Introduction
Chronic rhinosinusitis with nasal polyps (CRSwNP) is
a major health care problem that affects about 0.5 to 4% of the
general population (1). CRSwNP
leads to high management costs and poor quality of life (2). Histomorphological transformation in
nasal polyps (NPs) involves infiltration of inflammatory cells,
abnormal angiogenesis, remarkable edema, submucosal fibrosis, a
decreased number of mucous glands and mucosal epithelial
hyperplasia (3). Many researches
indicate that the increase in capillary and basilar membrane
permeability in NPs leading to severe edema and polyp growth
(4,5). However, the exact pathogenic
mechanisms underlying NPs are still largely unknown. Therefore,
further research of capillary may help to illuminate the
pathogenesis of NPs.
Exosomes, as an important communication tool among
various cells, are very popular in present clinical research
(6,7). Exosomes are small, 50–100 nm membrane
vesicles, which are released extracellularly after fusion of
multivesicular endosomes with the cell membrane (8). Exosomes exist in various body fluids
such as serum, urine, and breast milk (9–12).
Recently, some researches have shown that exosomes in human nasal
lavage fluid (NLF) can be as a new diagnostic indicator of upper
respiratory tract disease (13–15).
Exosomes containing abundant protein, mRNA, miRNA and some other
bioactive substances are involved in immunoregulation,
extracellular matrix remodeling, cellular signal transduction and
so on (16,17). Lee et al (18) have found that in hypoxia condition,
exosomes can promote pathological angiogenesis in a zebrafish tumor
model. Kalani et al (19)
also have shown that exosomes can regulate permeability of human
umbilical vein endothelial cells (HUVECs). Although there have been
many reports about exosomes, the role of exosomes in NPs are still
unknown.
A Disintegrin And Metalloproteases (ADAMs) are
family of proteases that are responsible for the liberation of a
variety of cell surface expressed proteins. Recently, some studies
have identified that ADAM10 can influence angiogenesis (20) and vascular permeability (21), which then inducing the genesis and
progression of some inflammatory diseases and cancers. In turn,
ADAM10 may be a potential therapeutic target in these diseases
(22,23). Particularly, ADAM10 has been
identified as a novel binding partner of VEGFR2 which influencing
the role of VEGF-VEGFR in vascular permeability, and ADAM10
regulates endothelial permeability by proteolysis of vascular
endothelial cadherin in the progressing of atherosclerosis
(24–26). Recently, ADAM10, but not other
metalloprotionases, has been identified in healthy nasal exosomes
in Lässer et al 2016 Journal of Translational Medicine
(15). However, the expression
level and the function of ADAM10 in NLF-derived exosomes from NPs
has not been demonstrated. In this study, exosomes were
successfully isolated from NLF, and we proved that the NLF-derived
exosomes from NPs promoted angiogenesis and vascular permeability.
Besides, we revealed that ADAM10 was highly expressed in
NLF-derived exosomes from NPs. Therefore, we speculated that ADAM10
may play an important role in the effects of NLF-derived exosomes
from NPs on angiogenesis and vascular permeability.
Materials and methods
Clinical specimens and cell lines
A total of 25 patients with CRSwNP were enrolled in
this prospective clinical study from the Affiliated Hospital of
Nantong University (Nantong, China). The diagnosis of CRSwNP was
made to the criteria established by the European Position Paper on
rhinosinusitis and nasal polyps guidelines (1). Each patient underwent routine workup
before operation, including a medical interview, a physical
examination, anterior rhinoscopy, nasal endoscopy, a computerized
tomography (CT) scan, blood tests, and skin prick tests (SPT). No
patient had a history of allergic rhinitis, asthma, or aspirin
sensitivity, and none had received systemic and/or topical nasal
steroids treatment at least 3 weeks before surgery. A total of 15
healthy volunteers were recruited to collect their NLF.
HUVECs (ScienCell Research Laboratories, Inc., San
Diego, CA, USA) were cultured in DMEM low glucose (HyClone, Logan,
UT, USA) and incubated at 37°C containing 5% CO2.
Written informed consent was obtained from all the NPs patients and
healthy volunteers, and the study was approved by the Medical
Ethics Committee of the Affiliated Hospital of Nantong
University.
NLF collection
NLF was collected from NP patients and healthy
volunteers respectively. We used an established method with minor
adjustments (27). In brief, 5 ml
of 0.9% saline was instilled in right nostril of the person while
the head leaned back at an angle about 30° and the soft palate
closed. The NLF was obtained by bending the head forward and
passively collecting the fluid. And then the procedure was repeated
in the left nostril. The NLF we collected was then passed through a
cell strainer (100 µm) and promptly centrifuged (3,000 × g, 20 min,
4°C) to remove crust and debris. The supernatant of NLF was stored
at −80°C before exosomes isolation.
Exosomes isolation and
purification
Exosomes were isolated from NLF of NP patients (NPs
exosomes) and healthy volunteers (normal exosomes) using a
previously described method (28,29)
with the modifications that included differential centrifugation of
NLF (6,000 × g for 30 min at 4°C and 10,000 × g for 60 min at 4°C)
followed by ultrafiltration (0.2-µm filter; Sarstedt,
Nümbrecht-Rommelsdorf, Germany) and qEV size-exclusion columns
(iZON Science, Christchurch, New Zealand). The supernatant of NLF
was then ultracentrifuged (100,000 × g) for 60 min at 4°C (Type 90
Ti Rotor; Beckman Coulter, Inc., Brea, CA, USA) to pellet the
exosomes. The exosome pellets were then washed once with PBS.
Electron microscopy
The exosome pellets were fixed with 2.5%
glutaraldehyde and centrifuged at 100,000 × g to remove the
glutaraldehyde. Afterwards, the pellets were stained by 3% aqueous
phosphotungstic acid and fixed on copper mesh formvar grids.
Samples were examined in a JEOL Transmission Electron Microscope
(JEM-1230; JEOL, Ltd., Tokyo, Japan).
Nanoparticle tracking analysis
(NTA)
NTA utilizes the properties of both light scattering
and Brownian motion in order to obtain the particle size
distribution of samples in liquid suspension. The NanoSight range
of instruments provides high resolution particle size,
concentration and aggregation measurements. Appropriate exosomes
concentration were used to assess the size distribution by Zeta
View (Particle Metrix GmbH, Meerbusch, Germany).
In vitro tube formation assay
Matrigel tube formation assays using growth
factor-reduced Matrigel (BD Biosciences, Franklin Lakes, NJ, USA).
HUVECs were seeded at 1.5×104 cells/well of 96-well
plates precoated with Matrigel and cocultured with exosomes for 6 h
at 37°C. Images of the tube formation were obtained using an
inverted microscope. Tubes were defined as elongated connecting
branches between 2 identifiable HUVECs.
In vitro permeability assay
HUVECs were seeded on transwell chamber (0.4-µm pore
size; Costar, Corning, NY, USA) in 24-well plates and grown until
reaching confluence. Fluorescein isothiocyanate (FITC)-dextran (1
mg/ml) (M, 40 000; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) was then added to the upper chamber. At the indicated time
points 50-µl samples were taken from the lower chamber and replaced
with the same volume of culture medium. A fluorescence plate reader
(Lambda Fluoro 320; MWG Biotech, Ebersberg, Germany) was using to
measure the fluorescent content of samples.
Cell viability assay
Cells were seeded into 96-well plates and assessed
by CCK-8 assay (Beyotime Institute of Biotechnology, Haimen,
China). The absorbance of each well was read on a microplate reader
(F-2500 Fluorescence Spectrophotometer; Hitachi, Ltd., Tokyo,
Japan) at 450 nm.
Uptake experiment
According to the manufacturer's instructions,
NLF-derived exosomes labeled by PKH67 (Sigma-Aldrich, St. Louis,
MO, USA) were added into HUVECs. HUVECs were then fixed and
processed an immunocytochemical analysis. Photographs were acquired
using a TCS SP-5 confocal microscope (Leica Microsystems, Wetzlar,
Germany), captured under 400 Hz with an image resolution of 512
×512 pixels, and then analyzed by Leica Application Suite 2.02.
Western blot analysis
Protein samples were separated using 10% SDS-PAGE
gel and transferred to polyvinylidene fluoride (PVDF) membranes,
and then blocked with 5% non-fat milk. The PVDF membranes were
incubated with primary antibody against ADAM10 (1:300;
D221496-0100; Sangon Biotech Co., Ltd., Shanghai, China), β-actin
(1:2,000; sc-47778) and CD63 (both from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), CD9 (Abcam, Cambridge, MA, USA),
respectively, overnight at 4°C. Afterwards, the membranes were
incubated with horseradish peroxidase-linked immunoglobulin G
(1:5,000; sc-2374; Santa Cruz Biotechnology, Inc.). Gray-scale
analysis was performed using Image J Software to examine the
protein bands.
Statistical analysis
Statistical analysis was performed using SPSS 19.0.
software (IBM Corp., Armonk, NY, USA). The results were presented
as mean ± standard deviation, and the statistical significance was
evaluated using analysis of variance with a Bonferroni post hoc
test. P-value <0.05 was considered as a statistically
significant difference.
Results
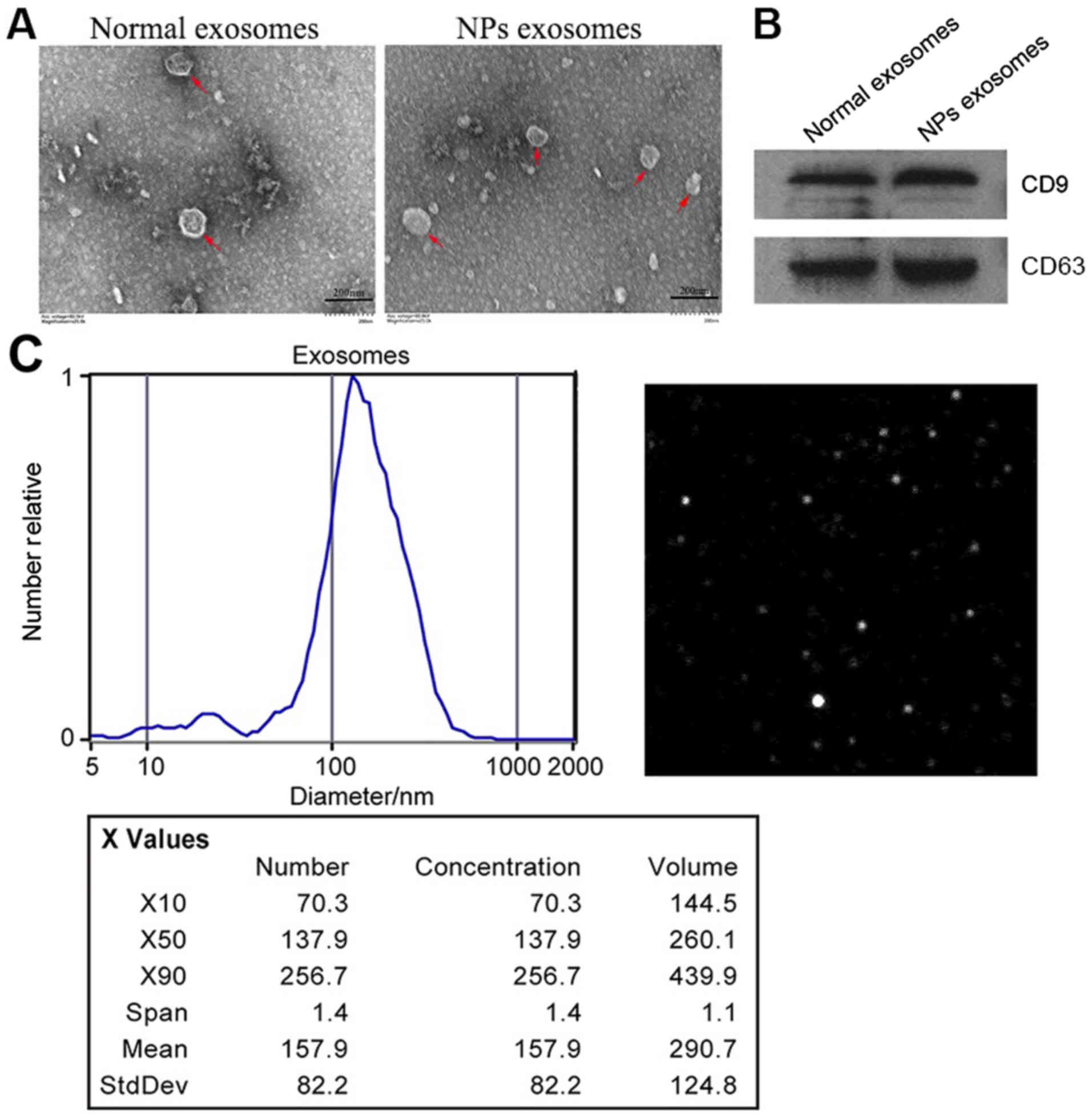
Identification of exosomes in NLF
Based on several reports indicating that exosomes
were present in NLF (13,14), we purified exosomes from the NLF of
NPs (NPs exo) and healthy donors (Normal exo). Electron microscopy
confirmed the presence of exosomes both in size and morphology
(Fig. 1A). Western blot analysis
revealed that the known exosomal markers tetraspanins CD63 and CD9
were highly expressed in NLF-derived exosomes (Fig. 1B). NTA showed vesicles with sizes
between 50 and 100 nm (Fig.
1C).
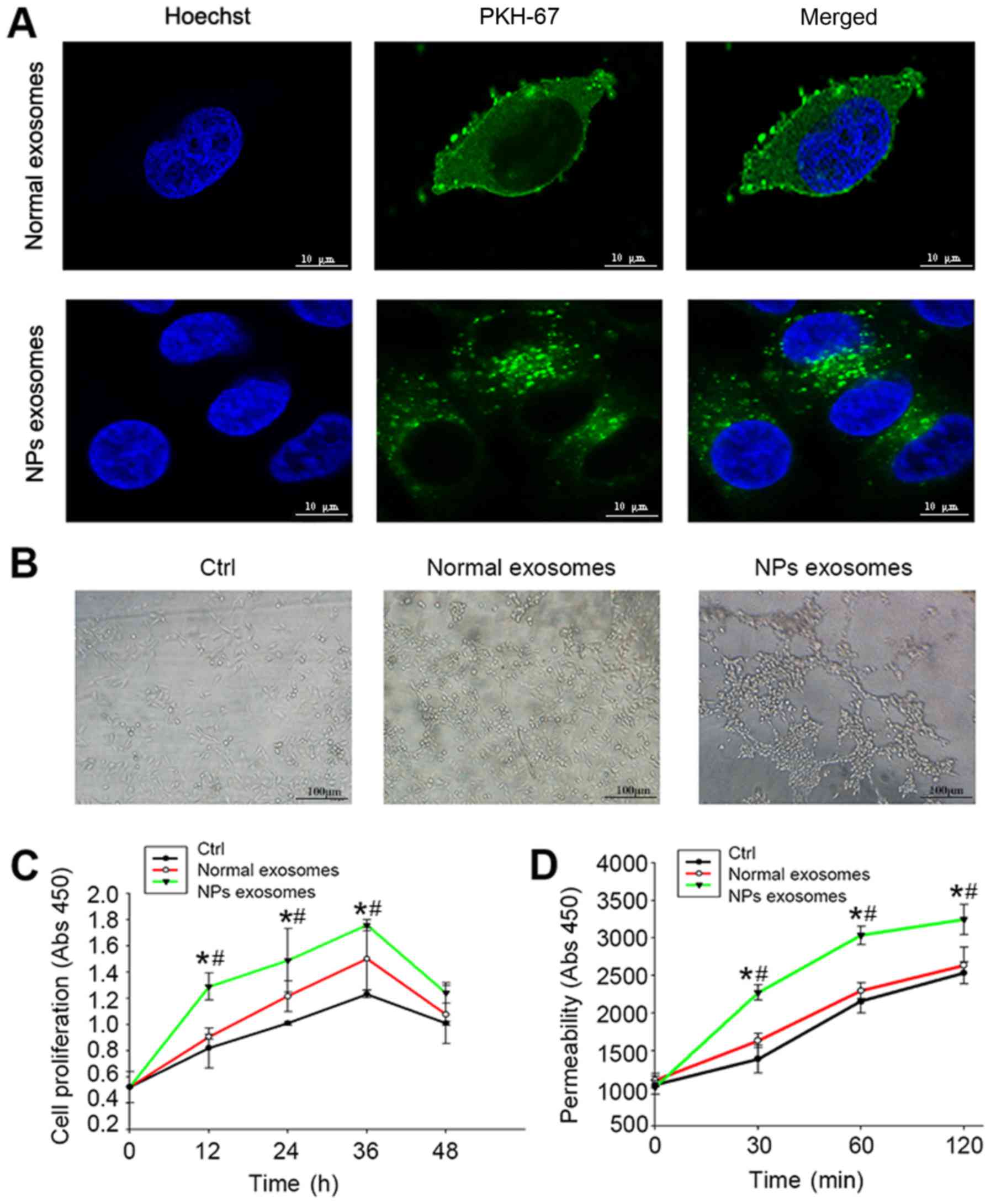
Influence of NLF-derived exosomes from
NPs on angiogenesis and permeability
Since the genesis of NPs was associated with the
abnormal angiogenesis and vascular permeability (3), we further evaluated effects of
NLF-derived exosomes from NPs on HUVECs. As shown in Fig. 2A, incubation of fluorescent
NLF-derived exosomes (green) resulted in transferring of
fluorescence to HUVECs. NLF-derived exosomes from NPs were
significantly more potent in stimulating tube formation (Fig. 2B), proliferation (Fig. 2C) and permeability (Fig. 2D) of HUVECs.
Expression of ADAM10 in NLF-derived
exosomes
Additional, we test to unravel the underlying
molecular mechanisms by which NLF-derived exosomes regulate
angiogenesis and vascular permeability. Previous studies have found
that ADAM10 can influence angiogenesis (20) and vascular permeability (21), and our group had conducted some
research on ADAM10 before (22).
Based on these data, we detected the expression of ADAM10 in
NLF-derived exosomes using western blot. Results showed that ADAM10
was enriched in NLF-derived exosomes from NPs compared to healthy
volunteers (Fig. 3).
Above data showed that exosomes existed in NLF.
NLF-derived exosomes from NPs promote angiogenesis and vascular
permeability. Furthermore, ADAM10 was highly expressed in
NLF-derived exosomes from NPs.
Discussion
NPs are characterized by angiogenesis and
interstitial edema with infiltration of inflammatory cells
(30,31). As previously described, the blood
vessels in NPs were immature and lacking normal innervations. The
modifications in these blood vessels suggest angiogenic processes
are important for the formation of NPs. And the interstitial edema
is probably due to excessive endothelial permeability (32).
To our knowledge, research on exosomes has been
expanding in many fields such as cancer (33,34),
inflammation (35), immunology
(36), diabetes (37) and so on. However, reports on
exosomes in NPs are rare. In our study, we found that exosomes exit
in NLF collected from NP patients. Isolated exosomes were
visualized using transmission electron microscope. Electron
micrographs of exosomes from NPs showed both the size and
morphology are similar to previous researches (8,9). And
then, these exosomes were identified by the western blot, showing
the common exosomal surface markers tetraspanins CD9 and CD63.
Furthermore, NTA showed vesicles with sizes between 50 and 100 nm.
Next, we investigated the influence of NLF-derived exosomes from
NPs on HUVECs. Confocal microscopy analysis showed that
PKH67-labeled (green) NLF-derived exosomes were uptaken by HUVECs
after co-culture for 3 h. However, it would have been more
convincing to also use a cell membrane marker to show that there
was green fluorescence inside cells. This is a limitation of our
study. Furthermore, CCK-8 assay, in vitro tube formation and
permeability assay respectively demonstrated that NPs exo
accelerated the proliferation, tube formation and permeability.
These results implied that NLF-derived exosomes from NPs had the
function of promoting angiogenesis and vascular permeability.
However, exosomes contain abundant protein, mRNA, miRNA and so on
(16,17), which bioactive substances are
related to the effect of NLF-derived exosomes from NPs on blood
vessels? Therefore, we tried to explore the molecular mechanisms
underlying the function of NLF-derived exosomes from NPs.
ADAMs are transmembrane proteins carrying an
NH2-terminal prodomain, preceding a metalloproteinase
(catalytic) domain, which is followed by a disintegrin, a
cysteine-rich, a transmembrane, and finally a cytoplasmic domain
(38). Previous researches have
shown that ADAMs are active in NPs. An immunohistochemical study
has found increased expression of ADAM33 protein in vessels and
stroma of NPs tissues (39).
Expression of ADAM8 was higher in NPs tissues than in normal nasal
mucosa, and there was a positive correlation between the strength
of ADAM8 immunostaining and the level of inflammation in NPs
tissues (40).
ADAM10 is a ubiquitous transmembrane metalloprotease
belonging to ADAM-family. It has been shown that ADAM10 is
associated with angiogenesis (20)
and vascular permeability (21),
and ADAM10 has been found in healthy nasal exosomes recently
(15). However, the expression
level of ADAM10 in NLF-derived exosomes from NPs has not been
demonstrated. In this study, we detected ADAM10 expression in
exosomes using western blot, showing that ADAM10 was overexpressed
in NLF-derived exosomes from NPs compared to the healthy
volunteers. Accordingly, we speculated that the role of NLF-derived
exosomes from NPs in promoting angiogenesis and vascular
permeability may be associated with ADAM10. However, this is just
our initial speculation. In the future, we plan to do some other
experiments such as inhibition of ADAM10 in the NLF both in
vivo (zebrafish model) and in vitro (human nasal
epithelial cells, hNECs) to validate our hypothesis. Besides,
ADAM10 is known associated with cell member. As for which cells did
release the exosomes with ADAM10 has not been known. Further
reseach on exosomes isolated from hNECs may answer the
question.
In conclusion, this study shows for the first time
that, NLF-derived exosomes from NPs promote angiogenesis and
vascular permeability and contain abundant ADAM10. Further research
on the effects of ADAM10-containing exosomes derived from NLF on
angiogenesis and vascular permeability may reveal the underlying
molecular mechanisms of NPs. Even though the presence of exosomes
in human NLF may be useful as a diagnostic indicator of upper
respiratory tract disease, it remains unclear whether these
extracellular components are causative. In the future, we plan to
do research on the correlation between NLF-derived exosomes and
severity of disease, which may help answering this question.
Acknowledgements
The authors would like to thank Professor Yiwen You
(Department of Otorhinolaryngology, Affiliated Hospital of Nantong
University, Jiangsu, China) for her general support and
assistance.
Funding
This study was supported by grants from Jiangsu
Provincial Commission of Health and Family Planning (grant no.
H201523) and Nantong Clinical Research Project (grant no.
HS2016001).
Availability of data and materials
All data analysed during the present study are
included in this published article, and the datasets are available
from the corresponding author on reasonable request.
Authors' contributions
JC and HY made substantial contributions to
conception and design, and gave final approval to the manuscript to
be published. HN and BY recruited the appropriate patients, and
collected nasal lavage fluid from them. YS, LB, DW and TZ conducted
the experiments and acquired the data. WZ and JZ analyzed and
interpreted data, and were the major contributors in writing the
manuscript. LC provided theoretical and technical support, and
revised the manuscript critically for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and healthy volunteers, and the study was approved by the
Medical Ethics Committee of the Affiliated Hospital of Nantong
University (Jiangsu, China; ethical review no. 2017-L088).
Consent for publication
Written informed consent was obtained from all
patients and healthy volunteers.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: EPOS 2012: European position paper on rhinosinusitis and nasal
polyps 2012. A summary for otorhinolaryngologists. Rhinology.
50:1–12. 2012.PubMed/NCBI
|
|
2
|
Wang C, Lou H, Wang X, Wang Y, Fan E, Li
Y, Wang H, Bachert C and Zhang L: Effect of budesonide transnasal
nebulization in patients with eosinophilic chronic rhinosinusitis
with nasal polyps. J Allergy Clin Immunol. 135:922–929.e6. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coste A, Rateau JG, Roudot-Thoraval F,
Chapelin C, Gilain L, Poron F, Peynegre R, Bernaudin JF and
Escudier E: Increased epithelial cell proliferation in nasal
polyps. Arch Otolaryngol Head Neck Surg. 122:432–436. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yukitatsu Y, Hata M, Yamanegi K, Yamada N,
Ohyama H, Nakasho K, Kojima Y, Oka H, Tsuzuki K, Sakagami M and
Terada N: Decreased expression of VE-cadherin and claudin-5 and
increased phosphorylation of VE-cadherin in vascular endothelium in
nasal polyps. Cell Tissue Res. 352:647–657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gosepath J, Brieger J, Lehr HA and Mann
WJ: Expression, localization, and significance of vascular
permeability/vascular endothelial growth factor in nasal polyps. Am
J Rhinol. 19:7–13. 2005.PubMed/NCBI
|
|
6
|
Record M, Subra C, Silvente-Poirot S and
Poirot M: Exosomes as intercellular signalosomes and
pharmacological effectors. Biochem Pharmacol. 81:1171–1182. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kulshreshtha A, Ahmad T, Agrawal A and
Ghosh B: Proinflammatory role of epithelial cell-derived exosomes
in allergic airway inflammation. J Allergy Clin Immunol.
131:1194–1203, 1203.e1-e14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Honegger A, Schilling D, Bastian S,
Sponagel J, Kuryshev V, Sültmann H, Scheffner M, Hoppe-Seyler K and
Hoppe-Seyler F: Dependence of intracellular and exosomal microRNAs
on viral E6/E7 oncogene expression in HPV-positive tumor cells.
PLoS Pathog. 11:e10047122015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
You B, Cao X, Shao X, Ni H, Shi S, Shan Y,
Gu Z and You Y: Clinical and biological significance of HAX-1
overexpression in nasopharyngeal carcinoma. Oncotarget.
7:12505–12524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You Y, Shan Y, Chen J, Yue H, You B, Shi
S, Li X and Cao X: Matrix metalloproteinase 13-containing exosomes
promote nasopharyngeal carcinoma metastasis. Cancer Sci.
106:1669–1677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakayama A: Proteomic analysis of urinary
exosomes. Rinsho Byori. 62:684–691. 2014.(In Japanese). PubMed/NCBI
|
|
12
|
Näslund TI, Paquin-Proulx D, Paredes PT,
Vallhov H, Sandberg JK and Gabrielsson S: Exosomes from breast milk
inhibit HIV-1 infection of dendritic cells and subsequent viral
transfer to CD4+ T cells. AIDS. 28:171–180. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lässer C, O'Neil SE, Ekerljung L, Ekström
K, Sjöstrand M and Lötvall J: RNA-containing exosomes in human
nasal secretions. Am J Rhinol Allergy. 25:89–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi EB, Hong SW, Kim DK, Jeon SG, Kim KR,
Cho SH, Gho YS, Jee YK and Kim YK: Decreased diversity of nasal
microbiota and their secreted extracellular vesicles in patients
with chronic rhinosinusitis based on a metagenomic analysis.
Allergy. 69:517–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lässer C, O'Neil SE, Shelke GV, Sihlbom C,
Hansson SF, Gho YS, Lundbäck B and Lötvall J: Exosomes in the nose
induce immune cell trafficking and harbour an altered protein cargo
in chronic airway inflammation. J Transl Med. 14:1812016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sampey GC, Saifuddin M, Schwab A, Barclay
R, Punya S, Chung MC, Hakami RM, Zadeh MA, Lepene B, Klase ZA, et
al: Exosomes from HIV-1-infected cells stimulate production of
pro-inflammatory cytokines through trans-activating response (TAR)
RNA. J Biol Chem. 291:1251–1266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Gu H, Qin D, Yang L, Huang W,
Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B and Fan GC:
Exosomal miR-223 contributes to mesenchymal stem cell-elicited
cardioprotection in polymicrobial sepsis. Sci Rep. 5:137212015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SL, Rouhi P, Dahl Jensen L, Zhang D,
Ji H, Hauptmann G, Ingham P and Cao Y: Hypoxia-induced pathological
angiogenesis mediates tumor cell dissemination, invasion, and
metastasis in a zebrafish tumor model. Proc Natl Acad Sci USA.
106:pp. 19485–19490. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalani A, Kamat PK, Chaturvedi P, Tyagi SC
and Tyagi N: Curcumin-primed exosomes mitigate endothelial cell
dysfunction during hyperhomocysteinemia. Life Sci. 107:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Isozaki T, Rabquer BJ, Ruth JH, Haines GK
III and Koch AE: ADAM-10 is overexpressed in rheumatoid arthritis
synovial tissue and mediates angiogenesis. Arthritis Rheum.
65:98–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blobel CP: Metalloprotease-disintegrins:
Links to cell adhesion and cleavage of TNF alpha and Notch. Cell.
90:589–592. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
You B, Shan Y, Shi S, Li X and You Y:
Effects of ADAM10 upregulation on progression, migration and
prognosis of nasopharyngeal carcinoma. Cancer Sci. 106:1506–1514.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chueh HW, Park SK, Hur DY and Bae WY:
Expression profile of ADAM10 and ADAM17 in allergic rhinitis. Int
Forum Allergy Rhinol. 5:1036–1041. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schulz B, Pruessmeyer J, Maretzky T,
Ludwig A, Blobel CP, Saftig P and Reiss K: ADAM10 regulates
endothelial permeability and T-cell transmigration by proteolysis
of vascular endothelial cadherin. Circ Res. 102:1192–1201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Donners MM, Wolfs IM, Olieslagers S,
Mohammadi-Motahhari Z, Tchaikovski V, Heeneman S, van Buul JD,
Caolo V, Molin DG, Post MJ and Waltenberger J: A disintegrin and
metalloprotease 10 is a novel mediator of vascular endothelial
growth factor-induced endothelial cell function in angiogenesis and
is associated with atherosclerosis. Arterioscler Thromb Vasc Biol.
30:2188–2195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reiss K, Cornelsen I, Husmann M, Gimpl G
and Bhakdi S: Unsaturated fatty acids drive disintegrin and
metalloproteinase (ADAM)-dependent cell adhesion, proliferation,
and migration by modulating membrane fluidity. J Biol Chem.
286:26931–26942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Howarth PH, Persson CG, Meltzer EO,
Jacobson MR, Durham SR and Silkoff PE: Objective monitoring of
nasal airway inflammation in rhinitis. J Allergy Clin Immunol. 115
3 Suppl 1:S414–S441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romancino DP, Paterniti G, Campos Y, De
Luca A, Di Felice V, d'Azzo A and Bongiovanni A: Identification and
characterization of the nano-sized vesicles released by muscle
cells. FEBS Lett. 587:1379–1384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hellquist HB: Nasal polyps update.
Histopathology. Allergy Asthma Proc. 17:pp. 237–242. 1996;
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Slavin RG: Nasal polyps and sinusitis.
Clin Allergy Immunol. 16:295–309. 2002.PubMed/NCBI
|
|
32
|
Watanabe K and Komatsuzaki A:
Ultrastructural findings of capillaries in nasal polyps. Rhinology.
30:49–56. 1992.PubMed/NCBI
|
|
33
|
Higginbotham JN, Demory BM, Gephart JD,
Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD,
McConnell RE, Tyska MJ and Coffey RJ: Amphiregulin exosomes
increase cancer cell invasion. Curr Biol. 21:779–786. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hood JL, San RS and Wickline SA: Exosomes
released by melanoma cells prepare sentinel lymph nodes for tumor
metastasis. Cancer Res. 71:3792–3801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martinez-Bravo MJ, Wahlund CJ, Qazi KR,
Moulder R, Lukic A, Rådmark O, Lahesmaa R, Grunewald J, Eklund A
and Gabrielsson S: Pulmonary sarcoidosis is associated with
exosomal vitamin D-binding protein and inflammatory molecules. J
Allergy Clin Immunol. 139:1186–1194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu M, Li Y, Shi J, Feng W, Nie G and Zhao
Y: Exosomes as extrapulmonary signaling conveyors for
nanoparticle-induced systemic immune activation. Small. 8:404–412.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bashratyan R, Sheng H, Regn D, Rahman MJ
and Dai YD: Insulinoma-released exosomes activate autoreactive
marginal zone-like B cells that expand endogenously in prediabetic
NOD mice. Eur J Immunol. 43:2588–2597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dreymueller D, Uhlig S and Ludwig A:
ADAM-family metalloproteinases in lung inflammation: Potential
therapeutic targets. Am J Physiol Lung Cell Mol Physiol.
308:L325–L343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Erbek SS, Erinanc H, Erbek S, Topal O and
Kiyici H: Expression of a disintegrin and metalloproteinase 33
protein in nasal polyposis: An immunohistochemical study. Am J
Rhinol Allergy. 24:79–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Erbek SS, Hizal E, Erinanc H and Erbek S:
Expression of a disintegrin and metalloproteinase 8 by inflammatory
cells in nasal polyps. Am J Rhinol Allergy. 27:1512013. View Article : Google Scholar : PubMed/NCBI
|