Introduction
Diabetes mellitus, mainly caused by various genetic
and environmental factors, is a chronic and systemic metabolic
syndrome. Diabetes results from a shortage in the amount of insulin
released from the pancreas in response to elevated blood glucose or
from a deficiency in the ability of fat and muscle cells to respond
to insulin (1). The representative
symptoms of diabetes are increased hunger, frequent urination, and
increased thirst, and untreated diabetes causes a variety of
complications including chronic kidney disease, stroke,
cardiovascular disease, and diabetic retinopathy (1–3).
Type 1 diabetes, also called insulin-dependent diabetes mellitus
(IDDM), occurs via an autoimmune reaction that attacks the β cells
of the pancreas that produce insulin (4). Type 2 diabetes, or
non-insulin-dependent diabetes (NIDDM), is caused by a deficiency
in the insulin-responsive system, and a strong connection between
type 2 diabetes and obesity has become clear (5). Currently, there are many different
classes of anti-diabetic drugs used in clinical practice to
decrease blood glucose levels, such as metformin, glucagon-like
peptide-1 receptor (GLP-1) agonists, and synthetic insulin analogs
(2,6). Nevertheless, many studies on natural
products are being carried out to discover potent anti-diabetic
lead compounds that have minimal side effects.
Morus alba L. (Moraceae), known as the white
mulberry tree, is cultivated in Asia, Europe, and India, and its
fruits, commonly known as mulberry, are widely cultivated as an
edible fruit (7). Mulberry has
been used as a traditional medicine in East Asia for the prevention
of insomnia, dizziness, and tinnitus as well as the alleviation of
high glucose levels (8,9). Previous researches have reported that
various chemical constituents and extracts from this natural source
exhibit useful pharmacological activities including
anti-inflammatory, antioxidant, and immunoregulative effects
(10–12). A recent study reported that
anthocyanin-rich mulberry extracts alleviate high glucose levels in
in vivo studies of glucose consumption and uptake, which was
attributed to AMPK/ACC/mTOR signaling (13). In addition, anti-hyperglycemic and
anti-hyperlipidemic effects of polysaccharides from the fruits of
M. alba have been reported, which provide a scientific
rationale for the development of this source as a new medication
candidate to treat diabetes (14).
However, anti-diabetic compounds/metabolites from M. alba
fruits have not yet been fully investigated. The present study
describes the protective effects of compounds isolated from M.
alba fruits against STZ-induced INS-1 cell death as well as its
molecular mechanisms in the apoptotic pathway.
Materials and methods
Extraction of M
alba fruits and isolation method
The fruits of M. alba were bought at the
Kyungdong Market (Woori Herb), Seoul, Korea, in January, 2014. A
voucher specimen (MA 1414) of the material was classified by one of
the authors (K.H. Kim) and was stored in the herbarium of the
School of Pharmacy, Sungkyunkwan University (Suwon, Korea). Dried
and pounded fruits of M. alba (10.0 kg) were extracted with
70% aqueous MeOH three times at room temperature and then filtered.
The filtrate was condensed in vacuo, affording a slurry
resultant (1.4 kg). The resultant residue was dissolved in
deionized water and successively partitioned with hexane,
CHCl3, EtOAc, and n-BuOH (800 ml ×3) until the
color of partitioned layer disappears, providing 27.8, 85.3, 32.9,
and 138.8 g, respectively. The CHCl3-soluble fraction
(85.0 g) was loaded to a silica gel (230–400 mesh) column and
fractionated using CHCl3-MeOH (40:1-1:1, gradient
system) to yield five fractions (CA-CE). Fraction CB (4.3 g) was
separated by RP-C18 silica gel (230–400 mesh) column
chromatography eluted with 70% MeOH/H2O to give eleven
fractions (CB1-CB11). Fraction CB1 (226 mg) was passed over
Sephadex LH-20 column chromatography eluted with 100% MeOH to give
six subfractions (CB1-1-CB1-6). Subfraction CB1-3 (33 mg) was
purified by semi-preparative reversed-phase HPLC using an isocratic
solvent system of 4% MeOH/H2O (Phenomenex Luna
Phenyl-hexyl, 250×10.0 mm, 5 µm, flow rate: 2 ml/min) to afford
compounds 1 (0.4 mg, tR=25.0 min) and 2 (4.4 mg,
tR=37.2 min). Fraction CB2 (750 mg) was
fractionated using silica gel (230–400 mesh) column chromatography
eluted with CHCl3-MeOH (40:1-5:1, gradient system) to
afford nine subfractions (CB2-1-CB2-9). Subfraction CB2-2 (176 mg)
was purified by semi-preparative reversed-phase HPLC using an
isocratic solvent system of 29% MeOH/H2O (Phenomenex
Luna Phenyl-hexyl, 250×10.0 mm, 5 µm, flow rate: 2 ml/min) to yield
compounds 3 (2.1 mg, tR=72.0 min) and 4 (1.6 mg,
tR=65.1 min). Subfraction CB2-3 (84 mg) was also
separated by semi-preparative reversed-phase HPLC using an
isocratic solvent system of 18% MeOH/H2O (Phenomenex
Luna Phenyl-hexyl, 250×10.0 mm, 5 µm, flow rate: 2 ml/min) to
afford compound 5 (1.3 mg, tR=40.5 min). Finally,
fraction CB4 was separated on silica gel (230–400 mesh) column
chromatography using CHCl3-MeOH (40:1-5:1, gradient
system) to give seven subfractions (CB4-1-CB4-7). Compound 6 (1.8
mg, tR=26.1 min) was purified from subfraction
CB4-2 (20.0 mg) by utilizing semi-preparative reversed-phase HPLC
with an isocratic solvent system of 58% MeOH/H2O
(Phenomenex Luna Phenyl-hexyl, 250×10.0 mm, 5 µm, flow rate: 2
ml/min).
Cell culture
INS-1 cell line, immortalized rat pancreatic islet
beta cells, were purchased from Biohermes (Shanghai, China) and
grown in RPMI-1640 (Cellgro, Manassas, VA, USA) supplemented with
10% FBS, 1% penicillin/streptomycin (Invitrogen Co., Grand Island,
NY, USA), 11 mM d-glucose, 10 mM HEPES, 2 mM l-glutamine, 1 mM
sodium pyruvate, and 0.05 mM 2-mercaptoethanol in an humidified
atmosphere supplying of 5% CO2 at 37°C.
Measurement of the level of
intracellular ROS
The levels of intracellular ROS were measured using
2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA;
Sigma, St Louis, MO, USA). Cells were plated into clear bottomed
96-well black plates at a density of 2×104 cells per
well and adhered for 24 h. After the pre-treatment with control or
indicated concentrations of compounds for 2 h, cells were then
exposed to 50 µM streptozotocin (STZ; Sigma) for additional 24 h.
After incubation, cells were stained 10 uM H2DCFDA for
30 min followed by washing with PBS three times. Green DCF
fluorescent intensity was measured at an excitation wavelength of
485 nm and an emission wavelength of 535 nm (Ex/Em) using a
fluorescent microplate reader (Tecan Infinite F200 Microplate
Fluorescence Reader; Tecan Zürich, Switzerland).
Assessment of cell viability
Cell viability were determined using Ez-Cytox cell
viability detection kit following manufacturer's instruction. In
brief, cells were grown in 96-well plate at a density of
2×104 cells per well for 24 h. Cells were then
pre-treated with control (0.5% DMSO) or the indicated
concentrations of compounds. After incubation for 2 h, cells were
exposed to 50 µM STZ for 24 h. Cells were then incubated with 10 µl
Ez-Cytox for additional 2 h. Cell viability was determined from the
absorbance at 450 nm using a microplate reader.
Tali-image based analysis of apoptotic
cells
Cells were plated in 6-well plates at a density of
3×105 cells per well and incubated for 24 h to adhere.
Cells were pre-treated with 50 and 100 µM compound 3 for 2 h and
exposed to 50 µM STZ for 24 h. The cells were then harvested and
washed with PBS. Cells were incubated with an Annexin V Alexa Fluor
488 in Annexin-binding buffer for 20 min followed by staining with
propidium iodide (PI). The percentage of apoptotic cells was
analyzed using a Tali image-based cytometer (Invitrogen, CA, USA).
In this analysis, the apoptotic cells were determined by the
percentage of Annexin V-positive cells on total counted-cells.
Western blot analysis
Cells were plated in 6-well plates at a density of
3×105 cells per well and incubated for 24 h to adhere.
Cells were pre-treated with 50 and 100 µM compound 3 for 2 h and
exposed to 50 µM STZ for 24 h. Cells were then lysed with RIPA
buffer supplemented with 1 mM phenylmethylsulfonyl fluoride
immediately before use. The equal amounts of protein were separated
by a sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto polyvinylidene difluoride membranes. The membranes
were incubated with primary antibodies against cleaved caspase-8,
cleaved caspase-9, cleaved caspase-3, B-cell lymphoma-2 (BCL-2),
PARP, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The
membranes were then incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies. Immunoreactive bands were
detected using ECL Advance western blotting detection reagents (GE
Healthcare, Chalfont, UK) and visualized using a FUSION Solo
Chemiluminescence System (PEQLAB Biotechnologie GmbH, Germany).
Statistical analysis
Differences between treatments were evaluated by
one-way analysis of variance (ANOVA) followed by a multiple
comparison test with a Bonferroni adjustment. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
The MeOH extract of M. alba fruits was
partitioned with hexane, CHCl3, EtOAc, and
n-BuOH. The CHCl3-soluble fraction was subjected
to a series of open-column chromatography and Sephadex-LH20 column
chromatography, and then purified by semi-preparative HPLC, to
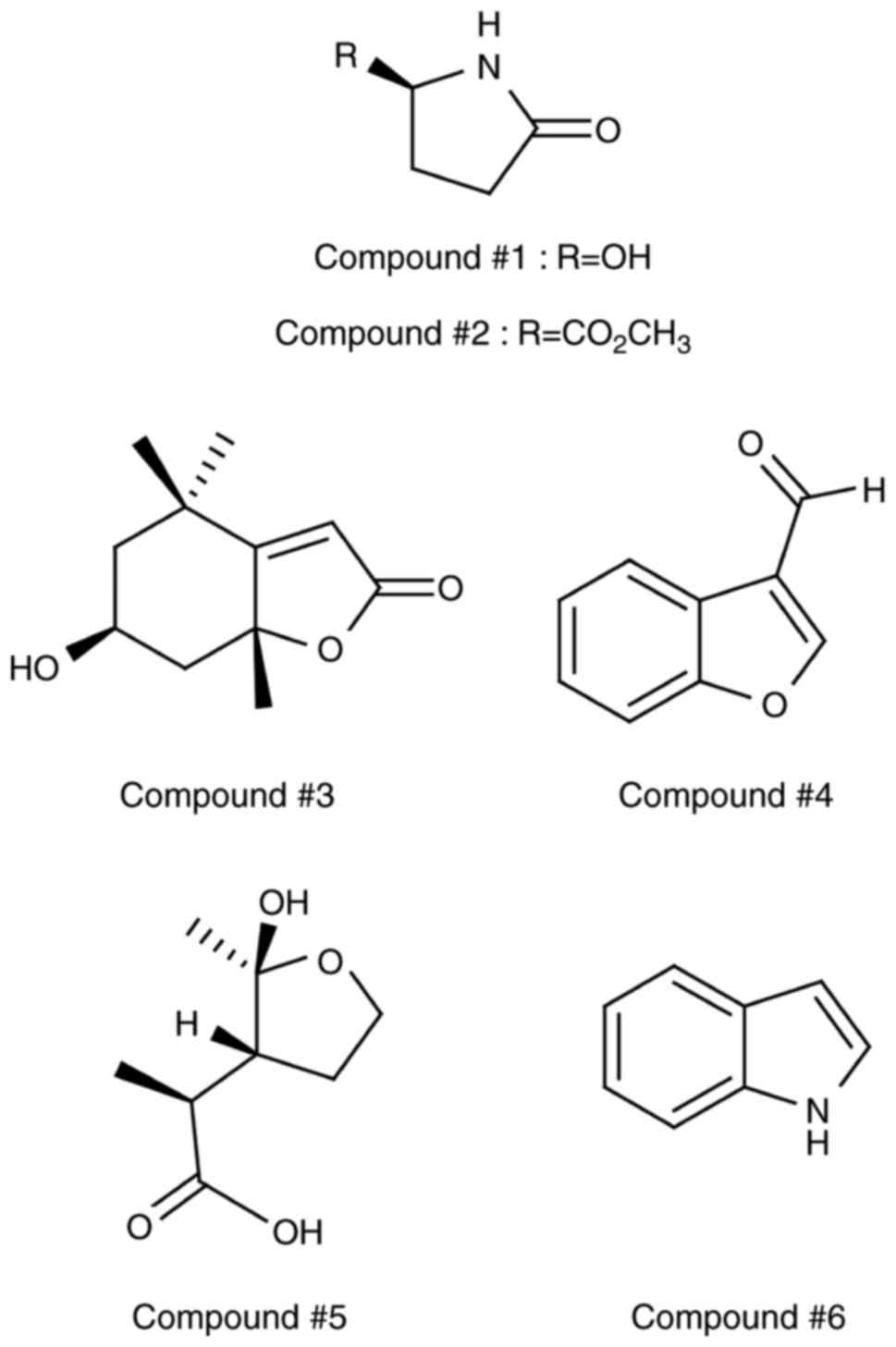
obtain six heterocyclic compounds (1–6)
(Fig. 1). The chemical structures
of the isolated compounds were unambiguously elucidated to be
(R)-5-hydroxypyrrolidin-2-one (1,15),
methyl (R)-pyroglutamate (2,16),
loliolide (3,17), 3-benzofurancarboxalde hyde
(4,18), odisolane (5,19),
and indole (6,20) by comparing their NMR spectroscopic
values and physical properties with previously reported literature
data.
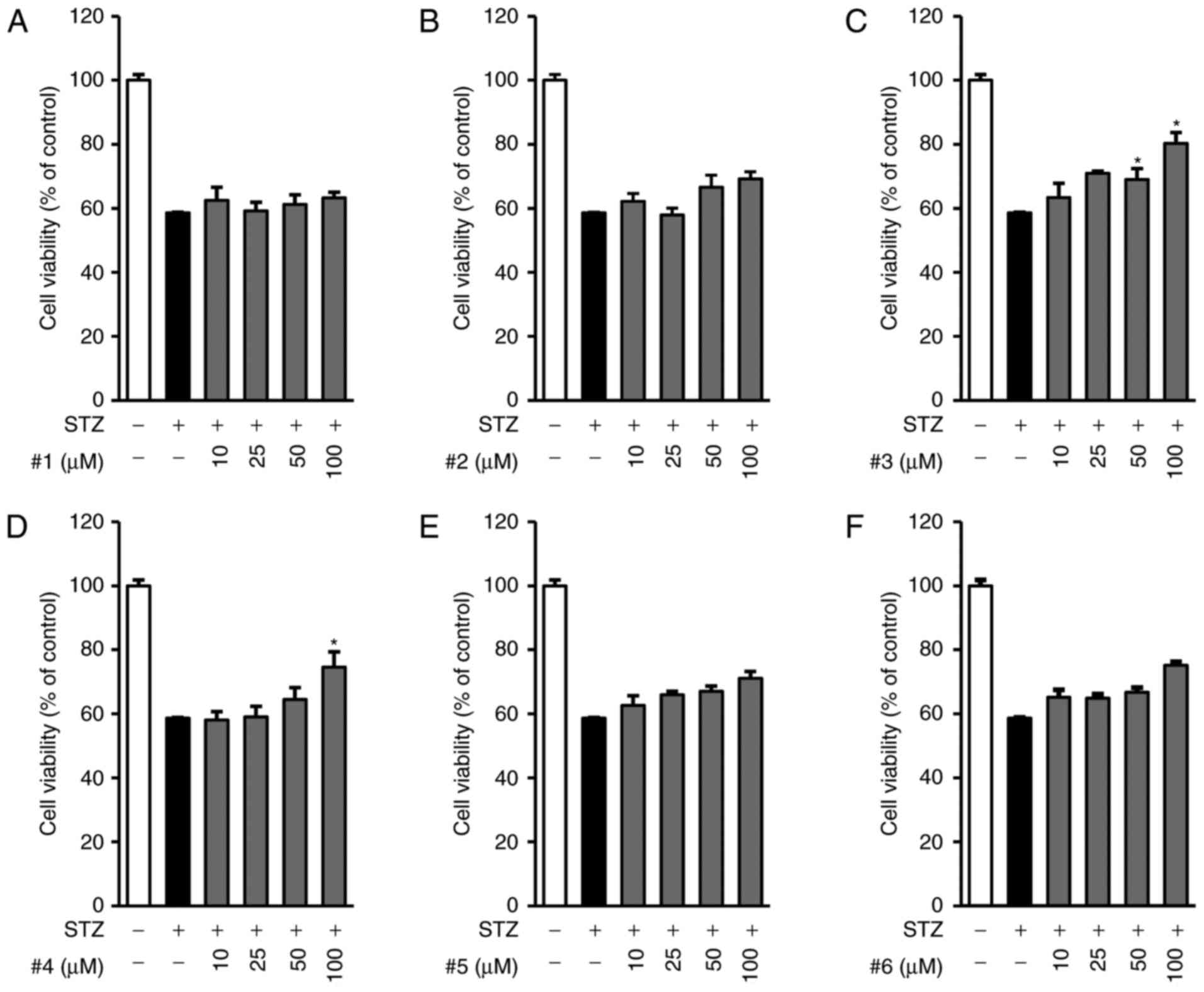
We first examined the effects of all compounds on
the cell viability decreased by STZ in INS-1 cells. Cells were
pre-treated with the indicated concentrations of compounds 1–6 (0
to 100 µM of each compound) for 2 h and further exposed to 50 µM
STZ for 24 h. As shown in Fig. 2,
the exposure to 50 µM STZ for 24 h decreased cell viability
(58.62±0.17%) compared to control treatment (100%). Among the six
compounds, compound 3 showed the strongest protective effect on
STZ-induced INS-1 cytotoxicity in a concentration-dependent manner,
whereas the other compounds showed minimal or no effect (Fig 2A-F). Compound 3 showed maximum
effect at the concentration of 100 µM, as indicated by increased
cell viability (80.27±3.48%).
The increase in the levels of intracellular ROS is a
key characteristic in STZ-induced pancreatic β-cell death (21). Therefore, we then examined the
antioxidative effects of compounds 1–6. Consistent with previous
studies, we found, as shown in Fig.
3, a remarkable increase in intracellular ROS after exposure to
STZ for 24 h. In contrast, compounds 3 (Fig. 3C) and 5 (Fig. 3E) significantly reduced the levels
of intracellular ROS that were increased by STZ in INS-cells,
whereas the other compounds were not effective (Fig. 3A, B, D, F). Based on the results of
both the cell viability assay and the ROS measurement studies, we
found that compound 3 was the most effective in preventing INS-1
cell death and the production of ROS induced by STZ. Therefore, we
further investigated not only the anti-apoptotic effect of compound
3 but also its underlying mechanism against STZ-induced apoptotic
cell death.
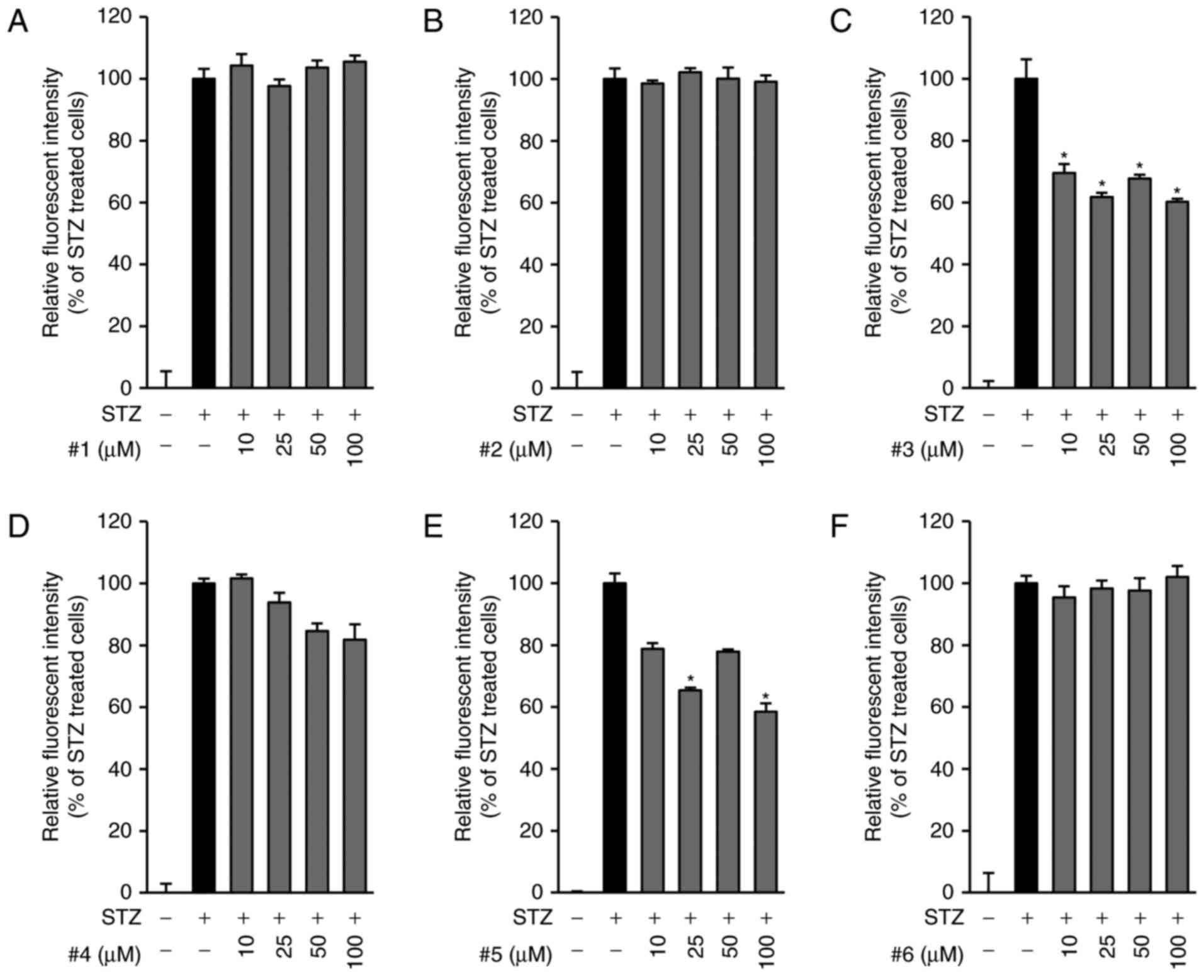 | Figure 3.Scavenging activities of compounds 1–6
against STZ-induced ROS production. INS-1 cells were exposed to 50
µM STZ for 24 h in the presence of compounds (A) 1, (B) 2, (C) 3,
(D) 4, (E) 5 and (F) 6 (0–100 µM) and stained with
H2DCFDA. Fluorescent intensities of DCF were measured
using a fluorescent microplate reader. The presence of compound 3
showed a strong protective effect on STZ-induced INS-1 cells. Mean
±SEM, *P<0.05 compared with STZ only treated cells. STZ,
streptozotocin; ROS, reactive oxygen species; H2DCFDA,
2′,7′-dichlorodihydrofluorescein diacetate; DCF,
dichlorofluorescein. |
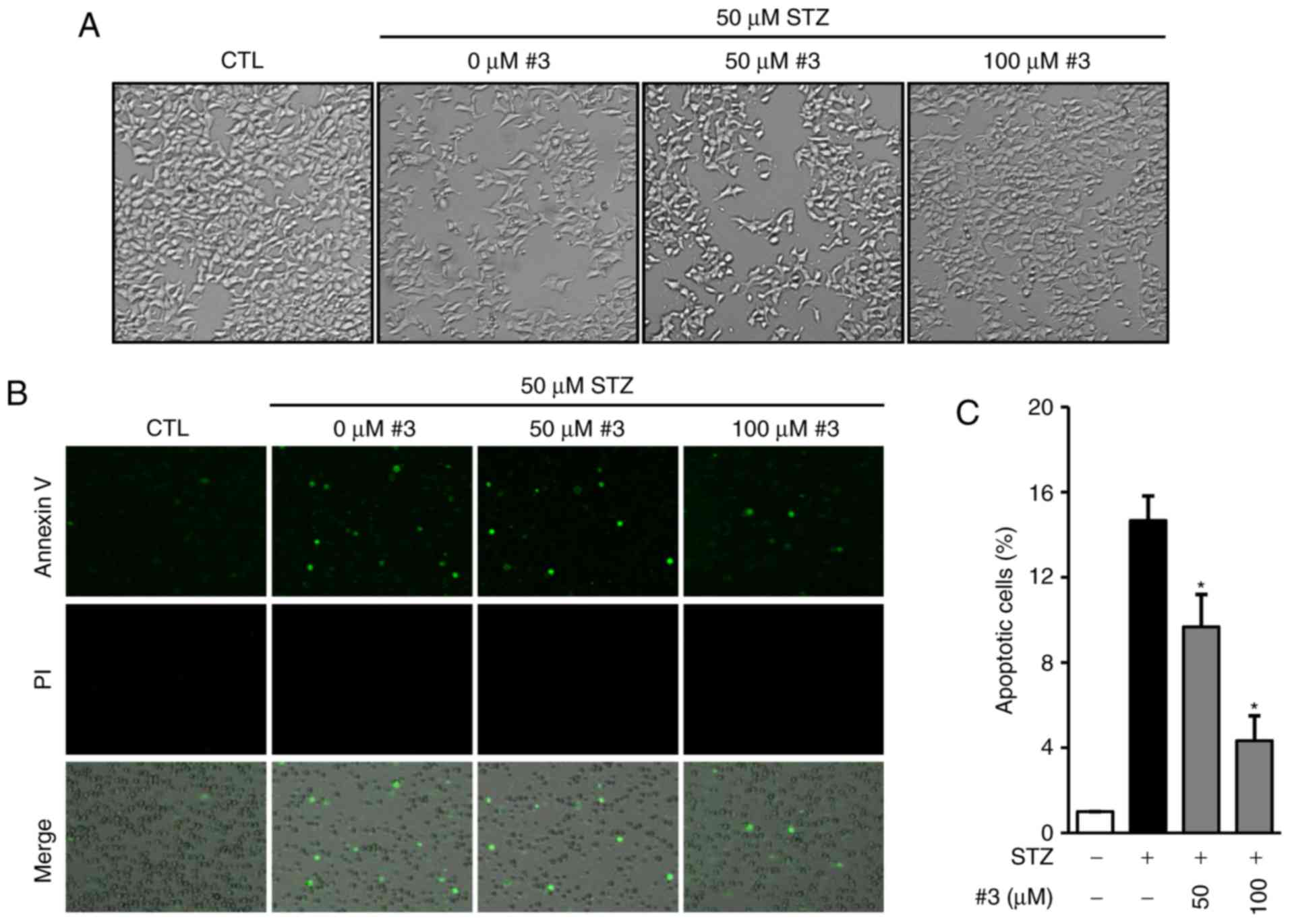
It has been reported that STZ induces apoptosis and
necrosis at low and high concentrations respectively and that both
apoptosis and necrosis contribute to the development of type 1
diabetes (22,23). Therefore, we examined the effect of
compound 3 against STZ-induced apoptosis in INS-1 cells. The
morphological images in Fig. 4A
show that the presence of compound 3 strongly prevented STZ-induced
apoptosis in INS-1 cells (Fig.
4A). To determine the anti-apoptotic effect, cells were stained
with Annexin-V Allexa 488 after exposure to 50 µM STZ in the
presence of 50 and 100 µM compound 3. The representative
photographs show that the treatment with compound 3 markedly
reduced the Annexin V-positive cells (Fig. 4B). In addition to this, we
quantitatively analyzed apoptotic cells by the percentage of
Annexin V-positive cells relative to total cells. As shown in
Fig. 4C, the percentage of
apoptotic cells dramatically increased by the exposure to STZ
(14.66±1.15%) while it was significantly reduced in the presence of
50 (9.66±1.52%) and 100 µM (4.33±1.15%) compound 3 (Fig. 4C).
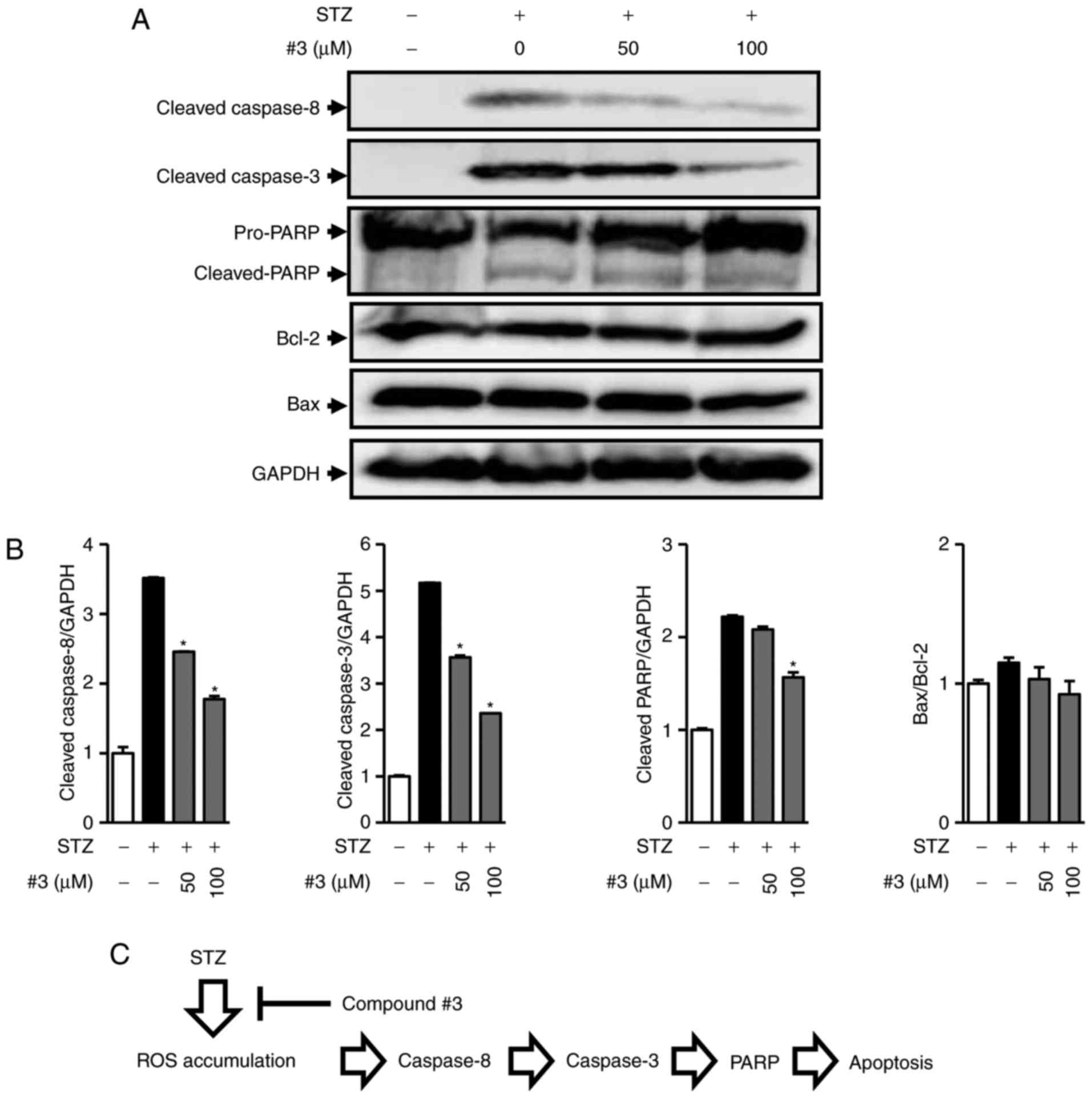
STZ induces apoptosis via activation of caspase-8
and caspase-3 and regulation of the protein expression of Bcl-2
family members in INS-1 cells (24). Moreover, we recently reported that
the inhibition of caspase-8 and caspase-3 by cirsimaritin prevented
apoptosis in INS-1 cells as well as increased Bcl-2 protein
expression (25). This suggests
that the inhibition of the caspase signaling pathway is a possible
target to protect pancreatic cell death in type 1 diabetes. We
further investigated to determine the underlying protective
mechanism of compound 3 against STZ-induced apoptotic INS-1 cell
death using western blot analysis for pro-apoptotic and
anti-apoptotic proteins. As shown in Fig. 5A, cleavage of caspase-8, caspase-3,
and PARP was markedly increased after treatment with 50 µM STZ,
whereas it decreased in the presence of 50 and 100 µM of compound 3
in a concentration-dependent manner (Fig. 5B). However, the ratio of Bax to
Bcl-2 indicating mitochondrial apoptotic pathway was altered
neither cisplatin only-nor cisplatin with compound 3-treated cells
(Fig 5A and B). This result
indicated that compound 3 exhibits anti-apoptotic activity via
blocking the activation of caspase-8 and caspase-3 and inducing
PARP cleavage (Fig. 5C).
In the present study, we found that compound 3
prevented STZ-induced apoptotic pancreatic β cell death via the
inhibition of the caspase signaling pathway and induction of Bcl-2
protein expression. Therefore, this study suggests that compound 3,
a strong bioactive natural compound from the extracts of M.
alba, may be a suitable therapeutic for type 1 diabetes.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT, and Future Planning
(NRF-2017R1A2B2011807). The present study was also supported by the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Science, ICT,
and Future Planning (2015R1C1A1A02037383).
References
|
1
|
Pantalone KM, Hobbs TM, Wells BJ, Kong SX,
Kattan MW, Bouchard J, Yu C, Sakurada B, Milinovich A, Weng W, et
al: Clinical characteristics, complications, comorbidities and
treatment patterns among patients with type 2 diabetes mellitus in
a large integrated health system. BMJ Open Diabetes Res Care.
3:e0000932015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Constantino MI, Molyneaux L,
Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, Twigg SM, Yue DK and
Wong J: Long-term complications and mortality in young-onset
diabetes: Type 2 diabetes is more hazardous and lethal than type 1
diabetes. Diabetes Care. 36:3863–3869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Emerging Risk Factors Collaboration, ;
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio
E, Ingelsson E, Lawlor DA, Selvin E, et al: Diabetes mellitus,
fasting blood glucose concentration, and risk of vascular disease:
A collaborative meta-analysis of 102 prospective studies. Lancet.
375:2215–2222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rother KI: Diabetes treatment-bridging the
divide. N Engl J Med. 356:1499–1501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malik VS, Popkin BM, Bray GA, Després JP
and Hu FB: Sugar-sweetened beverages, obesity, type 2 diabetes
mellitus, and cardiovascular disease risk. Circulation.
121:1356–1364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ripsin CM, Kang H and Urban RJ: Management
of blood glucose in type 2 diabetes mellitus. Am Fam Physician.
79:29–36. 2009.PubMed/NCBI
|
|
7
|
Khan MA, Rahman AA, Islam S, Khandokhar P,
Parvin S, Islam MB, Hossain M, Rashid M, Sadik G, Nasrin S, et al:
A comparative study on the antioxidant activity of methanolic
extracts from different parts of Morus alba L. (Moraceae). BMC Res
Notes. 6:242013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oki T, Kobayashi M, Nakamura T, Okuyama A,
Masuda M, Shiratsuchi H and Suda I: Changes in radical-scavenging
activity and components of mulberry fruit during maturation. J Food
Sci. 71:C18–C22. 2006. View Article : Google Scholar
|
|
9
|
Pawlowska AM, Oleszek W and Braca A:
Quali-quantitative analyses of Flavonoids of Morus nigra L. and
Morus alba L. (Moraceae) fruits. J Agric Food Chem. 56:3377–3380.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo C, Li R, Zheng N, Xu L, Liang T and He
Q: Anti-diabetic effect of ramulus mori polysaccharides, isolated
from Morus alba L., on STZ-diabetic mice through blocking
inflammatory response and attenuating oxidative stress. Int
Immunopharmacol. 16:93–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren C, Zhang Y, Cui W, Lu G, Wang Y, Gao
H, Huang L and Mu Z: A polysaccharide extract of mulberry leaf
ameliorates hepatic glucose metabolism and insulin signaling in
rats with type 2 diabetes induced by high fat-diet and
streptozotocin. Int J Biol Macromol. 72:951–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zelová H, Hanáková Z, Čermáková Z, Šmejkal
K, Dalĺ Acqua S, Babula P, Cvačka J and Hošek J: Evaluation of
anti-inflammatory activity of prenylated substances isolated from
Morus alba and Morus nigra. J Nat Prod. 77:1297–1303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan F and Zheng X: Anthocyanin-rich
mulberry fruit improves insulin resistance and protects hepatocytes
against oxidative stress during hyperglycemia by regulating
AMPK/ACC/mTOR pathway. J Funct Foods. 30:270–281. 2017. View Article : Google Scholar
|
|
14
|
Jiao Y, Wang X, Jiang X, Kong F, Wang S
and Yan C: Antidiabetic effects of Morus alba fruit polysaccharides
on high-fat diet- and streptozotocin-induced type 2 diabetes in
rats. J Ethnopharmacol. 199:119–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KH, Lee IK, Park KM, Kim WK and Lee
KR: Isolation of γ-Lactam Alkaloids from the Macrolepiota
neomastoidea. Bullet Korean Che Soc. 29:1591–1593. 2008. View Article : Google Scholar
|
|
16
|
Bateman L, Breeden SW and O'Leary P: New
chiral diamide ligands: Synthesis and application in allylic
alkylation. Tetrahedron: Asymmet. 19:391–396. 2008. View Article : Google Scholar
|
|
17
|
Kim MR, Lee SK, Kim CS, Kim KS and Moon
DC: Phytochemical constituents of Carpesium macrocephalum FR-et
SAV. Arch Pharm Res. 27:1029–1033. 2004. View Article : Google Scholar
|
|
18
|
Podea PV, Toşa MI, Paizs C and Irimie FD:
Chemoenzymatic preparation of enantiopure
L-benzofuranyl- and L-benzo[b]thiophenyl
alanines. Tetrahedron: Asymmet. 19:500–511. 2008. View Article : Google Scholar
|
|
19
|
Lee SR, Park JY, Yu JS, Lee SO, Ryu JY,
Choi SZ, Kang KS, Yamabe N and Kim KH: Odisolane, a novel oxolane
derivative, and antiangiogenic constituents from the fruits of
mulberry (Morus alba L.). J Agric Food Chem. 64:3804–3809. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siu J, Baxendale IR and Ley SV: Microwave
assisted Leimgruber-Batcho reaction for the preparation of indoles,
azaindoles and pyrroylquinolines. Org Biomol Chem. 2:160–167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen F, Xiong H, Wang J, Ding X, Shu G and
Mei Z: Antidiabetic effect of total flavonoids from Sanguis
draxonis in type 2 diabetic rats. J Ethnopharmacol. 149:729–736.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mythili MD, Vyas R, Akila G and
Gunasekaran S: Effect of streptozotocin on the ultrastructure of
rat pancreatic islets. Microsc Res Tech. 63:274–281. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saini KS, Thompson C, Winterford CM,
Walker NI and Cameron DP: Streptozotocin at low doses induces
apoptosis and at high doses causes necrosis in a murine pancreatic
beta cell line, INS-1. Biochem Mol Biol Int. 39:1229–1236.
1996.PubMed/NCBI
|
|
24
|
Kasono K, Yasu T, Kakehashi A, Kinoshita
N, Tamemoto H, Namai K, Ohno R, Ueba H, Kuroki M, Ishikawa S and
Kawakami M: Nicorandil improves diabetes and rat islet beta-cell
damage induced by streptozotocin in vivo and in vitro. Eur J
Endocrinol. 151:277–285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee D, Kim KH, Lee J, Hwang GS, Lee HL,
Hahm DH, Huh CK, Lee SC, Lee S and Kang KS: Protective effect of
cirsimaritin against streptozotocin-induced apoptosis in pancreatic
beta cells. J Pharm Pharmacol. 69:875–883. 2017. View Article : Google Scholar : PubMed/NCBI
|