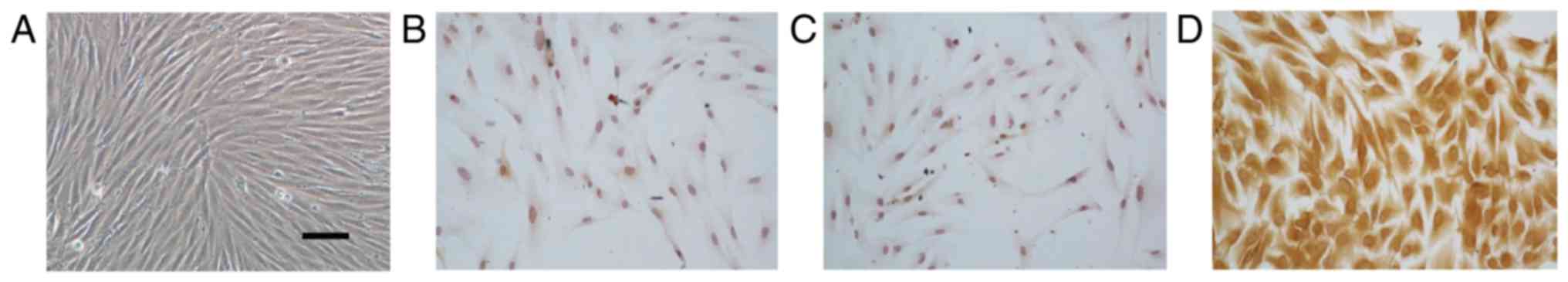
To identify NPDFs, we first isolated fibroblast
cells from nasal polyp tissue observe the cell morphologies. As
showed in Fig. 1A, NPDFs were
spindle and nest-like distributed. Immunocytochemistry staining of
primary NPDFs indicated that the isolated and cultured cells were
vimentin-positive and CK (pan)-negative, which was consistent with
the characteristics of fibroblasts (Fig. 1B-D).
Bleomycin A5 induces NPDF apoptosis in
a concentration- and time-dependent manner
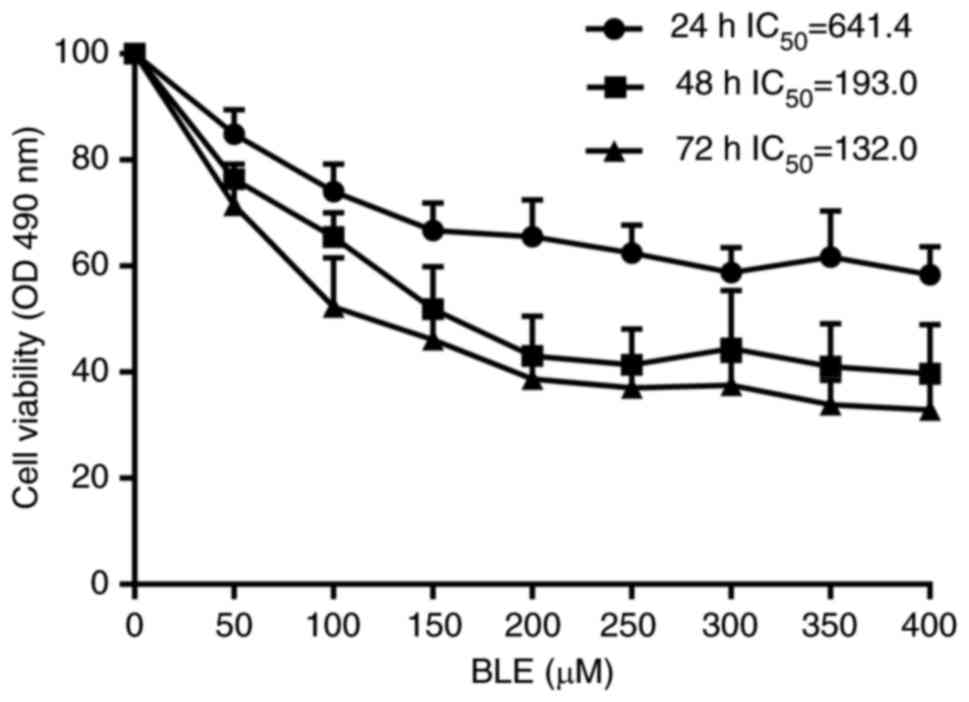
Next, we examined the effect of bleomycin A5 on NPDF
viability. Results showed that groups treated with higher
concentrations of bleomycin A5 had lower cell viability. This
phenomenon was especially visible at doses ranging from 0 to 200
µM. As the duration of bleomycin A5 exposure increased, cell
viability also decreased in all treated groups. In the 48 h group,
the IC50 = 193.0 (95% confidence intervals, 146.3 to
254.5) (Fig. 2). These results
indicated that the effect of bleomycin A5 on NPDFs was
concentration- and time-dependent.
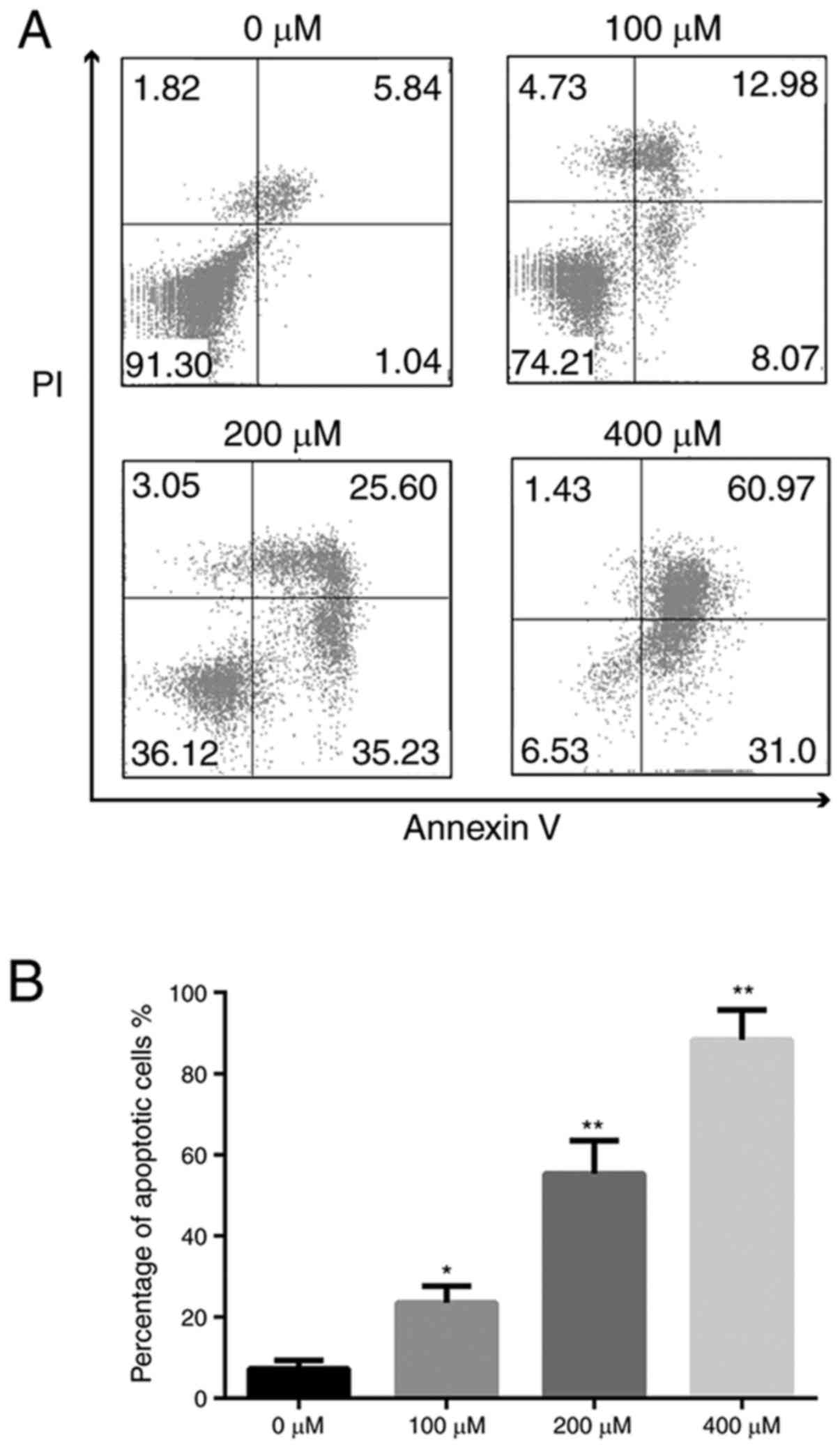
Flow cytometry indicated that the ratio of apoptotic
cells was 7.25±2.09% in the 0 µM group, 23.50±4.09% in 100 µM
group, 55.27±8.17% in the 200 µM group, and 88.32±7.39% in 400 µM
group (Fig. 3). NPDFs exposed to
bleomycin A5 exhibited increased cell apoptosis in
concentration-dependent manner. These results further confirmed
that bleomycin A5 can induce NPDF apoptosis in a dose-dependent
manner.
Caspases, PARP, and Bcl-2 family
participate in bleomycin A5-induced apoptosis in NPDFs
We next focused on identifying the pathways that
lead to NPDF apoptosis using bleomycin A5. To identify the genes
involved in apoptosis, we first conducted a PCR array using NPDFs
treated with bleomycin A5 for 48 h at different concentrations.
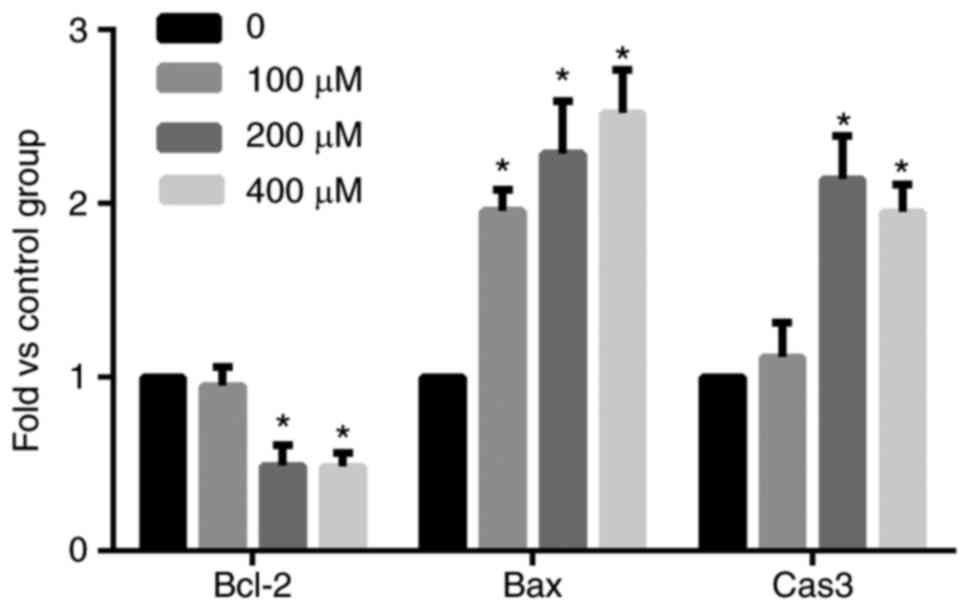
Using RT-qPCR, the mRNA levels of Bcl-2, Bax, and caspase-3
increased, and the difference was statistically significant
(P<0.05) after bleomycin A5 treatment for 48 h (Fig. 4).
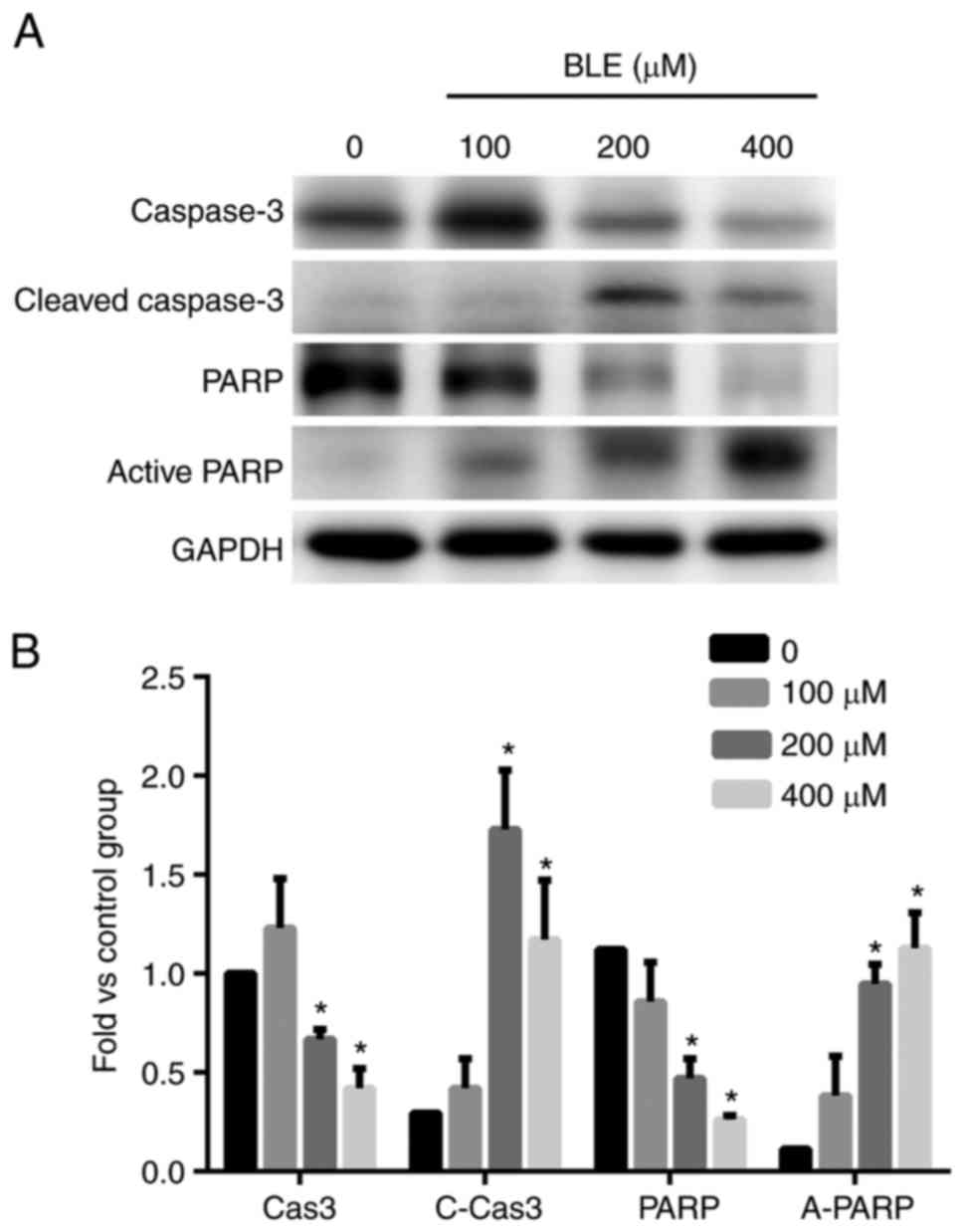
Next, we examined the changes in the protein levels
of these genes using western blot analysis. Even though the 100 µM
group seemed to show more caspase-3 expression than the control
group, the difference was not significant. However, the levels of
cleaved caspase-3 increased in a dose-dependent manner (Fig. 5). The levels of PARP, a zymolyte of
cleaved caspase-3, also declined as the concentration of bleomycin
A5 increased, and the level of active PARP increased in a
dose-dependent manner. These results indicated that the
concentrations of the apoptotic downstream effective proteins were
significantly higher in both the 200 and 400 µM groups than in the
control group (Fig. 5).
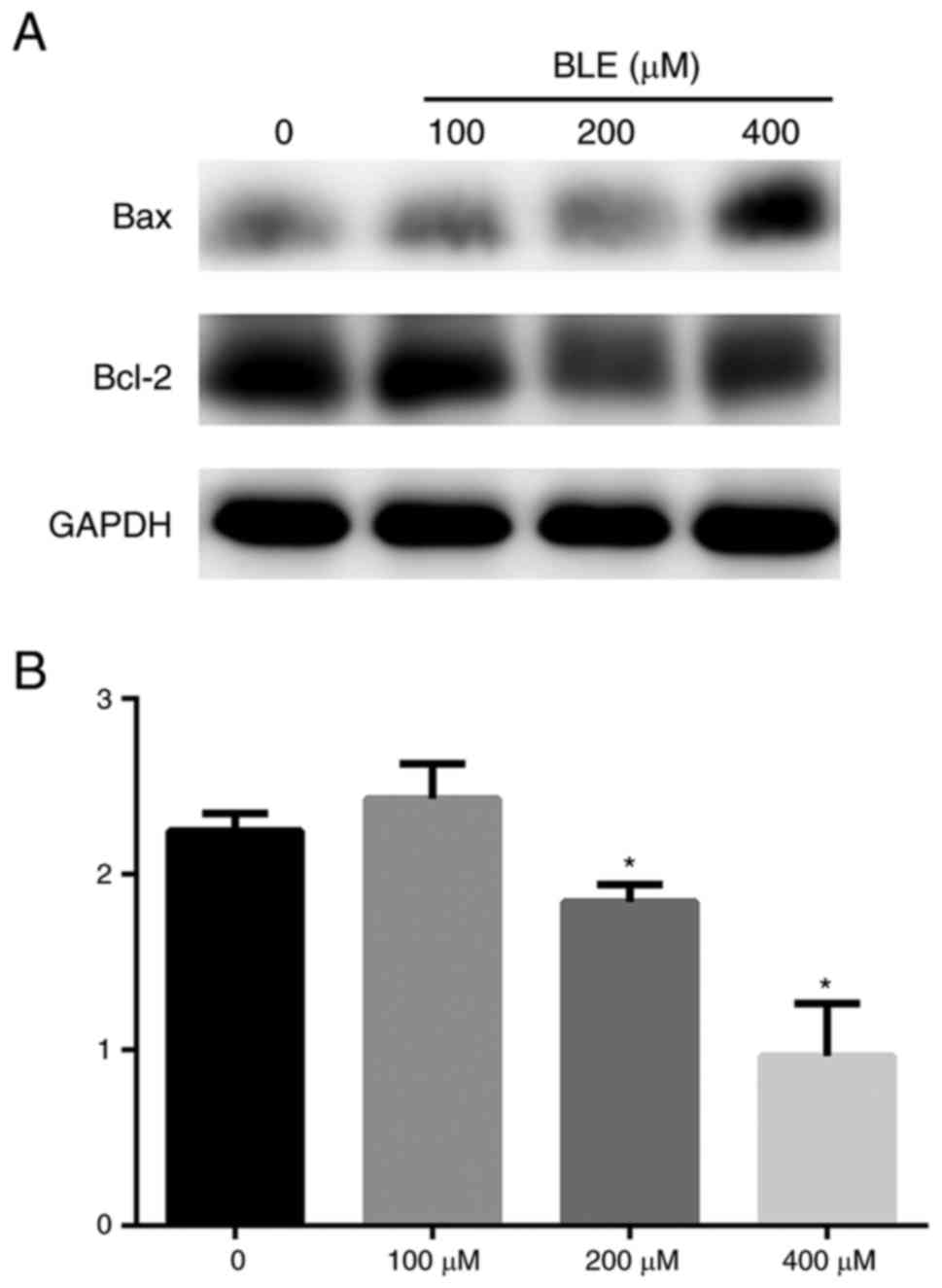
Bcl-2 and Bax are 2 important mitochondria-mediated
caspase-dependent apoptotic proteins. It has been proven that Bax
forms a complex with the anti-apoptotic protein Bcl-2 to balance
its apoptotic effects (15). The
ratio of Bcl-2/Bax was used to determine whether cells undergo
programmed cell death. The Bcl-2/Bax ratio was determined through
densitometry and normalized to the GAPDH levels of each group. The
results indicated that stimulation of NPDFs with bleomycin A5 in
vitro resulted in a dose-dependent decrease in the Bcl-2/Bax
ratio (Fig. 6). To further confirm
this conclusion, pro-apoptotic protein Bax was knockdown by siRNA
transfection. The Annexin V/PI staining shows that such knockdown
could significantly attenuate apoptosis in BLE-treated NPDFs
(Fig. 7).
Discussion
Recent studies have shown that cultures of human
dermal fibroblasts undergo reductions in lysyl oxidase
concentration as a result of the action of bleomycin. In addition,
fibroblast apoptosis and proliferation levels are both higher
during bleomycin-induced lung fribrosis (16). Results have demonstrated that
bleomycin, which mainly targets the fibroblasts in scar tissue, can
induce keloid fibroblast death and lead to a reduction in collagen
synthesis. For this reason, the intralesional injection of
bleomycin A5 into keloids and hypertrophic scars has been proposed
as a modality for scar treatment and scar prevention after surgery
(17). Because of the effects of
bleomycin on fibroblasts and its roles in the production of
cytokines and collagen in stromal fibroblasts in nasal polyp
tissue, we here propose that bleomycin A5 may have an inhibitory
effect on NPDFs. In fact, when we performed this study, we also
collected clinical data regarding the use of intralesional
bleomycin A5 in nasal polyps, and results showed that this
off-label use can cure nasal polyps and improve the nasal
ventilation function of patients suffering from nasal polyps
effectively (data not shown). In this study, we further isolated
NPDFs from surgical specimens of nasal polyp tissue and then used a
primary culture to investigate bleomycin A5-induced apoptosis in
NPDFs. Results showed that bleomycin A5 induced apoptosis in NPDFs
in a time- and concentration-dependent manner through activation of
bcl-2 family and caspase cascades.
Previous studies have shown bleomycin A5-induced
apoptosis in primary cultures of rat type II alveolar epithelial
cells and in human A549 cells to be concentration- and
time-dependent (18). To determine
whether bleomycin-A5 induced NPDF apoptosis is time- or
concentration-dependent in NPDFs, we choose different
concentrations of bleomycin A5 and durations of stimulus. We found
that at higher concentrations of bleomycin A5 and over longer
periods of exposure, the number of NPDFs with condensed cytoplasm
and nuclei increased and total number of cells decreased. Notably,
at 48 h, the number of cells undergoing apoptosis became stable
regardless of concentration. For this reason, we choose 48 h for
the rest of our experiments.
PARP is a DNA repair enzyme that is activated by
various environmental stimuli, including ROS, free radicals, and
peroxynitrite. During almost all forms of apoptosis, PARP can be
processed in vivo into its apoptotic 24 and 89 kDa fragments
by caspase-3 or −7 (19). In this
study, we found that bleomycin A5-treated NPDFs contained
significantly greater amounts of the active forms of caspase-3 and
underwent more cleavage of PARP at the protein levels. These
results indicated that increased activation of caspase cascades
mediated bleomycin A5 induced NPDF apoptosis.
Previous studies indicate that activation of the
pro-apoptotic Bcl-2 family is required for the development of
pulmonary fibrosis after the intratracheal instillation of
bleomycin in mice. Mice lacking Bid exhibited significantly less
pulmonary fibrosis in response to bleomycin than WT mice (20). It is here suggested that apoptosis
mediated by members of the Bcl-2 family may play an essential role
in apoptosis of lung cells (21).
The bcl-2 family is divided into 2 groups: Pro-apoptotic and
anti-apoptotic molecules. Pro-apoptotic proteins can induce
apoptosis through breakage of the mitochondrial membrane potential
(22). To the contrary, by binding
to specific site of Bax, the anti-apoptotic proteins retained
normal permeability and prevented the release of mitochondrial
pro-apoptotic factors into the cytoplasm (23). Thus, the balance of pro-apoptotic
and anti-apoptotic proteins is often used to regulate cell
apoptosis (17). In this study, we
analyzed the pro-apoptotic molecules Bax and the anti-apoptotic
molecule Bcl-2 using RT-PCR and Western blotting. These results
indicated that there was significantly more mRNA and protein
expression of the pro-apoptotic Bax molecules in bleomycin
A5-treated NPDFs than in untreated NPDFs. In contrast, expression
of Bcl-2 mRNA and protein was significantly lower in bleomycin
A5-treated NPDFs than in untreated NPDFs. And knockdown of Bax
result in decrease of apoptosis in BLE-treated NPDFs. This
pro-apoptotic change in the expression levels of members of the
Bcl-2 family, which are major regulators of apoptosis via the
intrinsic pathway, suggests that the induction of apoptosis in
NPDFs by bleomycin A5 is mediated by an intrinsic
mitochondria-mediated pathway.
In conclusion, this study demonstrated that
bleomycin A5 induces apoptosis of primary NPDFs in association with
increased expression of pro-apoptotic proteins, decreased
expression of genes in the anti-apoptotic Bcl-2 family and
increased expression of caspase cascades (caspase-3 and PARP) in a
time-, concentration-, and caspase-dependent manner. Intralesional
injection has already been used in this way in clinical settings
for the treatment of infantile hemangiomas, keloids, and
hypertrophic scar, and this approach may be a suitable alterative
therapeutic option for patients with nasal polyps, especially
recurrent, difficult-to-treat, and glucocorticoid insensitive
cases.
Acknowledgements
This study was supported by grants from National
Natural Science Foundation of China (no. 81500773), the Natural
Science Foundation of Guangdong Province of China (no.
2015A030310125), and the Natural Science Foundation of Guangdong
Province of China (no. 2014A030313158); Grant [2013]163 from Key
Laboratory of Malignant Tumor Molecular Mechanism and Translational
Medicine of Guangzhou Bureau of Science and Information Technology;
Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene
Regulation and Target Therapy of Guangdong Higher Education
Institutes.
References
|
1
|
Thomas M, Yawn BP, Price D, Lund V, Mullol
J and Fokkens W; European Position Paper on Rhinosinusitis and
Nasal Polyps Group, : EPOS primary care guidelines: European
position paper on the primary care diagnosis and management of
rhinosinusitis and nasal polyps 2007-a summary. Prim Care Respir J.
17:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hedman J, Kaprio J, Poussa T and Nieminen
MM: Prevalence of asthma, aspirin intolerance, nasal polyposis and
chronic obstructive pulmonary disease in a population-based study.
Int J Epidemiol. 28:717–722. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang QP, Escudier E, Roudot-Thoraval F,
Abd-Al Samad I, Peynegre R and Coste A: Myofibroblast accumulation
induced by transforming growth factor-beta is involved in the
pathogenesis of nasal polyps. Laryngoscope. 107:926–931. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park IH, Park SJ, Cho JS, Moon YM, Kim TH,
Lee SH and Lee HM: Role of reactive oxygen species in transforming
growth factor beta1-induced alpha smooth-muscle actin and collagen
production in nasal polyp-derived fibroblasts. Int Arch Allergy
Immunol. 159:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park HH, Park IH, Cho JS, Lee YM and Lee
HM: The effect of macrolides on myofibroblast differentiation and
collagen production in nasal polyp-derived fibroblasts. Am J Rhinol
Allergy. 24:348–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin JM, Park JH, Park IH and Lee HM:
Doxycycline inhibits TGF-β1-induced extracellular matrix production
in nasal polyp-derived fibroblasts. Int Forum Allergy Rhinol.
6:256–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ledon JA, Savas J, Franca K, Chacon A and
Nouri K: Intralesional treatment for keloids and hypertrophic
scars: A review. Dermatol Surg. 39:1745–1757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones CD, Guiot L, Samy M, Gorman M and
Tehrani H: The use of chemotherapeutics for the treatment of keloid
scars. Dermatol Reports. 7:58802015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hendricks T, Martens MF, Huyben CM and
Wobbes T: Inhibition of basal and TGF beta-induced fibroblast
collagen synthesis by antineoplastic agents. Implications for wound
healing. Br J Cancer. 67:545–550. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeowell HN, Marshall MK, Walker LC, Ha V
and Pinnell SR: Regulation of lysyl oxidase mRNA in dermal
fibroblasts from normal donors and patients with inherited
connective tissue disorders. Arch Biochem Biophys. 308:299–305.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wallach-Dayan SB, Izbicki G, Cohen PY,
Gerstl-Golan R, Fine A and Breuer R: Bleomycin initiates apoptosis
of lung epithelial cells by ROS but not by Fas/FasL pathway. Am J
Physiol Lung Cell Mol Physiol. 290:L790–L796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang R, Ibarra-Sunga O, Verlinski L, Pick
R and Uhal BD: Abrogation of bleomycin-induced epithelial apoptosis
and lung fibrosis by captopril or by a caspase inhibitor. Am J
Physiol Lung Cell Mol Physiol. 279:L143–L151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Zou J, Li B, Ren X and Shi J:
Eosinophil apoptosis in nasal polyposis tissue after bleomycin A5
local injection. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 18:279–281.
2004.(In Chinese). PubMed/NCBI
|
|
14
|
Park IH, Um JY, Hong SM, Cho JS, Lee SH,
Lee SH and Lee HM: Metformin reduces TGF-β1-induced extracellular
matrix production in nasal polyp-derived fibroblasts. Otolaryngol
Head Neck Surg. 150:148–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of
breast cancer cells to paclitaxel. Asian Pac J Cancer Prev.
15:8617–8622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsukui T, Ueha S, Abe J, Hashimoto S,
Shichino S, Shimaoka T, Shand FH, Arakawa Y, Oshima K, Hattori M,
et al: Qualitative rather than quantitative changes are hallmarks
of fibroblasts in bleomycin-induced pulmonary fibrosis. Am J
Pathol. 183:758–773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XQ, Liu YK, Qing C and Lu SL: A
review of the effectiveness of antimitotic drug injections for
hypertrophic scars and keloids. Ann Plast Surg. 63:688–692. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Zhang H, Soledad-Conrad V, Zhuang J
and Uhal BD: Bleomycin-induced apoptosis of alveolar epithelial
cells requires angiotensin synthesis de novo. Am J Physiol Lung
Cell Mol Physiol. 284:L501–L507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bleackley RC and Heibein JA: Enzymatic
control of apoptosis. Nat Prod Rep. 18:431–440. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Budinger GR, Mutlu GM, Eisenbart J, Fuller
AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY
and Chandel NS: Proapoptotic Bid is required for pulmonary
fibrosis. Proc Natl Acad Sci USA. 103:pp. 4604–4609. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kasper M and Barth K: Bleomycin and its
role in inducing apoptosis and senescence in lung cells-modulating
effects of caveolin-1. Curr Cancer Drug Targets. 9:341–353. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YB, Aon MA, Hsu YT, Soane L, Teng X,
McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, et al: Bcl-×L
regulates mitochondrial energetics by stabilizing the inner
membrane potential. J Cell Biol. 195:263–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prenek L, Boldizsár F, Kugyelka R, Ugor E,
Berta G, Németh P and Berki T: The regulation of the mitochondrial
apoptotic pathway by glucocorticoid receptor in collaboration with
Bcl-2 family proteins in developing T cells. Apoptosis. 22:239–253.
2017. View Article : Google Scholar : PubMed/NCBI
|