Introduction
Age-related brain health decline is an important
risk factor for neurodegenerative diseases, including Alzheimer's
disease (AD) and Parkinson's disease (PD), which are characterized
by cognitive impairment, behavioral alterations and motor
dysfunction. Inflammation contributes to cognitive decline, and
abnormalities in brain structure and metabolism (1). Astrocytes serve an important role in
modulating inflammation of the central nervous system. Astrocytosis
in the brain may be an early phenomenon in AD development (2,3), and
increased expression of glial fibrillary acidic protein (GFAP) by
astrocytes has been reported to be associated with an AD-like
pathology (4,5). Reactive astrocytes surrounding senile
plaques may be responsible for the ongoing inflammatory process in
AD via the release of cytokines and other toxic products (6), including interleukin (IL)-1β
(7,8) and IL-6 (9). Cyclooxygenase (COX)-2 is a
rate-limiting enzyme involved in the production of prostaglandins,
which is also involved in inflammatory mechanisms. The increase of
neuronal COX-2 signaling in the hippocampus may be an indicator of
dementia progression in early AD (10). Furthermore, the activation of COX-2
may be induced by IL-1β and other cytokines (11,12).
These inflammatory mediators may finally activate the nuclear
factor (NF)-κB signaling pathway (13). Conversely, agonists of peroxisome
proliferator-activated receptor (PPAR)-γ reduced COX-2 protein
expression, NF-κB activation and oxidative stress (14).
Previous studies have suggested that oxidative
stress is a prominent feature in patients with mild cognitive
impairment (MCI) and AD (15–17),
which exhibit high levels of the product of lipid peroxidation
[malondialdehyde (MDA)] and low activities of antioxidants
[superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px)].
In addition, oxidative stress is associated with the development of
neuronal death and neural dysfunction in AD and PD, which are two
common age-related neurodegenerative diseases (18). In addition, patients with PD
exhibited higher levels of inflammatory markers compared with
control individuals (19,20). Therefore, inhibiting inflammation
and oxidative stress may be an important strategy for the treatment
of age-related neurodegenerative diseases.
Bushen-Yizhi formula (BSYZ) is a Chinese medical
compound, which acts via numerous mechanisms. Our previous studies
have demonstrated that BSYZ may attenuate cognitive impairment in
AD-like animal models, and affect the modulation of numerous
targets (21,22). The present study aimed to
investigate the effects of BSYZ on learning and memory abilities in
senescence-accelerated mouse prone 8 (SAMP8) mice, and examined the
underlying molecular mechanisms involved in age-related
alterations, including inflammation, oxidative stress and neuronal
apoptosis, in the brains of mice.
Materials and methods
Preparation of BSYZ extracts
BSYZ extracts were provided by the School of Chinese
Materia Medica, Guangzhou University of Chinese Medicine
(Guangzhou, China), as described previously (22). The doses of BSYZ extracts were
expressed as the weight of original raw herbs in grams per kilogram
of body weight.
Animals and housing
Male SAMP8 mice (age, 3 months; weight, 35±5 g;
n=48), and age-matched senescence-accelerated mouse resistant 1
(SAMR1) mice (weight, 35±5 g; n=12) were purchased from the Animal
Research Center of Peking University (Beijing, China). All mice
were housed at the Experimental Animal Center of Guangzhou
University of Chinese Medicine under a controlled temperature
(20±2°C), a relative humidity of 55±2% and a 12-h light/dark cycle
with ad libitum access to food and water. Experimental
protocols were approved by the Animal Ethics Committee of Guangzhou
University of Chinese Medicine, and experiments were performed in
compliance with the Guide for the Care and Use of Laboratory
Animals (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf).
Drug administration
SAMP8 mice were randomly divided into four groups:
SAMP8 (vehicle, saline only; n=12), L-BSYZ (low dose BSYZ, 1.46
g/kg/day; n=10), M-BSYZ (middle dose BSYZ, 2.92 g/kg/day; n=10) and
H-BSYZ (high dose BSYZ, 5.84 g/kg/day; n=9). SAMR1 mice served as a
healthy control and were treated with saline (n=12). At the age of
6 months, when the SAMP8 mice show impaired memory (23), all mice received drug or saline by
oral administration once daily for 30 days.
Behavioral tests
After 30 days of drug administration, mice were
acclimated to the environment of the behavioral test room for 3
days. Morris water maze test and step-down test were performed to
investigate the learning and memory abilities of mice.
Morris water maze test
The equipment for the Morris water maze test
consisted of a black circular pool (diameter, 120 cm; height, 40
cm) and a camera monitor linked to video-tracking software Behavior
Sys (Guangzhou Feidi Biological Technology Co., Ltd., Guangzhou,
China). The test was performed as described by Chen et al
(24) with minor modifications.
Briefly, the test consisted of two phases: Hidden-platform training
and spatial probe trial. During hidden-platform training, the
platform (diameter, 8 cm) was located in one of four equal
quadrants of the pool and submerged 1 cm below the surface of the
water. The water was 30 cm in depth, 22–26°C and made opaque with
non-toxic white paint. Mice were given four trials a day (120 sec
each) for 5 days. The time to find the platform (the escape
latency) was recorded. If the mouse failed to find the platform
within 120 sec, the escape latency was recorded as 120 sec, and the
mouse was directed to the platform. Mice were allowed to stay on
the platform for 20 sec after finding or being guided to the
platform. At the end of the daily trial, mice were dried and
returned to their home cages. On the sixth day, memory was assessed
via a spatial probe trial where the platform was removed. Each
mouse was given only one trial for 120 sec from the starting point
opposite to the platform location. The number of mice crossing the
previous platform position and time spent in the target quadrant
were recorded.
Step-down test
The apparatus for the step-down test consisted of a
plastic testing box (12×12×18 cm) and a rubber platform placed in a
corner of the box (Guangzhou Feidi Biological Technology Co.,
Ltd.). The step-down test was conducted as described by Zhu et
al (25). The number of errors
and the latency were recorded using video tracking software
(Guangzhou Feidi Biological Technology Co. Ltd. China) to measure
memory learning and retention.
Brain sections and tissue
preparation
Following performance of the behavioral tests, 3
mice per group were used for preparation of brain sections,
according to a previously described protocol (22). Brain coronal sections were taken
for immunohistochemistry and histological examination. The
remaining mice were anesthetized (sodium pentobarbital, 50 mg/kg
body weight) and decapitated; the brains were rapidly removed,
divided into two parts (the hippocampus and cortex) on ice and
stored at −80°C. The cortexes and hippocampi were used for
biochemical analysis and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), respectively.
Immunohistochemistry
Immunohistochemical staining was performed as
previously described (22). The
following primary antibodies were used: GFAP (SC-9065; 1:200; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), COX-2 (12282; 1:200;
Cell Signaling Technology, Inc.), PPAR-γ (2435; 1:200; Cell
Signaling Technology, Inc.), NF-κB (6956; 1:200; Cell Signaling
Technology, Inc.) and B-cell lymphoma extra-large (Bcl-xL; 2764;
1:200, Cell Signaling Technology, Inc.). Following incubation with
primary antibodies at 4°C overnight, sections were washed in 0.1 M
phosphate buffered saline (PBS) and incubated with anti-rabbit or
anti-mouse horseradish peroxidase linked secondary antibodies (7074
or 7076; 1:2,000; Cell Signaling Technology, Inc.) for 1 h at 37°C.
Following washing in PBS, sections were incubated with
diaminobenzidine (PA110; Tiangen Biotech Co., Ltd., Beijing,
China), and counterstaining with hemalum (AR0005; Boster Biological
Technology, Pleasanton, CA, USA). Photomicrographic images were
captured using a Leica DM4000B microscope (Leica Microsystems,
Inc., Wetzlar, Germany) and Leica QWin plus version 4 software
(Leica Microsystems, Inc.). For semi-quantitative analysis, the
percentage of the positively stained area was measured at original
magnification ×200 using Leica QWin plus software (Leica
Microsystems, Inc.).
Histological examination
Terminal deoxynucleotidyl transferase dUTP nick-end
labeling (TUNEL) staining, hematoxylin and eosin (H&E) and
Nissl staining were performed according to the manufacturer's
protocol of the following kits: FragEL™ DNA
Fragmentation Detection kit (QIA33; Merck KGaA, Darmstadt,
Germany), Hematoxylin-Eosin kit (AR1180; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), and Nissl staining solution
(C0117; Beyotime Institute of Biotechnology, Haimen, China). Images
were captured using a Leica DM4000B microscope and Leica QWin plus
software (Leica Microsystems, Inc.).
Biochemical analysis
To determine MDA content, SOD and GSH-Px activities,
the cortexes of mice were homogenized with 0.9% saline (10 ml/g
sample weight) on ice and centrifuged at 3,000 × g for 10 min at
4°C. The supernatant was used for analysis according to the kits
protocols (A003-1, A001-1-1 and A005 for MDA, SOD and GSH-Px,
respectively; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China,). Absorbance (532, 550 and 420 nm wavelength for MDA, SOD
and GSH-Px, respectively) was measured using a universal microplate
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
RT-qPCR
Mice hippocampi were homogenized with RNAiso Plus
(D9108A; Takara Biotechnology Co., Ltd., Dalian, China) and total
RNA was extracted according to the manufacturer's protocols. After
quantifying total RNA with a NanoDrop 2000 spectrophotometer
(NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, ME, USA),
reverse transcription was performed using the
PrimeScript™ RT Master Mix (Perfect Real Time; RR036A;
Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol. Cycling conditions were as follows: 37°C for 15 min, 85°C
for 5 sec and 4°C. Amplification reactions were performed in a
total reaction volume of 25 µl using the SYBR® Premix
Ex Taq™ II (Tli RNaseH Plus; RR820A; Takara
Biotechnology Co., Ltd.) on a CFX96™ Real-Time PCR
Detection system (Bio-Rad Laboratories, Inc.). Cycling conditions
were as follows: An initial predenaturation step at 95°C for 30
sec, followed by 40 cycles at 95°C for 5 sec and 62°C for 30 sec,
then 95°C for 10 sec, melt curve step at 65 to 95°C (increments
0.5°C, each for 5 sec). Each sample was analyzed in triplicate.
Primers were designed and synthesized by Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Primer sequences were as follows: IL-1β
(IL1B), forward 5′-GAAATGCCACCTTTTGACAGTG-3′, reverse
5′-TGGATGCTCTCATCAGGACAG-3′; IL-6 (IL6), forward
5′-TCTATACCACTTCACAAGTCGGA-3′, reverse
5′-GAATTGCCATTGCACAACTCTTT-3′; and β-actin, forward
5′-GGCTGTATTCCCCTCCATCG-3′ and reverse
5′-CCAGTTGGTAACAATGCCATGT-3′. β-actin was used as housekeeping
gene. The relative amount of target gene expression was normalized
against housekeeping gene and calculated by the 2−∆∆Cq
method (26).
Statistical analysis
SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Differences in escape
latency in the Morris water maze test were analyzed by a repeated
measures analysis of variance, whereas the remaining data was
analyzed by one-way analysis of variance or a nonparametric test
(Kruskal-Wallis test) where appropriate. Data are expressed as the
mean ± standard error (experiments were repeated at least three
times, except the behavioral tests). The least significant
difference post hoc test was used to establish significance between
individual groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of BSYZ on learning and memory
of SAMP8 mice in the Morris water maze test
Spatial learning and memory in the SAMP8 mice was
determined using the Morris water maze test. In the hidden-platform
training phase, there were significant differences in escape
latency between the groups (F=4.612, P=0.003); however, there no
significant difference was observed among training days (F=0.164,
P=0.957) or in the interactive effect between group and training
day (F=1.549, P=0.086). Post hoc analysis revealed that the escape
latency in the SAMP8 group was significantly greater than the SAMR1
group (day 1–2, P<0.05; day 3–5, P<0.01). Following treatment
with low dose BSYZ, the escape latency on day 5 was significantly
reduced compared with the SAMP8 group (P<0.05). No significant
difference in escape latency was observed between the M-BSYZ/H-BSYZ
and SAMP8 groups (Fig. 1A).
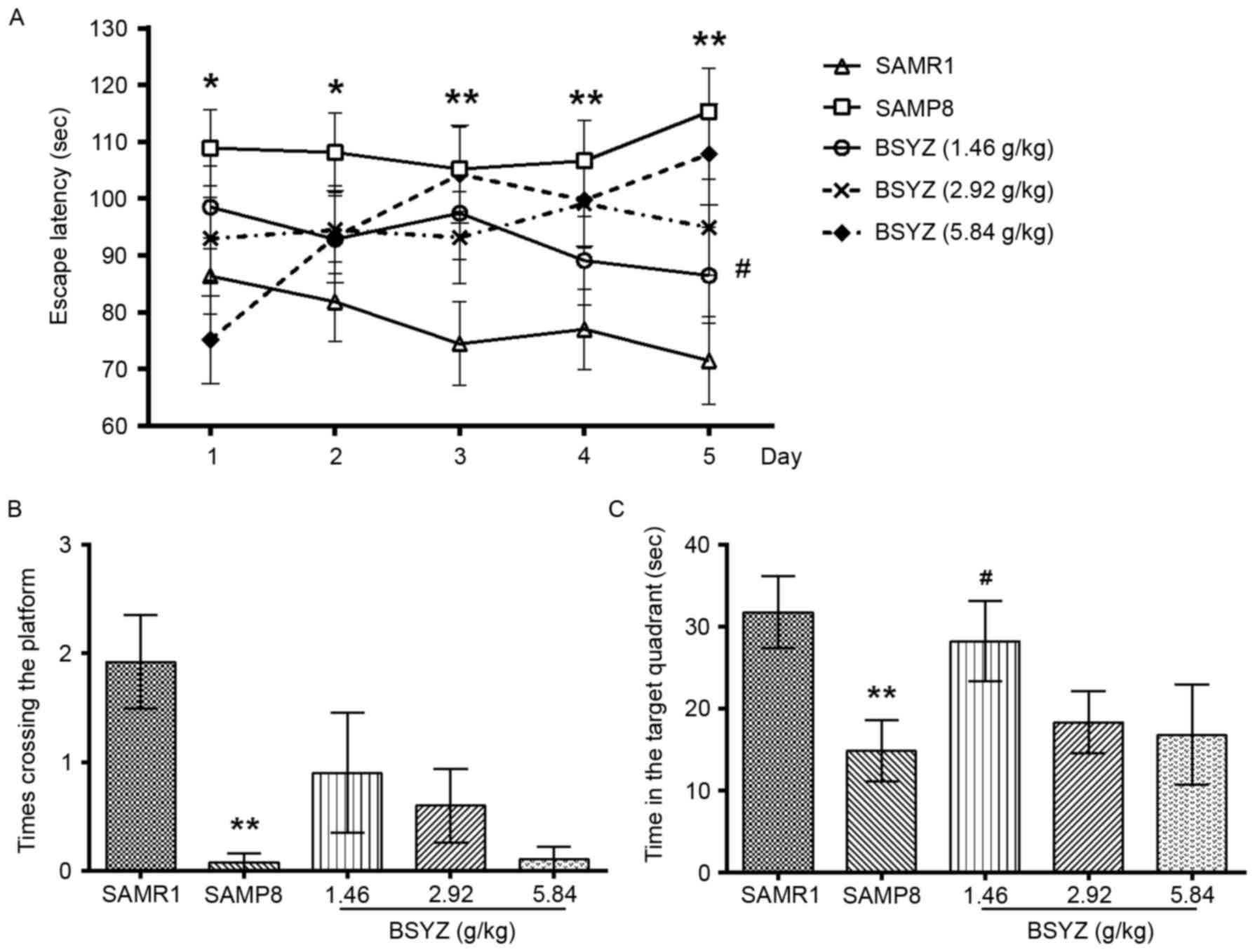 | Figure 1.Effects of BSYZ on the Morris water
maze test. (A) Escape latency to find the platform in the
hidden-platform training test. (B) Time crossing the platform and
(C) time spent in the target quadrant in the probe trial in SAMRI,
SAMP8, low dose BSYZ (1.46 g/kg), medium dose BSYZ (2.92 g/kg) and
high dose BSYZ (5.84 g/kg) mice. Data are presented as the mean ±
standard error (SAMR1, n=12; SAMP8, n=12; L-BSYZ, n=10; M-BSYZ,
n=10; H-BSYZ, n=9). *P<0.05 and **P<0.01 vs. SAMR1 group;
#P<0.05 vs. SAMP8 group. BSYZ, Bushen-Yizhi formula;
SAMP8, senescence-accelerated mouse prone 8; SAMRI,
senescence-accelerated mouse resistant 1. |
In the spatial probe trial phase, there were
significant differences in the times crossing the platform location
(Kruskal-Wallis Test: χ2=21.706, P<0.001) and the
time spent in the target quadrant (F=2.879, P=0.032) among the
groups. The time crossing the platform in the SAMP8 group was
significantly reduced compared with the SAMR1 group (P<0.01;
Fig. 1B). A similar result was
observed for time spent in the target quadrant between SAMP8 and
SAMR1 groups (P<0.01; Fig. 1C).
Compared with the SAMP8 mice, in the L-BSYZ treatment group, the
time spent in the target quadrant was significantly increased
(Fig. 1C; P<0.05); however, the
time crossing the platform between these two groups was not
significantly different (Fig.
1B).
Effects of BSYZ on learning and memory
of SAMP8 mice in the step-down test
Another method of assessing learning is measuring
passive avoidance using a step-down test. There were significant
differences in the number of errors made among the groups (F=3.313,
P=0.018). The number of errors in the SAMP8 group was significantly
increased compared with the SAMR1 group (P<0.01; Fig. 2A). Compared with the SAMP8 group,
administration of BSYZ at low and middle doses significantly
reduced the number of errors (P<0.05; Fig. 2A). No significant difference was
observed in latency between the groups (F=2.185, P=0.085; Fig. 2B).
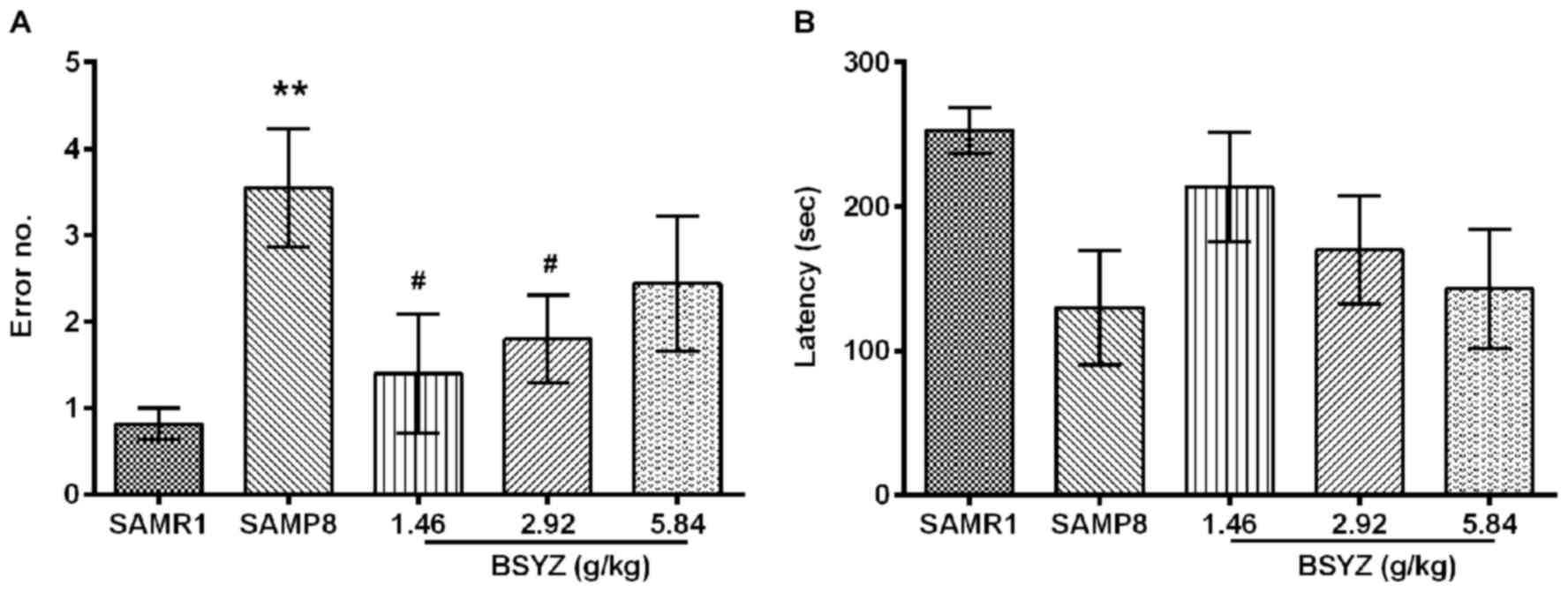 | Figure 2.Effects of BSYZ on the step-down
test. (A) Number of errors and (B) latency in SAMRI, SAMP8, low
dose BSYZ (1.46 g/kg), medium dose BSYZ (2.92 g/kg) and high dose
BSYZ (5.84 g/kg) mice. Data are presented as the mean ± standard
error (SAMR1, n=11; SAMP8, n=11; L-BSYZ, n=10; M-BSYZ; n=10,
H-BSYZ, n=9). **P<0.01 vs. SAMR1 group; #P<0.05
vs. SAMP8 group. BSYZ, Bushen-Yizhi formula; SAMP8,
senescence-accelerated mouse prone 8; SAMRI, senescence-accelerated
mouse resistant 1. |
Effects of BSYZ on GFAP expression in
the brains of SAMP8 mice
Immunohistochemical staining of GFAP was performed
in brain sections from the mice (Fig.
3A). There were significant differences in GFAP expression in
the hippocampus (F=62.220, P<0.001) and cortex (F=3.724,
P=0.042) among the groups. GFAP expression in the SAMP8 group was
significantly increased in the hippocampus and cortex compared with
the SAMR1 group (P<0.01; Fig.
3B). BSYZ treatment significantly reduced GFAP expression in
the brain compared with the SAMP8 group (hippocampus: All
P<0.01; cortex: P<0.05 for L-BSYZ and M-BSYZ groups; Fig. 3B).
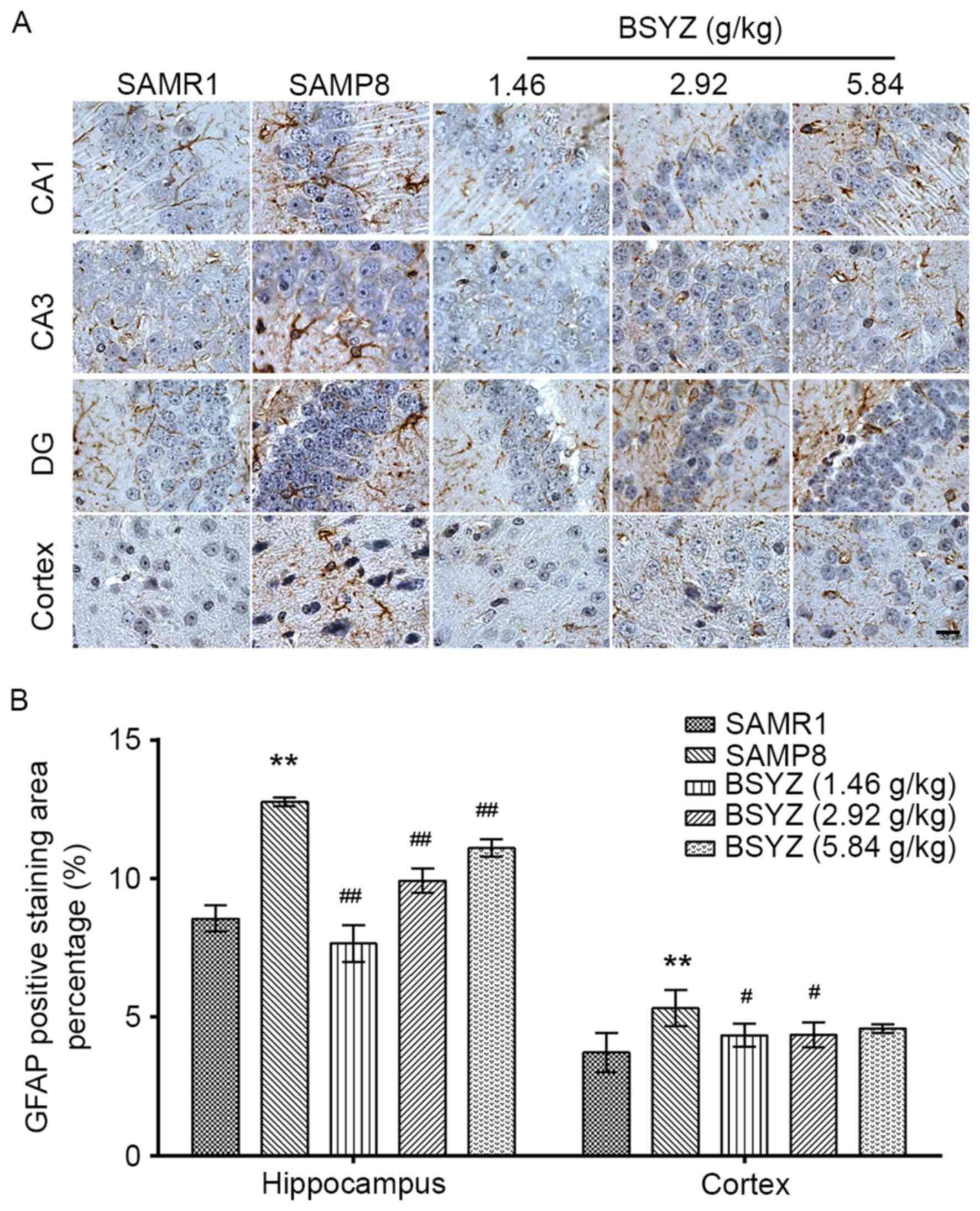 | Figure 3.Effects of BSYZ on GFAP expression in
brain tissue, as demonstrated by immunohistochemistry. (A) Images
of the immunohistochemical staining for GFAP in cortex, DG, CA3 and
CA1 tissues of SAMRI, SAMP8, low dose BSYZ (1.46 g/kg), medium dose
BSYZ (2.92 g/kg) and high dose BSYZ (5.84 g/kg) mice. Scale bar, 30
µm. (B) Percentage of positively stained GFAP cells. Data are
presented as the mean ± standard error (n=3/group). **P<0.01 vs.
SAMR1 group; #P<0.05 and ##P<0.01 vs.
SAMP8 group. BSYZ, Bushen-Yizhi formula; SAMP8,
senescence-accelerated mouse prone 8; SAMRI, senescence-accelerated
mouse resistant; GFAP, glial fibrillary acidic protein; DG, dentate
gyrus. |
Effects of BSYZ on COX-2 expression in
brains of SAMP8 mice
Immunohistochemical staining of COX-2 was performed
in brain sections from the mice (Fig.
4A). There were significant differences in COX-2 expression in
the hippocampus (F=4.413, P=0.026) and cortex (F=14.490,
P<0.001) among the groups. COX-2-positive staining in the SAMP8
group was significantly enhanced in the hippocampus and cortex
compared with the SAMR1 group (P<0.05 and P<0.01,
respectively; Fig. 4B). Following
treatment with BSYZ, COX-2 expression was significantly decreased
in the brain compared with in the SAMP8 group (hippocampus:
P<0.01, P<0.05 and P<0.05 for L-BSYZ, M-BSYZ and H-BSYZ
groups, respectively; cortex: All P<0.01; Fig. 4B).
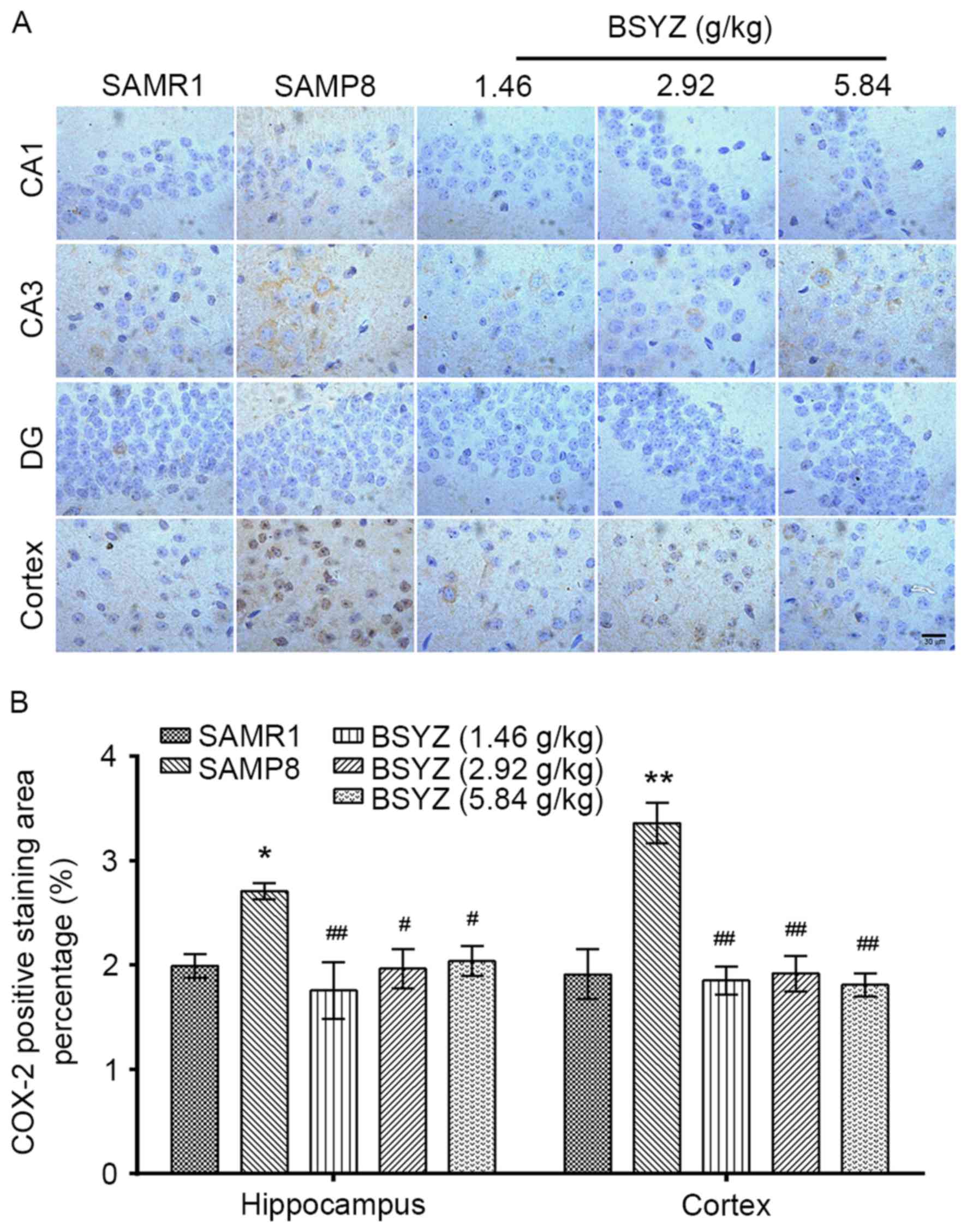 | Figure 4.Effects of BSYZ on COX-2 expression
in brain tissue, as demonstrated by immunohistochemistry. (A)
Images of immunohistochemical staining for COX-2 in cortex, DG, CA3
and CA1 tissues of SAMRI, SAMP8, low dose BSYZ (1.46 g/kg), medium
dose BSYZ (2.92 g/kg) and high dose BSYZ (5.84 g/kg) mice. Scale
bar, 30 µm. (B) Percentage of positively stained COX-2 cells. Data
are presented as the mean ± standard error (n=3/group). *P<0.05
and **P<0.01 vs. SAMR1 group; #P<0.05 and
##P<0.01 vs. SAMP8 group. BSYZ, Bushen-Yizhi formula;
SAMP8, senescence-accelerated mouse prone 8; SAMRI,
senescence-accelerated mouse resistant 1; COX-2, cyclooxygenase-2;
DG, dentate gyrus. |
Effects of BSYZ on PPAR-γ expression
in brains of SAMP8 mice
Immunohistochemical staining of PPAR-γ was performed
in brain sections from the mice (Fig.
5A). No significant differences in PPAR-γ expression were
observed in the hippocampus (F=1.911, P=0.185); however, there was
a significant difference in cortex expression (F=6.004, P=0.010)
among the groups. PPAR-γ expression in the SAMP8 group was
significantly reduced in the cortex compared with the SAMR1 group
(P<0.01; Fig. 5B). BSYZ
treatment significantly increased PPAR-γ expression in the cortex
compared with the SAMP8 group (P<0.01, P<0.05 and P<0.05
for L-BSYZ, M-BSYZ and H-BSYZ groups, respectively; Fig. 5B).
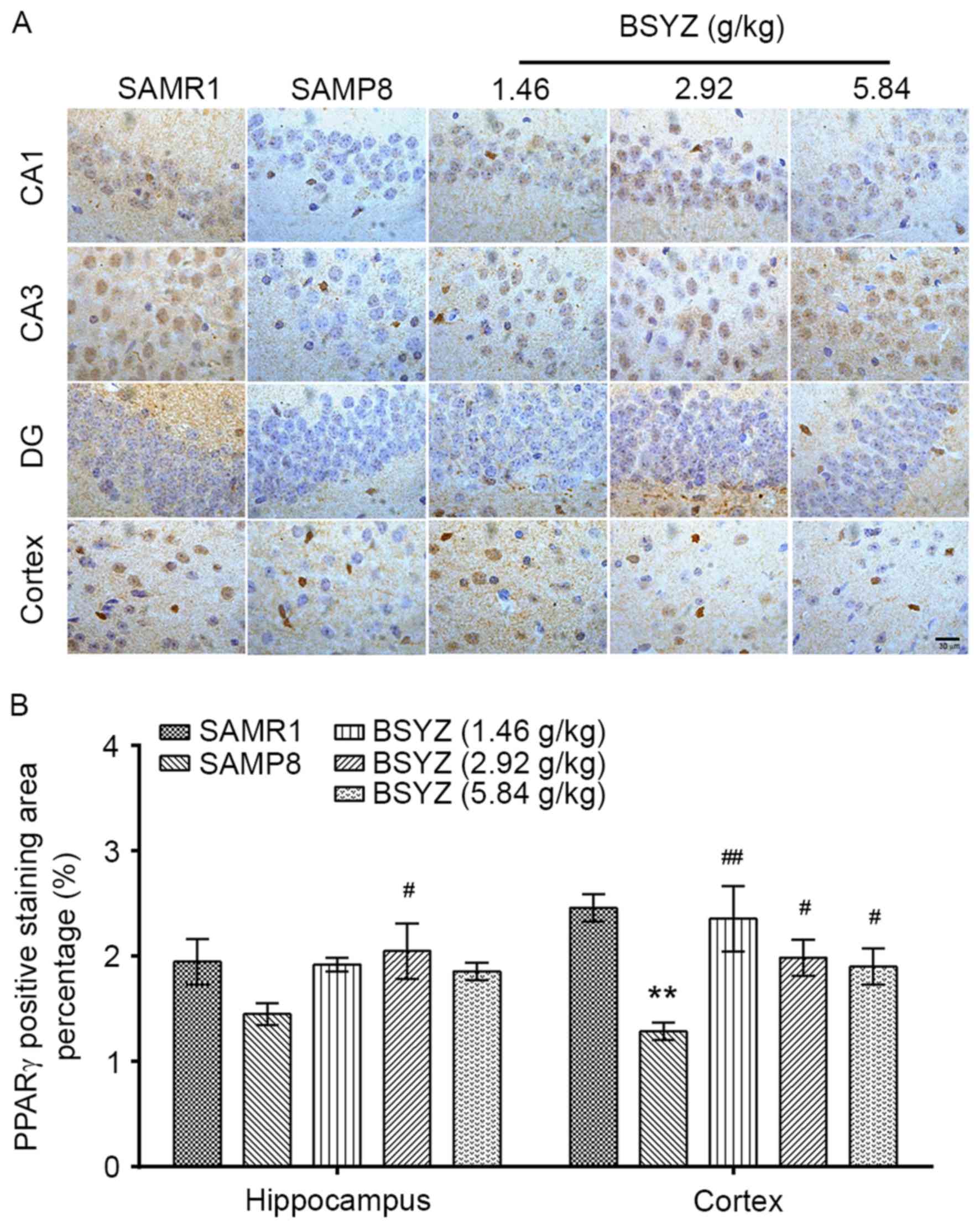 | Figure 5.Effects of BSYZ on PPAR-γ expression
in brain tissue, as demonstrated by immunohistochemistry. (A)
Images of the immunohistochemical staining for PPARγ in cortex, DG,
CA3 and CA1 tissues of SAMRI, SAMP8, low dose BSYZ (1.46 g/kg),
medium dose BSYZ (2.92 g/kg) and high dose BSYZ (5.84 g/kg) mice.
Scale bar: 30 µm. (B) Percentage of positively stained PPAR-γ
cells. Data are presented as the mean ± standard error (n=3/group).
**P<0.01 vs. SAMR1 group; #P<0.05 and
##P<0.01 vs. SAMP8 group. BSYZ, Bushen-Yizhi formula;
SAMP8, senescence-accelerated mouse prone 8; SAMRI,
senescence-accelerated mouse resistant 1; PPAR-γ, peroxisome
proliferator-activated receptor-γ; DG, dentate gyrus. |
Effects of BSYZ on NF-κB expression in
brains of SAMP8 mice
Immunohistochemical staining of NF-κB was performed
in brain sections from the mice (Fig.
6A). No significant differences in NF-κB expression were
observed in the hippocampus (F=2.471, P=0.112); however, there was
a significant difference in cortex expression (F=31.556,
P<0.001) among the groups. NF-κB expression in the SAMP8 group
was significantly increased in the hippocampus and in the cortex
compared with the SAMR1 group (P<0.05 and P<0.01,
respectively; Fig. 6B). However,
BSYZ treatment significantly decreased NF-κB expression in the
cortex compared with the SAMP8 group (P<0.01 for all BSYZ
groups; Fig. 6B).
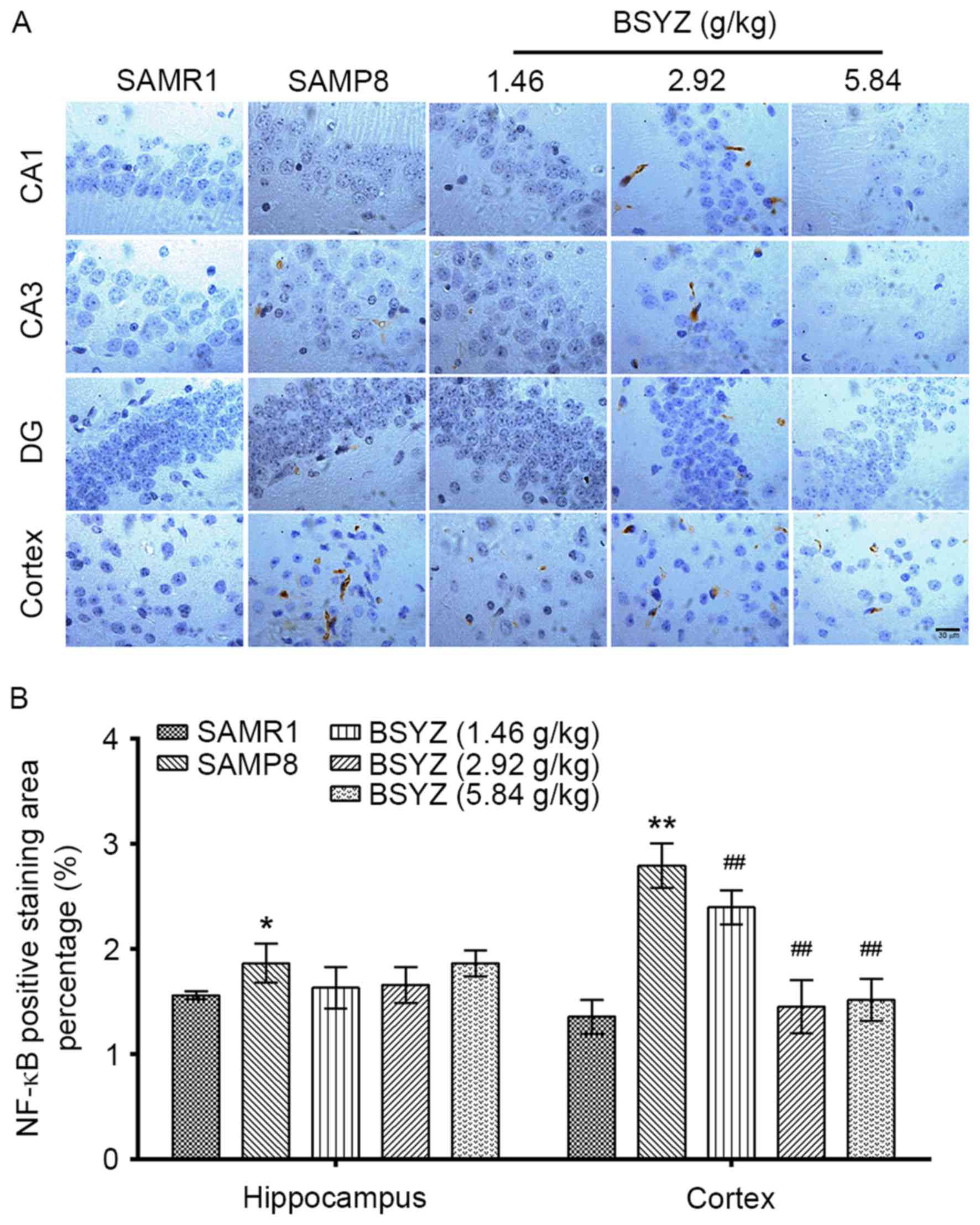 | Figure 6.Effects of BSYZ on NF-κB expression
in brain tissue, as demonstrated by immunohistochemistry. (A)
Images of the immunohistochemical staining for NF-κB in cortex, DG,
CA3 and CA1 tissues of SAMRI, SAMP8, low dose BSYZ (1.46 g/kg),
medium dose BSYZ (2.92 g/kg) and high dose BSYZ (5.84 g/kg) mice.
Scale bar, 30 µm. (B) Percentage of positively stained NF-κB cells.
Data are presented as the mean ± standard error (n=3/group).
*P<0.05 and **P<0.01 vs. SAMR1 group; ##P<0.01
vs. SAMP8 group. BSYZ, Bushen-Yizhi formula; SAMP8,
senescence-accelerated mouse prone 8; SAMRI, senescence-accelerated
mouse resistant 1; NF-κB, nuclear factor-κB; DG, dentate gyrus. |
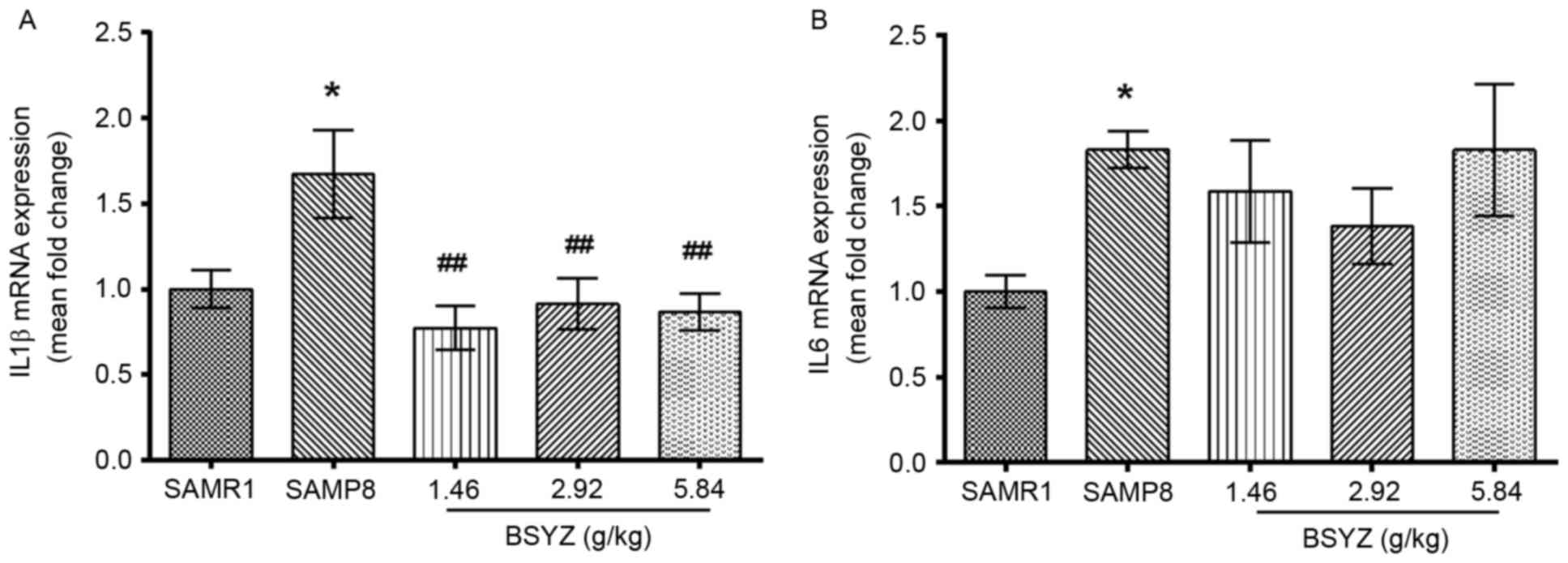
Effects of BSYZ on IL1B and IL6 mRNA
expression in brains of SAMP8 mice
The mRNA expression levels of genes encoding the
inflammatory cytokines IL-1β and IL-6, IL1B and IL6,
were measured by RT-qPCR. There was a significant difference in
IL1B mRNA expression levels among the groups (F=4.892,
P=0.011). The expression levels of IL1B in the SAMP8 group
were significantly higher compared with the SAMR1 group (P<0.05;
Fig. 7A). However, BSYZ treatment
significantly decreased IL1B expression compared with the
SAMP8 group (P<0.01 for all BSYZ groups; Fig. 7A). No significant difference in
IL6 expression was observed among the groups (F=1.994,
P=0.147; Fig. 7B). However,
IL6 was significantly increased in SAMP8 group compared with
the SAMR1 group (P<0.05; Fig.
7B).
Effects of BSYZ on oxidative stress in
SAMP8 mice
To assess the effects of BSYZ on oxidative stress of
SAMP8 mice, SOD and GSH-Px activities, and MDA content were
measured in mice brains (Fig. 8).
No significant difference in SOD activity was observed (F=1.901,
P=0.163); however, there were significant differences in MDA
content (F=5.458, P=0.006) and GSH-Px activity (F=5.618, P=0.006)
among the groups. Post hoc test demonstrated that MDA content in
the SAMP8 group was significantly greater compared with the SAMR1
group (P<0.01; Fig. 8B).
Conversely, the activities of SOD and GSH-Px in the SAMP8 mice were
significantly reduced compared with the SAMR1 group (P<0.05 and
P<0.01, respectively; Fig. 8A and
C). However, compared with the SAMP8 group, BSYZ treatment
significantly reversed SOD activity (P<0.05 in the L-BSYZ group;
Fig. 8A), MDA content (P<0.01
for all BSYZ groups; Fig. 8B) and
GSH-Px activity (P<0.01 for L-BSYZ and P<0.05 for M-BSYZ and
H-BSYZ groups, respectively; Fig.
8C).
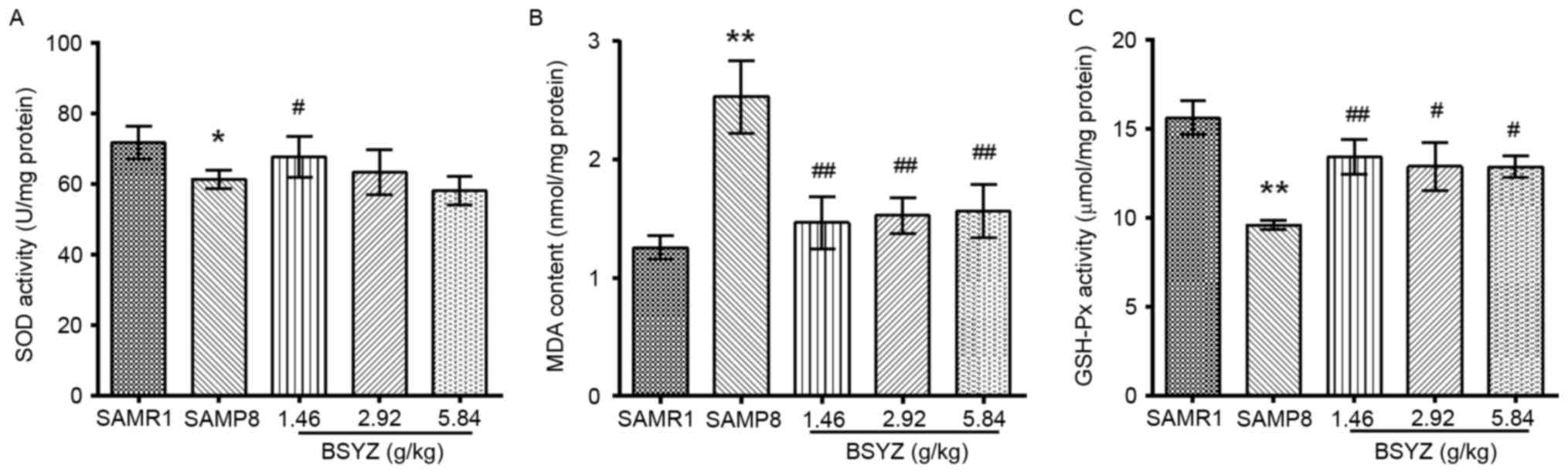 | Figure 8.Effects of BSYZ on SOD, MDA and
GSH-Px levels in the cortex of SAMP8 mice. (A) SOD activity, (B)
MDA content and (C) GSH-Px activity in the cortex of SAMRI, SAMP8,
low dose BSYZ (1.46 g/kg), medium dose BSYZ (2.92 g/kg) and high
dose BSYZ (5.84 g/kg) mice. Data are presented as the mean ±
standard error (n=4/group). *P<0.05 and **P<0.01 vs. SAMR1
group; #P<0.05 and ##P<0.01 vs. SAMP8
group. BSYZ, Bushen-Yizhi formula; SAMP8, senescence-accelerated
mouse prone 8; SAMRI, senescence-accelerated mouse resistant 1;
SOD, superoxide dismutase; MDA, malondialdehyde; GSH-Px,
glutathione peroxidase. |
Effects of BSYZ on neuronal apoptosis
in brains of SAMP8 mice
The immunohistochemical staining of Bcl-xL in the
brain tissue sections of mice is presented in Fig. 9A. Significant differences in Bcl-xL
expression were observed in the hippocampus (F=16.377, P<0.001)
and cortex (F=110.228, P<0.001) among the groups. Bcl-xL
expression in SAMP8 mice was significantly lower compared with the
SAMR1 mice (hippocampus, P<0.05; cortex, P<0.01; Fig. 9B). BSYZ treatment significantly
increased Bcl-xL expression in the hippocampus (P<0.05 for
L-BSYZ group; Fig. 9B) and cortex
(P<0.01, P<0.05 and P<0.05 for L-BSYZ, M-BSYZ and H-BSYZ
groups, respectively; Fig. 9B)
compared with the SAMP8 group.
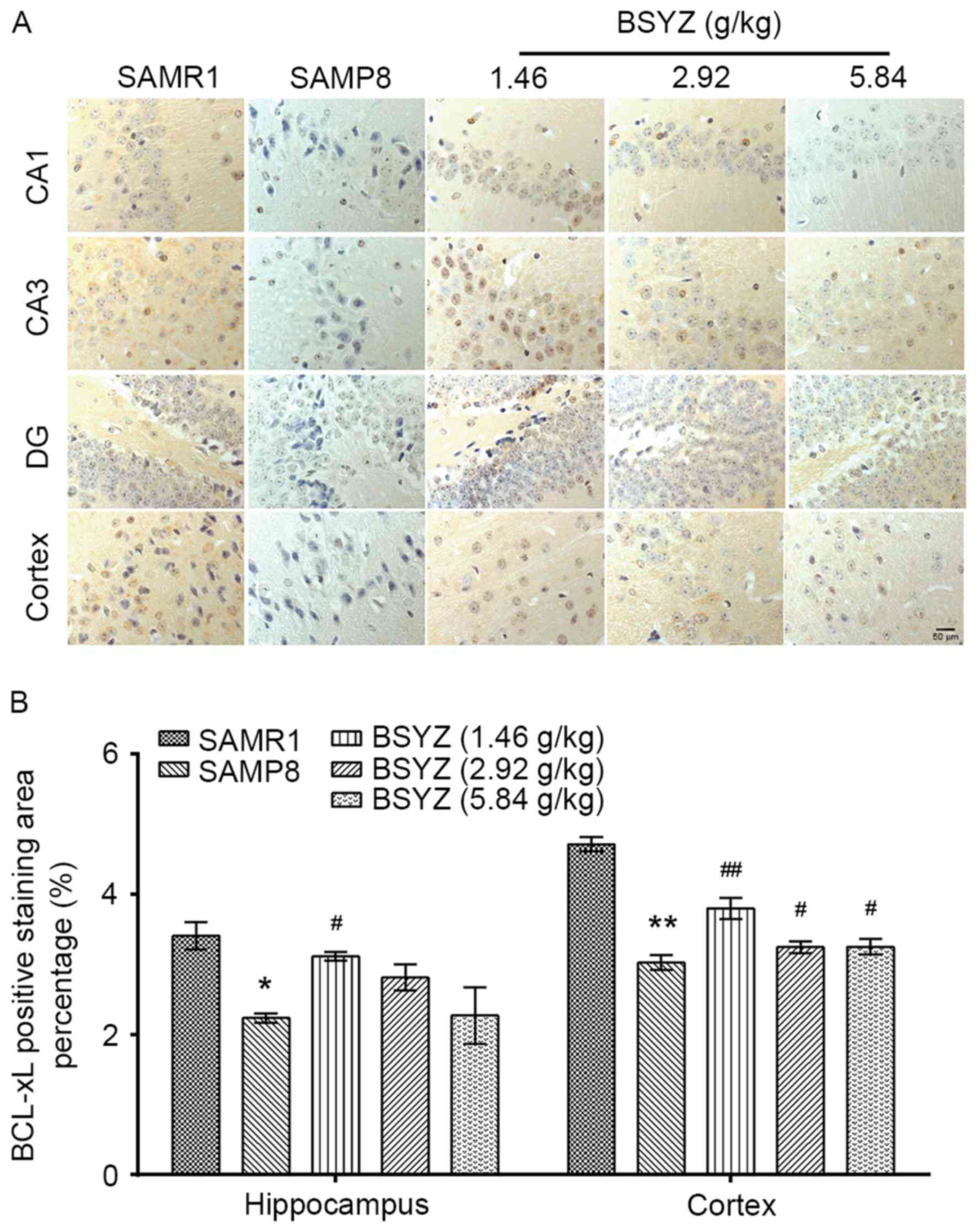 | Figure 9.Effects of BSYZ on Bcl-xL expression
in brain tissue, as demonstrated by immunohistochemistry. (A)
Images of immunohistochemical staining for Bcl-xL in cortex, DG,
CA3 and CA1 tissues of SAMRI, SAMP8, low dose BSYZ (1.46 g/kg),
medium dose BSYZ (2.92 g/kg) and high dose BSYZ (5.84 g/kg) mice.
Scale bar, 50 µm. (B) Percentage of positively stained Bcl-xL
cells. Data are presented as the mean ± standard error (n=3/group).
*P<0.05 and **P<0.01 vs. SAMR1 group; #P<0.05
and ##P<0.01 vs. SAMP8 group. BSYZ, Bushen-Yizhi
formula; SAMP8, senescence-accelerated mouse prone 8; SAMRI,
senescence-accelerated mouse resistant 1; Bcl-xL, B-cell lymphoma
extra-large; DG, dentate gyrus. |
The results of TUNEL staining in the brain tissue
sections of mice are presented in Fig. 10. There were significant
differences in the number of TUNEL-positive cells in the
hippocampus (F=26.507, P<0.001) and cortex (F=20.660,
P<0.001) among the groups. The number of TUNEL-positive cells in
the SAMP8 group was significantly increased compared with the SAMR1
group (P<0.01). Treatment with BSYZ resulted in a decrease in
the number of TUNEL-positive cells in the hippocampus and cortex
compared with the SAMP8 group (P<0.01).
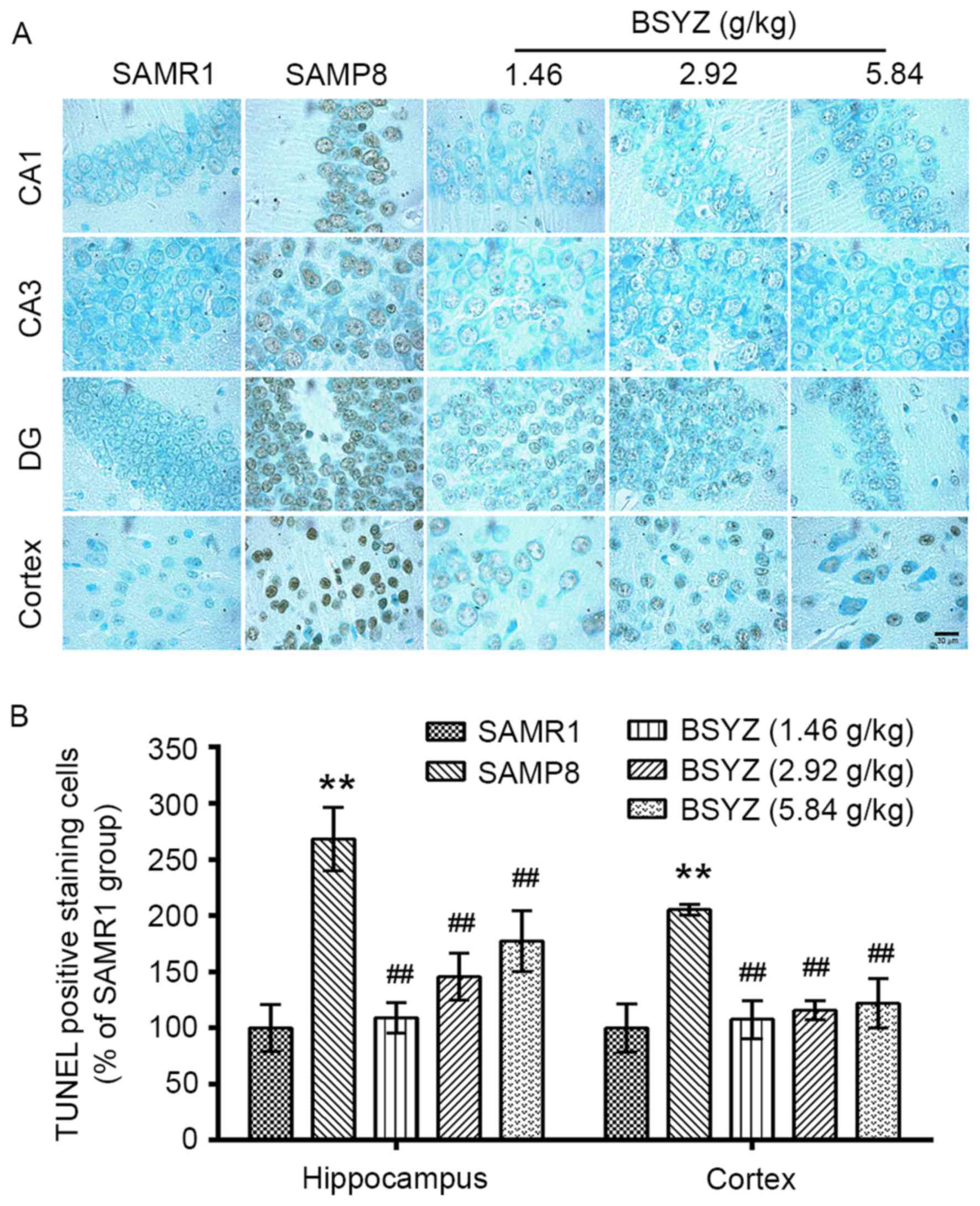 | Figure 10.Effects of BSYZ on TUNEL staining of
brain tissue. (A) Images of TUNEL staining in cortex, DG, CA3 and
CA1 tissues of SAMRI, SAMP8, low dose BSYZ (1.46 g/kg), medium dose
BSYZ (2.92 g/kg) and high dose BSYZ (5.84 g/kg) mice. Scale bar, 30
µm. (B) Percentage of positively stained TUNEL cells. Data are
presented as the mean ± standard error (n=3/group). **P<0.01 vs.
SAMR1 group; ##P<0.01 vs. SAMP8 group. BSYZ,
Bushen-Yizhi formula; SAMP8, senescence-accelerated mouse prone 8;
SAMRI, senescence-accelerated mouse resistant 1; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick-end labeling. |
Effects of BSYZ on histological
alterations in the brains of SAMP8 mice
As presented in Fig.
11A, irregular arrangement of neurons in the hippocampus and
unhealthy neurons (appearing as indistinct, darker or shrunken,
lacking a clear cell boundary, and possessing a small darkened
nucleus) were observed in brain tissue of SAMP8 mice. There were
significant differences in the number of unhealthy neurons in the
hippocampus (F=24.233, P<0.001) and cortex (F=17.947,
P<0.001) among the groups. In addition, the number of unhealthy
neurons in the SAMP8 group was significantly greater than in the
SAMR1 group (P<0.01; Fig.
11B). However, administration of BSYZ significantly decreased
the number of unhealthy neurons (hippocampus: P<0.01, P<0.01
and P<0.05 for L-BSYZ, M-BSYZ and H-BSYZ groups, respectively;
cortex: P<0.01 for L-BSYZ and M-BSYZ groups; Fig. 11B). Chromatolysis, a decrease in
Nissl-stained cells and shrinkage of cells was observed in the
brains of SAMP8 mice (Fig. 12A).
There were significant differences in the number of Nissl-positive
neurons in the hippocampus (F=13.428, P<0.001) and cortex
(F=8.273, P=0.003) among the groups. The number of Nissl-positive
neurons in the SAMP8 group was significantly reduced compared with
the SAMR1 group (P<0.01; Fig.
12B). However, administration of BSYZ significantly increased
the number of Nissl-positive neurons (hippocampus: P<0.01 for
L-BSYZ group; cortex: P<0.05 for L-BSYZ group; Fig. 12B) compared with the SAMP8
group.
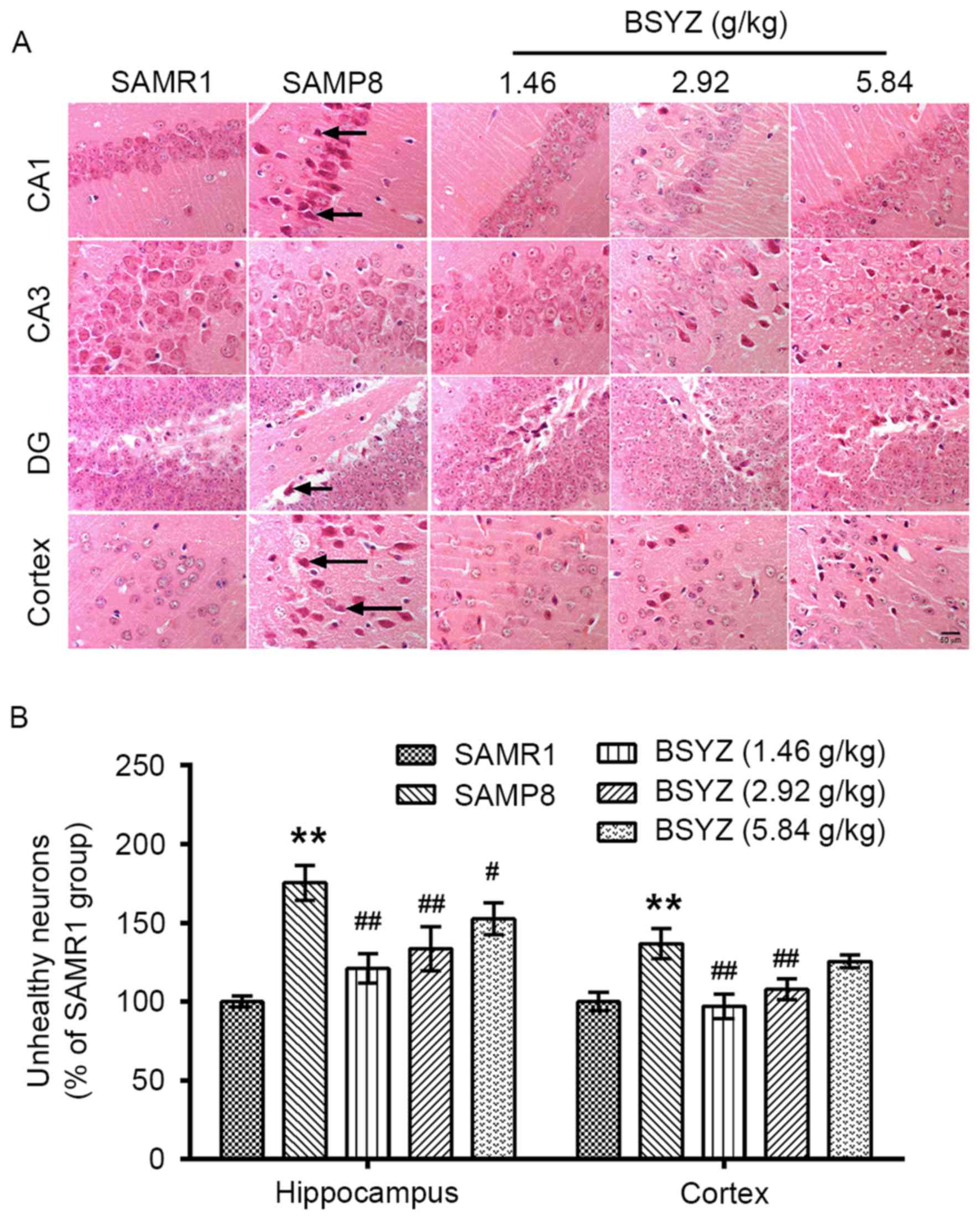 | Figure 11.Effects of BSYZ on H&E staining
of brain tissue. (A) Images of H&E staining in cortex, DG, CA3
and CA1 tissues of SAMRI, SAMP8, low dose BSYZ (1.46 g/kg), medium
dose BSYZ (2.92 g/kg) and high dose BSYZ (5.84 g/kg) mice. Black
arrows indicate unhealthy neurons. Scale bar: 50 µm. (B) Percentage
of positively stained unhealthy neurons. Data are presented as the
mean ± standard error (n=3/group). **P<0.01 vs. SAMR1 group;
#P<0.05 and ##P<0.01 vs. SAMP8 group.
BSYZ, Bushen-Yizhi formula; SAMP8, senescence-accelerated mouse
prone 8; SAMRI, senescence-accelerated mouse resistant 1; H&E,
hematoxylin and eosin; DG, dentate gyrus. |
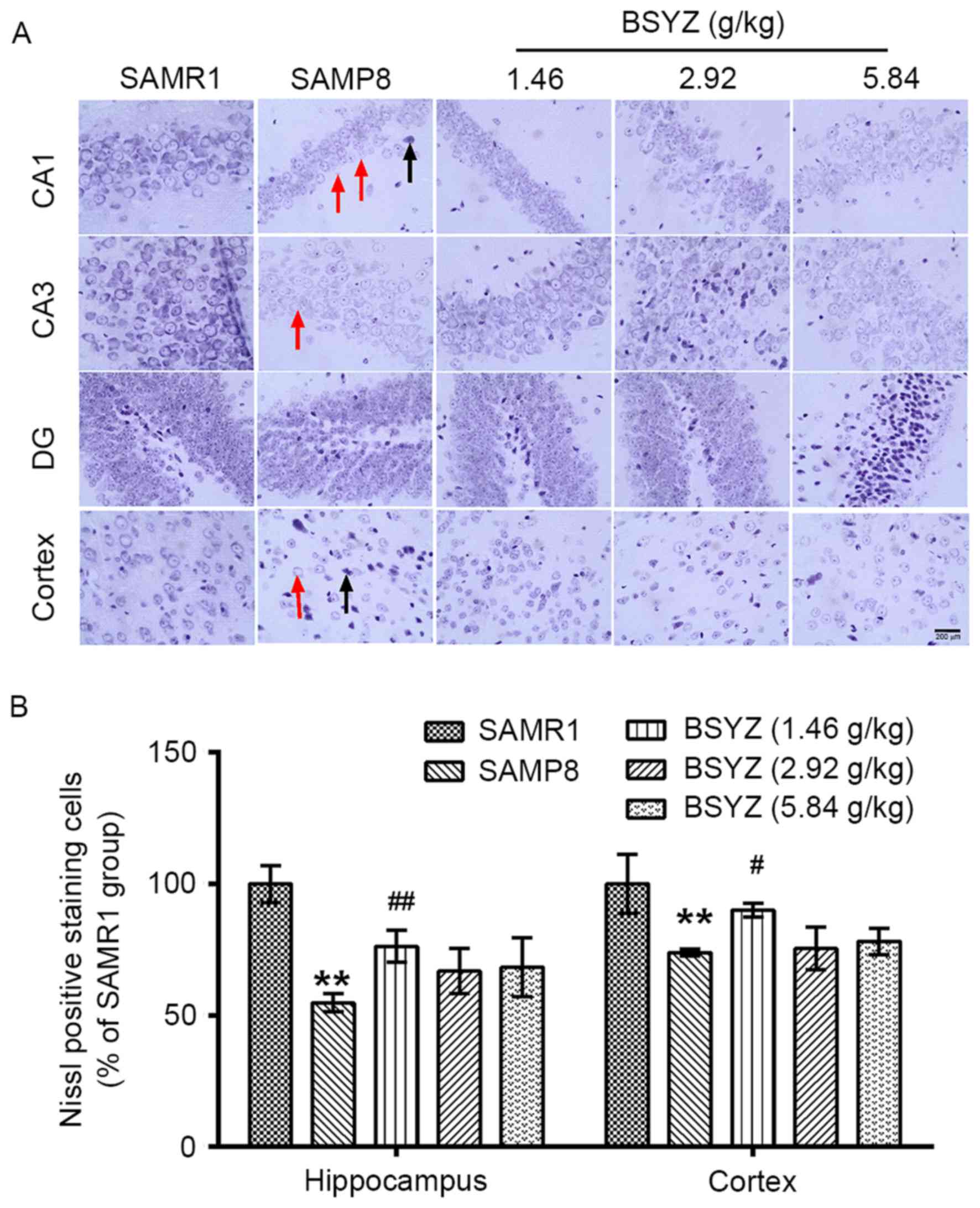 | Figure 12.Effects of BSYZ on Nissl staining of
brain tissue. (A) Images of Nissl staining in cortex, DG, CA3 and
CA1 tissues of SAMRI, SAMP8, low dose BSYZ (1.46 g/kg), medium dose
BSYZ (2.92 g/kg) and high dose BSYZ (5.84 g/kg) mice. Black arrows
indicate neuronal karyopyknosis, chromatolysis and shrinkage of
cells. Red arrows indicate a decrease in Nissl bodies. Scale bar,
200 µm. (B) Percentage of positively stained Nissl cells. Data are
presented as the mean ± standard error (n=3/group). **P<0.01 vs.
SAMR1 group; #P<0.05 and ##P<0.01 vs.
SAMP8 group. BSYZ, Bushen-Yizhi formula; SAMP8,
senescence-accelerated mouse prone 8; SAMRI, senescence-accelerated
mouse resistant 1; DG, dentate gyrus. |
Discussion
The present study demonstrated that BSYZ treatment
exerted a beneficial effect on learning and memory abilities in
SAMP8 mice, which was consistent with our previous studies in other
AD-like animal models (21,22).
In addition, BSYZ treatment demonstrated anti-inflammatory and
antioxidative effects, and decreased neuronal apoptosis in SAMP8
mice.
SAMP8 mice harbor AD-like cognitive and behavioral
alterations, and a neuropathological phenotype (27). In the present study, SAMP8 mice
demonstrated significant impairment of spatial learning and memory
in a Morris water maze test, in addition to deficiency of passive
avoidance response in a step-down test, compared with SAMR1 mice.
These results were similar to other studies (28,29).
Furthermore, administration of BSYZ improved cognitive impairment
in SAMP8 mice. Low dose BSYZ exhibited an improved
cognition-enhancing effect compared with the higher doses; however,
a further dose-effect study is warranted.
Inflammation is a risk factor in the course of
neurodegenerative diseases, including dementia. It has been
suggested that plasma levels of inflammatory proteins are increased
prior to the clinical onset of dementia, AD and vascular dementia
(30). Increased peripheral levels
of inflammatory markers are also associated with a modest increase
in the risk of all types of dementia (31).
Astrocytes are involved in the neuroinflammatory
response and become reactive in response to the majority of
pathological situations in the brain, including axotomy, ischemia,
infection and neurodegenerative diseases (13). Astrocyte reactivity was originally
characterized by morphological alterations, including hypertrophy
and process remodeling, and the overexpression of the intermediate
filament GFAP (13). It has been
reported that compared with in non-demented individuals,
significantly greater GFAP levels were observed in brain samples
from individuals diagnosed with AD, mixed dementia and
vascular-mediated dementia (32).
In addition, age-related increases in cellular hypertrophy of
GFAP-positive cells were observed in 6- and 10-month old SAMP8 mice
(33). Another study detected
higher levels of GFAP in the brains of SAMP8 mice compared with
SAMR1 mice via immunofluorescence staining and western blotting
(34). Therefore, the present
study detected GFAP expression in brain tissue sections from SAMP8
mice and investigated the effects of BSYZ on astrocyte activation.
Consistent with previous reports, hypertrophic astrocytes and
increased GFAP expression were observed in SAMP8 mice compared with
SAMRI mice, which were reduced following treatment with BSYZ. These
findings suggested that the effects of BSYZ on inflammation may be
mediated, at least partially, by reducing astrocyte activation in
the brain.
Astrocytes host a complex network of signaling
pathways, providing an abundance of potential molecular targets
(35), including the NF-κB
signaling pathway (13). Following
injection of oligomers of β-amyloid protein into the cortex of
rats, inflammatory markers, including COX-2, IL-1β and TNF-α were
expressed in reactive astrocytes, and GFAP and COX-2 proteins
co-localized with NF-κB (36). To
investigate whether BSYZ had effects on the regulation of
inflammatory markers, the present study measured COX-2, NF-κB and
IL1B expression in the brain tissue of
senescence-accelerated mice. In addition, other inflammatory
markers, including IL6 (37) and PPAR-γ, (38) which are associated with cognitive
function and are used as therapeutic targets, were detected. The
results from a previous study suggested that PPAR-γ agonists
affected modulation of the inflammatory response by reducing COX-2
protein expression and activating MAPKs and NF-κB in the
hippocampus of rats exposed to cerebral ischemia/reperfusion
(14). In the present study, the
levels of proinflammatory markers in SAMP8 mice were higher than in
SAMRI control mice. BSYZ treatment downregulated the expression
levels of COX-2 in the hippocampus and cortex of mice, and
significantly reduced the NF-κB expression in the cortex.
Conversely, BSYZ significantly increased PPAR-γ expression in the
cortex compared with untreated SAMP8 rats. In addition, BSYZ
treatment significantly downregulated IL1B; however, no
significant differences were observed in the mRNA expression levels
of IL6. These results suggested that BSYZ may exert an
anti-inflammatory effect by regulating numerous targets. However,
further research is required to support this theory.
Alterations in astrocyte function are associated
with oxidative stress. It has previously been reported that the
expression of certain oxidative stress markers is increased in aged
astrocytes (39). Oxidative damage
is associated with aging and age-related diseases. In the serum of
patients with MCI and AD, the activity of primary enzymatic
antioxidant defenses (SOD and GSH-Px) were decreased and production
of the MDA lipid peroxidation marker were increased, compared with
the age-matched control group (40). Similar alterations in oxidative
stress markers were observed in SAMP8 mice (41). Therefore, to assess the effects of
BSYZ on oxidative stress, the present study determined the SOD, MDA
and GSH-Px levels in the brains of SAMP8 mice. Results revealed
that oxidative damage in the cortex of SAMP8 mice was attenuated
following treatment with BSYZ. It has been reported that PPAR-γ
agonists may reduce oxidative stress (14). The ameliorative effects of BSYZ on
oxidative stress were consistent with the effects of BSYZ on
increasing PPAR-γ expression in the cortex.
It has previously been reported that PPAR-γ
overexpression prevents apoptosis by upregulating the
anti-apoptotic Bcl-2 family proteins, including Bcl-xL (42). SAMP8 mice demonstrated that with
age, there was a significant increase in the relative expression of
pancreatic genes involved in inflammation, oxidative stress and
apoptosis (43). Therefore, the
present study detected neuronal apoptosis in the brains of SAMP8
mice and protein expression of Bcl-xL. The results demonstrated
that the brains of SAMP8 mice exhibited histological alterations
and reduced Bcl-xL expression. Following treatment with BSYZ for 30
days, the number of neuronal apoptotic cells was decreased and the
expression levels of Bcl-xL were enhanced. These results suggested
that BSYZ may exert an anti-apoptotic effect.
In conclusion, the results of the present study
indicated that treatment with BSYZ improved cognitive function in
SAMP8 mice, and the underlying molecular mechanism may be
associated with anti-inflammatory, antioxidative and anti-apoptotic
effects. With these multi-target effects, BSYZ may be a potential
drug in treating dementia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81703901), the
Doctoral Fund of Education Ministry of China (grant no.
20134425110003), the Guangdong Provincial Major Science and
Technology for Special Program of China (grant no. 2012A080202017),
the Shandong Province Natural Science Foundation of China (grant
no. ZR2016HB56), the Foundation of Overseas Distinguished Taishan
Scholars of Shandong Province, and the Collaborative Innovation
Center for Research and Development of Traditional Chinese Medicine
in Mount Tai.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW and Y-BC designed the experiments. X-QH, H-PS,
S-YC and S-HF performed the experiments and X-QH and H-PS wrote the
manuscript. J-GZ revised the experimental design and manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Experimental protocols were approved by the Animal
Ethics Committee of Guangzhou University of Chinese Medicine, and
experiments were performed in compliance with the Guide for the
Care and Use of Laboratory Animals (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosano C, Marsland AL and Gianaros PJ:
Maintaining brain health by monitoring inflammatory processes: A
mechanism to promote successful aging. Aging Dis. 3:16–33.
2012.PubMed/NCBI
|
|
2
|
Carter SF, Schöll M, Almkvist O, Wall A,
Engler H, Långström B and Nordberg A: Evidence for astrocytosis in
prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: A
multitracer PET paradigm combining 11C-Pittsburgh compound B and
18F-FDG. J Nucl Med. 53:37–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choo IL, Carter SF, Schöll ML and Nordberg
A: Astrocytosis measured by 11C-deprenyl PET correlates
with decrease in gray matter density in the parahippocampus of
prodromal Alzheimer's patients. Eur J Nucl Med Mol Imaging.
41:2120–2126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wharton SB, O'Callaghan JP, Savva GM,
Nicoll JA, Matthews F, Simpson JE, Forster G, Shaw PJ, Brayne C and
Ince PG: MRC Cognitive Function and Ageing Neuropathology Study
Group: Population variation in glial fibrillary acidic protein
levels in brain ageing: Relationship to Alzheimer-type pathology
and dementia. Dement Geriatr Cogn Disord. 27:465–473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simpson JE, Ince PG, Lace G, Forster G,
Shaw PJ, Matthews F, Savva G, Brayne C and Wharton SB: MRC
Cognitive Function and Ageing Neuropathology Study Group: Astrocyte
phenotype in relation to Alzheimer-type pathology in the ageing
brain. Neurobiol Aging. 31:578–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Medeiros R and LaFerla FM: Astrocytes:
Conductors of the Alzheimer disease neuroinflammatory symphony. Exp
Neurol. 239:133–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mrak RE and Griffin WS: Interleukin-1,
neuroinflammation, and Alzheimer's disease. Neurobiol Aging.
22:903–908. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh S, Wu MD, Shaftel SS, Kyrkanides S,
LaFerla FM, Olschowka JA and O'Banion MK: Sustained interleukin-1β
overexpression exacerbates tau pathology despite reduced amyloid
burden in an Alzheimer's mouse model. J Neurosci. 33:5053–5064.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holmes C, Cunningham C, Zotova E,
Culliford D and Perry VH: Proinflammatory cytokines, sickness
behavior, and Alzheimer disease. Neurology. 77:212–218. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ho L, Purohit D, Haroutunian V, Luterman
JD, Willis F, Naslund J, Buxbaum JD, Mohs RC, Aisen PS and
Pasinetti GM: Neuronal cyclooxygenase 2 expression in the
hippocampal formation as a function of the clinical progression of
Alzheimer disease. Arch Neurol. 58:487–492. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mollace V, Colasanti M, Muscoli C, Lauro
GM, Iannone M, Rotiroti D and Nistico G: The effect of nitric oxide
on cytokine-induced release of PGE2 by human cultured astroglial
cells. Br J Pharmacol. 124:742–746. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Samy AS and Igwe OJ: Regulation of
IL-1β-induced cyclooxygenase-2 expression by interactions of Aβ
peptide, apolipoprotein E and nitric oxide in human neuroglioma. J
Mol Neurosci. 47:533–545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ben Haim L, Carrillo-de Sauvage MA,
Ceyzériat K and Escartin C: Elusive roles for reactive astrocytes
in neurodegenerative diseases. Front Cell Neurosci. 9:2782015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collino M, Aragno M, Mastrocola R,
Gallicchio M, Rosa AC, Dianzani C, Danni O, Thiemermann C and
Fantozzi R: Modulation of the oxidative stress and inflammatory
response by PPAR-gamma agonists in the hippocampus of rats exposed
to cerebral ischemia/reperfusion. Eur J Pharmacol. 530:70–80. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rinaldi P, Polidori MC, Metastasio A,
Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U
and Mecocci P: Plasma antioxidants are similarly depleted in mild
cognitive impairment and in Alzheimer's disease. Neurobiol Aging.
24:915–919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Torres LL, Quaglio NB, de Souza GT, Garcia
RT, Dati LM, Moreira WL, Loureiro AP, de Souza-Talarico JN, Smid J,
Porto CS, et al: Peripheral oxidative stress biomarkers in mild
cognitive impairment and Alzheimer's disease. J Alzheimers Dis.
26:59–68. 2011.PubMed/NCBI
|
|
17
|
López N, Tormo C, De Blas I, Llinares I
and Alom J: Oxidative stress in Alzheimer's disease and mild
cognitive impairment with high sensitivity and specificity. J
Alzheimers Dis. 33:823–829. 2013.PubMed/NCBI
|
|
18
|
Yan MH, Wang X and Zhu X: Mitochondrial
defects and oxidative stress in Alzheimer disease and Parkinson
disease. Free Radic Biol Med. 62:90–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rocha NP, Teixeira AL, Scalzo PL, Barbosa
IG, de Sousa MS, Morato IB, Vieira EL, Christo PP, Palotás A and
Reis HJ: Plasma levels of soluble tumor necrosis factor receptors
are associated with cognitive performance in Parkinson's disease.
Mov Disord. 29:527–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lindqvist D, Hall S, Surova Y, Nielsen HM,
Janelidze S, Brundin L and Hansson O: Cerebrospinal fluid
inflammatory markers in Parkinson's disease-associations with
depression, fatigue, and cognitive impairment. Brain Behav Immun.
33:183–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou XQ, Wu DW, Zhang CX, Yan R, Yang C,
Rong CP, Zhang L, Chang X, Su RY, Zhang SJ, et al: Bushen-Yizhi
formula ameliorates cognition deficits and attenuates oxidative
stressrelated neuronal apoptosis in scopolamine-induced senescence
in mice. Int J Mol Med. 34:429–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou XQ, Zhang L, Yang C, Rong CP, He WQ,
Zhang CX, Li S, Su RY, Chang X, Qin JH, et al: Alleviating effects
of Bushen-Yizhi formula on ibotenic acid-induced cholinergic
impairments in rat. Rejuvenation Res. 18:111–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Del Valle J, Bayod S, Camins A,
Beas-Zárate C, Velázquez-Zamora DA, González-Burgos I and Pallàs M:
Dendritic spine abnormalities in hippocampal CA1 pyramidal neurons
underlying memory deficits in the SAMP8 mouse model of Alzheimer's
disease. J Alzheimers Dis. 32:233–240. 2012.PubMed/NCBI
|
|
24
|
Chen Y, Wei G, Nie H, Lin Y, Tian H, Liu
Y, Yu X, Cheng S, Yan R, Wang Q, et al: β-Asarone prevents
autophagy and synaptic loss by reducing ROCK expression in
asenescence-accelerated prone 8 mice. Brain Res. 1552:41–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu L, Zhang L, Zhan L, Lu X, Peng J,
Liang L, Liu Y, Zheng L, Zhang F and Liu Q: The effects of Zibu
Piyin Recipe components on scopolamine-induced learning and memory
impairment in the mouse. J Ethnopharmacol. 151:576–582. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng XR, Zhou WX and Zhang YX: The
behavioral, pathological and therapeutic features of the
senescence-accelerated mouse prone 8 strain as an Alzheimer's
disease animal model. Ageing Res Rev. 13:13–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Orejana L, Barros-Miñones L, Jordán J,
Puerta E and Aguirre N: Sildenafil ameliorates cognitive deficits
and tau pathology in a senescence-accelerated mouse model.
Neurobiol Aging. 33:625.e11–e20. 2012. View Article : Google Scholar
|
|
29
|
He XL, Zhou WQ, Bi MG and Du GH:
Neuroprotective effects of icariin on memory impairment and
neurochemical deficits in senescence-accelerated mouse prone 8
(SAMP8) mice. Brain Res. 1334:73–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Engelhart MJ, Geerlings MI, Meijer J,
Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A,
Witteman JC and Breteler MM: Inflammatory proteins in plasma and
the risk of dementia: The rotterdam study. Arch Neurol. 61:668–672.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koyama A, O'Brien J, Weuve J, Blacker D,
Metti AL and Yaffe K: The role of peripheral inflammatory markers
in dementia and Alzheimer's disease: A meta-analysis. J Gerontol A
Biol Sci Med Sci. 68:433–440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kashon ML, Ross GW, O'Callaghan JP, Miller
DB, Petrovitch H, Burchfiel CM, Sharp DS, Markesbery WR, Davis DG,
Hardman J, et al: Associations of cortical astrogliosis with
cognitive performance and dementia status. J Alzheimers Dis.
6(595–604): discussion 673–681. 2004.PubMed/NCBI
|
|
33
|
Watanabe K, Tonosaki K, Kawase T, Karasawa
N, Nagatsu I, Fujita M and Onozuka M: Evidence for involvement of
dysfunctional teeth in the senile process in the hippocampus of
SAMP8 mice. Exp Gerontol. 36:283–295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernandez-Gómez FJ, Muñoz-Delgado E,
Montenegro MF, Campoy FJ, Vidal CJ and Jordán J: Cholinesterase
activity in brain of senescence-accelerated-resistant mouse SAMR1
and its variation in brain of senescence-accelerated-prone mouse
SAMP8. J Neurosci Res. 88:155–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Furman JL, Sama DM, Gant JC, Beckett TL,
Murphy MP, Bachstetter AD, Van Eldik LJ and Norris CM: Targeting
astrocytes ameliorates neurologic changes in a mouse model of
Alzheimer's disease. J Neurosci. 32:16129–16140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carrero I, Gonzalo MR, Martin B,
Sanz-Anquela JM, Arévalo-Serrano J and Gonzalo-Ruiz A: Oligomers of
β-amyloid protein (Aβ1-42) induce the activation of
cyclooxygenase-2 in astrocytes via an interaction with
interleukin-1β, tumour necrosis factor-α, and a nuclear factor κ-B
mechanism in the rat brain. Exp Neurol. 236:215–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eriksson UK, Pedersen NL, Reynolds CA,
Hong MG, Prince JA, Gatz M, Dickman PW and Bennet AM: Associations
of gene sequence variation and serum levels of C-reactive protein
and interleukin-6 with Alzheimer's disease and dementia. J
Alzheimers Dis. 23:361–369. 2011.PubMed/NCBI
|
|
38
|
Liu J, Wang LN and Jia JP: Peroxisome
proliferator-activated receptor-gamma agonists for Alzheimer's
disease and amnestic mild cognitive impairment: A systematic review
and meta-analysis. Drugs Aging. 32:57–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pertusa M, Garcia-Matas S, Rodriguez-Farré
E, Sanfeliu C and Cristofol R: Astrocytes aged in vitro show a
decreased neuroprotective capacity. J Neurochem. 101:794–805. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Padurariu M, Ciobica A, Hritcu L, Stoica
B, Bild W and Stefanescu C: Changes of some oxidative stress
markers in the serum of patients with mild cognitive impairment and
Alzheimer's disease. Neurosci Lett. 469:6–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakajima A, Aoyama Y, Nguyen TT, Shin EJ,
Kim HC, Yamada S, Nakai T, Nagai T, Yokosuka A, Mimaki Y, et al:
Nobiletin, a citrus flavonoid, ameliorates cognitive impairment,
oxidative burden, and hyperphosphorylation of tau in
senescence-accelerated mouse. Behav Brain Res. 250:351–360. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu JS, Lin TN and Wu KK: Rosiglitazone and
PPAR-gamma overexpression protect mitochondrial membrane potential
and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family
proteins. J Cell Physiol. 220:58–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cuesta S, Kireev R, Garcia C, Forman K,
Escames G, Vara E and Tresguerres JA: Beneficial effect of
melatonin treatment on inflammation, apoptosis and oxidative stress
on pancreas of a senescence accelerated mice model. Mech Ageing
Dev. 132:573–582. 2011. View Article : Google Scholar : PubMed/NCBI
|