Introduction
Acute lung injury (ALI) is one of the clinically
common emergency and severe diseases, and the infection is the
major cause of the disease (1)
G-bacillus infection causes acute lung injury (ALI), which is
primarily because lipopolysaccharide (LPS) activated cells release
a large amount of inflammatory factors (1). Studying LPS signal pathways and its
blocking effect has important theoretical and practical
significance to help understand the occurrence mechanism of ALI and
could help to identify new targets of ALI treatment (2).
Gram-negative bacterial infection is the primary
cause of the acute lung/acute respiratory distress syndrome
(ALI/ARDS) (3). LPS, as the main
pathogenic composition of Gram-negative bacteria, activates the
nuclear factor-κB (NF-κB) and/or mitogen-activated protein kinase
signaling molecules under the action of the receptor and regulatory
proteins through the signal transduction system, resulting in the
expression of various inflammatory factors (4). Such cascade amplification effect can
cause the systemic inflammatory response syndrome (SIRS) and
compensatory anti-inflammatory response syndrome and the excessive
SIRS will develop into the multiple organ dysfunction syndromes,
revealing ALI/ARDS in the lungs (5).
ALI and ARDS is one of the major diseases that
causes human death (6). ALI
primarily manifests in the many neutrophils of the lungs, affecting
production of inflammatory mediators and lung epithelial injury
(7). The host receptor recognizes
the LPS, which is the most important first step to stimulate the
cell signaling cascade (7). LPS
can stimulate and activate a variety of cells and combine it with
the CD14 receptor on the surface of the target cells. Toll-like
receptor 4 (TLR4) is the proximal trans-membrane receptor of the
LPS/CD14 complex, acting downstream of CD14, and transmitting the
LPS signal (8). Following the
pathogenic microorganisms and endogenous antigens are recognized by
TLR4 on the cell surface, and the NF-κB is activated through the
MyD88-dependent or MyD88-independent signal transduction pathways,
inducing the generation and release of tumor necrosis factor
(TNF)-α, interleukin (IL)-1, cyclooxygenase 2, intercellular
adhesion molecule-1 and other cell factors and chemical factors,
thus leading to the neutrophil infiltration, microvascular
endothelial cell injury and protein liquid leakage (9). It has been proven that CD14 and TLR4
are necessary for lipopolysaccharides to activate the immune signal
transduction pathways and activate NF-κB, while the activation of
the latter is the common method of inflammatory reactions (10).
Heat shock protein (HSP) is a kind of stress
protein, as well as an endogenous protective substance. HSP70 is a
major HSP family which is highly abundant in many organisms and
markedly expressed following cell stress (11). In addition, studies have indicated
that Rheum officinale serves a protective role for
ALI-induced LPS, which can inhibit nitric oxide (NO) generation and
inducible NO synthase activity, and reduce the activity of
phospholipase A2 and platelet activating factor, thus protecting
the lungs and reducing the effect of lung injury LPS-induced ALI
(12,13).
Dioscin, a saponin, is a naturally occurring steroid
found in plants (Fig. 1). As the
important raw material for the synthetic steroid hormone drugs and
steroidal contraceptives, dioscin is generally used for the
production of pregnenolone, progesterone, cortisol and other drugs
(14). In the past few decades,
the pharmacological effects of dioscin have been thoroughly studied
(15,16). Dioscin has an obvious antitumor
effect, and it also has the function of regulating blood-lipid,
anti-platelet aggregation and choleresis promotion, which is an
important drug for the treatment of cardiovascular disease,
encephalitis, skin diseases and tumors (16). Therefore, the aim of the present
paper was to investigate the effects of dioscin against ALI and its
possible mechanisms.
Materials and methods
Animal models
All animal protocols were approved by the Animal
Care and Use Committee of the Zhongshan Hospital of Xiamen
University (Xiamen, China). All experiments were conducted in
accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals. Male C57BL/6J mice
(8-weeks-old; 20–22 g; n=46) were purchased from Animal
Experimental Center of Xiamen University (Xiamen, China) and were
maintained in a laminar-flow housing apparatus under controlled
temperature (22–24°C), humidity and a 12 h light/dark regimen. Mice
had free access to food and water.
Experimental design and LPS-induced
ALI model
All mice were randomly assigned to five groups: Sham
(n=6), LPS model (n=10), 20 mg/kg dioscin (n=10), 40 mg/kg dioscin
(n=10) and 60 mg/kg dioscin (n=10). Mice were injected with 5 mg/kg
LPS to induce lung injury (intrathoracic injection). Mice in the
sham group were given PBS without LPS. Mice were treated with
dioscin (20, 40 and 60 mg/kg) following LPS-induced lung injury.
Left lung tissue samples measured using an electronic scale as wet
weight (W) and heated to 70°C for 48 h to determine the dry weight
(D). The water content of lung tissue was calculated with the W/D
weight ratio. The left lung was lavaged with 0.5 ml sterile saline
and 2 ml bronchoalveolar lavage fluid (BALF) was instilled. The
BALF in the respiratory system was collected to detect total
protein levels.
Hematoxylin and eosin staining
Right lung tissue samples were washed with ice-cold
PBS and were fixed in 4% paraformaldehyde (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) for 24 h and embedded in
paraffin. Then, the paraffin-embedded tissues samples were sliced
into 5 µm sections onto glass slides and stained with hematoxylin
and eosin (Beyotime Institute of Biotechnology, Haimen, China).
Tissues were imaged using a laser scanning confocal microscope
(Nikon Eclipse TE2000-U, Nikon Corporation, Tokyo, Japan). ALI
score was divided: 0=normal; 1=mild; 2=moderate; 3=severe; and
calculated for a total ALI score (17).
Isolation of alveolar macrophages
Lung tissue was lavaged with 1 ml of sterile PBS
through an intratracheal catheter and BALF was collected. BALF was
centrifuged at 1,000 × g for 10 min at 4°C and pelleted cells were
resuspended and cultured in a 60 mm culture dish in RPMI1640
supplemented with 10% fetal bovine serum, 1 mmol/l glutamine, 10
mmol/l 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid at 37°C
for 4 h. The cells adhering to the bottom of dish were washed twice
using PBS and total number of alveolar macrophages in BALF was
calculated using a cell counting chamber.
Quantification of indicators using
enzyme-linked immunosorbent assay (ELISA) kit
Mice were anaesthetized with 35 mg/kg pentobarbital
sodium and the venous blood of every mouse was collected from the
eye socket. Serum was collected following centrifugation at 10,000
× g for 10 min at 4°C. IL-1β (cat no. E-EL-M0037c), IL-6 (cat no.
E-EL-M0044c), TNF-α (cat no. E-EL-M0049c), NF-κB (cat no.
E-EL-M0838c), myeloperoxidase (cat no. E-EL-H1964c), interferon-γ
(cat no. E-CL-M0046c) and ICAM-1 (cat no. E-CL-M0445c) activities
were determined using a commercially available mouse ELISA kits
(Elabscience, Wuhan, China).
Western blotting assay
Lung tissue samples were collected from eye socket
under the condition of anesthesia and washed with ice-cold PBS.
Lung tissue samples (50 mg) were cut into pieces and immediately
lysed using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology). Protein content was determined using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology).
Proteins (50 µg) were subjected to 10% SDS-PAGE and then
transferred to a nitrocellulose membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membrane was blocked with 5% nonfat
milk in TBS with 0.1% Tween-20 for 1 h at 37°C and was incubated
with anti-COX-2 (cat no. sc-7951; 1:500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-TLR4 (cat no. sc-10741, 1:500; Santa
Cruz Biotechnology, Inc.), anti-MyD88 (cat no. sc-11356, 1:500;
Santa Cruz Biotechnology, Inc.), anti-NF-κB (cat no. sc-109, 1:500;
Santa Cruz Biotechnology, Inc.), anti-HSP70 (cat no. sc-59570,
1:500; Santa Cruz Biotechnology, Inc.) and anti-GAPDH (cat no.
E-AB-20079, 1:2,000; Elabscience) overnight at 4°C. Following three
washes, the membranes were incubated with goat anti-rabbit or mouse
IgG secondary antibody conjugated with horseradish peroxidase (cat
nos. sc-2004 or sc-2005; 1:5,000; Santa Cruz Biotechnology, Inc.)
at room temperature for 1 h. Membranes were visualized with
enhanced chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.)
and bands were quantified with Image Lab software (version 3.0;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All values are presented as mean ± standard error of
the mean. Differences were analyzed by one-way analysis of variance
with Tukey's post-hoc test, or by the unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significantly
difference.
Results
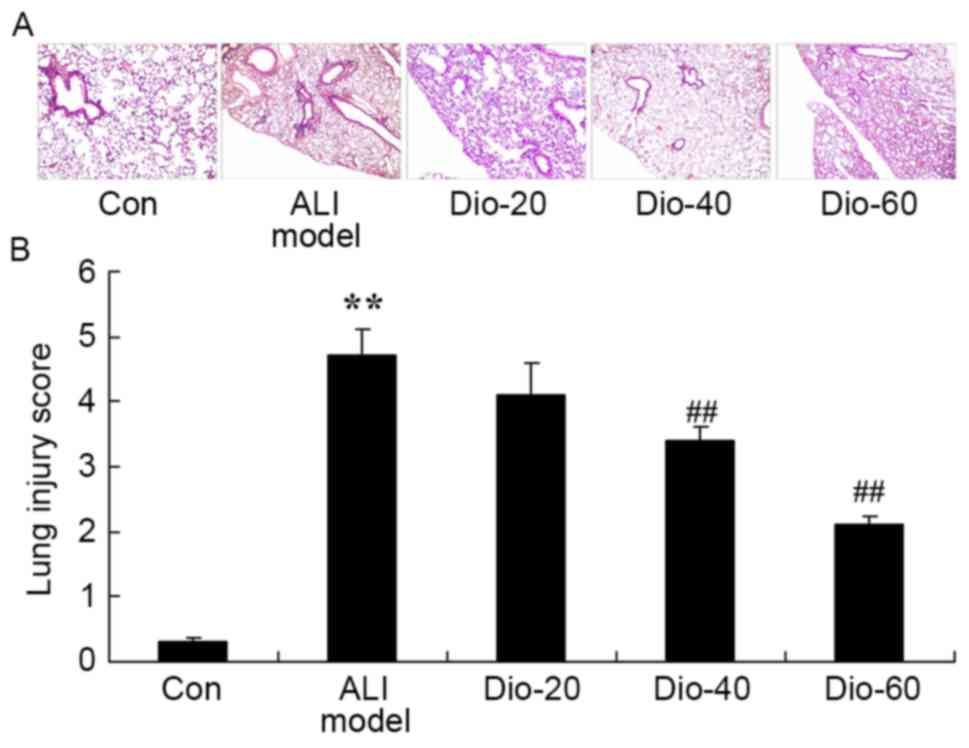
Dioscin decreases lung injury score in
ALI rats
As presented in Fig.
2, there was a significant increase in lung injury score of in
the ALI rat model group, compared with the control group. Under
these conditions, treatment with dioscin (40 and 60 mg/kg)
significantly inhibited the ALI-induced lung injury score in ALI
rats, compared with the ALI rat model group (Fig. 2).
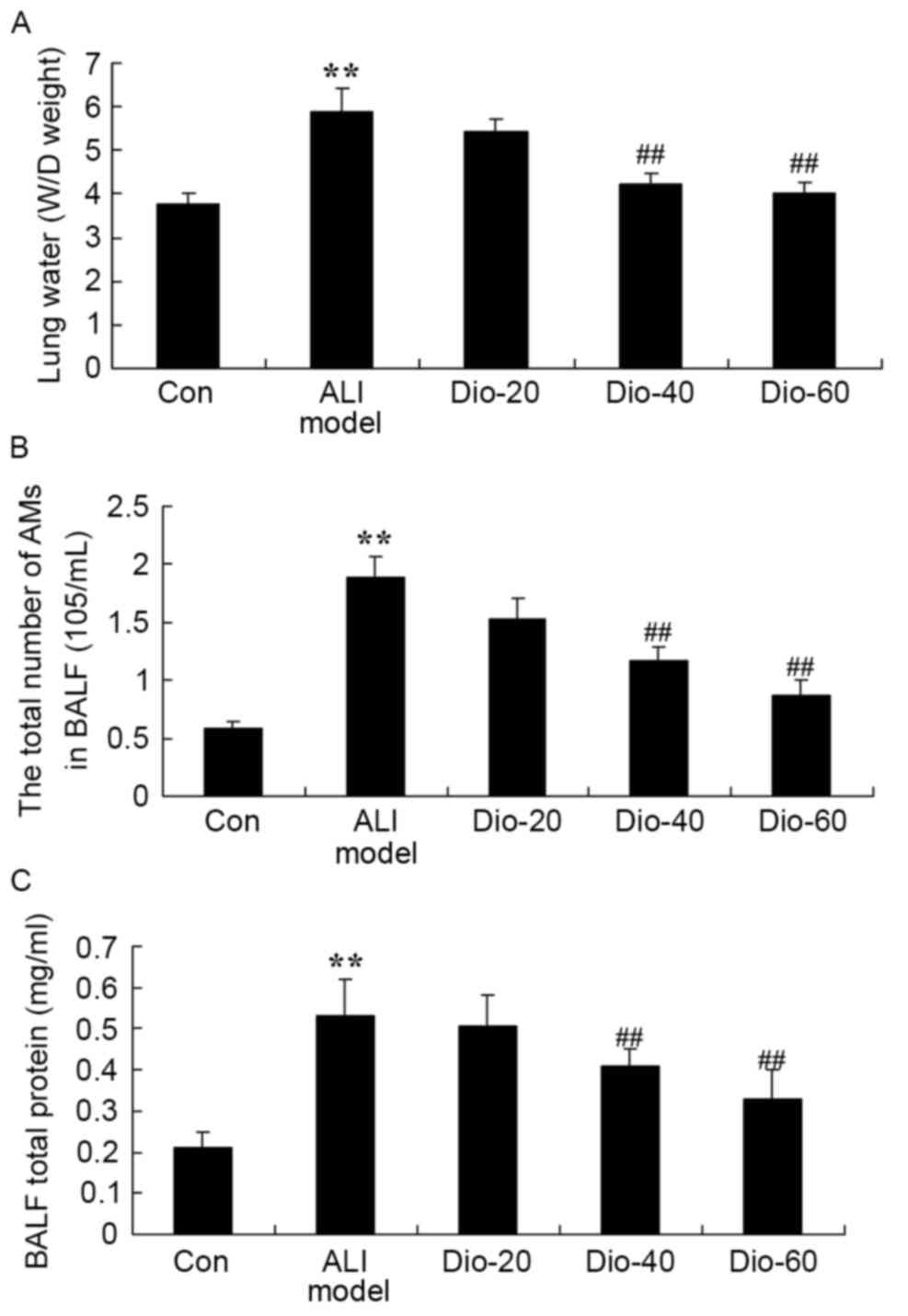
Dioscin decreases total number of
alveolar macrophages, water content of lung and total protein
concentration in ALI rats
Importantly, there were significant increases in
total number of alveolar macrophages, water content of lung and
total protein concentration in ALI rats, compared with the control
group (Fig. 3). The rat in
dioscin-treated (40 and 60 mg/kg) groups demonstrated a significant
reduction of these changes in lung tissue samples of ALI model rats
(Fig. 3).
Dioscin decreases the activity levels
of IL-1B, IL-6, TNF-α and NF-κB in ALI rats
To determine whether the anti-inflammation effect of
dioscin in ALI rats, IL-1B, IL-6, TNF-α and NF-κB activity levels
were measured in the current study. ALI significantly enhanced
IL-1B, IL-6, TNF-α and NF-κB activity levels in ALI rats, compared
with the control group (Fig. 4).
Treatment with 60 and 40 mg/kg dioscin significantly suppressed the
ALI-induced IL-1B, IL-6, TNF-α and NF-κB activity levels in ALI
rats, compared with the ALI model rat group (Fig. 4).
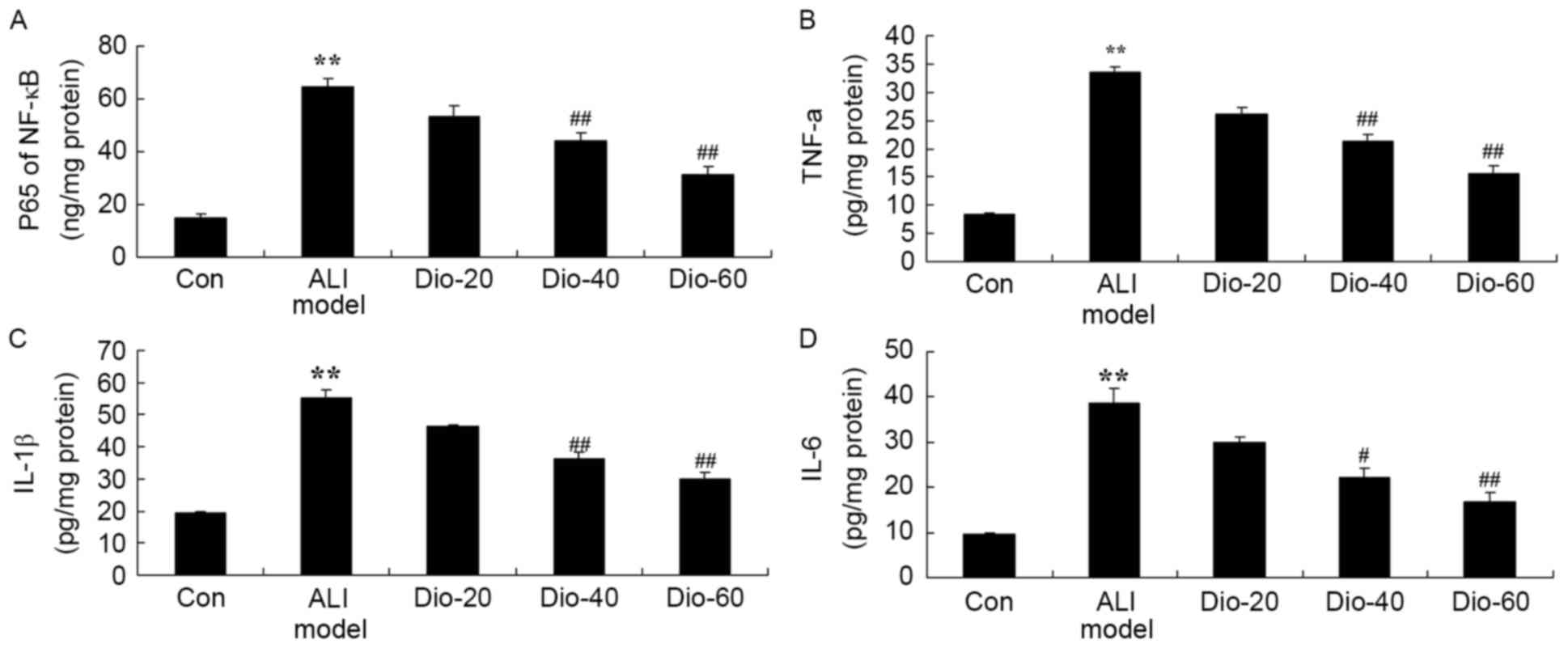 | Figure 4.Dioscin prevents the activity levels
of NF-κB, TNF-α, IL-1B and IL-6 in ALI rats. Dioscin prevents the
activity levels of (A) NF-κB, (B) TNF-α, (C) IL-1B and (D) IL-6 in
ALI rats, compared with the ALI model group. Con, control group;
Dio-20, 20 mg/kg dioscin group; Dio-40, 40 mg/kg dioscin group;
Dio-60, 60 mg/kg dioscin group. **P<0.01 vs. control group;
##P<0.01 vs. ALI model group. IL, interleukin; TNF-α,
tumor necrosis factor-α; NF-κB, nuclear factor-κB; ALI, acute lung
injury. |
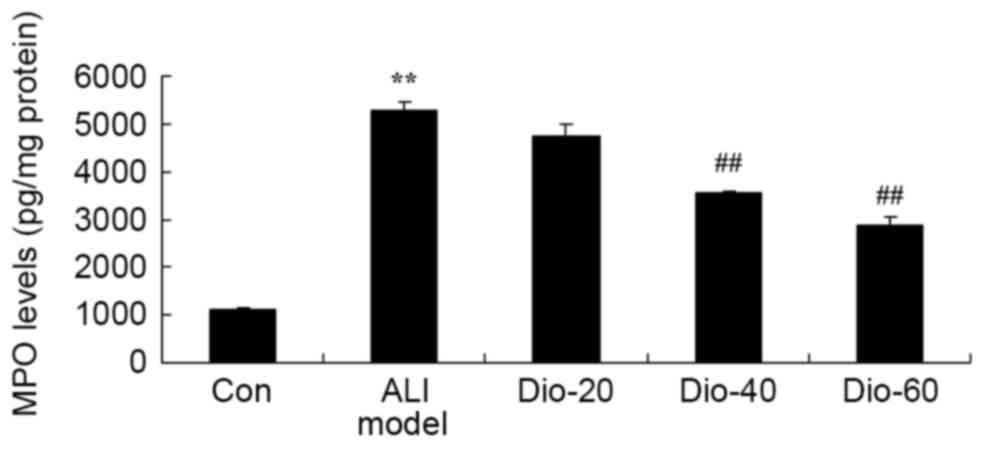
Dioscin decreases MPO level in ALI
rats
The authors determined the anti-inflammatory effects
of dioscin in ALI rats by measuring MPO level. As presented in
Fig. 5, the MPO level in all ALI
rats was significantly induced, compared with the control group.
However, compared with the ALI model group, treatment with 40 and
60 mg/kg dioscin was significantly different (Fig. 5).
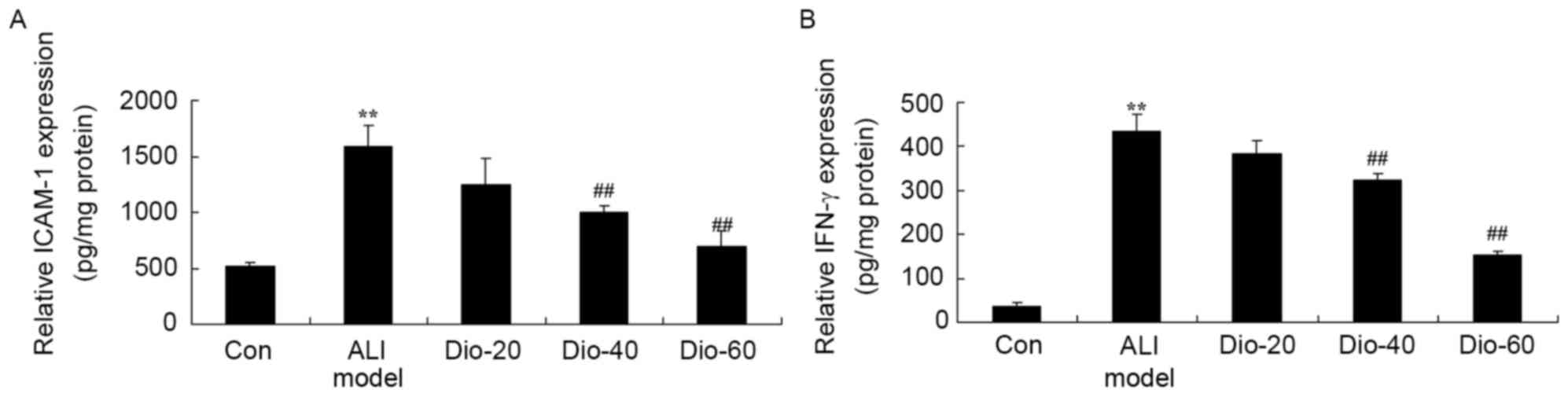
Dioscin decreases the IFN-γ and ICAM-1
levels in ALI rats
To further determine whether the anti-inflammatory
effect of dioscin in ALI rats, IFN-γ and TGF-β1 levels in ALI rat
were measured. As presented in Fig.
6, the activation of IFN-γ and ICAM-1 activity levels in ALI
rats was increased compared with the control group. In 40 and 60
mg/kg dioscin-treated groups, IFN-γ and ICAM-1 activity levels were
significantly decreased in ALI rats, compared with ALI model rat
group (Fig. 6).
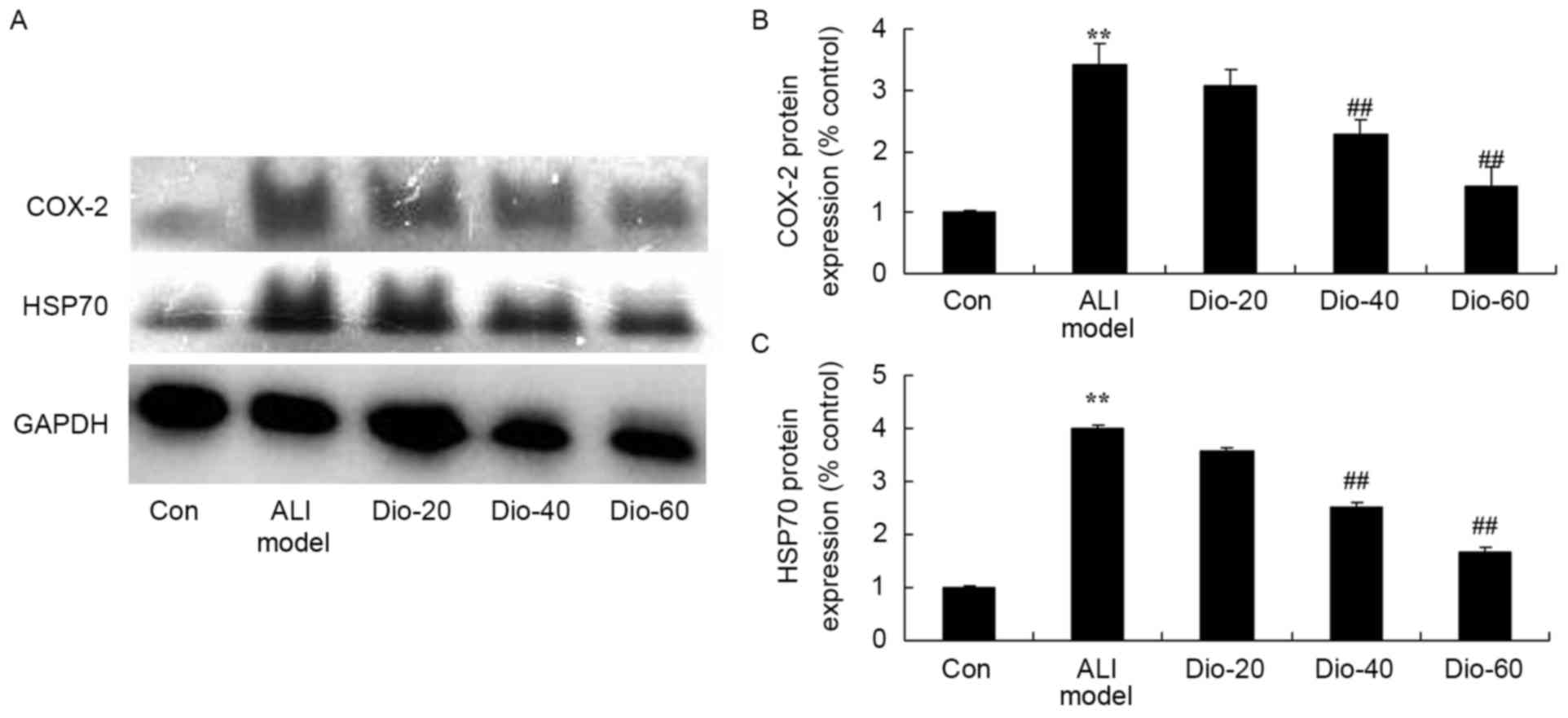
Dioscin decreases the COX-2 and HSP70
protein levels in ALI rats
To assess whether the anti-inflammatory effect of
dioscin on COX-2 and HSP70 protein expression level in ALI rats,
COX-2 protein expression level was measured using a western
blotting assay. COX-2 and HSP70 protein expression levels were
significantly induced by ALI, compared with the control group
(Fig. 7). Meanwhile, 40 and 60
mg/kg dioscin significantly suppressed the ALI-induced COX-2 and
HSP70 protein expression level in ALI rats, compared with the ALI
model rat group (Fig. 7).
Dioscin decreases the TLR4, MyD88 and
NF-κB protein levels in ALI rats
To assess whether the anti-inflammatory effect of
dioscin on TLR4, MyD88 and NF-κB protein levels using western
blotting assay. As presented in Fig.
8, ALI significantly induced TLR4, MyD88 and NF-κB protein
expression level in ALI rats, compared with the control group
(Fig. 8). 40 and 60 mg/kg dioscin
significantly suppressed TLR4, MyD88 and NF-κB protein levels in
ALI rats, compared with the ALI model rat group (Fig. 8).
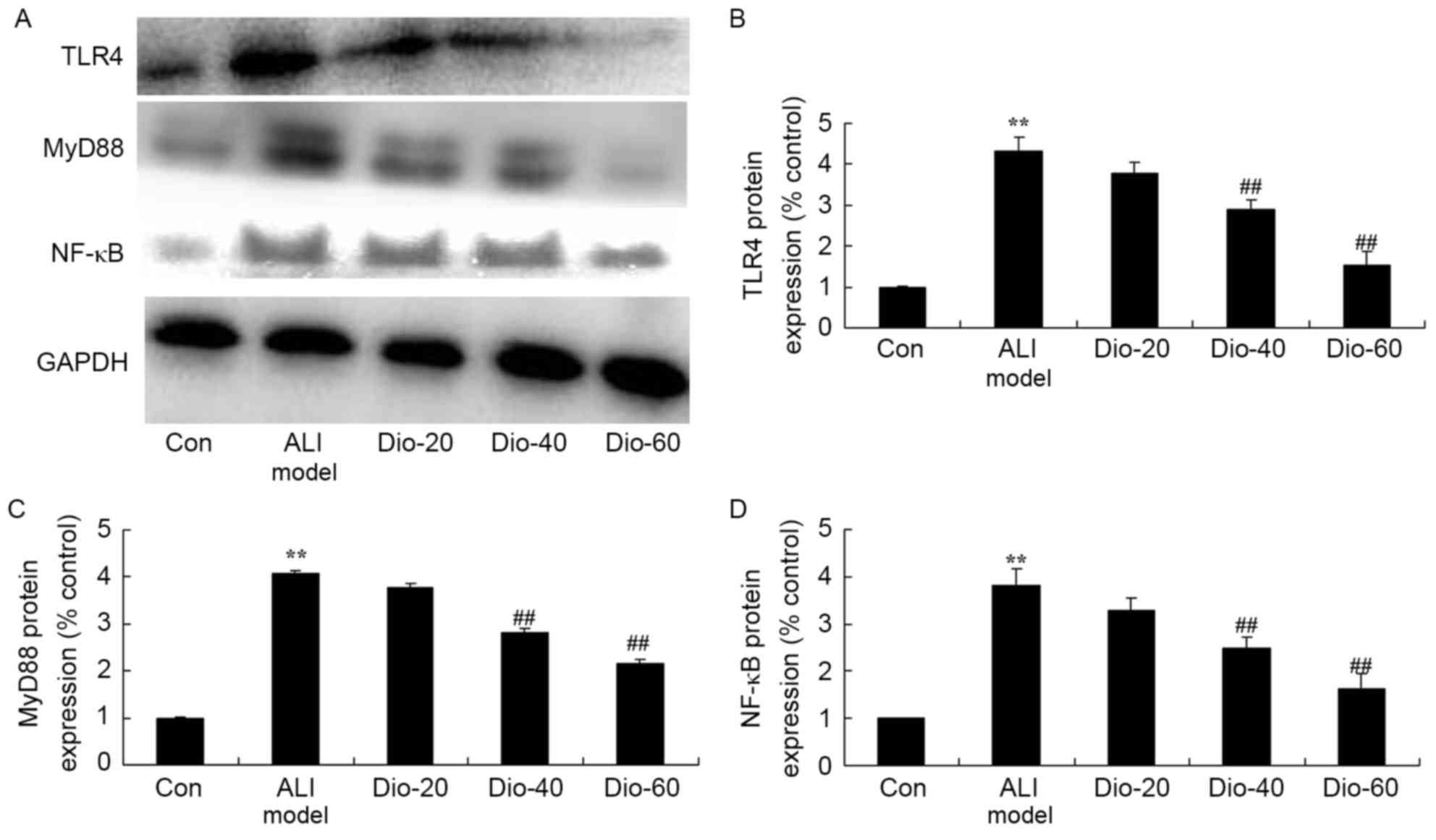 | Figure 8.Dioscin decreases TLR4, MyD88 and
NF-κB protein levels in ALI rats. Dioscin prevents the TLR4, MyD88
and NF-κB protein levels (A) by western blotting assays and (B-D)
statistical analysis of COX-2 and HSP70 protein levels in ALI rats.
Con, control group; Dio-20, 20 mg/kg dioscin group; Dio-40, 40
mg/kg dioscin group; Dio-60, 60 mg/kg dioscin group. **P<0.01
vs. control group; ##P<0.01 vs. ALI model group.
TLR4, Toll-like receptor 4; NF-κB, nuclear factor-κB; ALI, acute
lung injury; COX-2, cyclooxygenase-2; HSP70, heat shock protein
70. |
Discussion
ALI is a common severe condition, and Gram-negative
bacterial infection is the major cause of the disease (18). G-bacillus is the primary pathogenic
bacteria involved in the clinical infection. G-bacillus infection
causes the ALI, mainly because the LPS activated cells release a
large amount of inflammatory factors, so LPS is important to
mediate SIRS, as well as multiple organ dysfunction syndrome
(19). The alveolar macrophages
are the first defense line in the respiratory tract, and are also
the main effector cell of LPS (19). Studying the LPS signaling pathways
and its blocking effect has important theoretical and practical
significance to help understand the occurrence mechanism of ALI and
look for the new target in ALI treatment (5). In the present work, dioscin
significantly inhibited ALI score, total number of alveolar
macrophages, water content of lung and total protein concentration
in ALI rats. Tao et al (14) suggested that dioscin attenuates
hepatic ischemia-reperfusion injury via anti-inflammation and
apoptosis in rats.
TLR4, as the receptor of lipopolysaccharides (the
primary component of the outer wall of Gram-negative bacteria
cell), has an important role in the inflammatory response (20). Besides LPS, other endogenous
ligands can also activate the TLR4 receptor, such as the high-speed
transfer protein B1, HSP70, and other factors released from dead or
injured cells that can activate TLR4 and NF-κB, leading to the
release of inflammatory factors TNF-α, IL-1 and IL-6 (11). In the current study, the authors
demonstrated that dioscin significantly suppressed the ALI-induced
IL-1B, IL-6, TNF-α and NF-κB activity, inhibited MPO, IFN-γ and
ICAM-1 activity and decreased COX-2 protein expression in ALI model
rats. Wu et al (21)
reported that dioscin suppresses TNF-α-induced vascular cell
adhesion protein-1, ICAM-1 and the NF-κB pathway.
The activation of TLR4 leads the adaptor protein
containing the TIR structure domain in the cells, such as MyD88, to
the TLR4 intracellular structure domain (10). Thus, TLR4-mediated signaling
pathways can be divided into the MyD88-dependent and
MyD88-independent ones (22).
MyD88 was originally identified as the members of the 12 myeloid
differentiation initial response genes. MyD88, as the adaptor
protein, can mediate the signal transduction of 10 TLRs families
(23). MyD88-dependent signaling
pathway may be involved in the injury caused by ischemia
reperfusion, aggravating the organ damage. Some in vivo
tests have proved that the TLR4-mediated MyD88 signaling pathway
induces the immune response in ALI (24). In the present work, dioscin
significantly suppressed TLR4, MyD88 and NF-κB protein levels in
ALI. Liu et al (25)
exhibited that dioscin alleviates alcoholic liver fibrosis through
the TLR4/MyD88/NF-κB signaling pathway in hepatic stellate cell
activation. These data demonstrated that the anti-inflammatory
effect of dioscin on ALI through suppression of the
TLR4/MyD88/NF-κB signaling pathway.
There has been thorough research on the
TLR4-mediated MyD88 signaling pathways caused by pathogenic
microorganisms, but the TLR4-activated ligand, caused by damage,
requires further research (24).
It is reported that the endogenous ligand HSP70 and HMGBI can
activate TLR2 and TLR4 in the case of no pathogens, causing
inflammation (11). HSP is a
stress protein, as well as an endogenous protective material, and
according to the molecular weight, it can be divided into HSP100,
HSP90, HSP70, HSP60, HSP40 and small molecular weight HSP (26). HSP70 is a highly conserved protein
expressed in the majority of organisms. It is highly expressed
following cellular stress events and exerts protective effects
(27). In addition, it is worth
mentioning that inducing and increasing the expression of HSP70 in
lung tissue through the thermal pretreatment, drug or gene transfer
methods can reduce the animal's ALI inflammatory reaction,
apoptosis of lung tissue and pulmonary edema. Therefore, this
improves the blood oxygen content, reducing the mortality of
animals, so that HSP has a protective effect on ALI (27,28).
The current results indicated that dioscin significantly suppressed
the ALI-induced HSP70 protein expression level in ALI. Qi et
al (29) suggested that
dioscin inhibits renal ischemia/reperfusion injury via upregulation
of HSP70.
In conclusion, the present results indicated that
dioscin significantly inhibited ALI-induced lung injury score,
total number of alveolar macrophages, water content of lung and
total protein concentration in ALI rats via the inhibition of
inflammation, inhibiting the TLR4/MyD88 signaling pathway via
upregulation of HSP70. The findings suggested the therapeutic
potential of dioscin for ALI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Author's contributions
HZ, LY, XZ, YC and JC performed the animal and cell
experiments and analyzed the data. HZ designed the experiments and
wrote the manuscript. HZ and LY performed reverse
transcription-quantitative polymerase chain reaction and western
blot analyses.
Ethics approval and consent to
participate
All animal protocols were approved by the Animal
Care and Use Committee of the Zhongshan Hospital of Xiamen
University. All experiments were conducted in accordance with the
National Institutes of Health Guidelines for the Care and Use of
Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krupa A, Fol M, Rahman M, Stokes KY,
Florence JM, Leskov IL, Khoretonenko MV, Matthay MA, Liu KD, Calfee
CS, et al: Silencing Bruton's tyrosine kinase in alveolar
neutrophils protects mice from LPS/immune complex-induced acute
lung injury. Am J Physiol Lung Cell Mol Physiol. 307:L435–L448.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Q, Wang J, Hu M, Yang Y, Guo L and Xu
J, Lei C, Jiao Y and Xu J: Uncoupling protein 2 increases
susceptibility to lipopolysaccharide-induced acute lung injury in
mice. Mediators Inflamm. 2016:91542302016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bohman JK, Vogt MN and Hyder JA:
Retrospective report of contraindications to extracorporeal
membrane oxygenation (ECMO) among adults with acute respiratory
distress syndrome (ARDS). Heart Lung. 45:227–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu X, Liu N, Zhang YX, Cao J, Wu D, Peng
Q, Wang HB and Sun WC: The protective effects of HJB-1, a
derivative of 17-Hydroxy-Jolkinolide B, on LPS-Induced acute
distress respiratory syndrome mice. Molecules. 21:772016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rafat N, Dacho C, Kowanetz G, Betzen C,
Tönshoff B, Yard B and Beck G: Bone marrow-derived progenitor cells
attenuate inflammation in lipopolysaccharide-induced acute
respiratory distress syndrome. BMC Res Notes. 7:6132014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petroni RC, Biselli PJ, de Lima TM,
Theobaldo MC, Caldini ET, Pimentel RN, Barbeiro HV, Kubo SA,
Velasco IT and Soriano FG: Hypertonic saline (NaCl 7.5%) reduces
LPS-Induced acute lung Injury in rats. Inflammation. 38:2026–2035.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McKallip RJ, Ban H and Uchakina ON:
Treatment with the hyaluronic Acid synthesis inhibitor
4-methylumbelliferone suppresses LPS-induced lung inflammation.
Inflammation. 38:1250–1259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krupa A, Fudala R, Florence JM, Tucker T,
Allen TC, Standiford TJ, Luchowski R, Fol M, Rahman M, Gryczynski
Z, et al: Bruton's tyrosine kinase mediates FcγRIIa/Toll-like
receptor-4 receptor crosstalk in human neutrophils. Am J Respir
Cell Mol Biol. 48:240–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han LP, Li CJ, Sun B, Xie Y, Guan Y, Ma ZJ
and Chen LM: Protective effects of celastrol on diabetic liver
injury via TLR4/MyD88/NF-κB signaling pathway in Type 2 diabetic
rats. J Diabetes Res. 2016:26412482016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen S, Yuan J, Yao S, Jin Y, Chen G, Tian
W, Xi J, Xu Z, Weng D and Chen J: Lipopolysaccharides may aggravate
apoptosis through accumulation of autophagosomes in alveolar
macrophages of human silicosis. Autophagy. 11:2346–2357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Shan P, Srivastava A, Jiang G,
Zhang X and Lee PJ: An endothelial Hsp70-TLR4 axis limits Nox3
expression and protects against oxidant injury in lungs. Antioxid
Redox Signal. 24:991–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lunova M, Zizer E, Kucukoglu O, Schwarz C,
Dillmann WH, Wagner M and Strnad P: Hsp72 overexpression
accelerates the recovery from caerulein-induced pancreatitis. PLoS
One. 7:e399722012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aschkenasy G, Bromberg Z, Raj N,
Deutschman CS and Weiss YG: Enhanced Hsp70 expression protects
against acute lung injury by modulating apoptotic pathways. PLoS
One. 6:e269562011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tao X, Wan X, Xu Y, Xu L, Qi Y, Yin L, Han
X, Lin Y and Peng J: Dioscin attenuates hepatic
ischemia-reperfusion injury in rats through inhibition of
oxidative-nitrative stress, inflammation and apoptosis.
Transplantation. 98:604–611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Xu L, Zheng L, Yin L, Qi Y, Han X,
Xu Y and Peng J: Potent effects of dioscin against gastric cancer
in vitro and in vivo. Phytomedicine. 23:274–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu X, Zhai Z, Liu X, Li H, Ouyang Z, Wu C,
Liu G, Fan Q, Tang T, Qin A and Dai K: Dioscin inhibits osteoclast
differentiation and bone resorption though down-regulating the Akt
signaling cascades. Biochem Biophys Res Commun. 443:658–665. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu DD, Pan PH, Liu B, Su XL, Zhang LM, Tan
HY, Cao Z, Zhou ZR, Li HT, Li HS, et al: Inhibition of alveolar
macrophage pyroptosis reduces Lipopolysaccharide-induced acute lung
injury in mice. Chin Med J (Engl). 128:2638–2645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jones HD, Crother TR, Gonzalez-Villalobos
RA, Jupelli M, Chen S, Dagvadorj J, Arditi M and Shimada K: The
NLRP3 inflammasome is required for the development of hypoxemia in
LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol
Biol. 50:270–280. 2014.PubMed/NCBI
|
|
19
|
Haitsma JJ, Lachmann B and Papadakos PJ:
Additives in intravenous anesthesia modulate pulmonary inflammation
in a model of LPS-induced respiratory distress. Acta Anaesthesiol
Scand. 53:176–182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi M, Chen-Yoshikawa TF, Menju T,
Ohata K, Kondo T, Motoyama H, Hijiya K, Aoyama A and Date H:
Inhibition of Toll-like receptor 4 signaling ameliorates lung
ischemia-reperfusion injury in acute hyperglycemic conditions. J
Heart Lung Transplant. 35:815–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu S, Xu H, Peng J, Wang C, Jin Y, Liu K,
Sun H and Qin J: Potent anti-inflammatory effect of dioscin
mediated by suppression of TNF-α-induced VCAM-1, ICAM-1and EL
expression via the NF-κB pathway. Biochimie. 110:62–72. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Chen N, Liu JB, Wu JB, Zhang J,
Zhang Y and Jiang X: Protective effect of resveratrol against acute
lung injury induced by lipopolysaccharide via inhibiting the
myd88-dependent Toll-like receptor 4 signaling pathway. Mol Med
Rep. 10:101–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan Q, Wang H, Han X, Lin Y and Yang Y, Gu
L, Zhao J, Wang L, Huang L, Li Y and Yang Y: Baicalin inhibits
TLR7/MYD88 signaling pathway activation to suppress lung
inflammation in mice infected with influenza A virus. Biomed Rep.
2:437–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Q, Yi M, Guo Q, Wang C, Wang H, Meng
S, Liu C, Fu Y, Ji H and Chen T: Protective effects of polydatin on
lipopolysaccharide-induced acute lung injury through
TLR4-MyD88-NF-κB pathway. Int Immunopharmacol. 29:370–376. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu M, Xu Y, Han X, Yin L, Xu L, Qi Y,
Zhao Y, Liu K and Peng J: Dioscin alleviates alcoholic liver
fibrosis by attenuating hepatic stellate cell activation via the
TLR4/MyD88/NF-κB signaling pathway. Sci Rep. 5:180382015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lyons MM, Raj NN, Chittams JL, Kilpatrick
L and Deutschman CS: TAT-HSP70 attenuates experimental lung injury.
Shock. 43:582–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang ZJ, Zhou CY, Luo YJ and Xiong HW:
Expression of heat shock protein 70 in lung tissues of acute
paraquat poisoned rats and intervention of ulinastatin. World J
Emerg Med. 1:229–233. 2010.PubMed/NCBI
|
|
28
|
Lin HJ, Wang CT, Niu KC, Gao C, Li Z, Lin
MT and Chang CP: Hypobaric hypoxia preconditioning attenuates acute
lung injury during high-altitude exposure in rats via up-regulating
heat-shock protein 70. Clin Sci (Lond). 121:223–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi M, Zheng L, Qi Y, Han X, Xu Y, Xu L,
Yin L, Wang C, Zhao Y, Sun H, et al: Dioscin attenuates renal
ischemia/reperfusion injury by inhibiting the TLR4/MyD88 signaling
pathway via up-regulation of HSP70. Pharmacol Res. 100:341–352.
2015. View Article : Google Scholar : PubMed/NCBI
|