Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors and the third leading cause of cancer-associated
mortality worldwide (1).
Currently, resection and chemotherapy are commonly used to improve
the outcomes in patients with CRC; however, metastasis is an
obstacle to successful treatment of CRC (2). Among patients with CRC, ~50%
eventually develop distant metastases, resulting in poor outcomes
even when primary tumors are resectable (3). Distant metastasis is the predominant
cause of death in patients with CRC (4). Lungs are the most common
extra-abdominal site of metastasis (5). Diagnosis of lung metastasis in
patients with CRC remains dependent on radiology and biopsy
(6,7). Radiology-guided biopsy has
demonstrated limited sensitivity (8). Therefore, investigation of novel
biomarkers and molecular mechanisms underlying metastatic
progression in CRC is necessary for improving the survival of
patients with CRC, and may be beneficial for earlier diagnosis and
treatment of patients with lung metastasis.
Only ~2% of human genome sequences encode proteins,
while the remainder is divided into two groups according the length
of the sequence: Short noncoding RNA (<200 nucleotides); and
long noncoding RNA (lncRNA; >200 nucleotides) (9,10).
lncRNA regulate gene expression on transcriptional or
post-transcriptional levels (11,12).
Currently, an increasing number of studies have demonstrated that
lncRNA serve important roles in cancer progression and metastasis
as well as cellular processes, including cell proliferation and
apoptosis (13–15). Furthermore, it has been reported
that many lncRNA serve roles in the regulation of CRC (16). However, biological and pathological
functions of the majority of lncRNA in patients with CRC and lung
metastasis remain to be elucidated.
Therefore, in the present study, differentially
expressed lncRNA and mRNA in tissues from patients with CRC and
with or without lung metastasis were investigated in order to
identify novel diagnostic and prognostic markers for patients with
CRC and lung metastasis.
Materials and methods
Ethical approval
All samples were collected from patients in the
Department of Gastrointestinal Surgery, The First Affiliated
Hospital of Kunming Medical University (Kunming, China) between
January 2013 and December 2015. The project was reviewed and
approved by the Ethics Committee of The First Affiliated Hospital
of Kunming Medical University. All patients included in the present
study provided written informed consent prior to the surgery.
Samples
A total of 6 primary CRC samples were obtained from
3 male patients with CRC and lung metastasis (CRC + m) (age,
66.33±7.37 years) and 3 male patients with CRC without metastasis
(CRC - m) (age, 64.67±11.93 years). All patients were
histologically confirmed to have colorectal cancer and did not
receive any other forms of therapy at the time of enrollment. At
the time of surgery, all tissue samples were immediately frozen in
liquid nitrogen and stored at −80°C for further use.
RNA extraction and quality
control
Total RNA was extracted from each sample using a
homogenizer and TRIzol regent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol.
Subsequently, the quantity and quality of purified RNA were
assessed by NanoDrop ND-1000 (Thermo Fisher Scientific, Inc.). The
integrity of RNA was assessed by electrophoresis on a denaturing
1.5% agarose gel and staining with ethidium bromide for 30 min at
60°C.
Microarray analysis
Sample labeling and human lncRNA array hybridization
(Human LncRNA Expression Microarray v4.0, Arraystar, Inc.,
Rockville, MD, USA) were performed according to the manufacturer's
protocol. Total RNA were digested with RNase R (Epicentre;
Illumina, Inc., San Diego, CA, USA) to remove linear RNA and enrich
circular (circ)RNA. The enriched circRNA were amplified and
transcribed into fluorescent lncRNA using random primers (Arraystar
Super RNA Labeling kit; Arraystar, Inc.). Labeled lncRNA were
purified by RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA). The
concentration and specific activity of the labeled lncRNA (pmol
Cy3/µg lncRNA) were measured by NanoDrop ND-1000. A total of 1 µg
of each labeled lncRNA was fragmented by adding 5 µl 10X blocking
agent and 1 µl 25X fragmentation buffer (Arraystar Super RNA
Labeling kit; Arraystar, Inc.) and the mixture was heated at 60°C
for 30 min. Finally, 25 µl 2X hybridization buffer (GE Healthcare,
Chicago, IL, USA) was added to dilute the labeled lncRNA. A total
of 50 µl hybridization solution was applied onto a gasket slide and
assembled to the lncRNA expression microarray slide. The slides
were incubated for 17 h at 65°C in an Agilent Hybridization Oven
(Agilent Technologies, Inc., Santa Clara, CA, USA). The hybridized
arrays were washed using Agilent wash buffer 1 and Agilent wash
buffer 2 (Agilent Technologies, Inc., Santa Clara, CA, USA), fixed
and scanned using the Agilent Scanner G2505C (Agilent Technologies,
Inc.).
Data analysis
Scanned images were imported to the Feature
Extraction software (version 11.0.1.1) for raw data extraction
(Agilent Technologies, Inc.). When comparing two groups of profile
differences (such as metastasis vs. non-metastasis), the fold
change (i.e., the ratio of group averages) between the groups of
each lncRNA was computed. A t-test was used to determine the
differences between two groups. The lncRNA demonstrating fold
changes ≥2 and P<0.05 compared with the control were selected as
differentially expressed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) validation
A total of 20 pairs of primary CRC samples obtained
from patients with CRC + m and patients with CRC - m were used for
validation of the results. Following extraction of total RNA, the
RNA were reverse-transcribed to cDNA using 5× HiScript®
II qRT SuperMix II (R223-01, Vazyme, Piscataway, NJ, USA) according
to the manufacturer's protocol. Subsequently, qPCR was performed in
10 µl reactions, including 0.5 µl PCR forward primer (10 µM), 0.5
µl PCR reverse primer (10 µM), 2 µl cDNA, 5 µl 2×
QuantiFast® SYBR® Green PCR Master Mix
(204054, Qiagen, Inc.) and 2 µl double distilled water. The
following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 10 min; followed by final extension of 40
cycles of 95°C for 10 sec and 60°C for 60 sec. β-actin served as a
reference gene. Relative expression levels of each lncRNA was
calculated using the 2−ΔΔCq method (17). The statistical significance of the
difference was estimated by a t-test using SPSS v20.0 (IBM Corp.,
Armonk, NY, USA); P<0.05 was considered to indicate a
statistically significant difference. The primers used in the
present study were as follows: HOXA distal transcript antisense RNA
(HOTTIP) forward (F), 5′-CCTAAAGCCACGCTTCTTTG-3′ and reverse (R),
5′-TGCAGGCTGGAGATCCTACT-3′; RP11-79H23.3 F,
5′-GCAAGGAGAGTAATGCTGGA-3′ and R, 5′-CAATGAGGATGAGAAGAGGTC-3′;
urothelial cancer associated 1 (UCA1) F,
5′-GTCAACGGATTTGGTCTGTATT-3′ and R, 5′-AGTCTTCTGGGTGGCAGTGAT-3′;
metastasis asisassociated lung adenocarcinoma transcript1 (MALAT1)
F, 5′-GGTAACGATGGTGTCGAGGTC-3′ and R,
5′-CCAGCATTACAGTTCTTGAACATG-3′; LOC100507661 F,
5-CTCGGATCCTACCATCATGGCTCACTGCAACCTC-3′ and R,
5′-CCCTCTAGCGCCGCTTTTTATGCATCAAAAATAAAGGTG-3′; maternally expressed
3 (MEG3) F, 5′-CTGCCCATCTACACCTCACG-3′ and R,
5′-CTCTCCGCCGTCTGCGCTAGGGGCT-3′; and β-actin F,
5′-AGCACAGAGCCTCGCCTTTG-3′ and R, 5′-CTTCTGACCCATGCCCACCA-3′.
Gene ontology (GO) analysis
GO (geneontology.org) was used for functional analysis in
order to categorize genes and gene products into the following
categories: i) Implicated in biological processes (BP); ii)
demonstrating a molecular function (MF); and iii) associated with
cellular components (CC). GO was used to analyze biological
functions of associated lncRNA and gene targets. The GO term
enrichment technique used the P<0.05 threshold to assign
differentially expressed lncRNA to target genes.
Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway analysis
Pathway analysis is a functional analysis of genes
that allows for construction of network datasets (http://www.genome.jp/kegg/pathway.html).
KEGG analysis allowed for determination of biological pathways
enriched with differentially expressed genes and lncRNA. P<0.05
was considered to indicate a statistically significant difference
in gene expression.
Results
Differentially expressed lncRNA and
mRNA in tissues of patients with CRC + m
A genome-wide analysis was performed to profile
differences in lncRNA and mRNA expression between CRC tissues from
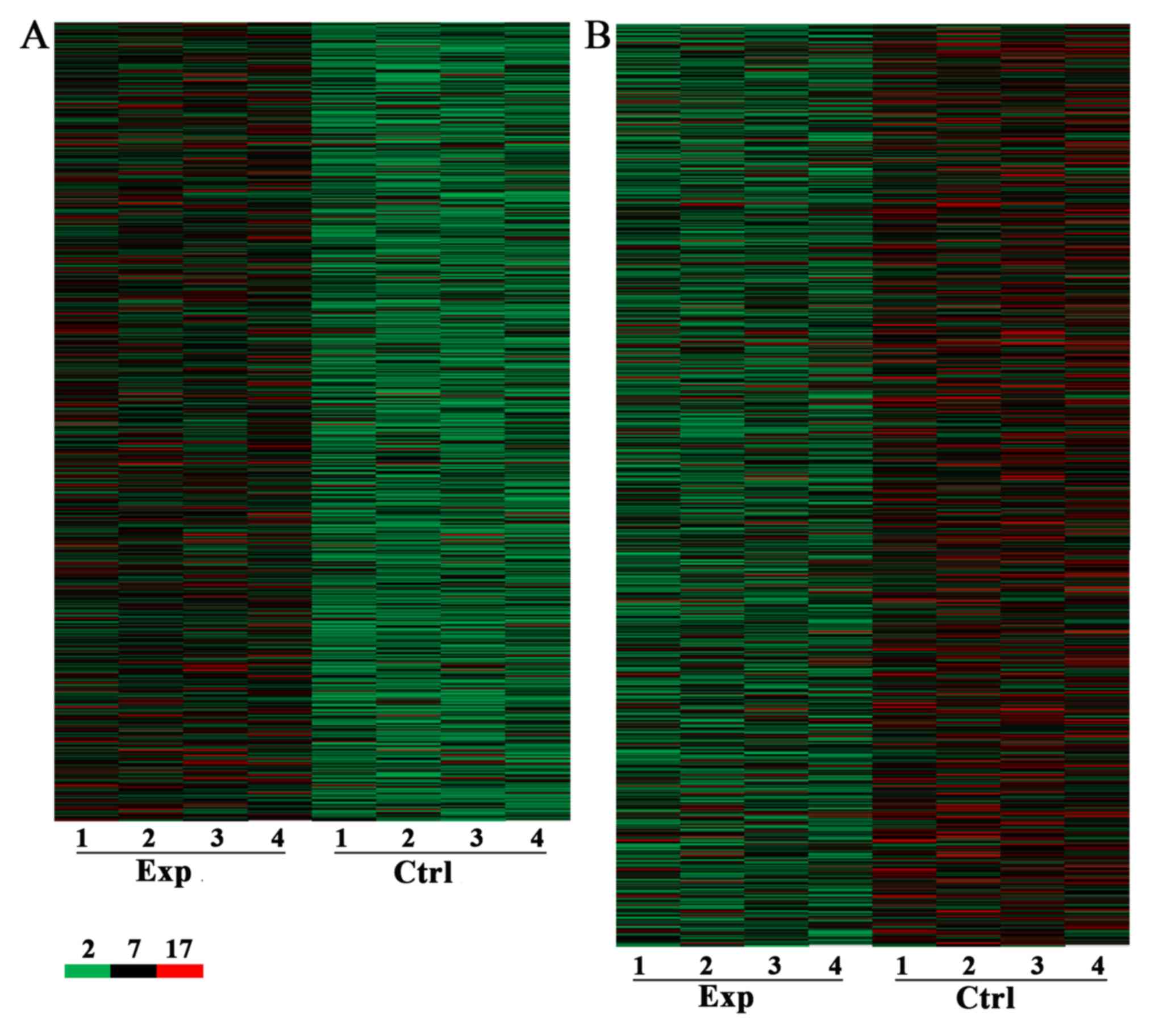
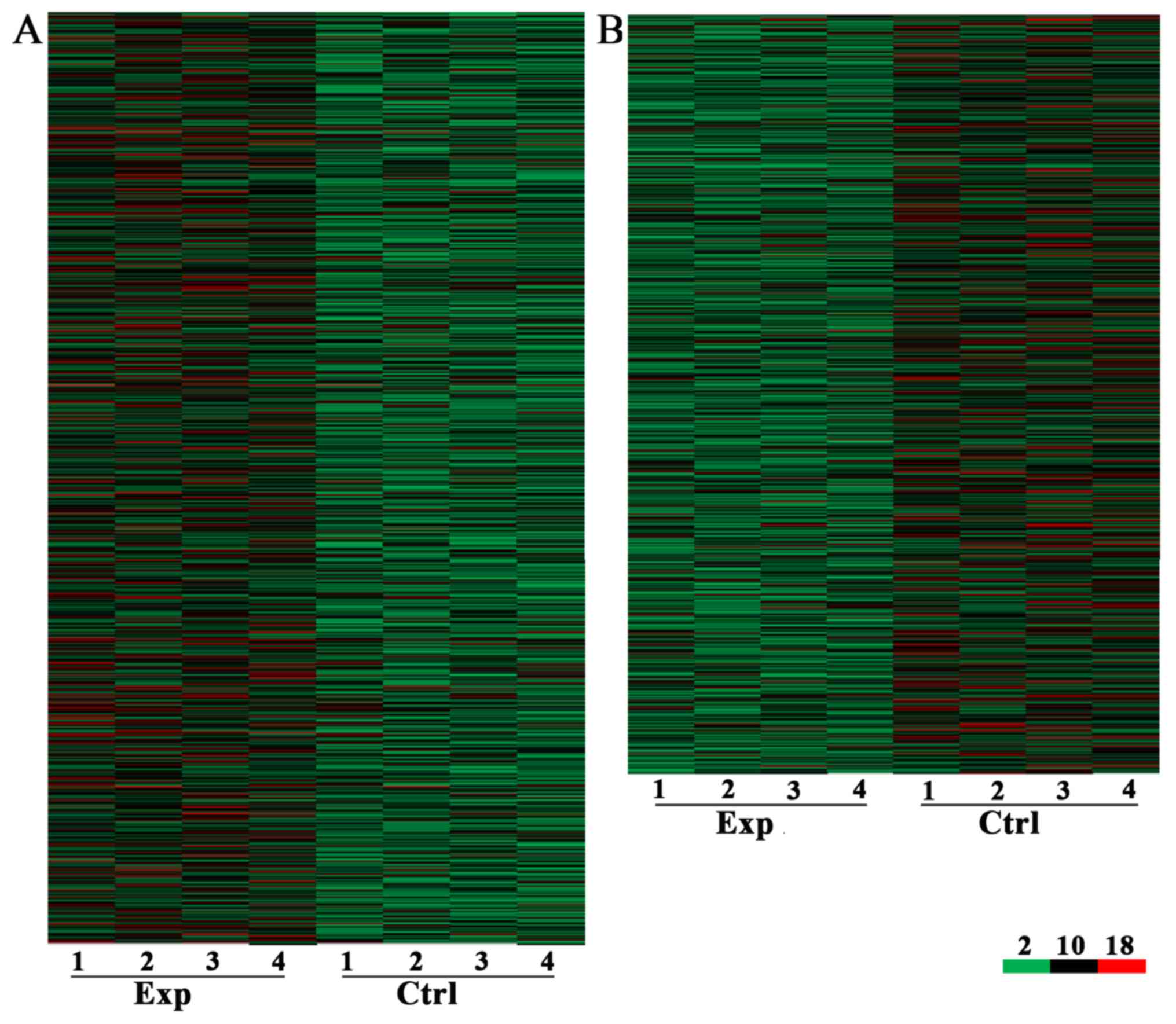
patients with CRC + m and CRC - m (Figs. 1 and 2). A total of 7,632 lncRNA and 6,185 mRNA
were identified to be differentially expressed with a fold change
≥2 and P<0.05, including 3,574 upregulated (Fig. 1A) and 4,058 downregulated (Fig. 1B) lncRNA. A total of 3,394 and
2,791 mRNA were upregulated (Fig.
2A) and downregulated (Fig.
2B), respectively, in the CRC + m group compared with the CRC -
m group.
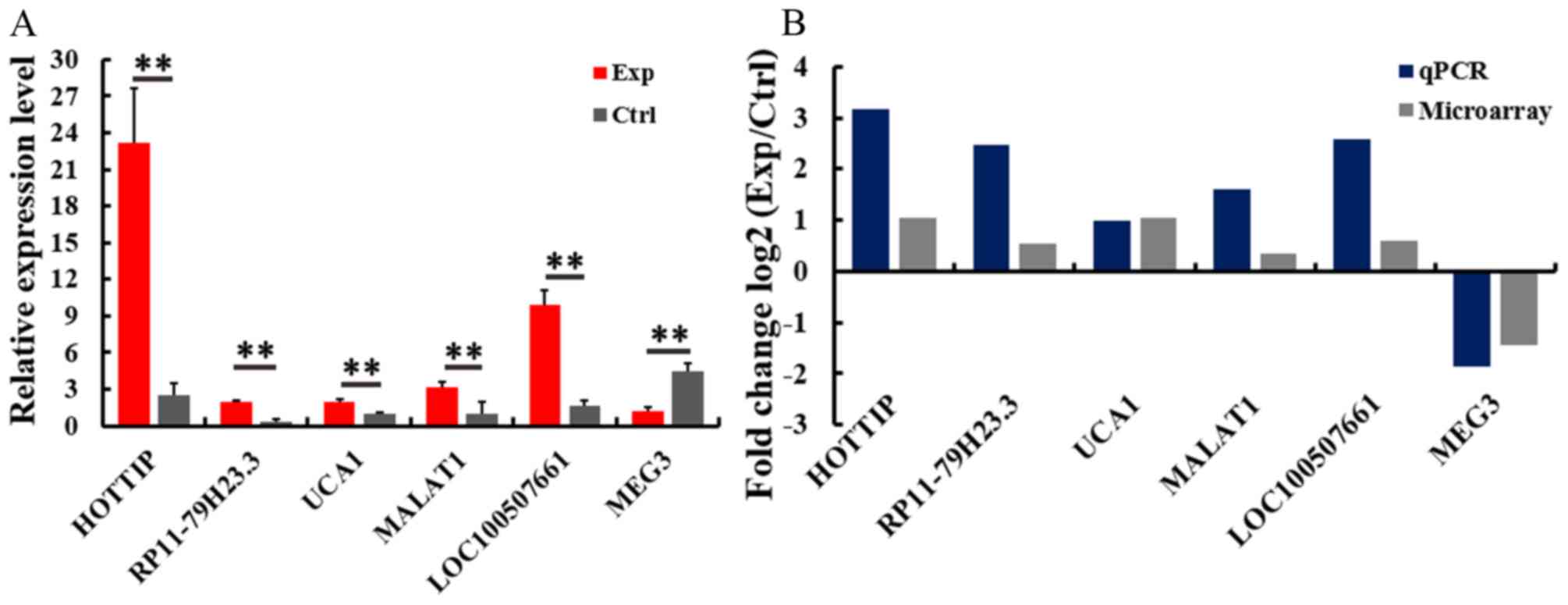
Validation of deregulated lncRNA
A total of six lncRNA were selected for RT-qPCR
verification of microarray results in 20 pairs of CRC samples. The
RT-qPCR assay demonstrated that the transcription of lncRNA HOTTIP,
RP11-79H23.3, UCA1, MALAT1 and LOC100507661 was significantly
upregulated, while the transcription of MEG3 was significantly
downregulated in the CRC + m group, compared with in the CRC - m
group (Fig. 3A). These results
were consistent with the microarray assay (Fig. 3B).
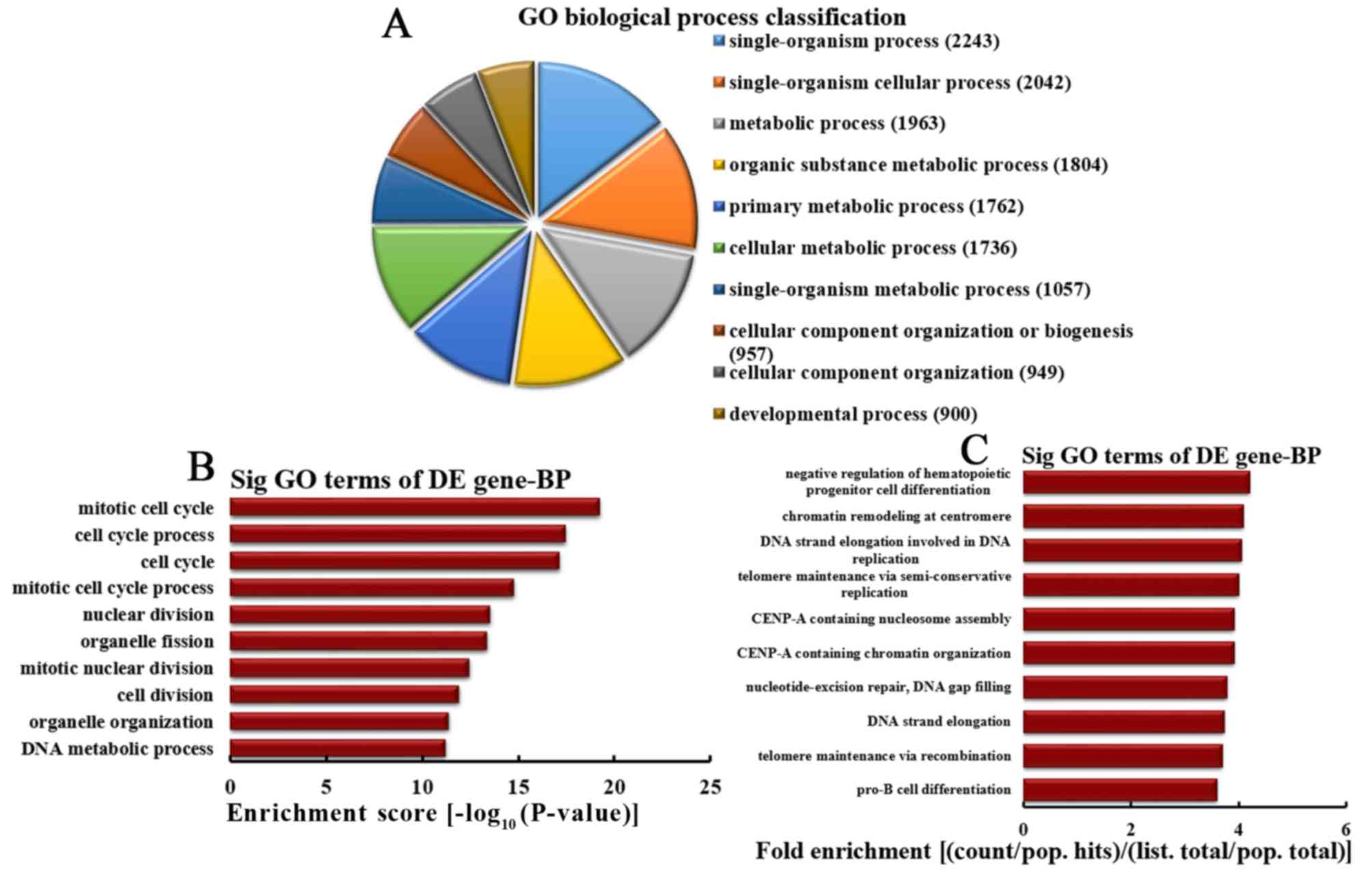
Analysis of upregulated mRNA
implicated in BPs
Based on the number of upregulated mRNA implicated
in BPs, the 10 most enriched BPs were classified (Fig. 4A). The classification included
2,243, 2,042, 1,963, 1,804, 1,762, 1,736, 1,057, 957, 949 and 900
mRNA involved in single-organism processes, single-organism
cellular processes, metabolic processes, organic substance
metabolic processes, primary metabolic processes, cellular
metabolic processes, single-organism metabolic processes, cellular
component organization or biogenesis, organization of cellular
components and developmental processes, respectively. A total of 10
most represented BPs were selected based on the enrichment score,
including mitotic cell cycle, cell cycle processes, cell cycle,
mitotic cell cycle processes, nuclear division, organelle fission,
mitotic nuclear division, cell division, organelle organization and
DNA metabolic processes (Fig. 4B).
The following 10 most represented BPs were also selected based on
fold enrichment: Negative regulation of hematopoietic progenitor
cell differentiation, chromatin remodeling at centromeres, DNA
strand elongation involved in DNA replication, telomere maintenance
via semi-conservative replication, centromere protein A (CENP-A)
containing nucleosome assembly, CENP-A containing chromatin
organization, nucleotide-excision repair, DNA gap filling, DNA
strand elongation, telomere maintenance via recombination and pro-B
cell differentiation (Fig.
4C).
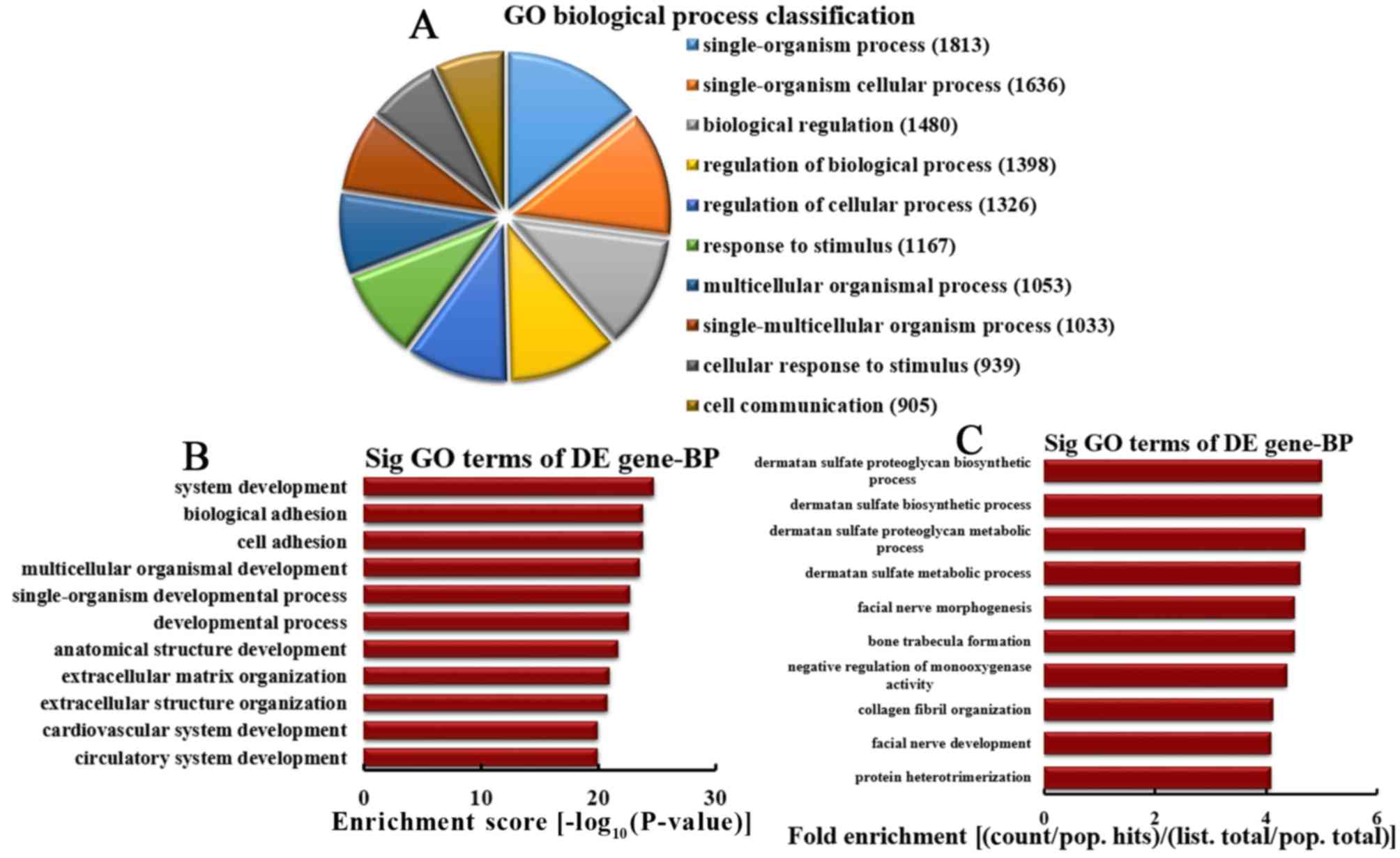
Analysis of downregulated mRNA
implicated in BPs
Based on the number of downregulated mRNA associated
with BPs, the 10 most enriched BPs were classified in the present
study (Fig. 5A). The BPs included
1,813, 1,636, 1,480, 1,398, 1,326, 1,167, 1,053, 1,033, 939 and 905
mRNA involved in single-organism processes, single-organism
cellular processes, biological regulations, regulation of
biological processes, regulation of cellular processes, response to
stimuli, multicellular organism-associated processes,
single-multicellular organism-associated processes, cellular
response to stimuli and cell communication, respectively. Based on
the enrichment score, the 10 most enriched BPs were selected, as
follows: System development, biological adhesion, cell adhesion,
multicellular organismal development, single-organism developmental
process, developmental process, anatomical structure development,
extracellular matrix organization, extracellular structure
organization, and cardiovascular and circulatory system development
(Fig. 5B). Based on fold
enrichment analysis, the 10 most represented BPs were selected,
including dermatan sulfate proteoglycan biosynthetic processes,
dermatan sulfate biosynthetic processes, dermatan sulfate
proteoglycan metabolic processes, dermatan sulfate metabolic
processes, facial nerve morphogenesis, bone trabecula formation,
negative regulation of monooxygenase activity, collagen fibril
organization, facial nerve development and protein
heterotrimerization (Fig. 5C).
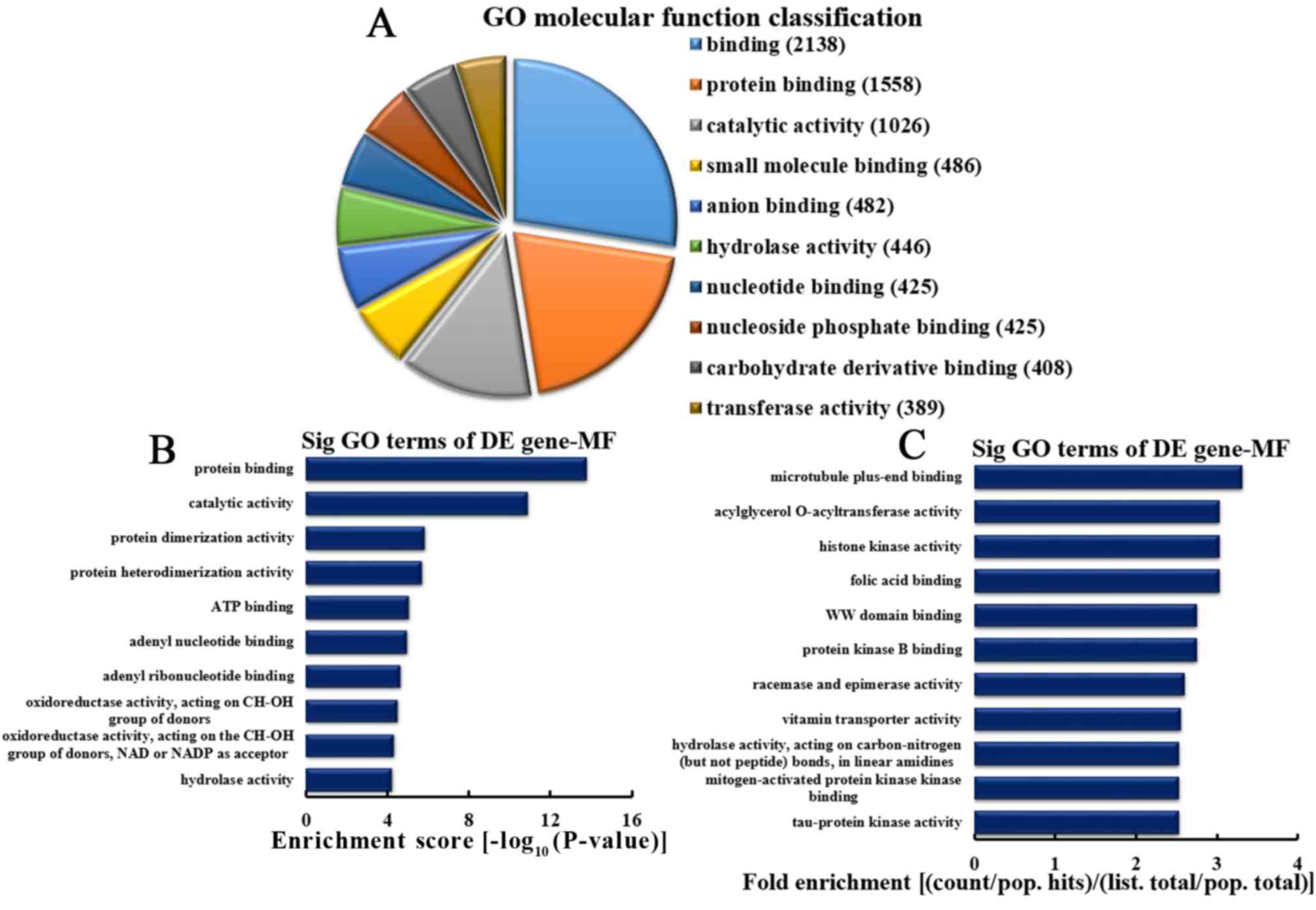
Analysis of upregulated mRNA
implicated in MFs
Based on the number of upregulated mRNA serving MFs,
the 10 most enriched MFs were selected (Fig. 6A). The categories included 2,138,
1,558, 1,026, 486, 482, 446, 425, 425, 408 and 389 mRNA involved in
binding, protein binding, catalytic activity, small molecule
binding, anion binding, hydrolase activity, nucleotide binding,
nucleoside phosphate binding, carbohydrate derivative binding and
transferase activity, respectively. Based on the enrichment score,
the 10 most represented MFs were selected, as follows: Protein
binding, catalytic activity, protein dimerization and
heterodimerization activities, adenosine 5′-triphosphate and adenyl
nucleotide binding, adenyl ribonucleotide binding, oxidoreductase
activity acting on CH-OH group of donors, oxidoreductase activity
acting on CH-OH group of donors or nicotinamide-adenine
dinucleotide or nicotinamide-adenine dinucleotide phosphate
acceptors, and mRNA with hydrolase activity (Fig. 6B). Based on the fold enrichment,
the 10 most represented MFs included microtubule plus-end binding,
acylglycerol O-acyltransferase activity, histone kinase activity,
folic acid binding, WW domain binding, protein kinase B binding,
racemase and epimerase activity, vitamin transporter activity,
hydrolase activity on carbon-nitrogen (but not peptide) bonds in
linear amidines, mitogen-activated protein kinase kinase binding
and tau-protein kinase activity (Fig.
6C).
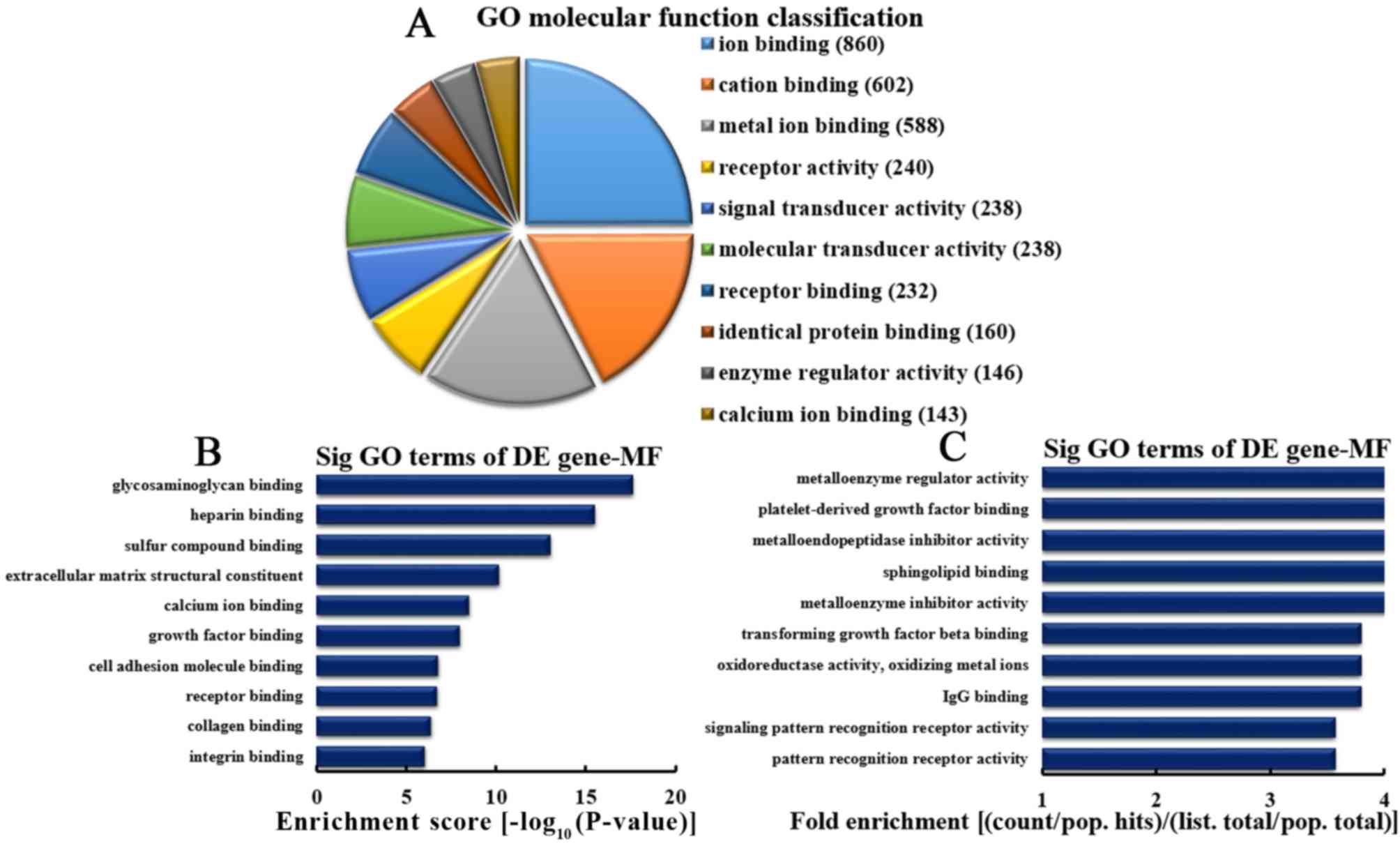
Analysis of downregulated mRNA
implicated in MFs
Based on the number of downregulated mRNA associated
with MFs, the 10 most represented MFs were as follows: 860, 602,
588, 240, 238, 238, 232, 146, 160 and 143 mRNA involved in ion
binding, cation binding, metal ion binding, receptor activity,
signal transduction, molecular transduction, receptor binding,
identical protein binding, enzyme regulatory activity and calcium
ion binding, respectively (Fig.
7A). Based on enrichment scores, the 10 most represented MF
were selected (Fig. 7B). These MFs
included mRNA involved in the binding of glycosaminoglycan,
heparin, sulfur binding, calcium ion, growth factor, cell adhesion
molecules, receptor, collagen, integrin and extracellular matrix
structural constituents. Based on fold enrichment analysis, the 10
most enriched MFs included the following: Metalloenzyme regulation,
platelet-derived growth factor (PDGF) binding, metalloendopeptidase
inhibition, sphingolipid binding, metalloenzyme inhibition,
transforming growth factor β binding, oxidoreductase activity,
oxidizing metal ions, immunoglobulin G binding, signaling pattern
recognition receptor and pattern recognition receptor activities
(Fig. 7C).
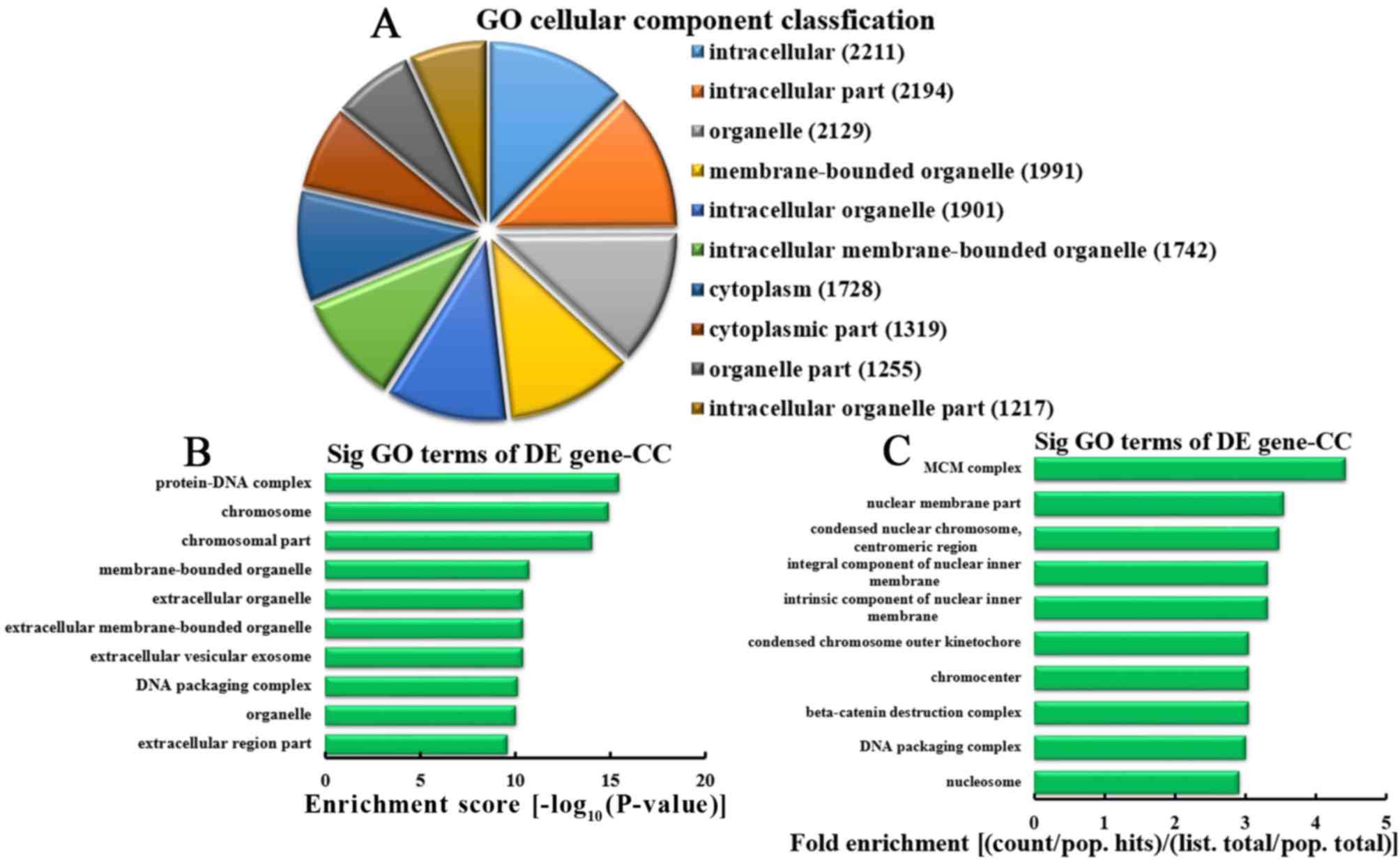
Analysis of upregulated mRNA
associated with CCs
According to the number of upregulated mRNA
associated with CC, the 10 most enriched CCs were classified
(Fig. 8A). A total of 2,211,
2,194, 2,129, 1,991, 1,901, 1,742, 1,728, 1,319, 1,255 and 1,217
mRNA were involved in intracellular, intracellular parts,
organelles, membrane-bounded organelles, intracellular organelles,
intracellular membrane-bounded organelles, cytoplasm, cytoplasmic
parts, organelle parts and intracellular organelle parts,
respectively. According to the enrichment score, the 10 most
represented CCs were selected, as follows: Protein-DNA complexes,
chromosomes, chromosomal parts, membrane-bounded organelles,
extracellular organelles, extracellular membrane-bounded
organelles, extracellular vesicular exosomes, DNA packaging
complexes, organelle and extracellular region parts (Fig. 8B). Based on the fold enrichment,
the 10 most represented CCs were selected: MCM complex, nuclear
membrane parts, condensed nuclear chromosomes, centromeric regions,
integral components of inner nuclear membranes, intrinsic
components of inner nuclear membranes, outer kinetochores of
condensed chromosomes, chromocenters, β-catenin destruction
complexes, DNA packaging complexes and nucleosomes (Fig. 8C).
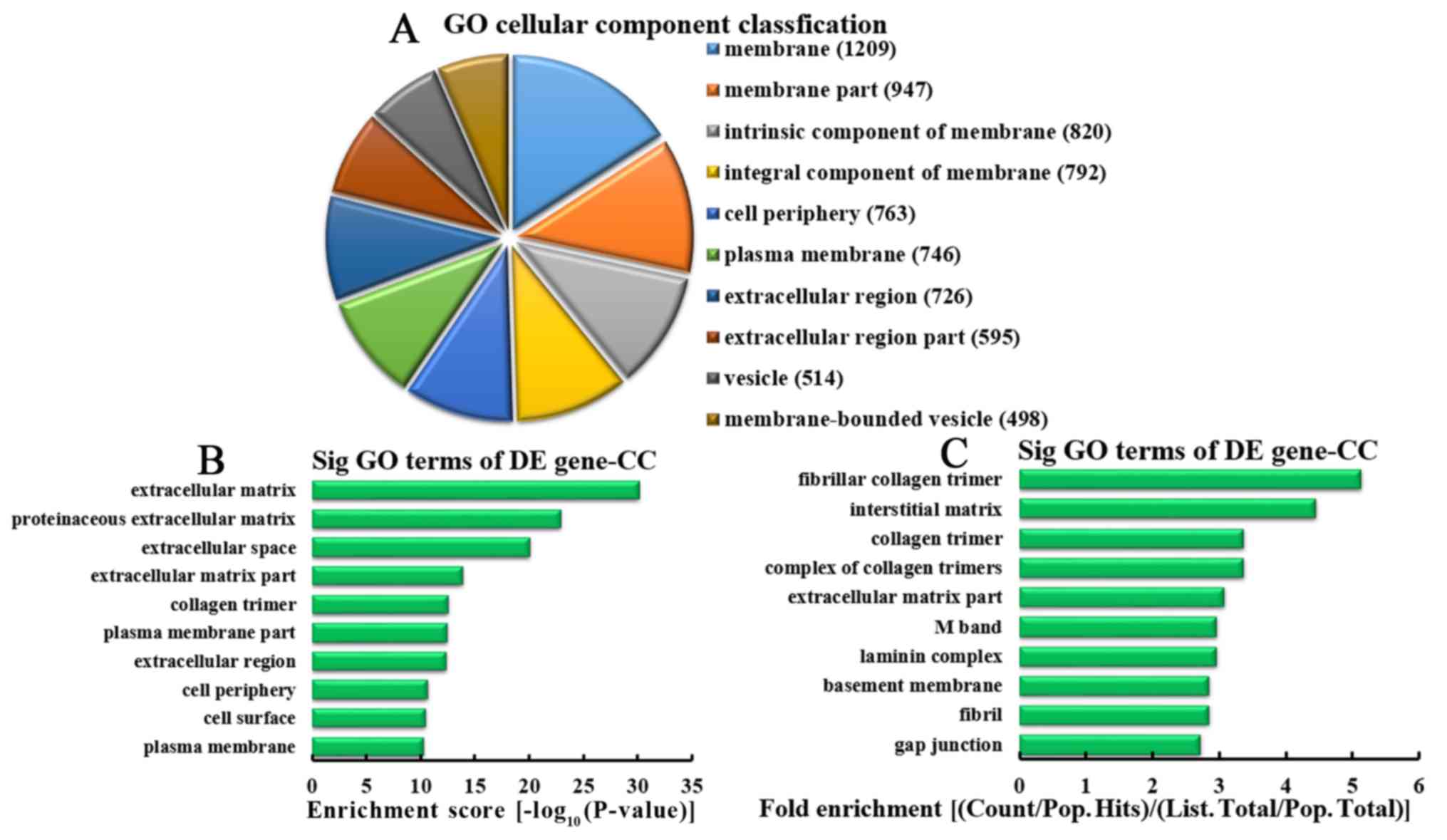
Analysis of downregulated mRNA
associated with CCs
Based on the number of downregulated mRNA associated
with CCs, the 10 most represented CCs were classified, as follows:
1,209, 947, 820, 792, 763, 746, 726, 595, 514 and 498 mRNA involved
in membranes, membrane parts, intrinsic components of membrane,
integral components of membrane, cell periphery, plasma membranes,
extracellular regions, parts of extracellular regions, mRNA
involved in vesicles and mRNA involved in membrane-bound vesicles,
respectively (Fig. 9A). According
to enrichment score, the 10 most represented CCs were selected,
including: Extracellular matrices, proteinaceous extracellular
matrices, extracellular space, extracellular matrix parts, collagen
trimers, plasma membrane parts, extracellular regions, cell
periphery, cell surface and plasma membranes (Fig. 9B). Based on the fold enrichment,
the 10 most represented CCs were selected, including fibrillar
collagen trimers, interstitial matrices, collagen trimers,
complexes of collagen trimers, parts of extracellular matrices, M
bands, laminin complexes, basement membranes, fibrils and gap
junctions (Fig. 9C).
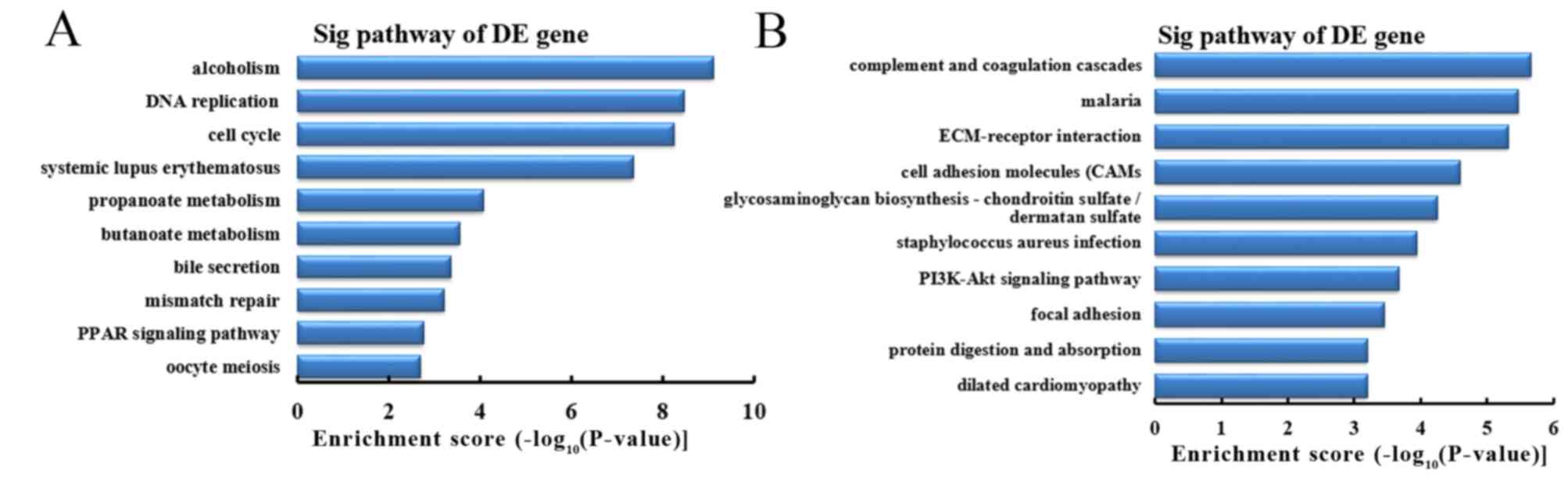
Analysis of differentially expressed
mRNA involved in signaling pathways
To further investigate the function of
differentially expressed mRNA in CRC tissues between the CRC + m
group and CRC - m group, the KEGG database was used for pathway
analysis. The results demonstrated that the upregulated mRNA in the
CRC + m group compared with the CRC - m group were primarily
associated with 58 signaling pathways. Downregulated mRNA in the
CRC + m group compared with the CRC - m group were primarily
associated with 32 signaling pathways. Based on the enrichment
score, the 10 most represented pathways among the upregulated mRNA
were as follows: Alcoholism, DNA replication, cell cycle, systemic
lupus erythematosus, propanoate and butanoate metabolism, bile
secretion, mismatch repair, peroxisome proliferator-activated
receptor signaling pathway and oocyte meiosis (Fig. 10A). Based on the enrichment score,
the 10 most represented pathways among downregulated mRNA included
complement and coagulation cascades, malaria, extracellular
matrix-receptor interaction, cell adhesion molecules,
glycosaminoglycan biosynthesis, staphylococcus aureus infection,
phosphoinositide 3-kinase (PI3K)-Akt signaling pathway, focal
adhesion, protein digestion and absorption, and dilated
cardiomyopathy (Fig. 10B).
Discussion
CRC is major public health problem globally
(18). Metastatic CRC develops on
average within 3 years in ~50% of cases and lungs are a site of
distant relapse (19,20). Patients affected by metastatic CRC
experience untimely diagnosis and poor prognosis. Therefore, it is
necessary to determine the molecular mechanism underlying CRC with
lung metastasis. In the present study, lncRNA and mRNA were
differentially expressed in CRC tissues from patients with CRC + m
compared with patients with CRC - m. GO and KEGG pathway analyses
were conducted to predict the potential function and associated
regulatory mechanism of mRNA in CRC tissues from patients with
pulmonary metastasis.
A total of 7,632 lncRNA (3,574 upregulated and 4,058
downregulated) and 6,185 mRNA (3,394 upregulated and 2,791
downregulated) were differentially expressed in CRC tissues from
patients with CRC + m compared with samples from patient with CRC -
m. A previous study indicated that 2,636 lncRNA were differentially
expressed in the CRC tissues from patients with liver metastasis,
including 1,600 upregulated and 1,036 downregulated, >2-fold
compared with the CRC tissues without metastasis, and 1,584 mRNA
(548 upregulated and 1,036 downregulated) were differentially
expressed (8). It has also been
reported that, in tumor and metastatic lymph node tissues, 53
lncRNA exhibited upregulated transcription levels and 337 lncRNA
exhibited downregulated transcription, whereas 102 and 406 mRNA
exhibited up- and downregulated expression levels, respectively
(21). A total of 762 lncRNA (390
up- and 372 downregulated) were differentially expressed between
CRC tissues and healthy tissues (22). To the best of our knowledge, the
present study is the first to report differentially expressed
lncRNA and mRNA in CRC tissues of patients with lung
metastasis.
Using GO analysis in the present study, it was
determined that the upregulated mRNA were associated with cell
division (BPs), protein kinase B binding (MFs) and intracellular
parts (CCs), while the downregulated mRNA were associated with cell
adhesion, PDGF binding and membrane parts. It has been previously
reported that upregulated differentially expressed genes were
significantly enriched in cell division-associated processes in CRC
tissues (23).
SH2-domain-containing 5 inositol phosphatase has been hypothesized
to serve a tumor initiating role by enhancing cell migration and
invasion through protein kinase B activation in colonic epithelial
cells (24). In silico
analyses of deregulated proteins in the secretome of metastatic CRC
cells demonstrated an increased abundance of proteins involved in
cell adhesion, and host-related carinoembryonic antigen cell
adhesion molecule 1 was hypothesized to promote metastasis of CRC
(25). PDGF was highly expressed
in early and late stages of primary CRCs and was elevated in
platelets of patients with CRC (26,27).
Inhibition of mechanistic target of rapamycin kinase and PDGF
prevented liver metastasis of CRC by regulating the tumor
microenvironment (28).
In the present study, KEGG pathway analysis
demonstrated that the Wnt signaling pathway was upregulated while
the PI3K-Akt signaling pathway was downregulated in CRC + m tissues
compared with the CRC - m group. A previous study demonstrated that
90% of CRC cases occur due to the activation of the Wnt signaling
pathway (29). The Wnt signaling
pathway serves a role in regulating embryonic development,
including body axis patterning, cell fate specification and cell
migration (30). For example,
fatty acid synthase knockdown attenuated the Wnt signaling pathway
by downregulating distinctive genes, including Wnt5a, Wnt5b and
Frizzled-2, which at least partly contributed to the decrease in
metastasis of CRC (31). Hcrcn81
has been demonstrated to induce initiation and progression of
carcinogenesis through regulation of the Wnt signaling pathway and
serve a role in the carcinogenesis of CRC (32). It has been reported that
Ras-related protein Rab-11A-family interacting proteins promoted
migration and invasion of CRC cells through the upregulation of
expression of matrix metallopeptidase 7 by activation of the
PI3K/Akt signaling pathway (33).
Ribonuclease inhibitor had been demonstrated to suppress
proliferation and metastasis in CRC cells through inhibition of the
PI3K/Akt pathway (34).
Odontogenic ameloblast-associated protein suppresses human CRC by
inactivating PI3K/Akt signaling (35). MicroRNA (miR)-92a is involved in
lymph node metastasis in patients with CRC through the phosphatase
and tensin homolog-regulated PI3K/Akt signaling pathway (36). miR-302a overexpression has been
demonstrated to suppress proliferation and invasion of CRC cells by
reducing the expression of associated proteins through the
inhibition of the mitogen-activated protein kinase and PI3K/Akt
signaling pathways (37).
Therefore, the above studies combined with the results of the
present study suggest that metastatic CRC is due to the interactive
effects of multiple lncRNA engaged in modulation of a multi-gene
system.
In conclusion, the present study identified a series
of differentially expressed lncRNA and mRNA in patients with CRC
with or without lung metastasis. The potential roles of lncRNA and
mRNAs were predicted by bioinformatics analyses (38). The differentially expressed lncRNA
identified in the present study may provide novel targets for
elucidation of the molecular mechanisms underlying the development
of metastatic CRC and for the diagnosis and prognosis of metastatic
CRC.
Acknowledgements
The present study was supported by the fund of
Yunling Scholar, the joint funds of Yannan Provincial Science and
Technology Department and Kunming Medical University (grant nos.
2017FE467-038 and −130), Project of the Department of Health in
Yunnan Province (grant nos. 2016NS002 and 2016NS003), and The
General Joint Project of Yunnan Provincial Science and Technology
Department and Kunming Medical University (grant no.
2015FB024).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah SA, Haddad R, Al-Sukhni W, Kim RD,
Greig PD, Grant DR, Taylor BR, Langer B, Gallinger S and Wei AC:
Surgical resection of hepatic and pulmonary metastases from
colorectal carcinoma. J Am Coll Surg. 202:468–475. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin PC, Lin JK, Lin CC, Wang HS, Yang SH,
Jiang JK, Lan YT, Lin TC, Li AF, Chen WS and Chang SC: Carbohydrate
antigen 19-9 is a valuable prognostic factor in colorectal cancer
patients with normal levels of carcinoembryonic antigen and may
help predict lung metastasis. Int J Colorectal Dis. 27:1333–1338.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cejas P, López-Gómez M, Aguayo C, Madero
R, de Castro Carpeño J, Belda-Iniesta C, Barriuso J, Moreno Garcia
V, Larrauri J, López R, et al: KRAS mutations in primary colorectal
cancer tumors and related metastases: A potential role in
prediction of lung metastasis. PLoS One. 4:e81992009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitry E, Guiu B, Cosconea S, Jooste V,
Faivre J and Bouvier AM: Epidemiology, management and prognosis of
colorectal cancer with lung metastases: A 30-year population-based
study. Gut. 59:1383–1388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warwick R and Page R: Resection of
pulmonary metastases from colorectal carcinoma. Eur J Surg Oncol.
33 Suppl 2:S59–S63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee WS, Yun SH, Chun HK, Lee WY and Yun H:
Clinical usefulness of chest radiography in detection of pulmonary
metastases after curative resection for colorectal cancer. World J
Surg. 31:1502–1506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen D, Sun Q, Cheng X, Zhang L, Song W,
Zhou D, Lin J and Wang W: Genome-wide analysis of long noncoding
RNA (lncRNA) expression in colorectal cancer tissues from patients
with liver metastasis. Cancer Med. 5:1629–1639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
ENCODE Project Consortium, . An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheetham SW, Gruhl F, Mattick JS and
Dinger ME: Long noncoding RNAs and the genetics of cancer. Br J
Cancer. 108:2419–2425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arase M, Horiguchi K, Ehata S, Morikawa M,
Tsutsumi S, Aburatani H, Miyazono K and Koinuma D: Transforming
growth factor-β-induced lncRNA-Smad7 inhibits apoptosis of mouse
breast cancer JygMC(A) cells. Cancer Sci. 105:974–982. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang K, Long B, Zhou LY, Liu F, Zhou QY,
Liu CY, Fan YY and Li PF: CARL lncRNA inhibits anoxia-induced
mitochondrial fission and apoptosis in cardiomyocytes by impairing
miR-539-dependent PHB2 downregulation. Nat Commun.
5:35962014.PubMed/NCBI
|
|
15
|
Yang Y, Li H, Hou S, Hu B, Liu J and Wang
J: The noncoding RNA expression profile and the effect of lncRNA
AK126698 on cisplatin resistance in non-small-cell lung cancer
cell. PLoS One. 8:e653092013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye LC, Zhu X, Qiu JJ, Xu J and Wei Y:
Involvement of long non-coding RNA in colorectal cancer: From
benchtop to bedside (Review). Oncol Lett. 9:1039–1045. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sargent DJ, Patiyil S, Yothers G, Haller
DG, Gray R, Benedetti J, Buyse M, Labianca R, Seitz JF, O'Callaghan
CJ, et al: End points for colon cancer adjuvant trials:
Observations and recommendations based on individual patient data
from 20,898 patients enrolled onto 18 randomized trials from the
ACCENT group. J Clin Oncol. 25:4569–4574. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guerrera F, Mossetti C, Ceccarelli M,
Bruna MC, Bora G, Olivetti S, Lausi PO, Solidoro P, Ciccone G,
Ruffini E, et al: Surgery of colorectal cancer lung metastases:
Analysis of survival, recurrence and re-surgery. J Thorac Dis.
8:1764–1771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang P, Xu ZP, Chen T and He ZY: Long
noncoding RNA expression profile analysis of colorectal cancer and
metastatic lymph node based on microarray data. Onco Targets Ther.
9:2465–2478. 2016.PubMed/NCBI
|
|
22
|
Xue Y, Ma G, Gu D, Zhu L, Hua Q, Du M, Chu
H, Tong N, Chen J, Zhang Z and Wang M: Genome-wide analysis of long
noncoding RNA signature in human colorectal cancer. Gene.
556:227–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang B, Li C and Zhao J: Identification
of key pathways and genes in colorectal cancer using bioinformatics
analysis. Med Oncol. 33:1112016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoekstra E, Das AM, Willemsen M, Swets M,
Kuppen PJ, van der Woude CJ, Bruno MJ, Shah JP, Ten Hagen TL,
Chisholm JD, et al: Lipid phosphatase SHIP2 functions as oncogene
in colorectal cancer by regulating PKB activation. Oncotarget.
7:73525–73540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arabzadeh A, Chan C, Nouvion AL, Breton V,
Benlolo S, DeMarte L, Turbide C, Brodt P, Ferri L and Beauchemin N:
Host-related carcinoembryonic antigen cell adhesion molecule 1
promotes metastasis of colorectal cancer. Oncogene. 32:849–860.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moench R, Grimmig T, Kannen V, Tripathi S,
Faber M, Moll EM, Chandraker A, Lissner R, Germer CT, Waaga-Gasser
AM and Gasser M: Exclusive inhibition of PI3K/Akt/mTOR signaling is
not sufficient to prevent PDGF-mediated effects on glycolysis and
proliferation in colorectal cancer. Oncotarget. 7:68749–68767.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peterson JE, Zurakowski D, Italiano JE Jr,
Michel LV, Connors S, Oenick M, D'Amato RJ, Klement GL and Folkman
J: VEGF, PF4 and PDGF are elevated in platelets of colorectal
cancer patients. Angiogenesis. 15:265–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuge R, Kitadai Y, Shinagawa K, Onoyama M,
Tanaka S, Yasui W and Chayama K: mTOR and PDGF pathway blockade
inhibits liver metastasis of colorectal cancer by modulating the
tumor microenvironment. Am J Pathol. 185:399–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Subramaniyan B, Jagadeesan K, Ramakrishnan
S and Mathan G: Targeting the interaction of Aurora kinases and
SIRT1 mediated by Wnt signaling pathway in colorectal cancer: A
critical review. Biomed Pharmacother. 82:413–424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Huang D, Wang Z, Wu C, Zhang Z,
Wang D, Li Z, Zhu T, Yang S and Sun W: LMO2 attenuates tumor growth
by targeting the Wnt signaling pathway in breast and colorectal
cancer. Sci Rep. 6:360502016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Xi Q and Wu G: Fatty acid synthase
regulates invasion and metastasis of colorectal cancer via Wnt
signaling pathway. Cancer Med. 5:1599–1606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Jiang T, Shi L and He K: hcrcn81
promotes cell proliferation through Wnt signaling pathway in
colorectal cancer. Med Oncol. 33:32016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu CL, Wang JZ, Xia XP, Pan CW, Shao XX,
Xia SL, Yang SX and Zheng B: Rab11-FIP2 promotes colorectal cancer
migration and invasion by regulating PI3K/AKT/MMP7 signaling
pathway. Biochem Biophys Res Commun. 470:397–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang Y, Liu P, Tian Y, Xu Y, Ren F, Cui X
and Fan J: Overexpression of ribonuclease inhibitor defines good
prognosis and suppresses proliferation and metastasis in human
colorectal cancer cells via PI3K/AKT pathway. Clin Transl Oncol.
17:306–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu M, Mu Y, Qi Y, Qin S, Qiu Y, Cui R and
Zhong M: Odontogenic ameloblast-associated protein (ODAM) inhibits
human colorectal cancer growth by promoting PTEN elevation and
inactivating PI3K/AKT signaling. Biomed Pharmacother. 84:601–607.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ke TW, Wei PL, Yeh KT, Chen WT and Cheng
YW: MiR-92a promotes cell metastasis of colorectal cancer through
PTEN-mediated PI3K/AKT pathway. Ann Surg Oncol. 22:2649–2655. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wei ZJ, Tao ML, Zhang W, Han GD, Zhu ZC,
Miao ZG, Li JY and Qiao ZB: Up-regulation of microRNA-302a
inhibited the proliferation and invasion of colorectal cancer cells
by regulation of the MAPK and PI3K/Akt signaling pathways. Int J
Clin Exp Pathol. 8:4481–4491. 2015.PubMed/NCBI
|
|
38
|
Huang M, Zhong Z, Lv M, Shu J, Tian Q and
Chen J: Comprehensive analysis of differentially expressed profiles
of lncRNAs and circRNAs with associated co-expression and ceRNA
networks in bladder carcinoma. Oncotarget. 7:47186–47200.
2016.PubMed/NCBI
|