Introduction
The escape of tumor cells from the immune system is
important for the occurrence, development, recurrence, and
metastasis of lung cancer (1,2).
Tumor cells protect themselves from being recognized and attacked
by the immune system by modifying surface antigens and altering the
microenvironment of the tissue (3,4).
Therefore, an imbalance between immune cells and cytokines at the
tumor site and in the peripheral blood often occurs (5). The proportional and functional
alterations of T cell subsets in the tumor microenvironment is a
key factor that contributes to the immune escape of tumor cells,
especially in cluster of differentiation
(CD)4+CD25+ forkhead box protein P3
(foxp3)+ T regulatory cells (Treg cells) (6,7).
Treg cells serve an immunosuppressive function in
the tumor microenvironment; as a result, the body is unable to
produce an effective immune response (8), resulting in tumor deterioration
(9,10). The proportion of Treg cells in the
peripheral blood and tumor tissue is increased in patients with
tumors. Furthermore, the number of Treg cells is correlated with
the degree of tumor progression and prognosis (11,12).
In a clinical study, it was demonstrated that the clinical stage of
non-small-cell lung cancer (NSCLC) was positively correlated with
the Treg cell ratio (13). Treg
cells surround the tumor in a cluster, whereas they are scattered
in normal paracarcinoma tissue (14). The results of a previous study
indicated that Treg is chemoattracted to tumor tissue and promotes
tumor growth via its immunosuppressive effect (15). Verma et al (16) reported that the response of
neoplastic cells to neoadjuvant chemotherapy is associated with
Treg levels in the peripheral circulation. The postoperative level
of Treg cells was considered to be an independent of the prognosis
(17).
Foxp3 is a specific transcription factor of Treg
cells and serves an important role in regulating the development
and function of Treg cells (18).
Foxp3 is necessary to maintain the immunosuppressive effect of Treg
cells (18). The role of foxp3 in
tumorigenesis and tumor progression is conflicting, both tumor
suppressive and promoting functions have been reported (19,20)
It has been reported that treatment with foxp3-knockout-Treg cells
reduces the incidence of tumors in animal experiments and foxp3
serves an important role in lung tumorigenesis (21). It has been demonstrated that foxp3
overexpression facilitates the proliferation and invasiveness of
cervical tumor cells, resulting in the development and metastasis
of cervical cancer (22).
Similarly, Treg cell infiltration in tumor tissue is negatively
correlated with the prognosis of NSCLC (23). In contrast, foxp3 is also known as
a potential tumor suppressor gene., Foxp3 inhibition decreases cell
proliferation, migration, and invasion, as well as the secretion of
inhibitory cytokines, suggesting that foxp3 as inhibitor for tumor
development of lung adenocarcinoma (24). It was also demonstrated that the
level of foxp3+Treg cells is positively correlated with the
prognosis of specific tumors (25,26).
Ladoire et al (27)
reported that the expression level of foxp3 in tumor cells is
positively correlated with prognosis of patients with breast
cancer. Hanke et al (28)
proved that the quantity of foxp3+Treg cells in the tumor is
associated with the prognosis of lymph node-negative colon cancer
patients. However, whether foxp3 exhibits tumor suppressive and
promoting functions in NSCLC is unclear.
In order to clarify the association between the
expression of foxp3 in Treg cells and NSCLC, a tumor cell and
immune cell co-culture model was used to study the interaction
between lung cancer cells and Treg cells in vitro.
Materials and methods
Cell culture
The human NSCLC cell line 95D was purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in RPMI-1640 medium (cat. no. SH30809.01B; HyClone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; cat. no. SH30087.01;
HyClone) and 1% penicillin-streptomycin (cat. no. SH30010;
HyClone).
Cell isolation
A total of 8 healthy volunteers, 4 male and 4
female, 25–30 years old, were selected from 9th December 2013 to
15th December 2013. The blood samples were collected from the
cubital vein of the forearm. Peripheral blood mononuclear cells
were isolated from 10 ml whole blood samples of healthy volunteers
using density gradient centrifugation as described previously
(29).
CD4+CD25+CD127dim/− cells were
obtained by positive magnetic cell sorting using the
CD4+CD25+CD127dim/− Regulatory T
Cell Isolation kit II (cat. no. 130-094-775; Miltenyi Biotec GmbH,
Bergisch Gladbach, Germany) according to the manufacturer's
protocol. Briefly, the non-CD4+ and CD127high
cells were removed using magnetic beads and CD25+ cells
were collected using CD25 positive selection magnetic beads.
CD4+CD25+CD127dim/− cells were
cultured for 48 h in X-VIVO15 (Biowhittaker, Inc., Walkersville,
MD, USA) supplemented with 2 mM L-glutamine (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 20 mg/ml gentamicin (Gibco;
Thermo Fisher Scientific, Inc.), 0.1 mg/ml rapamycin (Gibco; Thermo
Fisher Scientific, Inc.), 500 U/ml interleukin (IL)-2 (Gibco;
Thermo Fisher Scientific, Inc.) and 5% autologous serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in an atmosphere containing
5% CO2. The present study was approved by the Medical
Ethics Committee of the Third Affiliated Hospital of Southern
Medical University (Guangzhou, China). Written informed consent was
obtained from all volunteers.
Cell co-culture system
Transwell co-culture experiments were performed in
24-well plates using inner wells with 0.4-µm pores to physically
separate Treg cells and 95D cells. The 95D cells (2×105
cells/well) were grown in the outer wells using 1.5 ml RPMI-1640
medium containing 10% FBS. Subsequently, isolated human Treg cells
(4×105 cells/well) were added to the inner wells in 500
ml RPMI-1640 medium. Following 14 days incubation at 37°C, Treg
cells and 95D cells were harvested and washed with PBS for the
following experiments.
Cell transfection
For gene expression analysis, Treg cells
(1×105 cells/well) were seeded in 6-well plates and
transfected with 1 µg pcDNA3.1-Foxp3 (Shanghai GenePharma Co.,
Ltd., Shanghai, China) per well using Lipofectamine®
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The subsequent
experiments were performed 48 h after transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the 95D and Treg cells
using a TRIzol reagent kit obtained from Invitrogen (Thermo Fisher
Scientific, Inc.). RNA was eluted in 50 ml RNase-free water and
preserved at −70°C. The total RNA was reverse transcribed using a
Transcriptor First Strand cDNA Synthesis kit (Takara Biotechnology
Co., Ltd., Dalian, China). The conditions were as follows: 42°C for
15 min and 85°C for 10 min. To analyze gene expression, the qPCR
mixture system containing primers, cDNA templates, and SYBR Green
qPCR Master Mix (Thermo Fisher Scientific, Inc.) was used as
described previously (24). The
qPCR conditions were as follows: 30 sec at 95°C followed by 40
cycles of 15 sec at 95°C for denaturation 30 sec at 60°C for
annealing and 25 sec at 72°C for extension. Fold changes in gene
expression were calculated using the 2−ΔΔCq method
(30). 18S was used as an internal
control. The primers used in the present study were listed as
follows: Foxp3, forward 5′-CGGATTTCGATGTTCGGTAC-3′ and reverse
5′-GCCATTGTCAGGTCCTGAGT-3′; matrix metalloproteinase (MMP)-9,
forward 5′-CTATAGCATCAGTAAGTGGTT-3′ and reverse
5′-GAGTAGGAACTGACCTAT-3′; 18S, forward 5′-AGAAACGGCTACCACATC-3′ and
reverse 5′-TACTCATTCCAATTACCAGACTC-3′.
Flow cytometry
The 95D cells were seeded in 6-well plates
(2×105 cells/well). After co-culturing with foxp3
over-expressed Treg cells for 48 h at 37°C, 95D cells were
collected. Cell apoptosis was assessed using n Annexin
V-fluorescein isothiocyanate cell apoptosis detection kit (cat. no.
KGA106; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) on a flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). All data were
analyzed using SPSS 16.0 software (SPSS, Inc., Chicago, IL,
USA).
MTS assay
95D cells were seeded in 96-well plates
(2×103 cells/well) for cultivation. Cells were
co-cultured with wild type Treg or Foxp3 over-expressed Treg cells
for 24 h as described above. During the last 4 h of each day of
culture, MTS was added to the medium. Formazan crystals were
dissolved using dimethyl sulfoxide and the absorbance was measured
at 450 nm using a microplate reader (Multiskan MK3; Thermo Fisher
Scientific, Inc.).
Transwell assay
The invasiveness of 95D cells was assessed using a
Transwell assay. The Transwell chamber (cat. no. 353097; BD
Biosciences) was covered with a Matrigel (cat. no. 356234; BD
Biosciences) basement membrane. The lower and upper chambers were
separated with a polycarbonate membrane. The lower and upper
chambers were filled with RPMI-1640 medium. The 95D cells
(2×105 cells/well) were seeded in the upper chamber and
cultured in a 5% CO2 incubator at 37°C for 24 h. The
cell coating was removed from the upper chamber with a cotton ball
48 h later. The cells that had migrated to the reverse side of the
membrane were stained with 0.05% crystal violet. The membrane was
observed (magnification ×200) under an inverted microscope (Olympus
Corporation, Tokyo, Japan). Five fields of view were randomly
selected by two independent pathologists. The cells were counted in
each field of view and the mean was calculated. The experiments
were performed in triplicate.
Western blotting
Total protein was extracted from Treg cells using
radioimmunoprecipitation assay buffer (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 50 µg protein was separated by 10%
SDS-PAGE and transferred onto a polyvinylidene difluoride membrane.
Subsequently, the membrane was blocked with 5% skim milk at room
temperature for 1 h and incubated with primary antibodies against
MMP-9 (cat. no. ab73734; 1:500; Abcam, Cambridge, MA, USA) and
GAPDH (cat. no. ab9485; 1:500; Abcam) at 4°C overnight. Following
washing with PBST three times, the membrane was incubated with a
horseradish peroxidase conjugated anti-rabbit IgG antibody (cat.
no. ab191866; 1:500; Abcam) for 1 h at 37°C. Finally, the protein
expression was detected by enhanced chemiluminescence reagent
(Thermo Fisher Scientific, Inc.). Chemiluminescent detection was
performed using Bio-Rad ChemiDoc™ MP Imaging System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell adhesion assay
Fibronectin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was pre-coated on microtiter plates and 95D cells
(2×105/ml) were added. Non-adherent cells were washed
away and the adherent cells were fixed and stained by DAPI staining
as described previously (31).
Finally, the cells were observed under an inverted fluorescence
microscope (magnification, ×100) and absorbance was measured using
a microtiter plate reader.
Wound healing assay
At 24 h following cell co-culture, a wound-healing
assay was performed. The 95D cells (1×105 cells/well)
were seeded into a 24-well plate at 60–70% confluence. A lesion was
created using a plastic pipette tip and cells were washed twice
with PBS buffer. The monolayer was then maintained in serum-free
RPMI-1640 medium and cultured at 37°C for 24 h. Five randomly
selected fields at the border of the lesion were viewed under an
inverted microscope (magnification, ×100; IX71; Olympus
Corporation).
Statistical analysis
All data analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. Differences among groups were compared
using a t-test or one-way analysis of variance (ANOVA). Tukey's
post hoc test was used for one-way ANOVA. P<0.05 was considered
to indicate a statistically significant difference.
Results
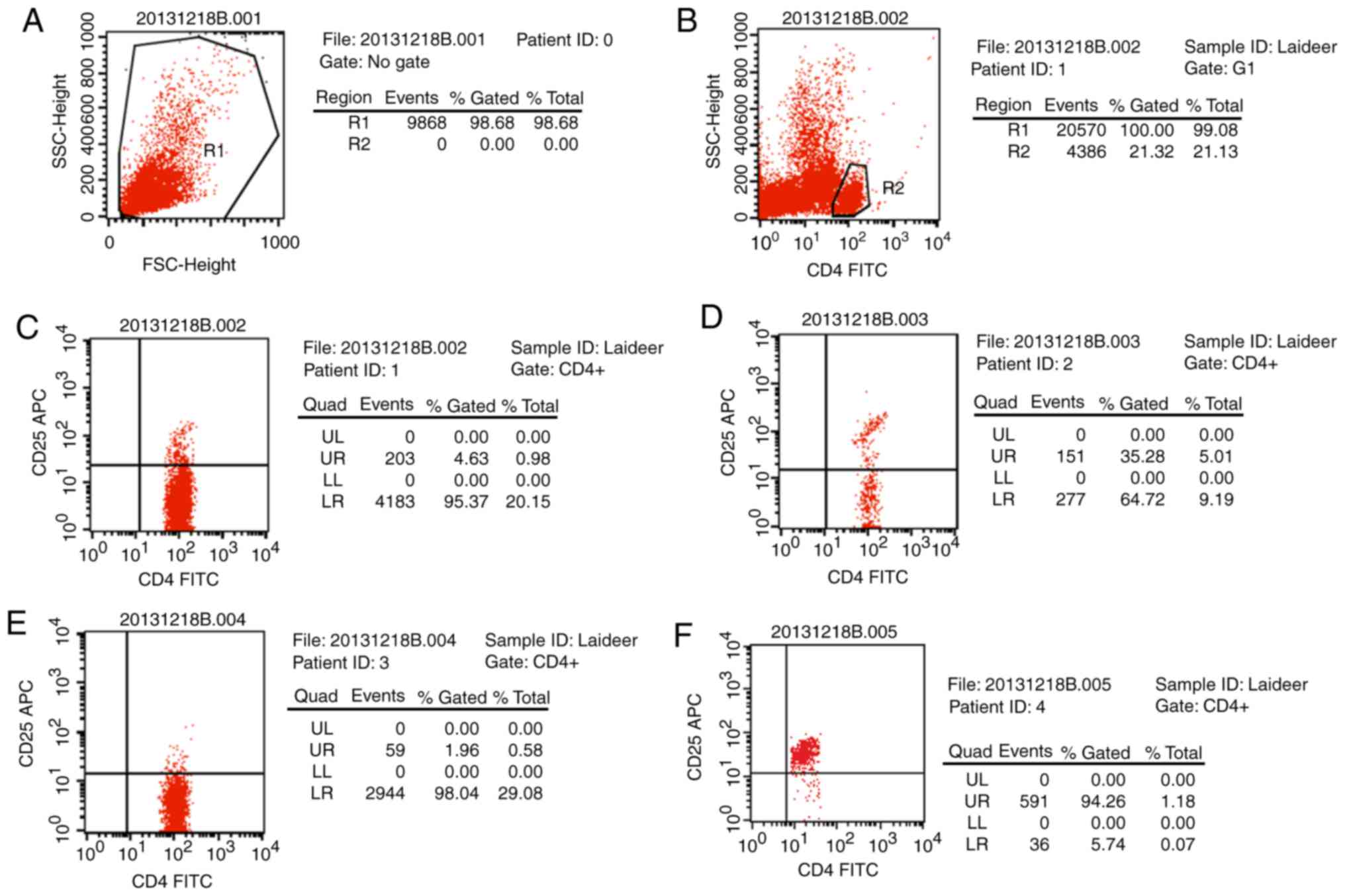
Treg cell isolation
Magnetic beads were applied to isolate Treg cells
from peripheral blood CD4+ T cells were separated out
and were demonstrated to account for ~21.32% of all T cells. Then
CD25 beads were used to extract CD4+CD25+ T
cells (4.63%) and further isolate
CD4+CD25+CD127dim/− T cells
(Fig. 1). The cell ratio reached
94.26% following purification, which was deemed suitable for the
following experiments.
Influence of Treg and 95D cell
co-culture on gene expression
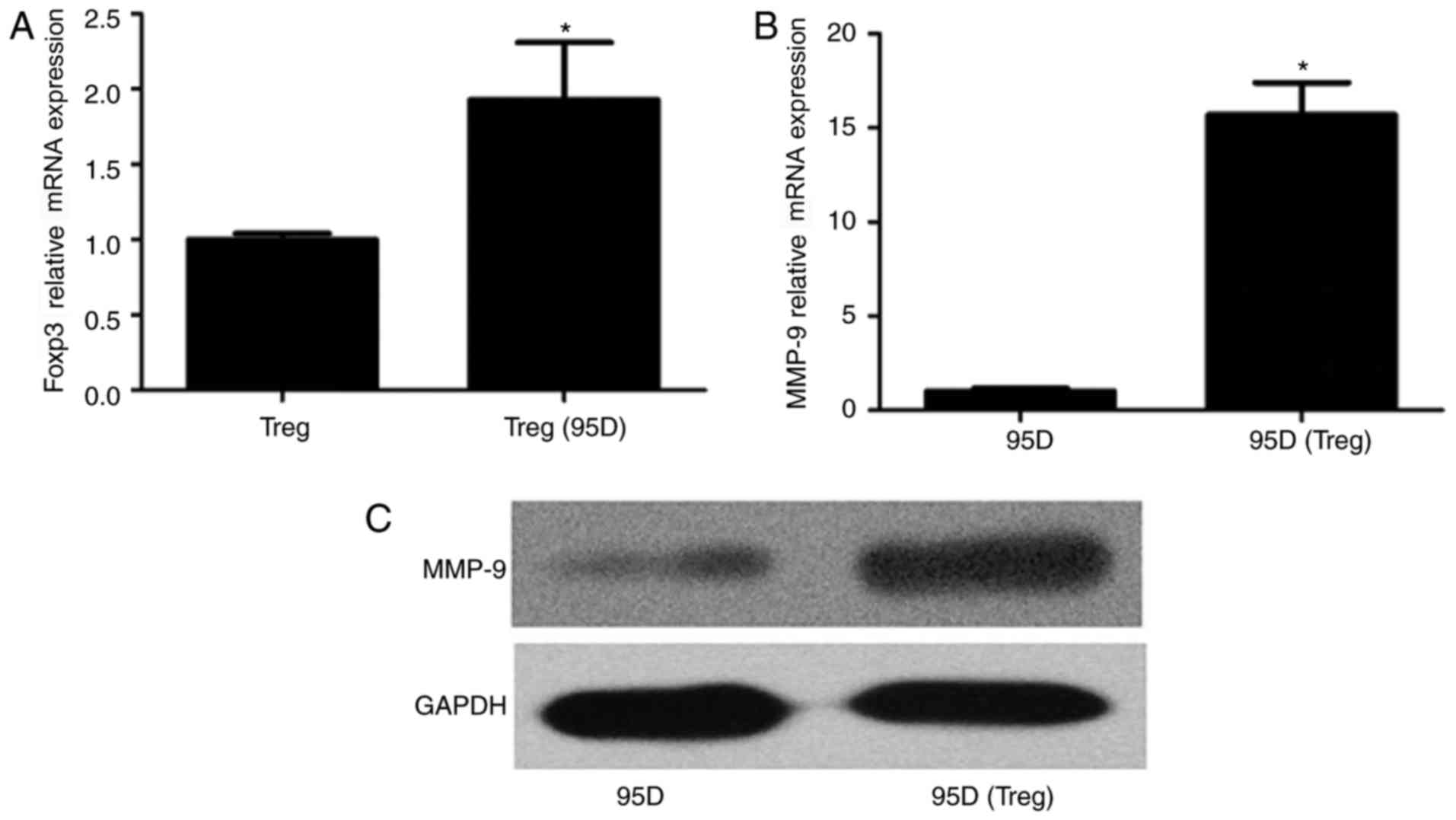
To investigate the influence of cell co-culture on
foxp3 expression in Treg cells and MMP-9 expression in 95D cells,
cells were incubated together for 36 h and total RNA was extracted.
qPCR revealed that foxp3 gene expression was significantly
increased in Treg cells following co-culture with 95D cells
(P<0.05; Fig. 2A). Similarly,
MMP-9 mRNA expression was significantly increased in 95D cells
following co-culture with Treg cells compared with the 95D only
group (P<0.05; Fig. 2B).
Western blotting results revealed that the expression of foxp3 and
MMP-9 proteins followed the same trend as the mRNA (Fig. 2C).
Effect of cell co-culture system on
95D cell behavior
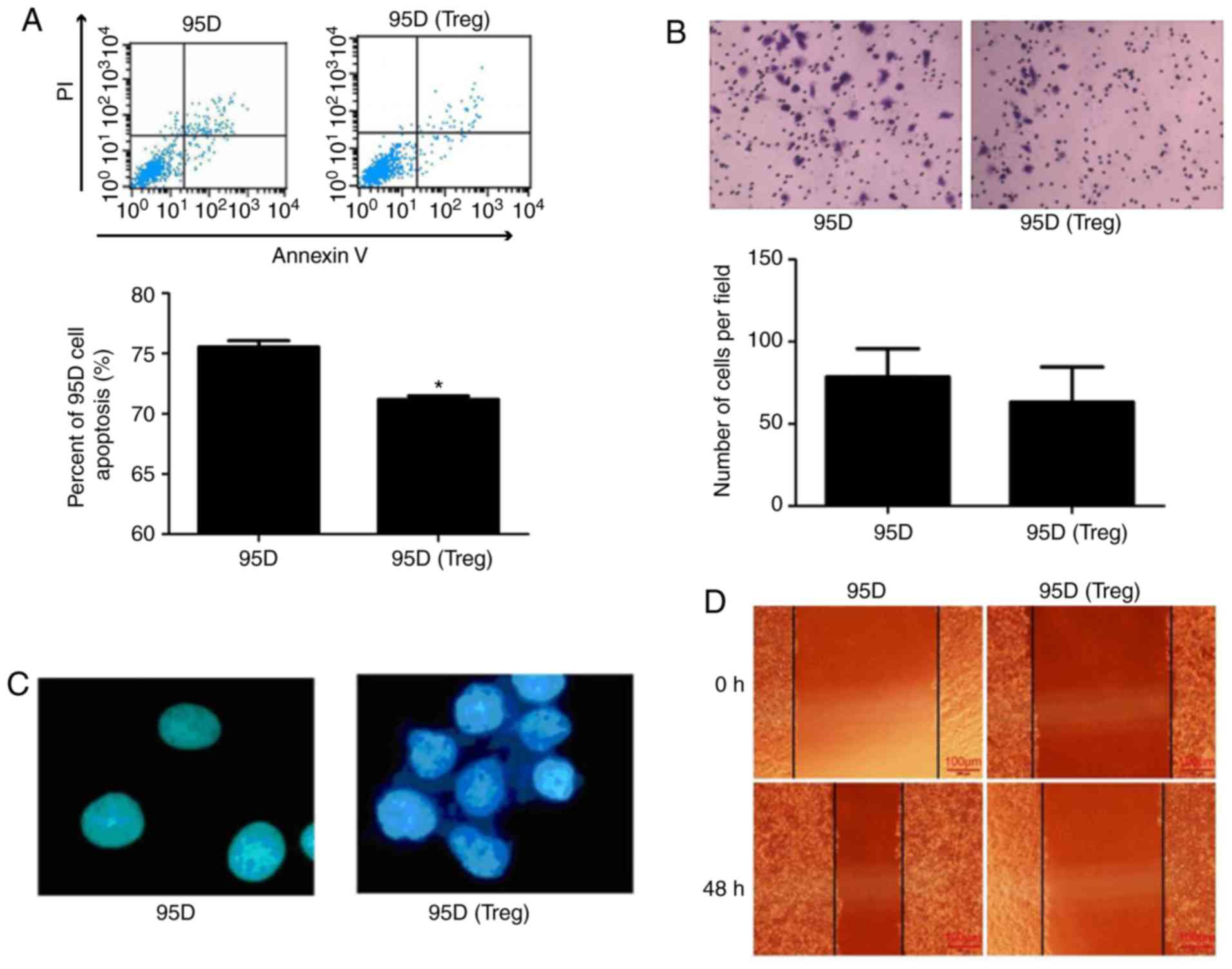
The effect of cell co-culture on 95D cell behavior
was investigated. Flow cytometry demonstrated that apoptosis was
significantly reduced in 95D cells following co-culture with Treg
cells (P<0.05; Fig. 3A).
However, the Transwell assay revealed that co-culture failed had no
significant effect on the invasive ability of 95D cells (Fig. 3B). A cell adhesion assay
demonstrated that the adhesion of 95D cells was markedly enhanced
following co-culture with Treg cells (Fig. 3C). In addition, a wound-healing
assay revealed that 95D cell migration was attenuated by Treg cell
co-culture (Fig. 3D).
Effect of foxp3 on cell
co-culture
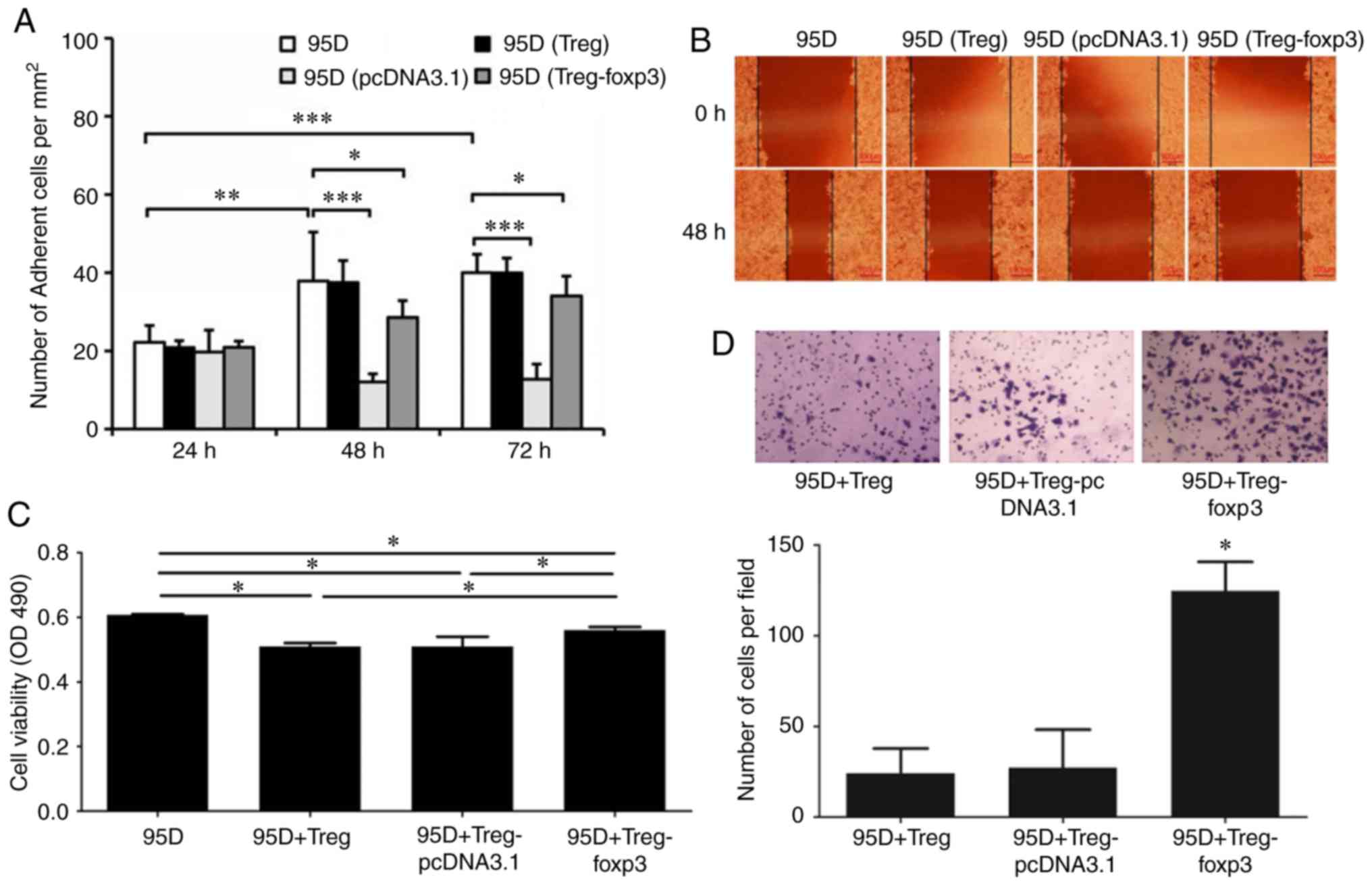
Foxp3 serves a crucial role in the regulation and
development of Treg cells. Based on this, a plasmid overexpressing
foxp3 was constructed and transfected into Treg cells to observe
its effect on the behavior of 95D cells. A cell adhesion assay
demonstrated that the adhesive ability of 95D cells decreased
following co-culture with foxp3 overexpressing Treg cells (Fig. 4A). A wound healing assay
demonstrated that the migration of 95D cells was decreased
following co-culture with foxp3 overexpressing Treg cells (Fig. 4B). An MTS assay demonstrated that
the viability of 95D cells was significantly reduced following
co-culture with wild type Treg cells, while it was significantly
increased following co-culture with foxp3 overexpressing Treg cells
(P<0.05; Fig. 4C). In addition,
the Transwell assay demonstrated that the invasive ability of 95D
cells was significantly enhanced by co-culture with foxp3
overexpressing Treg cells (P<0.05; Fig. 4D).
Discussion
The tumor microenvironment influences the
interactions between cells and ultimately affects the outcome of
tumor (32). Treg cells are
important components of the tumor immune microenvironment, which
inhibits the antitumor immune function and is associated with tumor
immune escape by suppressing the function of activated T cells
(33). The immunosuppressive
effect of Treg cells depends on intercellular contact (34) and the impact of cytokines (35). The results of the present study
suggest that 95D cells promote foxp3 expression in Treg cells,
indicating that Treg cells have a significant synergistic effect
with 95D cells. However, Treg cells inhibit the apoptosis of 95D
cells and enhanced the adhesive ability of 95D cells. The results
indicate that Treg cells promote tumor development in the NSCLC
microenvironment; these results are similar to a previous study, in
which it was demonstrated that Treg cell invasion was negatively
correlated with prognosis in patients with esophageal cancer who
received neoadjuvant radiotherapy and chemotherapy (17).
Foxp3 is a recognized marker of Treg cells and
maintaining the growth and immunosuppressive function of Treg
cells. Foxp3-knockout-Treg cells lost the immunosuppressive effects
observed in wild type Treg cells, being altered from
immunoregulatory cells to proinflammatory cells (36). The expression of foxp3 in Treg
cells is regulated by transcription factor activation and chromatin
molecule modification (37).
Previous studies have demonstrated that T-cell receptor (TCR)
signaling important for the expression of foxp3 in Treg cells
(38). The expression of CD25 is
upregulated by the TCR signaling pathway, which activates IL-2
signaling and the downstream signal transducer and activator of
transcription 5A (39). CD28 binds
with its ligand, CD80/CD86, to induce foxp3 expression (40). The effect of epigenetic regulation
and nuclear factor (NF)-κB signaling pathway on foxp3 expression is
also associated with the TCR signaling pathway (41). The TCR-responsive enhancer in the
first intron of the foxp3 gene is dependent on a cyclic-AMP
response element binding protein (CREB)/activating transcription
factor site overlapping a CpG island (42). The methylation of the island
inhibits CREB binding and downregulates the transcription of foxp3
(42). The TCR signaling pathway
is able to upregulate the foxp3 transcription via the demethylation
of CpG islands (42). Conserved
noncoding sequences (CNS) 2 demethylation also serves an important
role in the stabilization of foxp3; upregulation of CNS2 expression
inhibits DNA methyltransferase and therefore stabilizes the Treg
cell phenotype (42). The NF-κB
signaling pathway regulates the CNS3 region via activation of its
constituent element, c-Rel, and the TCR signaling pathway, thereby
promoting foxp3 transcription and Treg cell differentiation
(43). It was observed in the
present study that tumor cells promote the expression of foxp3 in
Treg cells, suggesting that they may be associated with cytokines
secreted by tumor cells and function between cells. Tumor cells
secrete a large number of cytokines, including transforming growth
factor (TGF)-β, IL-10, prostaglandin-2, retinoic acid A, and
indoleamine-2,3-dioxygenase (44).
Cytokines induce the expression of foxp3 in Treg cells and enhance
the immunosuppressive function (45). For example, TGF-β was reported to
regulate the sustained expression of foxp3 and stabilize the number
and immunosuppressive functions of Treg cells in vivo and
in vitro (38).
In the present study, the apoptosis of 95D cell
decreased following co-culture, suggesting that foxp3 may be
expressed in tumor cells (46).
The TCR signaling pathway is important for the regulation of foxp3
and is o key apoptotic associated pathway. Associated cell factors
bind with the TNF domain on the tumor cell surface to initiate
apoptosis via the TCR signaling pathway (47). Following the activation of Treg
cells, the expression of the associated signal molecules is also
increased, including glucocorticoid-induced TNF receptor (GITR) and
cytotoxic T lymphocyte antigen 4 and TCR-inducible costimulatory
receptor (48). Nocentini et
al (49) demonstrated that
GITR, a member of the TNF receptor family, is associated with
TCR-mediated cell death, and attenuates anti-CD3 monoclonal
antibody-induced apoptosis. Zhang et al (50) also revealed that co-culture of
tumor cells with peripheral blood mononuclear cells upregulates
GITR expression.
In the present study, Treg cell infiltration
promoted tumor cell growth and reduced cell apoptosis. The
influence of foxp3 expression by tumor cells remains unclear. Tan
et al (51) suggested that
overexpression of foxp3 in tumor cells inhibited tumor growth and
promoted cell apoptosis. Studies also obtained similar results in
glioma (52), gastric cancer
(53), breast cancer (54) and other associated tumors (55). It was reported that endogenous
foxp3 overexpression inhibited gastric cancer cell proliferation
and facilitated apoptosis by upregulating microRNA-146a/b and
negatively regulating the NF-κB signaling pathway (56). The role of foxp3 varies in
different tumors cells and tumor microenvironments; however,
mutations of foxp3 are observed in certain tumors. For example, if
foxp3 loses some exons in MCF-7 cells, the variants lose the
ability to suppress gene expression in cancer cells (57).
MMPs regulate the movement of hematopoietic stem
cells and degrade a variety of extracellular matrix proteins
(58). MMP-9 serves a crucial role
in tumorigenesis and development by forming vascular endothelial
growth factor receptor 2/fetal liver kinase 1 receptor in
endothelial cells through remodeling the extracellular matrix and
promoting germination and growth of novel vessels (59). Studies have indicated that MMP-9
expression is associated with tumor progression and prognosis
(60–62). Similarly, MMP-9 expression in
immunosuppressive macrophages in the tumor microenvironment also
contributes to tumor invasion and metastasis (60). MMP-9 is able to promote cell
migration via activating mitogen-activated protein kinase and
phosphoinositide 3-kinase pathways (63). It has been demonstrated that MMP-9
expression is also associated with the activation of TGF-β
(64). In dendritic cells, the
MMP-9 inhibitor decreased the activation of latent TGF-β1 (65). Overexpression of MMP-9 was detected
in laryngeal cancer and serves a critical role in the development
of tolerogenic dendritic cells, therefore serving a key role in
tumor survival (66).
The results of the present study demonstrate that
the expression of MMP-9 in tumor cells, tumor invasion and foxp3
levels were increased in the co-cultured Treg cells. The effect of
foxp3 on MMP-9 expression may be associated with certain cytokines
[e.g., IL-17 (67)] and
phosphorylation. Foxp3 downregulation reduces the expression of
MMP-9 and MMP-2, thereby inhibiting tumor invasion (68). The tumor microenvironment contains
a variety of cytokines, including IL-17, IL-1, IL-6, IL-10, and
TGF-β. IL-17A is a proinflammatory cytokine that induces the
expression of VEGF, MMPs and C-X-C motif chemokine 8 and therefore
promotes neovascularization (69).
IL-17A inhibition in the tumor microenvironment enhances the
cytotoxicity of tumor-infiltrating lymphocytes (70). In addition, it was demonstrated
that foxp3 phosphorylation downregulates MMP-9 and reduces the
aggressiveness of tumor cells and affecting the NF-κB function
(57). Phosphorylation of the
Tyr-342 fragment in foxp3 serves an important role in
downregulating the expression of MMP-9 and S-phase
kinase-associated protein 2 (57).
Morawski et al (71) also
demonstrated that cell cycle-dependent kinase 2 has a z regulatory
effect on the stability and activity of foxp3 via foxp3
phosphorylation.
In the present study, cells were seeded in a
Transwell plate to detect the invasiveness of 95D cells. The
results suggested that the invasive ability of 95D cells was
similar following co-culture with Treg cells. However, the
expression of MMP-9 in tumor cells was increased and apoptosis was
decreased following co-culture. It was hypothesized that invasion
may be associated with the duration of co-culture. In the foxp3
overexpression model group, 95D cells exhibited the highest
viability and it was hypothesized that this may be due to the short
co-culture time and the intercellular contact inhibition (72).
In conclusion, the results of the present study
demonstrate that tumor cell viability and invasiveness are enhanced
by co-culture with foxp3-overexpressing Treg cells and that foxp3
in the tumor microenvironment promotes tumor cell growth. Further
investigation of the foxp3 phenotype in Treg cells and the
associated mechanism in tumor cells may provide a novel method for
the treatment and prevention of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JP and YL designed this work. ZY and LX performed
the the experiments and wrote the manuscript. JW and JL drafted
statistical methods and analyzed the data. JP, DL and QY helped
with data collection and data interpretation. All authors read and
approved the manuscript for publication.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Third Affiliated Hospital of Southern Medical
University (Guangzhou, China). Written informed consent was
obtained from all volunteers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang J, Jia Y, Zhao S, Zhang X, Wang X,
Han X, Wang Y, Ma M, Shi J and Liu L: BIN1 reverses PD-L1-mediated
immune escape by inactivating the c-MYC and EGFR/MAPK signaling
pathways in non-small cell lung cancer. Oncogene. 36:6235–6243.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGranahan N, Rosenthal R, Hiley CT, Rowan
AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P,
Herrero J, et al: Allele-Specific HLA loss and immune escape in
lung cancer evolution. Cell. 171:1259–1271.e11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schafer CC, Wang Y, Hough KP, Sawant A,
Grant SC, Thannickal VJ, Zmijewski J, Ponnazhagan S and Deshane JS:
Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung
cancer by metabolic reprogramming of immune cells in the tumor
microenvironment. Oncotarget. 7:75407–75424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan R, Sethi P, Jyoti A, McGarry R and
Upreti M: Investigating the radioresistant properties of lung
cancer stem cells in the context of the tumor microenvironment.
Radiat Res. 185:169–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor JG and Gribben JG: Microenvironment
abnormalities and lymphomagenesis: Immunological aspects. Semin
Cancer Biol. 34:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Pan K and Xia JC: Interaction of
indoleamine-2,3-dioxyagnase and CD4+CD25+ regulatory T cells in
tumor immune escape. Ai Zheng. 28:184–187. 2009.PubMed/NCBI
|
|
7
|
Qu Y, Zhang B, Zhao L, Liu G, Ma H, Rao E,
Zeng C and Zhao Y: The effect of immunosuppressive drug rapamycin
on regulatory CD4+CD25+Foxp3+T cells in mice. Transpl Immunol.
17:153–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long SA and Buckner JH: CD4+FOXP3+ T
regulatory cells in human autoimmunity: More than a numbers game. J
Immunol. 187:2061–2066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY,
Xiao YS, Xu Y, Li YW and Tang ZY: Intratumoral balance of
regulatory and cytotoxic T cells is associated with prognosis of
hepatocellular carcinoma after resection. J Clin Oncol.
25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizukami Y, Kono K, Kawaguchi Y, Akaike H,
Kamimura K, Sugai H and Fujii H: Localisation pattern of Foxp3+
regulatory T cells is associated with clinical behaviour in gastric
cancer. Br J Cancer. 98:148–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei T, Zhang J, Qin Y, Wu Y, Zhu L, Lu L,
Tang G and Shen Q: Increased expression of immunosuppressive
molecules on intratumoral and circulating regulatory T cells in
non-small-cell lung cancer patients. Am J Cancer Res. 5:2190–2201.
2015.PubMed/NCBI
|
|
12
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WJ, Tao Z, Gu W and Sun LH: Variation
of blood T lymphocyte subgroups in patients with non-small cell
lung cancer. Asian Pac J Cancer Prev. 14:4671–4673. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schneider T, Kimpfler S, Warth A, Schnabel
PA, Dienemann H, Schadendorf D, Hoffmann H and Umansky V: Foxp3(+)
regulatory T cells and natural killer cells distinctly infiltrate
primary tumors and draining lymph nodes in pulmonary
adenocarcinoma. J Thorac Oncol. 6:432–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue L, Chen J, Peng JZ, Chen BS, Hua P and
Yang YQ: Clinical significance of tumor interstitial T lymphocyte
subset activity in non-small-cell lung cancer. Nan Fang Yi Ke Da
Xue Xue Bao. 29:2456–2458. 2009.(In Chinese). PubMed/NCBI
|
|
16
|
Verma C, Eremin JM, Robins A, Bennett AJ,
Cowley GP, El-Sheemy MA, Jibril JA and Eremin O: Abnormal T
regulatory cells (Tregs: FOXP3+, CTLA-4+), myeloid-derived
suppressor cells (MDSCs: Monocytic, granulocytic) and polarised T
helper cell profiles (Th1, Th2, Th17) in women with large and
locally advanced breast cancers undergoing neoadjuvant chemotherapy
(NAC) and surgery: Failure of abolition of abnormal treg profile
with treatment and correlation of treg levels with pathological
response to NAC. J Transl Med. 11:162013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vacchelli E, Semeraro M, Enot DP, Chaba K,
Poirier Colame V, Dartigues P, Perier A, Villa I, Rusakiewicz S,
Gronnier C, et al: Negative prognostic impact of regulatory T cell
infiltration in surgically resected esophageal cancer
post-radiochemotherapy. Oncotarget. 6:20840–20850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JH, Ko JS, Shin Y, Cho JY, Oh HA,
Bothwell AL and Lee SK: Intranuclear interactomic inhibition of
FoxP3 suppresses functions of Treg cells. Biochem Biophys Res
Commun. 451:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Liu R, Ribick M, Zheng P and Liu
Y: FOXP3 as an X-linked tumor suppressor. Discov Med. 10:322–328.
2010.PubMed/NCBI
|
|
20
|
Katoh H, Zheng P and Liu Y: Signalling
through FOXP3 as an X-linked tumor suppressor. Int J Biochem Cell
Biol. 42:1784–1787. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Granville CA, Memmott RM, Balogh A,
Mariotti J, Kawabata S, Han W, Lopiccolo J, Foley J, Liewehr DJ,
Steinberg SM, et al: A central role for Foxp3+ regulatory T cells
in K-Ras-driven lung tumorigenesis. PLoS One. 4:e50612009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo Q, Zhang S, Wei H, Pang X and Zhang H:
Roles of Foxp3 in the occurrence and development of cervical
cancer. Int J Clin Exp Pathol. 8:8717–8730. 2015.PubMed/NCBI
|
|
23
|
O'Callaghan DS, Rexhepaj E, Gately K,
Coate L, Delaney D, O'Donnell DM, Kay E, O'Connell F, Gallagher WM
and O'Byrne KJ: Tumour islet Foxp3+ T-cell infiltration predicts
poor outcome in nonsmall cell lung cancer. Eur Respir J.
46:1762–1772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Li D, Yang W, Fu H, Liu Y and Li Y:
Overexpression of the transcription factor FOXP3 in lung
adenocarcinoma sustains malignant character by promoting G1/S
transition gene CCND1. Tumour Biol. 37:7395–7404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tzankov A, Meier C, Hirschmann P, Went P,
Pileri SA and Dirnhofer S: Correlation of high numbers of
intratumoral FOXP3+ regulatory T cells with improved survival in
germinal center-like diffuse large B-cell lymphoma, follicular
lymphoma and classical Hodgkin's lymphoma. Haematologica.
93:193–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Badoual C, Hans S, Rodriguez J, Peyrard S,
Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P,
Fridman WH, et al: Prognostic value of tumor-infiltrating CD4+
T-cell subpopulations in head and neck cancers. Clin Cancer Res.
12:465–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ladoire S, Arnould L, Mignot G, Coudert B,
Rébé C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, et
al: Presence of Foxp3 expression in tumor cells predicts better
survival in HER2-overexpressing breast cancer patients treated with
neoadjuvant chemotherapy. Breast Cancer Res Treat. 125:65–72. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanke T, Melling N, Simon R, Sauter G,
Bokemeyer C, Lebok P, Terracciano LM, Izbicki JR and Marx AH: High
intratumoral FOXP3+ T regulatory cell (Tregs) density is
an independent good prognosticator in nodal negative colorectal
cancer. Int J Clin Exp Pathol. 8:8227–8235. 2015.PubMed/NCBI
|
|
29
|
Zhang T, Shao B and Liu GA: Rosuvastatin
promotes the differentiation of peripheral blood monocytes into M2
macrophages in patients with atherosclerosis by activating PPAR-γ.
Eur Rev Med Pharmacol Sci. 21:4464–4471. 2017.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin H, Guo C, Wang Y, Liu D, Lv Y, Lv F
and Lu Z: Fengycin inhibits the growth of the human lung cancer
cell line 95D through reactive oxygen species production and
mitochondria-dependent apoptosis. Anticancer Drugs. 24:587–598.
2013.PubMed/NCBI
|
|
32
|
Del Monte U and Statuto M: Drop of
connexins: A possible link between aging and cancer? Exp Gerontol.
39:273–275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
AlHilli MM, Hopkins MR and Famuyide AO:
Endometrial cancer after endometrial ablation: Systematic review of
medical literature. J Minim Invasive Gynecol. 18:393–400. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakaguchi S: Naturally arising CD4+
regulatory t cells for immunologic self-tolerance and negative
control of immune responses. Annu Rev Immunol. 22:531–562. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zelenay S, Lopes-Carvalho T, Caramalho I,
Moraes-Fontes MF, Rebelo M and Demengeot J: Foxp3+ CD25-CD4 T cells
constitute a reservoir of committed regulatory cells that regain
CD25 expression upon homeostatic expansion. Proc Natl Acad Sci USA.
102:pp. 4091–4096. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hansmann L, Schmidl C, Kett J, Steger L,
Andreesen R, Hoffmann P, Rehli M and Edinger M: Dominant Th2
differentiation of human regulatory T cells upon loss of FOXP3
expression. J Immunol. 188:1275–1282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu L, Barbi J and Pan F: The regulation of
immune tolerance by FOXP3. Nat Rev Immunol. 17:703–717. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marie JC, Letterio JJ, Gavin M and
Rudensky AY: TGF-beta1 maintains suppressor function and Foxp3
expression in CD4+CD25+ regulatory T cells. J Exp Med.
201:1061–1067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsang JY, Camara NO, Eren E, Schneider H,
Rudd C, Lombardi G and Lechler R: Altered proximal T cell receptor
(TCR) signaling in human CD4+CD25+ regulatory T cells. J Leukoc
Biol. 80:145–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tone Y, Furuuchi K, Kojima Y, Tykocinski
ML, Greene MI and Tone M: Smad3 and NFAT cooperate to induce Foxp3
expression through its enhancer. Nat Immunol. 9:194–202. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jana S, Jailwala P, Haribhai D, Waukau J,
Glisic S, Grossman W, Mishra M, Wen R, Wang D, Williams CB and
Ghosh S: The role of NF-kappaB and Smad3 in TGF-beta-mediated Foxp3
expression. Eur J Immunol. 39:2571–2583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim HP and Leonard WJ: CREB/ATF-dependent
T cell receptor-induced FoxP3 gene expression: A role for DNA
methylation. J Exp Med. 204:1543–1551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng Y, Josefowicz S, Chaudhry A, Peng
XP, Forbush K and Rudensky AY: Role of conserved non-coding DNA
elements in the Foxp3 gene in regulatory T-cell fate. Nature.
463:808–812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee KJ, Moon JY, Choi HK, Kim HO, Hur GY,
Jung KH, Lee SY, Kim JH, Shin C, Shim JJ, et al: Immune regulatory
effects of simvastatin on regulatory T cell-mediated tumour immune
tolerance. Clin Exp Immunol. 161:298–305. 2010.PubMed/NCBI
|
|
45
|
Haxhinasto S, Mathis D and Benoist C: The
AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+
cells. J Exp Med. 205:565–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Feng Y, van der Veeken J, Shugay M,
Putintseva EV, Osmanbeyoglu HU, Dikiy S, Hoyos BE, Moltedo B,
Hemmers S, Treuting P, et al: A mechanism for expansion of
regulatory T-cell repertoire and its role in self-tolerance.
Nature. 528:132–136. 2015.PubMed/NCBI
|
|
47
|
Richter MV and Topham DJ: The alpha1beta1
integrin and TNF receptor II protect airway CD8+ effector T cells
from apoptosis during influenza infection. J Immunol.
179:5054–5063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ghourbani Gazar S, Andalib A, Hashemi M
and Rezaei A: CD4+Foxp3+ Treg and its
ICOS+ subsets in patients with myocardial infarction.
Iran J Immunol. 9:53–60. 2012.PubMed/NCBI
|
|
49
|
Nocentini G, Giunchi L, Ronchetti S,
Krausz LT, Bartoli A, Moraca R, Migliorati G and Riccardi C: A new
member of the tumor necrosis factor/nerve growth factor receptor
family inhibits T cell receptor-induced apoptosis. Proc Natl Acad
Sci USA. 94:pp. 6216–6221. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang NN, Chen JN, Xiao L, Tang F, Zhang
ZG, Zhang YW, Feng ZY, Jiang Y and Shao CK: Accumulation mechanisms
of CD4(+)CD25(+)FOXP3(+) regulatory T cells in EBV-associated
gastric carcinoma. Sci Rep. 5:180572015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tan B, Anaka M, Deb S, Freyer C, Ebert LM,
Chueh AC, Al-Obaidi S, Behren A, Jayachandran A, Cebon J, et al:
FOXP3 over-expression inhibits melanoma tumorigenesis via effects
on proliferation and apoptosis. Oncotarget. 5:264–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang B, Dou Y, Xu X, Wang X, Xu B, Du J,
Wang Q, Li Q and Wang J: Endogenous FOXP3 inhibits cell
proliferation, migration and invasion in glioma cells. Int J Clin
Exp Med. 8:1792–1802. 2015.PubMed/NCBI
|
|
53
|
Zhang L, Xu J, Zhang X, Zhang Y, Wang L,
Huang X and Xu Z: The role of tumoral FOXP3 on cell proliferation,
migration, and invasion in gastric cancer. Cell Physiol Biochem.
42:1739–1754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moreno Ayala MA, Gottardo MF, Imsen M,
Asad AS, Bal de Kier Joffé E, Casares N, Lasarte JJ, Seilicovich A
and Candolfi M: Therapeutic blockade of Foxp3 in experimental
breast cancer models. Breast Cancer Res Treat. 166:393–405. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tang J, Yang Z, Wang Z, Li Z, Li H, Yin J,
Deng M, Zhu W and Zeng C: Foxp3 is correlated with VEGF-C
expression and lymphangiogenesis in cervical cancer. World J Surg
Oncol. 15:1732017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu R, Liu C, Chen D, Yang WH, Liu X, Liu
CG, Dugas CM, Tang F, Zheng P, Liu Y and Wang L: FOXP3 controls an
miR-146/NF-kB negative feedback loop that inhibits apoptosis in
breast cancer cells. Cancer Res. 75:1703–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nakahira K, Morita A, Kim NS and
Yanagihara I: Phosphorylation of FOXP3 by LCK downregulates MMP9
expression and represses cell invasion. PLoS One. 8:e770992013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Endres M, Kneitz S, Orth MF, Perera RK,
Zernecke A and Butt E: Regulation of matrix metalloproteinases
(MMPs) expression and secretion in MDA-MB-231 breast cancer cells
by LIM and SH3 protein 1 (LASP1). Oncotarget. 7:64244–64259. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Heissig B, Hattori K, Dias S, Friedrich M,
Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et
al: Recruitment of stem and progenitor cells from the bone marrow
niche requires MMP-9 mediated release of kit-ligand. Cell.
109:625–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ishibashi M, Fujimura T, Hashimoto A, Haga
T, Onami K, Tsukada A, Kambayashi Y, Hidaka T, Furudate S, Shimada
R and Aiba S: Successful treatment of MMP-9-expressing angiosarcoma
with low-dose docetaxel and bisphosphonate. Case Rep Dermatol.
4:5–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870.
2017.PubMed/NCBI
|
|
62
|
Zhang S, Wu M, Zhao Y, Gu R, Peng C, Liu
J, Zhu Q and Li Y: Correlation of MMP-9 and p53 protein expression
with prognosis in metastatic spinal tumor of lung cancer. Oncol
Lett. 14:5452–5456. 2017.PubMed/NCBI
|
|
63
|
Dufour A, Sampson NS, Zucker S and Cao J:
Role of the hemopexin domain of matrix metalloproteinases in cell
migration. J Cell Physiol. 217:643–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dayer C and Stamenkovic I: Recruitment of
matrix metalloproteinase-9 (MMP-9) to the fibroblast cell surface
by Lysyl hydroxylase 3 (LH3) triggers transforming growth factor-β
(TGF-β) activation and fibroblast differentiation. J Biol Chem.
290:13763–13778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mirastschijski U, Schnabel R, Claes J,
Schneider W, Agren MS, Haaksma C and Tomasek JJ: Matrix
metalloproteinase inhibition delays wound healing and blocks the
latent transforming growth factor-beta1-promoted myofibroblast
formation and function. Wound Repair Regen. 18:223–234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang BQ, Zhang CM, Gao W, Wang XF, Zhang
HL and Yang PC: Cancer-derived matrix metalloproteinase-9
contributes to tumor tolerance. J Cancer Res Clin Oncol.
137:1525–1533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Benevides L, Cardoso CR, Tiezzi DG, Marana
HR, Andrade JM and Silva JS: Enrichment of regulatory T cells in
invasive breast tumor correlates with the upregulation of IL-17A
expression and invasiveness of the tumor. Eur J Immunol.
43:1518–1528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ma C, Peng C, Lu X, Ding X, Zhang S, Zou X
and Zhang X: Downregulation of FOXP3 inhibits invasion and immune
escape in cholangiocarcinoma. Biochem Biophys Res Commun.
458:234–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Miossec P, Korn T and Kuchroo VK:
Interleukin-17 and type 17 helper T cells. N Engl J Med.
361:888–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hayata K, Iwahashi M, Ojima T, Katsuda M,
Iida T, Nakamori M, Ueda K, Nakamura M, Miyazawa M, Tsuji T and
Yamaue H: Inhibition of IL-17A in tumor microenvironment augments
cytotoxicity of tumor-infiltrating lymphocytes in tumor-bearing
mice. PLoS One. 8:e531312013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Morawski PA, Mehra P, Chen C, Bhatti T and
Wells AD: Foxp3 protein stability is regulated by cyclin-dependent
kinase 2. J Biol Chem. 288:24494–24502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Batson J, Astin JW and Nobes CD:
Regulation of contact inhibition of locomotion by Eph-ephrin
signalling. J Microsc. 251:232–241. 2013. View Article : Google Scholar : PubMed/NCBI
|