Introduction
A previous epidemiological study determined that
Helicobacter pylori infection may be associated with chronic
gastritis (CG), peptic ulcers (PU) and other gastric diseases
(1). The International Agency for
Research on Cancer officially recognized H. pylori as an
oncogenic factor in patients with human gastric cancer (GC)
(2). H. pylori is a
microaerophilic Gram-negative bacterium, and H. pylori
lipopolysaccharides (LPS) are responsible for the toxicity and
contribute to the pathogenesis of CG, PU and GC. However, H.
pylori LPS has several unique characteristics, including fewer
fatty acid residues and absence of 4-phosphate groups; therefore,
it has a distinctive activity by induction of cytokines (3,4).
Exposure to H. pylori LPS leads to a marked increase in NO
and proinflammatory cytokine levels, including interleukin (IL)-8
and toll-like receptor (TLR4) in gastric mucosa (5,6).
Ebselen [2-phenyl-1, 2-benzisoselenazol-3 (2H)-one]
is a seleno-organic compound that has an activity similar to
glutathione peroxidase (GPX). A previous study identified that
ebselen may have antioxidant and anti-inflammatory activity
(7), while another determined that
ebselen may inhibit airway inflammation induced by inhalational LPS
(8). However, the underlying
molecular mechanism of its action remains to be elucidated.
The aim of the present study was to investigate the
effect of ebselen on inflammation mediated by H. pylori LPS
in vitro and to determine the underlying molecular
mechanisms. The effect of ebselen on proliferation and migration of
GC cells was investigated in vitro. The findings of the
present study may aid in the identification of the possible
association between ebselen and H. pylori LPS-induced
inflammation in GC cells.
Materials and methods
Cell culture
Human GC cell lines AGS and MGC-803 were obtained
from the Shanghai Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China). Cells at a density
of 1×106/ml were cultured in 25 cm2 cell
culture flasks at 37°C in a humidified atmosphere of 5%
CO2 with RPMI-1640 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal calf serum (FCS;
Gibco; Thermo Fisher Scientific, Inc.) with 50 U/ml penicillin and
50 µg/ml streptomycin.
Extraction of LPS
LPS was extracted using hot phenol-water extraction,
as previously described (9). A
suspension of 500 mg biomass in 50 ml distilled water was mixed
with 50 ml 90% phenol and mixed at 68°C for 20 min. The mixture was
subsequently cooled to 4°C and centrifuged at 2,800 × g 4°C for 1
h, the resulting supernatant was separated. Subsequently, the
phenol layer was extracted with 50 ml distilled water. Pooled
supernatants in the aqueous phase were purified by dialysis using
cellulose membranes for 24 h, following centrifugation at 2,800 ×
g, 4 times for 30 min the aqueous phase was separated. Crude LPS
was resuspended in phosphate buffer containing 10 µg/ml
deoxyribonuclease and 100 µg/ml ribonuclease. The solution was
incubated for 16 h at 37°C and 750 ml 90% phenol in distilled water
was added. Following dialysis for 10 min, the solution was
centrifuged at 4,500 × g at 4°C for 30 min and the aqueous phase
was separated and maintained at −20°C.
Analysis of cell viability
Cell viability was quantified using the Cell
Counting Kit (CCK)-8 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), which determined the number of viable cells based
on the reduction of water-soluble formazan by the dehydrogenases
present in viable cells. Cells were seeded at a density of
2×104/well in 96-well plates, with 100 µl medium/well
and were incubated with 0, 5, 10, 15, 20, 25, 50, 75, and 100
µmol/l ebselen (Sigma-Aldrich, Merck Millipore, Darmstadt, Germany)
for 24 h. Three wells of each group were used in repeat
experiments. After 24 h post-attachment, 10 µl WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium]
solution was added to each well and cells were incubated at 37°C
for 1 h. Absorbance was read at 450 nm on a microplate reader
(SpectraFluor; Tecan, Inc., Zürich, Switzerland).
Western blotting
Cells were seeded at a density of
2×106/well in 90-mm dishes, and 0 or 200 ng/ml LPS was
added. The cells were subsequently incubated with or without 20
µmol/l ebselen for 10, 20, 30, 40, 50 min, 1, 2, 5 or 10 h. Total
proteins were extracted by RIPA cell lysis buffer containing 1
mmol/l PMSF (Beyotime Institute of Biotechnology, Shanghai, China)
with a centrifugation at 12,000 × g for 10 min at 4°C. The protein
concentrations were determined using a BCA kit (Beyotime Institute
of Biotechnology). A total of 50 mg protein per lane was
electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide
electrophoresis gel and transferred onto a nitrocellulose (NC)
membrane. Following three washes with TBST (10 min each), the
membrane was blocked by 5% non-fat milk for 1 h at room
temperature. Then the membranes were incubated with the primary
antibody [GPX2, cat. no. ab137431; 1:1,000; GPX4, cat. no.
ab125066, 1:1,000; and TLR4, cat. no. ab22048, 1:1,000; all from
Abcam, Cambridge, MA, USA; p38 mitogen-activated protein kinase
(p38 MAPK), cat. no. 9212, 1:1,000; and phosphorylated (p)-p38
MAPK, cat. no. 4511, 1:1,000; both from Cell Signaling Technology,
Inc., Danvers, MA, USA] at 4°C overnight. The membraned were washed
with TBST for 3 times, 10 min each, incubated with secondary
antibody [goat anti-rabbit IgG-peroxidase antibody; cat. no. A0545;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; goat anti-mouse IgG
(Fc specific)-peroxidase antibody; cat. no. A0168; Sigma-Aldrich;
Merck KGaA] with 1:5,000 dilution at room temperature for 1 h. The
membranes were subsequently washed with TBST for 3 times, 10 min
each, the membrane was washed three times with TBST and detected
with enhanced chemiluminescence (Beyotime Institute of
Biotechnology, Haimen, China).
ELISA
Cells were seeded at a density of
2×104/well in 96-well plates and cultured overnight,
followed by serum starvation for 24 h prior to every experiment.
Cells were incubated with 200 or 200 ng/ml LPS and 20 µmol/l
ebselen for 1–5 h. The supernatants obtained by centrifugation at
2,000 × g, 4°C for 20 min were quantified using the human IL-8
ELISA kit according to the manufacturer's protocol (R&D
Systems, Inc., Minneapolis, MN, USA).
Quantification of reactive oxygen
species (ROS) generation
Intracellular ROS accumulation was detected using
fluorescence microscopy. Cells were plated at a density of
2×104/well in a 96-well plate and cultured in RPMI-1640
medium supplemented with 10% FCS. The culture medium was renewed
when the cells reached 80% confluence. Cells were treated with 200
or 200 ng/ml LPS and 20 µmol/l ebselen at 37°C for 1–4 h. The
supernatants were quantified using the Cellular ROS Detection Assay
kit (Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are expressed as mean ± standard deviation.
CCK-8 data was analyzed using two-way analysis of variance (ANOVA)
followed by the Dunnett's test. Western blot analysis, ROS levels
and IL-8 assays, were analyzed using one-way ANOVA followed by a
Newman-Keuls post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Ebselen modulates the viability of GC
cells
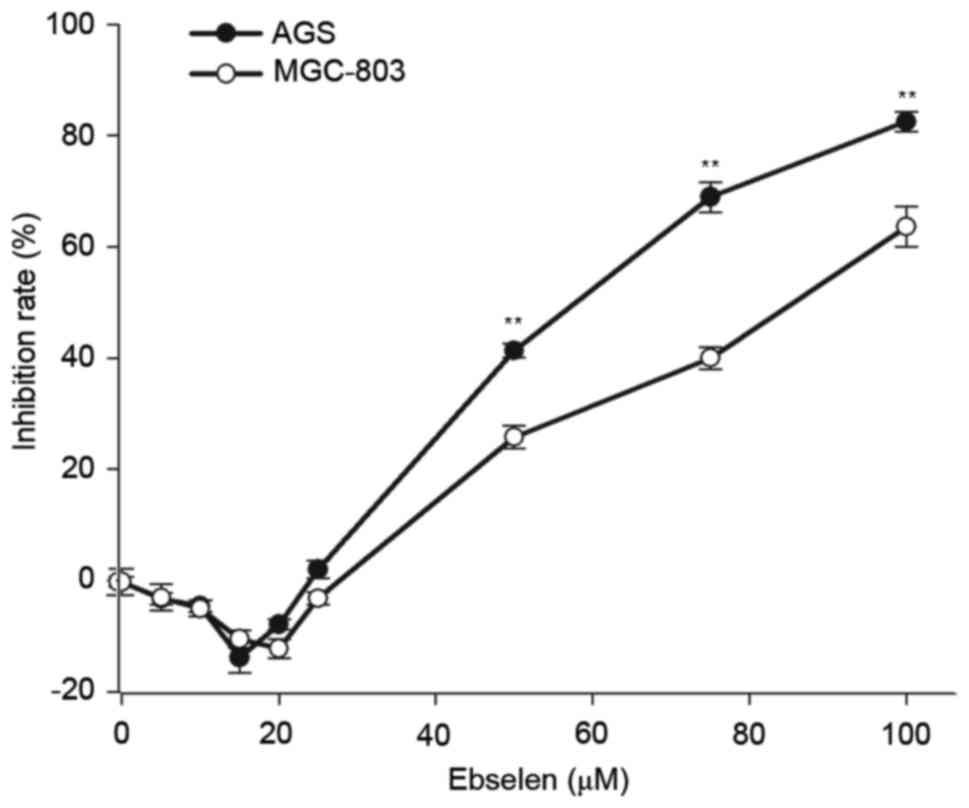
In order to determine the effect of ebselen on GC
cell viability, AGS and MGC-803 cells were treated with various
concentrations of ebselen for 24 h, before the cell viability rate
was detected by CCK-8 assay. The assay revealed that different
concentrations of ebselen produced different effects on GC cell
viability (F=18.204; P<0.001; Fig.
1). Ebselen promoted cell viability at low concentrations,
whereas at high concentrations it significantly inhibited cell
growth. The effect on cell viability was dose-dependent, with a
minimal effect at 20 µmol/l; therefore, this concentration was
selected for subsequent experiments.
Ebselen inhibits IL-8 production
induced by H. pylori LPS
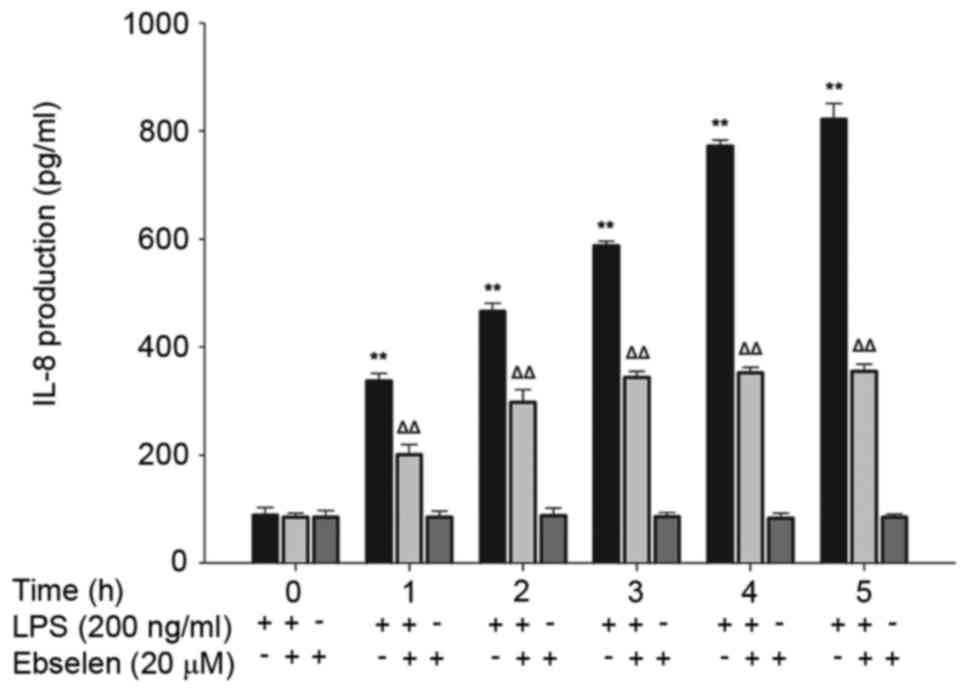
Production of IL-8 in AGS cells treated with 0 and
200 ng/ml H. pylori LPS for 0–4 h was detected in the
absence or presence of 20 µmol/l ebselen (Fig. 2). Ebselen treatment did not result
in any significant differences in IL-8 release at any time point
compared with the 0 h control (Fig.
2). Stimulation with H. pylori LPS significantly
increased IL-8 production in a time-dependent manner (P<0.01;
Fig. 2); however, this effect was
suppressed by ebselen treatment (Fig.
2). The 1 h treatment with 200 ng/ml H. pylori LPS
enhanced the production of IL-8 in AGS cells in a time-dependent
manner. Simultaneous addition of 20 µmol/l ebselen inhibited the
production of IL-8 in HP-LPS promoted cells. However, in a certain
period of time (3 h), this effect achieved leveled off.
Ebselen inhibits ROS enhanced by H.
pylori LPS via activation of GPX signaling
The underlying mechanism that affects IL-8
production was then investigated. ROS are essential components of
the innate immune response against intracellular bacteria, and are
closely associated with IL-8. Therefore, the effect of ebselen on
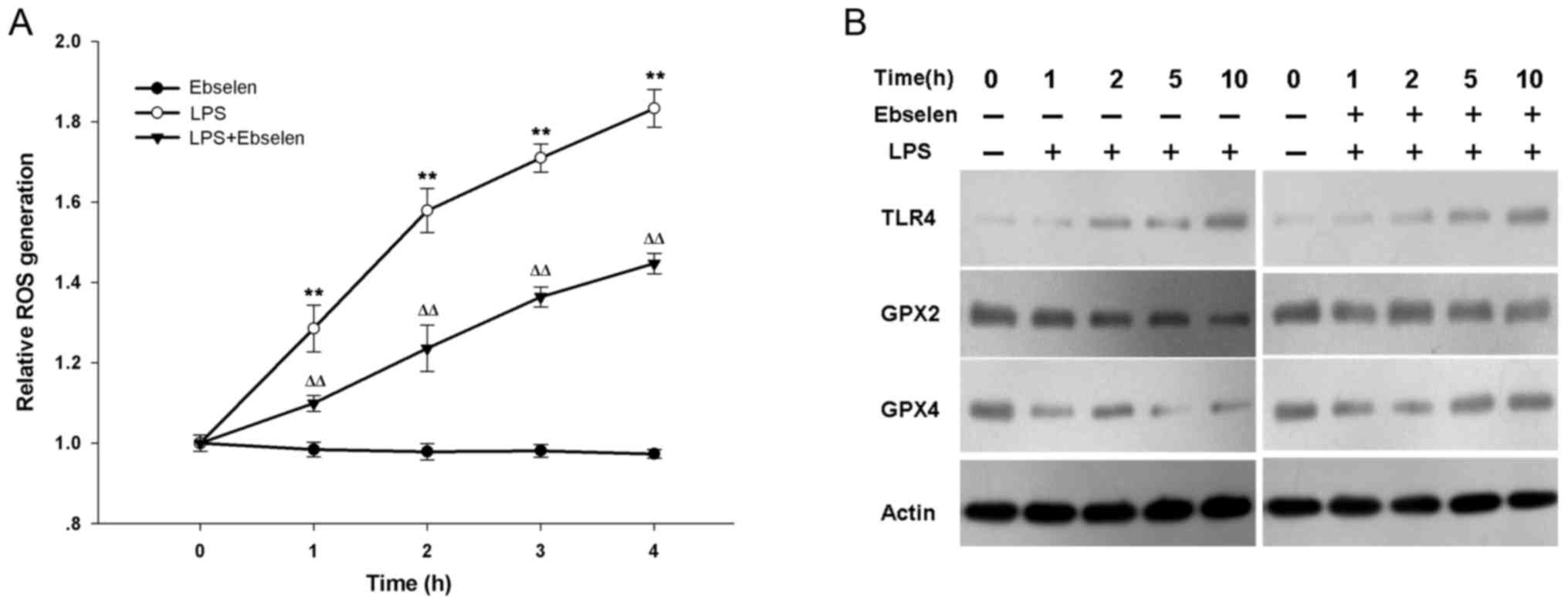
H. pylori LPS-induced ROS was investigated. The levels of
ROS generation in ebselen-treated, LPS-treated, and
LPS+ebselen-treated AGS cells was measured (Fig. 3A). LPS-treated cells produced more
ROS at each time point from 1 h than ebselen-treated and
LPS+ebselen-treated cells (Fig.
3A; LPS vs. ebselen, P=0.001 at 1 h, P<0.001 at 2, 3 and 4
h; LPS vs. LPS+ebselen, P=0.006 at 1 h, P=0.002 at 2 h, P<0.001
at 3 and 4 h). Ebselen treatment alone did not inhibit spontaneous
ROS generation with increased treatment time (Fig. 3A). In order to determine whether
ebselen affected TLR or GPX signaling, the effect of LPS and
ebselen on AGS cells was investigated (Fig. 3B). H. pylori LPS treatment
visibly increased TLR4 protein expression levels with time in AGS
cells; however, treatment with ebselen+LPS had no significant
effect on TLR4 expression compared with LPS alone (Fig. 3B). H. pylori LPS treatment
alone reduced GPX2 and GPX4 expression, however co-treatment with
ebselen treatment prevented this effect (Fig. 3B).
Ebselen blocks H. pylori LPS-induced
phosphorylation of p38 MAPK
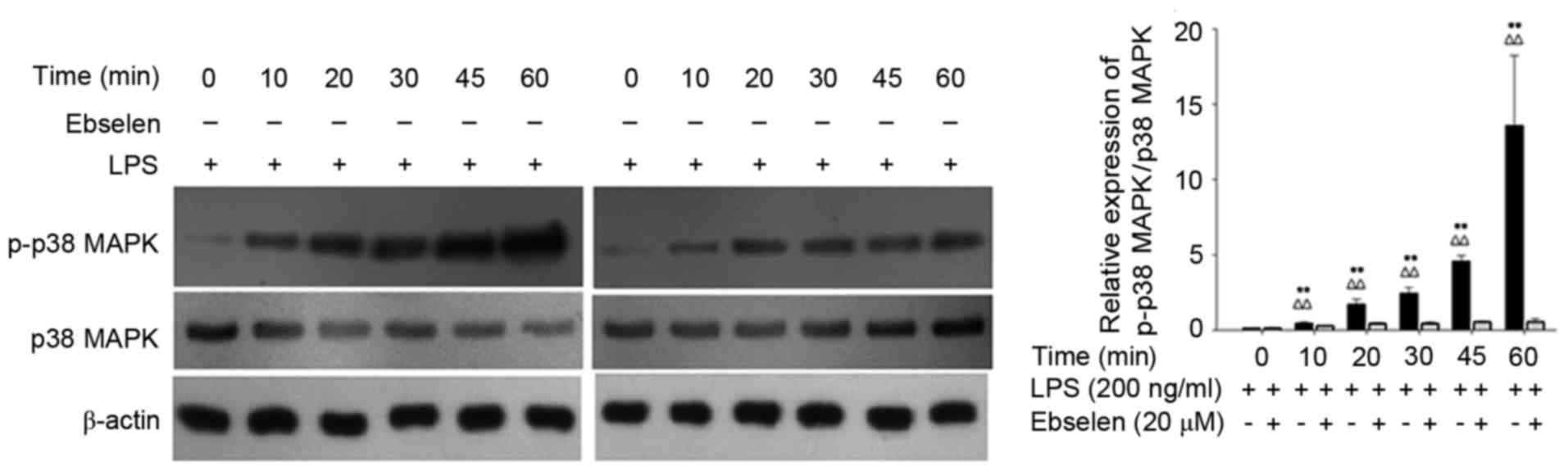
The effect of ebselen on p-p38 MAPK expression
levels induced by H. pylori LPS treatment was also
investigated. Western blotting confirmed that phosphorylation of
p38 MAPK was significantly greater in AGS cells treated with LPS
compared with cells treated with LPS+ebselen (P<0.01; Fig. 4). These findings suggested that
ebselen inhibited the phosphorylation of p38 MAPK induced by H.
pylori LPS treatment and may be capable of reducing IL-8
expression levels by inhibiting the phosphorylation of p38
MAPK.
Discussion
Several toxic substances associated with H.
pylori, including LPS, perform a key role in gastroduodenal
diseases. Cytokine induction is triggered by a ROS signal, but the
identity of the specific TLR responsible for the recognition of
H. pylori LPS remains to be determined. Previous studies
have implicated TLR4 (10–13), whereas others have suggested a role
for TLR2, therefore the exact mechanism is not fully understood.
Previous studies have reported the anti-inflammatory activity of
ebselen, particularly in human cancer cells (14,15).
Ebselen affects extracellular signal-regulated kinase (ERK), c-Jun
N-terminal kinase (JNK) and p38 MAPK signaling which are involved
in various cellular processes, such as proliferation,
differentiation and apoptosis (16–19).
The impact of ebselen on these pathways is currently under
investigation.
The present study determined that H. pylori
LPS was important for ROS and IL-8 production in GC cells. LPS
significantly increased ROS and IL-8 production, suggesting that it
is a major virulence factor for H. pylori-associated mucosal
inflammation. This effect was inhibited by the anti-inflammatory
drug ebselen. Additionally, the present study determined that
ebselen had a dose-dependent effect on GC cell viability. In AGS
cells, TLR4 expression was increased, but GPX2 and GPX4 expression
was decreased by H. pylori LPS. In further analysis, ebselen
was demonstrated to inhibit the H. pylori LPS-induced
downregulation of GPX2/4 expression. However, ebselen treatment did
not affect TLR4 expression, suggesting that ebselen may inhibit
H. pylori LPS-induced ROS production via GPX2/4 expression
as opposed to TLR4 signaling. This may be due to the fact that
ebselen acts as an oxidant at redox-modulatory sites, which mimics
the activity of endogenous GPX, therefore partially restores GPX
antioxidant capacity (20,21). Furthermore, the present study
demonstrated that phosphorylation of p38 MAPK was significantly
increased following H. pylori LPS treatment and that
LPS-induced phosphorylation of p38 MAPK was inhibited by treatment
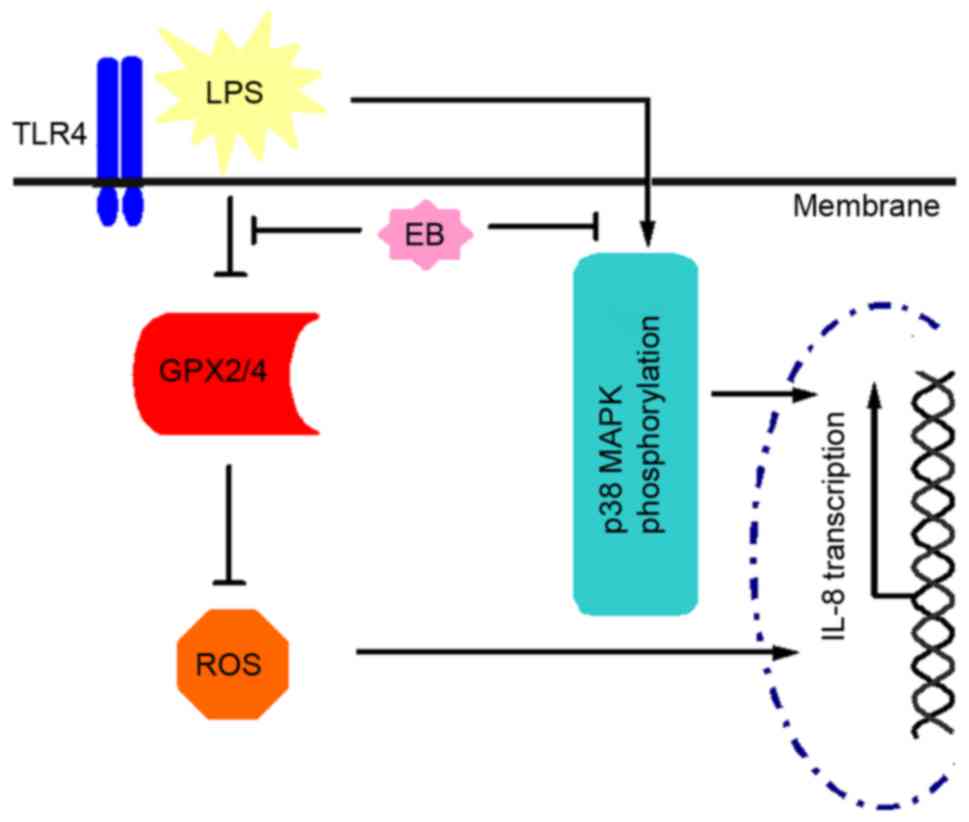
with ebselen. Therefore, the present study hypothesizes that
ebselen disrupted the H. pylori LPS-activated ROS/IL-8
pathway by restoring GPX2/4 expression and blocking the generation
of IL-8 by inhibiting phosphorylation of p38 MAPK, as presented in
Fig. 5.
The present study revealed that the
anti-inflammatory activity of ebselen may be associated with its
antioxidative properties. Therefore, ebselen may be a potential
therapeutic agent to mediate H. pylori LPS-induced cell
damage.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81472242
and 81570549), Shanghai Municipal Health Bureau Key Disciplines
Grant (grant no. ZK2015A24), Natural Science Foundation of the
Science and Technology Commission of Shanghai Municipality, (grant
nos. 14ZR1431600 and 14411973700) and the Shanghai Municipal Health
Bureau (grant no. 20134100).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MS and YW conceived and designed the experiments, LX
and CG performed the experiments, GL and JW analyzed the data, TW
and WM contributed the reagents and materials, and LX wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peek RM Jr and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nat Rev
Cancer. 2:28–37. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schistosomes, liver flukes and
Helicobacter pylori. IARC working group on the evaluation of
carcinogenic risks to humans; Lyon, 7–14 June 1994. IARC Monogr
Eval Carcinog Risks Hum. 61:1–241. 1994.PubMed/NCBI
|
|
3
|
Hynes SO, Ferris JA, Szponar B, Wadström
T, Fox JG, O'Rourke J, Larsson L, Yaquian E, Ljungh A, Clyne M, et
al: Comparative chemical and biological characterization of the
lipopolysaccharides of gastric and enterohepatic
Helicobacter. Helicobacter. 9:313–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esmaeilli D, Mobarez AM, Salmanian AH and
Hosseini AZ: Bioactivity and immunological evaluation of LPS from
different serotypes of Helicobacter pylori. Iran J Microbio.
5:142–146. 2013.
|
|
5
|
Ogawa T, Asai Y, Sakai Y, Oikawa M, Fukase
K, Suda Y, Kusumoto S and Tamura T: Endotoxic and immunobiological
activities of a chemically synthesized lipid A of Helicobacter
pylori strain 206-1. FEMS Immunol Med Microbiol. 36:1–7. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lepper PM, Triantafilou M, Schumann C,
Schneider EM and Triantafilou K: Lipopolysaccharides from
Helicobacter pylori can act as antagonists for Toll-like
receptor 4. Cell Microbiol. 7:519–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marthandan S, Hyland P, Pawelec G and
Barnett Y: An investigation of the effects of the antioxidants,
ebselen or N-acetyl cysteine on human peripheral blood mononuclear
cells and T cells. Immun Ageing. 10:72013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haddad el-B, McCluskie K, Birrell MA,
Dabrowski D, Pecoraro M, Underwood S, Chen B, De Sanctis GT, Webber
SE, Foster ML and Belvisi MG: Differential effects of ebselen on
neutrophil recruitment, chemokine and inflammatory mediator
expression in a rat model of lipopolysaccharide-induced pulmonary
inflammation. J Immunol. 169:974–982. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernardová K, Babica P, Marsálek B and
Bláha L: Isolation and endotoxin activities of lipopolysaccharides
from cyanobacterial cultures and complex water blooms and
comparison with the effects of heterotrophic bacteria and green
alga. J Appl Toxicol. 28:72–77. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uno K, Kato K, Atsumi T, Suzuki T,
Yoshitake J, Morita H, Ohara S, Kotake Y, Shimosegawa T and
Yoshimura T: Toll-like receptor (TLR)2 induced through TLR4
signaling initiated by Helicobacter pylori cooperatively
amplifies iNOS induction in gastric epithelial cells. Am J Physiol
Gastrointest Liver Physiol. 293:G1004–G1012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chochi K, Ichikura T, Kinoshita M, Majima
T, Shinomiya N, Tsujimoto H, Kawabata T, Sugasawa H, Ono S, Seki S
and Mochizuki H: Helicobacter pylori augments growth of
gastric cancers via the lipopolysaccharide-toll-like receptor 4
patyway whereas its lipopolysaccharide attenuates antitumor
activities of human mononuclear cells. Clin Cancer Res.
14:2909–2917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawahara T, Teshima S, Oka A, Sugiyama T,
Kishi K and Rokutan K: Type I Helicobacter pylori
lipopolysaccharice stimulates toll-like receptor 4 and activates
mitogen oxidase 1 in gastric pit cell. Infect Immun. 69:4382–4389.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith SM, Moran AP, Duggan SP, Ahmed SE,
Mohamed AS, Windle HJ, O'Neill LA and Kelleher DP: Tribbles 3: A
novel regulator of TLR2-mediated signaling in response to
Helicobacter pylori lipopolysaccharide. J Immunol.
186:2462–2471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Azad GK, Singh V, Mandal P, Singh P, Golla
U, Baranwal S, Chauhan S and Tomar RS: Ebselen induces reactive
oxygen species (ROS)-mediated cytotoxicity in Saccharomyces
cerevisiae with inhibition of glutamate dehydrogenase being a
target. FEBS Open Bio. 4:77–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parnham MJ and Sies H: The early research
and development of ebselen. Biochem Pharmacol. 86:1248–1253. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshizumi M, Fujita Y, Izawa Y, Suzaki Y,
Kyaw M, Ali N, Tsuchiya K, Kagami S, Yano S, Sone S and Tamaki T:
Ebselen inhibits tumor necrosis factor-alpha-induced c-Jun
N-terminal kinase activation and adhesion molecule expression in
endothelial cells. Exp Cell Res. 292:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshizumi M, Kogame T, Suzaki Y, Fujita Y,
Kyaw M, Kirima K, Ishizawa K, Tsuchiya K, Kagami S and Tamaki T:
Ebselen attenuates oxidative stress-induced apoptosis via the
inhibition of the c-Jun N-terminal kinase and activator protein-1
signalling pathway in PC12 cells. Br J Pharmacol. 136:1023–1032.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma V, Tewari R, Sk UH, Joseph C and
Sen E: Ebselen sensitizes glioblastoma cells to tumor necrosis
Factor (TNFalpha)-induced apoptosis through two distinct pathways
involving NF-kappaB downregulation and Fas-mediated formation of
death inducing signaling complex. Int J Cancer. 123:2204–2212.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang CF, Shen HM and Ong CN: Ebselen
induces apoptosis in HepG-2 cells through rapid depletion of
intracellular thiols. Arch Biochem Biophys. 374:142–152. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo Z, Liang L, Sheng J, Pang Y, Li J,
Huang L and Li X: Synthesis and biological evaluation of a new
series of ebselen derivatives as glutathione peroxidase (GPX)
mimics and cholinesterase inhibitors against Alzheimer's disease.
Bioorg Med Chem. 22:1355–1361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Yun JW and Lei XG: Glutathione
peroxidase mimic ebselen improves glucose-stimulated insulin
secretion in murine islets. Antioxid Redox Signal. 20:191–203.
2014. View Article : Google Scholar : PubMed/NCBI
|