Introduction
Aging is the universal and gradual process of
recession of all bodily organs. It has been reported that the
decline of male physiological and sexual functions is associated
with aging of the male reproductive system (1). The testes are a vital organ of the
male reproductive system, and have the ability to
synthesize/excrete testosterone and produce sperm
(spermatogenesis). Therefore, it is important to delay the aging of
the testes in order to improve the physiological and sexual
function in elderly men.
It has been reported that natural aging may be
modeled by D-galactose (D-gal) in vitro and in vivo
(2–4). D-gal has been widely used to generate
an aging model. The D-gal-induced model is regarded as the ideal
model to investigate the process of aging, and to assay potential
compounds for their effects against organismal aging. Ginseng is a
tonic drug in traditional Chinese medicine. Ginsenoside Rg1 is a
principal anti-aging active ingredient of ginseng. Previous studies
(2–5) have revealed that ginsenoside Rg1 may
defer the senescence of stem cells, and protect the immune organs,
hemopoietic organs and brain from D-gal-induced aging via
antioxidant and anti-inflammatory mechanisms. However, the effects
of ginsenoside Rg1 on the testes in D-gal-induced mice have not
been reported.
In recent decades (6,7), a
variety of aging associated molecules and their corresponding
signal pathway was determined. The p19-p53-p21 pathway has been
considered to be an important regulatory pathway associated with
cellular senescence (8). Our
previous study demonstrated that the oxidative stress response to
senescence in D-gal-induced mice activated the p19-p53-p21 pathway
(2,4,5).
Furthermore, p53, p21 and p19 proteins have also been suggested to
be associated with cellular senescence, and their expression can be
used as a measure of the degree of cellular senescence (2–5).
In the present study, testicular aging was
established to observe the alterations in testicular microstructure
and function in vivo in D-gal-induced mice. The protective
effect of ginsenoside Rg1 was investigated. In addition, the
preliminary underlying mechanism was explored. The present study
aimed to identify a drug to delay the decline of the males
reproductive system by attenuating the retrogression of testicular
function.
Materials and methods
Animals
6-8-week old male C57BL/6 mice (20–25 g) were
purchased from the Medical and Laboratory Animal Center of
Chongqing (no. SCXK yu. 2012-0001), and housed in specific pathogen
free conditions at a temperature of 20–25°C, a 50–70% humidity, a
light/dark cycle of 12/12 h, and with free access to water and
food. All procedures complied with the guiding principles for the
care and use of animals and were approved by the Committee on
Ethics of Animal Experimentation at Chongqing Medical University
(Chongqing, China). The 60 mice were divided into four groups at
random (control, Rg1, Rg1 + D-gal and D-gal). In the D-gal group,
D-gal (120 mg/kg/day) was injected subcutaneously into the mice
daily for 42 days. In the D-gal + Rg1 group, Rg1 (20 mg/kg/day) was
injected intraperitoneally concomitantly for 28 days, from day 15
of the D-gal injections. In the control group, saline was given in
the same volume subcutaneously and intraperitoneally, respectively.
In the Rg1 group, saline at the same volume as the D-gal injection
was injected subcutaneously for 42 days, and Rg1 (20 mg/kg/day) was
injected intraperitoneally for 28 days from day 15 of saline
injection. On the 43rd day, the mice were sacrificed by cervical
dislocation.
Reagents
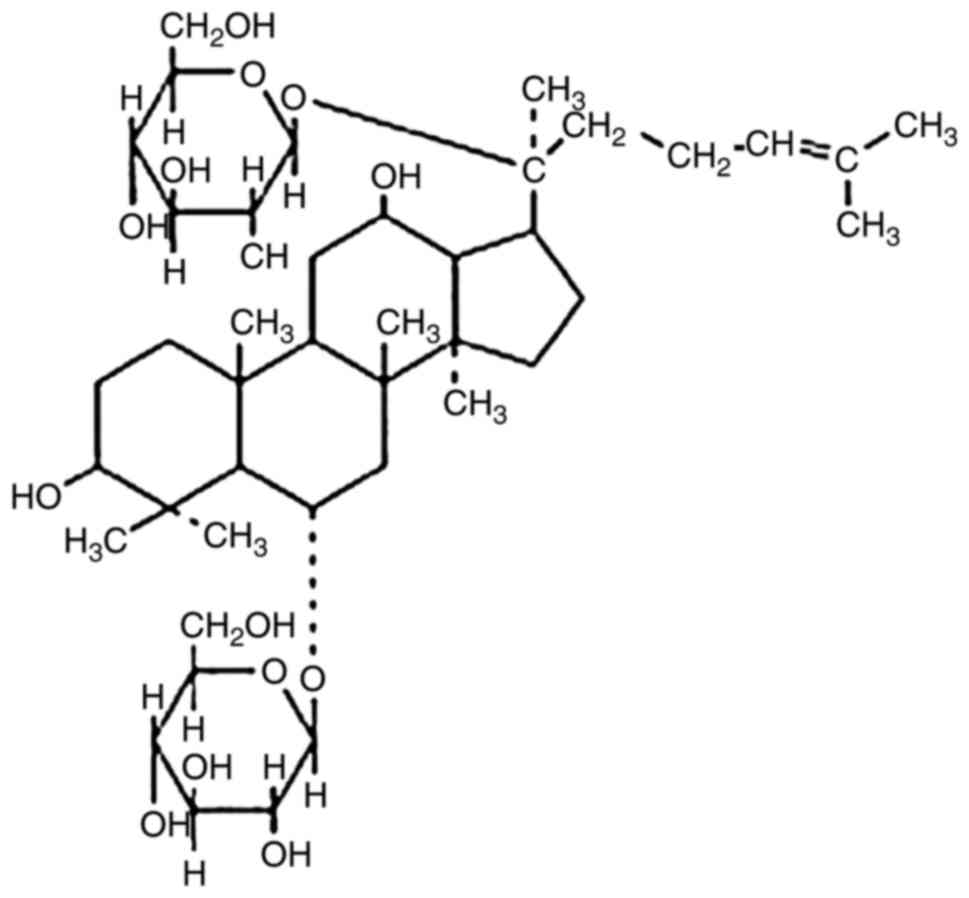
Ginsenoside Rg1 (cat. no. RSZD-121106; purity,
98.3%) was purchased from Xi'an Haoxuan Bio-Tech Co., Ltd. (Xi'an,
China) (Fig. 1), dissolved in PBS
at a concentration of 20 mg/ml, and sterilized by ultrafiltration.
D-gal (purity, >99%) was acquired from Shanghai Bioengineering
Co., Ltd. (Shanghai, China). The superoxide dismutase (SOD) kit,
methane dicarboxylic aldehyde (MDA) kit, total antioxidant capacity
(TAC) kit, primary antibody dilution buffer and goat anti-rabbit
(cat. no. A0208) and goat anti-mouse (cat. no. A0216) secondary
antibodies were obtained from Beyotime Institute of Biotechnology
(Haimen, China). The interleukin (IL)-1β kit (cat. no. EMC001B),
IL-6 kit (cat. no. EMC004) and tumor necrosis factor (TNF)-α kit
(cat. no. EMC102a) were purchased from Neobioscience Co., Ltd.
(Shenzhen, China). The senescence-associated β-galactosidase
(SA-β-gal) staining kit was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The terminal deoxynucleotidyl
transferase (TdT) dUTP nick end labeling (TUNEL) Apoptosis Assay
kit was purchased from Jiamay Biotech (Beijing, China).
Anti-S-phase kinase-associated protein (p19) rabbit polyclonal
(cat. no. 10272-2-AP), anti-cellular tumor antigen p53 (p53) and
anti-cyclin-dependent kinase inhibitoru 1 (p21) mouse monoclonal
antibodies (cat. nos. 10442-1-AP and 60214-1-Ig) were obtained from
ProteinTech Group, Inc. (Chicago, IL, USA).
Assessment of testosterone in the
serum
Mouse serum obtained from the angular vein was
centrifuged (3,000 × g, 10 min, 4°C). The level of serum
testosterone was analyzed using an automatic biochemical analyzer
(cat. no. DXI800; Beckman Coulter, Inc., Brea, CA, USA).
Analysis of testis index and
structure
Testis index was calculated using the equation
testis index=testes weight (mg)/body weight (g), and the testes was
stored in a solution of 4% paraformaldehyde (pH 7.4) for 24 h at
4°C, dehydrated in graded (50–100%) alcohol and embedded in
paraffin. Sections (5–8 µm) were cut and stained with hematoxylin
and eosin (hematoxylin staining for 5 min at room temperature;
eosin staining for 3 min at room temperature). The pathological
structure of the testes and the differential counts of
spermatogenetic cells were measured using a microscope image
analysis system (Olympus Corporation, Tokyo, Japan) (9).
Detection of senescence and apoptosis
of spermatogenetic cells
The SA-β-gal staining and apoptosis detection were
performed according to the manufacturers' protocols. Frozen
sections (10-µm; −25°C) of mouse testis tissue were prepared for
SA-β-gal staining. Senescent cells appeared blue under light
microscopy (magnification ×40). Paraffin-embedded sections (4-µm)
were prepared for TUNEL staining. The pre-processing included
dewaxing, anhydration and antigen retrieval and blocking using
normal goat serum for 30 min to prevent nonspecific staining.
Antigen retrieval was performed by immersing sections in citrate
buffer and then heating the sections for 15 min at 90°C. Following
cooling, the sections were washed three times in PBS and then
immersed in 3% hydrogen peroxide for 15 min at room temperature.
Following this, the sections were again washed using PBS. The
sections were stained with TUNEL Staining Solution (solution A:
solution B, 1:9) for 60 min at room temperature. The sections were
color-developed with diaminobenzidine and the nuclei were stained
with hematoxylin for 5 min at room temperature. At least three
different fields of vision per section and per animal were observed
and analyzed with Image Pro Plus 6.0 software (Media Cybernetics
Inc., Rockville, MD, USA).
Measurement of oxidation-associated
biomarkers and inflammatory cytokines
The testes of each mouse were weighed and
homogenized with cold saline, and centrifuged (10,000 × g, 4°C, 20
min) to obtain the supernatant for further experiments. The SOD
activity, MDA content and TAC were evaluated by chemical
colorimetric analysis, according to the manufacturer's protocols.
The levels of inflammatory cytokines (IL-1β, IL-6 and TNF-α) in
each group were measured by ELISA analysis, according to the
manufacturer's protocol.
Senescence-associated protein
analysis
Tissue homogenate in each group was prepared as
described above. The total protein was extracted using
radioimmunoprecipitation assay lysis buffer mixed 1% protease
inhibitor cocktail (Beyotime Institute of Biotechnology), and the
concentration was measured by a bichinchoninic acid assay. Samples
containing 40 µg proteins were separated via 12% SDS-PAGE and
transferred to polyvinylidene fluoride membranes. The membranes
were blocked using skimmed milk powder (5%, dissolved in TBS-Tween
20) for 2 h at room temperature, and the membranes were then
incubated overnight at 4°C with anti-p53, anti-p21 and anti-p19
antibodies (1:1,000 diluted in primary antibody dilution buffer).
The membranes were then incubated with secondary antibodies (1:800
diluted in TBS-Tween 20) for 90 min at room temperature. The
membranes were visualized using an enhanced chemiluminescence
detection system (Pierce; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). β-actin (1:1,000 diluted in antibody dilution buffer) was
used as an internal control. Integral optical density was
quantified using Image Lab 5.2.1 (Bio Rad Laboratories, Inc.,
Hercules, CA, USA).
Measurement of variation in semen
parameters
Sperm was collected from the cauda epididymis and
vas deferens. The epididymis was dissected in PBS and incubated for
10 min in a constant temperature incubator (37°C, 5%
CO2) to allow the sperm to be released. A total of 10 µl
diluted sperm from each group was placed in a counting chamber
under a light microscope to determine the sperm density and
survival rate (10). A total of 5
µl diluted sperm from each group was mixed with 5 µl eosin on
microslides for 30 sec at room temperature, and a microscope (×200)
was then used to investigate sperm viability (dead sperm appeared
red). A total of 10 µl sperm was used to produce the smears. Sperm
smears were dried at room temperature, stained with crystal violet
(cat. no. C0121; Beyotime Institute of Biotechnology) for 1–3 min
and washed using water for 3–5 min. The sperm morphology of each
group was then determined.
Statistical analysis
All data are presented as mean ± standard deviation.
All statistical analyses were performed using one-way analysis of
variance followed by the Fisher's least significant difference test
with SPSS v20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Rg1 affects the general condition of
D-gal-induced aging mice
Following the injection of D-gal, the mice developed
withered and caducous hair, decreased skin elasticity,
listlessness, a tendency to cluster together in groups, decreased
food intake, and decreased activity and responsiveness. The control
group, Rg1 group and Rg1 + D-gal group exhibited none of these
signs. There were no complications detected in any of the mice. In
previous studies, complications were not identified in other organs
(4,5,11,12).
Rg1 affects the serum testosterone
level in D-gal-induced aging mice
Testosterone is one of the steroid hormones produced
primarily by Leydig cells in the testes. Testosterone is
responsible for the development of the male reproductive system and
is involved in the maintenance of male secondary sexual
characteristics. Serum testosterone levels have observed to decline
gradually with the aging process (13,14).
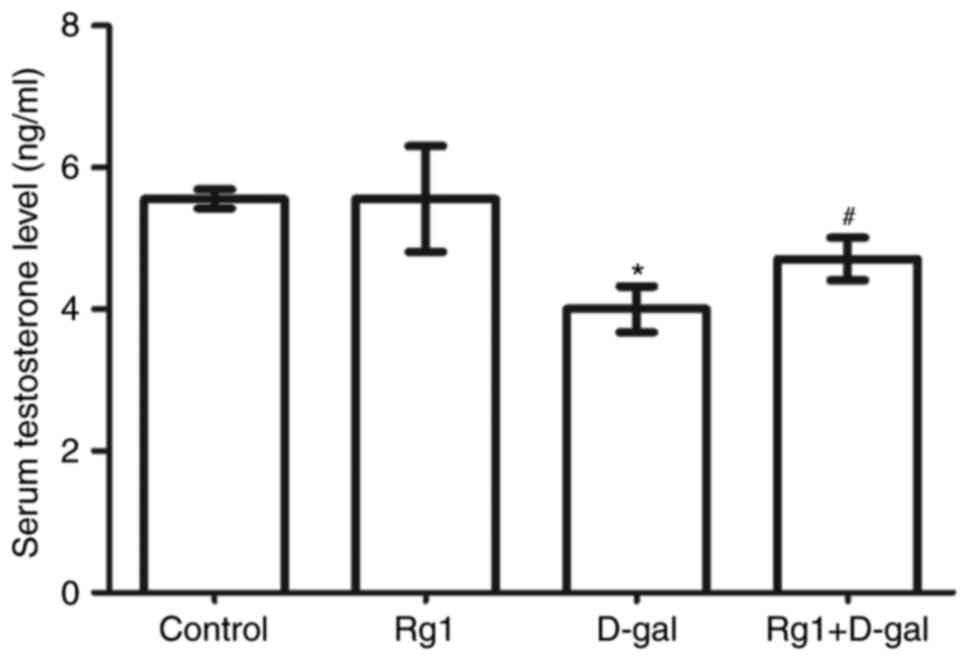
The results of the present study (Fig.
2) demonstrated that the level of serum testosterone was
significantly decreased in the D-gal-induced group. Following
treatment with Rg1, the level of serum testosterone significantly
increased compared with D-gal-induced group. This result
demonstrated that Rg1 was able to significantly improve the ability
of Leydig cells to secrete testosterone in D-gal-induced aging
mice.
Rg1 affects the index and microscopic
structure of testes
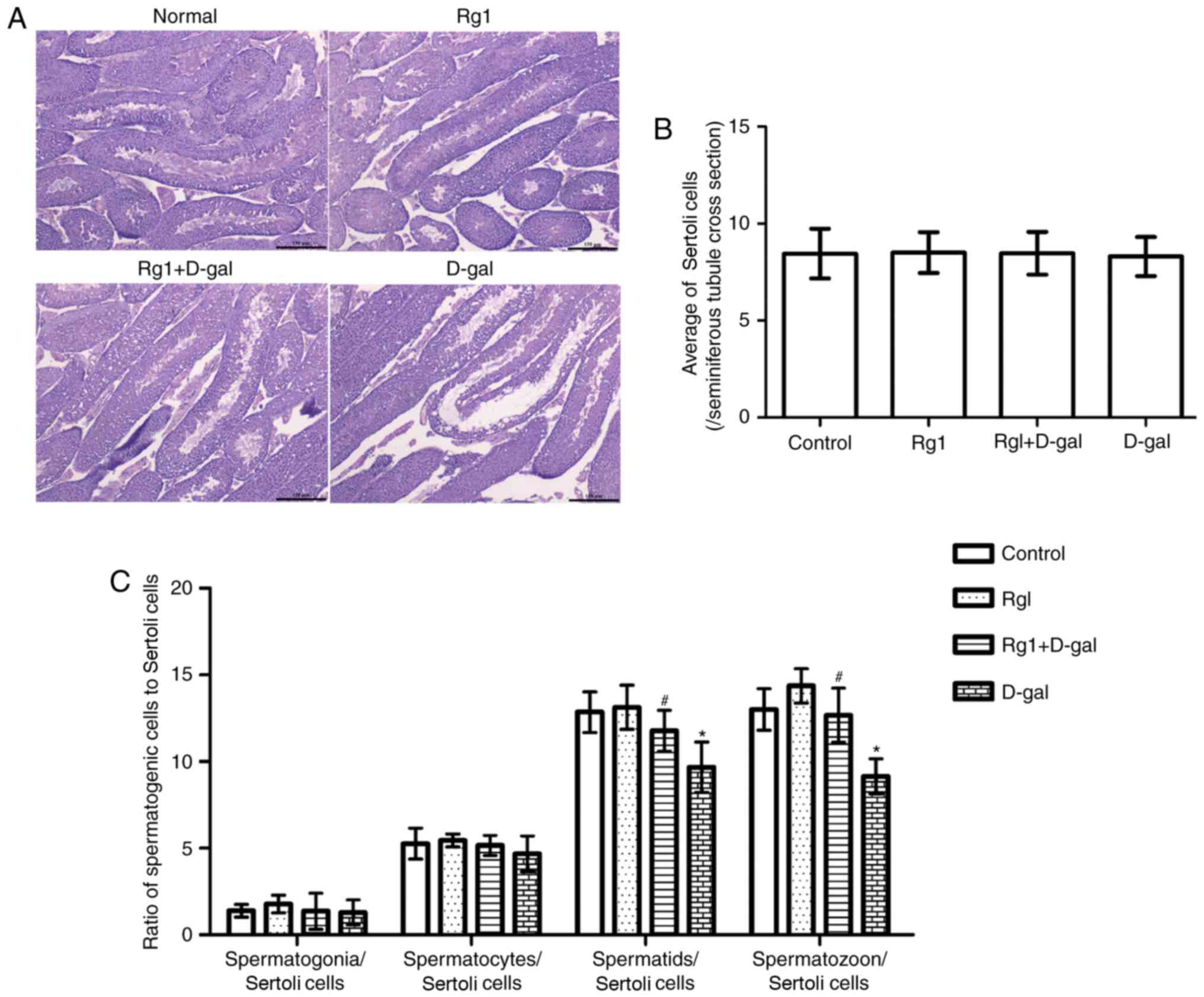
A previous study (9) reported that spermatogenetic
malfunction may occur during the aging process. Classification and
counting of spermatogenetic cells is of diagnostic importance for
spermatogenetic malfunction. Tissue examination (Fig. 3A) demonstrated the integrity of the
spermatogenetic epithelium and the regular arrangement of
spermatogenetic cells in the control group. Compared with the
control, structural abnormalities of the seminiferous tubule and a
disordered arrangement of spermatogenetic was observed in the D-gal
group. In the Rg1 + D-gal group, the pathological alteration of
abnormal structure of the seminiferous tubule was not noted. No
significant differences were observed in morphology or organ index
among the four groups (D-Gal group, 0.0060±0.00081; Rg1 + D-Gal
group, 0.0065±0.0012; Rg1 group, 0.0063±0.00037; and control group,
0.0064±0.00048). Subsequently, the differential counts of
spermatogenetic cells were measured. The results (Fig. 3B and C) revealed that
spermatogenetic cell/Sertoli cell number was decreased in the D-gal
group, and increased following treatment with Rg1.
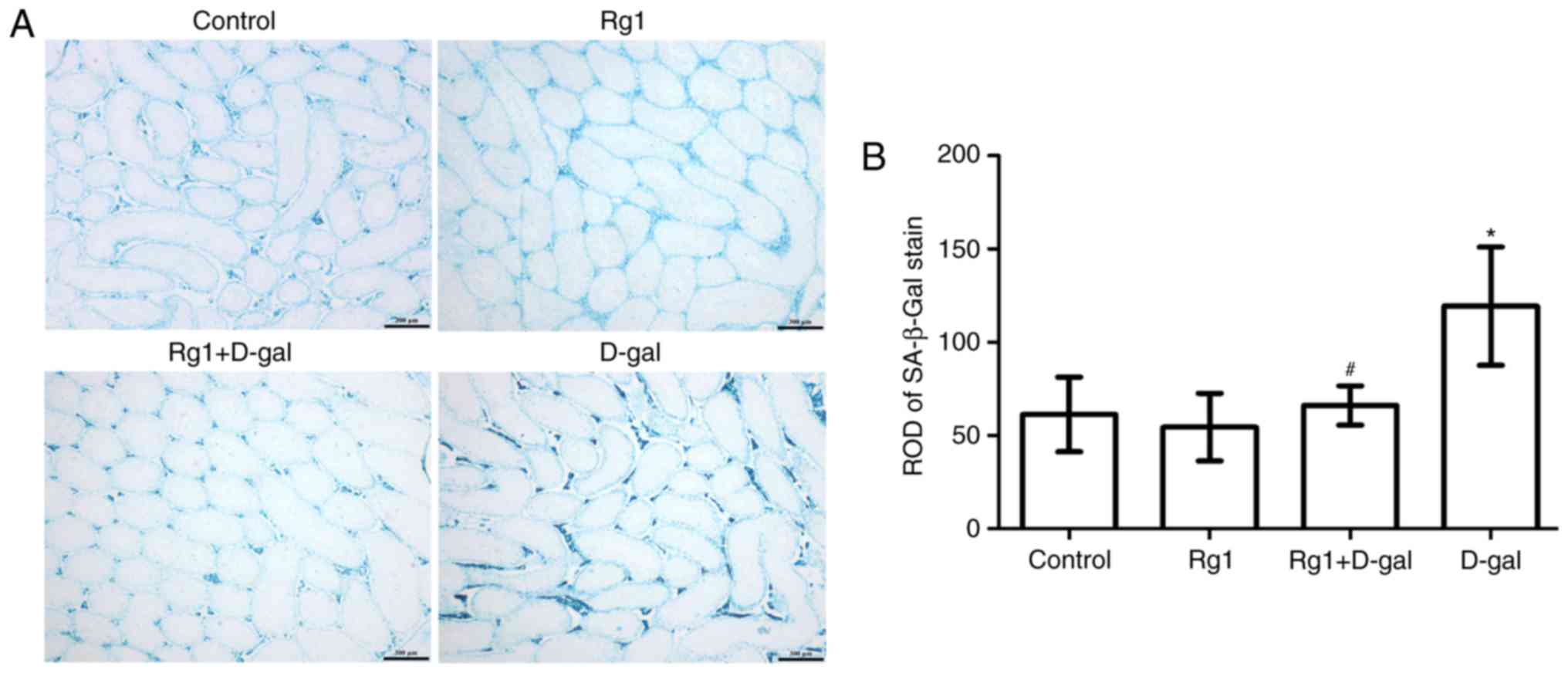
Rg1 affects SA-β-Gal staining in the
testes of aged mice
SA-β-gal is a widely-used biomarker for aging cells.
By adjusting to pH 6, blue molecules (Fig. 4A) may be visualized in the
cytoplasm of the aging cell (2).
The intensity of SA-β-gal staining was evaluated via the relative
optical density (ROD) value of SA-β-gal-positive cells (Fig. 4A and B). The results demonstrated
that the ROD value of the SA-β-gal staining was increased in the
D-gal-induced group. By contrast, the ROD value of the SA-β-gal
staining was decreased following treatment with Rg1 in the Rg1 +
D-gal group. These results suggested that Rg1 may protect the
testes against senescence.
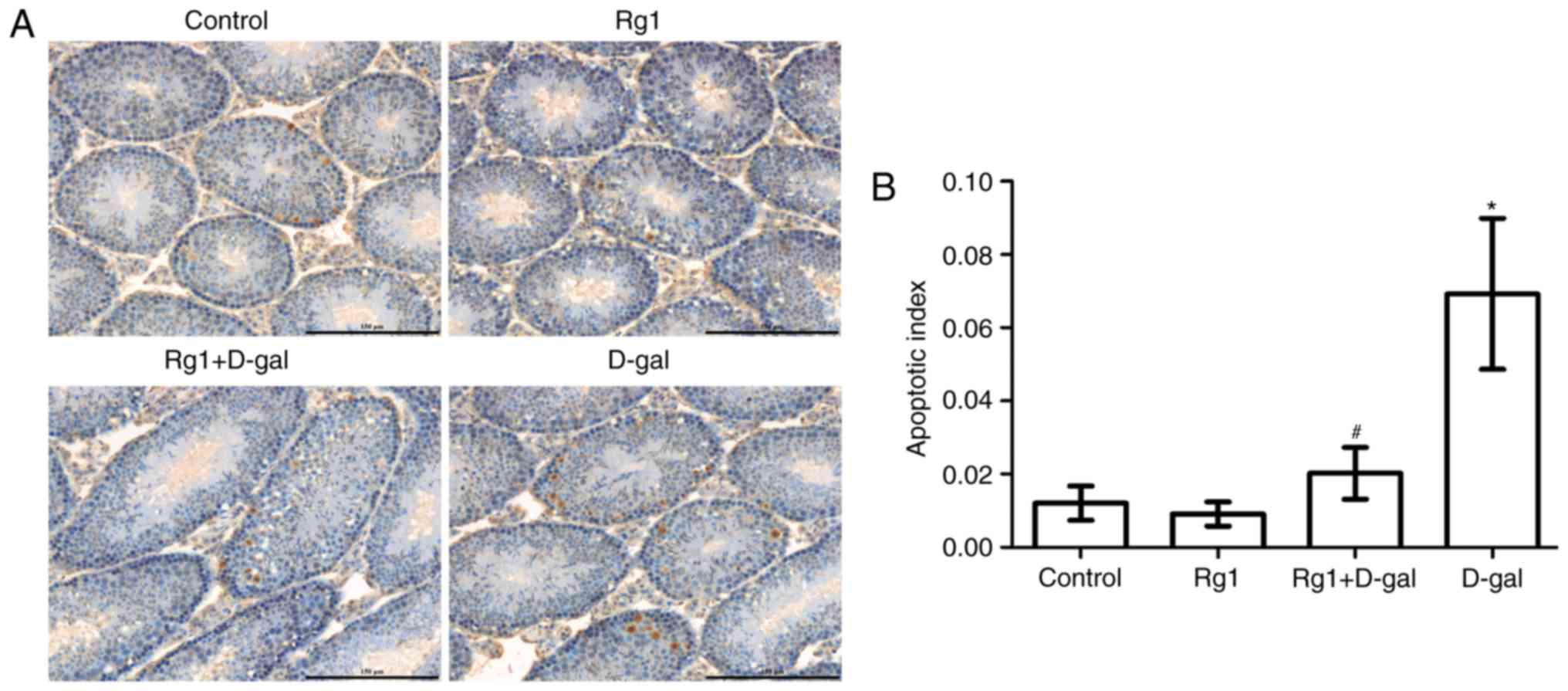
Rg1 reduces spermatocyte apoptosis in
aged mice
Previous studies have indicated that the
degeneration of spermatogenesis is caused by apoptosis (15,16).
TUNEL staining is a commonly used method, which has been proven to
be useful for detecting DNA fragmentation resulting from apoptotic
signaling cascades. TUNEL staining relies on the DNA ends that can
be identified by TdT and the addition of dUTPs, which is a
secondarily labeled marker catalyzed by an enzyme. In the present
study, TUNEL was used to detect the level of spermatogenetic
cellular apoptosis; positive cells displayed brown-yellow granules
in the nucleus (Fig. 5A). Compared
with the control group, positive cell number and the apoptotic
index were increased in the D-gal group (P<0.05). The apoptotic
index and the level of spermatocyte apoptosis were decreased
following treatment with Rg1 (Fig.
5B).
Rg1 affects oxidation-associated
biomarkers in aged mice
Oxidative stress is one of the causes of senescence.
A previous study reported that the oxidation-reduction system may
be damaged by aging (17). SOD is
an enzyme that removes the free radicals generated by oxidative
stress in aging. Thus, SOD activity is an important target for
estimating antioxidant capacity. MDA may reflect the level of
oxidative injury in the body, and level of MDA is an important
target for estimating the level of oxidative damage. In the present
study, the results demonstrated that SOD activity and TAC were
decreased significantly, and MDA content was elevated
significantly, in the D-gal group compared with the control.
Conversely, in the Rg1 + D-gal group, SOD activity and TAC were
significantly increased compared with the D-gal group. In addition,
MDA content was decreased in the Rg1 + D-gal group (Table I). These results demonstrated that
another factor of the protective effect of Rg1 on the testes may be
an increase in the activity of SOD and a decreased in the oxidation
product MDA.
 | Table I.Effect of Rg1 on the levels of SOD,
MDA and TAC in the testes of D-gal-injected mice (mean ± standard
deviation; n=14). |
Table I.
Effect of Rg1 on the levels of SOD,
MDA and TAC in the testes of D-gal-injected mice (mean ± standard
deviation; n=14).
| Group | SOD (U/mg) | MDA (µmol/mg) | TAC (mM) |
|---|
| Control |
4.65±0.61 |
3.34±0.43 |
0.25±0.01 |
| Rg1 |
4.65±0.67 |
3.35±0.67 |
0.25±0.03 |
| Rg1 + D-gal |
3.82±0.37b |
4.40±0.39b |
0.17±0.01c |
| D-ga1 |
2.72±0.30a |
5.46±0.63a |
0.11±0.01a |
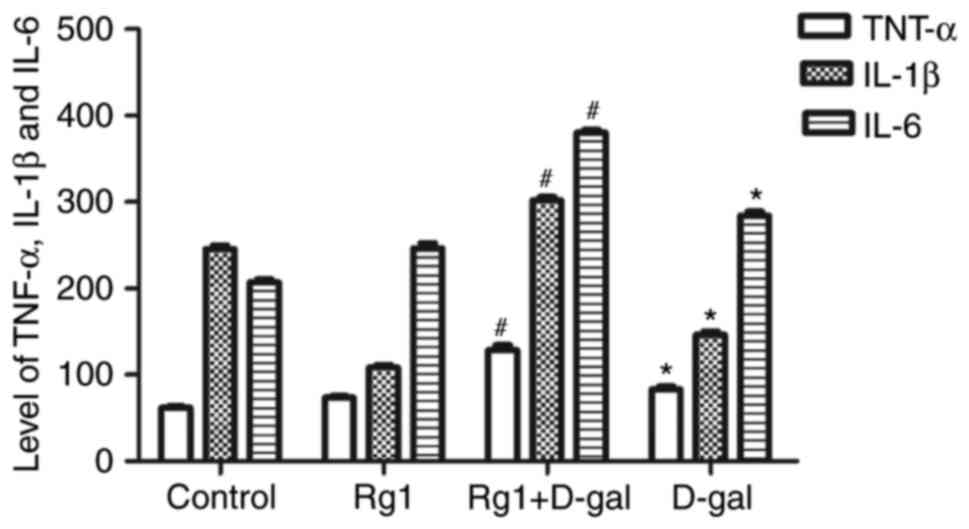
Rg1 affects the levels of inflammatory
cytokines in the testes of aged mice
Chronic inflammation is increased and the secretion
of inflammatory cytokines is upregulated during the aging process.
It has been reported that inflammatory cytokines, including TNF-α,
IL-1β and IL-6, are increased during natural aging (18). The results of the present study
demonstrated that the levels of TNF-α, IL-1β and IL-6 were
significantly increased in the testes of the D-gal group. However,
compared with the D-gal group, the levels of these three
inflammatory cytokines were significantly decreased in the Rg1 +
D-gal group (Fig. 6). These
results indicated that the effect of D-gal on the levels of
inflammatory cytokines may affect the aging of the testes, and that
Rg1 was able to reduce the levels of these inflammatory
cytokines.
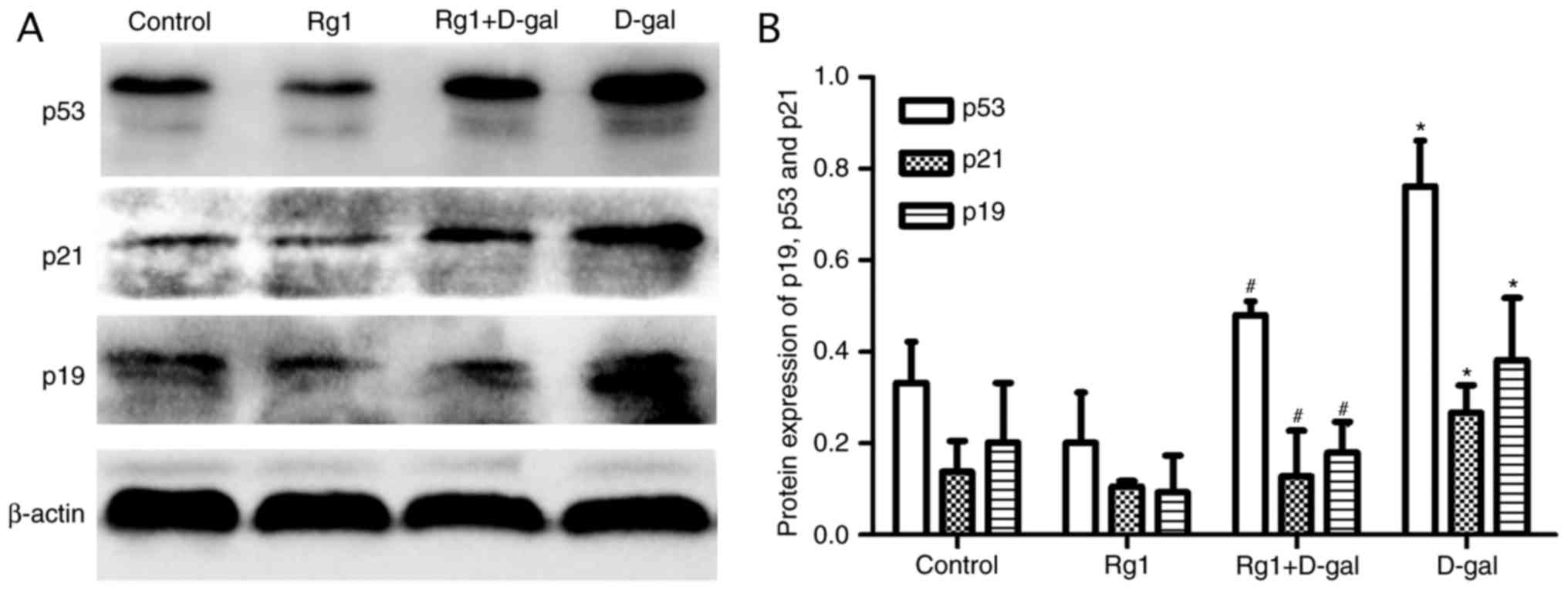
Rg1 affects p19, p21 and p53
expression in the testes of aged mice
The p19/p53/p21 pathway serves an important role in
aging. Following oxidative damage, the p19/p53/p21 pathway is
activated and the expression of the proteins is upregulated
(8). According to the western blot
analysis in the present study, the levels of p19, p21 and p53 in
the D-gal group were significantly increased compared with the
control group. Following treatment with Rg1, the levels of these
proteins were decreased significantly (Fig. 7). The results of the present study
demonstrated that the p19/p53/p21 pathway was downregulated
following treatment with Rg1.
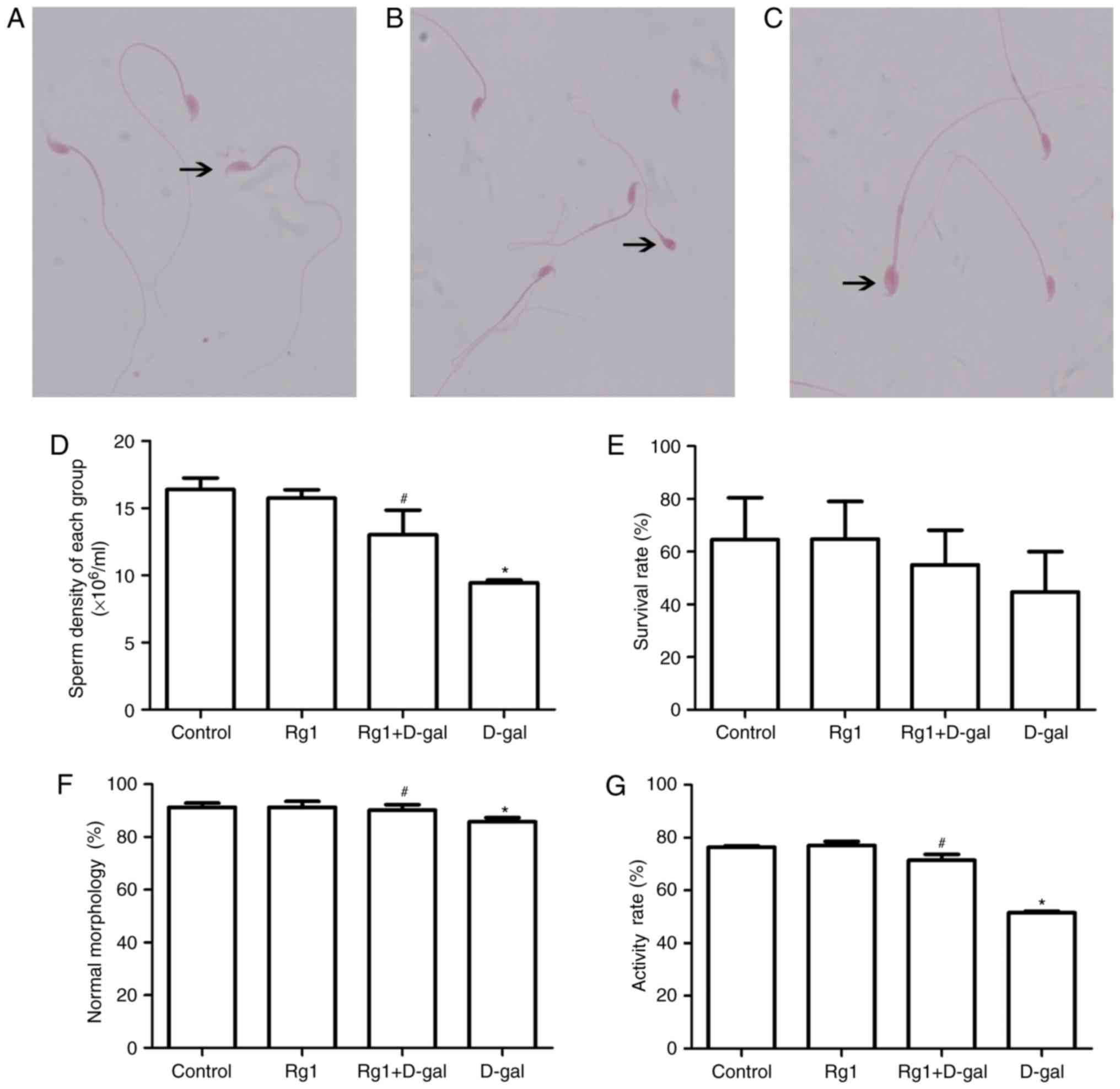
Rg1 affects sperm parameters in the
cauda epididymis and vas deferens in aged mice
One of the most common ways of tracking male
fertility is semen analysis (10).
Therefore, male reproductive status and spermatogenesis monitoring
are of importance (Fig. 8). Sperm
parameters, including sperm density, normal sperm morphology
percentage and activity rate; are meaningful to monitor male
reproductive function. In the present study, the sperm density
(Fig. 8D), survival rate (Fig. 8E), normal sperm morphology
percentage (Fig. 8F) and activity
rate (Fig. 8G) were significantly
decreased in the aging model. Following treatment with Rg1, sperm
parameters improved significantly (Fig. 8). These results suggested that the
reproductive function of aging males may be improved by treatment
with Rg1.
Discussion
Aging is an unavoidable and complex biological
process, which primarily refers to the alterations of biological
structures and the degradation of functions resulting from a
variety of factors (19–22). It has been reported that the normal
40–50-year old male may begin to exhibit reproductive functional
decline, and that physiological function additionally declines,
causing a negative impact on quality of life (23–25).
The testes are an important part of the male reproductive system.
The function of the testes broadly reflects the state of the male
reproductive system (1,23). Therefore, it is of important to
identify anti-aging drugs to delay the aging of the testes.
In the natural aging process, alterations in
morphology and function may be observed in the male testes. In
particular, testicular senescence manifests as testicular volume
loss and a decrease in weight. In addition, the disordered
arrangement of seminiferous tubules and spermatogenetic cells, and
disturbances in spermatogenesis, have been investigated. The
ability of the testes to secrete testosterone and sperm quality
additionally decline (1). D-gal is
the principal component of lactose, and lactose acts as a
metabolite in glycometabolism. However, the excessive accumulation
of D-gal causes metabolic disorders, eventually leading to aging
(5,26). Previous studies (2,5,12)
have demonstrated that ginsenoside Rg1 may protect against aging of
the brain, liver, kidney and thymus. However, the effect of Rg1 on
the testes remains unclear and the precise mechanism has not been
elucidated. Therefore, in the present study, D-gal was used to
generate an aging model to examine the role and mechanism of Rg1 in
protecting the testes of aging mice. The results of the present
study demonstrated that the classification and counting of
spermatogenetic cells, including spermatocyte/supporting cells,
spherical spermatid/supporting cells and long spermatid/supporting
cells; were decreased in the D-gal group, which had been increased
following treatment with Rg1. In the D-gal group, sperm survival
rate, activity and normal sperm rate, and the level of serum
testosterone were decreased significantly compared with the control
group. Following treatment with Rg1, the improvements in sperm
parameters and the level of serum testosterone were significant. By
contrast, the alterations in weight and morphology in the D-gal and
D-gal + Rg1 groups were not significant. These results indicated
that D-gal is induced aging of testicular function, although it
exerted no effect on weight and morphology. Rg1 was able to improve
the function of the testes. The reasons for these results required
further investigation. SA-β-gal staining is based on the
upregulation of the level of SA-β-gal activity as a result of
aging. Senescent cells with high enzymatic activity at pH 6.0 were
stained blue. SA-β-gal staining has been used to detect normal
cellular aging (2). Apoptosis
serves an important role in maintaining testicular spermatogenetic
function (16). TUNEL staining has
been previously used to detect the apoptosis of spermatogenetic
cells (16). In the present study,
the absorbance of SA-β-gal-positive cells and the apoptosis index
of spermatogenetic cells were increased in the D-gal group, and
decreased following treatment with Rg1. These results further
indicated that Rg1 was able to protect against D-gal-induced aging
of the testes.
The free radical theory of aging states that the
aging of an organism is frequently a consequence of the
accumulation of cellular free radical damage over time (17). For the majority of biological
structures, free radical damage is associated with oxidative
damage. Under normal circumstances, oxidation and antioxidation are
in a dynamic balance (17,20). MDA, as the end product of lipid
oxidation, exerts a cytotoxic effect that increases with the degree
of lipid oxidation, indicating that the level of MDA may be an
important target to measure membrane lipid peroxidation. SOD is a
type of antioxidant enzyme that may slow the process of aging by
clearing free radicals (27). The
activity of SOD reflects the antioxidant ability of an organism. In
the present study, the activity of SOD and TAC in the D-gal-induced
group was significantly decreased, and the MDA content was
significantly increased. The results of the present study
demonstrated that D-gal may lead to aging by causing oxidative
damage. By contrast, following treatment with Rg1, the activity of
SOD and TAC increased compared with the D-gal group, and MDA
decreased. This protective effect may be associated with the
antioxidant effect of Rg1.
Oxidative stress regulates and promotes the
expression of the senescence-associated proteins p19, p53 and p21
(28–30). The activity of the tumor suppressor
protein p53 is increased (31) and
p53 induces p21 expression, which has an association with cell
cycle arrest triggering cellular senescence (29). p19 is encoded by the INK4A locus,
and is involved in the p53 pathway by inhibiting E3
ubiquitin-protein ligase Mdm2 activity (32). The increased expression of p19 is
one mechanism through which p53 is activated (33). The results of the present study
demonstrated that the reduction of p19, p21 and p53 protein were
significant compared with the aging group. Therefore, it may be
hypothesized that Rg1 may exert its protective effect against
testicular senescence by downregulating the p19/p53/p21 pathway.
The release of cell-associated inflammatory factors was observed in
the present study. It has been previously demonstrated that these
factors may lead to chronic inflammation of the body, which is the
crux of the inflammatory aging theory (34,35).
TNF-α, IL-1, IL-6 and other inflammatory factors may be used as
serum markers of inflammatory senescence. The present study
demonstrated that the levels of TNF-α, IL-1 and IL-6 in testicular
tissues were significantly decreased following treatment with Rg1,
which may be the underlying mechanism of the anti-aging effect of
Rg1.
In conclusion, the present study examined the
protective effect of Rg1 on D-gal-induced testicular aging, and it
was determined that the underlying mechanism of Rg1 anti-aging in
the testes of D-gal mice is antioxidant and anti-inflammatory. It
is of importance to identify drugs that protect against the effects
of aging on male reproductive function.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 30973818
and 81673748).
References
|
1
|
Gunes S, Hekim GN, Arslan MA and Asci R:
Effects of aging on the male reproductive system. J Assist Reprod
Genet. 33:441–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu W, Jing P, Wang L, Zhang Y, Yong J and
Wang Y: The positive effects of ginsenoside Rg1 upon the
hematopoietic microenvironment in a D-galactose-induced aged rat
model. BMC Complement Altern Med. 15:1192015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Liu J, Cai S, Liu D, Jiang R and
Wang Y: Protective effects of ginsenoside Rg1 on aging
Sca-1+ hematopoietic cells. Mol Med Rep. 12:3621–3628.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Cai D, Yao X, Zhang Y, Chen L, Jing
P, Wang L and Wang Y: Protective effect of ginsenoside Rg1 on
hematopoietic stem/progenitor cells through attenuating oxidative
stress and the Wnt/β-catenin signaling pathway in a mouse model of
d-galactose-induced aging. Int J Mol Sci. 17:2016.
|
|
5
|
Fan Y, Xia J, Jia D, Zhang M, Zhang Y,
Huang G and Wang Y: Mechanism of ginsenoside Rg1 renal protection
in a mouse model of d-galactose-induced subacute damage. Pharm
Biol. 54:1815–1821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DiLoreto R and Murphy CT: The cell biology
of aging. Mol Biol Cell. 26:4524–4531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Finkel T: The metabolic regulation of
aging. Nat Med. 21:1416–1423. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wright WW, Fiore C and Zirkin BR: The
effect of aging on the seminiferous epithelium of the brown Norway
rat. J Androl. 14:110–117. 1993.PubMed/NCBI
|
|
10
|
Cao XW, Lin K, Li CY and Yuan CW: A review
of WHO laboratory manual for the examination and processing of
human semen (5th edition). Zhonghua Nan Ke Xue. 17:1059–1063.
2011.(In Chinese). PubMed/NCBI
|
|
11
|
Dong Z, Xu M, Huang J, Chen L, Xia J, Chen
X, Jiang R, Wang L and Wang Y: The protective effect of Ginsenoside
Rg1 on aging mouse pancreas damage induced by D-galactose. Exp Ther
Med. 14:616–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia JY, Fan YL, Jia DY, Zhang YY, Li J,
Jing PW, Wang L and Wang YP: Protective effect of Angelica sinensis
polysaccharide against liver injury induced by D-galactose in aging
mice and its mechanisms. Zhonghua Gan Zang Bing Za Zhi. 24:214–219.
2016.(In Chinese). PubMed/NCBI
|
|
13
|
Golan R, Scovell JM and Ramasamy R:
Age-related testosterone decline is due to waning of both
testicular and hypothalamic-pituitary function. Aging Male.
18:201–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Russo SJ, Murrough JW, Han MH, Charney DS
and Nestler EJ: Neurobiology of resilience. Nat Neurosci.
15:1475–1484. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsueh AJ, Eisenhauer K, Chun SY, Hsu SY
and Billig H: Gonadal cell apoptosis. Recent Prog Horm Res.
51:433–456. 1996.PubMed/NCBI
|
|
16
|
Wang C, Sinha Hikim AP, Lue YH, Leung A,
Baravarian S and Swerdloff RS: Reproductive aging in the Brown
Norway rat is characterized by accelerated germ cell apoptosis and
is not altered by luteinizing hormone replacement. J Androl.
20:509–518. 1999.PubMed/NCBI
|
|
17
|
Harman D: About ‘Origin and evolution of
the free radical theory of aging: A brief personal history,
1954–2009’. Biogerontology. 10:7832009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brüünsgaard H and Pedersen BK: Age-related
inflammatory cytokines and disease. Immunol Allergy Clin North Am.
23:15–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Young A: Ageing and physiological
functions. Philos Trans R Soc Lond B Biol Sci. 352:1837–1843. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu BP and Chung HY: Adaptive mechanisms to
oxidative stress during aging. Mech Ageing Dev. 127:436–443. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Everitt A and Meites J: Aging and
anti-aging effects of hormones. J Gerontol. 44:B139–B147. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakamoto Y, Ichimura T, Tsuruzoe S and
Nakao M: Aging and DNA methylation. Nihon Ronen Igakkai Zasshi.
42:137–143. 2005.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Araujo AB, Mohr BA and McKinlay JB:
Changes in sexual function in middle-aged and older men:
Longitudinal data from the Massachusetts Male Aging Study. J Am
Geriatr Soc. 52:1502–1509. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brahem S, Mehdi M, Elghezal H and Saad A:
The effects of male aging on semen quality, sperm DNA fragmentation
and chromosomal abnormalities in an infertile population. J Assist
Reprod Genet. 28:425–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gray A, Feldman HA, McKinlay JB and
Longcope C: Age, disease, and changing sex hormone levels in
middle-aged men: Results of the massachusetts male aging study. J
Clin Endocrinol Metab. 73:1016–1025. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu X, Zhou Y, Wu T and Hao L: Ameliorative
effect of black rice anthocyanin on senescent mice induced by
D-galactose. Food Funct. 5:2892–2897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Raamsdonk JM and Hekimi S: Deletion of
the mitochondrial superoxide dismutase sod-2 extends lifespan in
Caenorhabditis elegans. PLoS Genet. 5:e10003612009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang L, Zhang XH, Ji B, Yao H, Ling XM,
Guo ZJ, Deng HZ and Wu XR: Yifuning postpones ovarian aging through
antioxidant mechanisms and suppression of the Rb/p53 signal
transduction pathway. Mol Med Rep. 14:888–896. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gambino V, De Michele G, Venezia O,
Migliaccio P, Dall'Olio V, Bernard L, Minardi SP, Della Fazia MA,
Bartoli D, Servillo G, et al: Oxidative stress activates a specific
p53 transcriptional response that regulates cellular senescence and
aging. Aging Cell. 12:435–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yue Z, Rong J, Ping W, Bing Y, Xin Y, Feng
LD and Yaping W: Gene expression of the p16(INK4a)-Rb and
p19(Arf)-p53-p21(Cip/Waf1) signaling pathways in the regulation of
hematopoietic stem cell aging by ginsenoside Rg1. Genet Mol Res.
13:10086–10096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Itahana K, Dimri G and Campisi J:
Regulation of cellular senescence by p53. Eur J Biochem.
268:2784–2791. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carnero A, Hudson JD, Price CM and Beach
DH: p16INK4A and p19ARF act in overlapping pathways in cellular
immortalization. Nat Cell Biol. 2:148–155. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacobs JJ, Keblusek P, Robanus-Maandag E,
Kristel P, Lingbeek M, Nederlof PM, van Welsem T, van de Vijver MJ,
Koh EY, Daley GQ and van Lohuizen M: Senescence bypass screen
identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified
in a subset of human breast cancers. Nat Genet. 26:291–299. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulop T, Witkowski JM, Pawelec G, Alan C
and Larbi A: On the immunological theory of aging. Interdiscip Top
Gerontol. 39:163–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De la Fuente M and Miquel J: An update of
the oxidation-inflammation theory of aging: The involvement of the
immune system in oxi-inflamm-aging. Curr Pharm Des. 15:3003–3026.
2009. View Article : Google Scholar : PubMed/NCBI
|