Introduction
Blunt chest trauma frequently occurs in patients
with poly-trauma, including victims of vehicular accidents, and is
frequently associated with hemorrhagic shock (HS) (1). Blunt chest trauma with HS (THS) is
associated with a high risk of acute lung injury (ALI) and acute
respiratory distress syndrome (ARDS), which results in considerable
morbidity and mortality (2).
Previous studies have demonstrated that ALI due to THS is
associated with a progressive inflammatory response during the
development of the ALI (3,4). Toll-like receptors (TLRs) are innate
immune receptors that serve a crucial role in the regulation of
inflammatory and innate immune responses. Among these receptors,
Toll-like receptor 4 (TLR4) is specifically recognized to be a
candidate gene that is involved with distinct forms of ALI
(5). TLR4 may activate multiple
intracellular signaling systems, including the p38
mitogen-activated protein kinase (p38MAPK) and nuclear factor-κB
(NF-κB) pathways, and induce the expression of pro-inflammatory
cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6
(IL-6), IL-1β and additional pro-inflammatory mediators involved in
ALI. MAPKs are well known as important mediators of
inflammation-induced tissue injury. Notably, MAPK activation has
been studied in inflammation-associated lung dysfunction and has
been demonstrated to contribute to ALI. In addition, p38MAPK is
known to be important for the expression of components of activator
protein-1 (AP-1) (6,7). However, the potential involvement of
TLR4-mediated MAPK phosphorylation following THS-induced ALI has
not been examined.
Penehyclidine hydrochloride (PHC), a novel
anti-cholinergic drug which is derived from hyoscyamine, has been
reported to attenuate systemic inflammatory responses and exhibit
anti-apoptotic properties (8).
Increasing basic and clinical evidence has indicated that PHC may
suppress pro-inflammatory cytokine production. Zhan et al
(9) reported that PHC
preconditioning may exert protective effects by inhibiting
inflammatory factor production and suppressing p38MAPK activation
in septic mice. Additionally, Li et al (10) reported that treatment with PHC
reduced the level of pro-inflammatory cytokines and suppressed
lipid peroxidation in rats with ARDS. Li et al (11) discovered that PHC exhibited marked
effects on inhibiting the upregulation of inflammatory molecules
downstream of the TLR4 signaling pathway in patients with traumatic
ALI. Although a number of studies have addressed the therapeutic
potential of PHC, whether PHC can protect against ALI from THS
remains unknown. A 2012 study (12) reported the protective effect of PHC
on pulmonary contusion induced by blunt chest trauma in rats; in
that study, a rat model of blunt chest trauma alone was used.
In the present study, a novel combination rat model
of THS was used. The present study elucidated the effects of PHC on
THS-induced ALI, which inhibited TLR4 signaling and inflammation in
the rats. The important proteins and transcription factors of the
TLR4 signaling pathway (p-p38MAPK, NF-κB and AP-1) were
additionally examined.
Materials and methods
Animals and reagents
A total of 30 healthy male Sprague-Dawley rats (aged
8 weeks, weighing 240–280 g) were obtained from Hunan Institute for
Biological Sciences (Hunan, China; certificate no. SCXX 2009–0004)
and maintained under specific pathogen-free conditions. Rats were
housed in conditions of a 12/12 h light/dark cycle, a mean room
temperature of 22–24°C, and a humidity of 50–60%. All animal
experiments complied with the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (Bethesda,
MD, USA), and were approved by the Bioethics Committee of Renmin
Hospital of Wuhan University (Wuhan, China). PHC was purchased from
Chengdu List Pharmaceutical Co., Ltd. (Chengdu, China). TLR4 and
phosphorylated (p)-p38MAPK antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). ELISA kits were
obtained from BD Biosciences (BD Pharmingen; BD Biosciences, San
Jose, CA, USA) and myeloperoxidase (MPO) kits were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
THS model
The present study used a novel combination rat model
of THS previously described by Raghavendran et al (13) and Knöferl et al (14). Rats were anesthetized with
intraperitoneal sodium pentobarbital (50 mg/kg) injections and
subjected to sham or THS surgery. The femoral artery and vein were
cannulated with polyethylene tubing for continuous invasive
pressure monitoring and to establish venous access. Heart rate (HR)
and mean arterial blood pressure (MAP) were determined using
Philips IntelliVue MP40 (Philips Healthcare, Andover, MA, USA).
Blunt chest trauma was induced in anesthetized rats at a fixed
chest impact energy of 2.45 J, as described previously (12). Following 1–3 min, HS was induced by
blood withdrawal through the tubing, which was not attached to the
monitor until the MAP reached 35–40 mmHg. This pressure was
maintained for 1 h. During the next 1 h, the rats were resuscitated
by transfusion of the withdrawn blood, and the withdrawn blood
volume was infused twice in the form of Ringer's lactate solution
(Baxter Healthcare Co., Ltd., Tianjin, China).
Experimental protocols
A total of 30 rats were randomly assigned to three
equal groups (n=10 rats/group): The sham group, THS group and PHC
group. In the PHC group, the rats were infused with PHC at 2 mg/kg
for 30 min prior to the induction of blunt chest trauma. The sham
control and THS rats received the same volume of 0.9% normal saline
solution. The sham control animals were subjected to the same
experimental procedures, including cannulation of the femoral
artery and vein, although no blunt chest trauma or HS was induced.
All animals were sacrificed under anesthesia via intraperitoneal
injection of sodium pentobarbital (50 mg/kg) and exsanguination
from the right carotid artery at 6 h post-THS challenge. Blood
samples and lung tissue specimens were harvested.
Blood gas analysis and lactic
acid
Arterial blood was assayed following collection from
the right carotid artery (1.0 ml each) when the animal was
sacrificed by exsanguination. Arterial blood samples were analyzed
for pH, partial pressure of oxygen (PaO2),
PaCO2, PaO2/fraction of inspired oxygen
(FiO2) and lactic acid, which were immediately
determined using an i-STAT Portable Clinical Analyzer (Abbott Point
of Care Inc., Princeton, NJ, USA).
Measurement of the lung wet/dry weight
(W/D) ratio and MPO activity
The water content of the lungs was determined by
calculating the W/D ratio of lung tissues at 6 h post-THS
challenge. The right lower lobe of the lung was dissected free from
nonpulmonary tissues, and weighed and dried in an oven at 60°C for
72 h, followed by reweighing. The W/D ratios are reported as a
measure of pulmonary edema.
MPO activity was determined as an index of
neutrophil accumulation in the lungs. Frozen (4°C) lung tissues
were homogenized and centrifuged at 1,500 × g for 10 min at 4°C.
Following weighing, the lungs were homogenized, centrifuged (1,000
× g for 30 min at 4°C) and resuspended in 50 mM
KH2PO4 buffer (Ph 6.0; cat. no. p0662;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) with 0.5%
hexadecyltrimethylammonium bromide (cat. no. H5882; Sigma-Aldrich;
Merck KGaA). Subsequently, samples were sonicated for 1 min at 4°C
and 20 KHz, and then incubated at 60°C for 2 h. The absorbance of
visible light at 460 nm was measured and the MPO activity was
calculated in units/gram of lung tissue. The MPO content was
determined by following the manufacturer's protocol for the MPO
assay kit. The results are expressed as units/gram of
protein/minute (U/g).
Transmission electron microscopy
(TEM)
The fragments of the right middle-lung tissue were
cut into 1-mm-thick slices, immersion-fixed in 2.5% buffered
glutaraldehyde at 0–4°C for 2 h, buffered in PBS three times, fixed
with 1% osmic acid for 1 h at room temperature, washed with
distilled water and dehydrated with dimethylketone. Following
embedding in Epon-812, they were cut into ultrathin sections (60
nm) using an LKB-V ultramicrotome (LKB Produkter AB; Bromma,
Stockholm, Sweden) and stained with 1% uranyl acetate for 30 min at
room temperature and lead citrate for 15 min at room temperature.
The sections were examined using a Hitachi H-600 transmission
electron microscope (Hitachi, Ltd., Tokyo, Japan).
Hematoxylin and eosin (H&E)
staining
All animals were sacrificed via exsanguination from
the right carotid artery at 6 h post-THS, and lung tissue samples
were harvested immediately. The right middle-lung specimens were
fixed in 10% formalin at 4°C for 24 h, sectioned (5 µm) and then
stained with H&E at room temperature. Following this, paraffin
sections were incubated at 60°C for 30 min, twice immersed in
xylene for 15 min at room temperature and then treated with a
descending ethanol series (100, 95, 90, 85 and 75%) for 5 min each
at room temperature. The sections were then treated with 0.5%
hematoxylin for 1–5 min at room temperature and then rinsed in tap
water for 1 min. Sections were incubated with PBS for 8 sec until a
blue color was observed, and then the sections were washed using
tap water for 1 min and then distilled water for 8 sec. Sections
were then stained with 1% eosin for 3 min at room temperature and
then washed with tap water. Following this, sections were treated
with an ascending ethanol series (75, 85, 90 and 95%) for 1 min
each at room temperature. Sections were then analyzed and graded
using the index of quantitative assessment (IQA) for the presence
of interstitial neutrophilic infiltrates, intra-alveolar hemorrhage
and pulmonary edema using a TEM microscope (magnification, ×200;
BX51; Olympus Corporation, Tokyo, Japan) according to the
aforementioned protocol. Upon viewing ~10 fields/sector under low
and high power, each section was assigned a numerical histological
IQA of lung injury using the following criteria: 0, normal; +1,
focal epithelial edema, pleural-based lesions occupying <25% of
the lung; +2, diffuse swelling with villi necrosis, more extensive
fibrosis involving 26–50% of the lung and fibrotic regions; +3,
diffuse pathology, neutrophil infiltration and widespread fibrosis
involving 51–70% of the lung; and +4, major widespread injury with
massive neutrophil infiltration and hemorrhage, and widespread
fibrosis involving >70% of the lung.
Western blot analysis
Western blot analysis was used to determine TLR4
(rabbit anti-mouse) and p-p38MAPK kinase (rabbit anti-mouse)
activity in the lungs. Lung tissue samples were thawed and
suspended in homogenization buffer (25 mM Tris-HCl, pH 7.6, 1%
NP-40, 0.5% sodium deoxycholate and 0.1% SDS) and homogenized. The
homogenate was centrifuged at 3,000 × g at 4°C for 10 min, and the
supernatant was centrifuged again at 10,000 × g at 4°C for 10 min.
Solubilized protein concentrations were determined using a
bicinchoninic acid (BCA) protein assay kit and equal amounts of
protein (10 µm/mg) were loaded per well on a 10% SDS-PAGE gel.
Subsequently, the proteins were transferred onto a polyvinylidene
difluoride membrane. Blots were blocked overnight at 4°C with TBS
containing 0.1% Tween-20 and 5% non-fat milk, and subsequently
incubated with rabbit anti-TLR4 (1:500; cat. no. ab13556; Abcam,
Shanghai, China) and p-p38 MAPK rabbit mAb (1:1,000; cat. no.
9215s; Cell Signaling Technology, Inc.) separately at room
temperature for 1 h followed by incubation with secondary antibody
goat anti-rabbit IgG-HRP (1:2,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h.
The blots were stripped with stripping buffer [2-metaptoethanol (35
µl), 10% SDS (1 ml), Tris (0.5 M, pH 6.7, 625 µl) and
dH2O (3.34 ml)] and reprobed with rabbit anti-rat GAPDH
(1:2,000; cat. no. 51332; Cell Signaling Technology, Inc.) at room
temperature for 1 h. The immunore active proteins were detected and
densitometric analysis was performed using the Odyssey®
Fc Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Immunofluorescence staining and
quantitative analysis
The lungs of rats were flushed with ice-cold PBS,
and then fixed with 10% formalin overnight at 4°C.
Paraffin-embedded lung sections (5 µm) were stained using the
streptavidin-biotin complex immunofluorescence technique for
p-p38MAPK detection as follows: The sections were then dewaxed in
xylene three times for 5 min each at room temperature and
rehydrated in a descending series of ethanol (100 and 95%) at room
temperature for 10 min each. Following deparaffinization, sections
were brought to a boil in 10 mM sodium citrate buffer (pH 6.0) and
then maintained at a sub-boiling temperature for 10 min. Slides
were then cooled for 30 min at room temperature. Sections were then
blocked using blocking buffer [1X PBS/5% normal goat serum (cat.
no. 5425; Cell Signaling Technology, Inc.)/0.3% Triton X-100] for 1
h at room temperature to minimize non-specific staining and then
incubated overnight at 4°C with p-p38MAPK rabbit mAb (1:50; cat.
no. 8632; Cell Signaling Technology, Inc.). Following washing with
PBS three times at room temperature for 5 min each, sections were
incubated with anti-rabbit IgG (1:1,000; cat. no. 8889; Cell
Signaling Technology, Inc.) for 30 min at room temperature in the
dark. Red staining in the nucleus and cytoplasm was considered to
be an indicator of positive expression. The mean optical densities
of p-p38 MAPK-positive cells from each section were analyzed by
image cytometry using HIPAS-2000 image analysis software (Wuhan
Qianli Technical Imaging Co. Ltd., Wuhan, China). Using the
HIPAS-2000 software, the results were evaluated semi-quantitatively
according to the optical density values of positive expression.
Nuclear protein extraction and
electrophoretic mobility shift assays
Nuclear extracts were prepared from lung tissues
using Nuclear Extraction Reagent, according to the manufacturer's
protocol (Viagene Biotech, Inc., Tampa, FL, USA), and aliquots were
incubated with γ-32P-ATP-labelled oligonucleotides
containing the binding sites for NF-κB
(5′-AGTTGAGGGGACTTTCCCAGGC-3′) and AP-1
(5′-CGCTTGATGAGTCAGCCGGAA-3′) (Pierce; Thermo Fisher Scientific,
Inc.). The protein content was measured via the BCA assay.
DNA-protein complexes were separated on a 4% nondenaturing
polyacrylamide gel at 90 V for 1.5 h. The gels were dried,
autoradiographed, and quantified using phosphor-imager analysis
(Santa Cruz Biotechnology, Inc.).
Determination of TNF-α, IL-6 and IL-1β
in the serum
Following collection of arterial blood samples, the
blood was immediately separated by centrifugation at 1,500 × g for
15 min at 4°C. The serum was divided into aliquots and stored at
−80°C until the assay. Cytokines (TNF-α, IL-6 and IL-1β) were
measured using commercially available ELISA kits (cat. nos. 558532,
550319 and 559603, respectively; BD Biosciences), according to the
manufacturer's protocol. The absorbance of each well was read at
450 nm using an ELISA plate reader.
Statistical analysis
The data are presented as the mean ± standard
deviation. Data analysis was performed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). Differences associated with the
primary sources of variation were tested with one-way analysis of
variance (ANOVA). When the F-statistic was significant in the ANOVA
comparisons, differences between individual means were tested for
significance using the Bonferroni test. The Bonferroni test is a
post hoc test that adjusts the α for multiple comparisons. Each
experiment was replicated thrice. P<0.05 was considered to
indicate a statistically significant difference.
Results
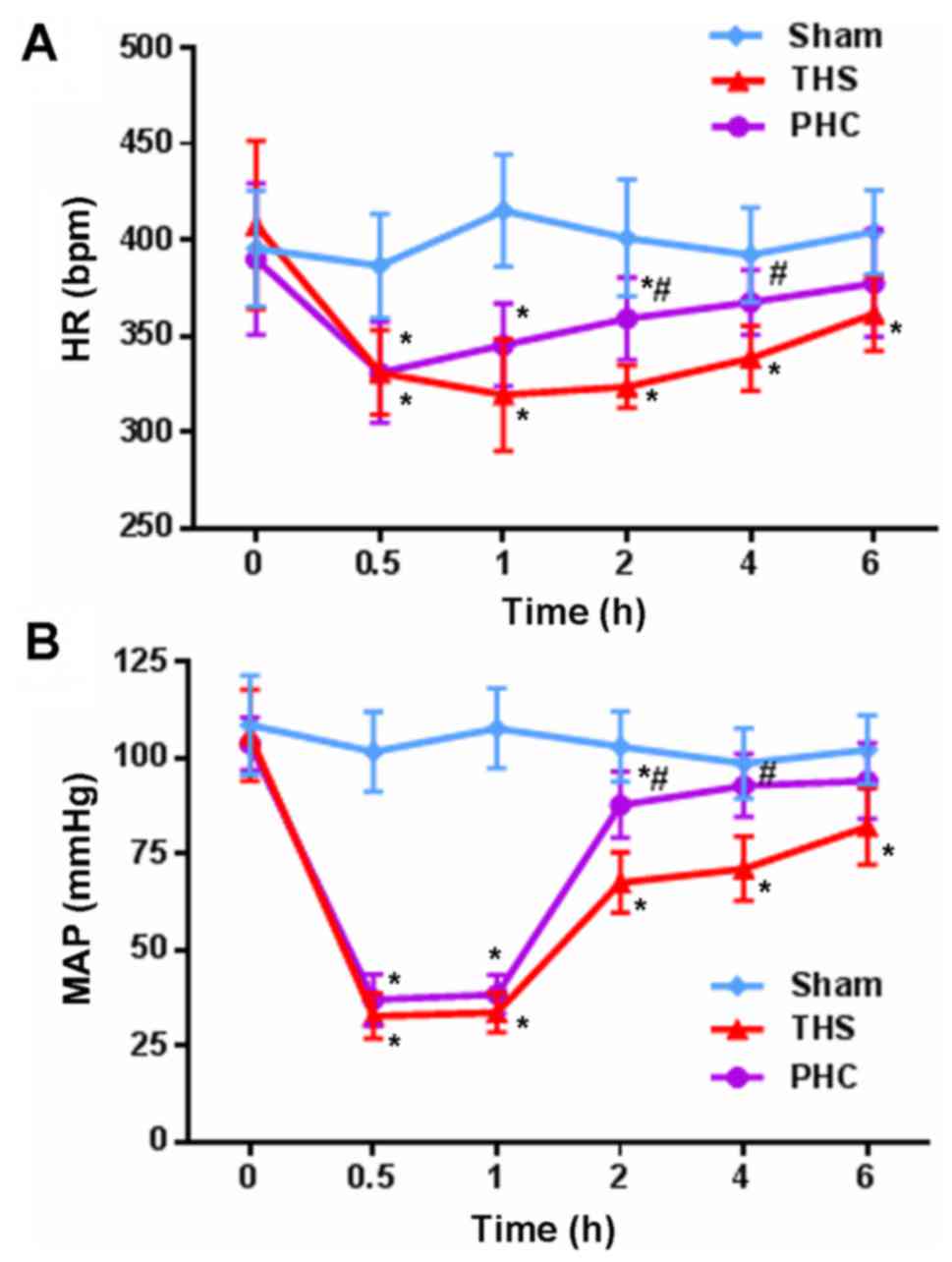
Hemodynamics (HR and MAP)
HR and MAP in the three groups of rats were observed
at the different time points. No significant alterations in MAP and
HR were observed in the sham control group at 6 h. At 2 and 4 h,
MAP and HR were decreased significantly in the THS rats (P<0.05)
compared with those in the sham controls, whereas fluctuations in
MAP and HR were markedly attenuated following the infusion of 2
mg/kg of PHC (Fig. 1).
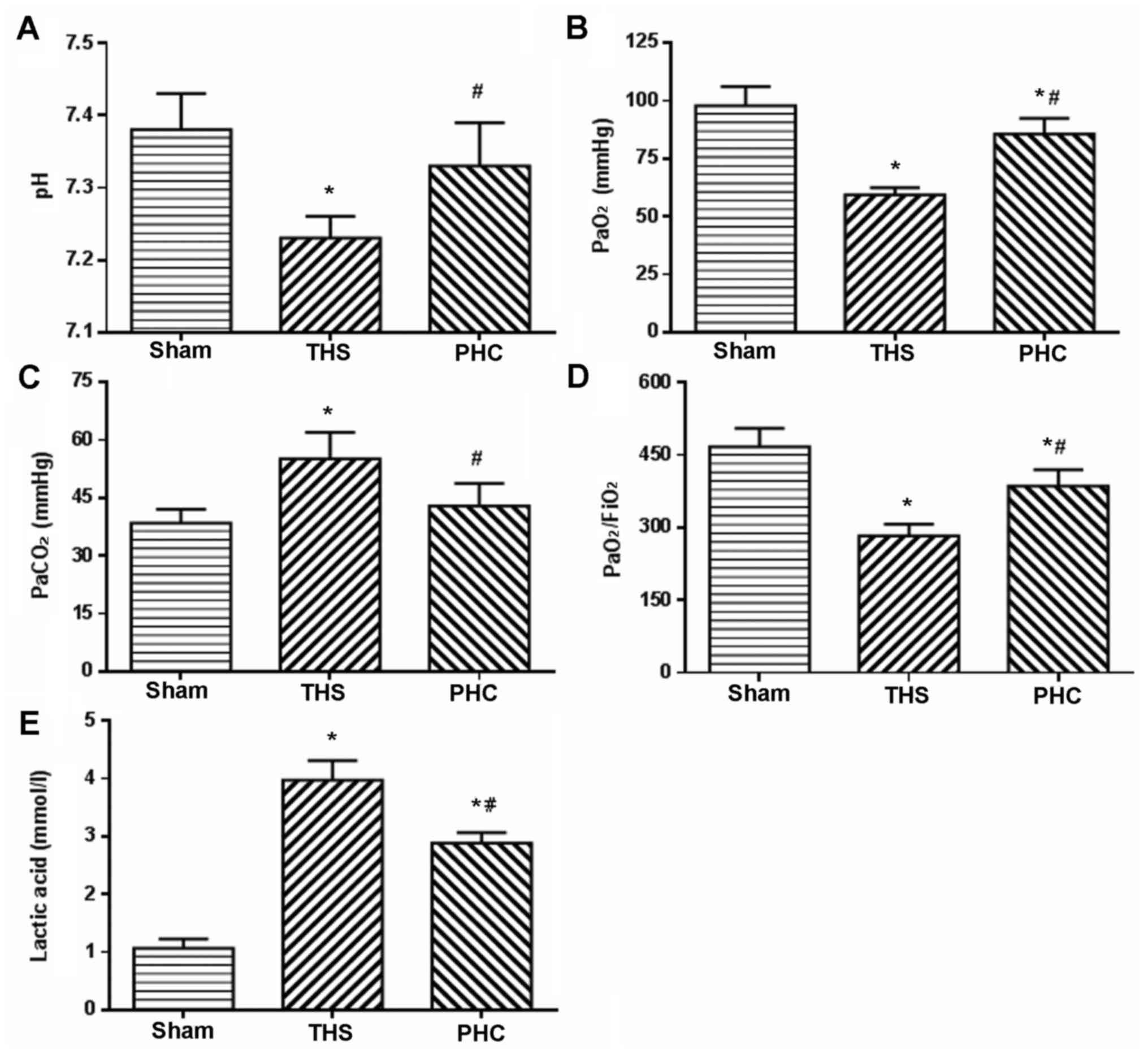
Effects of PHC on blood gas and lactic
acid in THS-induced ALI rats
PaO2/FiO2, as an evaluation
index of gas exchange, was measured to determine the degree of lung
injury at 6 h post-THS challenge. The rats in the THS group
exhibited a significant decrease in arterial blood PaO2,
and notable increases in arterial blood PaCO2 and lactic
acid (P<0.05). Furthermore, the pH and the
PaO2/FiO2 ratio in the arterial blood were
decreased (P<0.05). Pretreatment with PHC efficiently reversed
the decreases in PaO2, pH and
PaO2/FiO2, and attenuated the increases in
PaCO2 and Lac induced by the THS challenge (Fig. 2).
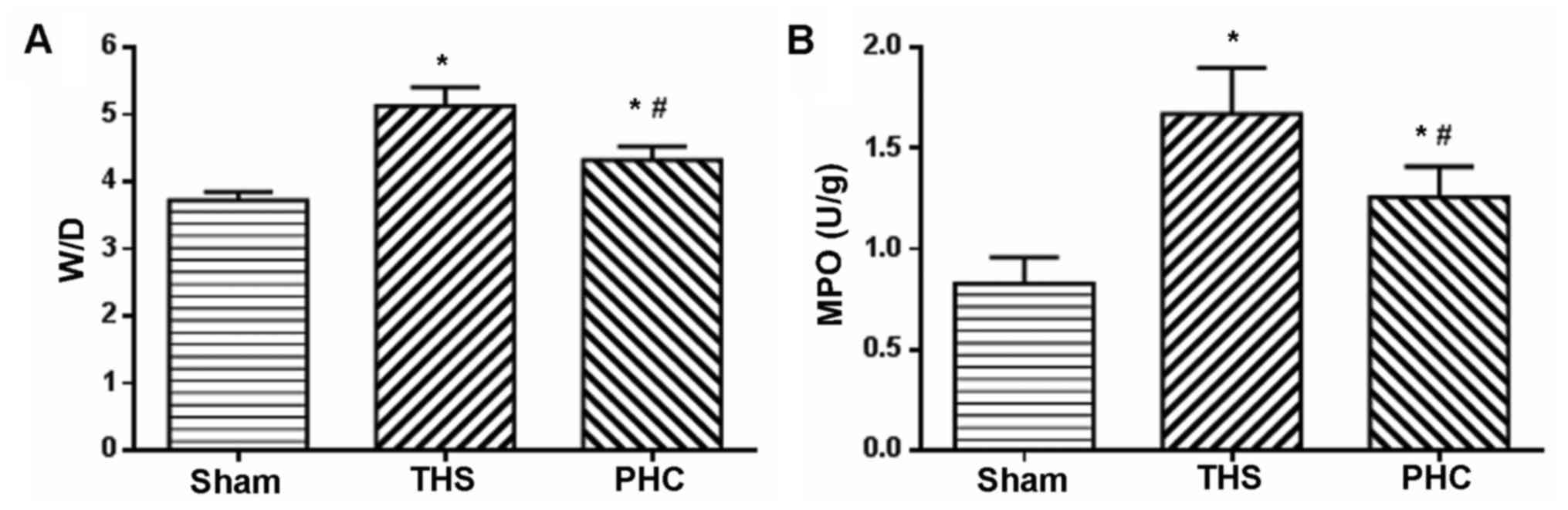
Effects of PHC on the lung W/D ratio
and MPO activity in THS-induced ALI rats
Neutrophil accumulation in the lung tissue and organ
edema were evaluated by MPO assay and the W/D ratio, respectively.
As presented in Fig. 3, the lung
W/D ratio and MPO activity were significantly elevated at 6 h
following ALI due to the THS challenge, compared with those of the
sham control animals (P<0.05). Treatment with PHC caused a
significant decrease in the W/D ratio and MPO activity (P<0.05
vs. THS) (Fig. 3).
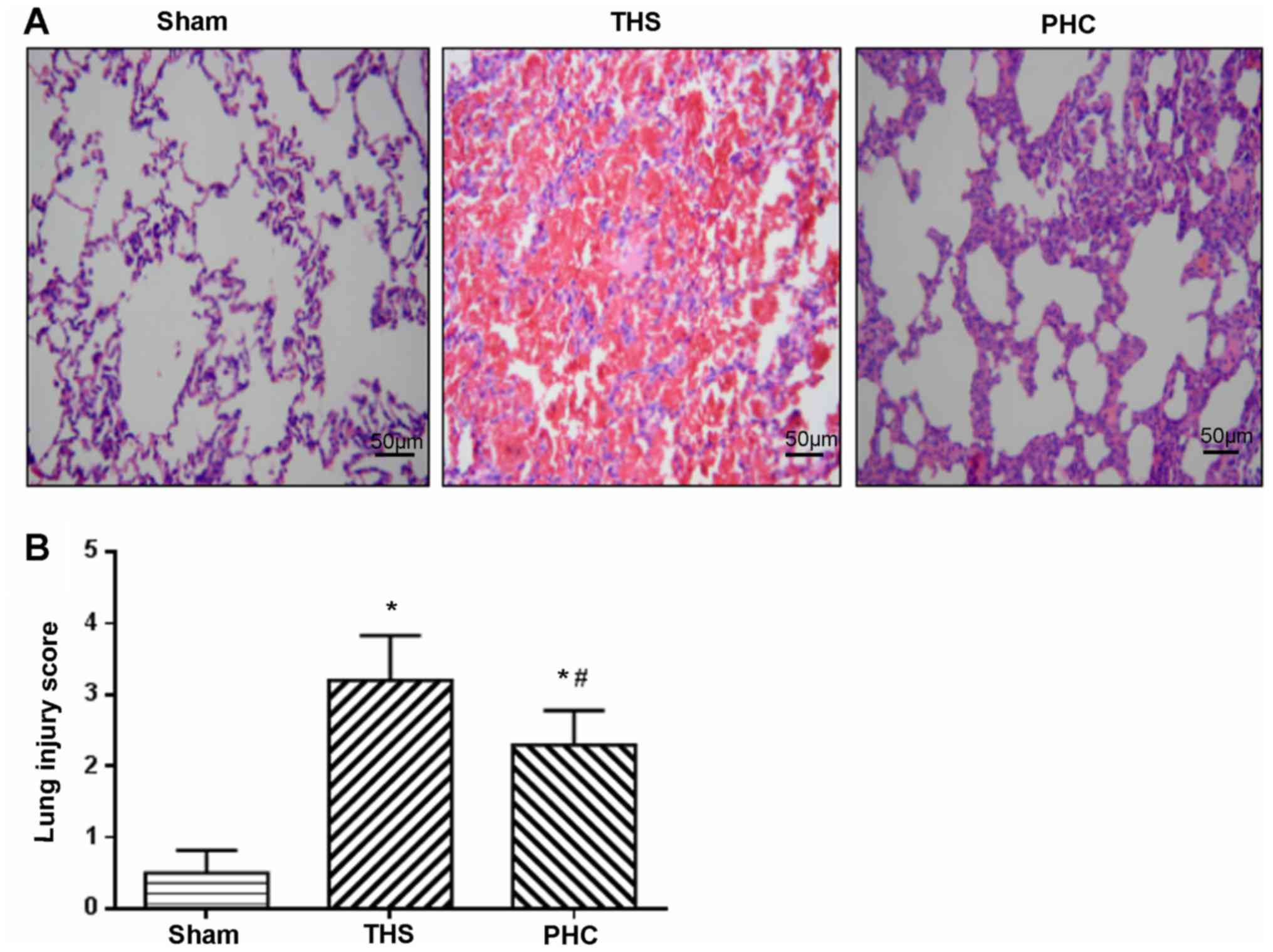
Effects of PHC on THS-mediated lung
pathological alterations
As presented in Fig.
4A, the hematoxylin and eosin staining microscopic findings in
the lung sections revealed a normal lung parenchyma in the sham
group. In the THS group, the normal alveolar structure of the rat
lung was disrupted, with severe hemorrhage and congestion with
infiltrating leukocytes. By contrast, the rats in the PHC group
exhibited significantly less hemorrhaging and leukocyte
infiltration compared with the THS group. The IQA scores were 0.5,
3.2 and 2.3 in the sham, THS and PHC groups, respectively (Fig. 4B). The IQA scores in the THS group
were increased compared with those in the sham control group
(P<0.05), and the PHC group (P<0.05).
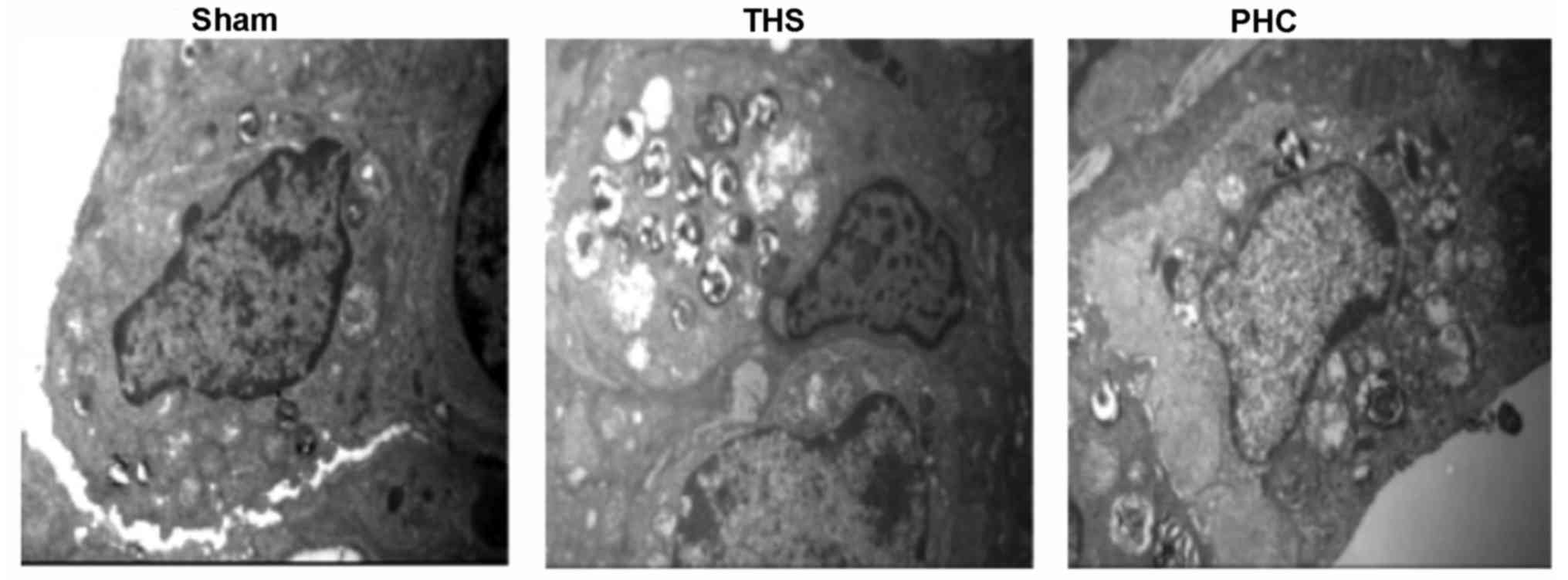
In Fig. 5, electron
microscopy of the THS rat tissues demonstrated ridge dissolution
and mitochondrial vacuolization in certain vascular endothelial
cells and alveolar epithelial cells, in addition to emptied
lamellar bodies. Furthermore, in the THS group, mitochondria in
type II alveolar cells appeared swollen, osmiophilic lamellar
bodies were emptied, and the cellular ridge had lodged and
disappeared. Additionally, the conjunctions between the alveolar
epithelial cells and the capillary endothelial cells were damaged,
as indicated by gaps, in the THS rats. In comparison, pretreatment
with PHC resulted in a marked attenuation of these pathological
alterations.
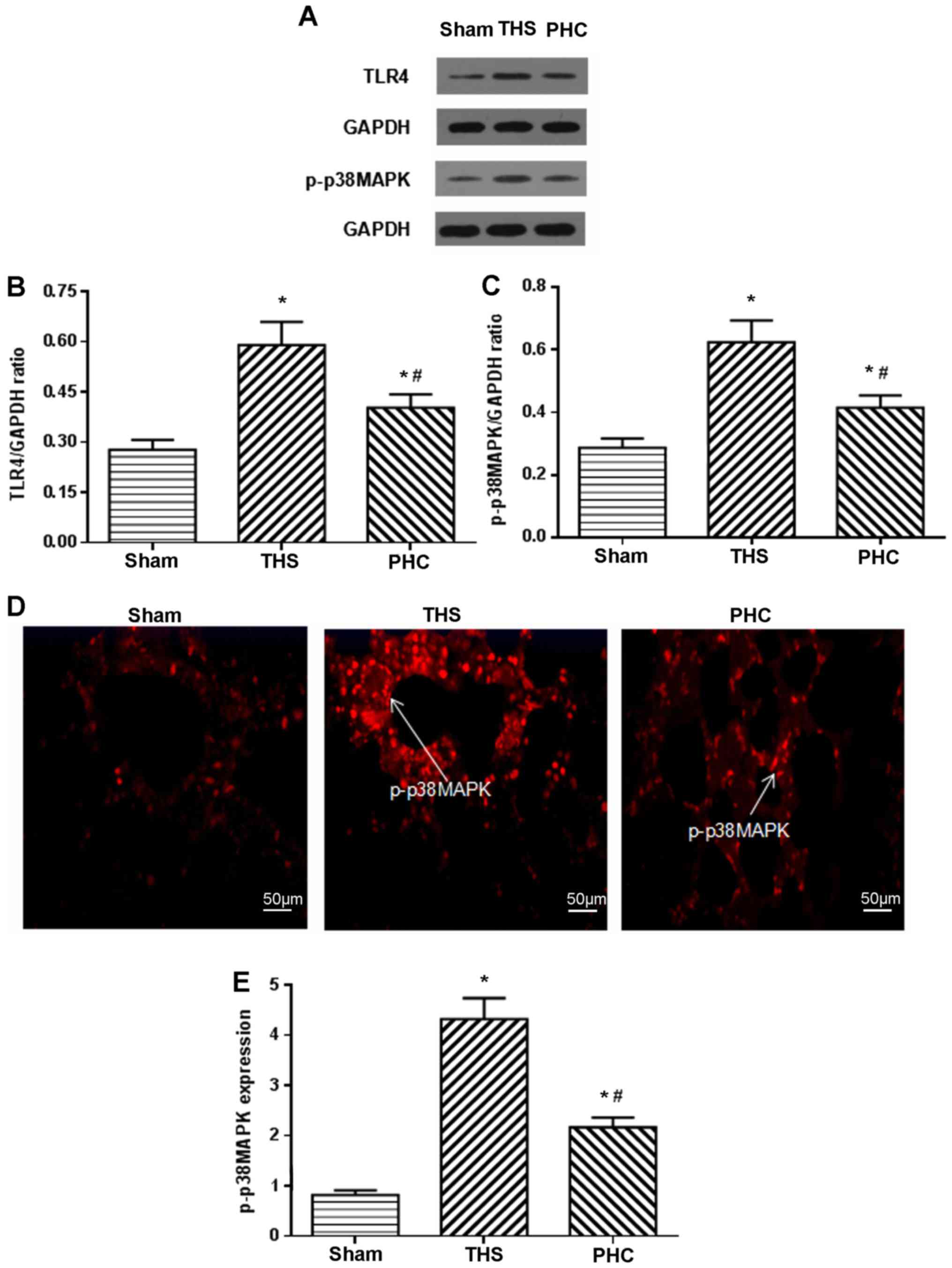
Effects of PHC on TLR4 and p-p38MAPK
protein expression in lung tissue, assessed by western blotting and
immunofluorescence analysis
The present study assayed the effects of PHC on the
activation of TLR4 and p-p38MAPK by western blotting. Following the
THS challenge, TLR4 and p-p38MAPK expression increased, and PHC
decreased the THS-induced expression of TLR4 and p-p38MAPK
(Fig. 6A-C). p-p38MAPK expression
in lung tissues was measured by immunofluorescence analysis. In the
sham group, positive cells were distributed in clumps with red
colorization. In the THS rats, p-p38MAPK-positive cells were
distributed throughout the visual fields, including alveolar
macrophages, vascular endothelial cells, bronchial epithelial cells
and interstitial cells, and were stained red. p-p38MAPK expression
in tissues was increased following the THS challenge compared with
that in the sham controls (P<0.05). Compared with the THS group,
PHC administration inhibited p-p38MAPK expression in tissues
(Fig. 6D and E).
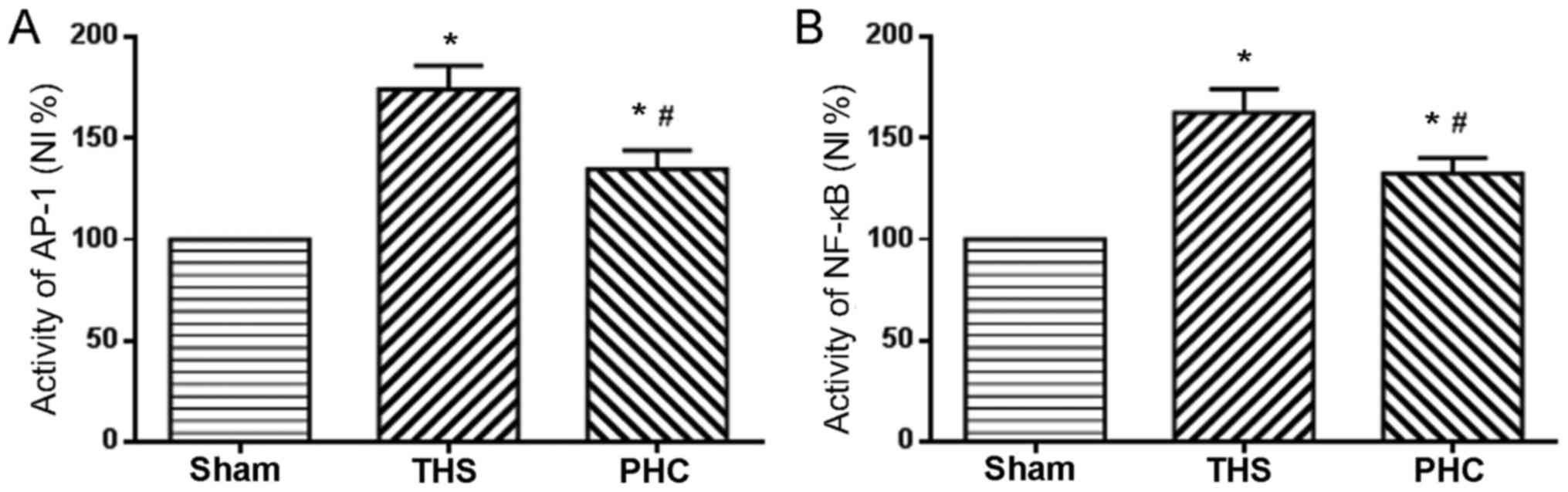
Effects of PHC on NF-κB and AP-1
activation in THS-induced ALI rats
NF-κB, a transcription factor known to regulate
inflammatory gene expression in macrophages, is additionally
considered to be a marker of classically activated pro-inflammatory
macrophages. The role of PHC in the activation of NF-κB and AP-1
nuclear binding activity, due to THS-induced ALI, was analyzed. In
response to THS, the DNA binding activity of NF-κB and AP-1 in lung
tissues was significantly increased compared with those in sham
group rats (P<0.05). PHC administration efficiently attenuated
the increases in NF-κB and AP-1 activity induced by THS (P<0.05;
Fig. 7).
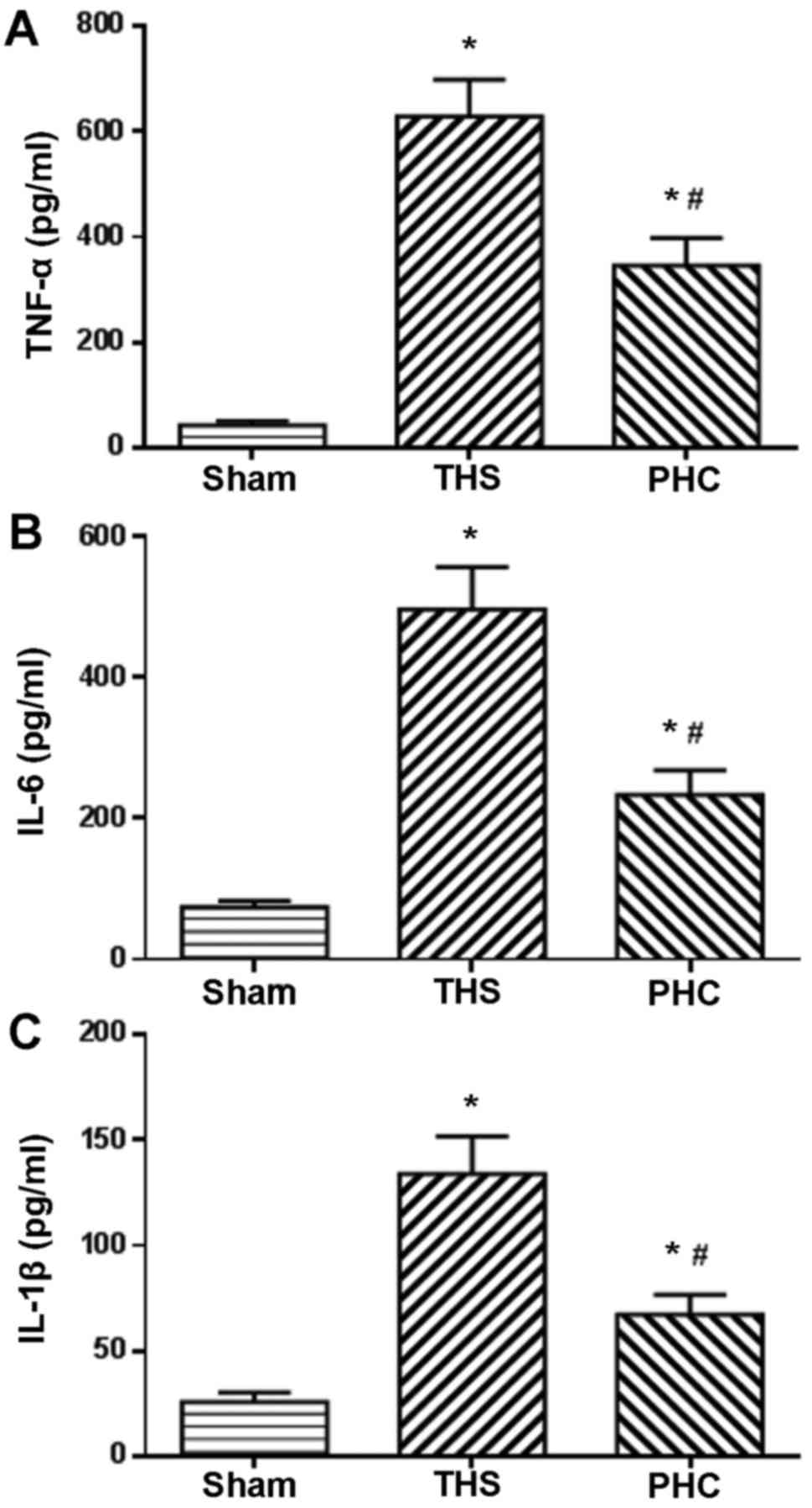
Effect of PHC on serum TNF-α, IL-6 and
IL-1β expression levels in THS-induced ALI rats
The serum expression levels of TNF-α, IL-6 and IL-1β
were evaluated by ELISA, since the enhanced expression of these
pro-inflammatory cytokines has been demonstrated to be associated
with the activation of innate immunity. The serum TNF-α, IL-6 and
IL-1β expression levels were significantly increased post-THS
challenge, as presented in Fig. 8.
Treatment with PHC significantly reduced the serum expression
levels of TNF-α, IL-6 and IL-1β induced by THS (P<0.05; Fig. 8).
Discussion
ALI and ARDS following blunt chest trauma are
important contributors to increased morbidity and mortality among
patients worldwide (15). HS is a
principal cause of mortality in the context of blunt chest trauma
(16). ALI and ARDS are well-known
common causes of pathogenesis following THS, and lead to an
uncontrollable systemic inflammatory response (17,18).
The present study used a novel rat model of THS and investigated
the effects of PHC on the TLR4 signaling pathway during ALI,
including inflammation and lung damage. At 6 h post-THS challenge,
the rats exhibited marked hypotension, arterial hypoxemia,
leukocytosis and alveolar edema in the interstitial capillaries,
and alveolar hemorrhage in histological assessments, which is
consistent with the findings of Wu et al (12). Additionally,
PaO2/FiO2 ratios ≤300 mmHg were measured in
THS rats, indicating that the replication of the two-hit-induced
ALI model in the rats was successful.
Hoth et al (19) demonstrated that pulmonary
inflammatory responses and systemic inflammatory responses are
important characteristics post-THS-induced pulmonary contusion,
including activated innate immune responses and an overwhelming
release of pro-inflammatory cytokines, particularly TLR4, which
serves a pivotal role in the host defense against invading
pathogenic microorganisms and the recognition of
pathogen-associated molecular patterns. A number of studies have
demonstrated that TLR4 is an important pattern recognition receptor
involved in the internal and external-derived inflammatory
factor-mediated pulmonary inflammatory response (20,21).
TLR4 activation has been demonstrated to be involved in the
pathogenesis of ALI and in the activation of numerous intracellular
signaling systems, including the p38MAPK and NF-κB pathways, all of
which are crucial regulators of inflammatory responses (22,23).
Barrenschee et al (24)
reported that activation of p38MAPK by TLR4 upregulated
inflammation-associated gene expression and stimulated the release
of inflammatory cytokines. Yang et al (25) revealed that TLR4 mediated the
activation of p38MAPK and contributed to high mobility group
protein B1-induced ALI following liver ischemia/reperfusion injury.
Furthermore, AP-1 is an important transcription factor required for
the expression of numerous pro-inflammatory cytokines involved in
the pathogenesis of ALI. Therefore, the present study aimed to
determine potential therapeutic avenues for ALI with respect to the
TLR4 signaling pathway. The results of the present study suggested
that TLR4 may serve an important role in the pathophysiological
mechanisms of THS-induced ALI and inflammation, and that p38MAPK,
NF-κB and AP-1 may be involved in TLR4 signaling pathway-mediated
lung inflammatory processes during THS.
PHC is a novel anticholinergic agent with
antimuscarinic and antinicotinic activity, although it has little
effect on HR and myocardial oxygen consumption. Animal and clinical
studies have demonstrated that PHC selectively blocks muscarinic
acetylcholine receptors 1 and 2 (M1 and M2),
and the nicontinic acetylcholine receptor, with fewer M2
receptor-associated cardiovascular side effects compared with other
hyoscyamine medicines marketed in China (26,27).
Previous studies reported that PHC may have potential positive
effects on lung/liver/renal injury, sepsis/septic shock, and
ischemia/reperfusion injury (28–32).
In the present study, it was demonstrated that pretreatment with
PHC attenuated the development of lung damage, as indicated by a
significantly elevated MAP, a stabilized HR, improved pulmonary
oxygenation, and decreased MPO activity, W/D ratio and serum levels
of TNF-α, IL-6 and IL-1β, in addition to the activation of their
transcription factors, NF-κB and AP-1, in the lung. These are
important advances regarding the effects of PHC.
A number of studies have confirmed that PHC exerts
anti-inflammatory, anti-apoptotic and antioxidative stress effects
in animal injury models. In 2009, Shen et al (33) reported that PHC administration
markedly attenuated the upregulation of the lung inflammatory
response and decreased lung vascular leakage in a rat model of
lipopolysaccharide-induced ALI. In 2010, Wang et al
(34) confirmed that PHC may
alleviate lung injury by inhibiting the apoptosis regulator
Bax/apoptosis regulator Bcl-2 signaling pathway in traumatic ALI.
In 2011, Zhan et al (9)
revealed that PHC was able to reduce inflammatory cytokine
expression in septic mice. In 2012, Shu et al (35) demonstrated that PHC may decrease
the expression levels of pro-inflammatory cytokines in the plasma
following cardiopulmonary bypass. In 2013, Wang et al
(36) discovered that PHC
attenuated oxidative stress, the inflammatory response and
apoptosis induced by renal ischemia/reperfusion in rats. The
findings of the present study that the pro-inflammatory cytokine
(TNF-α, IL-6 and IL-1β) responses induced by THS were significantly
attenuated by PHC, indicated that PHC has potent anti-inflammatory
effects in ALI due to THS.
In conclusion, the results of the present study
suggested that the activation of the TLR4 signaling pathway is a
potentially important pathophysiological mechanism during the
development of ALI following THS. These results indicate that PHC
has anti-inflammatory properties and exerts a protective effect
against the damage in ALI caused by THS, by inhibiting the TLR4
signaling pathway. Therefore, activation of the TLR4 signaling
pathway may be one of the pathophysiological mechanisms of ALI, and
PHC therapy may be a treatment option for inflammatory diseases,
including ALI, in the future.
Acknowledgements
The authors would like to thank Shenzhen IVY-Valued
Biotechnology Co. Ltd. (Shenzhen, China) for English editing
service.
Funding
This research was supported by the National Natural
Science Foundation of China (grant nos. 81671891 and 81401574) and
the Natural Science Foundation of Hubei Province of China (grant
no. 2016CFB251).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW, XS and ZX designed the study. XW, BZ, YL and EW
performed the experiments. HL, LZ and QM analyzed the data. XW and
HL wrote the manuscript.
Ethics approval and consent to
participate
All animal experiments complied with the Guide for
the Care and Use of Laboratory Animals of the National Institutes
of Health, and were approved by the Bioethics Committee of Renmin
Hospital of Wuhan University (Wuhan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PHC
|
penehyclidine hydrochloride
|
|
ALI
|
acute lung injury
|
|
THS
|
blunt chest trauma with hemorrhagic
shock
|
|
HS
|
hemorrhagic shock
|
|
AP-1
|
activator protein-1
|
|
TNF
|
tumor necrosis factor
|
|
NF-κB
|
nuclear factor-κB
|
|
IL
|
interleukin
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
TLRs
|
Toll-like receptors
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
MPO
|
myeloperoxidase
|
|
IQA
|
index of quantitative assessment
|
References
|
1
|
Hildebrand F, Weuster M, Mommsen P, Mohr
J, Fröhlich M, Witte I, Keibl C, Ruchholtz S, Seekamp A, Pape HC,
et al: A combined trauma model of chest and abdominal trauma with
hemorrhagic shock-description of a new porcine model. Shock.
38:664–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erickson SE, Martin GS, Davis JL, Matthay
MA and Eisner MD: NIH NHLBI ARDS Network: Recent trends in acute
lung injury mortality: 1996–2005. Crit Care Med. 37:1574–1579.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reino DC, Palange D, Feketeova E, Bonitz
RP, Xu DZ, Lu Q, Sheth SU, Peña G, Ulloa L, De Maio A, et al:
Activation of toll-like receptor 4 is necessary for trauma
hemorrhagic shock-induced gut injury and polymorphonuclear
neutrophil priming. Shock. 38:107–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Z, Li Y, Liu B, Deperalta DK, Zhao T,
Chong W, Duan X, Zhou P, Velmahos GC and Alam HB: Synergistic
effects of hypertonic saline and valproic acid in a lethal rat
two-hit model. J Trauma Acute Care Surg. 74:991–997. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bosmann M, Russkamp NF and Ward PA:
Fingerprinting of the TLR4-induced acute inflammatory response. Exp
Mol Pathol. 93:319–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao
J, Chen L, Cui L and Qiao H: Protective effect of shikonin in
experimental ischemic stroke: Attenuated TLR4, p-p38MAPK, NF-κB,
TNF-α and MMP-9 expression, up-regulated claudin-5 expression,
ameliorated BBB permeability. Neurochem Res. 39:97–106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joh EH, Gu W and Kim DH: Echinocystic acid
ameliorates lung inflammation in mice and alveolar macrophages by
inhibiting the binding of LPS to TLR4 in NF-κB and MAPK pathways.
Biochem Pharmacol. 84:331–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han XY, Liu H, Liu CH, Wu B, Chen LF,
Zhong BH and Liu KL: Synthesis of the optical isomers of a new
anticholinergic drug, penehyclidine hydrochloride (8018). Bioorg
Med Chem Lett. 15:1979–1982. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhan J, Liu Y, Zhang Z, Chen C, Chen K and
Wang Y: Effect of penehyclidine hydrochloride on expressions of
MAPK in mice with CLP-induced acute lung injury. Mol Biol Re.
38:1909–1914. 2011. View Article : Google Scholar
|
|
10
|
Li H, Qian Z, Li J, Han X and Liu M:
Effects of early administration of a novel anticholinergic drug on
acute respiratory distress syndrome induced by sepsis. Med Sci
Monit. 11:BR319–BR325. 2011.
|
|
11
|
Li BQ, Sun HC, Nie SN, Shao DB, Liu HM and
Qian XM: Effect of penehyclidine hydrochloride on patients with
acute lung injury and its mechanisms. Chin J Traumatol. 13:329–335.
2010.PubMed/NCBI
|
|
12
|
Wu XJ, Xia ZY, Wang LL, Luo T, Zhan LY,
Meng QT and Song XM: Effects of penehyclidine hydrochloride on
pulmonary contusion from blunt chest trauma in rats. Injury.
43:232–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raghavendran K, Davidson BA, Helinski JD,
Marschke CJ, Manderscheid P, Woytash JA, Notter RH and Knight PR: A
rat model for isolated bilateral lung contusion from blunt chest
trauma. Anesth Analg. 101:1482–1489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Knöferl MW, Angele MK, Diodato MD,
Schwacha MG, Ayala A, Cioffi WG, Bland KI and Chaudry IH: Female
sex hormones regulate macrophage function after trauma-hemorrhage
and prevent increased death rate from subsequent sepsis. Ann Surg.
235:105–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Du DY, Hu X, Xia DK, Xiang XY,
Huang C, Zhou JH and Jiang JX: Prevalence and mortality of severe
chest trauma in Three Gorges Area of China. Zhongguo Yi Xue Ke Xue
Yuan Xue Bao. 34:567–572. 2012.(In Chinese). PubMed/NCBI
|
|
16
|
Seitz DH, Perl M, Liener UC, Tauchmann B,
Braumüller ST, Brückner UB, Gebhard F and Knöferl MW: Inflammatory
Alterations in a novel nombination model of blunt chest trauma and
hemorrhagic Shock. J Trauma. 70:189–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhatia M, Zemans RL and Jeyaseelan S: Role
of chemokines in the pathogenesis of acute lung injury. Am J Respir
Cell Mol Biol. 46:566–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wohlauer M, Moore EE, Silliman CC, Fragoso
M, Gamboni F, Harr J, Accurso F, Wright F, Haenel J, Fullerton D
and Banerjee A: Nebulized hypertonic saline attenuates acute lung
injury following trauma and hemorrhagic shock via inhibition of
matrix metalloproteinase-13. Crit Care Med. 40:2647–2653. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoth JJ, Wells JD, Brownlee NA, Hiltbold
EM, Meredith JW, McCall CE and Yoza BK: Toll-like receptor
4-dependent responses to lung injury in a murine model of pulmonary
contusion. Shock. 31:376–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reino DC, Pisarenko V, Palange D, Doucet
D, Bonitz RP, Lu Q, Colorado I, Sheth SU, Chandler B, Kannan KB, et
al: Trauma hemorrhagic shock-induced lung injury involves a
gut-lymph-induced TLR4 pathway in mice. PLoS One. 6:e148292011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feinman R, Deitch EA, Aris V, Chu HB,
Abungu B, Caputo FJ, Galante A, Xu D, Lu Q, Colorado I, et al:
Molecular signatures of trauma-hemorrhagic shock-induced lung
injury. Shock. 28:360–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen C, Wang Y, Zhang Z, Wang C and Peng
M: Toll-like receptor 4 regulates heme oxygenase-1 expression after
hemorrhagic shock induced acute lung injury in mice: Requirement of
p38 mitogen-activated protein kinase activation. Shock. 31:486–492.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu HP, Hwang TL, Hsieh PW and Lau YT: Role
of estrogen receptor-dependent upregulation of p38 MAPK/heme
oxygenase 1 in resveratrol-mediated attenuation of intestinal
injury after trauma-hemorrhage. Shock. 35:517–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barrenschee M, Lex D and Uhlig S: Effects
of the TLR2 agonists MALP-2 and Pam3Cys in isolated mouse lungs.
PLoS One. 5:e138892010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Z, Deng Y, Su D, Tian J, Gao Y, He Z
and Wang X: TLR4 as receptor for HMGB1-mediated acute lung injury
after liver ischemia/reperfusion injury. Lab Invest. 93:792–800.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang YA, Zhou WX, Li JX, Liu YQ, Yue YJ,
Zheng JQ, Liu KL and Ruan JX: Anticonvulsant effects of
phencynonate hydrochloride and other anticholinergic drugs in soman
poisoning: Neurochemical mechanisms. Life Sci. 78:210–223. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao HT, Liao Z, Meng X, Yan X, Chen S and
Mo Z: Effects of the selective muscarinic receptor antagonist
penehyclidine hydrochloride on the respiratory tract. Pharmazie.
64:337–341. 2009.PubMed/NCBI
|
|
28
|
Li J, Li J, Zhang L, Huang Y, Pan JH and
Chen KZ: Penehyclidine prevents nuclear factor-кappaB activation in
acute lung injury induced by lipopolysaccharide. J Pharm Pharmaco.
60:1197–1205. 2008. View Article : Google Scholar
|
|
29
|
Cai DS, Jin BB, Pei L and Jin Z:
Protective effects of penehyclidine hydrochloride on liver injury
in a rat cardiopulmonary bypass model. Eur J Anaesthesiol.
27:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He SS, Lin CS, Gu MN, Chen DT, Zong SL and
Chen Y: Protective effects of penehyclidine hydrochloride against
acute renal injury induced by hemorrhagic shock and
lipopolysaccharides in rats. Nan Fang Yi Ke Da Xue Xue Bao.
31:899–902. 2011.(In Chinese). PubMed/NCBI
|
|
31
|
Zhan J, Wang YL, Wang CY, Li JG, Zhang ZZ
and Jia BH: Protective effects of penehyclidine hydrochloride on
septic mice and its mechanism. Shock. 28:727–732. 2007.PubMed/NCBI
|
|
32
|
Zhang Y, Leng YF, Xue X, Zhang Y, Wang T
and Kang YQ: Effects of penehyclidine hydrochloride in small
intestinal damage caused by limb ischemia-reperfusion. World J
Gastroentero. 17:254–259. 2011. View Article : Google Scholar
|
|
33
|
Shen W, Gan J, Xu S, Jiang G and Wu H:
Penehyclidine hydrochloride attenuates LPS-induced acute lung
injury involvement of NF-kappaB pathway. Pharmacol Res. 60:296–302.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang LL, Zhan LY, Wu XJ and Xia ZY:
Effects of penehyclidine hydrochloride on apoptosis of lung tissues
in rats with traumatic acute lung injury. Chinese J Traumatol.
13:15–19. 2010.
|
|
35
|
Shu LJ and Wei XC: Effect of Penehyclidine
hydrochloride in systemic inflammatory response syndrome caused by
cardiopulmonary bypass. Sichuan Da Xue Xue Bao Yi Xue Ban.
43:543–546. 2012.PubMed/NCBI
|
|
36
|
Wang YP, Li G, Ma LL, Zheng Y, Zhang SD,
Zhang HX, Qiu M and Ma X: Penehyclidine hydrochloride ameliorates
renal ischemia-reperfusion injury in rats. J Surg Res. 186:390–397.
2014. View Article : Google Scholar : PubMed/NCBI
|