Introduction
Mast cells have been generally considered to be
specifically associated with allergic reactions. Increasing
functions of mast cells have been identified, which indicate their
relevance as effector cells and regulatory cells in innate and
adaptive immunity (1,2). However, the role of mast cells in the
immunopathogenesis of periodontitis remains to be elucidated.
Toll-like receptors (TLRs) are a type of recognition receptor with
pathogen-associated molecule patterns, and are important in innate
immune responses (3). As an
important member of the TLR family, TLR4 is a receptor found in the
lipopolysaccharide of cell walls in Gram-negative bacteria. Our
previous study revealed that the presence of mast cells and
degranulated mast cells were significantly increased in human
chronic periodontitis tissues, indicating that the recruitment and
degranulation of mast cells appeared to be associated with human
periodontal disease (4,5). However, at present, the association
between TLRs and periodontitis remain to be fully elucidated. In
the present study, toluidine blue and immunohistochemical stainings
were used to identify mast cells. However,
double-immunofluorescence was used for the identification of
tryptase-TLR4 double-positive mast cells in gingival tissues. The
aim of the present study was to examine the expression of TLR4 on
mast cells in the gingival tissues of patients with human chronic
periodontitis, to analyze the correlation between the expression of
TLR4 on mast cells and periodontitis severity, and to investigate
the potential roles of mast cell TLR4 in periodontitis using double
immunofluorescence staining.
Materials and methods
Study subjects and periodontitis case
selection
In the present study, subjects aged between 25–65
years who visited the Dental Clinic of the Affiliated Liwan
Stomatological Hospital of Jinan University (Guangzhou, China) were
recruited. The enrolled subjects consisted of 37 men (47±11.7 years
old) and 31 women (43±12.1 years old) and there were no significant
differences in gender or age between each exprimental group. All
subjects were free of diabetes or other systemic diseases; had not
received any antibiotic treatment within the last 6 months, had no
known drug or food allergy history, had not been pregnant or
received any contraception therapy, and had not received
periodontal surgery within the last year. The inclusion criteria
were based on the American Academy of Periodontology (4) guidelines for the classification of
periodontal disease and conditions in 1999 (6). All subjects signed informed consent.
The present study was approved by the ethics committee of the
Medical School of Jinan University.
Grouping
The patients were divided into three groups, as
follows: i) Healthy control group (n=20), comprising individuals
with no periodontal pockets, no periodontal attachment loss, no
alveolar bone absorption on X-ray examination, no gingival
inflammation; ii) mild periodontitis group, comprising patients
with moderate periodontitis, gingival inflammation, bleeding on
probing, pocket depths ≤4 mm, attachment loss ≤2 mm and alveolar
bone loss of no more than one third of the root length on X-ray
examination, with or without halitosis; iii) severe periodontal
group, comprising patients diagnosed with severe periodontitis
presented with pocket depths >6 mm, attachment loss ≥5 mm,
alveolar bone loss of more than half or even two thirds of the root
length on X-ray examination, furcation involvement on multiple
teeth, loose teeth, periodontitis and possibly periodontal abscess
presence.
Tissue specimens
The 23 tissue specimens from the mild periodontitis
group, were obtained from those who required crown lengthening
surgery; the 25 specimens in the severe periodontitis group were
obtained from teeth with no reservation value, or with poor
prognosis; the 20 clinically healthy specimens were harvested from
the membranes of premolars extracted for orthodontic reasons.
Tissue specimen collection, processing
and observation
The gingival biopsy specimens were fixed in 4%
neutral buffered formalin for a minimum of 48 h prior to being cut
into 5-µm-thick buccolingual serial sections, stained with
hematoxylin and eosin, and observed under an optical microscope.
The observation and grading of gingival inflammation was perfored
by two pathologists in a blinded-manner. The average grade was then
determined. The grade of inflammatory severity of the periodontal
tissues was based on the scale provided by Huang et al
(7) as follows: 0, no
inflammation; 1, mild inflammation comprising focal inflammatory
cell infiltrate; 2, moderate inflammation comprising focal or
patchy inflammatory cell infiltrate; 3, severe inflammation
comprising diffusive infiltration of inflammatory cells in gingival
tissues.
SP immunohistochemical staining of
TLR4 and processing
The SP immunohistochemical staining was performed to
stain the gingival specimen sections. The dilution of I-anti-TLR4
antibody (cat. no. BA1717, Wuhan Boster Biological Technology,
Ltd., Wuhan, China) was 1:100. The sections were stained using the
SP immunohistochemical staining test kit (cat. no. PV9002; OriGene
Technologies, Beijing, China), applied the DAB (DAB staining kit;
OriGene Technologies) as the chromogenic reagents and substituted
the I-antibody with PBS as the negative control. The specimens were
then counterstained with hematoxylin. In the results of the
histochemical staining, a positive signal was shown as small, brown
granules, located in the nucleus or the cytoplasm. The number of
positive cells was counted in each section. The total number of
cells and number of positive cells in each of the tissue sections
were counted in five representative visual fields (magnification,
×400) and the positive ratio (%) of the cells was determined. The
positive ratio was the average percentage of postive cells in the
total cells.
SP immunohistochemical staining of
mast cells and TLR4, and processing
Double immunofluorescence staining was performed to
stain the gingival specimen sections. The dilution of the I
anti-tryptase antibody (cat. no. ab134932, Abcam, Cambridge, MA,
USA) was 1:200. The dilution of the I anti-TLR4 antibody (cat. no.
TA309486, OriGene Technologies) was 1:100. The dilution of goat
anti-mouse IgG (H+L) Alex Flour® 555 (cat. no. 4409;
Cell Signaling Technology, Inc., Dallas, TX, USA), goat anti rabbit
IgG (H+L) Alex Flour® 488 (cat. no. 4412; Cell Signaling
Technology, Inc.) was 1:200. Immunofluorescence positive signals
showed as green fluorescence on mast cells and red fluorescence on
TLR4, which were located in the nucleus or cytoplasm. When TLR4 and
mast cells were superimposed in a single image, staining appeared
yellow. Two pathologists observed the sections under an
immunofluorescence microscope. They counted the numbers of
TLR4-positive mast cells and calculated the average. The
TLR4-positive mast cells were counted in five visual fields
(magnification, ×400), which showed the highest yellow
fluorescence.
Statistical analysis
All data are presented as the mean ± standard
deviation and were analyzed using SPSS 13.0 (SPSS, Inc., Chicago,
IL, USA. Differences in gingival histopathological examination data
between different groups were analyzed using Wilcoxon's signed rank
test. The expression of TLR4 and the number of TLR4-positive mast
cells in multiple groups were compared using completely randomized
one-way analysis of variance. Multiple comparisons were compared
using the least significant difference test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Histopathological analysis
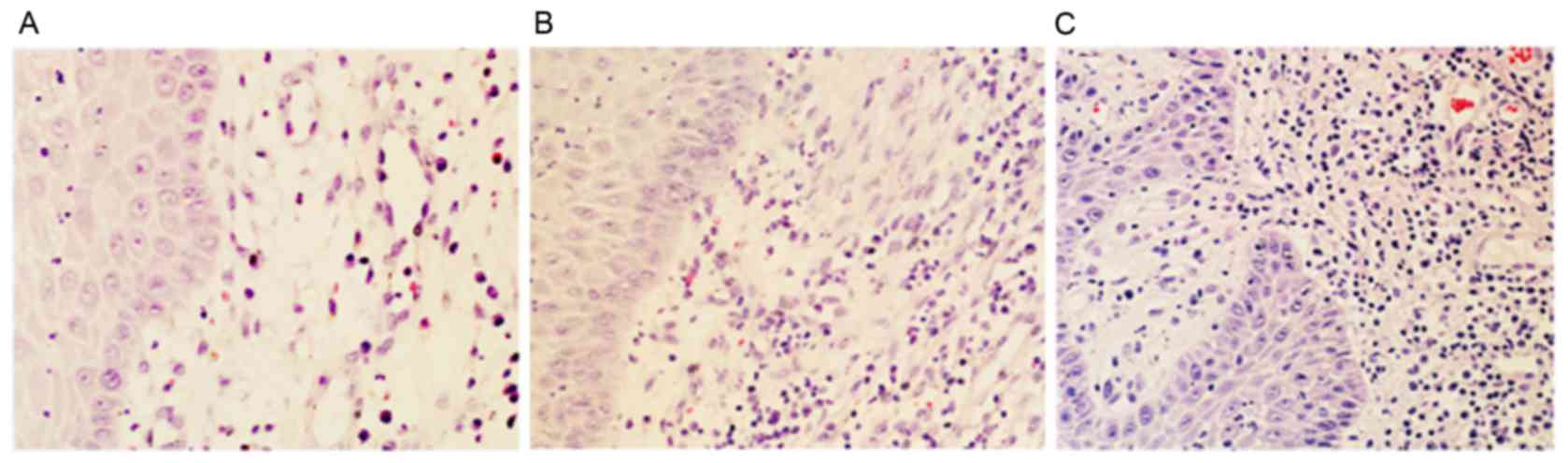
The histological results of the gingival tissues
from different groups are shown in Fig. 1. No marked inflammatory
infiltration was found in the healthy gingival tissues (Fig. 1A). The specimens in the mild
periodontitis group showed moderate inflammatory cell infiltration
(Fig. 1B). The speciments in the
severe periodontitis group manifested with intense inflammatory
cell infiltration, predominantly comprising lymphocytes, plasma
cells and mast cells, and with a discrete presence of macrophage
cells and polymorphonuclear cells (Fig. 1C). The severity of the grading of
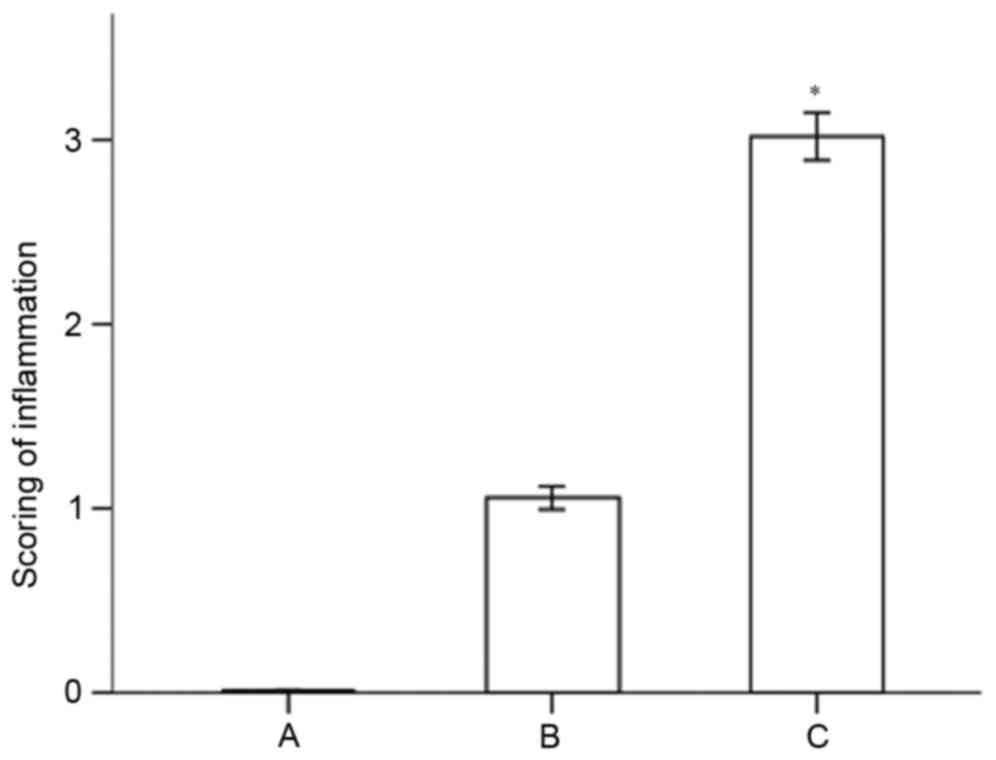
inflammation in the specimens from the different groups is shown in
Fig. 2. The results indicated that
the histological score of the periodontal tissues in the severe
periodontitis group was significantly higher, compared with that in
the moderate periodontitis group (P<0.05).
Gingival TLR4 immunohistochemical
staining
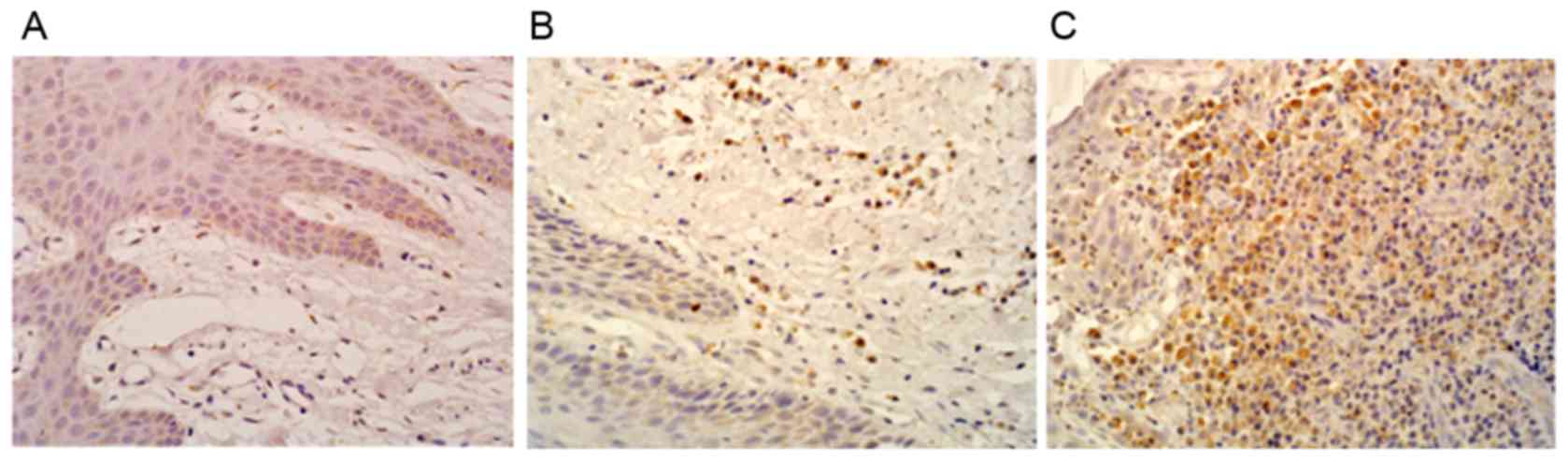
The results of the gingival TLR4 immunohistochemical
staining are shown in Fig. 3. The
positive signal was observed as small brown granules, located in
the nucleus or the cytoplasm. Only a few scattered TLR4 were
observed in the healthy gingival tissues (Fig. 3A), whereas a higher number of
TLR4-positive cells were observed in the mild periodontitis group
(Fig. 3B) and the expression of
TLR4 was even higher in the severe periodontitis group (Fig. 3C). The TLR4-positive cell ratios in
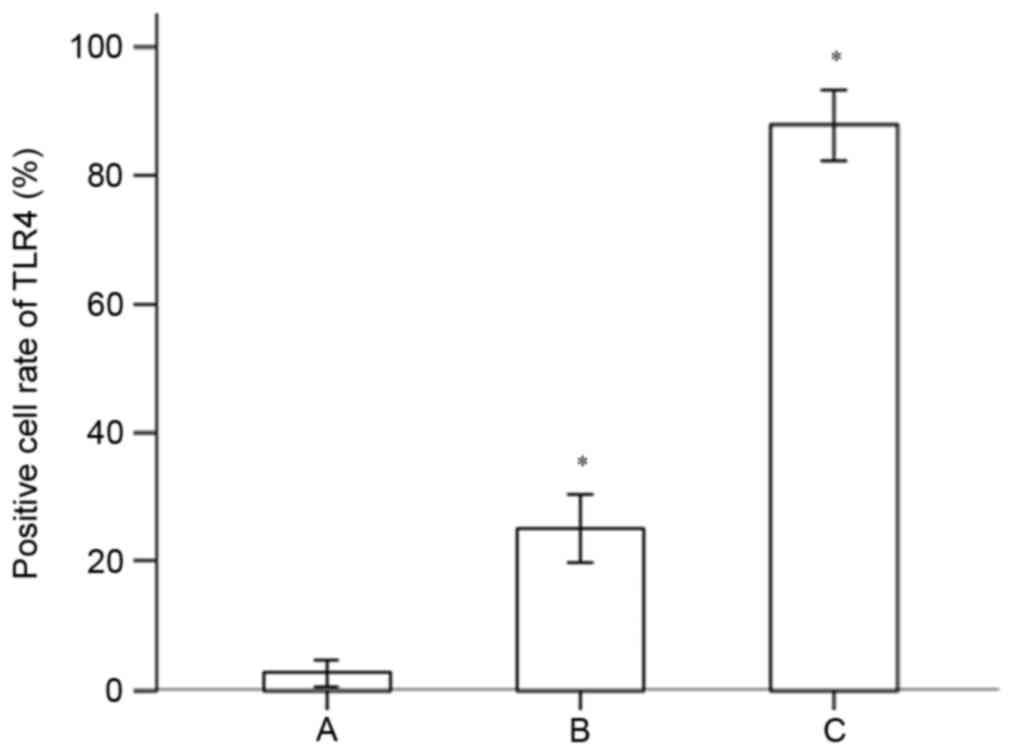
the gingival specimens from the different groups are shown in
Fig. 4. The results indicated that
the expression of TLR4 in the gingival tissues of the chronic
periodontitis group was significantly higher, compared with that of
the normal control group (P<0.05). The expression of TLR4 in the
gingival tissues of the advanced chronic periodontitis was
significantly higher, compared with that of the mild chronic
periodontitis group (P<0.05).
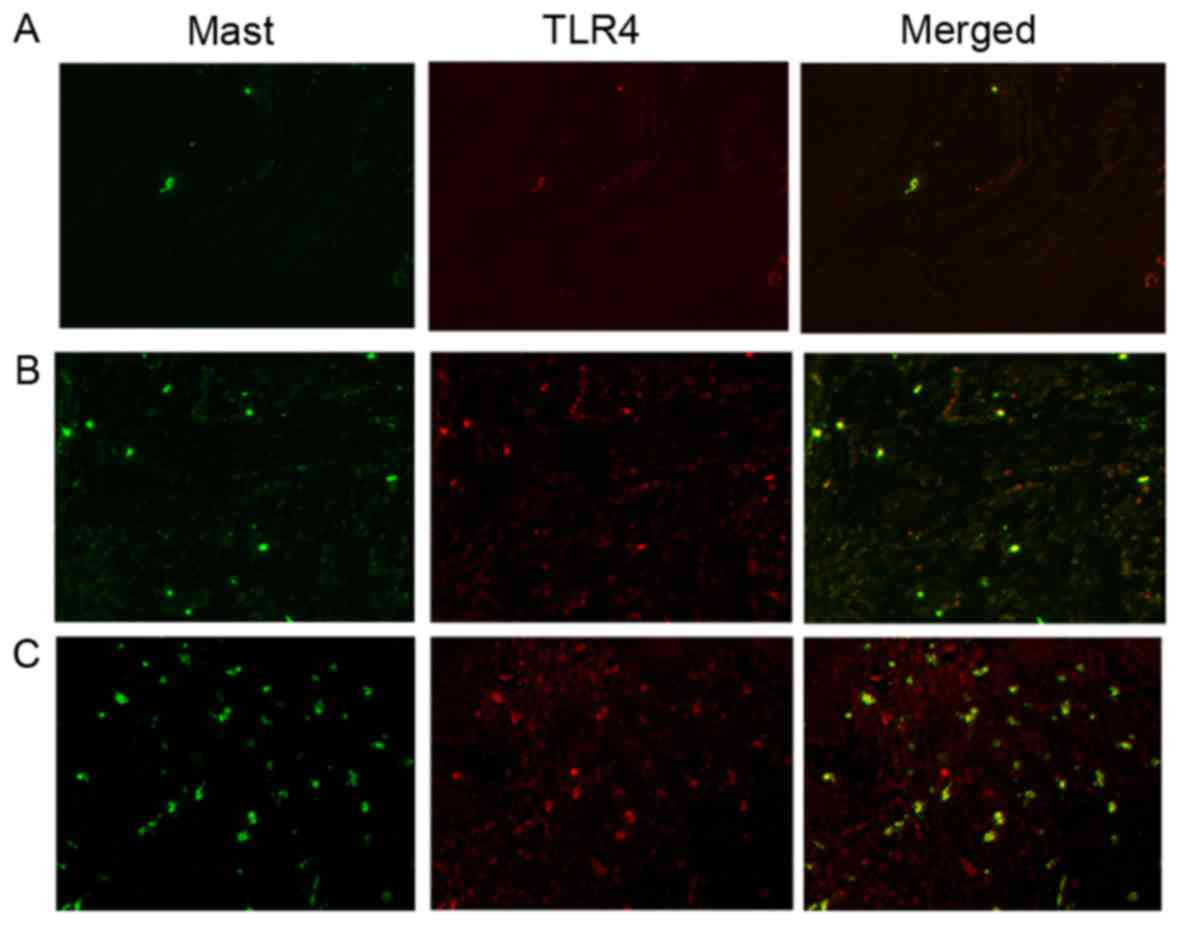
Immunohistochemical staining of mast
cells and TLR4
Immunofluorescence-positive signals were shown as
green fluorescence of mast cells and red fluorescence of TLR4,
which was located in the nucleus or cytoplasm. When the TLR4 and
mast cells were superimposed on each other, it appeared as yellow
fluorescence. The immunohistochemical staining of the mast cells
and TLR4 is shown in Fig. 5. A few
scattered mast cells with TLR4 expression were observed in the
healthy gingival tissues (Fig.
5A), whereas the number of mast cells and TLR4-positive mast
cells in the gingival tissues was significantly increased in the
mild periodontitis group (Fig.
5B). An increased number of mast cells and TLR4-positive mast
cells were observed in the severe periodontitis gingival tissues
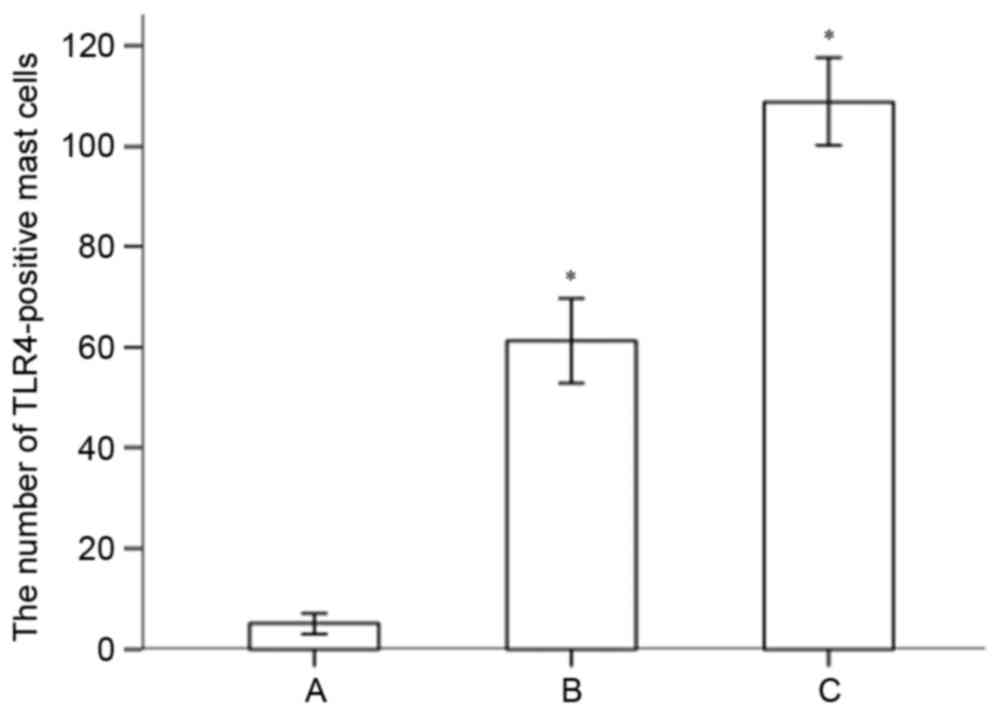
(Fig. 5C). The numbers of
TLR4-positive mast cells in the gingival specimens of the different
groups are shown in Fig. 6. The
expression of TLR4 on mast cells in the gingival tissues of the
chronic periodontitis group was significantly higher, compared with
that in the normal control (P<0.05). The number of TLR4-positive
mast cells in the advanced chronic periodontitis group was
significantly higher, compared with that in the mild chronic
periodontitis group (P<0.05).
Discussion
TLRs are widespread biological pattern recognition
receptors in mammalian cells. It can identify several conserved
microbial surface structures, including bacterial
lipopolysaccharides, peptidoglycan, lipoprotein, bacterial DNA and
double-stranded RNA. By upregulating costimulatory molecule
expression on the surface of antigen presenting cells (APCs), TLR
signals, in addition to the inflammatory cytokines secreted by
APCs, can induce T and B lymphocytes to differentiate into effector
T and B lymphocytes, and adjust the adaptive immune response
(8,9). A previous study found that
neutrophils, lymphocytes, macrophages and monocytes all exihibited
TLR expression, and these TLRs were able identify different
pathogens, rapidly activate innate immunity following pathogen
invasion, trigger the inflammatory response and kill invading
pathogens (10). As an important
member of the TLR family, TLR4 is present in the lipopolysaccharide
of Gram-negative bacteria walls (11). Periodontal diseases predominantly
result from chronic pathological injuries, which are imposed on the
periodontal tissues by gram-negative anaerobic bacteria, including
Porphyromonas gingivalis. Studies have shown that the
expression levels of TLR2, TLR3, TLR4 and TLR9 are significantly
higher in tissues of patients with chronic periodontitis, compared
with levels in the tissues of control individuals (12,13).
TLR2 polymorphisms were found to be closely associated with
aggressive periodontitis in a Japanese population (14). TLR2 and TLR4 were also shown to be
important in mediating osteoclast differentiation of alveolar bone
in response to dyslipidemia (15).
In periodontitis, a previous study found that the expression of
TLR4 was identified predominantly in connective tissues, and that
no TLR4 was expressed in the relatively healthy gingival tissues of
patients with periodontitis or in healthy gingival tissues,
indicating that TLR4 was involved in the periodontitis immune
response (16). These results
suggested that the expression level of TLR4 on human gingival
fibroblasts reflects the extent of inflammation in the gingival
tissues (17). Using
immunohistochemistry, the present study found that the expression
of TLR4 in the gingival tissue of patients with chronic
periodontitis was significantly higher, compared with that in the
tissue of the normal control group. The expression of TLR4 in the
gingival tissues of patients with advanced chronic periodontitis
was significantly higher, compared with that in tissues of patients
with mild chronic periodontitis, indicating a close association
betwen the expression of TLR4 and the severity of chronic
periodontitis.
In previous years, the unique immune properties of
mast cells have been increasingly acknowledged. In addition to the
fact that mast cells are important in allergy and autoimmunity,
mast cells are key effector and regulatory cells, and can
initialize a rapid and continues immune response, which signifies
their importance in innate and adaptive immunity (18,19).
The TLRs on the mast cell surface can identify a wide range of
pathogen products and damage-associated molecular patterns
(20). Mast cells activation can
release pre-formed mediators such as histamine from their granules,
as well as release de novo synthesized lipid mediators, cytokines,
and chemokines. On receiving the corresponding stimulus, mast cells
instantly release mediators, and initialize the innate immune
response. Subsequently, they induce the inflammatory response or
are involved in defence responses (21,22).
Bacterial components and inflammatory cytokines can regulate mast
cell expression of TLR4. Following activation by TLRs, mast cells
are not only engaged in the process of antibacterial defence and
the inflamatory response, but they also affect the process of
allergic reactions (23). A
previous study found that activated TLRs promoted the generation of
Th1-type cytokines and induced Th1 cell polarization (24). The TLR4 receptors on the surface of
human and mouse mast cells can synergistically regulate the mast
cell signaling pathway mediated by FcRI, and can promote mast cell
degranulation and the release of Th2 cytokines (25). The present study showed that TLR4
was expressed on mast cells in human gingival tissues from
individuals with chronic periodontitis. The results showed that the
expression of TLR4 on mast cells in the gingival tissues of chronic
periodontitis was significantly higher, compared with that in mild
chronic periodontitis, suggesting that mast cell TLR4 in human
chronic periodontitis may be involved in the gingival inflamatiory
reaction and is important in disease progression. However the
mechanisms underlying how mast cells regulate periodontal disease
via the identification of pathogenic bacterial antigens by TLR4
remain to be elucidated, and how mast cells regulate the onset and
progression of periodontal disease through TLR4 signal transduction
requires further investigation.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science
Foundation of China (grant no. 81702020) and by Guangdong
Provincial Science Foundation (grant no. 2015A030310107).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author contributions
HS designed the present study and wrote the
manuscript. HB and DQ performed experiments and analyzed data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All subjects signed informed consent. The present
study was approved by the Ethics Committee of the Medical School of
Jinan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shea-Donohue T, Stiltz J, Zhao A and
Notari L: Mast cells. Curr Gastroenterol Rep. 12:349–357. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Alcaide P, Liu L, Sun J, He A,
Luscinskas FW and Shi GP: Regulation of endothelial cell adhesion
molecule expression by mast cells, macrophages, and neutrophils.
PLoS One. 6:e145252011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mackern Oberti JP, Breser ML, Nuñez N,
Maccioni M, Rodríguez N, Wantia N, Ertl T, Miethke T and Rivero VE:
Chemokine response induced by Chlamydia trachomatis in prostate
derived CD45+ and CD45-cells. Reproduction. 142:427–437. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan Q, LVFL, Huang B, Cheng Y, Liu M and
Huang SG: Pathophysiological significance of mast cell
degranulation in periodontitis. J Pract Stomatol. 28:316–319.
2012.(In Chinese).
|
|
5
|
Huang S, Lu F, Chen Y, Huang B and Liu M:
Mast cell degranulation in human periodontitis. J Periodontol.
84:248–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Armitage GC: Development of a
classification system for periodontal diseases and conditions. Ann
Periodontol. 4:1–6. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S, Lu F, Zhang Z, Yang X and Chen Y:
The role of psychological stress-induced hypoxia-inducible
factor-1α in rat experimental periodontitis. J Periodontol.
82:934–941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwasaki A and Medzhitov R: Toll-like
receptor control of the adaptive immune responses. Nat Immunol.
5:987–995. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Wu X, Chen ZC, Liao JD, Gu JY, et
al: Comparison of Toll-like receptor-expressing cells from cord
blood and adult peripheral blood granulocytes.
Zhongguobinglishenglizazhi. 29:676–681. 2013.(In Chinese).
|
|
10
|
Hedayat M, Netea MG and Rezaei N:
Targeting of Toll-like receptors: A decade of progress in combating
infectious diseases. Lancet Infect Dis. 11:702–712. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei JH, Mo BW and Huang JW: Effect of
TLR4/NF-κB on airway inflammation and airway remodeling in rat
model of asthma. Chin J Pathophysiol. 27:962–967. 2011.(In
Chinese).
|
|
12
|
Wara-aswapati N, Chayasadom A, Surarit R,
Pitiphat W, Boch JA, Nagasawa T, Ishikawa I and Izumi Y: Induction
of toll-like receptor expression by Porphyromonas
gingivalis. J Periodontol. 84:1010–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buduneli N, Özçaka Ö and Nalbantsoy A:
Salivary and plasma levels of Toll-like receptor 2 and Toll-like
receptor 4 in chronic periodontitis. J Periodontol. 82:878–884.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi M, Chen Z, Watanabe K, Kobayashi
H, Nakajima T, Kimura A and Izumi Y: Toll-like receptor 2 gene
polymorphisms associated with aggressive periodontitis in Japanese.
Open Dent J. 5:190–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomofuji T, Ekuni D, Azuma T, Irie K, Endo
Y, Kasuyama K, Yoneda T and Morita M: Involvement of toll-like
receptor 2 and 4 in association between dyslipidemia and osteoclast
differentiation in apolipoprotein E deficient rat periodontium.
Lipids Health Dis. 12:12013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Oliveira NF, Andia DC, Planello AC,
Pasetto S, Marques MR, Nociti FH Jr, Line SR and De Souza AP: TLR2
and TLR4 gene promoter methylation status during chronic
periodontitis. J Clin Periodontol. 38:975–983. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang PL, Oido-Mori M, Fujii T, Kowashi Y,
Kikuchi M, Suetsugu Y, Tanaka J, Azuma Y, Shinohara M and Ohura K:
Heterogeneous expression of Toll-like receptor 4 and downregulation
of Toll-like receptor 4 expression on human gingival fibroblasts by
Porphyromonas gingivalis lipopolysaccharide. Biochem Biophys
Res Commun. 288:863–867. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bischoff SC and Krämer S: Human mast
cells, bacteria, and intestinal immunity. Immunol Rev. 217:329–337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dietrich N, Rohde M, Geffers R, Kröger A,
Hauser H, Weiss S and Gekara NO: Mast cells elicit proinflammatory
but not type I interferon responses upon activation of TLRs by
bacteria. Proc Natl Acad Sci USA. 107:pp. 8748–8753. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dawicki W and Marshall JS: New and
emerging roles for mast cells in host defense. Curr Opin Immunol.
19:31–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang Y and Huang T: Effects of mast cell
degranulation on plaque stabilization in apolipoprotein E-Knock out
mice. Chin J Arteriosclerosis. 26:1987–1988. 2010.(In Chinese).
|
|
22
|
Shelburne CP and Abraham SN: The mast cell
in innate and adaptive immunity. Adv Exp Med Biol. 716:162–185.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pietrzak A, Wierzbicki M, Wiktorska M and
Brzezińska-Błaszczyk E: Surface TLR2 and TLR4 expression on mature
rat mast cells can be affected by some bacterial components and
proinflammatory cytokines. Mediators Inflamm. 2011:4274732011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaisho T and Akira S: Toll-like receptor
function and signaling. J Allergy Clin Immunol. 117:979–988. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Novak N, Bieber T and Peng WM: The
immunoglobulin E-Toll-like receptor network. Int Arch Allergy
Immunol. 151:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|