Introduction
Cataract is a major ophthalmological disorder that
is characterized by the opacification of the eye lens (1). Cataract is an age-related disorder,
and is one of the main causes of severe visual impairment or
blindness in the aging population (2). Age-related cataract can lower the
quality of life and affect the global economy, rendering the
disease a significant public health issue (3). The causes of cataract are numerous
and complex, and include drug-induced alterations, developmental
abnormalities, ultraviolet radiation exposure, trauma and metabolic
disorders (4). Previous studies
have reported that the apoptosis of human lens epithelial (HLE)
cells contributed to all types of cataract, with the exception of
congenital disorders (5,6). Furthermore, oxidative stress and the
formation of oxygen free radicals are also involved in cataract,
and have been identified as a major risk factor for cataract
development (4,7). Previous studies have demonstrated
that hydrogen peroxide (H2O2) could induce
the apoptosis of HLE cells (8,9);
however, the molecular mechanisms implicated in
H2O2-induced HLE cell apoptosis have yet to
be fully elucidated.
MicroRNAs (miRNAs) have attracted attention in the
efforts to elucidate the pathophysiological mechanisms implicated
in various diseases or cancers (10). miRNAs are a class of small
non-coding RNA molecules, 19–22 nucleotides long, which can target
the 3′untranslated region of mRNAs to induce translational
repression or mRNA degradation (11). miRNAs have been reported to
participate in the regulation of several physiological and
pathological processes, including cell proliferation, apoptosis,
senescence and stress response (12). Previous studies have suggested that
the aberrant expression of miRNAs may be implicated in the
pathogenesis of age-related diseases, including the progression of
cataract (3,13). One study reported that miRNA
(miR)-125b was able to inhibit the apoptosis of HLE cells in
age-related cataract by targeting p53 (14). Another study demonstrated that the
let-7b miRNA precursor induced HLE cell apoptosis by directly
regulating the expression of leucine-rich repeat-containing G
protein-coupled receptor 4 in cataract (15). However, the information available
on miRNA expression in cataract-related tissues is limited.
Previous studies have revealed that the downregulation of miR-181a
was able to significantly inhibit the
H2O2-induced apoptosis of cardiomyocytes
(16), that miR-181a could inhibit
the migration, proliferation and epithelial-mesenchymal transition
(EMT) of lens epithelial cells (11). Therefore, it may be hypothesized
that miR-181a serves a crucial role in the apoptosis of HLE cells
induced by H2O2 and in the development of
cataract in humans.
In the present study, RNA interference (RNAi) was
performed using short hairpin (sh)RNA-based stable gene knockdown
(17) to silence the expression of
miR-181a in human HLE-B3 cells. The effects of miR-181a knockdown
were evaluated on HLE-B3 proliferation and apoptosis in the
presence of H2O2. In addition, the molecular
mechanisms underlying the effects of miR-181a downregulation on
HLE-B3 cell apoptosis were investigated. Elucidation of the
functional roles of miR-181a may offer novel insight to decipher
the complex regulatory mechanisms underlying the pathogenesis of
cataract.
Materials and methods
Cell culture
Human HLE-B3 lens epithelial cells was were
purchased from Jennio Biotech Co., Ltd. (Guangzhou, China) and the
human 293T cell line was obtained from the Cell Bank of the Chinese
Academy of Sciences (Shanghai, China). HLE-B3 and 293T cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin
(all from both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Cell cultures were maintained in a humidified incubator
at 37°C in a 5% CO2 atmosphere.
Generation of lentivirus-based RNAi
plasmid
The hsa-miR-181a-5p sequence was obtained from the
miRBase database (http://www.mirbase.org). The lentivirus-based RNAi
transfer plasmid pLKO. 1-puro-miR-181a (miR-181a-shRNA) targeting
miR-181a (5′-AACAUUCAACGCUGUCGGUGAGU-3′) and the control plasmid
pLKO. 1-puro were prepared using the pLKO. 1-puro plasmid obtained
from the State Key Laboratory of Oncogenes and Related Genes,
Shanghai Cancer Institute, Shanghai Jiao Tong University (Shanghai,
China). To generate the pLKO. 1-puro-miR-181a, a miR-181a sponge
was constructed using annealed oligonucleotides for tandem
miR-181a-binding sites, as previously described (18). Briefly, the miR-181a sponge
oligonucleotides (forward,
5′-CCGGACTCACCGACACGCATGAATGTTCCGGACTCACCGACACGCATGAATGTTCCGGACTCACCGACACGCATGAATGTTTTTTTT−3′
and reverse
5′-AATTAAAAAAAACATTCATGCGTGTCGGTGAGTCCGGAACATTCATGCGTGTCGGTGAGTCCGGAACATTCATGCGTGTCGGTGAGT-3′)
were annealed using 50 pmol forward and reverse oligonucleotides,
and 10X polymerase chain reaction (PCR) buffer (Takara Bio, Inc.,
Otsu, Japan), and cloned after the U6 promoter in the
AgeI/EcoRI-digested pLKO.1-puro vector for the
construction of pLKO.1-puro-miR-181a. The annealing conditions were
as follows: 94°C for 3 min; followed by 55 cycles at 80°C for 30
sec with −1°C/cycle. The constructs were verified prior to use by
sequencing by Sangon Biotech Co., Ltd. (Shanghai, China).
Lentiviral production
Lentivirus production and infection of the targeted
cells were performed as previously described (19). Briefly, prior to transfection,
25×106 293T cells were plated onto 60×15 mm Petri dishes
and grown to 80% confluence. The cells were then co-transfected
with 3 µg psPAX2 packaging plasmid and 1 µg pMD2.G envelope plasmid
(both from Invitrogen; Thermo Fisher Scientific, Inc.), along with
4 µg either pLKO.1-puro empty vector or pLKO.1-puro-miR-181a
plasmid using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) as the transfection reagent, according to the
manufacturer's protocol. Cells were cultured in serum-free DMEM for
6 h, following which the medium was replaced with DMEM supplemented
with 10% FBS. The culture supernatants containing the lentiviral
particles were collected at 48 and 72 h post-transfection. The
supernatants were mixed, filtered by 0.45-µm filter and
concentrated by ultracentrifugation at 4,000 × g for 20 min at 4°C.
Viral supernatants were then aliquoted and stored at −80°C as a
viral stock.
Stable transduction with
shRNA-encoding lentivirus
HLE-B3 cells were divided into the following 3
experimental groups: Normal control, negative control and
shRNA-transfected groups. A total of 1×105 cells were
seeded in complete DMEM medium in a 24-well plate 24 h prior to
transduction. Untreated HLE-B3 cells were used as the normal
control group. The supernatant of the lentiviral particles
containing the empty pLKO.1-puro vector was used to transduce the
negative control cells for 24 h at 37°C, followed by the addition
of fresh complete DMEM medium, HLE-B3 cells were infected with the
supernatant of the recombinant lentivirus containing the
miR-181a-shRNA for 24 h at 37°C, followed by the addition of fresh
complete DMEM medium. Cells stably expressing the shRNA were
obtained by puromycin selection at ~72 h using DMEM containing 2
µg/ml puromycin until the HLE-B3 cells all died in the control
group. Confirmation of miR-181a knockdown was performed by reverse
transcription-quantitative PCR (RT-qPCR).
Cell proliferation assay
Cell proliferation was evaluated by the colorimetric
water-soluble tetrazolium salt assay, Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according
to the manufacturer's protocol immediately following confirmation
of successful knockdown (20).
Briefly, control or stably transfected HLE-B3 cells were seeded
(2×104 cells/well) in 96-well round bottom plates
immediately following transfection. Following overnight incubation
at 37°C in serum-free medium, the cells were challenged with or
without 200 µM H2O2 for 24 h at 37°C.
Subsequently, 10 µl CCK-8 solution was added to each well and cells
were incubated for another 4 h at 37°C. The number of viable cells
was assessed by measuring the optical density values of the samples
at 450 nm using a microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Flow cytometric analysis of
apoptosis
Flow cytometry was used to assess cell apoptosis
using an Annexin V-fluorescein isothiocyanate (FITC)/propidium
iodide (PI) apoptosis detection kit (BD Biosciences, San Jose, CA,
USA), according to the manufacturer's protocol. Briefly, control
and stably transfected HLE-B3 cells were seeded (4×105
cells/well) in 6-well plates. Cells were cultured in serum-free
medium overnight at 37°C and subsequently challenged with or
without 200 µM H2O2 for an additional 24 h at
37°C. Cells were trypsinized, collected by centrifugation at 500 ×
g at 4°C for 6 min, washed with phosphate-buffered saline (PBS) and
resuspended in 1X binding buffer. For apoptosis detection, 5 µl
annexin V-FITC and 5 µl PI were added to a culture tube containing
100 µl cell suspension and incubated for 15 min in a dark container
at room temperature (25°C). Subsequently, 400 µl 1X binding buffer
was added to each culture tube and the samples were assessed using
a FACSCalibur flow cytometer with CellQuest software (version 5.1;
BD Biosciences) within 1 h. Apoptotic cell populations were
detected by flow cytometric analysis and the fractions of cell
population were analyzed in different quadrants through the
quadrant statistics, with the results being calculated as
percentages of apoptotic cells.
RT-qPCR
Total RNA from normal and stably transfected HLE-B3
cells (5×106 cells), with or without
H2O2 treatment, was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The purity and concentration of RNA
were determined using a NanoDrop spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA)
and an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa
Clara, CA, USA). RT-qPCR was performed to evaluate the expression
of the apoptosis-related genes caspase-3 (CASP3) and B-cell
lymphoma-2-assciated X protein (BAX), and of the potential
target genes for miR-181a c-MET, cyclooxygenase 2
(COX-2) and snail family transcriptional repressor 2
(SNAI2). β-actin was used as the internal control to
normalize gene expression levels. Sequences of the specific primers
used in the present study are presented in Table I. All primers were designed and
synthesized by Takara Biotechnology Co., Ltd. (Dalian, China).
 | Table I.Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of the primers used for
reverse transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| CASP3 | F:
GTGCTATTGTGAGGCGGTTGT |
|
| R:
TCCATGTATGATCTTTGGTTC |
| c-MET | F:
AGAAGGCTAAAGGAAACGAA |
|
| R:
GGACCGTCAAGAAGTAAATAAA |
| COX-2 | F:
CCCTGAGCATCTACGGTTTG |
|
| R:
CAGTATTAGCCTGCTTGTCT |
| SNAI2 | F:
ATTTATGCAATAAGACCTATTCT |
|
| R:
AGGCTCACATATTCCTTGTCACA |
| BAX | F:
CTGACGGCAACTTCAACTGGG |
|
| R:
GGAGTCTCACCCAACCACCCT |
| β-actin | F:
AGCGGGAAATCGTGCGTG |
|
| R:
CAGGGTACATGGTGGTGGTGCC |
First-strand cDNA was synthesized using the
PrimeScript First-Strand cDNA Synthesis kit (Takara Biotechnology
Co., Ltd.) with Oligo-dT primers in a 20 µl reaction mixture
containing 0.5 µg DNase-treated RNA, 4 µl 5X PrimeScript RT Master
Mix and RNase-free water to a total volume of 20 µl, according to
the manufacturer's protocol. The reaction was incubated at 37°C for
15 min and at 85°C for 5 sec. qPCR was performed on cDNA using
Power SYBR Green PCR Master Mix in a 7500 Fast Real-Time PCR system
(both from Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The PCR reaction volume
was 20 µl, containing 10 µl 2X SYBR Premix Ex Taq, 10 µM of each
primer, and diluted cDNA to a total volume of 20 µl. The
thermocycling conditions were as follows: Initial denaturation at
50°C for 3 min and at 95°C for 30 min, followed by 40 cycles at
95°C for 10 sec and annealing at 60°C for 30 sec. The specificity
was verified by melting curve analysis (60–95°C) following the 40
cycles of amplification. Each sample was analyzed in triplicate.
The quantification cycle (Cq) was set within the exponential phase
of the PCR and relative gene expression was calculated using the
2−ΔΔCq method (21).
For quantification of miRNAs, a TaqMan MicroRNA
reverse transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used according to the manufacturer's
protocol. The primers used for miR-181a were as follows: miR-181a
forward, 5′-GCGGCGAACATTCAACGATG-3′ and reverse,
5′-GTGCAGGGTCCGAGG−3′. Relative gene expression was calculated
using the 2−ΔΔCq method (21).
Measurement of malondialdehyde (MDA),
superoxide dismutase (SOD) and catalase (CAT) levels
Control and stably transfected HLE-B3 cells were
seeded (4×105 cells/well) in 6-well plates. Cells were
cultured in serum-free medium overnight at 37°C and subsequently
challenged with 200 µM H2O2 or with nothing
for an additional 24 h at 37°C. Cells were harvested and lysed by
two rounds of sonication in 100 µl PBS (3 times for 10 sec) in an
ice-water bath, incubated at −80°C for 30 min in between.
Concentrations of MDA, SOD and CAT were measured using commercially
available MDA ELISA kits (cat no. ml027131), SOD ELISA kit (cat no.
ml026976), and CAT ELISA kit (cat no. ml026352) (all from Shanghai
Enzyme-linked Biological Technology, Co., Ltd., Shanghai, China),
according to the manufacturer's protocol. The absorbance of each
sample was measured at 450 nm using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Statistical analysis
All statistical analyses were performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation of 3 independent
experiments. The statistical significance of the differences
between groups was assessed using Student's t-test for pair-wise
comparisons or one-way analysis of variance followed by the
Student-Newman-Keuls post hoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Lentiviral knockdown of miR-181a in
HLE-B3 cells
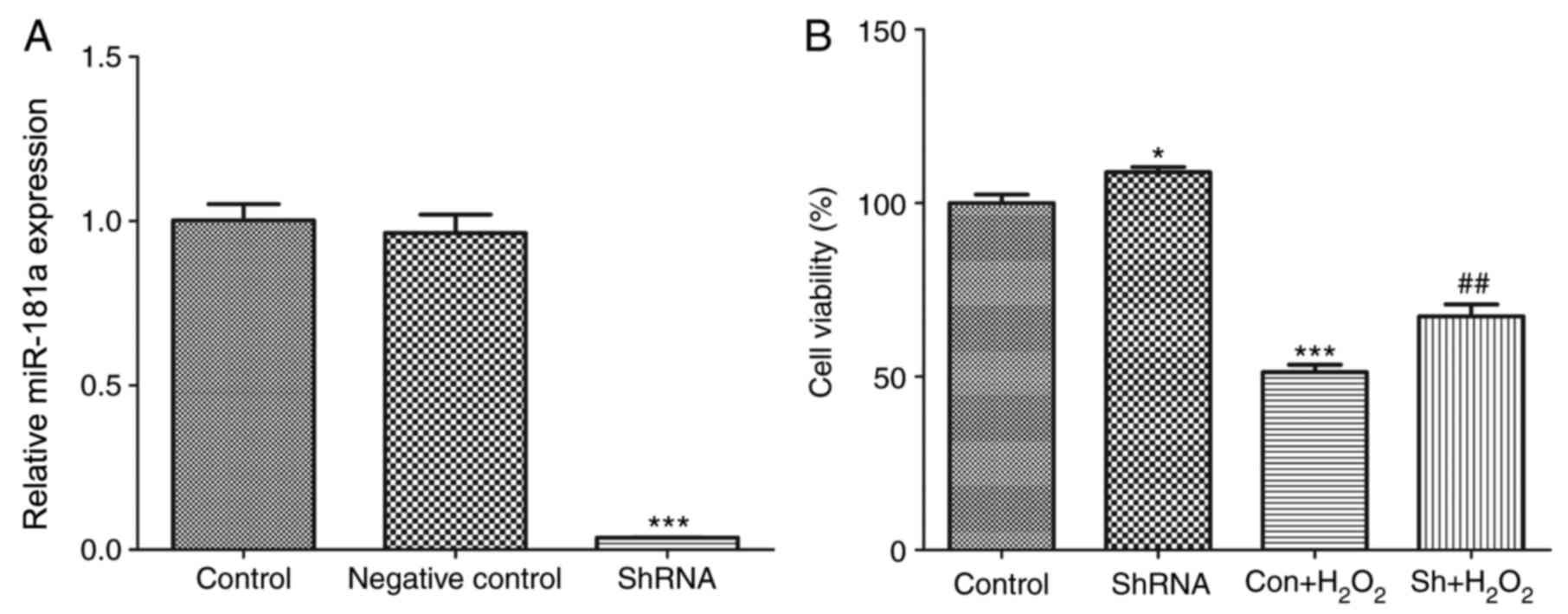
Knockdown of miR-181a expression in HLE-B3 cells was
performed using pLKO.1-puro-miR-181a lentiviral particles that
express a miR-181a-targeting shRNA, whereas an empty pLKO.1-puro
vector was used as a negative control. To test the knockdown
efficiency, the expression of miR-181a was examined using RT-qPCR
48 h post-infection. The expression levels of miR-181a in
miR-181a-shRNA-transfected cells were significantly decreased
compared with expression levels in the normal control and negative
control cells (P<0.001; Fig.
1A). These findings indicated that the construction of the
HLE-B3 cell line in which miR-181a expression was stably silenced
was successful.
miR-181a knockdown enhances HLE-B3
cell proliferation following H2O2
treatment
To assess the effects of shRNA-mediated miR-181a
silencing on cell proliferation and survival following
H2O2 treatment, the growth of HLE-B3 cells
was investigated in vitro. miR-181a knockdown significantly
enhanced the proliferation of HLE-B3 cells compared with normal
control cells (P<0.05; Fig.
1B). HLE-B3 cells treated with H2O2 (200
µM) exhibited a decrease in viability compared with untreated
control cells (P<0.001; Fig.
1B). Notably, knockdown of miR-181a expression partially
rescued H2O2-treated HLE-B3 cell viability,
as compared with the untransfected
H2O2-treated group (P<0.05; Fig. 1B); however, the proliferation of
shRNA-transfected H2O2-treated cells remained
significantly reduced compared with the control group (P<0.01;
Fig. 1B). These findings suggested
that shRNA-mediated miR-181a silencing may partially protect the
viability of HLE-B3 cells against oxidative stress-induced
compromise.
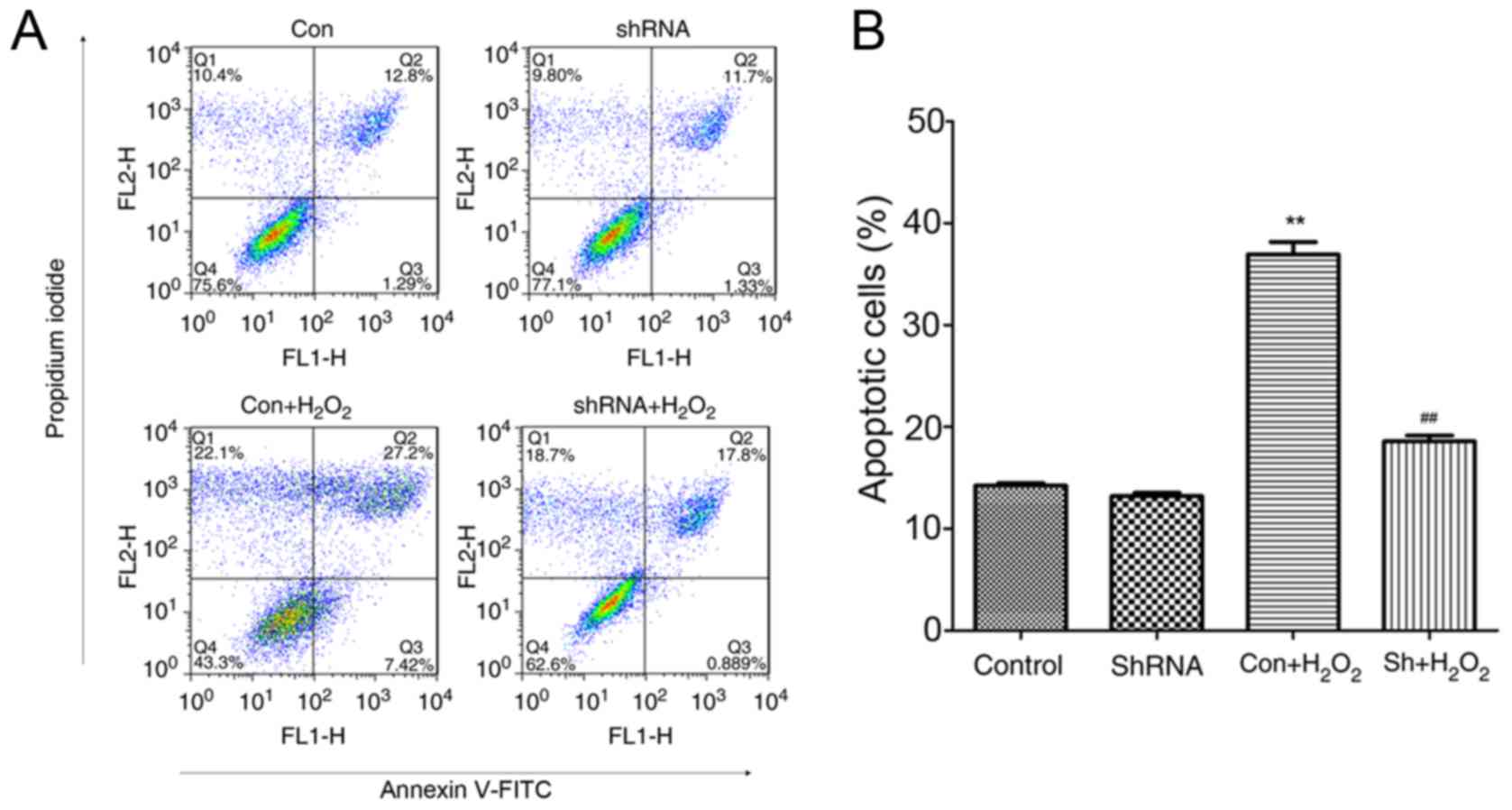
miR-181a knockdown counteracts
H2O2-induced apoptosis in HLE-B3 cells
Decreased cell viability is closely associated with
cell apoptosis (22); therefore,
the present study examined whether the downregulation of miR-181a
expression in HLE-B3 cells may exert a protective effect against
H2O2-induced apoptosis. HLE-B3 cells infected
with miR-181a-shRNA and treated with H2O2
appeared to be less susceptible to
H2O2-induced damage, as indicated by the
lower apoptotic rates compared with untransfected
H2O2-treated cells (P<0.05; Fig. 2). However, no significant
difference in the apoptotic rate was detected between the control
and the shRNA-treated groups (P>0.05). These findings suggested
that the downregulation of miR-181a expression in HLE-B3 cells may
exert a protective effect against
H2O2-induced apoptosis.
Alterations in gene expression
following miR-181a knockdown in HLE-B3 cells
To assess the potential effects of shRNA-mediated
miR-181a silencing on gene expression, mRNA expression levels of
the apoptosis-associated genes CASP3 and BAX, and of
the potential target genes for miR-181a c-MET, COX-2 and
SNAI2 were detected. The results demonstrated that there
were no significant differences in shRNA vs. con without treatment.
The present results demonstrated that H2O2
exposure significantly increased the expression of CASP3,
BAX and COX-2 compared with expression levels in the
normal control group (P<0.001; Fig.
3A). Notably, following knockdown of miR-181a expression in
H2O2-treated cells, the mRNA expression
levels of BAX and COX-2 were significantly suppressed
compared with in untransfected H2O2-treated
cells (P<0.01 and P<0.001 respectively; Fig. 3A). Conversely, cells treated with
miR181a-shRNA with or without H2O2
co-treatment exhibited no significant effects on the mRNA
expression levels of c-MET or SNAI2 (P>0.05;
Fig. 3B). These results suggested
that shRNA-mediated miR-181a silencing may affect the expression of
apoptosis-associated genes CASP3 and BAX, and of the
putative COX-2 miR-181a target gene.
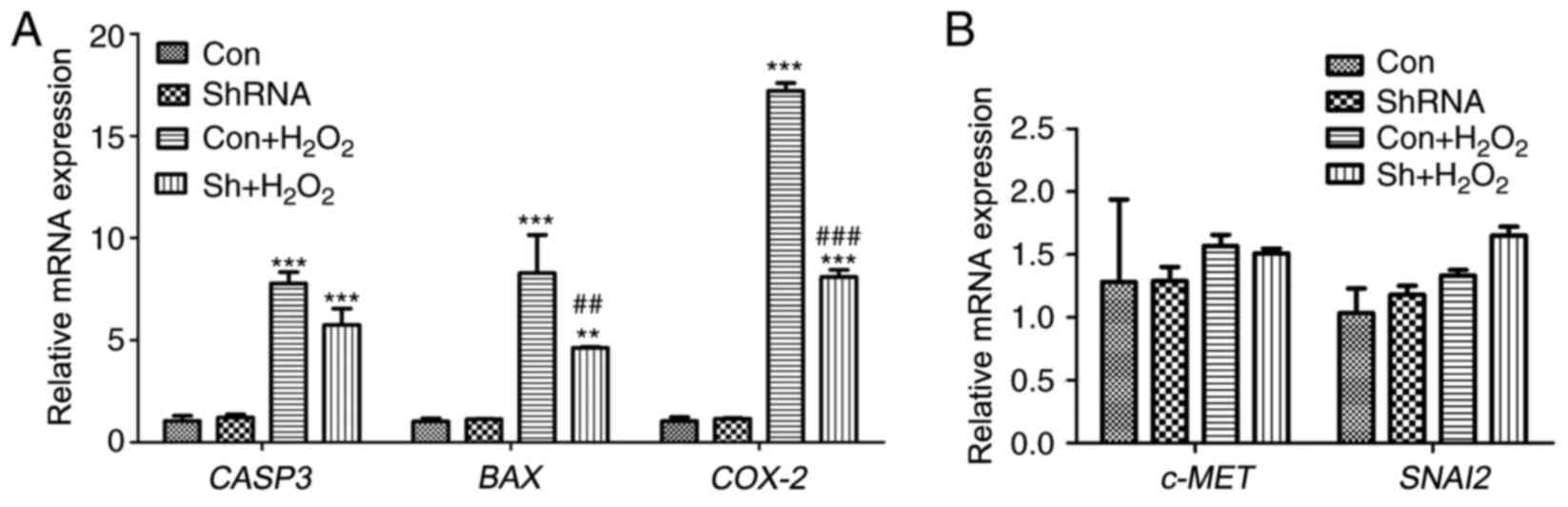 | Figure 3.Alterations in gene expressions
following miR-181a knockdown in HLE-B3 cells were assessed using
reverse transcription-quantitative polymerase chain reaction. (A)
Relative mRNA expression of the apoptosis-associated genes CASP3
and BAX, and of the potential miR-181a target gene COX-2. (B)
Relative mRNA expression of the potential miR-181a target genes
c-MET and SNAI2. Data are expressed as the mean ± standard
deviation; **P<0.01, ***P<0.001 vs. con;
##P<0.01, ###P<0.001
Sh+H2O2 vs. con+H2O2.
BAX, B-cell lymphoma-2-associated X protein; CASP3, caspase-3; con,
control; COX-2, cyclooxygenase 2; H2O2,
hydrogen peroxide; HLE, human lens epithelial; miR, microRNA; sh,
short hairpin; SNAI2, snail family transcriptional repressor 2. |
Effects of miR-181a silencing on MDA,
SOD and CAT expression levels
MDA is a product of lipid peroxidation and its
intracellular levels are indicative of oxidative damage (14), whereas SOD and CAT are endogenous
antioxidative enzymes (23). The
levels of MDA, SOD and CAT in HLE-B3 cells from the various
experimental groups are presented in Fig. 4. The results demonstrated that
there were no significant differences in shRNA vs. con without
treatment. The results demonstrated that in the presence of
H2O2, the intracellular levels of MDA and CAT
were significantly increased in HLE-B3 cells compared with
expression in untreated control cells (P<0.001; Fig. 4A and C, respectively). Conversely,
SOD expression levels were significantly reduced in cells following
H2O2 treatment compared with untreated
control cells (P<0.001; Fig.
4B). Notably, the levels of MDA were significantly suppressed
in H2O2-treated cells following miR-181a
knockdown compared with in untransfected
H2O2-treated cells (P<0.01; Fig. 4A). Conversely, miR-181a-shRNA
treatment exhibited no significant effects on the intracellular
levels of SOD and CAT in the H2O2 co-treated
group compared with the expression levels in the untransfected
H2O2-treated cells (P>0.05; Fig. 4B and C, respectively). These
results suggested that miR-181a knockdown may suppress the
production of MDA following exposure to oxidative stress in
vitro.
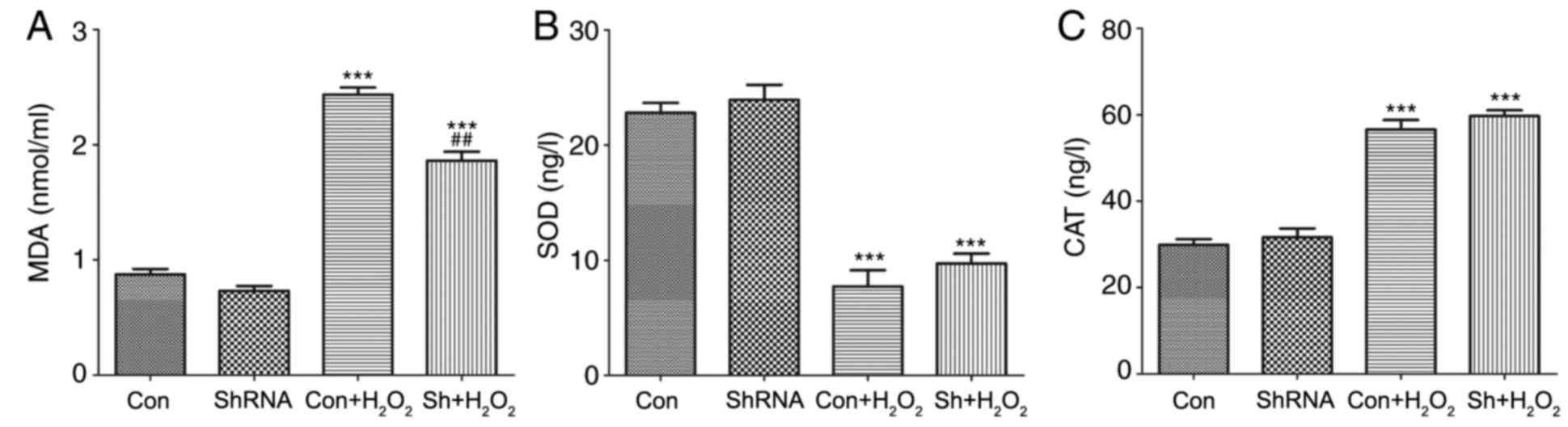 | Figure 4.Levels of MDA, SOD and CAT were
measured in HLE-B3 cells using ELISA kits. Intracellular expression
levels of (A) MDA, (B) SOD and (C) CAT were measured in cells
treated with or without miR-181a-shRNA and with or without
H2O2 co-treatment. Data are expressed as the
mean ± standard deviation; ***P<0.001 vs. con;
##P<0.01 vs. con + H2O2. CAT,
catalase; con, control; H2O2, hydrogen
peroxide; HLE, human lens epithelial; miR, microRNA; MDA,
malondialdehyde; sh, short hairpin; SOD, superoxide dismutase. |
Discussion
Cataract is among the leading causes of blindness
worldwide, and 90% of cataract-related cases of blindness occur in
developing countries (4). miRNAs
have garnered attention as a prominent class of gene expression
regulators, and mounting evidence supports the implication of
miRNAs in the pathophysiology of human cataract (12). The results of the present study
suggested that the downregulation of miR-181a expression using
RNAi-mediated suppression may protect or rescue HLE-B3 cells in
vitro from undergoing apoptosis following
H2O2 exposure. The molecular mechanisms
underlying the effects of miR-181a knockdown in human lens cells
may involve the suppression of CASP3, COX-2 and BAX
expression, and the inhibition of MDA production.
Oxidative stress serves a crucial role in the
regulation of several cellular events, including oxidative damage
and cell death (24). Reactive
oxygen species, including H2O2, may induce
irreversible oxidative modifications to nucleic acids, lipids and
proteins, and may lead to cellular damage and cell necrosis or
apoptosis (25). A previous study
observed a significant decrease in HLE-B3 cell viability in the
presence of 200 µM H2O2 compared with control
untreated cells (22); therefore.
200 µM H2O2 was used in the present study to
treat HLE-B3 cells and to investigate the effects of silencing
miR-181a expression. The present results demonstrated that
following transduction with a lentiviral vector encoding
miR-181a-shRNA, the expression of miR-181a was successfully
silenced. Notably, miR-181a was revealed to partially rescue the
compromise in HLE-B3 cell viability following oxidative stress
exposure compared with viability in
H2O2-treated cells.
Compromises in cell viability are closely associated
to cell apoptosis, and the present study examined whether miR-181a
downregulation in HLE-B3 cells following H2O2
exposure affected apoptotic signaling pathways. CASP3 mediates
apoptosis thorough the regulation of several crucial events during
apoptosis (26). During apoptosis,
BAX proteins undergo conformational changes and translocate to the
mitochondrial outer membrane, thus allowing the release of
proapoptotic factors from the intermembrane space (27). A previous study reported that Grx2
was able to protect cells against
H2O2-induced compromises in cell viability
and against apoptosis through the inhibition of proapoptotic
signaling, including BAX activation and CASP3 release (22). Similarly, the present results
demonstrated that the downregulation of miR-181a suppressed the
mRNA expression of BAX and CASP3 in HLE-B3 cells.
These results suggested that miR-181a may serve a significant role
in the pathogenesis of cataract, through the regulation of
BAX and CASP3 expression in lens cells. Furthermore,
the intracellular levels of MDA, SOD and CAT were investigated; MDA
levels are indicative of oxidative damage (16), whereas SOD and CAT are
antioxidative enzymes (23). The
present results revealed that miR-181a knockdown suppressed the
generation of MDA, thus suggesting that miR-181a silencing may
inhibit oxidative damage associated with apoptosis in lens
cells.
Several potential target genes have been identified
for miR-181a: For example, a previous study demonstrated that
miR-181a-5p was downregulated in hepatocellular cancer and
suggested that miR-181a-5p may inhibit tumor motility and invasion
by directly targeting the expression of c-Met (28). Another study suggested that
miR-181a may suppress salivary adenoid cystic carcinoma metastasis
by targeting the extracellular signal-regulated kinase/SNAI pathway
(29). Furthermore, miRNA-181a has
been reported to inhibit the proliferation, migration and EMT of
lens epithelial cells by directly targeting SNAI2 and
COX-2 expression (11).
Therefore, the present study investigated the expression of three
potential target genes for miR-181a, namely c-MET, COX-2 and
SNAI2 in HLE-B3 cells. The results demonstrated that
miR-181a knockdown using RNAi significantly downregulated the mRNA
expression of COX-2 in H2O2-treated
HLE-B3 cells compared with in untransfected
H2O2-treated cells. However,
H2O2 treatment and miR-181a silencing did not
exert a significant effect on the mRNA expression levels of
c-MET and SNAI2. The present data suggested that the
downregulation of miR-181a expression may inhibit HLE cell
apoptosis by targeting COX-2 expression. Further studies are
required to fully elucidate the molecular mechanisms and the target
genes that are implicated in the protective effects of miR-181a
knockdown.
In conclusion, the results of the present study
suggested that the downregulation of miR-181a expression may
protect or rescue HLE-B3 cells from undergoing apoptosis following
H2O2 exposure in vitro. The molecular
mechanisms underlying the protective effects of miR-181a silencing
may involve the downregulation of CASP3, BAX and
COX-2 expression, and the inhibition of lipid peroxidation.
Further studies are required to fully elucidate the functional
roles of miR-181a and may provide the foundation for a better
understanding of the miRNA-mediated regulation of cataract
development in the eye with eventual implications in the diagnosis
and treatment of cataract.
References
|
1
|
Asbell PA, Dualan I, Mindel J, Brocks D,
Ahmad M and Epstein S: Age-related cataract. Lancet. 365:599–609.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoffmann A, Huang Y, Suetsugu-Maki R,
Ringelber CS, Tomlinson CR, Del Rio-Tsonis K and Tsonis PA:
Implication of the miR-184 and miR-204 competitive RNA network in
control of mouse secondary cataract. Mol Med. 18:528–538. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khee SG, Yusof YA and Makpol S: Expression
of senescence-associated microRNAs and target genes in cellular
aging and modulation by tocotrienol-rich fraction. Oxid Med Cell
Longev. 2014:7259292014.PubMed/NCBI
|
|
4
|
Chien KH, Chen SJ, Liu JH, Chang HM, Woung
LC, Liang CM, Chen JT, Lin TJ, Chiou SH and Peng CH: Correlation
between microRNA-34a levels and lens opacity severity in
age-related cataracts. Eye (Lond). 27:883–888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li WH, Kang ZF and Li L: Research of
controlling genes of lens epithelial cell apoptosis and cataract.
Int J Ophthalmol. 10:88–89. 2010.
|
|
6
|
Li Y, Liu S, Zhang F, Jiang P, Wu X and
Liang Y: Expression of the microRNAs hsa-miR-15a and hsa-miR-16-1
in lens epithelial cells of patients with age-related cataract. Int
J Clin Exp Med. 8:2405–2410. 2014.
|
|
7
|
Khan L, Khan RA, Ahmed W, Rauf A, Khan WM,
Khan W, Durrani SA and Qayum: Frequency, causes and cutting-edge
treatment of cataract: A review. American J Biomed Life Sci.
3:25–28. 2015. View Article : Google Scholar
|
|
8
|
Yang Y, Sharma R, Cheng JZ, Saini MK,
Ansari NH, Andley UP, Awasthi S and Awasthi YC: Protection of HLE
B-3 cells against hydrogen peroxide-and naphthalene-induced lipid
peroxidation and apoptosis by transfection with hGSTA1 and hGSTA2.
Invest Ophthalmol Vis Sci. 43:434–445. 2002.PubMed/NCBI
|
|
9
|
Bai J, Zheng Y, Dong L, Cai X, Wang G and
Liu P: Inhibition of p38 mitogen-activated protein kinase
phosphorylation decreases H2O2-induced
apoptosis in human lens epithelial cells. Graefes Arch Clin Exp
Ophthalmol. 253:1933–1940. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka Y, Tsuda S, Kunikata H, Sato J,
Kokubun T, Yasuda M, Nishiguchi KM, Inada T and Nakazawa T:
Profiles of extracellular miRNAs in the aqueous humor of glaucoma
patients assessed with a microarray system. Sci Rep. 4:50892014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong N, Xu B and Tang X: miRNA-181a
inhibits the proliferation, migration and epithelial-mesenchymal
transition of lens epithelial cells. Invest Ophthalmol Vis Sci.
56:993–1001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu C, Lin H, Wang Q, Chen W, Luo H, Chen W
and Zhang H: Discrepant expression of microRNAs in transparent and
cataractous human lenses. Invest Ophthalmol Vis Sci. 53:3906–3912.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng CH, Liu JH, Woung LC, Lin TJ, Chiou
SH, Tseng PC, Du WY, Cheng CK, Hu CC, Chien KH and Chen SJ:
MicroRNAs and cataracts: Correlation among let-7 expression, age
and the severity of lens opacity. Br J Ophthalmol. 96:747–751.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Q, Zhao J, Min X, Wang M, Luo W, Wu D,
Yan Q, Li J, Wu X and Zhang J: MicroRNA-125b inhibits lens
epithelial cell apoptosis by targeting p53 in age-related cataract.
Biochim Biophys Acta. 1842:2439–2447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong Y, Zheng Y, Xiao J, Zhu C and Zhao M:
MicroRNA let-7b induces lens epithelial cell apoptosis by targeting
leucine-rich repeat containing G protein-coupled receptor 4 (Lgr4)
in age-related cataract. Exp Eye Res. 147:98–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Huang H, Fan Y, Kong B, Hu H, Hu
K, Guo J, Mei Y and Liu WL: Effects of downregulation of
microRNA-181a on H2O2-induced H9c2 cell
apoptosis via the mitochondrial apoptotic pathway. Oxid Med Cell
Longev. 2014:9603622014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu G and Luo J: A primer on using pooled
shRNA libraries for functional genomic screens. Acta Biochim
Biophys Sin (Shanghai). 44:103–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hatakeyama H, Murata M, Sato Y, Takahashi
M, Minakawa N, Matsuda A and Harashima H: The systemic
administration of an anti-miRNA oligonucleotide encapsulated
pH-sensitive liposome results in reduced level of hepatic
microRNA-122 in mice. J Control Release. 173:43–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stewart SA, Dykxhoorn DM, Palliser D,
Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, et
al: Lentivirus-delivered stable gene silencing by RNAi in primary
cells. RNA. 9:493–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu H, Xing K and Lou MF: Glutaredoxin 2
prevents H2O2-induced cell apoptosis by
protecting complex I activity in the mitochondria. Biochim Biophys
Acta. 1797:1705–1715. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang CK, Lin Y, Su H and Ye D:
Forsythiaside protects against hydrogen peroxide-induced oxidative
stress and apoptosis in PC12 cell. Neurochem Res. 40:27–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bergamini CM, Gambetti S, Dondi A and
Cervellati C: Oxygen, reactive oxygen species and tissue damage.
Curr Pharm Des. 10:1611–1626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: A metabolic by-product or a common
mediator of ageing signals? Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galluzzi L, López-Soto A, Kumar S and
Kroemer G: Caspases connect cell-death signaling to organismal
homeostasis. Immunity. 44:221–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Colin J, Garibal J, Clavier A, Szuplewski
S, Risler Y, Milet C, Gaumer S, Guénal I and Mignotte B: Screening
of suppressors of bax-induced cell death identifies
glycerophosphate oxidase-1 as a mediator of debcl-induced apoptosis
in drosophila. Genes Cancer. 6:241–253. 2015.PubMed/NCBI
|
|
28
|
Korhan P, Erdal E and Atabey N:
miR-181a-5p is downregulated in hepatocellular carcinoma and
suppresses motility, invasion and branching-morphogenesis by
directly targeting c-Met. Biochem Biophys Res Commun.
450:1304–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He Q, Zhou X, Li S, Jin Y, Chen Z, Chen D,
Cai Y, Liu Z, Zhao T and Wang A: MicroRNA-181a suppresses salivary
adenoid cystic carcinoma metastasis by targeting the MAPK-Snai2
pathway. Biochim Biophys Acta. 1830:5258–5266. 2013. View Article : Google Scholar : PubMed/NCBI
|