Introduction
Increasing evidence has demonstrated that general
anesthetic agents may cause long-term impairment to the central
nerve system (1). Ikonomidou et
al (2) demonstrated that
exposure to NMDA receptor blockades, including ketamine, during
gestational days 17–19 or postnatal days 3–7 in rats may induce
neuronal apoptotic degeneration in the developing brain. Hayashi
et al (3) reported that the
majority of general anesthetics could cause irreversible cognitive
defects associated with neuronal apoptosis.
It has been reported that, when exposed to general
anesthetics, the developing brain may experience neuronal apoptosis
and long-term defects in learning and memory (4,5). The
fetal brain is susceptible to noxious stimulus (6) and ~2% of pregnant women undergo
non-obstetric surgeries during gestation (7), the majority of which are performed
under general anesthetic (8).
Propofol is one of the most commonly used general anesthetics and
easily passes through the placental barrier (9). It has been demonstrated that propofol
exposure in early life can cause long-term cognitive deficits
(6) associated with impairment of
the hippocampal neurons (10). A
previous study by our group demonstrated that maternal exposure to
propofol in early or late pregnancy impairs learning and memory in
rat offspring (11,12). However, the effect of propofol
exposure during middle pregnancy remains to be elucidated.
It has been demonstrated that the
N-methyl-D-aspartate (NMDA) receptor in the hippocampus serves an
important role in the formation and maintenance of learning and
memory. The NMDA receptor contains 7 subunits. The functional
properties of the NMDA receptor are determined by the NDMA receptor
2B subunit (NR2B) (13). NR2B
knockout results in cognitive function impairments, whereas
transfection with the NR2B gene promotes the formation of long-term
potentiation (LTP) and enhances learning and memory in mice
(14,15). NR2B must be transported to the
neuronal membrane by kinesin family member 17 (KIF17), a
neuron-specific molecular motor in neuronal dendrites, in order to
serve its function (16).
Selective transport is accomplished by direct interaction between
the KIF17 tail and a PDZ domain of mLin-10 (Mint1/X11), a
constituent of a large protein complex including mLin-2 (CASK),
mLin-7 (MALS/Velis) and the NR2B subunit (17). There is an ATP binding site in the
head of the molecular motor KIF17, which provides energy for KIF17
to transport NR2B on microtubules (16). When vesicles containing NR2B are
transported to the end of dendrite by KIF17, NR2B will be released
and bind to the neuronal membrane (18). The release depends on regulation of
the calmodulin-dependent protein kinase II (CaMKII) (18). Costa et al (19) reported that the decrease in
NR2B/NR2A is associated with KIF17 decline in the Ts65Dn mouse
brain. Guillaud et al (20)
also demonstrated that NR2B on the synaptic membrane (M-NR2B)
decreased with KIF17 decline in mice. KIF17 knockout results in a
marked decline of M-NR2B; however, total NR2B (T-NR2B) was not
affected (21). This confirms that
KIF17 regulates the levels of M-NR2B and affects learning and
memory (22).
In the present study, the effects of maternal
propofol exposure during middle pregnancy on the expression of
KIF17 and NR2B protein, including T-NR2B and M-NR2B, were
investigated in order to ascertain the role of KIF17 in learning
and memory impairment in offspring rats exposed to propofol during
middle pregnancy.
Materials and methods
Animals
Sprague-Dawley rats (8–9 weeks old; weight, 200–270
g; 34 females and 16 males) were purchased from the Animal Science
Research Department of Jiangxi Traditional Chinese Medicine College
(JZDWNO, 2011-0030; Nanchang, Jiangxi, China). Rats were housed at
22–25°C and 55±5% humidity, with a 12 h light/dark cycle and free
access to food and water. Following a Morris water maze (MWM) test,
these parental rats that could find the platform no more than once
during 6 consecutive days' training trials were excluded (4 females
and 1 male). Then 30 female and 15 male parental rats were randomly
assigned to control, P4 and P8 group. The learning and memory of
the parental rats were analyzed and showed no significant
difference among three groups (P>0.05). Propofol infusion for 4
h (P4), propofol infusion for 8 h (P8) and saline control (C)
groups (n=10). Female and male parental rats were housed together
to allow for mating (2 females per male). All protocols were
approved by the institutional review board of the First Affiliated
Hospital of Nanchang University on the Use of Animals in Research
and Teaching.
Propofol exposure
On gestational day 14 (G14), 20 mg/kg of propofol
(200 mg/20 ml; AstraZeneca, Basiglio, Italy) was injected into
pregnant rats in the propofol exposure groups via the caudal vein
at a rate of 20 mg/kg/h of continuous infusion for 4 or 8 h. The
dose of propofol was selected based on a previous study (12). Equal volumes of saline were
administered to rats in the control group. The propofol infusion
time was selected for a number of reasons; i) a previous study
(12) demonstrated that neuronal
damage is highest when general anesthetic exposure time is between
6 and 8 h, whereas 2 h exposure has no significant difference; ii)
the majority of surgical procedures require 2–4 h of anesthesia or
more; and iii) our previous study (12) demonstrated that maternal exposure
to propofol (at the same dosage used in present study) for 2 h
during early gestation had no significant effect on learning and
memory in rat offspring, whereas exposure for 4 or 8 h caused
significant impairments without affecting the levels of blood gases
in pregnant rats.
Electrocardiograms, saturation of pulse oximetry
(SpO2) and tail non-invasive blood pressure were
monitored during propofol exposure. If SpO2 was <95%
and/or the systolic blood pressure was <80% of baseline for
>5 min, the rat was removed from the study and another pregnant
rat was selected to replace it, thereby preventing toxicity from
maternal ischemia or hypoxia in rat offspring.
Blood gases analysis
To determine whether propofol exposure at G14 causes
disturbances in maternal blood gases, blood was harvested from the
caudal vein at the end of propofol anesthesia and was analyzed
using a blood gas analyzer (ABL77; Radiometer Medical ApS,
Brønshøj, Denmark) for pH, partial pressure (P)O2,
PCO2, HCO3−, Na+, K+,
Ca2+, and base excess.
MWM
Spatial memory and learning were assessed in rat
offspring using an MWM system (Beijing Sunny Instruments Co., Ltd.,
Beijing, China) beginning on postnatal day 30 (P30) as previously
described (11). The trials began
at 9:00 am on each testing day. The MWM is a black circular steel
pool (diameter = 50 cm and height = 60 cm) that was filled with
water at 24±1°C. A platform was hidden 1 cm below the water surface
in the second (target) quadrant of the MWM system. Each rat was put
into the pool and allowed to search for the platform once per day
for 6 consecutive days. The time taken for the rats to find the
platform was recorded as escape latency (learning ability). If they
were able to find the platform within 120 sec, rats were allowed to
stay on the platform for 30 sec. However, if a rat did not find the
platform within 120 sec, it was guided to the platform and allowed
to remain there for 30 sec, with the escape latency recorded as 120
sec. On day 7, the platform was removed. The rats were then allowed
to perform the spatial probe test (memory test) for 120 sec. The
number of times that the rat swam across the site where the
platform was hidden (platform-crossing times) and the duration
spent in the target quadrant (target quadrant time) were recorded
using a video connected to a computer (ZH0065; Beijing Sunny
Instruments Co., Ltd., Beijing, China). The mean value of the
latencies, platform-crossing times and target quadrant times of
offspring born to the same mother were calculated as the final
results.
Hippocampus harvest
The day following the spatial probe test, rats were
sacrificed by cervical dislocation. Hippocampus tissues of
offspring were harvested and stored at −80°C for western blot
analysis.
Western blot analysis
Total hippocampus protein was extracted by
homogenizing hippocampal tissues (n=10 from each group) in lysis
buffer (78510; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing a protease inhibitor cocktail (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). The hippocampal membrane protein was
extracted using a membrane protein extraction kit (E231-01; Vazyme
Biotech Co., Ltd., Nanjing, China) according to the manufacturer's
protocol. Protein concentrations were determined using a
bicinchoninic acid protein assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A total of 50 µg protein/lane was separated by
8% SDS-PAGE and transferred onto a polyvinylidene fluoride
membrane. The membranes were blocked with a 5% non-fat dry milk
buffer at room temperature for 1 h and then incubated overnight at
4°C with the following primary antibodies: rabbit polyclonal
anti-KIF17 (sc-50455; 1:2,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), rabbit polyclonal anti-NR2B (4207S; 1:500; Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit polyclonal
anti-β-actin (T0022-25UG; 1:2,000; Affinity Biosciences, Cambridge,
UK) and anti-Na+/K+ ATPase antibody (ab33655;
1:2,000; Abcam, Cambridge, MA, USA). The membranes were incubated
with horseradish peroxidase-conjugated polyclonal anti-IgG antibody
(HS101-01; 1:500; Beijing TransGen Biotech Co., Ltd., Beijing,
China) and developed using a SuperSignal™ West Pico PLUS
Chemiluminescent Substrate (34577; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Images were scanned using an Image Master II
scanner (GE Healthcare, Chicago, IL, USA) and the optical densities
of bands were quantitatively analyzed by using ImageJ 1.38
(National Institutes of Health, Bethesda, MD, USA). The results
were expressed relative to β-actin or Na+/K+
ATPase.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA) was used
to analyze the data. The escape latency was analyzed by repeated
measurement two-way analysis of variance with prenatal treatment as
between-subjects independent factors and day as repeated factors
with the least significant difference post hoc test. Data from
protein and blood gases were analyzed by one-way analysis of
variance with the least significant difference post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Physical features of the
offspring
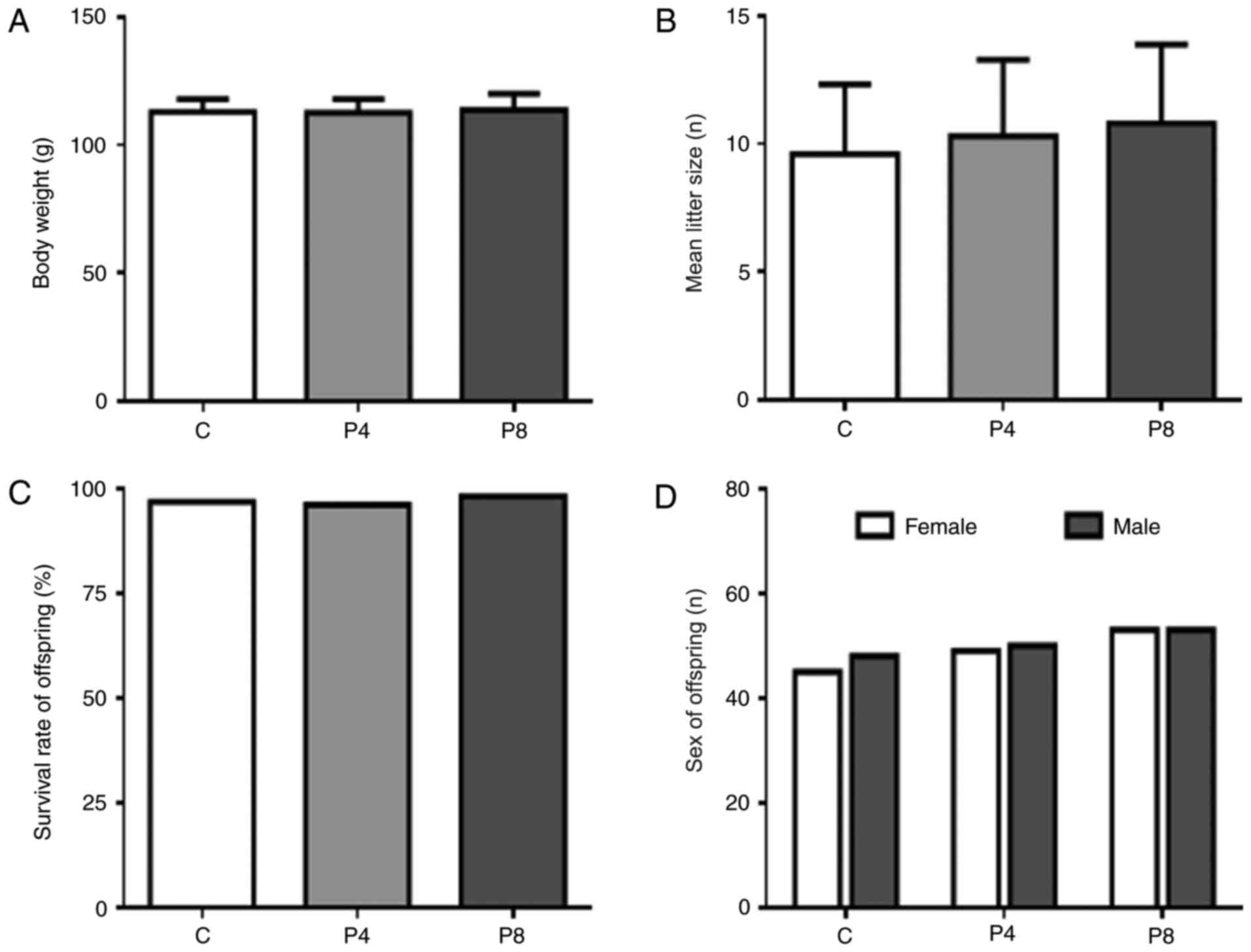
On day P30, no significant differences in mean body
weight were observed between the control and propofol exposure
groups (Fig. 1A). Propofol
exposure in middle pregnancy had no significant effect on birth
rate, offspring survival rate or sex ratio (Fig. 1B-D, respectively). Maternal
propofol exposure also had no evident influence on physical
development of offspring. Dyskinesia was not observed in either of
the three groups. These results indicate that propofol exposure on
day G14 does not affect offspring survival, sex or basic physical
development, which suggests that the differences in learning and
memory observed in the present study were induced by maternal
propofol exposure rather than physical differences.
Blood gases
To investigate whether propofol exposure on day G14
causes disturbances in maternal blood gases, caudal vein blood was
collected from pregnant rats following propofol anesthesia. No
significant differences in blood gases were observed between the
propofol exposure and control groups (Table I). These results suggest that the
differences in learning and memory observed in the present study
were induced by maternal propofol exposure rather than internal
environment disturbance in pregnant rats.
 | Table I.Blood gases in pregnant rats (n=10;
mean ± standard deviation). |
Table I.
Blood gases in pregnant rats (n=10;
mean ± standard deviation).
| Parameter | Control group | P8 group | P-value |
|---|
| pH |
7.36±0.13 |
7.35±0.19 | 0.290 |
| PO2
(mmHg) |
46.33±3.65 |
44.50±5.59 | 0.140 |
| PCO2
(mmHg) |
53.70±4.12 |
51.79±9.67 | 0.146 |
|
HCO3− (mmol/l) |
29.43±1.19 |
26.31±3.37 | 0.220 |
| BE(B) (mmol/l) |
3.41±0.87 |
3.53±1.12 | 0.558 |
| Ca2+
(mmol/l) |
1.33±0.12 |
1.41±0.26 | 0.520 |
| K+
(mmol/l) |
4.35±0.71 |
4.61±0.63 | 0.631 |
| Na+
(mmol/l) | 136.71±1.78 | 135.12±1.52 | 0.518 |
The body temperature, heart rate, respiratory rate,
blood pressure and SPO2 of maternal rats were monitored during
propofol infusion. No significant differences were identified in
these indexes
Impaired learning and memory in
offspring
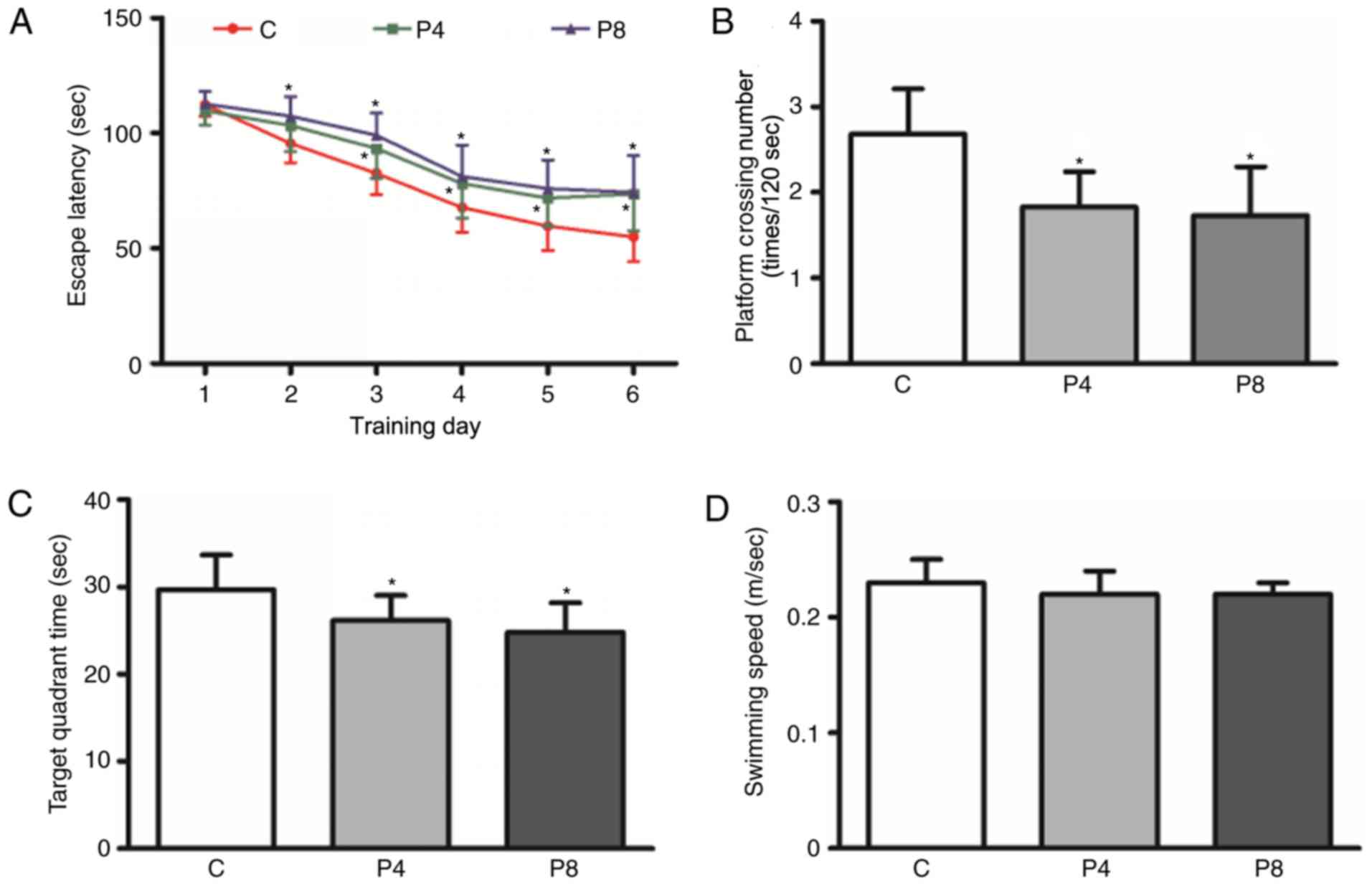
The results of the MWM test demonstrated that the
escape latency of rats in P4 and P8 groups was significantly
prolonged compared with the C group (P<0.05; Fig. 2A). The escape latency in the P8
group was longer compared with the P4 group, although no
significant difference was observed between the two groups
(Fig. 2A). The platform-crossing
times and target quadrant time in the P4 and P8 groups were
significantly lower compared with the C group (P<0.05; Fig. 2B and C) and were slightly lower in
the P8 group compared with the P4 group (Fig. 2B and C). No significant difference
in swimming speed was observed between the 3 groups (Fig. 2D). Taken together, these data
suggest that exposure of pregnant rats to propofol induced learning
and memory impairments in rat offspring.
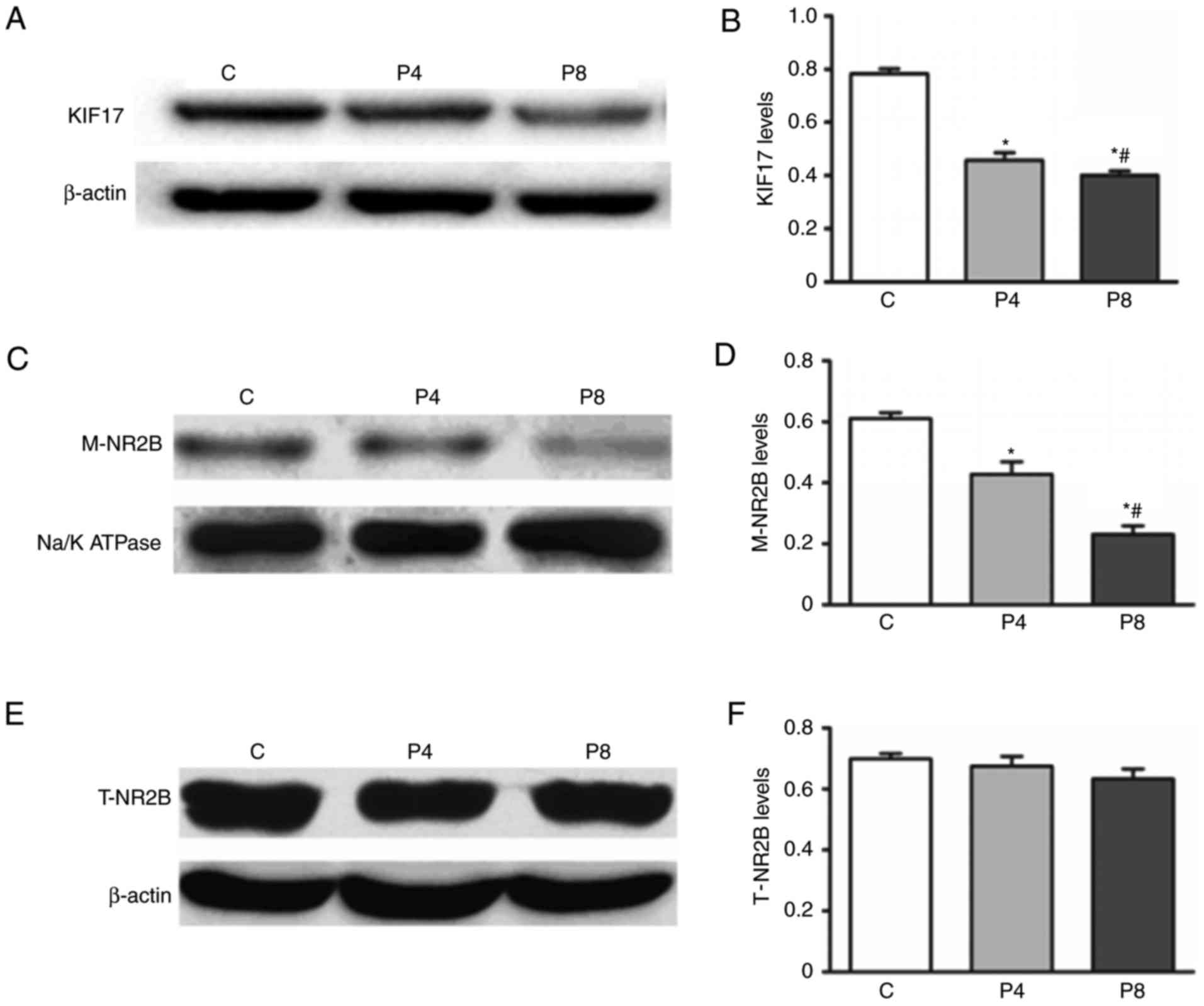
Decreased KIF17 protein levels in the
hippocampus of offspring rats
Expression of KIF17 and NR2B in the hippocampus of
offspring rats was assessed using western blotting (Fig. 3). Levels of KIF17 protein in the
hippocampus of offspring rats in the P8 and P4 groups were
significantly decrease compared with the C group (P<0.05;
Fig. 3A and B). The expression of
KIF17 protein in the P8 group was significantly lower compared with
P4 group (P<0.05; Fig. 3C and
B), suggesting that pregnant rat exposure to propofol decreases
the expression of KIF17 protein in the hippocampus of offspring
rats.
Decreased levels of M-NR2B were
observed in propofol treatment groups
No significant differences in the hippocampus
expression of T-NR2B protein were identified between the three
groups (Fig. 3E and F). However,
the expression of M-NR2B was significantly lower in group P8
compared with group P4 and expression in group P4 was significantly
lower compared with group C (P<0.05; Fig. 3D and E). These results suggest that
propofol-induced learning and memory impairments in offspring are
associated with a decrease in M-NR2B protein in the
hippocampus,
Correlation analysis
The levels of M-NR2B in the hippocampus of offspring
rats were positively correlated KIF17 expression (r=0.877;
P=0.001), which suggests that KIF17 serves a role in transporting
NR2B to its target cell membrane.
Discussion
The results of the present study demonstrate that
exposure to propofol during middle pregnancy causes learning and
memory defects as well as decreased hippocampal expression of KIF17
and M-NR2B in offspring rats. The body temperature, heart rate,
respiratory rate, blood pressure and SPO2 of maternal
rats were monitored during propofol infusion. No significant
changes were identified in these indexes. Blood gases were analyzed
following propofol infusion and no significant changes were
observed. Therefore, the learning and memory impairments observed
in the present study were not due to pathological disorders.
Propofol had no significant effect on the physical development of
the offspring and no significant differences in body weight or
dyskinesia were observed between the groups, suggesting that the
learning and memory impairments were induced by maternal propofol
exposure rather than physical differences. These results are
similar to a previous study, in which it was reported that propofol
infusion on day G18 caused brain damage and permanent learning and
memory dysfunction in offspring rats (1).
Preclinical evidence has demonstrated that general
anesthetic agents may accelerate apoptosis and inhibit the
proliferation of neuron progenitor cells, thus causing irreversible
neurological damage to receptors (23,24).
Combined use of two or more anesthetic agents resulted in more
severe damage compared with a single anesthetic alone (25,26).
Propofol is able to induce neuronal apoptosis in young rats and
cause brain dysfunction by activating the γ-aminobutyric acid
receptor (27,28). Neonatal or juvenile exposure to
propofol has been reported to cause learning and memory impairments
in adult rats by inducing neurodegeneration and a reduction in the
expression of neurotransmitters and brain-derived neurotrophic
factor (BDNF) (29–33). In addition, prolonged exposure to
propofol in aged rats also causes long-term learning and memory
impairment (34,35). An in vitro study
demonstrated that propofol damages the neural structure in a
dose-dependent manner (36).
Our previous study demonstrated the offspring of
pregnant rats exposed to anesthesia for 2 h had no deficit in
learning and memory; however, prolonged exposure of 4 h could
resulted in significant impairments (12). The second trimester is regarded as
the safest period during pregnancy and the majority of
non-obstetric surgeries are performed during this period under
general anesthesia (37,38). However, the effect of maternal
exposure to propofol during the second trimester on the learning
and memory in offspring remains to be elucidated. The results of
the present study demonstrate that exposure to propofol caused
learning and memory impairment in offspring rats. It is therefore
necessary to consider the underlying mechanisms responsible.
NMDA receptors are divided into 7 subunits, of which
NR2B is the most important for learning and memory formation
(39). NR2B gene knockout causes
changes in synaptic plasticity and serious deficits in learning and
memory, whereas NR2B overexpression is beneficial for LTP, thus
promoting learning and memory (14,15).
The combination of phosphorylated cyclic adenosine
monophosphate response element (cAMP) binding protein (CREB) with
the cAMP response element on NR2B will increase the expression of
NR2B (40,41), and then NR2B will potentiate the
transcription of memory related-downstream genes, including
immediate early genes c-fos and c-jun. Transcription
of these genes potentiates synaptic reconstruction, promotes LTP
and inhibits long-term depression in the hippocampus, thus
affecting learning and memory development (42,43).
In the present study, maternal propofol exposure during middle
pregnancy significantly decreased the expression of M-NR2B protein
in the hippocampus of offspring rats, whereas T-NR2B expression was
unaffected. These data suggest that maternal propofol exposure
during the second trimester may impair learning and memory in
offspring by inhibiting NR2B transport to the neuronal membrane as
opposed to inhibiting the expression of NR2B in the hippocampus.
This is possible as NR2B protein must be transported to the cell
membrane in order to perform its regulatory function in learning
and memory (44). It is necessary
to consider how maternal propofol exposure may inhibit
transportation of NR2B to the neuronal membrane.
KIF17, a kinetic protein that is responsible for the
transportation of NR2B to the cell membrane by combining with the
vesicle via Mint-1 protein, moves along the microtubules towards
the cell membrane (17). Vesicles
containing NR2B are released into the dendritic cell membrane to
exert learning and memory regulating functions (17,18).
Previous studies have demonstrated that KIF17 expression may affect
the levels of M-NR2B and thus influence synaptic plasticity and
cognition (21,22). Yin et al (44) confirmed that KIF17 (−/−) mice lost
the ability to transport NR2B, resulting in a decrease in NR2B in
the synaptic membrane and causing impaired synaptic plasticity and
spatial memory. The results of the present study demonstrated that
to propofol for 4 and 8 h during middle pregnancy decreased the
expression of KIF17 in the hippocampus of offspring rats, which was
associated with a downregulation in M-NR2B.
In the present study, KIF17 and M-NR2B expression
was significantly lower in the P8 group compared with the P4 group,
whereas no significant differences in learning and memory were
observed between the 2 groups. Memory includes spatial memory,
recognition memory, episodic memory, emotional memory and semantic
memory. Though the MWM test is recognized as a useful for assessing
memory in rodents, it mainly reflects spatial learning and memory
(45). In addition, the learning
and memory tasks in the MWM test are relatively simple. To overcome
this limitation, multiple behavioral tests, including open field
test, fear conditioning test and object recognition tests should be
used in future studies.
Although learning and memory are mainly regulated by
the hippocampus, other areas of the brain, including the cerebral
cortex, serve an important role (46). In order to gain a more
comprehensive understanding of the underlying mechanisms of memory
defects, changes in KIF17 and NR2B in other cerebral areas should
be investigated in future studies. Our previous study demonstrated
that propofol exposure during late gestation impaired the learning
and memory of rat offspring via the BDNF-tropomyosin receptor
kinase B (TrkB) pathway (11).
Interactions between the BDNF-TrkB signaling pathway and NR2B serve
a critical role in memory (47).
However, whether the BDNF-TrkB signaling pathway serves a role in
the learning and memory defects induced by maternal exposure to
propofol during middle pregnancy requires further
investigation.
In conclusion, maternal propofol exposure during
middle pregnancy may impair learning and memory function in
offspring rats by suppressing the expression of KIF17 in the
hippocampus, thus inhibiting translocation of NR2B to the cellular
membrane. This suggests KIF17 may be a potential therapeutic target
for the memory deficits in offspring caused by maternal propofol
exposure.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460175) and the
Natural Science Foundation of Jiangxi Province of China (grant no.
20171ACB20030).
References
|
1
|
Xiong M, Li J, Alhashem HM, Tilak V, Patel
A, Pisklakov S, Siegel A, Ye JH and Bekker A: Propofol exposure in
pregnant rats induces neurotoxicity and persistent learning deficit
in the offspring. Brain Sci. 4:356–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikonomidou C, Bosch F, Miksa M, Bittigau
P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L and
Olney JW: Blockade of NMDA receptors and apoptotic
neurodegeneration in the developing brain. Science. 283:70–74.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayashi H, Dikkes P and Soriano SG:
Repeated administration of ketamine may lead to neuronal
degeneration in the developing rat brain. Paediatr Anaesth.
12:770–774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jevtovic-Todorovic V and Olney JW: PRO:
Anesthesia-induced developmental neuroapoptosis: Status of the
evidence. Anesth Analg. 106:1659–1663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loepke AW and Soriano SG: An assessment of
the effects of general anesthetics on developing brain structure
and neurocognitive function. Anesth Analg. 106:1681–1707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGowan FX Jr and Davis PJ:
Anesthetic-related neurotoxicity in the developing infant: Of mice,
rats, monkeys and, possibly, humans. Anesth Analg. 106:1599–602.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van De Velde M and De Buck F: Anesthesia
for non-obstetric surgery in the pregnant patient. Minerva
Anestesiol. 73:235–240. 2007.PubMed/NCBI
|
|
8
|
Baldwin EA, Borowski KS, Brost BC and Rose
CH: Antepartum nonobstetrical surgery at ≥23 weeks' gestation and
risk for preterm delivery. Am J Obstet Gynecol. 212:232.e1–e5.
2015. View Article : Google Scholar
|
|
9
|
Chidambaran V, Costandi A and D'Mello A:
Propofol: A review of its role in pediatric anesthesia and
sedation. CNS Drugs. 29:543–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krzisch M, Sultan S, Sandell J, Demeter K,
Vutskits L and Toni N: Propofol anesthesia impairs the maturation
and survival of adult-born hippocampal neurons. Anesthesiology.
118:602–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong L, Luo F, Zhao W, Feng Y, Wu L, Lin
J, Liu T, Wang S, You X and Zhang W: Propofol exposure during late
stages of pregnancy impairs learning and memory in rat offspring
via the BDNF-TrkB signalling pathway. J Cell Mol Med. 20:1920–1931.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Q, Luo F, Zhao W, Li B, Tang Y and
Hu Y: Effect of prolonged anesthesia with propofol during early
pregnancy on cognitive function of offspring rats. Chin J
Anesthesiol. 34:1051–1053. 2014.
|
|
13
|
Williams JM, Guévremont D, Kennard JT,
Mason-Parker SE, Tate WP and Abraham WC: Long-term regulation of
N-methyl-D-aspartate receptor subunits and associated synaptic
proteins following hippocampal synaptic plasticity. Neuroscience.
118:1003–1013. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niimi K, Takahashi E and Itakura C:
Improved short-term memory and increased expression of NR2B
observed in senescence-accelerated mouse (SAM) P6. Exp Gerontol.
43:847–852. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Engelhardt J, Doganci B, Jensen V,
Hvalby Ø, Göngrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JN,
Seeburg PH, et al: Contribution of hippocampal and
extra-hippocampal NR2B-containing NMDA receptors to performance on
spatial learning tasks. Neuron. 60:846–860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yildiz A and Selvin PR: Kinesin: Walking,
crawling or sliding along? Trends Cell Biol. 15:112–120. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Setou M, Nakagawa T, Seog DH and Hirokawa
N: Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA
receptor-containing vesicle transport. Science. 288:1796–1802.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guillaud L, Wong R and Hirokawa N:
Disruption of KIF17-Mint1 interaction by CaMKII-dependent
phosphorylation: A molecular model of kinesin-cargo release. Nat
Cell Biol. 10:19–29. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costa AC, Scott-McKean JJ and Stasko MR:
Acute injections of the NMDA receptor antagonist memantine rescue
performance deficits of the Ts65Dn mouse model of Down syndrome on
a fear conditioning test. Neuropsychopharmacology. 33:1624–1632.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guillaud L, Setou M and Hirokawa N: KIF17
dynamics and regulation of NR2B trafficking in hippocampal neurons.
J Neurosci. 23:131–140. 2003.PubMed/NCBI
|
|
21
|
Yin X, Takei Y, Kido MA and Hirokawa N:
Molecular motor KIF17 is fundamental for memory and learning via
differential support of synaptic NR2A/2B levels. Neuron.
70:310–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong RW, Setou M, Teng J, Takei Y and
Hirokawa N: Overexpression of motor protein KIF17 enhances spatial
and working memory in transgenic mice. Proc Natl Acad Sci USA.
99:pp. 14500–14505. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong C, Rovnaghi CR and Anand KJ: Ketamine
alters the neurogenesis of rat cortical neural stem progenitor
cells. Criti Care Med. 40:2407–2416. 2012. View Article : Google Scholar
|
|
24
|
Tang XM, Qin Y, Liao CJ, Xie YB and Lan
YY: Effects of propofol on expression of hippocampal survivin and
Caspase-3 in newborn rats. Zhonghua Er Ke Za Zhi. 50:361–365.
2012.(In Chinese). PubMed/NCBI
|
|
25
|
Schubert H, Eiselt M, Walter B, Fritz H,
Brodhun M and Bauer R: Isoflurane/nitrous oxide anesthesia and
stress-induced procedures enhance neuroapoptosis in intrauterine
growth-restricted piglets. Intensive care Med. 38:1205–1214. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou X, Liu F, Zhang X, Patterson TA,
Callicott R, Liu S, Hanig JP, Paule MG, Slikker W Jr and Wang C:
Inhalation anesthetic-induced neuronal damage in the developing
rhesus monkey. Neurotoxicol Teratol. 33:592–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pain L, Angst MJ, LeGourrier L and
Oberling P: Effect of a nonsedative dose of propofol on memory for
aversively loaded information in rats. Anesthesiology. 97:447–453.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kahraman S, Zup SL, McCarthy MM and Fiskum
G: GABAergic mechanism of propofol toxicity in immature neurons. J
Neurosurg Anesthesiol. 20:233–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karen T, Schlager GW, Bendix I, Sifringer
M, Herrmann R, Pantazis C, Enot D, Keller M, Kerner T and
Felderhoff-Mueser U: Effect of propofol in the immature rat brain
on short- and long-term neurodevelopmental outcome. PLoS One.
8:e644802013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu D, Jiang Y, Gao J, Liu B and Chen P:
Repeated exposure to propofol potentiates neuroapoptosis and
long-term behavioral deficits in neonatal rats. Neurosci Lett.
534:41–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen B, Deng X, Wang B and Liu H:
Persistent neuronal apoptosis and synaptic loss induced by multiple
but not single exposure of propofol contribute to long-term
cognitive dysfunction in neonatal rats. J Toxicol Sci. 41:627–636.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu D, Li L and Yuan W: Neonatal anesthetic
neurotoxicity: Insight into the molecular mechanisms of long-term
neurocognitive deficits. Biomed Pharmacother. 87:196–199. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee JE, Rayyan M, Liao A, Edery I and
Pletcher SD: Acute dietary restriction acts via TOR, PP2A, and Myc
signaling to boost innate immunity in Drosophila. Cell Rep.
20:479–490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bekker AY and Weeks EJ: Cognitive function
after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol.
17:259–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Culley DJ, Baxter M, Yukhananov R and
Crosby G: The memory effects of general anesthesia persist for
weeks in young and aged rats. Anesth Analg. 96:1004–1009, table of
contents. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vutskits L, Gascon E, Tassonyi E and Kiss
JZ: Clinically relevant concentrations of propofol but not
midazolam alter in vitro dendritic development of isolated
gamma-aminobutyric acid-positive interneurons. Anesthesiology.
102:970–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tran KM: Anesthesia for fetal surgery.
Semin Fetal Neonatal Med. 15:40–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reddy SV: Effect of general anesthetics on
the developing brain. J Anaesthesiol Clin Pharmacol. 28:6–10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW,
Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al: Roles of NMDA NR2B
subtype receptor in prefrontal long-term potentiation and
contextual fear memory. Neuron. 47:859–872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dalle S, Quoyer J, Varin E and Costes S:
Roles and regulation of the transcription factor CREB in pancreatic
β-cells. Curr Mol Pharmacol. 4:187–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suzuki A, Fukushima H, Mukawa T, Toyoda H,
Wu LJ, Zhao MG, Xu H, Shang Y, Endoh K, Iwamoto T, et al:
Upregulation of CREB-mediated transcription enhances both short-
and long-term memory. J Neurosci. 31:8786–8802. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barco A, Alarcon JM and Kandel ER:
Expression of constitutively active CREB protein facilitates the
late phase of long-term potentiation by enhancing synaptic capture.
Cell. 108:689–703. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pittenger C, Huang YY, Paletzki RF,
Bourtchouladze R, Scanlin H, Vronskaya S and Kandel ER: Reversible
inhibition of CREB/ATF transcription factors in region CA1 of the
dorsal hippocampus disrupts hippocampus-dependent spatial memory.
Neuron. 34:447–462. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yin X, Feng X, Takei Y and Hirokawa N:
Regulation of NMDA receptor transport: a KIF17-cargo
binding/releasing underlies synaptic plasticity and memory in vivo.
J Neurosci. 32:5486–5499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vorhees CV and Williams MT: Assessing
spatial learning and memory in rodents. ILAR J. 55:310–332. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He J, Zhao C, Liu W, Huang J, Liang S,
Chen L and Tao J: Neurochemical changes in the hippocampus and
prefrontal cortex associated with electroacupuncture for learning
and memory impairment. Int J Mol Med. 41:709–716. 2018.PubMed/NCBI
|
|
47
|
Nakai T, Nagai T, Tanaka M, Itoh N, Asai
N, Enomoto A, Asai M, Yamada S, Saifullah AB, Sokabe M, et al:
Girdin phosphorylation is crucial for synaptic plasticity and
memory: A potential role in the interaction of BDNF/TrkB/Akt
signaling with NMDA receptor. J Neurosci. 34:14995–15008. 2014.
View Article : Google Scholar : PubMed/NCBI
|