Introduction
Candida species are ubiquitous fungi and the
most common fungal pathogens affecting humans. Candida, in
particular, affects high-risk patients who are either
immunocompromised or critically ill. More than 100 species of
Candida exist, but only a few are recognized as causing
disease in humans. Candida glabrata and Candida
albicans account for 70 to 80% of yeasts isolated from patients
with invasive candidiasis. In recent decades, C. glabrata
has become important because of its increasing world-wide incidence
and because of its developing resistance to antifungals, including
azoles, in the clinic.
Azole antifungal agents such as fluconazole and
voriconazole are approved therapy for candidemia and invasive
candidiasis caused by C. glabrata (1). Mechanisms of azole resistance include
i) increased drug export (efflux pumps), ii) lost drug targets
(e.g., altered drug target binding site), iii) up-regulated
homeostatic stress-response pathways to deal with azole-associated
damage, and iv) changed biosynthetic pathways (particularly sterol
synthesis) that circumvent or attenuate the effects of azole
inhibition (2).
The cellular toxicity of azole antifungals occurs
primarily through their ability to affect the fungal cell membrane
by inhibiting the biosynthesis of ergosterol, the principal sterol
in the fungal cell membrane, leading to the depletion of ergosterol
and an accumulation of possibly toxic sterol intermediates in the
cytoplasmic membrane. Azole antifungal compounds (imidazoles and
triazoles) inhibit cytochrome P-450 dependent sterol
14α-demethylase (Erg11p), an enzyme that catalyzes the oxidative
removal of the 14α-methyl group of lanosterol in the ergosterol
biosynthetic pathway (2). Fungal
cells exposed to azoles must either synthesize more endogenous
ergosterol or import exogenous sterol if they are to survive
antimycotic treatment.
Sterol uptake appears to confer resistance to
antifungal drugs since mutant strains of Candida species
lacking AUS1 and TIR3, the sterol influx transporters
(3), or UPC2, the
transcription factor that controls AUS1 and TIR3
expression (3–5), exhibit reduced uptake of cholesterol
and are hypersensitive to azoles (6,7),
whereas enhanced UPC2 or AUS1 expression and
cholesterol uptake have been implicated in the azole-resistant
phenotype (6,7). Furthermore, several studies have even
suggested that pathogenic fungi can scavenge free sterols for the
cell membrane, including cholesterol, resulting in resistance to
polyene and azole antifungals (8).
Increased uptake of exogenous cholesterol may be associated with
drug resistance in clinical isolates of bile-dependent C.
glabrata cells as well as in sterol auxotrophic C.
glabrata strains (9). These
observations indicate that the ability to scavenge exogenous
sterols, when ergosterol biosynthesis is defective or is blocked by
antimycotic agents, may play an important role in increased azole
resistance in pathogenic fungal species.
Besides sterol uptake, alteration in sterol
biosynthesis seems to also affect fungal susceptibility to
antifungals. ERG11 is one of the critical genes in the
ergosterol biosynthetic pathway, and its encoding protein product
Erg11p (or CYP51p depending on nomenclature) is the major target
enzyme of azole antifungals. Mutations in ERG11 are the most
common mechanism of azole resistance in C. albicans
(10). Over-expression of
ERG11 has been attributed to decreases in azole activity in
C. albicans. Alterations in other enzymes of the ergosterol
biosynthetic pathway, particularly ERG3 (C-5 sterol
desaturase), ERG5 (C-22 sterol desaturase), ERG6
(C-24 sterol methyl-transferase), and ERG25 (C-4 sterol
methyloxidase), which are up-regulated with inhibition of Erg11p,
have also been documented to reduce azole susceptibility of C.
albicans. In C. glabrata, depletion of the ergosterol
content in a CgERG1 mutant increases the levels of
susceptibility to azoles, while complementation of the
CgERG1 mutation restores drug sensitivity to wild-type
levels (8). In both C.
albicans and C. glabrata, regulation of sterol synthesis
is vital to azole susceptibility.
In this study, we assessed the role of two
protective mechanisms in conferring antifungal susceptibility in
C. glabrata. We showed that expression of the genes involved
in both sterol uptake and sterol synthesis is up-regulated in C.
glabrata when ergosterol is depleted by azole treatment and
when ergosterol biosynthesis is defective. We further corroborated
that pathogenic fungi can accumulate exogenous sterols from the
environment as a protective strategy to survive under azole and
hypoxic stress. These findings suggest that sterol uptake and
sterol synthesis may act coordinately and collaboratively to
sustain growth and to mediate antifungal tolerance in C.
glabrata through dynamic gene expression in response to azole
pressure and environmental changes.
Materials and methods
Cell culture and drug treatment
All C. glabrata strains (Table I) used in the present study were
cultured on YPD agar containing 1% Bacto yeast extract (Difco
Laboratories, Detroit, MI, USA), 2% Bacto peptone (Difco
Laboratories), and 2% glucose (Sigma-Aldrich, St. Louis, MO, USA).
NCCLS84, Cg1660, CgTn201S, and CgTn201Su/aus1 were also grown on
minimal (MIN) agar containing 0.67% Yeast Nitrogen Base without
amino acids (Difco Laboratories) plus 2% glucose. The ura3
mutants Cg84u, Cg1660u, and CgTn201Su were grown in MIN medium
supplemented with 20 µg/ml of uracil (Sigma-Aldrich) or were
selected on a MIN plus uracil agar plate containing 0.1%
5-fluoroorotic acid (FOA) (Lancaster, Pelham, NH, USA). YEPG agar
was used for drug sensitivity assay, which contained 1% Bacto yeast
extract (Difco Laboratories), 2% Bacto peptone (Difco
Laboratories), 3% glycerol (Invitrogen/Life Technologies, Carlsbad,
CA, USA), 1% ethanol (Warner-Graham, Inc., Cockeysville, MD), and
2% agar (Difco Laboratories).
 | Table I.Candida glabrata strains used
in this study. |
Table I.
Candida glabrata strains used
in this study.
| Strain | Parental
strain | Genotype or
description | Reference or
source |
|---|
| NCCLS84 |
| Wild-type
(ATCC90030)a | ATCCa |
| Cg84u | NCCLS84 | Cgura3 | (8) |
| Cg1660 |
| Clinical
isolate | FHCRCb |
| Cg1660u | Cg1660 | Cgura3 | (8) |
| CgTn201S | Cg1660u | Cgura3
Cgerg1::Tn5<Cm URA3> | (8) |
| CgTn201Su | CgTn201S | Cgura3
Cgerg1::Tn5<Cm ura3> | Present study |
| CgTn201Su/aus1 | CgTn201Su | Cgura3
Cgerg1::Tn5<Cm ura3> | Present study |
|
|
|
Cgaus1∆::URA3 |
|
Fluconazole (Euroasian Chemicals Private Ltd.,
Mumbai, India) was added to cultures of each strain at a final
concentration of 200 µg/ml, followed by continued incubation with
shaking for 2 h. Cell cultures without fluconazole treatment served
as controls. After incubation, the cells were harvested, and the
cell pellets were stored at −80°C until the subsequent isolation of
RNA.
The BBL GasPak Plus gas generator envelope (Becton
Dickinson Microbiology Systems, Sparks, MD, USA) or the BBL GasPak
Pouch Anaerobic Systems (Becton Dickinson Microbiology Systems,
Cockeysville, MD, USA) was used for cultures grown under conditions
of low oxygen tension (hypoxia). To mimic the host environment of
animals and humans, C. glabrata cells were also grown in the
presence of 5% CO2 under low oxygen conditions using an
InvivO2 400 workstation at 37°C with shaking at 200 rpm (Ruskinn
Technology Ltd., Bridgend, UK). For analysis of sterol uptake and
exogenous sterol utilization, 2 mg of ergosterol or cholesterol was
dissolved in a mixture of 50% ethanol and 50% Tween-80
(Sigma-Aldrich) to give a 2 mg/ml stock solution, which was used to
supplement the media with a final sterol concentration of 20 µg/ml.
For comparison, the same final concentration of ethanol-Tween-80
without sterol was used.
Drug sensitivity assay
The susceptibility of the C. glabrata strains
Cg1660, CgTn201S, and CgTn201Su/aus1 to fluconazole and
voriconazole was determined on MIN and YEPG agar media using an
E-test (AB Biodisk, Solna, Sweden) according to the manufacturer's
instructions. In brief, logarithmic-phase cells were harvested and
adjusted to the desired concentrations by counting the number of
cells with a hemocytometer. From each cell suspension, 200 µl
(1×106 cells/ml) was plated in duplicate on MIN and YEPG
agar. All plates were incubated under conditions of low oxygen
tension at 37°C for 3 days. Growth inhibition zones (minimum
inhibitory concentration, MIC) to fluconazole and voriconazole were
measured.
RT-qPCR analysis
Total RNA was extracted from C. glabrata
(Cg84u) logarithmic-phase cultures grown in YPD broth using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's instructions. RNA was
converted to cDNA using the High Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) as previously described (11). Primers and probes were designed in
our laboratory using the primer analysis software Primer Express
3.0 (Applied Biosystems; Thermo Fisher Scientific, Inc.). TaqMan
probes were synthesized by Applied Biosystems (Thermo Fisher
Scientific, Inc.) and primers were synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.). The sequences of TaqMan probes
and forward and reverse primers, as well as the gene numbers for
all genes assessed in this study, are listed in Table II. To study gene expression, the
amplification was detected in real time using both SYBR Green
chemistry (SYBR-Green PCR Master Mix; Applied Biosystems; Thermo
Fisher Scientific, Inc.) and TaqMan chemistry (TaqMan Universal PCR
Master Mix; Applied Biosystems; Thermo Fisher Scientific, Inc.) as
described previously (11).
 | Table II.Primers and TaqMan probes for RT-qPCR
analysis of gene expression used in this study. |
Table II.
Primers and TaqMan probes for RT-qPCR
analysis of gene expression used in this study.
| Gene | Primer and probe
sequence (5′→3′) | Gene
numbera |
|---|
| ACT1 |
|
|
| F |
TTGGACTCTGGTGACGGTGTTA | CAGL0K12694g |
| R |
AAAATAGCGTGTGGCAAAGAGAA |
|
| P |
CCACGTTGTTCCAATTTACGCCGG |
|
| RDN5.8 |
|
|
| F |
CTTGGTTCTCGCATCGATGA | CAGL0L13387r |
| R |
GGCGCAATGTGCGTTCA |
|
| P |
ACGCAGCGAAATGCGATACGTAATGTG |
|
| ERG2 |
|
|
| F |
TCCCAGGTATGACCCATCATC | CAGL0L10714g |
| R |
TGCGAAGGAGTTTTGATCCAT |
|
| P |
ACAAAAGGGCTACGCAAAGCAATACGC |
|
| ERG3 |
|
|
| F |
TGCACTGGCCTCGTGTCTAC | CAGL0F01793g |
| R |
TAACCGTCGACTGGGTGGAA |
|
| P |
TGGTTGGTCTGCACTCCATTCGCC |
|
| ERG4 |
|
|
| F |
CCCTCAATTAGGTGTCGTCATGT | CAGL0A00429g |
| R |
GGCACGATTAATTCTTCACCCTTA |
|
| P |
CCACTGGCTGTACGCTAACGCTTGTG |
|
| ERG10 |
|
|
| F |
GCCAGAACCCCAATTGGTT | CAGL0L12364g |
| R |
TGCAATGACACCTAGGTCAACAG |
|
| P |
TTCCAAGGTGCGTTGGCCTCCA |
|
| ERG11 |
|
|
| F |
TGTCTTGATGGGTGGTCAACA | CAGL0E04334g |
| R |
CTGGTCTTTCAGCCAAATGCA |
|
| P |
CTTCCGCTGCTACCTCCGCTTGG |
|
| PDR16 |
|
|
| F |
CCTGGAGACGTGAATTTGGAAT | CAGL0J07436g |
| R |
ACAGCAACCAAATCCGATGTAA |
|
| P |
CTCGTCACCATTTTCTTCACCCAAATGG |
|
| PDR16 |
|
|
| F |
TTGGCCTGGAGACGTGAATT | CAGL0J07436g |
| R |
GTTTACCACTTTCATTCTCTACAGCAA |
|
| P |
TTGGGTGAAGAAAATGGTGACGAGGTTACA |
|
| AUS1 |
|
|
| F |
CCAAGCCACTGCAGGTGAA | CAGL0F01419g |
| R |
GGCGTGAAACAGGGACTTGA |
|
| P |
CGGTGCCCCAACGTCGGGTATC |
|
| SUT1 |
|
|
| F |
GTTGATGGCATTACATGGCAAT | CAGL0I04246g |
| R |
AGTAAAGGAGTTGGATGATGAGTGAA |
|
| P |
ACCAATTCCTATCGCCTCCAATGCCA |
|
| SUT2 |
|
|
| F |
AGGGCCTTCAAGGTATCGAAGT | CAGL0L09383g |
| R |
TCGGTTTTTGGATCACACCAA |
|
| P |
TTGCCTCTCCAAAACAGAAACTACCCTCCC |
|
| ECM22 |
|
|
| F |
CAATTACAAGAGCATGCAAACATTG | CAGL0C01199g |
| R |
GGAGTTAGCCTGACCATGAGTATTATT |
|
| P |
TGCATCAGAAACAGCATATCCAACGACTGT |
|
| UPC2A |
|
|
| F |
AAAATAGTACAGGAGCAACGGAGACT | CAGL0C01199g |
| R |
TGGTTGCACCTGGAGATGAA |
|
| P |
CTGTCGCCTTCTCTGAATCTGCTTACACCC |
|
| UPC2B |
|
|
| F |
GGTCGCAAGTGCATTGTTGT | CAGL0F07865g |
| R |
TCAGTCGCATTTGATGTATCTTTAGG |
|
| P |
CGTGGAATAATCACGATCCTCACATGCA |
|
Microarray hybridization and data
analysis
DNA microarray analysis was used to identify genes
with altered expression in the C. glabrata erg1 mutant
strain CgTn201S and the wild-type strain Cg1660. Total RNA was
isolated from the log phase culture of C. glabrata grown in
YPD by using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
and the RNeasy MiniElute Cleanup kit (Qiagen, Valencia, CA, USA).
RNA was assessed for quality using the Agilent 2100 Bioanalyzer
with the RNA 6000 Nano Reagent Kit. Pin-spotted 70-mer
oligonucleotide in-house arrays fabricated at the National
Institute of Allergy and Infectious Diseases (NIAID) were used for
analysis of the pair strains. In brief, a total of 5,908 70-mer
oligonucleotides were purchased from Institut Pasteur (Paris,
France) and were used for microarray printing at the NIAID
Microarray Research Facility. Expression of each open reading frame
(ORF) was measured by hybridization to a specific 70-mer
oligonucleotide. Thirty micrograms of total RNA from the
erg1 mutant strain CgTn201S and the wild-type strain Cg1660
was reverse-transcribed to cDNA to incorporate the fluorescent
Cy3-dUTP and Cy5-dUTP (GE Healthcare, Piscataway, NJ, USA),
respectively. The labeled cDNA of the paired mutant/wild-type
strain was combined and used for microarray hybridization. Six
microarrays were performed for analysis of the erg1
mutant/wild-type pair, including two with reciprocal labeling. The
microarrays were prehybridized at 42°C in prehybridization buffer
[5 × SSC (1 × SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1%
bovine serum albumin (BSA), 0.1% SDS] for 30 to 60 min and then
hybridized to the labeled cDNA in 50 µl of hybridization buffer
[25% formamide, 5 × SSC, 0.2% SDS, 20 µg/ml poly
(dA)40-60, 200 µg/ml Cot-1 DNA (Invitrogen; Thermo
Fisher Scientific, Inc.), 80 µg/ml yeast tRNA] overnight at 42°C.
The microarrays were washed three times in wash buffer A (1 × SSC,
0.05% SDS) and wash buffer B (0.1 × SSC). The in-house arrays were
scanned with a GenePix 4000B scanner (Molecular Devices, Sunnyvale,
CA, USA). All microarray data archive and statistical calculations
were performed on the ‘processed signal’ data by using the
Web-based mAdb analysis system provided by the Bioinformatics and
Molecular Analysis group (BIMAS) at the Center for Information
Technology (CIT), National Institutes of Health. The data were
filtered with the parameters that included genes present in four or
more arrays and each array with 80% or more genes present. The data
set of the paired strains was then analyzed by Student's t-test.
The genes with P-values less than 0.001 and with at least 1.5-fold
altered gene expression were then selected. The final data set
included all genes with altered expression in the pair.
Cloning of CgAUS1
The full nucleotide sequence of the C. glabrata
AUS1 homolog (CgAUS1) was obtained from the Candida
Genome Database (www.candidagenome.org). A 1,087 bp partial ORF of the
CgAUS1 gene was obtained by PCR with PfuUltra High-Fidelity
DNA polymerase (Stratagene, La Jolla, California, USA), primer set
CgAUS1S (5′-TTGAAGTTGCCTCTTGACTC-3′) and CgAUS1AS
(5′-AAGGCAACAAACACAGCGGCAG-3′), and the total genomic DNA of Cg1660
was used as the template. The PCR parameters were 2 min at 95°C,
followed by 30 cycles of 95°C for 30 sec, 53°C or 55°C for 30 sec,
and 72°C for 1 min 30 sec, and then extended at 72°C for 10 min.
The 1,087 bp PCR product, which was used as a probe for Southern
blotting, was then cloned into pCR-Blunt II-TOPO (Invitrogen;
Thermo Fisher Scientific, Inc.) to produce plasmid pCgAUS1.
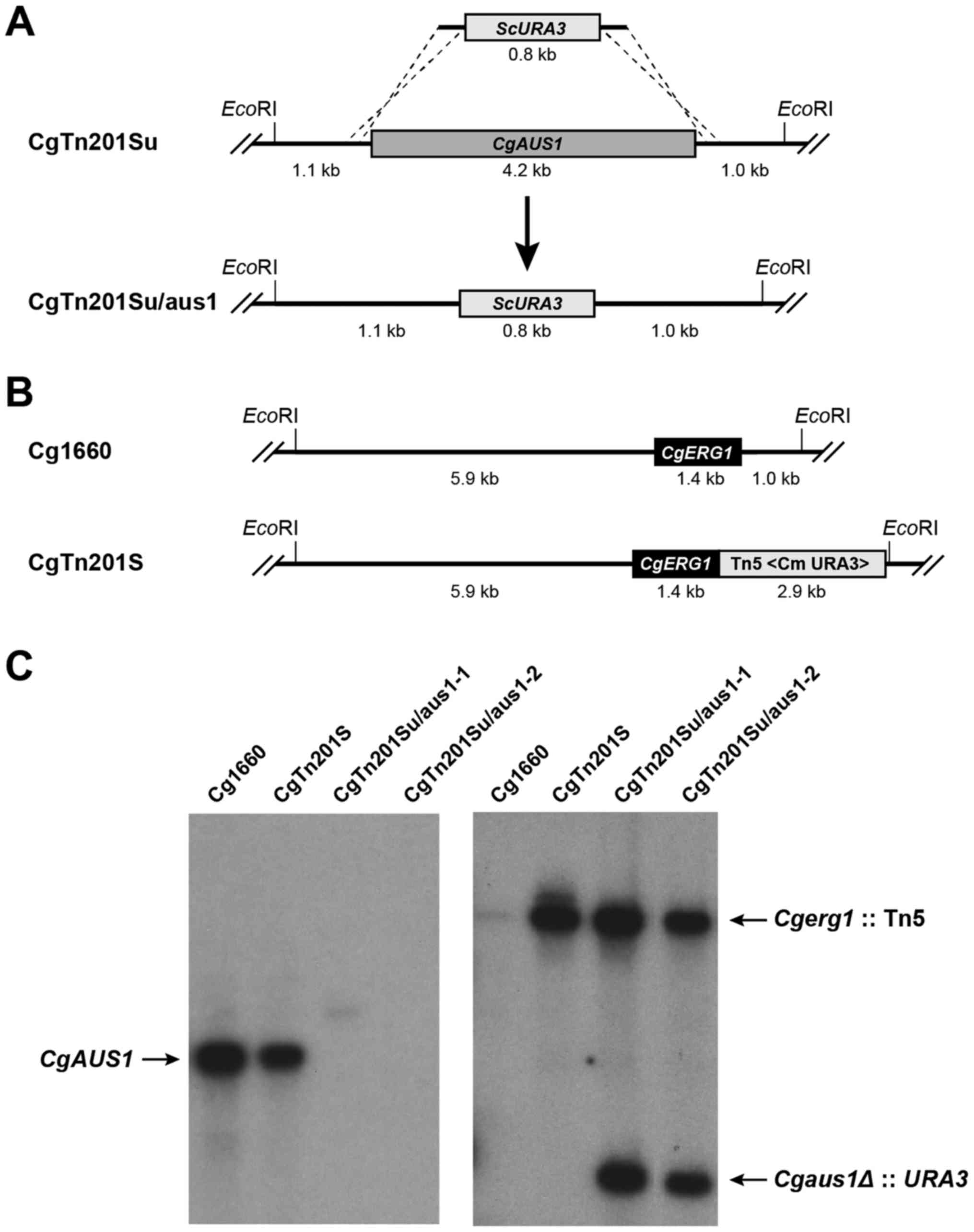
Construction of the Candida glabrata
Cgerg1 and Cgaus1∆ double mutant
The Cgerg1 mutant was generated in our
laboratory as described previously (8). Gene deletion of Cgaus1 was
performed as described by Vermitsky et al (12). Briefly, the primer pair CgAUS1D1S
(TTGAAATTCTCGGAAAGAAACATCAAATCAAAAAATTTTAACCTTCTAAAACTTGTTCTTTTTTTGGGAAATATAAGATGTCGAAAGCTACATATAAGGA)
and CgAUS1D2AS
(AGTAGATTAAAGAAAAGTGTTAAATTTAAGAATAAAATGGAATTGTTATTTCATTAAAAGCTTGTAGGAGTCACTCTTATTAGTTTTGCTGGCCGCATCT)
was used to generate the CgAUS1 deletion cassette using
YEP24 as the template to amplify Saccharomyces cerevisiae
URA3, which served as the selection marker. An additional round
of PCR using the primer pair CgAUS1D3S
(CCCCAACTATCAATTTTCTTTAAATCAAGGAAAATCTATTACATTCGCTATTAATCTCTACTATCTTTATCTTAGTTTTTTGAAATTCTCGGAAAGAAAC)
and CgAUS1D4AS
(ATTATATTTTAAATTTTGTTGTATAGCTTTTTTGCTGTAAAGGTGAAAAAACCGGGAATTTTGAGCATTAGTATTAGTAGATTAAAGAAAAGTGTTAAAT)
extended the 5′ and 3′ ends with about a 160 bp homologous region
on each end of the CgAUS1 deletion cassettes to increase the
efficiency of homologous recombination. S. cerevisiae URA3
was transformed into CgTn201Su (Cgura3 Cgerg1::Tn5<Cm
ura3>) to construct the Cgerg1/aus1 double mutant
CgTn201Su/aus1 via a successful double crossover homologous
recombination, which resulted in replacement of the CgAUS1
ORF with S. cerevisiae URA3 (Fig. 1A). The CgERG1 locus in the
clinical isolate Cg1660 and its transposon mutant CgTn201S
(Cgerg1) are shown in Fig.
1B. Transformants were selected on MIN medium, and the deletion
of CgAUS1 was confirmed by Southern blot analysis of the
EcoRI-digested genomic DNA (Fig.
1C). The purified 1.2-kb ScURA3 probe detected a single
signal in the Cgerg1 mutant CgTn201S; two signals were
detected in the two putative Cgerg1/aus1 mutants
CgTn201Su/aus1-1 and CgTn201Su/aus1-2, while there was no signal in
the parental strain Cg1660 (Fig.
1C, right panel). The 1087-bp CgAUS1 probe detected a
single 6.3-kb signal in Cg1660 and CgTn201S, but not in the two
putative Cgerg1/aus1 mutants CgTn201Su/aus1-1 and
CgTn201Su/aus1-2 (Fig. 1C, left
panel).
Southern blot analysis
Candida glabrata genomic DNA was isolated
from cultures grown in YPD overnight by using the MasterPure Yeast
Purification Kit (Epicentre, Madison, WI, USA). Purified DNA
fragments were recovered using the Strataprep Gel DNA Extraction
kit (Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA).
Hybond-N nylon membranes (Amersham, Arlington Heights, IL, USA)
were used for Southern hybridization analyses. DNA probes were
labeled with [α-32P] dCTP or [α-32P] dATP (MP
Biomedical, Solon, OH, USA) by using the Prime-It II kit
(Stratagene; Agilent Technologies, Inc.). DNA cloning and
hybridization analyses were done according to the standard
protocol.
Other techniques and reagents
DNA sequencing was done using the DNA sequencing kit
with a dRhodamine dye terminator (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and an ABI automatic DNA sequencing system
(Perkin-Elmer, Foster City, CA, USA). For sequencing of PCR
products, PfuUltra DNA polymerase (Stratagene; Agilent
Technologies, Inc.) was used for PCR amplification to minimize the
rate of PCR-introduced mutations. The PCR products were cleaned
with the Strataprep PCR Purification kit (Stratagene; Agilent
Technologies, Inc.) and were used as templates for DNA
sequencing.
Bioinformatic and statistical
analysis
The Gene Ontology (GO) enrichment analysis and the
network visualization of the biological functions related to the
differentially expressed genes were performed using Cytoscape
software with associated plug-ins (13). To create a functional network by
selecting the ClueGO: Function, GO: Biological process, all the
network evidence and only the terms with various levels of
significance (P<0.1-<0.0005) were taken into consideration
using the plug-in ClueGO (14),
which was followed by enrichment of the functional network with the
plug-in CluePedia (15).
Results of RT-qPCR for each gene are presented as
the fold-change over the expression in the sample not treated with
fluconazole, which was set as 1. The difference in triplicate
Cq values (∆Cq) was used to calculate the
difference between untreated and azole-treated samples. Student's
t-test was used to analyze the statistical significance of
differences between untreated and fluconazole-treated C.
glabrata cells, and P-values were adjusted using Holm's
adjustment for multiple testing. Values of P<0.05 were taken to
indicate significance.
Results
Azole stress up-regulates the genes
involved in sterol uptake and biosynthesis
Ergosterol biosynthetic genes encode the enzymes in
the sterol synthetic pathway leading to ergosterol biosynthesis in
yeast. It is of interest to understand whether the inhibition of
ergosterol biosynthesis by azoles alters endogenous sterol
synthesis in C. glabrata through changes in the expression
of ergosterol biosynthetic genes. We found that mRNA levels of
CgERG2, CgERG3, CgERG4, CgERG10, and CgERG11 were
strikingly increased in C. glabrata following fluconazole
treatment. As seen in Table III,
the extent of inducible up-regulation of ERG gene expression
by fluconazole in these cells ranged from more than 5-fold in
CgERG3 to 19-fold in CgERG10 compared with the
untreated controls. Interestingly, we also showed a 3.7-fold
increase in mRNA expression of CgPDR16 in
fluconazole-treated C. glabrata cells (Table III); PDR16p is an ATP-binding
cassette (ABC) transporter involved in the sterol biosynthetic
process and phospholipid transport in S. cerevisiae
(16).
 | Table III.Upregulated expression of sterol
biosynthetic and sterol transporter genes in Candida
glabrata under fluconazole stress. |
Table III.
Upregulated expression of sterol
biosynthetic and sterol transporter genes in Candida
glabrata under fluconazole stress.
| Gene | mRNA levels |
|---|
| ERG2 | 11.79a |
| ERG3 |
5.04a |
| ERG4 | 11.08a |
| ERG10 | 19.07a |
| ERG11 |
5.87a |
| PDR16 |
3.73a |
| AUS1 |
2.51b |
| SUT1 |
2.60b |
| SUT2 |
0.97 |
| UPC2A |
4.71a |
| UPC2B |
1.60b |
AUS1 and UPC2 (or its paralog
ECM22) have been demonstrated to be responsible for sterol
uptake in S. cerevisiae (17). We next addressed whether inhibition
of sterol synthesis by azoles alters the expression of these genes
in C. glabrata. Sequencing data have shown that C.
glabrata is more closely related to Saccharomyces
cerevisiae than to C. albicans, and some genes are
functionally exchangeable between C. glabrata and S.
cerevisiae. Therefore, to identify AUS1 and
ECM22/UPC2 orthologs in C. glabrata, we selected the
S. cerevisiae AUS1, ECM22, and UPC2 genes as queries
to perform homology searches. A BLAST search against the C.
glabrata open reading frame (ORF) nucleotide and amino acid
sequences (www.candidagenome.org) predicted CAGL0F01419g
(CgAUS1) to be the ortholog of AUS1. We also
performed a BLAST search against C. glabrata ORF nucleotide
sequence alone, which predicted CAGL0C01199g and CAGL0F07865g to be
possible orthologs of ECM22 or UPC2. The amino acid
sequences encoded by these two genes are particularly highly
homologous to Upc2p or Ecm22p. We refer to CAGL0C01199g and
CAGL0F07865g as UPC2A (ECM22) and UPC2B
(ECM22), respectively. This is in concordance with the work
of Nagi et al (4) and
Whaley et al (18). Our
results demonstrate a transcriptional up-regulation of over
two-fold and over four-fold in CgAUS1 and CgUPC2A
expression, respectively, in our model following fluconazole
challenge (Table III). These
data clearly show that inhibition of ergosterol synthesis by azoles
induce the expression of sterol transporter genes and likely lead
to enhanced sterol uptake in C. glabrata.
Besides its role in anaerobic sterol uptake,
UPC2 has been shown to be an activator of ergosterol
biosynthetic genes in yeast (4,18).
As the up-regulation of CgUPC2 expression was induced by
fluconazole in C. glabrata, this result prompted us to
address whether azoles alter the expression of other genes encoding
transactivators responsible for sterol synthesis or uptake in
fungi. It has been demonstrated that SUT1 is a transcription
factor involved in sterol uptake and synthesis in aerobically
growing S. cerevisiae cells (19). Therefore, to identify SUT1
ortholog in C. glabrata, we selected the S. cerevisiae
SUT1 gene as queries to perform homology searches. A BLAST
search against the C. glabrata ORF nucleotide sequence
(www.candidagenome.org) predicted
CAGL0I04246g and CAGL0L09383g to be possible orthologs of
SUT1. The amino acid sequences encoded by these two genes
are particularly highly homologous to Sut1p. We refer to
CAGL0I04246g and CAGL0L09383g as CgSUT1 and CgSUT2,
respectively. In the current model, we show that the expression of
CgSUT1, but not CgSUT2, was significantly increased
by fluconazole in C. glabrata (Table III).
Up-regulated expression of the genes
involved in sterol uptake and biosynthesis in Candida glabrata erg1
mutant
To further assess whether ergosterol depletion
affects the genes involved in sterol uptake and sterol synthesis in
C. glabrata, we used DNA microarray to analyze gene
expression in a C. glabrata erg1 mutant with defective
ergosterol biosynthesis. Transcriptional profiling of the
microarrays revealed 35 genes up-regulated and 4 genes
down-regulated in the C. glabrata erg1 mutant CgTn201S
compared to the parental wild-type strain Cg1660. Particularly,
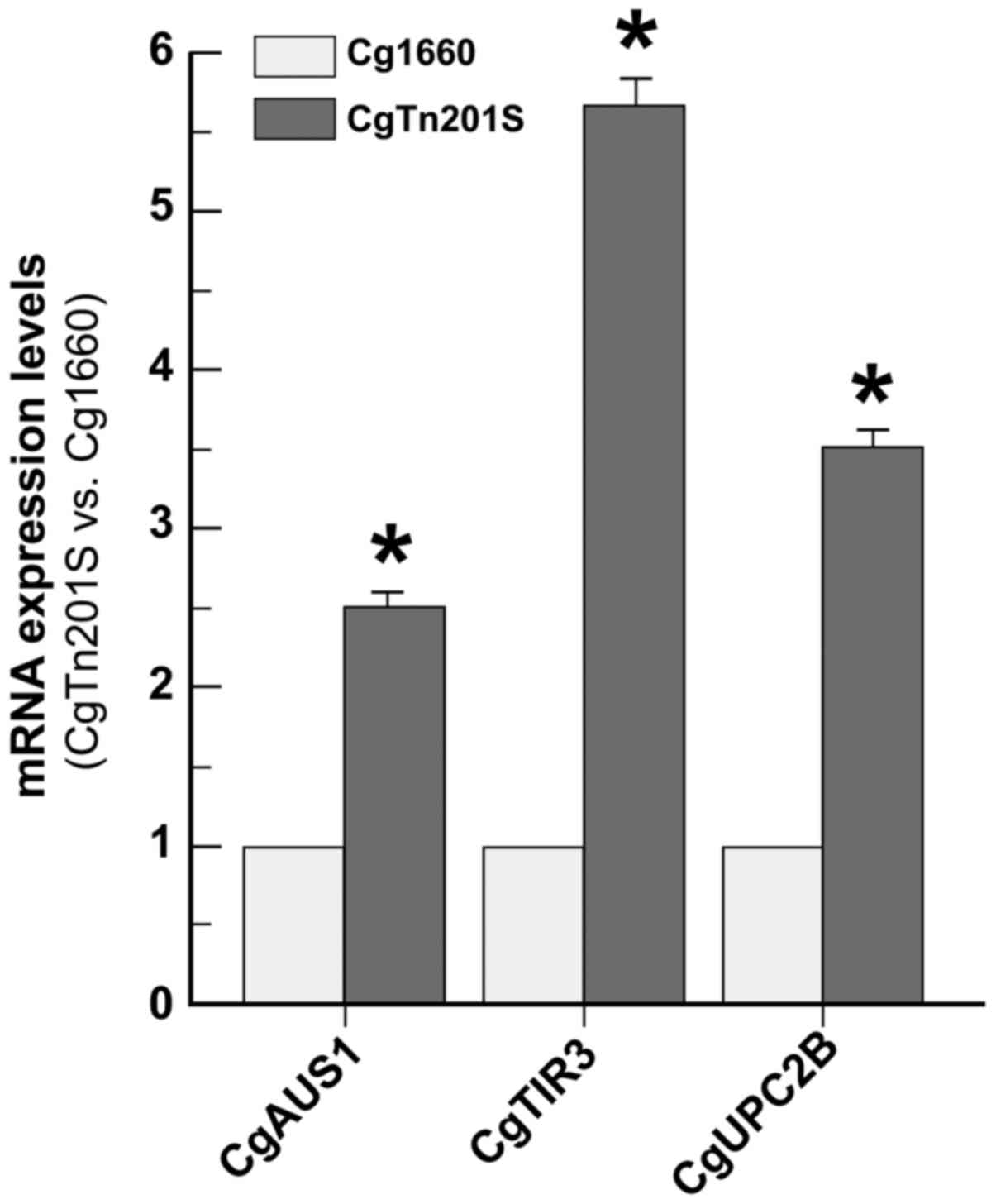
C. glabrata erg1 mutant had 2.5-fold up-regulation of
CgAUS1 mRNA, 5.6-fold up-regulation of CgTIR3 mRNA,
and 3.5-fold up-regulation of CgUPC2B mRNA over the
wild-type cells (Fig. 2). To a
lesser extent, we also found that the up-regulation of CgERG2,
CgERG3, CgERG5, CgERG6, CgERG7, CgERG10, CgERG11, and
CgERG29 in the C. glabrata erg1 mutant cells ranged
from 1.5-to 2.8-fold mRNA expression among these genes (Table IV), which is similar to our data
from the C. glabrata cells in which ergosterol synthesis was
inhibited by fluconazole (Table
III). Additionally, we show a 3-fold increase in mRNA
expression of HES1/KES1, which is involved in
ergosterol biosynthesis, oxysterol binding, and sterol transport
(Table IV). These data highlight
the notion that blocking ergosterol synthetic pathway leading to
ergosterol depletion causes increased sterol uptake activity and
sterol biosynthesis in C. glabrata.
 | Table IV.Candida glabrata genes up- and
down-regulated ≥1.5-fold in response to CgERG1 disruption
(Cgerg1 mutation) in Cg1660 host. |
Table IV.
Candida glabrata genes up- and
down-regulated ≥1.5-fold in response to CgERG1 disruption
(Cgerg1 mutation) in Cg1660 host.
| C. glabrata
designation | S.
cerevisiae homologue | Description | Fold
expressiona |
|---|
| Upregulated
genes |
|
|
|
|
CAGL0F01419g | AUS1 | ATP-binding
cassette transporter involved in sterol uptake | 2.5006 |
|
CAGL0C03872g | TIR3/YIL011w | Putative GPI-linked
cell wall protein involved in sterol uptake | 5.6547 |
|
CAGL0F07865g | UPC2B | Transcription
factor transcriptionally regulates ergosterol biosynthetic genes
and sterol transporter genes | 3.4979 |
|
CAGL0L10714g | ERG2 | C-8 sterol
isomerase participates in ergosterol biosynthesis | 2.0136 |
|
CAGL0F01793g | ERG3 | C-5 sterol
desaturase participates in ergosterol biosynthesis | 2.7363 |
|
CAGL0M07656g | ERG5 | C-22 sterol
desaturase participates in ergosterol biosynthesis | 2.3415 |
|
CAGL0H04653g | ERG6 | C-24 sterol
methyltransferase participates in ergosterol biosynthesis | 2.1176 |
|
CAGL0J10824g | ERG7 | Lanosterol synthase
participates in ergosterol biosynthesis | 2.31 |
|
CAGL0L12364g | ERG10/POT14 | Acetyl-CoA
C-acetyltransferase participates in ergosterol biosynthesis | 2.2113 |
|
CAGL0E04334g | ERG11 | Lanosterol
14-a-demethylase involved in ergosterol biosynthesis | 2.8477 |
|
CAGL0K03927g | ERG29 | Roles in ergosterol
biosynthesis, mitochondrion organization, etc | 1.5044 |
|
CAGL0J03916g | HES1/KES1 | Roles in ergosterol
biosynthesis, oxysterol binding, sterol transport, etc | 2.9779 |
|
CAGL0J00297g | YHR045w | Possible roles in
iron, amino acid, and carbohydrate metabolisms | 1.5889 |
|
CAGL0A01089g | YPL272c | Alcohol
O-acetyltransferase with role in alcohol metabolic process | 4.0299 |
|
CAGL0I01408g | CYC1 | Cytochrome-c
isoform 1 involved in mitochondrial electron transport | 2.6164 |
|
CAGL0L03828g | CYB5 | Cytochrome b5
involved in oxidation-reduction process | 1.5483 |
|
CAGL0K10868g | CTA1 | Catalase A involved
in cellular response to oxidative stress | 2.7464 |
|
CAGL0K12100g | HEM13 | Coproporphyrinogen
III oxidase involved in heme biosynthesis | 2.3296 |
|
CAGL0G03905g | ISA1 | Regulation of ROS
metabolic process and biotin biosynthetic process | 1.5708 |
|
CAGL0H04851g | PPZ1 | Protein phosphatase
Z involved in cation homeostasis and cell wall integrity | 1.633 |
|
CAGL0L07480g | NRG1/NRG2 | Transcription
factor activity, sequence-specific DNA binding activity | 1.613 |
|
CAGL0F01485g | TIR4 | Putative GPI-linked
cell wall mannoprotein of the Srp1p/Tip1p family | 5.4181 |
|
CAGL0H09614g | TIR1 | Putative GPI-linked
cell wall protein | 6.3371 |
|
CAGL0C00110g | FLO1 | Member of the FLO
family of cell wall flocculation proteins | 1.9697 |
|
CAGL0M04125g | YNL320w | Roles in cell
polarity, endoplasmic reticulum, mitochondrion, etc | 1.5089 |
|
CAGL0E00187g | YMR317w | Putative
adhesin-like protein; belongs to adhesin cluster IV | 4.3814 |
|
CAGL0G04499g | SET4/YJL105w | Ortholog of S.
cerevisiae: SET4 and YJL105w | 1.9778 |
|
CAGL0F08965g | MSC7/YHR039c | Roles in cytosol,
endoplasmic reticulum, nucleus localization, etc | 1.6906 |
|
CAGL0C00209g | DAN1/YJR151c | Putative
adhesin-like cell wall protein; predicted GPI-anchor | 4.6475 |
|
CAGL0G10175g | DAN1/YJR151c | Adhesin-like
protein; predicted GPI anchor | 4.7887 |
|
CAGL0K04279g | SCM4/YGR049w | Ortholog(s) have
mitochondrial outer membrane localization | 1.5542 |
| Downregulated
genes |
|
|
|
|
CAGL0H03971g | YCP4/PST2 | Roles in cellular
response to oxidative stress, mitochondrion, etc | 1.5484 |
|
CAGL0M05995g | PET10 | Roles in lipid
metabolism, respiratory growth, and ATP/ADP exchange | 1.5975 |
|
CAGL0G05566g | FMP45 | In mitochondria;
role in ascospore formation, cellular response to drug, etc | 1.5862 |
|
CAGL0L10142g | RSB1/YOR049c | Sphingolipid
transporter; involved in fatty acid transport | 2.1631 |
Besides the genes for sterol uptake and synthesis,
23 other genes demonstrated dominant and reproducible expression in
C. glabrata erg1 mutant with the average level of the six
microarrays ranging from 1.5-fold to 6.3-fold greater than the
wild-type strain (Table IV).
These differentially expressed genes were associated with multiple
different cellular metabolisms and biological processes, such as
iron, amino acid, and carbohydrate metabolisms, alcohol metabolism,
reactive oxygen species (ROS) metabolism, heme biosynthesis, biotin
biosynthesis, mitochondrion organization, mitochondrial electron
transport, oxidation-reduction process, cation homeostasis, cell
wall integrity, and in-cell polarity, endoplasmic reticulum,
mitochondrion, nucleus localization, and so forth (Table IV). Furthermore, mRNA expression
of 4 genes was down-regulated 1.5- to 2.2-fold lower than the
parental wild-type cells (Table
IV). These down-regulated genes were enriched in several
cellular and metabolic processes, including lipid metabolism,
sphingolipid and fatty acid transport, respiratory growth, ATP/ADP
exchange, and cellular response to oxidative stress (Table IV).
Potential coordination between sterol
biosynthesis and sterol uptake in Candida glabrata erg1 mutant
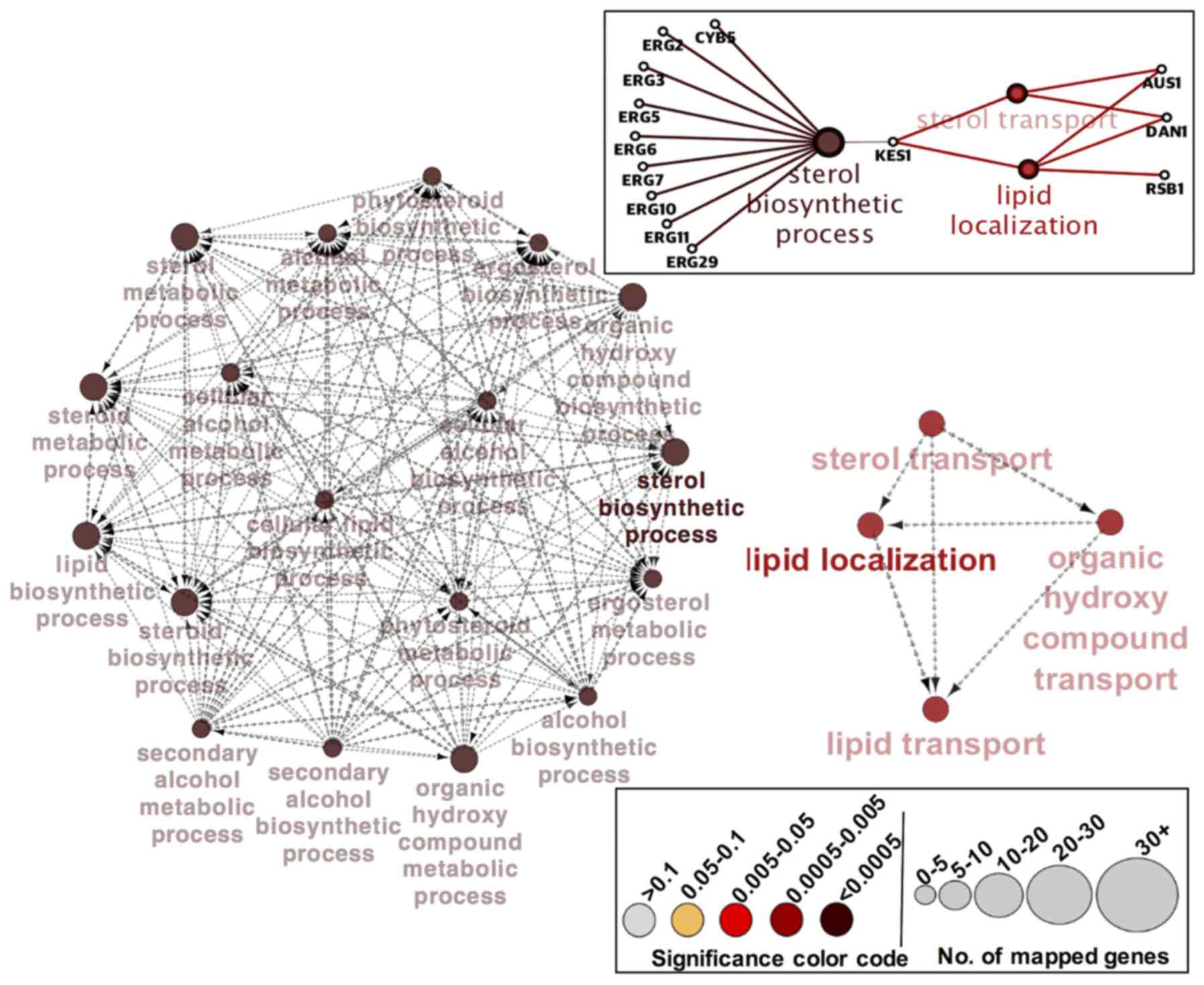
To further explore the functional themes of the
above 35 differentially expressed genes in C. glabrata erg1
mutant, we used Cytoscape, a bioinformatics software for
visualizing functional and molecular interaction networks. The
functional interaction networks in Fig. 3 support our interpretation that the
ergosterol biosynthetic process is the major biological function
affected in C. glabrata mutant strain lacking ERG1.
To compensate for the defective sterol biosynthetic pathway in the
cell, and overcome the imbalance in cellular levels of sterol,
cells increase the sterol uptake machinery and ergosterol
biosynthesis processes in order to survive under azole and
environmental stress. As shown in Fig.
3, nine genes (ERG2, ERG3, ERG5, ERG6, ERG7, ERG10, ERG11,
ERG29, and CYB5) comprise the largest network cluster
and contribute towards the enrichment of the ‘sterol biosynthetic
process.’ While three genes (AUS1, DAN1, and RSB1) in
the other smaller functional network cluster are enriched with
‘lipid localization’ in combination with GO-term ‘sterol transport’
(Fig. 3). KES1 and
HES1 are the genes bridging the two major clusters by
establishing a potential coordination between the ‘sterol
biosynthetic process’ and ‘lipid localization/sterol transport’
(Fig. 3). The enriched processes
identified with the Cytoscape software and GO database using the
differentially expressed genes in C. glabrata erg1 mutant
are shown in Table V.
 | Table V.Functional enrichment with associated
genes identified by using Cytoscape and GO database. |
Table V.
Functional enrichment with associated
genes identified by using Cytoscape and GO database.
| Enriched
process | Associated
gene | P-value |
|---|
| Sterol biosynthetic
process | CYB5, ERG2,
ERG3, ERG5, ERG6, ERG7, ERG10, ERG11, ERG29 | <0.0005 |
| Lipid
localization | AUS1, DAN1,
RSB1 | 0.0005 to
0.005 |
Candida glabrata aus1 deletant is
defective in sterol uptake and has greater susceptibility to azoles
under hypoxic conditions
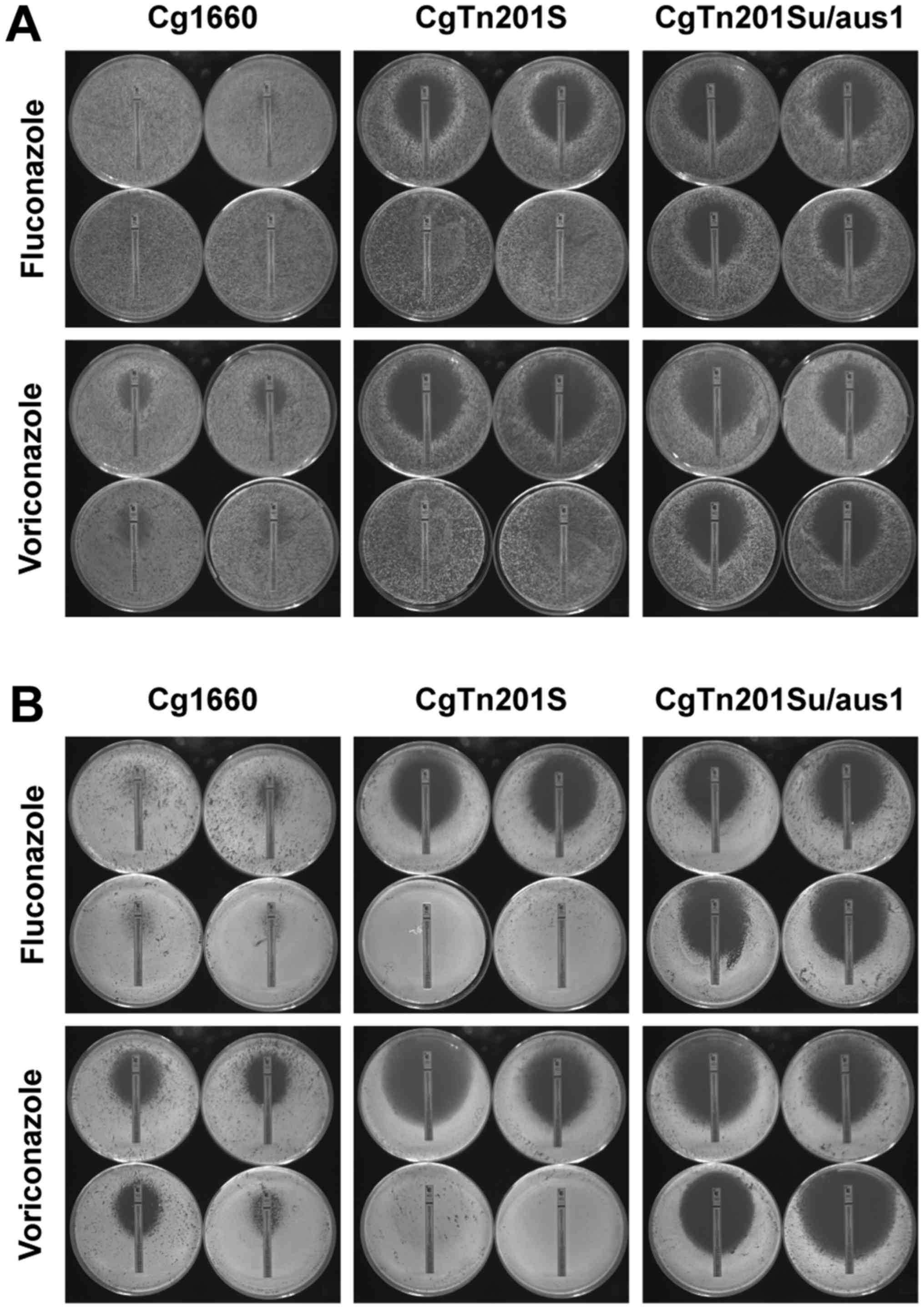
Lastly, we validated whether C. glabrata
cells accumulate sterols from the environment through the Aus1p
transporter and whether sterol uptake confers resistance to azoles
in C. glabrata. To distinguish the uptake of exogenous
sterol from that of endogenous ergosterol biosynthesis, we
generated a C. glabrata erg1/aus1Δ double mutant strain and
used the C. glabrata erg1 mutant strain CgTn201S and the
wild-type strain Cg1660 as the controls. As seen in Fig. 4A and B and Tables VI and VII, in the presence of exogenous
cholesterol or ergosterol, C. glabrata erg1 mutant cells
showed much lower susceptibility to fluconazole and voriconazole
compared to those cells in the absence of exogenous sterols,
suggesting that C. glabrata erg1 mutant cells are capable of
accumulating sterols from the medium through the wild-type sterol
transporter CgAus1p; but not through ergosterol synthesis because
the cells were defective in ergosterol synthetic pathway. In
contrast, in the presence of exogenous cholesterol or ergosterol,
the C. glabrata erg1/aus1Δ double mutant shows much higher
susceptibility to fluconazole and voriconazole than the C.
glabrata erg1 mutant, indicating that C. glabrata
erg1Δaus1Δ double mutant cells are not able to uptake and
synthesize sterols due to defective conditions in both the CgAus1p
transporter and ergosterol biosynthesis in the cells. We also
tested whether the loss of CgAus1p in C. glabrata sensitizes
the pathogen to polyene amphotericin B and the echinocandins
anidulafungin and caspofungin, and our E-test results reveal that
C. glabrata cells lacking CgAus1p do not show altered
susceptibility to non-azole antifungals in the presence or absence
of exogenous sterols (data not shown). These observations confirm
the findings of Zavrel et al (20) that CgAus1p is responsible for
taking up exogenous cholesterol or ergosterol in C. glabrata
under hypoxic stress when sterol synthesis is either absent or
insufficient, and further suggest that sterol uptake plays an
important role in the development of azole resistance in yeast
under ergosterol starvation conditions, such as under azole drug
pressure or when defective in sterol biosynthesis.
 | Table VI.Inhibitory effect of azoles in
Candida glabrata wild-type, erg1 mutant, and
erg1/aus1∆ double mutant on MIN agar medium with sterol
supplement under hypoxic condition. |
Table VI.
Inhibitory effect of azoles in
Candida glabrata wild-type, erg1 mutant, and
erg1/aus1∆ double mutant on MIN agar medium with sterol
supplement under hypoxic condition.
|
| E-test on MIN agar
medium |
|---|
|
|
|
|---|
| Straina | Detergent (0.5%
EtOH and 0.5% Tween-80) | Cholesterol (20
µg/ml) | Ergosterol (20
µg/ml) | Fluconazole MIC
(µg/ml) | Voriconazole MIC
(µg/ml) |
|---|
| Cg1660 | − | − | − | >256 | 4.0 |
| Cg1660 | + | − | − | >256 | 4.0 |
| Cg1660 | + | + | − | >256 | 12 |
| Cg1660 | + | − | + | >256 | 12 |
| CgTn201S | − | − | − | 0.50 | 0.064 |
| CgTn201S | + | − | − | 0.50 | 0.064 |
| CgTn201S | + | + | − | >256 | >32 |
| CgTn201S | + | − | + | >256 | >32 |
|
CgTn201Su/Cgaus1 | − | − | − | 0.50 | 0.064 |
|
CgTn201Su/Cgaus1 | + | − | − | 0.50 | 0.064 |
|
CgTn201Su/Cgaus1 | + | + | − | 0.50 | 0.064 |
|
CgTn201Su/Cgaus1 | + | − | + | 0.50 | 0.064 |
 | Table VII.Inhibitory effect of azoles in
Candida glabrata wild-type, erg1 mutant, and
erg1/aus1∆ double mutant on YEPG agar medium with sterol
supplement under hypoxic condition. |
Table VII.
Inhibitory effect of azoles in
Candida glabrata wild-type, erg1 mutant, and
erg1/aus1∆ double mutant on YEPG agar medium with sterol
supplement under hypoxic condition.
|
| E-test on YEPG agar
medium |
|---|
|
|
|
|---|
| Straina | Detergent (0.5%
EtOH and 0.5% Tween-80) | Cholesterol (20
µg/ml) | Ergosterol (20
µg/ml) | Fluconazole MIC
(µg/ml) | Voriconazole MIC
(µg/ml) |
|---|
| Cg1660 | − | − | − | >256 | 1.0 |
| Cg1660 | + | − | − | >256 | 1.0 |
| Cg1660 | + | + | − | >256 | 1.0 |
| Cg1660 | + | − | + | >256 | 6.0 |
| CgTn201S | − | − | − | 1.0 | 0.064 |
| CgTn201S | + | − | − | 1.0 | 0.064 |
| CgTn201S | + | + | − | >256 | >32 |
| CgTn201S | + | − | + | >256 | >32 |
|
CgTn201Su/Cgaus1 | − | − | − | 1.0 | 0.064 |
|
CgTn201Su/Cgaus1 | + | − | − | 1.0 | 0.064 |
|
CgTn201Su/Cgaus1 | + | + | − | 1.0 | 0.064 |
|
CgTn201Su/Cgaus1 | + | − | + | 1.0 | 0.064 |
Discussion
Yeast develops strategies to grow and survive in
different unfavorable environments. In the present study, we
observed that C. glabrata acquires two protective mechanisms
to allow the pathogen to grow and survive under azole and hypoxic
stress: Through increasing endogenous sterol synthesis or through
importing exogenous sterols. Our data revealed that the expression
of both ergosterol biosynthesis and sterol metabolism regulator
genes, as well as sterol influx transporter genes, are
significantly increased in C. glabrata under fluconazole
stress. Likewise, the sterol influx transporter genes
(CgAUS1 and CgTIR3) and the ergosterol biosynthetic
genes (CgERG2, CgERG3, CgERG5, CgERG6, CgERG7, CgERG10,
CgERG11, and CgERG29) are markedly up-regulated in yeast
when ergosterol biosynthesis is suppressed either under hypoxia or
due to defective sterol synthesis. We also confirmed that
CgAUS1 in the cell is responsible for importing exogenous
cholesterol and ergosterol, thus allowing C. glabrata to
survive under low oxygen tension conditions or under azole
pressure. The presence of both in vivo suggests an
underlying mechanism for azole resistance in clinical practice.
Nakayama and colleagues show that CgAUS1
protects C. glabrata cells against azoles in the presence of
serum (21). The composition of
serum is complicated, and it includes many different molecules,
minerals, and nutrients other than cholesterol. It is possible
that, besides importing cholesterol via the sterol transporter
CgAUS1, C. glabrata cells may also take up other molecules
and nutrients from serum that are necessary to their survival
amidst azole treatment. In our experiments, as in those of Zavrel
et al (20), we did not use
serum but solubilized cholesterol and ergosterol using the
detergent Tween 80. This allowed for the incorporation of exogenous
sterol into the fungal cell. Our results, together with the data
discussed above, reinforce the understanding of how both enhanced
endogenous sterol synthesis and increased exogenous sterol uptake
by yeast are integral to conferring resistance to azole therapy in
fungal infections. Resistance to azole treatment not only limits
the usefulness of this class of drugs, it also drives the survival
of intrinsically low-susceptibility C. glabrata cells that
become increasingly resistant following prolonged treatment with
azole therapeutic agents.
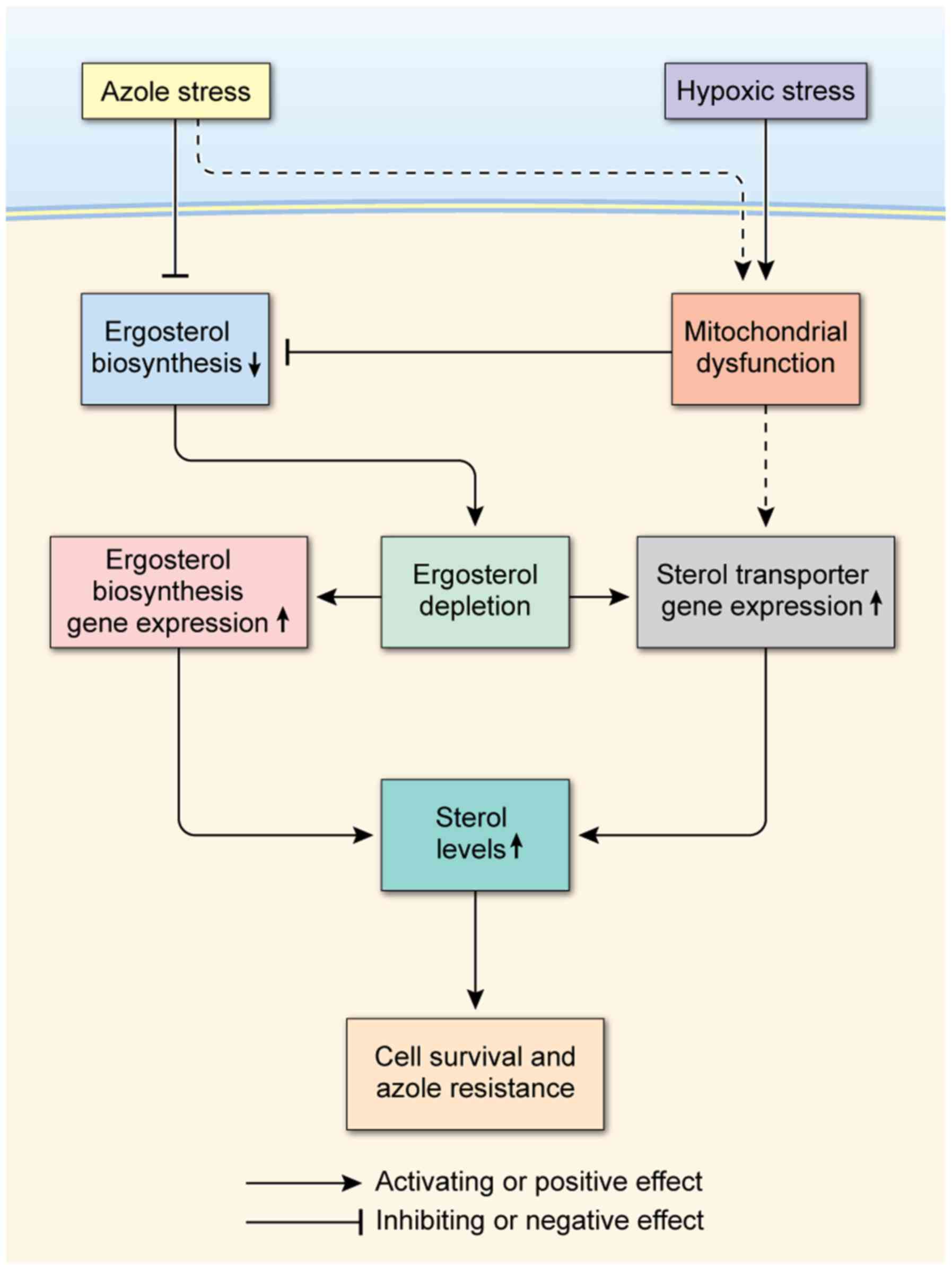
Combining our data in this study, we propose a
hypothetical model in which sterol uptake and sterol biosynthesis
act coordinately and collaboratively to mediate azole antifungal
resistance in C. glabrata under azole and hypoxic stress as
shown in Fig. 5. In this model,
azole and hypoxic stresses deplete ergosterol directly or
indirectly by inhibiting ergosterol biosynthesis. Hypoxia and
azoles cause mitochondrial dysfunction by depriving the
mitochondria of oxygen and inducing the accumulation of toxic
sterol-intermediates, ultimately leading to the reduction of
ergosterol biosynthesis. In response, ergosterol depletion triggers
up-regulation of the genes involved in sterol transport and
ergosterol biosynthesis, leading to increases in sterol levels.
Because both sterol uptake and sterol biosynthesis increase sterol
levels through distinct mechanisms, they both may function together
to maintain sterol levels and sustain cell growth. Such a
cooperative mechanism may serve to integrate the roles of sterol
transport and sterol biosynthesis culminating in cell survival,
which may underlie the mechanism of azole antifungal resistance in
C. glabrata.
Collectively, this study shows the up-regulation of
the genes involved in ergosterol biosynthesis and sterol transport
in C. glabrata cells in which sterol synthesis is defective
or is abrogated by fluconazole treatment. We also corroborate that
the sterol influx transporter CgAus1p imports exogenous cholesterol
or ergosterol, which contributes to the development of clinical
resistance to azole antifungals in C. glabrata. These
findings demonstrate that sterol uptake and sterol biosynthesis may
act coordinately and collaboratively to sustain growth and to
mediate antifungal resistance in C. glabrata through dynamic
gene expression in response to azole stress and environmental
challenges.
Acknowledgements
The authors would like to thank Ms. Cindy Clark for
carefully reviewing the manuscript.
Funding
This study was supported by the Intramural Research
Program of the National Institute of Allergy and Infectious
Diseases, National Institutes of Health.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
QQL conceived of the project, conducted the
studies, performed drug sensitivity assay, RT-qPCR and Southern
blot analysis, cloning of CgAUS1, construction of
Cgerg1 and Cgaus1 double mutant, as well as other
experiments, carried out the analysis and interpretation of data,
wrote the manuscript, and is the primary author of this paper. HFT
conceived the project, performed microarray hybridization and data
analysis, and critically reviewed the manuscript. AM performed
bioinformatics and statistical analysis. BAW, JAN, and YF analyzed
and interpreted data and edited the manuscript. JEB participated in
the design and coordination of the study, critically reviewed the
manuscript, and edited the final version of the paper. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pappas PG, Kauffman CA, Andes DR, Clancy
CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez
JA, Walsh TJ, et al: Executive summary: Clinical practice guideline
for the management of candidiasis: 2016 update by the infectious
diseases society of America. Clin Infect Dis. 62:409–417. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodrigues CF, Silva S and Henriques M:
Candida glabrata: A review of its features and resistance.
Eur J Clin Microbiol Infect Dis. 33:673–688. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inukai T, Nagi M, Morita A, Tanabe K,
Aoyama T, Miyazaki Y, Bard M and Nakayama H: The mannoprotein TIR3
(CAGL0C03872g) is required for sterol uptake in Candida
glabrata. Biochim Biophys Acta. 1851:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagi M, Nakayama H, Tanabe K, Bard M,
Aoyama T, Okano M, Higashi S, Ueno K, Chibana H, Niimi M, et al:
Transcription factors CgUPC2A and CgUPC2B regulate ergosterol
biosynthetic genes in Candida glabrata. Genes Cells.
16:80–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagi M, Tanabe K, Ueno K, Nakayama H,
Aoyama T, Chibana H, Yamagoe S, Umeyama T, Oura T, Ohno H, et al:
The Candida glabrata sterol scavenging mechanism, mediated
by the ATP-binding cassette transporter Aus1p, is regulated by iron
limitation. Mol Microbiol. 88:371–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silver PM, Oliver BG and White TC: Role of
Candida albicans transcription factor Upc2p in drug
resistance and sterol metabolism. Eukaryot Cell. 3:1391–1397. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacPherson S, Akache B, Weber S, De Deken
X, Raymond M and Turcotte B: Candida albicans zinc cluster
protein Upc2p confers resistance to antifungal drugs and is an
activator of ergosterol biosynthetic genes. Antimicrob Agents
Chemother. 49:1745–1752. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai HF, Bard M, Izumikawa K, Krol AA,
Sturm AM, Culbertson NT, Pierson CA and Bennett JE: Candida
glabrata erg1 mutant with increased sensitivity to azoles and
to low oxygen tension. Antimicrob Agents Chemother. 48:2483–2489.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bard M, Sturm AM, Pierson CA, Brown S,
Rogers KM, Nabinger S, Eckstein J, Barbuch R, Lees ND, Howell SA
and Hazen KC: Sterol uptake in Candida glabrata: Rescue of
sterol auxotrophic strains. Diagn Microbiol Infect Dis. 52:285–293.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flowers SA, Colón B, Whaley SG, Schuler MA
and Rogers PD: Contribution of clinically derived mutations in
ERG11 to azole resistance in Candida albicans. Antimicrob
Agents Chemother. 59:450–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li QQ, Skinner J and Bennett JE:
Evaluation of reference genes for real-time quantitative PCR
studies in Candida glabrata following azole treatment. BMC
Mol Biol. 13:222012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vermitsky JP, Earhart KD, Smith WL,
Homayouni R, Edlind TD and Rogers PD: Pdr1 regulates multidrug
resistance in Candida glabrata: Gene disruption and
genome-wide expression studies. Mol Microbiol. 61:704–722. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bindea G, Galon J and Mlecnik B: CluePedia
Cytoscape plugin: Pathway insights using integrated experimental
and in silico data. Bioinformatics. 29:661–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van den Hazel HB, Pichler H, do Valle
Matta MA, Leitner E, Goffeau A and Daum G: PDR16 and PDR17, two
homologous genes of Saccharomyces cerevisiae, affect lipid
biosynthesis and resistance to multiple drugs. J Biol Chem.
274:1934–1941. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marek M, Milles S, Schreiber G, Daleke DL,
Dittmar G, Herrmann A, Müller P and Pomorski TG: The yeast plasma
membrane ATP binding cassette (ABC) transporter Aus1: Purification,
characterization, and the effect of lipids on its activity. J Biol
Chem. 286:21835–21843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whaley SG, Caudle KE, Vermitsky JP,
Chadwick SG, Toner G, Barker KS, Gygax SE and Rogers PD: UPC2A is
required for high-level azole antifungal resistance in Candida
glabrata. Antimicrob Agents Chemother. 58:4543–4554. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ness F, Bourot S, Régnacq M, Spagnoli R,
Bergès T and Karst F: SUT1 is a putative Zn[II]2Cys6-transcription
factor whose upregulation enhances both sterol uptake and synthesis
in aerobically growing Saccharomyces cerevisiae cells. Eur J
Biochem. 268:1585–1595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zavrel M, Hoot SJ and White TC: Comparison
of sterol import under aerobic and anaerobic conditions in three
fungal species, Candida albicans, Candida glabrata, and
Saccharomyces cerevisiae. Eukaryot Cell. 12:725–738. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakayama H, Tanabe K, Bard M, Hodgson W,
Wu S, Takemori D, Aoyama T, Kumaraswami NS, Metzler L, Takano Y, et
al: The Candida glabrata putative sterol transporter gene
CgAUS1 protects cells against azoles in the presence of serum. J
Antimicrob Chemother. 60:1264–1272. 2007. View Article : Google Scholar : PubMed/NCBI
|