Introduction
The thyroid hormone triiodothyronine (T3)
and its prohormone, thyroxine (T4) are produced by the
thyroid gland and serve an important role in metabolism in the
body, including in the central nervous system (1,2). The
hippocampus has a high density of thyroid hormone receptors and
adult onset of hypothyroidism causes damage to morphology and
function in the hippocampus (3),
suggesting that it is an important target for thyroid hormones in
the brain (4). Hypothyroidism also
reduces the proliferation of cells in the hippocampal dentate gyrus
in the adult brain (5,6). Adult-onset hypothyroidism in rats
facilitates the hyper-phosphorylation of tau protein and reduces
the synaptic plasticity marker proteins, including neurogranin,
extracellular signal-regulated kinases, glycogen synthase kinase 3β
and phosphorylated cAMP response element-binding protein (7).
Cyclooxygenase (COX) is responsible for the
formation of prostanoids, including thromboxane and prostaglandins,
and provides anti-inflammatory functions. COX-1 is produced
constitutively, whereas COX-2 production is inducible following
insult, including inflammation (8,9).
COX-2 has been investigated since it is also constitutively
expressed in the brain (10,11),
particularly in the cerebral cortex and hippocampus (12). Of particular interest, the
expression of COX-2 in the hippocampus regulates adult hippocampal
neurogenesis in the dentate gyrus in normal and brain damage
conditions. However, there are no reports that have determined the
correlation between hypothyroidism and COX-2 expression in the
hippocampus, although hypothyroidism affects hippocampal function
in rats. The present study therefore investigated the effects of
hypothyroidism on the levels of COX-2 and certain pro-inflammatory
cytokines including interleukin (IL)-1β, IL-6 and tumor necrosis
factor-α (TNF-α) in the hippocampus to elucidate the roles of
COX-2-associated neuro-inflammation and hippocampal neurogenesis in
hypothyroidism model rats.
Materials and methods
Experimental animals
A total of 20 male Sprague-Dawley rats (6-week-old;
body weight, 180–200 g) were purchased from Orient Bio, Inc.
(Seongnam, Korea). They were housed under standard conditions with
adequate temperature (22°C) and humidity (60%) control, a 12-h
light: 12-h dark cycle and free access to food and water. The
handling and care of the animals conformed to the guidelines
established to comply with current international laws and policies
stated in the NIH Guide for the Care and Use of Laboratory Animals,
NIH Publication, 1996 (13) and
were approved by the Institutional Animal Care and Use Committee of
Seoul National University (Seoul, Korea). All the experiments were
conducted with an effort to minimize the number of animals used and
the suffering caused by the procedures employed in the present
study.
Experimental design
To investigate the effects of
2-mercapto-1-methyl-imidazole (methimazole)-induced hypothyroidism
on COX-2 expression in the rat hippocampus, rats were randomly
divided into euthyroid and hypothyroid groups (n=10 in each group).
At 7 weeks of age, 0.03% methimazole (~12 mg/day; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was administered to the hypothyroid
group in drinking water for 5 weeks to prevent thyroid hormone
synthesis by inhibited coupling and iodination (14).
Serum levels of thyroid-stimulating
hormones (TSH) and thyroid hormones
To confirm the hypothyroid state, blood specimens at
morning (9:00-11:00 a.m.) were drawn from the vehicle and
hypothyroid groups following sacrifice at the age of 12 weeks for
analysis. The serum circulating TSH, free T3 and
T4 levels were measured to determine thyroid function in
these rats using Accu Bind Vast enzyme-linked immunosorbent assay
(ELISA) kit (cat. no. 8025-300D; Monobind, Inc., Lake Forest, CA,
USA).
Tissue processing for histology
For histology, the animals (n=5 in each group) were
anesthetized with 1 g/kg urethane (Sigma-Aldrich; Merck KGaA) at
various time points following surgery. Animals were perfused
transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4),
followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were
removed and post-fixed in the same fixative for 12 h prior to
undergoing cryoprotection via overnight storage in 30% sucrose.
Serial coronal brain sections (30 µm) were produced using a
cryostat (Leica Microsystems GmbH, Wetzlar, Germany) and collected
into 6-well plates containing PBS. To ensure that the histochemical
and immunohistochemical data were comparable between groups,
sections were carefully processed under parallel conditions. Tissue
sections located 120 µm apart from each other were selected from an
area between 3.00 mm and 4.08 mm posterior to the bregma, as
defined by a rat brain atlas (15).
Immunohistochemistry for COX-2, Ki67
and doublecortin (DCX)
A total of four sections from tissue sections
located 120 µm apart from each other were sequentially incubated
with 0.3% hydrogen peroxide (H2O2) in PBS for
30 min and 10% normal horse serum (cat. no. S-2000; Vector
Laboratories, Inc., Burlingame, CA, USA) in 0.05 M PBS for 30 min.
Sections were then incubated with a rabbit anti-COX-2 antibody
(cat. no. 160106; 1:200; Cayman, Ann Arbor, MI, USA), rabbit
anti-Ki67 antibody (cat. no. ab15580; 1:1,000; Abcam, Cambridge,
UK), or goat anti-DCX antibody (cat. no. sc-8066; 1:50; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at room
temperature. Sections were then incubated for 2 h at 25°C with
biotinylated horse anti-rabbit IgG (cat. no. BA-1100; 1:200; Vector
Laboratories, Inc.) or horse anti-goat IgG (cat. no. BA-9500;
1:200; Vector Laboratories, Inc.), followed by a
streptavidin-peroxidase complex (cat. no. SA-5004; 1:200; Vector
Laboratories, Inc.). Immunostaining was visualized by reaction with
diaminobenzidine in 0.1 M Tris-HCl buffer (pH 7.2). Sections were
dehydrated and mounted on gelatin-coated slides in Canada balsam
(Kanto Chemical Co., Ltd., Tokyo, Japan).
To quantify the COX-2 immunoreactivity, analysis of
the dentate gyrus and hippocampal CA3 region was performed using an
image analysis system (Optimas version 6.5; Cyber Metrics
Corporation, Scottsdale, AZ, USA) and ImageJ software version 1.5
(National Institutes of Health, Bethesda, MD, USA). Digital images
of the mid-point of the dentate gyrus and hippocampal CA3 region
were captured with a BX51 light microscope (Olympus Corporation,
Tokyo, Japan) equipped with a digital camera (DP72; Olympus
Corporation) connected to a computer monitor. Images were
calibrated into an array of 512×512 pixels corresponding to a
tissue area of 1,200×900 µm (primary magnification, ×100). Each
pixel resolution was 256 gray levels and the intensity of COX-2
immunoreactivity was evaluated by relative optical density (ROD),
which was obtained following transformation of the mean gray level
using the formula: ROD = log (256/mean gray level). ROD of
background staining was determined in unlabeled portions of the
sections using Photoshop CC 2015 software (Adobe Systems, Inc., San
Jose, CA, USA) and this value was subtracted to correct for
nonspecific staining, using ImageJ. Data are expressed as a
percentage of the euthyroid group values (set to 100%).
Ki67- and DCX-positive cell counts were performed
for each section of the dentate gyrus using an image analysis
system equipped with a computer-based CCD camera (Optimas version
6.5; Cyber Metrics Corporation, Scottsdale, AZ, USA). Cell counts
from all the sections of all the rats were averaged.
ELISA for IL-1β, IL-6 and TNF-α
To confirm changes in TNF-α, IL-1β and IL-6 level in
the hippocampus, animals in the euthyroid and hypothyroid groups
(n=5 per group) were sacrificed and used for ELISA analysis.
Following sacrifice and removal of the hippocampus, the hippocampal
tissues were homogenized in ice-cold 50 mM sodium phosphate buffer
(pH 7.4) containing 0.1 mM EDTA using a glass-Teflon homogenizer
(Heidolph Silent Crusher M; Heidolph Instruments GmbH, Schwabach,
Germany). The supernatant was separated by centrifugation at 1,000
× g for 20 min at 4°C. IL-1β, IL-6 and TNF-α were measured in the
supernatant of homogenized hippocampal tissue by using ELISA kits
(cat. nos. MBS175941, MBS355410 and MBS2502004 respectively;
BioSource International, Inc., Camarillo, CA, USA). The procedures
were carried out according to the manufacturer's protocols. IL-1β,
IL-6 and TNF-α were determined from a standard curve and their
levels were expressed in ng/mg total protein.
Statistical analysis
The data are presented as mean ± standard error of
the mean. Differences among the means were statistically analyzed
by a Student's t-test, using GraphPad Prism version 5.01 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of hypothyroidism on
phenotypes in blood and organs
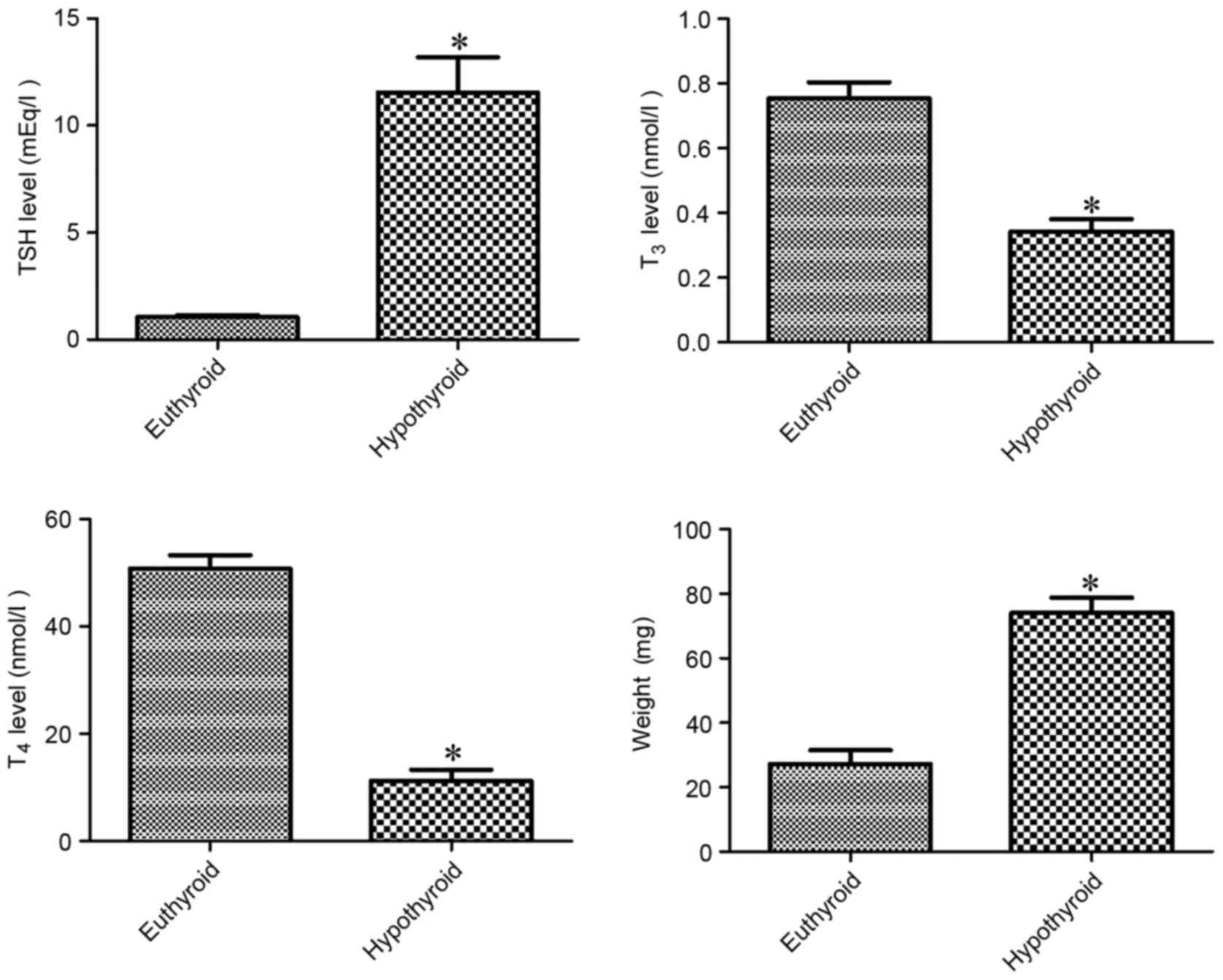
At 12 weeks of age, hypothyroidism significantly
decreased serum T3 and T4 levels by 45.4 and
22.1%, respectively, compared with vehicle-treated group. By
contrast, TSH level was significantly higher (11-fold) in the
hypothyroid rats compared with the vehicle-treated group. The
weight of the thyroid gland was significantly higher (2.72-fold) in
the hypothyroid rats compared with the vehicle-treated group
(Fig. 1).
Effects of hypothyroidism on COX-2
expression in the hippocampus
At 12 weeks of age, COX-2 immunoreactivity was
detectable in the granule cell layer, polymorphic layer and CA4
region of the dentate gyrus (Fig.
2A) in addition to the stratum pyramidale of the hippocampal
CA3 region (Fig. 2B). COX-2
immunoreactivity was significantly higher in these regions in the
hypothyroid rats compared with the euthyroid rats (Fig. 2C).
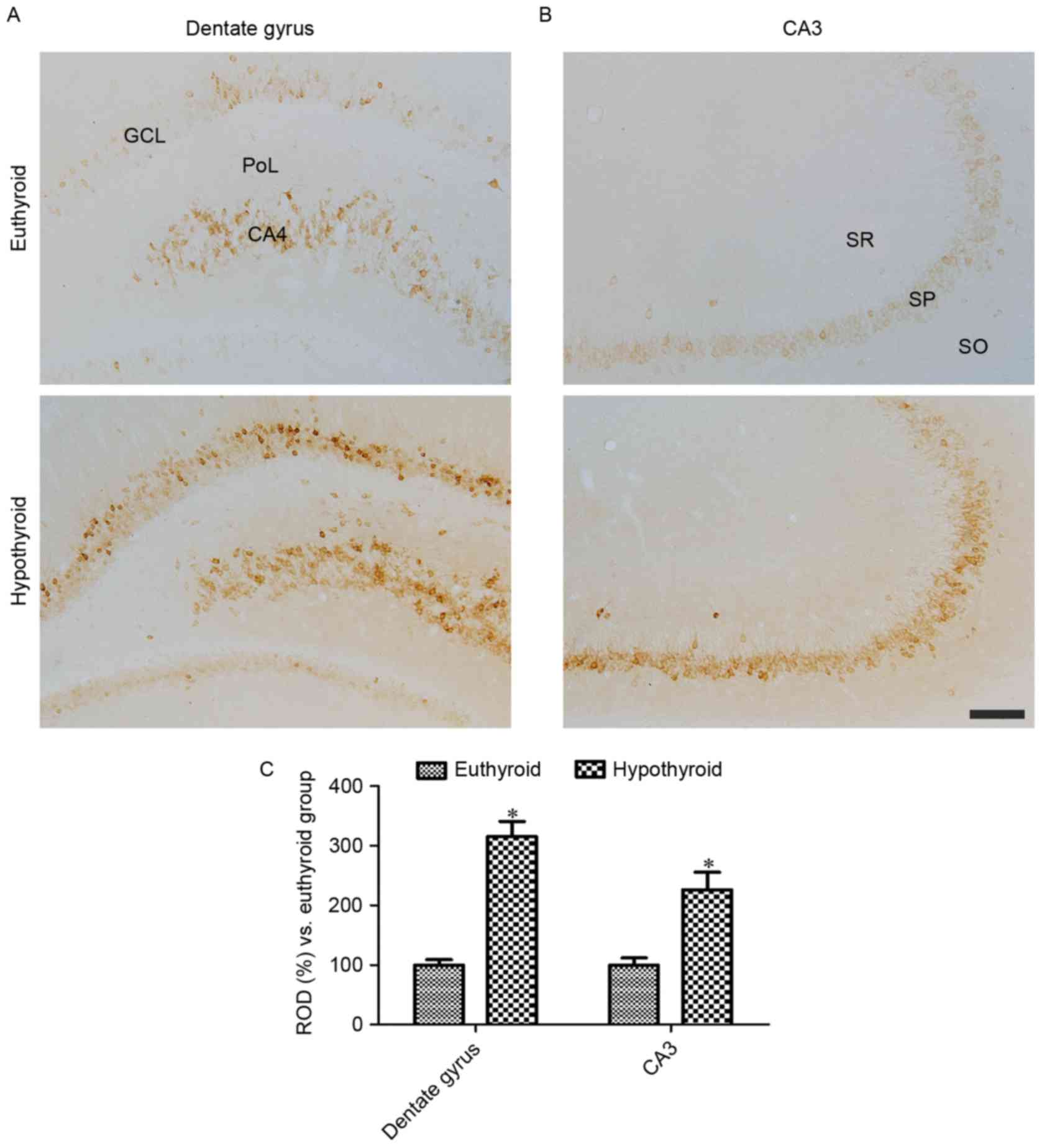 | Figure 2.COX-2 immunoreactivity in the (A)
dentate gyrus and (B) CA3 region of euthyroid and hypothyroid rats.
COX-2 immunoreactivity is identified in the GCL, PoL and CA4 region
of dentate gyrus in addition to in the SP of the CA3 region. COX-2
immunoreactivity is dense in the hypothyroid rats compared with the
euthyroid rats. Scale bar, 100 µm. (C) The ROD, expressed as a
percentage of the value in the euthyroid group of COX-2
immunoreactivity in the dentate gyrus and hippocampal CA3 region
per section. Differences between the means were analyzed using
Student's t-test (n=5 per group; *P<0.05 vs. euthyriod). The
bars represent the mean ± standard error of the mean. GCL, granule
cell layer; PoL, polymorphic layer; SP, SO, stratum oriens; SR,
stratum radiatum; SP, stratum pyramidale; ROD, relative optical
density. |
Effects of hypothyroidism on
pro-inflammatory cytokines
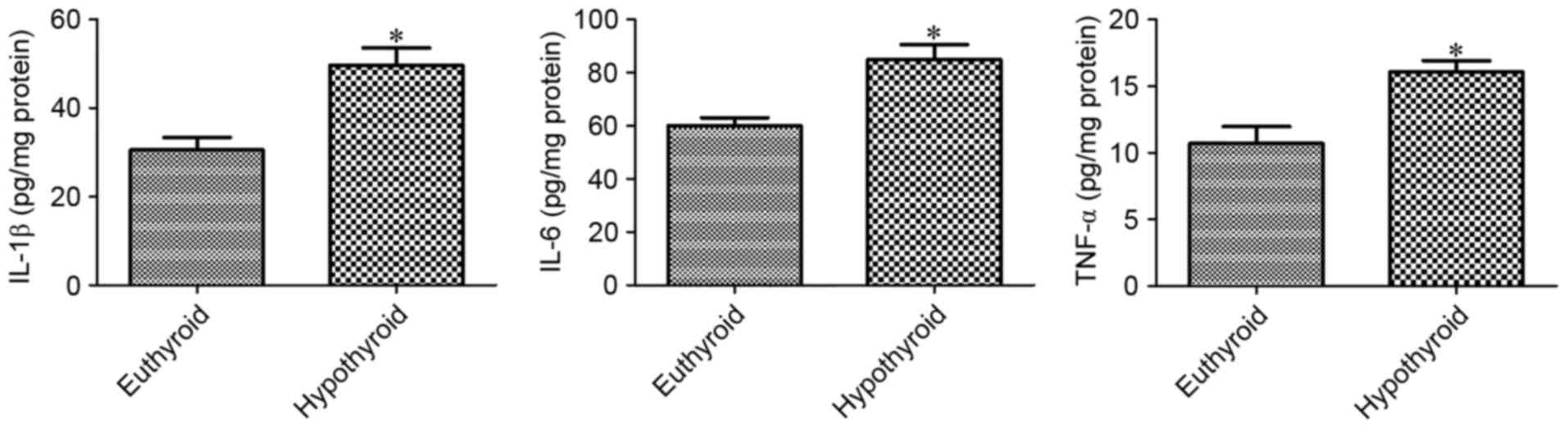
At 12 weeks of age, hypothyroidism significantly
increased the levels of IL-1β, IL-6 and TNF-α, as detected by
ELISA, compared with the euthyroid group (Fig. 3).
Effects of hypothyroidism on cell
proliferation
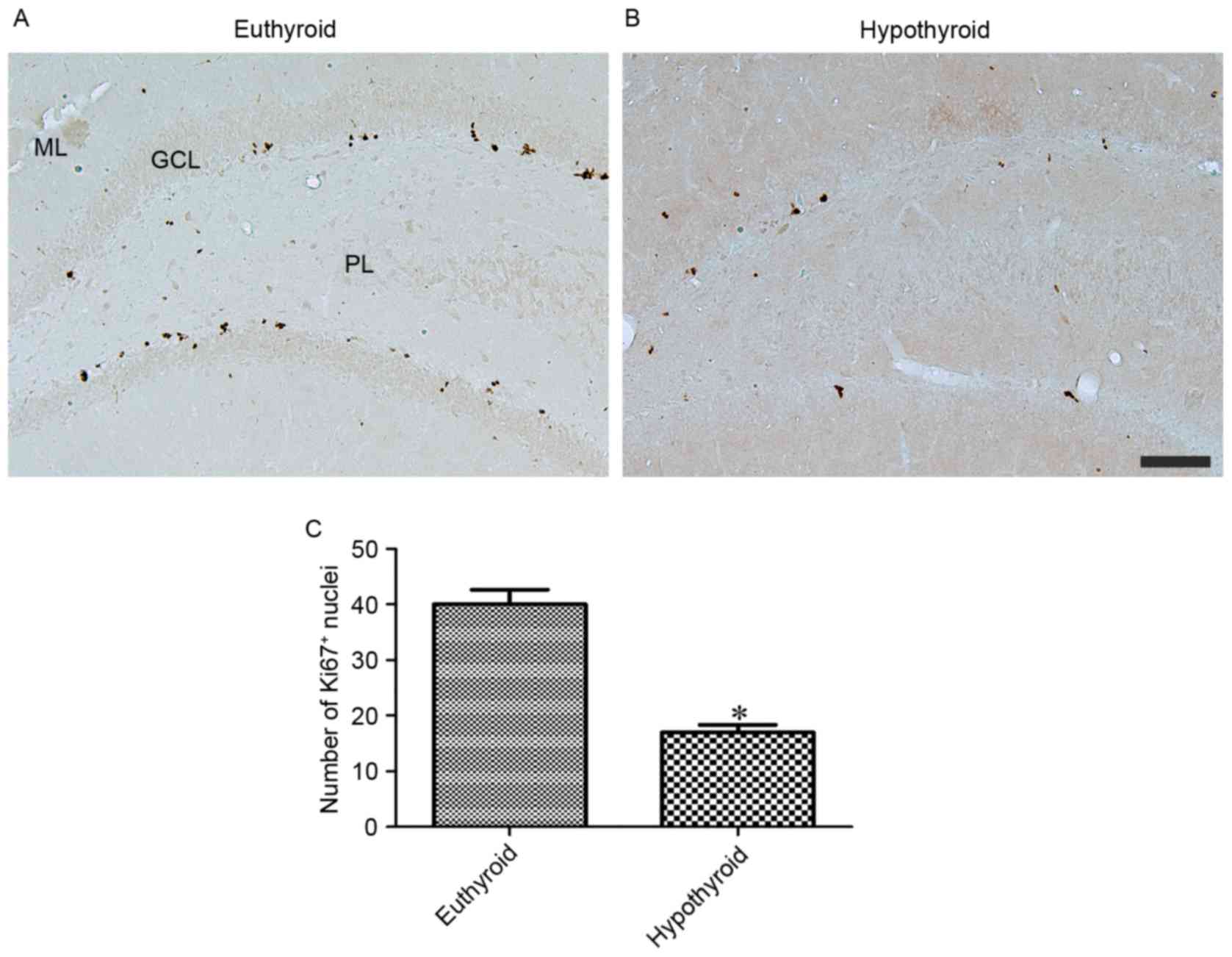
At 12 weeks of age, Ki67 immunoreactivity was
observed in the nuclei located in the subgranular zone of the
dentate gyrus (Fig. 4A and B).
Hypothyroidism significantly reduced the number of Ki67-positive
nuclei in the dentate gyrus compared with the euthyroid group
(Fig. 4C).
Effects of hypothyroidism on
neuroblast differentiation
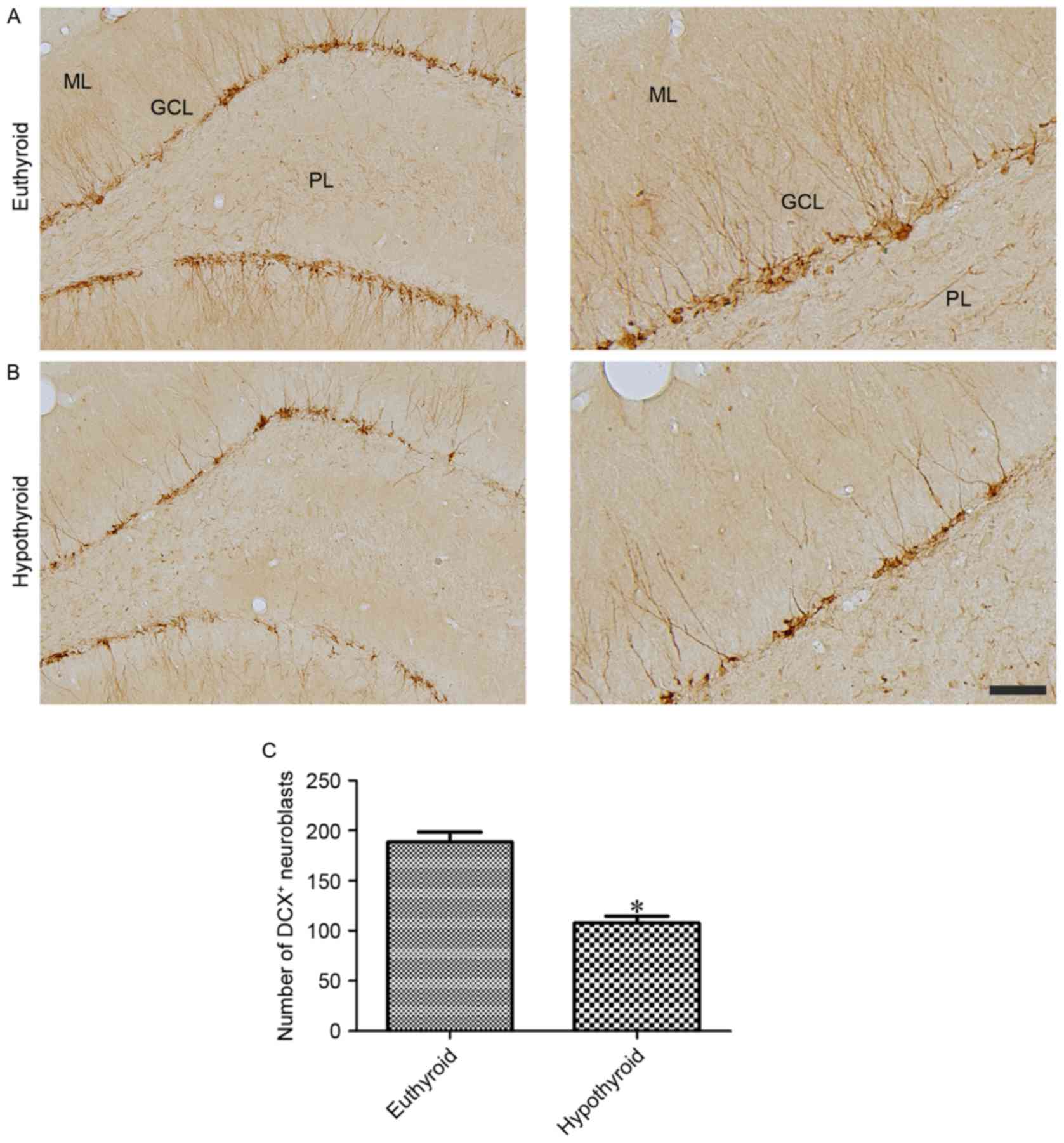
DCX-immunoreactive neuroblasts were observed in the
cytoplasm and dendrites located in the subgranular zone of the
dentate gyrus and extending to the molecular layer of the dentate
gyrus, respectively (Fig. 5A and
B). Hypothyroidism significantly reduced the number of
DCX-immunoreactive neuroblasts and the complexity of dendrites
compared with the euthyroid group (Fig. 5C).
Discussion
Adult hypothyroidism increases amyloid β precursor
protein (APP) gene expression and facilitates the amyloidogenic
pathway of APP processing in the rat hippocampus (16,17).
In Alzheimer patients, localized hypothyroidism has been identified
in the hippocampus (18,19). In the present study, hypothyroidism
was induced by methimazole and the hypothyroid state was assessed
by serum TSH, T3 and T4 levels in addition to
weight of the thyroid gland. Methimazole treatment significantly
reduced serum T3 and T4 levels, while serum
TSH level in addition to thyroid gland weight were significantly
increased.
The changes in COX-2 levels in the hippocampus was
investigated as COX-2 has dual functions in the synaptic plasticity
and inflammation in the hippocampus. In certain types of brain
injury, COX-2 serves multiple roles in the regulation of adult
hippocampal neurogenesis in the dentate gyrus (20–22).
Knockdown of COX-2 significantly reduces the number of
proliferating cells and differentiated neuroblasts in the dentate
gyrus compared with wild-type littermates (21,22).
In a previous study (23), we
demonstrated that the blocking constitutive COX-2 in the
hippocampus by celecoxib, a COX-2 inhibitor, significantly
decreased cell proliferation and neuroblast differentiation in the
dentate gyrus. Hypothermia protects neurons from ischemic damage
and produces a dramatic increase in COX-2 immunoreactivity in the
granule cells of the dentate gyrus within 4 h after ischemia
compared with normothermic animals (24). However, the present study observed
a significant reduction in cell proliferation and neuroblast
differentiation in the dentate gyrus. This result is supported by
previous studies (5,6,25)
that indicate that postnatal or adult onset hypothyroidism
decreases neurogenesis in the dentate gyrus. Thus, it is
hypothesized that the upregulation of COX-2 may be closely
associated with an increase in pro-inflammatory cytokines,
including IL-1β, IL-6 and TNF-α in the hippocampal homogenates.
The present study noted a significant increase in
IL-1β expression and IL-6 and TNF-α levels in the hippocampal
homogenates. This result is consistent with that of a previous
study (7), which demonstrated that
hypothyroidism induced by propylthiouracil for 5 weeks
significantly increased the mRNA levels of pro-inflammatory
cytokines, including IL-1β, IL-6 and TNF-α in the hippocampus.
Patients with hypothyroidism also present a significantly higher
level of the inflammatory marker C-reactive protein (26). In addition, acute inflammation by
lipopolysaccharide significantly increases the mRNA levels of COX-2
and reduces neurogenesis in the dentate gyrus (27). In a previous study (23), we demonstrated that treadmill
exercise significantly increased COX-2 expression in the dentate
gyrus of control and type 2 diabetic rats. In addition, treadmill
exercise in COX-2 knockout mice significantly increased
neurogenesis in the dentate gyrus (28). With colleagues, we have also
demonstrated that COX-2 immunoreactivity was significantly
increased in the hippocampus 3 weeks after streptozotocin treatment
(29), while cell proliferation
and neuroblast differentiation were significantly decreased at 3
weeks after streptozotocin treatment (30). The induction of COX-2 subsequently
increases the synthesis of prostaglandin E2
(PGE2). The administration of PGE2analogue
increases the cell proliferation and differentiated neuroblasts in
the subgranular zone (31). In
addition, mRNA levels of G-protein coupled E-prostanoid receptors
are highly expressed during neurogenesis (32). However, previous studies (33–35)
have demonstrated that neuro-inflammation reduces neurogenic
deficits. In addition, IL-6 suppresses neurogenesis and reduces
hippocampal volume, skewing neural stem cells toward gliogenesis
(36–38).
In conclusion, adult-onset hypothyroidism
significantly increases activation of the COX-2-mediated
inflammation pathway and reduces the cell proliferation and
neuroblast differentiation in the hippocampus. The present study
proposed that COX-2-mediated inflammation is important as a
candidate target for hippocampal impairment in the hypothyroidism.
Future studies on the effect on the brain using a selective drug
inhibitor or a genetic model of COX-2 are required for a better
understanding of the importance of COX-2 in the hypothyroid
condition.
Acknowledgements
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
NRF-2015R1D1A1A01059314) and partially supported by the Research
Institute for Veterinary Science, Seoul National University.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chan S and Kilby MD: Thyroid hormone and
central nervous system development. J Endocrinol. 165:1–8. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warner A and Mittag J: Thyroid hormone and
the central control of homeostasis. J Mol Endocrinol. 49:R29–R35.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koromilas C, Liapi C, Schulpis KH,
Kalafatakis K, Zarros A and Tsakiris S: Structural and functional
alterations in the hippocampus due to hypothyroidism. Metab Brain
Dis. 25:339–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Jong FJ, den Heijer T, Visser TJ, de
Rijke YB, Drexhage HA, Hofman A and Breteler MM: Thyroid hormones,
dementia, and atrophy of the medial temporal lobe. J Clin
Endocrinol Metab. 91:2569–2573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambrogini P, Cuppini R, Ferri P, Mancini
C, Ciaroni S, Voci A, Gerdoni E and Gallo G: Thyroid hormones
affect neurogenesis in the dentate gyrus of adult rat.
Neuroendocrinology. 81:244–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Desouza LA, Ladiwala U, Daniel SM, Agashe
S, Vaidya RA and Vaidya VA: Thyroid hormone regulates hippocampal
neurogenesis in the adult rat brain. Mol Cell Neurosci. 29:414–426.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaalal A, Poirier R, Blum D, Gillet B, Le
Blanc P, Basquin M, Buée L, Laroche S and Enderlin V: PTU-induced
hypothyroidism in rats leads to several early neuropathological
signs of Alzheimer's disease in the hippocampus and spatial memory
impairments. Hippocampus. 24:1381–1393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao C, Matsumura K, Yamagata K and
Watanabe Y: Induction by lipopolysaccharide of cyclooxygenase-2
mRNA in rat brain; its possible role in the febrile response. Brain
Res. 697:187–196. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Graham SH and Hickey RW: Cyclooxygenases
in central nervous system diseases: A special role for
cyclooxygenase 2 in neuronal cell death. Arch Neurol. 60:628–630.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dubois RN, Abramson SB, Crofford L, Gupta
RA, Simon LS, Van De Putte LB and Lipsky PE: Cyclooxygenase in
biology and disease. FASEB J. 12:1063–1073. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamagata K, Andreasson KI, Kaufmann WE,
Barnes CA and Worley PF: Expression of a mitogen-inducible
cyclooxygenase in brain neurons: Regulation by synaptic activity
and glucocorticoids. Neuron. 11:371–386. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. National Acadamies Press;
Washington, DC: 1996
|
|
14
|
Cooper DS: Antithyroid drugs. N Engl J
Med. 311:1353–1362. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. Elsevier Academic Press; Cambridge, MA:
2007
|
|
16
|
Ghenimi N, Alfos S, Redonnet A, Higueret
P, Pallet V and Enderlin V: Adult-onset hypothyroidism induces the
amyloidogenic pathway of amyloid precursor protein processing in
the rat hippocampus. J Neuroendocrinol. 22:951–959. 2010.PubMed/NCBI
|
|
17
|
O'Barr SA, Oh JS, Ma C, Brent GA and
Schultz JJ: Thyroid hormone regulates endogenous amyloid-beta
precursor protein gene expression and processing in both in vitro
and in vivo models. Thyroid. 16:1207–1213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo L and Stopa EG: Thyrotropin releasing
hormone inhibits tau phosphorylation by dual signaling pathways in
hippocampal neurons. J Alzheimers Dis. 6:527–536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sampaolo S, Campos-Barros A, Mazziotti G,
Carlomagno S, Sannino V, Amato G, Carella C and Di Iorio G:
Increased cerebrospinal fluid levels of 3,3′,5′-triiodothyronine in
patients with Alzheimer's disease. J Clin Endocrinol Metab.
90:198–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumihashi K, Uchida K, Miyazaki H,
Kobayashi J, Tsushima T and Machida T: Acetylsalicylic acid reduces
ischemia-induced proliferation of dentate cells in gerbils.
Neuroreport. 12:915–917. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nam SM, Kim JW, Yoo DY, Choi JH, Kim W,
Jung HY, Won MH, Hwang IK, Seong JK and Yoon YS: Comparison of
pharmacological and genetic inhibition of cyclooxygenase-2: Effects
on adult neurogenesis in the hippocampal dentate gyrus. J Vet Sci.
16:245–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sasaki T, Kitagawa K, Sugiura S,
Omura-Matsuoka E, Tanaka S, Yagita Y, Okano H, Matsumoto M and Hori
M: Implication of cyclooxygenase-2 on enhanced proliferation of
neural progenitor cells in the adult mouse hippocampus after
ischemia. J Neurosci Res. 72:461–471. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang IK, Yi SS, Yoo KY, Park OK, Yan B,
Kim IY, Kim YN, Song W, Moon SM, Won MH, et al: Effects of
treadmill exercise on cyclooxygenase-2 in the hippocampus in type 2
diabetic rats: Correlation with the neuroblasts. Brain Res.
1341:84–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamashita A, Kunimatsu T, Yamamoto T and
Yoshida K: Hypothermic, but not normothermic, ischemia causes a
drastic increase in cyclooxygenase-2 immunoreactive granule cells
in rat dentate gyrus after 4 hours of ischemic reperfusion. Arch
Histol Cytol. 70:197–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Blomgren K, Kuhn HG and
Cooper-Kuhn CM: Effects of postnatal thyroid hormone deficiency on
neurogenesis in the juvenile and adult rat. Neurobiol Dis.
34:366–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dizdarevic-Bostandic A, Burekovic A,
Velija-Asimi Z and Godinjak A: Inflammatory markers in patients
with hypothyroidism and diabetes mellitus type 1. Med Arch.
67:160–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Y, Matsuwaki T, Yamanouchi K and
Nishihara M: Glucocorticoids suppress the protective effect of
cyclooxygenase-2-related signaling on hippocampal neurogenesis
under acute immune stress. Mol Neurobiol. 54:1953–1966. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nam SM, Kim JW, Yoo DY, Choi JH, Kim W,
Jung HY, Won MH, Hwang IK, Seong JK and Yoon YS: Effects of
treadmill exercise on neural stem cells, cell proliferation, and
neuroblast differentiation in the subgranular zone of the dentate
gyrus in cyclooxygenase-2 knockout mice. Neurochem Res.
38:2559–2569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nam SM, Yi SS, Yoo DY, Kim W, Choi JH,
Hwang IK, Seong JK and Yoon YS: Changes in cyclooxygenase-2
immunoreactivity in the hippocampus in a model of
streptozotocin-induced type 1 diabetic rats. J Vet Med Sci.
74:977–982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi JH, Hwang IK, Yi SS, Yoo KY, Lee CH,
Shin HC, Yoon YS and Won MH: Effects of streptozotocin-induced type
1 diabetes on cell proliferation and neuronal differentiation in
the dentate gyrus; correlation with memory impairment. Korean J
Anat. 42:41–48. 2009.
|
|
31
|
Uchida K, Kumihashi K, Kurosawa S,
Kobayashi T, Itoi K and Machida T: Stimulatory effects of
prostaglandin E2 on neurogenesis in the dentate gyrus of the adult
rat. Zoolog Sci. 19:1211–1216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tamiji J and Crawford DA: Prostaglandin
E(2) and misoprostol induce neurite retraction in Neuro-2a cells.
Biochem Biophys Res Commun. 398:450–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ekdahl CT, Claasen JH, Bonde S, Kokaia Z
and Lindvall O: Inflammation is detrimental for neurogenesis in
adult brain. Proc Natl Acad Sci USA. 100:pp. 13632–13637. 2003;
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Monje ML, Toda H and Palmer TD:
Inflammatory blockade restores adult hippocampal neurogenesis.
Science. 302:1760–1765. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Zhang M, Kang X, Jiang C, Zhang H,
Wang P and Li J: Thrombin-induced microglial activation impairs
hippocampal neurogenesis and spatial memory ability in mice. Behav
Brain Funct. 11:302015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakanishi M, Niidome T, Matsuda S, Akaike
A, Kihara T and Sugimoto H: Microglia-derived interleukin-6 and
leukaemia inhibitory factor promote astrocytic differentiation of
neural stem/progenitor cells. Eur J Neurosci. 25:649–658. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balasubramaniam B, Carter DA, Mayer EJ and
Dick AD: Microglia derived IL-6 suppresses neurosphere generation
from adult human retinal cell suspensions. Exp Eye Res. 89:757–766.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng H, Sun L, Jia B, Lan X, Zhu B, Wu Y
and Zheng J: HIV-1-infected and immune-activated macrophages induce
astrocytic differentiation of human cortical neural progenitor
cells via the STAT3 pathway. PLoS One. 6:e194392011. View Article : Google Scholar : PubMed/NCBI
|