Introduction
The tyrosinase (TYR) gene family are derived from a
common ancestor, subcategorized into TYR-related protein (TRP)-l
and TRP-2 during evolution. TRP-1 and TRP-2 are members of the
family of dinuclear copper-binding proteins and contain two highly
conserved copper-binding regions (1–3).
TRP-1 and TRP-2 are similar to TYR, which also belongs to the TYR
gene family, in terms of protein sequences, exhibiting the
conserved structure of the TYR family (4,5).
TRP-2 participates in the synthesis of melanin, which interacts
with various proteins and is present in human and animal skin and
hair, in addition to the bark of certain plants and insect shells.
In animals with woolly coats, TRP-2 may serve an important role in
the early stages of TYR catalytic activity in melanin formation and
is one of the key enzymes involved in melanin synthesis. For
certain diseases, including albinism and vitiligo, TRP-2 exhibits
important research significance (6).
TRP-2 contains at least eight exons and seven
introns, and has a gene length of 60 kb. All of the eight exons
code for the TRP-2 protein (7).
The TRP-2 carboxyl-terminal and membrane structure domain serve a
key role in melanosome production (8). Valverde et al (9) demonstrated that the molecular weight
of TRP-2 is 75 kDa. Dopa red pigment compounds may be isomerized to
the colorless eumelanin medium, 5,6-dihydroxyindole-2-carboxylic
acid (DHICA), by TRP-2, and hence, TRP-2 has been named dopachrome
tautomerase (Dct) (10). Of the
relevant members of the family, TRP-2 directly participates in the
regulation of melanin production and melanocyte growth, survival
and function (11). TRP-2 also
controls the proportion of DHICA and 5,6-dihydroxyindole in
melanocytes, which are important proteins that regulate animal coat
color.
Current studies of TRP-2 focus on humans, mice, and
rabbits. In humans, TRP-2 is a cytoplasmic antigen expressed in
skin melanocytes and melanoma cells and is associated in the
pathogenesis of vitiligo. TRP-2 autoantibodies may immunoreact with
either TRP-2 or TYR as cross antigens, forming a solid foundation
for research on the mechanism and treatment of vitiligo. In mice,
studies on TRP-2 focus on its effect on melanin formation and thus
its role in color regulation. Research has focused on white-coated,
black-eyed iris rabbits for TRP-2 gene cloning and analysis. In the
present study, the association between TRP-2 expression was
investigated in sheep skin and various coat colors and the function
of TRP-2 was determined via deep sequencing of skin transcriptome,
immunohistochemistry, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR), cell transfection and western blotting.
This work may provide a theoretical basis for research on the
underlying regulatory mechanisms of animal coat color.
Materials and methods
Experimental animals and sample
collection
Sheep housing and care and skin sample collection
for experimental use were conducted in accordance with the
International Guiding Principles for Biomedical Research Involving
Animals (12). The study was
approved by the Experimental Animals Ethical Committee of Shanxi
Agricultural University (Shanxi, China).
During the study phases, the studied sheep were kept
in the following conditions: 12 h light/dark cycle ratio, 25±2°C,
50±5% humidity and acceptable ventilation conditions. In all
stages, food and water were supplied freely to sheep. Following
shaving and disinfection, nine Dorset sheep (Shanxi Hunyuan Sheep
Yard, Jinzhong, China) were subjected to a skin biopsy using
cutisectors. The sheep were all 1-year-old males and consisted of
three sheep for each of the black, white and black-white coat
colors. Every effort was made to minimize the suffering and number
of animals used in the study. A total of 3 biopsies were collected
from each of the black and white sheep, whereas three from
dark-colored areas and three from light-colored areas were obtained
in black-white sheep. A single sample was used for deep sequencing
of skin transcriptome at Beijing Genomics Institute (Guangdong,
China), one for RNA isolation and the last was fixed in 10%
formalin overnight at 4°C, processed, embedded in paraffin and
sectioned at 5 µm thickness.
RNA preparation, PCR and RT-qPCR. Total RNA was
isolated from 12 sheep skin samples using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc. Waltham, MA,
USA). Total RNA concentrations were determined using a NanoDrop
2000 spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA), and electrophoresis
performed using an 0.8% agarose gel to confirm the integrity of
total RNA. Complementary strand cDNA was synthesized following the
instructions of the reverse transcription kit (cat no. RR014A;
Takara Biotechnology, Co., Ltd., Dalian, China). Following
completion of RT, the samples were stored at −20°C for later use.
PCR was performed using these specific primers (Table I) and an appropriate amount of cDNA
(5 ng/µl) as the template. Conditions of the PCR were as follows:
Preheating at 95°C for 5 min, followed by 35 cycles of shuttle
heating at 95°C for 30 sec, annealing temperature at 60°C for 30
sec and extending temperature at 72°C for 20 sec. The PCR products
were detected by 1% agarose gel electrophoresis: After running at
220 V for 10 min.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Sequence | Size |
|---|
| TRP-2 |
|
|
| F1 |
GGTTCTAAAGCCATGAGCCCT | 1586 bp |
| R1 |
CTAGAGCAAGGCGTGAGCAT |
|
| F2 |
GTCCTTCGCTTTGCCCTACT | 175 bp |
| R2 |
GACTCGGCGGTTGTAGTCAT |
|
| 18S |
|
|
| F |
AGTCCCTGCCCTTTGTACACA | 198 bp |
| R |
TTATTGCTTAAGAATACGCGTAG |
|
The levels of gene mRNA were measured using
SYBR®Premix Ex TaqTMII (Tli RNaseH Plus, cat no.
DRR081A; Takara Biotechnology, Co., Ltd.). The cDNA synthesis for
RT-qPCR analysis of TRP-2 expression in sheep skin was performed
using the NCode mRNA q-RT-PCR kit, according to the manufacturer's
protocol (Invitrogen; Thermo Fisher Scientific, Inc.). In brief,
total RNA was polyadenylated using poly A polymerase and adenosine
triphosphate. Complementary DNA was synthesized using the NCode
universal reverse primer, and RT-qPCR was performed using a TRP-2
primer. All reactions were performed in triplicate on the
Stratagene Mx3005P Real-Time QPCR system. Conditions for RT-qPCR
were as follows: Preheating at 95°C for 5 min, followed by 40
cycles of shuttle heating at 95°C for 30 sec, annealing temperature
at 60°C for 30 sec, extending temperature at 72°C for 20 sec.
Quantification of TRP-2 transcriptional abundance was compared
using the 2−ΔΔCq cyclic thresholding method (13), and the abundance of TRP-2 relative
to 18S was determined. RT-qPCR was performed as previously
described (14–17). All primer sequences are listed in
Table I.
Immunohistochemical analysis
The optimal conditions for the immunohistochemistry
procedure were previously described (18–20).
The present study used the streptavidin-biotin-enzyme complex
(SABC) system and peroxidase (Biomeda staining kit; Dako, Agilent
Technologies, Inc., Santa Clara, CA, USA), which is an indirect
immunohistochemistry method using a streptavidin-biotin complex
that allows signal amplification. Free-floating sections were
washed in 0.1 M PBS and incubated in 3% hydrogen peroxide for 15
min at room temperature to block the activity of endogenous
peroxidase. Following washing with 0.1 M PBS, the sections were
boiled in 0.01 M citric acid for 10 min, followed by a 20 min
immersion in PBS containing 5% bull serum albumin (cat no. C500626;
Sangon Biotech, Co., Ltd., Shanghai, China) at 37°C. The sections
were incubated overnight at 4°C with anti-TRP-2 (Dct H-150; cat no.
sc-25544; 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
and then at room temperature for 0.5 h. Following three washes in
0.1 M PBS for 5 min each, the sections were incubated with
biotinylated anti-rabbit IgG as a secondary antibody (cat no.
BA1003; 1:100; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) at 37°C for 20–30 min. Following washing with PBS, the
sections were incubated with avidin-biotin-peroxidase (1:200; SABC
Elite; Wuhan Boster Biological Technology, Ltd.). The products of
the immunoreaction were visualized by incubating the sections in a
staining solution containing 0.04% 3,3′-diaminobenzidine, 0.06%
hydrogen peroxide and 0.06% nickel sulfate at room temperature for
5–10 min. Nuclei were counterstained with hematoxylin at room
temperature for 5 min, and the sections were observed under a light
microscope (DM3000 b; Leica Microsystems GmBH, Wetzlar, Germany).
For the negative controls, PBS was substituted for the primary
antibody (21).
Optical density of TRP-2
collection
A total of 15 immunohistological sections were
prepared for each coat color. Using the image analysis system
Image-pro plus version 6. 0 (Media Cybernetics, Inc., Rockville,
MD, USA) to check the staining intensity: Objects were counted and
characterized using manual and automatic measurement tools, and
optical density; objects of interest were noted and density
parameters, negative staining=0, positive staining=1, were employed
for analysis. Three positive TRP-2 data were collected from
melanocytes for each section and were used for statistical
analysis.
Expression vector construction for
TRP-2
An oligonucleotide sequence corresponding to the
TRP-2 sequence was synthesized and ligated into a mammalian
expression vector, pLV.ExBi.P/Puro-CMV-eGFP-MCS-TYP2 by Cyagen
Biosciences, Inc. (Guangzhou, China). The vector contained a
cytomegalovirus promoter, which drives the expression of TRP-2 and
green fluorescent protein (GFP). The TRP-2 sequence in the
constructs was identical to the endogenous sequence. It was
demonstrated that this plasmid was capable of simultaneously
expressing TRP-2 and GFP in the present study. The GFP expression
unit was used to monitor TRP-2 expression. The TRP-2 construct was
transformed into Escherichia coli DH5α (cat. no. CW0808; CW
Biotech, Beijing, China) and the positive colonies were verified by
sequencing (Beijing Genomics Institute, Beijing, China).
Culture of sheep melanocytes and
transfection of TRP-2
The skin of newborn sheep was removed using sterile
techniques and digested with 0.25% dispase II (cat no. 17105041;
Thermo Fisher Scientific, Inc.) at 4°C for 12–14 h. The epidermis
was then separated from the dermis using scalpel and forceps, cut
into sections, and treated with 0.05% trypsin-0.01% EDTA at 37°C
for 10 min. The melanocytes were seeded (4.5×106
cells/ml) in a melanocyte basal medium supplemented with 0.2 µg/ml
cholera toxin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 2.5
µg/ml fungizone (cat. no. V900919-1G; Sigma-Aldrich; Merck KGaA),
0.05 mg/ml gentamicin (cat. no. G1272; Sigma-Aldrich; Merck KGaA),
0.5 µg/ml hydrocortisone (cat. no. A610506; Sangon Biotech, Co.,
Ltd.), 50 µg/ml bovine pituitary extract (cat. no. 13028014;
Invitrogen; Thermo Fisher Scientific, Inc.), 1 ng/ml basic
fibroblast growth factor (cat. no. 13256029; Thermo Fisher
Scientific, Inc.), 10 ng/ml tissue plasminogen activator (cat no.
T0831; Sigma-Aldrich; Merck KGaA) and 5 µg/ml insulin (cat. no.
I8040; Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). The cell culture plate was coated with 0.01% rat
tail collagen (cat. no. C7661; Sigma-Aldrich; Merck KGaA).
Vector-TRP-2 and empty vector (pLV.ExBi.P/Puro-CMV-eGFP-MCS, Cyagen
Biosciences, Inc.) transfections were performed at 60–80% cell
confluence, in accordance with the operation manual of the kit
(cat. no. 11668027; Thermo Fisher Scientific, Inc.); normal
untransfected melanocytes served as a control. The transfection
reagent was Lipofectamine® and 3 ug/ml of each vector
was used for transfection. Following 12 h of culture at 37°C, the
medium was replenished and the cells were observed under an
inverted microscope (Leica Microsystems, Inc., Germany). The
extracted cell Total RNA and proteins were subjected to RT-qPCR and
western blot analysis, respectively.
Western blotting
Total protein was extracted from melanocytes by
using a total protein extraction radioimmunoprecipitation assay
lysis buffer (cat. no. CW23345; CW Biotech). Protein concentrations
were measured using an Enhanced Bicinchoninic Acid Protein Assay
kit (cat. no. P0009, Beyotime Institute of Biotechnology, Haimen,
China) using pure bovine serum albumin (cat. no. ST023-50 g;
Beyotime Institute of Biotechnology) as a reference. Extracts were
denatured at 95°C for 5 min. For 10% SDS-PAGE, 100 µg total protein
sample was deposited in each well. The separated protein was
transferred to a nitrocellulose membrane, which was blocked with 5%
skim milk at room temperature for 1 h and then incubated with a
TRP-2 mouse monoclonal antibody (TRP2 C9; 1:500; cat. no. sc-74439;
Santa Cruz Biotechnology, Inc.) and a GAPDH rabbit polyclonal
antibody as an internal reference (1:3,000; cat no. sc-25778; Santa
Cruz Biotechnology, Inc.) at 4°C overnight. To reveal TRP-2
interference in melanogenesis associated transcription factor
(MITF), which is an important transcription factor located upstream
of TRP-2 involved in the melanogenesis pathway that is associated
with pigmentation, MITF protein expression levels (total and
phosphorylated protein) were measured by incubating with a MITF
mouse monoclonal antibody (MITF 21D1418; 1:200; cat. no. sc-52938;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. On the second
day, the membrane was transferred to room temperature for 30 min
and washed three times In TBST with 0.1% Tween-20 for 10 min each.
Horse radish peroxidase-conjugated anti-mouse IgG (1:10,000; cat.
no. BA1001; Wuhan Boster Biological Technology, Ltd.) was added to
cover the membrane and incubated at 37°C with horizontal shaking
for 1 h. Following washing six times with TBST for 5 min each, the
membrane was developed using the highly sensitive, enhanced
chemiluminescence solution (cat. no. CW00495; CW Biotech)
consisting of solutions A and B at 1:1 ratio followed by film
exposure. The resulting image was scanned. The TRP-2, MITF and
GAPDH immunoblot results were analyzed using the Image-ProPlus
version 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA)
to measure the area and gray value of each target band.
Semi-quantitative analysis was performed by comparing the target
protein and the internal reference using the following equations:
Protein content=area of the band × average gray scale;
semi-quantitative target protein content=target protein
content/GAPDH protein content. All data were presented as mean ±
standard error. Univariate analysis of variance and a Tukey's post
hoc test were performed using SPSS software, version 19.0 (IBM
SPSS, Armonk, NY, USA), and P<0.05 was considered to indicate a
statistically significant difference.
Determination of melanin content
Constitutive and TRP-2 over-expressive melanin
contents were compared on the third day following transfection.
Melanin content was determined as previously described (22). Melanocytes were harvested, rinsed
with PBS, and counted. Melanin was solubilized in 0.2 mol/l NaOH,
resulting in a cell count of 106 cells/ml, and measured
spectrophotometrically at an absorbance of 475 nm against a
standard curve of known synthetic melanin (Sigma-Aldrich; Merck
KgaA) concentrations. Melanin content was expressed as
µg/106 cells. All experiments were performed in
triplicate.
Statistical analysis
All experiments were performed in triplicate. Data
are presented as the mean ± standard deviation. Differences in the
protein abundance of TRP-2 in sheep skin samples of various coat
colors were determined by univariate analysis of variance, followed
by a Tukey's post hoc test using SPSS software, version 19.0 (IBM
SPSS). P<0.05 was considered to indicate a statistically
significant difference.
Results
Transcriptome sequencing and analysis
of black and white sheep skins
A total of 74,533 and 90,006 unigenes were assembled
from the reads obtained from black and white sheep skin,
respectively. Genes encoding for the ribosomal proteins and
keratin-associated proteins were the most highly expressed. A total
of 2,235 known genes were differentially expressed in black versus
white sheep skin, with 479 genes upregulated and 1,756 genes
downregulated. A total of 845 novel genes were differentially
expressed in black sheep skin compared with white sheep skin, 107
of which were upregulated, including 2 highly expressed genes
exclusively expressed in black sheep skin, whereas 738 were
downregulated. A total of 49 known coat color genes were expressed
in sheep skin, 12 of which revealed high expression in black sheep
skin (Table II). A number of
these upregulated genes, including TRP-2 (Dct), membrane-associated
transporter protein, TYR and TRP-1 are members of melanosome
components and their precursor ontology category.
 | Table II.Differentially expressed known coat
color genes in black vs. white sheep skin. |
Table II.
Differentially expressed known coat
color genes in black vs. white sheep skin.
| Symbol | Gene name | Differential
expression | Function |
|---|
| Edn3 | Endothelin 3 | 4.903 | Growth and
differentiation factor |
| Dct
(Trp-2) | Dopachrome
tautomerase | 6.023 | Melanosomal
enzyme |
| Gpnmb | Glycoprotein
NMB | 4.281 | Apparent
melanosomal component |
| Matp | Membrane-associated
transporter protein | 32.75 | Apparent
transporter |
| Rab38 | RAB38, member RAS
oncogene family | 2.206 | Targeting of
Trp1 |
| Si | Silver | 25.2 | Melanosome
matrix |
| Tyr | TYR | 61.79 | Melanosomal
enzyme |
| Trp-1 | TYR-related protein
1 | 284.17 | Melanosomal
protein |
| Mlph | Melanophilin | 27.75 | Melanosome
transport |
| Ggt1 |
Glutamyltranspeptidase 1 | 5.747 | Glutathione
metabolism (pheomelanin synthesis) |
| Mc1r | Melanocortin 1
receptor | 9.315 |
Eumelanin/pheomelanin switch (among
others) |
| Mgrn1 | Mahogunin, ring
finger 1 | 2.814 | Melanin color, CNS
role. E3 ubiquitin ligase |
TRP-2 mRNA expression
In the skins of white sheep, light-colored and
dark-colored regions of piebald sheep, and black sheep, the
expression levels of the TRP-2 gene following PCR amplification was
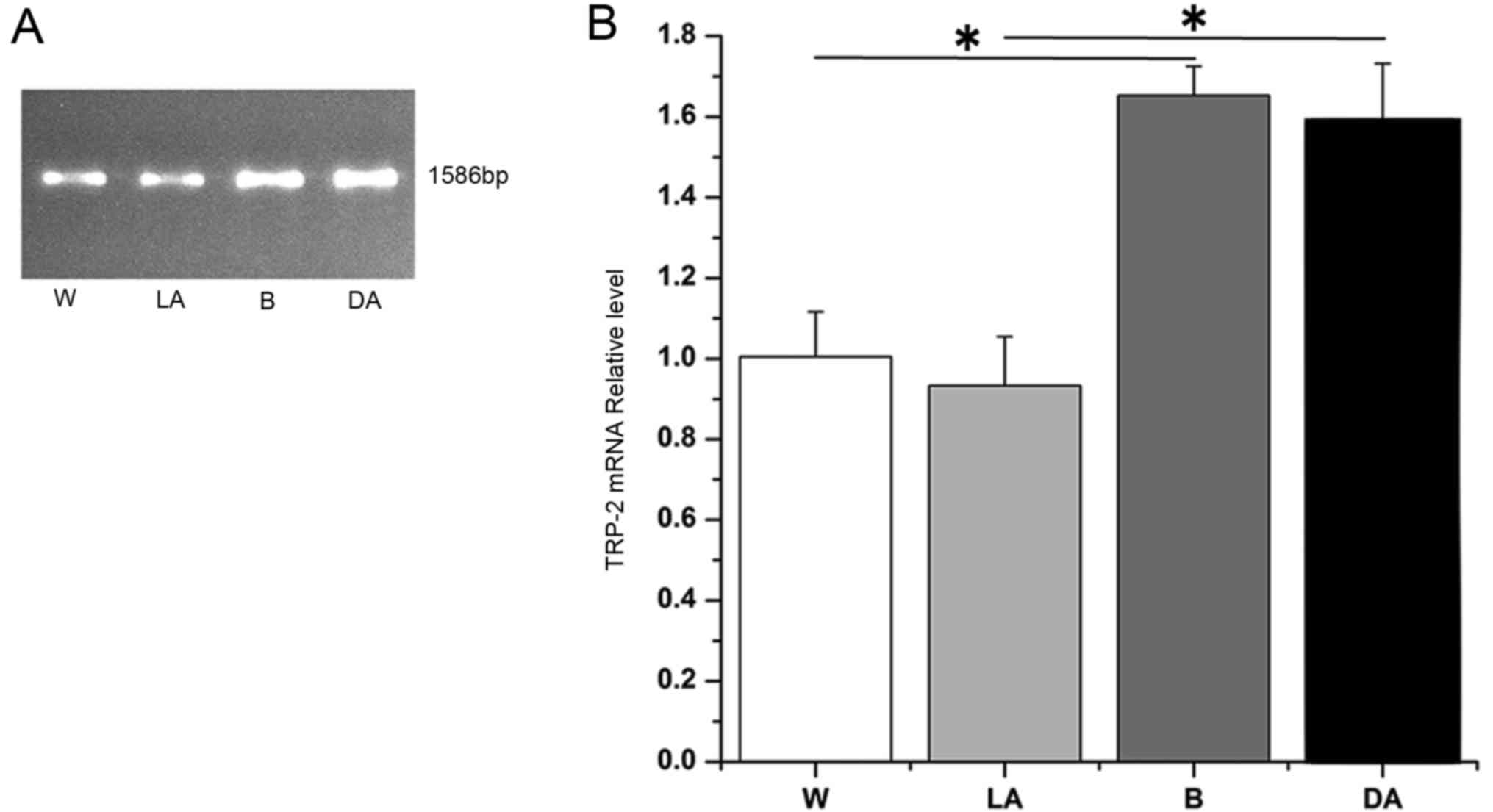
determined, by 0.8% gel electrophoresis. The results revealed that
the four groups produced TRP-2 bands without any nonspecific
background. TRP-2 was 1,586 bp (representative data shown, Fig. 1A). Following sequencing, 4 TRP-2
sequences consistently aligned with the NCBI-published sequences
from Bos taurus, Capra hircus, Camelus bactrianus, Sus scrofa, Homo
sapiens and Balaenoptera acutorostrata scammoni via Basic Local
Alignment Search Tool, demonstrating alignment scores of 100, 100,
98, 99, 99 and 99%, respectively (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome).
Following evaluation of mRNA integrity, the RT-qPCR amplification
curve and melting curve revealed optimal results and the target
gene, TRP-2, and reference genes did not produce nonspecific
amplification or primer dimers (data not shown). The results of the
data analysis demonstrated that TRP-2 mRNA expression levels in
black sheep, dark-colored regions of piebald sheep and
light-colored regions in piebald sheep was 1.75, 1.67 and 0.94
times increased compared with white sheep (Fig. 1B). TRP-2 mRNA expression levels in
black sheep and dark-colored regions in piebald sheep were
significantly increased compared white sheep and light-colored
regions in piebald sheep (Fig. 1B;
P<0.05). However, the difference in the expression levels of
black sheep and dark-colored regions was not significant (Fig. 1B). The difference in expression
levels between white sheep and light-colored regions in piebald
sheep was also not significant (Fig.
1B). These findings suggested that differences in coat color of
sheep may be associated with the TRP-2 mutation.
Immunolocalization and TRP-2
expression levels in sheep of various coat colors
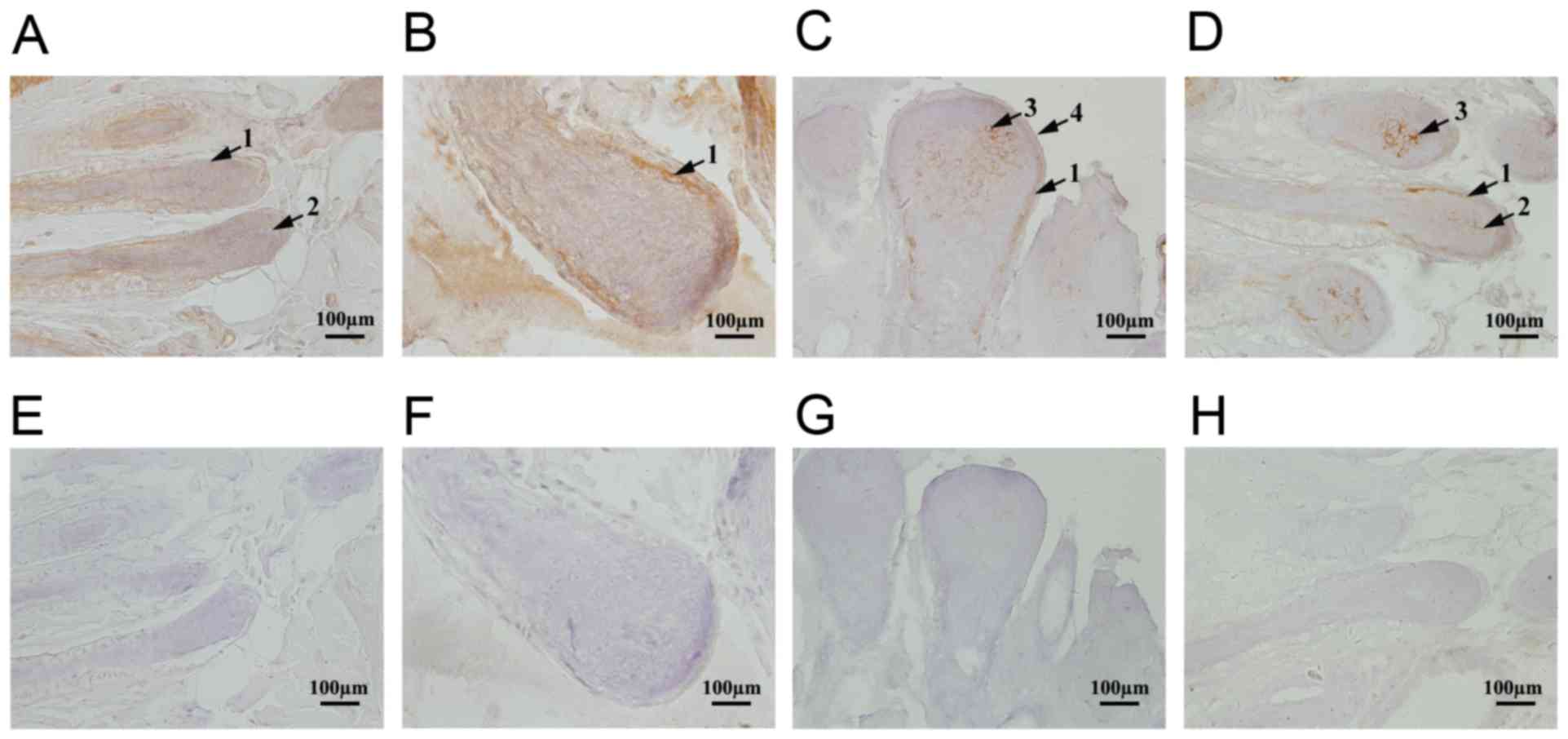
Immunohistochemical assays were performed to
determine the localization of TRP-2 in sheep coats of various
colors. TRP-2 was expressed in the epidermis, inner root sheath and
dermal papilla of skins (representative data shown, Fig. 2A-D). Different expression levels of
TRP-2 were observed in various coat colors and in the same sheep at
regions of different coat colors. In white sheep, TRP-2 staining
was weakly positive (Fig. 2A),
whereas that in black was strongly positive, particularly in the
dermal papilla and inner root sheath melanocytes (Fig. 2C). TRP-2 staining intensity was
similar between single-colored sheep and those with various colored
regions (Fig. 2A-D). TRP-2
expression levels were lowest in white-coated sheep and highest in
black-coated sheep (Fig. 2A and
C). Furthermore, the levels of TRP-2 in dark-colored areas were
significantly higher compared with light-colored areas in piebald
sheep (Fig. 2D and B). Melanin
synthesis was associated with TRP-2 expression. The control group
demonstrated no positive staining (Fig. 2E-H).
Optical density analysis of the
immunohistochemical results
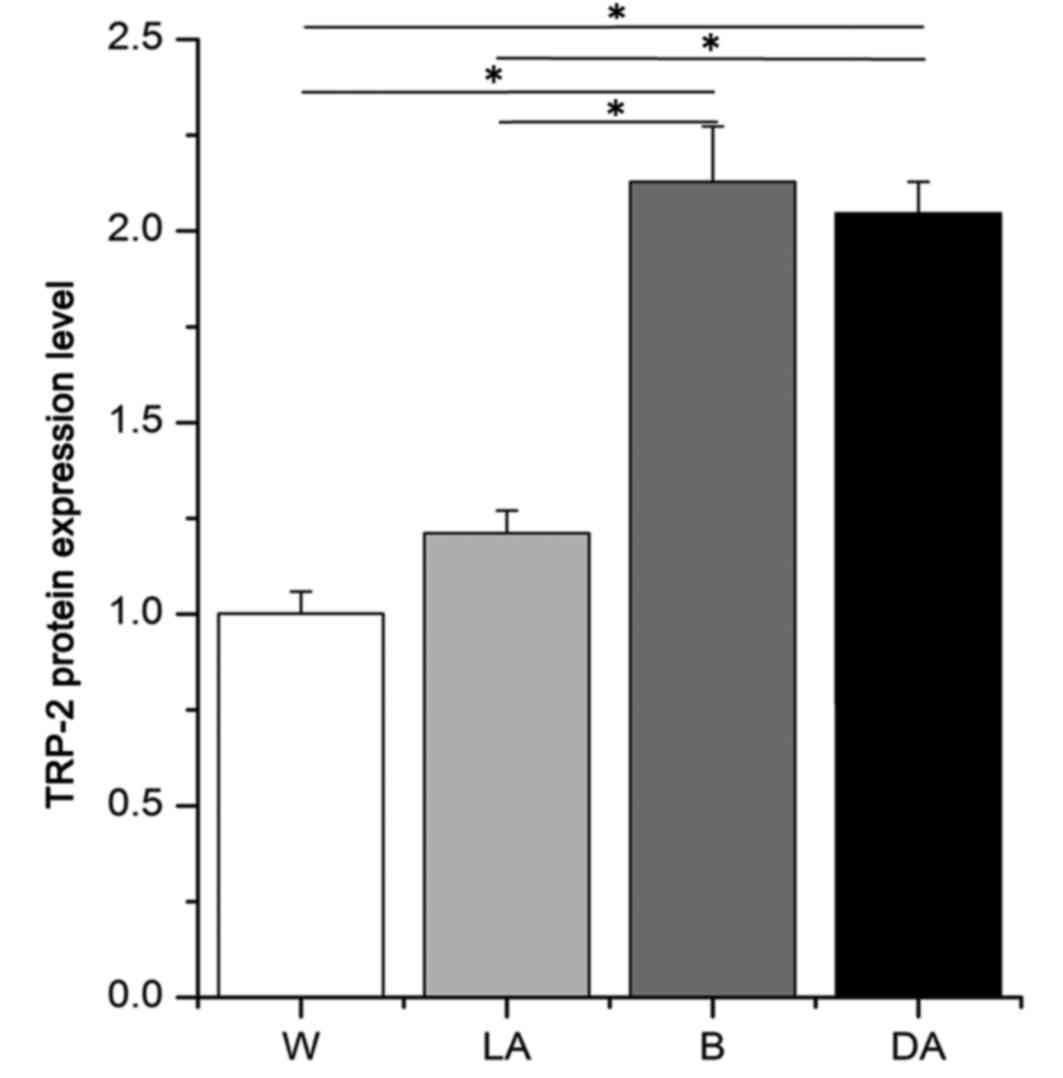
Average optical density values for TRP-2 were
2.13±0.14, 2.04±0.08, 1.21±0.06 and 1.00±0.06 in black skin, black
dots, white dots and white skin, respectively (Fig. 3). These values indicated that TRP-2
was expressed at 1.76- and 2.13-fold increased levels in black skin
versus light-colored areas in piebald skin and white skin and 1.68-
and 2.04-fold higher in dark-colored areas versus light-colored
areas in piebald skin and white skin, respectively. No significant
difference in TRP-2 expression between black skin and dark-colored
areas in piebald skin was observed (Fig. 3).
Detection of melanin following
transfection of sheep melanocytes with TRP-2
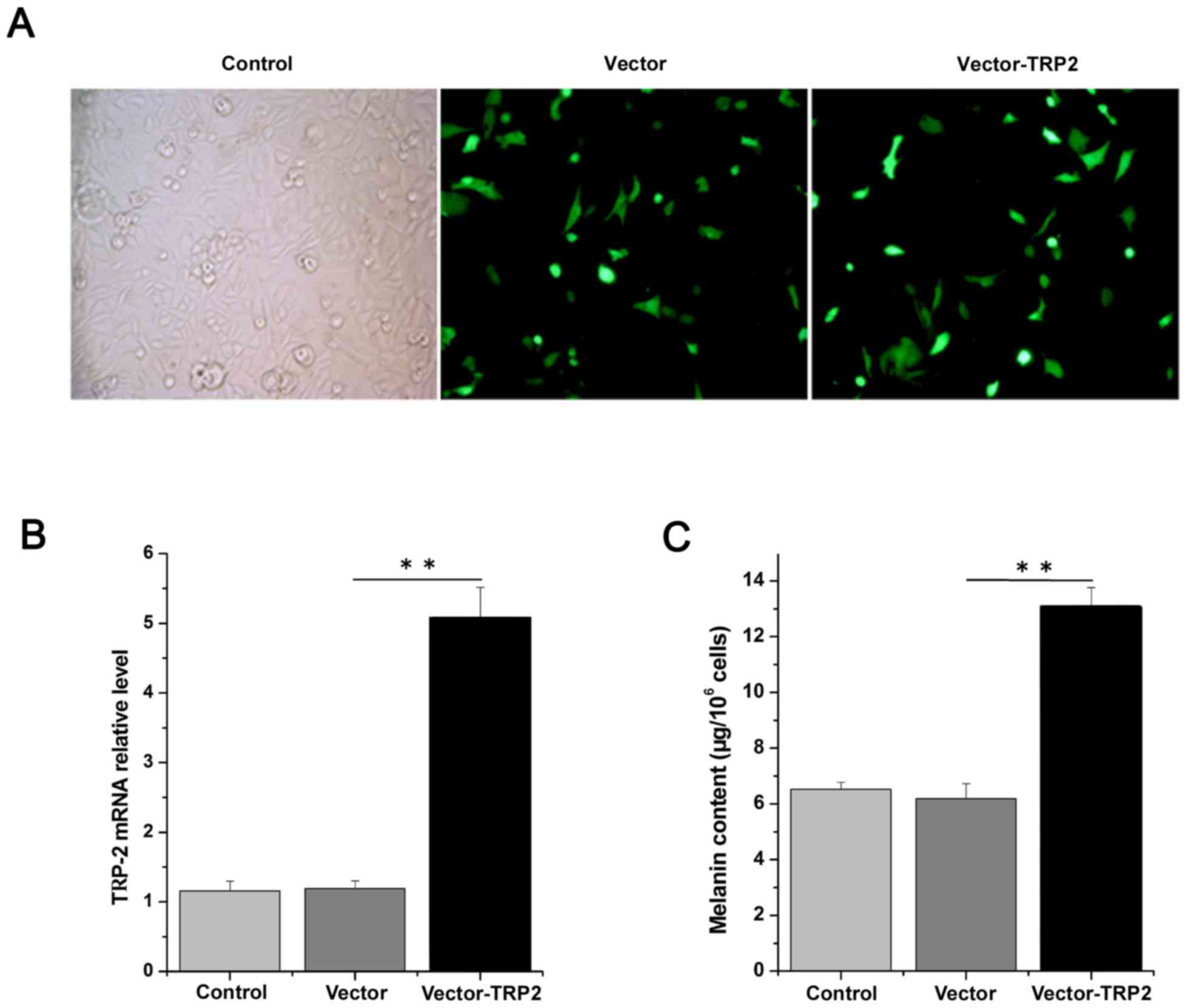
To determine the effects of TRP-2, TRP-2 was
overexpressed by transfecting logarithmic-phase primary melanocytes
with the TRP-2 plasmid. The melanocytes exhibited good growth
conditions, with the morphology revealing polygon and dendritic
protrusions. Following transfection, melanocytes demonstrated
normal growth status and high transfection efficiency (Fig. 4A). Cells transfected with the
pLV.ExBi.P/Puro-CMV-eGFP-MCS-TYP2 construct demonstrated a 5-fold
higher expression of TRP-2 compared with cells transfected with the
pLV.ExBi.P/Puro-CMV-eGFP-MCS vector control (Fig. 4B). The melanin content was
6.54±0.24, 6.19±0.54 and 13.08±0.69 µg/106 cells in the
control, vector without TRP-2 and vector-TRP-2 group, respectively,
suggesting that the overexpression of TRP-2 produced more melanin
compared with the other groups (Fig.
4C). These data support the hypothesis that TRP-2
overexpression may enhance melanin content and influence coat color
synthesis. In addition, western blotting results demonstrated that
TRP-2 protein levels were significantly increased 1.38-fold in
cells overexpressing TRP-2 compared with those transfected with the
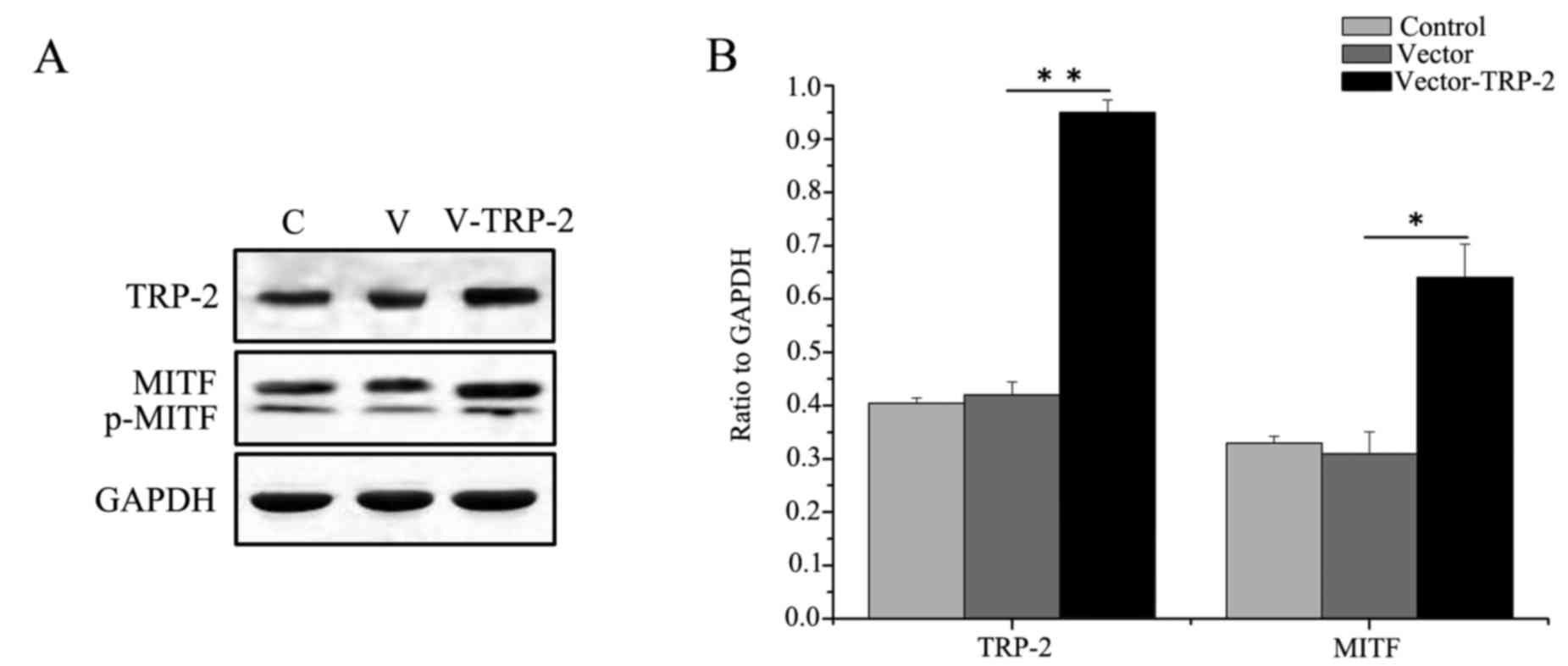
vector control (Fig. 5A).
It was demonstrated that MITF was significantly
increased in the TYP2-overexpressing cells compared with the
control group (Fig. 5A and B),
suggesting that TRP-2 regulates expression of MITF.
Discussion
In mammals, the development of skin accessory
organs, including hair follicles and hair shafts, involves cycles
of growth (anagen), regression (catagen) and rest (telogen)
(23). Hair follicles have a
complicated shape and structure and a three-dimensional
conformation. They control hair cyclical growth and renew
themselves. Once hair follicles are formed in the embryo, dermal
papilla and the first half of the hair follicle do not alter
further following birth, whereas the other hair follicles, along
with stromal cells and nutrients, undergo cyclic alterations
(24). In the human mature
follicle, dopamine-positive melanocytes are easily detected around
the hair bulb base layer and dermal papilla, and moderately
differentiated melanocytes may be detected in the base layer of
sebaceous glands. Dopamine-negative melanocytes that cannot produce
melanin distribute in the middle of the outer root sheath or base,
whereas others are distributed in the peripheral region of the hair
bulb and the adjacent matrix. It has previously been demonstrated
that immature melanoblasts may exist in the mature follicles, and
no dopa oxidase activity occurs in the absence of pigmented hair
follicles; however, in certain dopamine-negative melanocytes, a
small amount of TYR is detected. Similarly, the expression of
mast/stem cell growth factor receptor, B-cell lymphoma 2 and
non-pigmented melanocytes also exist in hair follicles which do not
express TRP-1 and TRP-2 (25).
The present study revealed that TRP-2 was expressed
in various coat colors of adult sheep skins. TRP-2 existed in
melanocytes at the root sheath in white sheep and in light-colored
areas in piebald sheep. TRP-2 was expressed in melanocytes of
dermal papilla and hair root sheaths in black sheep and
dark-colored areas in piebald sheep, which was consistent with
previous study (26). These
results suggested that melanocytes of hair follicles, dermal
papilla and root sheaths in black sheep and back-colored regions in
piebald sheep may produce melanin. On the contrary, the cells
around hair root sheath in white sheep and light-colored regions
may be dopa-negative melanocytes, which are not involved in melanin
production.
The synthesis, transport, and distribution of
melanin in the mammalian skin, hair follicles and eyes results in
the appearance of various colors (16,27).
In melanocytes, specific organelles called melanosomes produce
melanin. TYR serves a vital role in this process and is responsible
for catalyzing three different reactions of melanin: Catalysis to
dopa, oxidation to dopaquinone, hydroxylation to dihydroxyindole.
In addition, TRP-2 participates in melanin synthesis in certain
reactions: TRP-2, which possesses the activity of dopa pigment
isomerase, catalyzes dopa pigments into DHICA (28). The present in vitro study
suggested that overexpression of TRP-2 affects melanin production.
In HeLa (29) and COS (26) cells, TRP-2 expression may lead to
the extraction of dopa pigment from these cells into DHICA, which
is the product of TRP-2 and may be used as substrates of TRP-1.
TRP-2 is an important protein that affects the formation of
melanin, and its expression and activity determines the speed of
melanin production and yield. Tyrosine hydroxyl is converted to
dopamine by TYR, whereas dobutamine is oxidized into dopaquinone
and further into various derivatives. Melanin is a dopaquinone,
indole-5 or 6 quinone and dopa pigment polymer. TRP-2 has become an
important protein to study melanin traits and albinism.
TRP-2 shares 40% identity with TYR and is a useful
marker for melanocyte differentiation. From dopaquinone, the
eumelanin and pheomelanin pathways diverge. TRP-1 and TRP-2 are two
crucial enzymes involved in eumelanogenesis. Pheomelanin is derived
from conjugation by glutathione or thiol-containing cysteine.
Therefore, pheomelanin is more photolabile and produces as
by-products, hydrogen peroxide, hydroxyl radicals and superoxide,
which result in further DNA damage. Individual melanocytes
typically synthesize both eumelanins and pheomelanins, with the
ratio of the two being determined by a balance of variables,
including pigment enzyme expression and availability of tyrosine
and sulfhydryl-containing reducing agents in the cells (30).
In the present study, it was demonstrated that the
TRP-2 protein was expressed in all coat colors of sheep, and the
expression levels of the TRP-2 protein in black sheep and
dark-colored regions in piebald sheep were significantly increased
compared with white sheep and light-colored regions. Expression was
most increased and decreased in sheep with black and white coats,
respectively. TRP-2 expression quantity and pigment production or
coat color formation were associated. However, there was a direct
association between black or white coats of sheep and TRP-2
expression. In conclusion, TRP-2 was expressed in sheep of all coat
colors. To a certain extent, TRP-2 expression levels reflected the
numbers of mature melanocytes in sheep of various coat colors,
indicating an important role for TRP-2 in the regulation of melanin
production and coat color formation in sheep. Whether the
expression of TRP-2 affects eumelanin and pheomelanin formation in
different coat-colored sheep skins require further investigation.
The findings of the present study may provide novel insights for
clinical research into skin diseases associated with pigmentation
disorders.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by the National
Natural Science Foundation of China (grant nos. 31772690 and
31302049), and the Science and Technology Innovation Foundations of
Shanxi Colleges and Universities (grant no. 20171115).
Availability of data and materials
Not applicable.
Authors' contributions
HW and XH designed the study. LX, BZ, RF and YL
conducted the studies and participated in data collection. TC and
YD performed the statistical analysis and assisted in the study
design. JL performed statistical analysis and helped draft the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Experimental
Animals Ethical Committee of Shanxi Agricultural University
(Shanxi, China). Experimental protocols were conducted in
accordance with the International Guiding Principles for Biomedical
Research Involving Animals (12).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iwata M, Corn T, Iwata S, Everett MA and
Fuller BB: The relationship between tyrosinase activity and skin
color in human Foreskins. J Invest Dermatol. 95:91990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Commo S, Gaillard OS Thibaut S and Bernard
B: Absence of TRP-2 in melanogenic melanocytes of human hair.
Pigment Cell Res. 17:488–497. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pomerantz SH and Ances IG: Tyrosinase
activity in human skin. Influence of race and age in newborns. J
Clin Invest. 55:1127–1131. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu L, Cai YQ, Jue TU and Chen ML: Cloning
and analysis of Trp1 and Trp2 genes from white hair black eyes
rabbit iris. Acta Laborat Anim Scientia Sin. 20:43–48. 2012.
|
|
5
|
Hearing VJ: The melanosome: The perfect
model for cellular responses to the environment. Pigment Cell Res.
13 Suppl 8:S23–S24. 2000. View Article : Google Scholar
|
|
6
|
Zhu Y and Xu A: The research progress of
vitiligo animal model. Int J Dermatol Venereal. 39:120–123.
2013.
|
|
7
|
del Marmol V, Ito S, Jackson I,
Vachtenheim J, Berr P, Ghanem G, Morandini R, Wakamatsu K and Huez
G: TRP-1 expression correlates with eumelanogenesis in human
pigment cells in culture. FEBS Lett. 327:307–310. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao L, Dong CS, He XY, He JP, Geng JJ, Fan
RW, Zhu ZW and You RL: Gene expression levels of alpaca tyrosinase
gene family in individuals of different colors. Chin J Ani Veter
Sci. 39:895–899. 2008.(In Chinese).
|
|
9
|
Valverde P, Healy E, Jackson I, Rees JL
and Thody AJ: Variants of the melanocyte-stimulating hormone
receptor gene are associated with red hair and fair skin in humans.
Nat Genet. 11:328–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q, Gao T, Li C, Shen Z and Li Q:
Cloning and expression of human tyrosinase related protein-2 cDNA.
J Fourth Military Med Univ. 23:629–632. 2002.(In Chinese).
|
|
11
|
Jiménez-Cervantes C, Solano F, Kobayashi
T, Urabe K, Hearing VJ, Lozano JA and García-Borrón JC: A new
enzymatic function in the melanogenic pathway. The
5,6-dihydroxyindole-2-carboxylic acid oxidase activity of
tyrosinase-related protein-1 (TRP1). J Biol Chem. 269:17993–8000.
1994.PubMed/NCBI
|
|
12
|
(CIOMS) International Guiding Principles
for Biomedical Research Involving Animals, . Altern Lab Anim.
12:ii1985.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong C, Wang H, Xue L, Dong Y, Yang L, Fan
R, Yu X, Tian X, Ma S and Smith GW: Coat color determination by
miR-137 mediated down-regulation of microphthalmia-associated
transcription factor in a mouse model. RNA. 1679–1686. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goswami S, Tarapore RS, Teslaa JJ,
Grinblat Y, Setaluri V and Spiegelman VS: MicroRNA-340-mediated
degradation of microphthalmia-associated transcription factor mRNA
is inhibited by the coding region determinant-binding protein. J
Biol Chem. 285:20532–20540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Z, He J, Jia X, Jiang J, Bai R, Yu X,
Lv L, Fan R, He X, Geng J, et al: MicroRNA-25 functions in
regulation of pigmentation by targeting the transcription factor
MITF in alpaca (Lama pacos) skin melanocytes. Domest Anim
Endocrinol. 38:200–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Segura MF, Hanniford D, Menendez S, Reavie
L, Zou X, Alvarez-Diaz S, Zakrzewski J, Blochin E, Rose A,
Bogunovic D, et al: Aberrant miR-182 expression promotes melanoma
metastasis by repressing FOXO3 and microphthalmia-associated
transcription factor. Proc Natl Acad Sci USA. 106:pp. 1814–1819.
2009; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Dong Y, Chen W, Hei J and Dong C:
Expression and localization of nerve growth factor (NGF) in the
testis of alpaca (llama pacos). Folia Histochem Cytobiol. 49:55–61.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tibary A and Vaughan J: Reproductive
physiology and infertility in male South American camelids: A
review and clinical observations. Small Ruminant Res. 61:283–298.
2006. View Article : Google Scholar
|
|
20
|
Yan YP, Dong CS, HE JP, Ren YH, He XY, Bai
R and Xie JS: Expression and localization of transforming growth
factor-β1 in Alpaca testis. Chin J Animal Veter Sci. 39:97–102.
2008.(In Chinese).
|
|
21
|
Ayer-Lelievre C, Olson L, Ebendal T,
Hallböök F and Persson H: Nerve growth factor mRNA and protein in
the testis and epididymis of mouse and rat. Proc Natl Acad Sci USA.
85:pp. 2628–2632. 1988; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen T, Wang H, Liu Y, Zhao B, Zhao Y, Fan
R, Wang P and Dong C: Ocular albinism type 1 regulates
melanogenesis in mouse melanocytes. Int J Mol Sci. 17:E15962016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alonso L and Fuchs E: The hair cycle. J
Cell Sci. 119:391–393. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Müller-Röver S, Handjiski B, van der Veen
C, Eichmüller S, Foitzik K, McKay IA, Stenn KS and Paus R: A
comprehensive guide for the accurate classification of murine hair
follicles in distinct hair cycle stages. J Invest Dermatol.
117:3–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horikawa T, Norris DA, Johnson TW, Zekman
T, Dunscomb N, Bennion SD, Jackson RL and Morelli JG: DOPA-negative
melanocytes in the outer root sheath of human hair follicles
express premelanosomal antigens but not a melanosomal antigen or
the melanosome-associated glycoproteins tyrosinase, TRP-1, and
TRP-2. J Invest Dermatol. 106:28–35. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Budd PS and Jackson IJ: Structure of the
mouse tyrosinase-related protein-2/dopachrome tautomerase
(Tyrp2/Dct) gene and sequence of two novel slaty alleles. Genomics.
29:35–43. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Luca M, D'Anna F, Bondanza S, Franzi AT
and Cancedda R: Human epithelial cells induce human melanocyte
growth in vitro but only skin keratinocytes regulate its proper
differentiation in the absence of dermis. J Cell Biol.
107:1919–1926. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Winder AJ, Wittbjer A, Odh G, Rosengren E
and Rorsman H: The mouse brown (b) locus protein functions as a
dopachrome tautomerase. Pigment Cell Res. 7:305–310. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yasumoto K, Yokoyama K, Takahashi K,
Tomita Y and Shibahara S: Functional analysis of
microphthalmia-associated transcription factor in pigment
cell-specific transcription of the human tyrosinase family genes. J
Biol Chem. 272:503–509. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Land EJ and Riley PA: Spontaneous redox
reactions of dopaquinone and the balance between the eumelanic and
phaeomelanic pathways. Pigment Cell Melanoma Res. 13:273–277. 2000.
View Article : Google Scholar
|