Introduction
Chronic arrhythmia is a serious threat to human
health, and anti-arrhythmic medication and the fitting of
electronic pacemakers are the standard treatments at present
(1). A biological pacemaker based
on cell and gene technology would be an improved clinical treatment
(2). However, the lack of ideal
seed cells hinders the development of biological pacemakers.
Adipose-derived stem cells (ADSCs) are easily obtained and
amplified, and exhibit weak immunogenicity and strong
differentiation (3). The
expression of cardiac troponin I was detected in differentiated
ADSCs isolated from the myocardial cells of mice aged 1–2 weeks
(4). Furthermore, it has been
reported that ADSCs are able to differentiate into pacemaker-like
cells in vitro (5).
Therefore, it may be hypothesized that ADSCs may have the potential
to become ideal seed cells for the development of biological
pacemakers.
T-box (TBX) 18 is a biomarker for pacemaker
progenitor cells and is associated with the development of the
heart, including the formation of the venous pole (6). Gene tracing technology has
demonstrated that the majority of cells in the sinoatrial node head
originate from TBX18+ progenitor cells, indicating that
TBX18 is an important transcription factor in the development of
the embryonic sinoatrial node (7).
Additionally, TBX18 is able to promote the development and
differentiation of embryonic epicardial cells (8). It has also been reported that
co-culture of white adipose-derived stem cells (WASCs) with newborn
mouse myocardial cells transfected with TBX18 promotes WASC
differentiation into pacemaker-like cells (9). Furthermore, one study also
demonstrated that stem cells isolated from brown adipose tissues
exhibit a high myocardial differentiation capacity and
spontaneously differentiate into functional cardiomyocytes in
vitro (10). Our previous
study demonstrated that brown adipose-derived stem cells (BASCs)
spontaneously differentiated into pacemaker cells in vitro,
and TBX18 mRNA and protein expression was elevated during the
differentiation process (11).
Based on these findings, it was hypothesized that transduction of
BASCs with the TBX18 gene may further promote their differentiation
into pacemaker-like cells. WASCs were also transduced with TBX18 as
they also have the ability to differentiate into myocardial cells.
TBX18-induced differentiation ability was assessed in BASCs and
WASCs to identify a more ideal seed cell line for the development
of biological pacemakers.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) was
purchased from Corning Incorporated (Corning, NY, USA) and fetal
bovine serum (FBS) was purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Penicillin, streptomycin,
amphotericin B were purchased from Gibco (Thermo Fisher Scientific,
Inc.), and bovine serum albumin (BSA) was purchased from
Sigma-Aldrich (cat. no. 0245C789; Merck KGaA, Darmstadt, Germany)
and TBX18 primary antibody (rabbit; cat. no. sc-17867) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
TBX3 (mouse; cat. no. ab89220;), sarcomeric α-actinin (Sr; mouse;
cat. no. ab9465) and hyperpolarization-activated cyclic
nucleotide-gated channel 4 (HCN4; rat; cat. no. ab32675) primary
antibodies were purchased from Abcam (Cambridge, MA, UK). β-actin
primary antibody (WB0196; 1:1,000; Shanghai Wei Biotechnology Co.,
Ltd., Shanghai, China), FITC-goat anti-mouse secondary antibody
(GB22301; 1:1,000; Shanghai Wei Biotechnology Co., Ltd.) was used
in immunofluorescence of Sr and CY3-goat anti-rat IgG (GB; GB21302;
1:1,000; Shanghai Wei Biotechnology Co., Ltd.) was used in
immunofluorescence of HCN4. DAPI-Staining-solution was purchased
from Wuhan Boster Biological Technology, Ltd., Wuhan, China (cat.
no. AR1176). An TRIquick total RNA extraction kit (cat. no. R1100)
was purchased from Beijing Solarbio Science and Technology Co.,
Ltd. (Beijing, China). Adenovirus (AD)-cherry-TBX18
(1.06×1010 PFU/ml) and AD-green fluorescent protein
(AD-GFP; 2.03×1010 PFU/ml) were prepared by Shanghai
Genechem Co., Ltd. (Shanghai, China).
Isolation and culture of stem
cells
All animal experiments were approved by the ethics
committee of the Second Military Medical University (Shanghai,
China). Healthy male C57BL/6 mice (n=25; weight, 30 g) were
obtained from the Laboratory Animal Center of the Second Military
Medical University, and they were kept at room temperature, with
free access to water and food (SLACOM; cat. no. 201404001; Shanghai
Pu Lu Teng Biotechnology Co., Ltd., Shanghai, China), 12-h
light/dark cycle and humidity 40–50%. Brown and white adipose
tissues were respectively isolated from the shoulder blade and
groin of the mice at 3–4 weeks. Tissues were digested in a solution
containing 0.1% BSA and 0.01% collagenase type II (Sigma-Aldrich;
Merck KGaA) in a 37°C water bath for 45 min. Tissue residues were
filtered by centrifugation at 800 × g for 5 min at 4°C. The
supernatant was discarded and cells were resuspended in DMEM
containing 15% FBS, 0.5% penicillin, 0.5%streptomycin and
0.5%amphotericin B, at 37°C with 5% CO2. The medium was
replaced every two days and cells were subcultured when 80–90%
confluence was reached.
Flow cytometry analysis
Third-generation WASCs and BASCs
(>106/ml) were harvested, centrifuged at 230 × g for
5 min at 4°C, resuspended in PBS, transferred to 1 ml EP tubes and
centrifuged again at 230 × g for 5 min at 4°C. The supernatant was
discarded and WASCs and BASCs were resuspended in the blocking
reagent containing 10% goat FBS and PBS for 30 min at room
temperature, and then incubated with indirect labeling primary
antibody CD29 (rabbit; cat. no. ab179471; 1:100), direct labeling
primary antibody CD90 (cat. no. ab226; 1:100), indirect labeling
primary antibody CD34 (rabbit; cat. no. ab81289; 1:100) and direct
labeling primary antibody CD45 (cat no. ab33916; 1:100; all Abcam)
monoclonal antibodies for 30 min at 4°C in the dark, respectively.
Then the corresponding secondary antibodies, CY3-goat anti-rabbit
IgG (GB21303; 1:100; Shanghai Wei Biotechnology Co., Ltd.,
Shanghai, China), FITC-goat anti-rabbit IgG (GB22303; 1:100;
Shanghai Wei Biotechnology Co., Ltd.) were added for 30 min at 4°C
in the dark. Additionally, a blank group was set up; cells were
centrifuged at 230 × g for 5 min at 4°C, the supernatant was
removed, and cells were washed with PBS twice and resuspended in
PBS prior to analysis using a flow cytometer (BD FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA) with CellQuest Pro version
5.2.1 software.
Multipotential differentiation of
BASCs and WASCs
Third-generation BASCs and WASCs under logarithmic
growth phase were cultured in a complete medium (DMEM containing
15% FBS, 0.5% penicillin, 0.5% streptomycin and 0.5% amphotericin
B). When cells had reached 100% confluence, the medium was
discarded by careful suction. Tri-lineage differentiation medium
(adipogenesis; cat. no. MUBMD-90031; osteogenesis; cat. no.
MUBMX-90021; and chondrogenesis; cat. no. MUBMD-9004; Cyagen
Biosciences Guangzhou, Inc., Guangzhou, China) was used to confirm
mesenchymal origin. Induction medium was replaced every three days
(adipogenesis, 7 days; osteogenesis, 21 days; chondrogenesis, 21
days) at 37°C with 5% CO2. Subsequently, the induced
cells were fixed in 4% paraformaldehyde for 10 min, at room
temperature and rinsed with PBS. Cells were stained with 0.5% Oil
Red O for 10 min to detect lipids and with 1% alizarin red S (pH
7.2) for 8 min to detect calcium deposition. The accumulation of
chondrocyte matrix was detected by Alcian Blue solution (anhydrous
ethanol 80 ml, glacial acetic acid 20 ml, Alcian Blue powder 0.1 g)
staining for 10 min (pH 2.5), all the experimental conditions were
at a room temperature. All above reagents were purchased from
Cyagen Biosciences Guangzhou, Inc. Cells were observed using an
Olympus IX70 microscope (Olympus Corporation, Tokyo, Japan) at
magnification, ×200.
Transduction of BASCs and WASCs with
TBX18
Adenovirus (AD)-TBX18 (1.06×1010 PFU/ml)
and AD-green fluorescent protein (AD-GFP; 2.03×1010
PFU/ml) were prepared by Shanghai Genechem Co., Ltd. (Shanghai,
China). When cells had reached 95% confluence and following medium
replacement, adipose stem cells were randomized into the control
group (WASC/BASC), blank virus genome group (GFP-WASC/BASC) and the
target gene group (TBX18-WASC/BASC). Prior to transduction with
AD-TBX18 and AD-GFP, cells were cultured in 100% DMEM for 12 h,
then complete medium (DMEM containing 15% FBS, 0.5% penicillin,
0.5% streptomycin and 0.5% amphotericin B), at 37°C with 5%
CO2. Cell transduction efficiency was tested at a
multiplicity of infection (MOI) of 10, 25, 40, 50 and 70; a MOI of
50 was subsequently selected as the transduction degree. Cells were
observed and imaged (Olympus IX70; Olympus Corporation) 48 h
post-transduction by fluorescence microscopy.
Transmission electron microscopy
(TEM)
At 48 h post-transduction collected cell samples
were washed three times with PBS and digested with 0.25% trypsin
(Gibco; Thermo Fisher Scientific, Inc.). Digested cells were
resuspended in a centrifuge tube containing serum medium (85% DMEM
and 15% FBS) and centrifuged at 230 × g for 5 min at 4°C. The
supernatant was discarded and cells were rapidly fixed in 2%
glutaraldehyde acetone solution at room temperature and subjected
to gradient dehydration. Epoxy resin (Epon812) was used for
embedding at 37°C for 3 h, and the sections cut at 70 nm, selected,
located and double-stained with uranyl acetate for 20 min and lead
citrate for 5 min, both at room temperature. The ultrastructures
were subsequently observed by TEM (magnification, ×10,000; Hitachi
h7650; Hitachi, Ltd., Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TBX18, TBX3, Sr and HCN4 mRNA expression was
detected by RT-qPCR. Total RNA was extracted with a TRIquick total
RNA extraction kit (R1100; Beijing Solarbio Science and Technology
Co., Ltd.) according to the manufacturer's protocol. A First Strand
cDNA synthesis kit (K1622; Invitrogen; Thermo Fisher Scientific,
Inc.) and a Quantity Nova SYBR-Green PCR kit (208052; Qiagen GmbH,
Hilden, Germany) were used for qPCR, according to the
manufacturer's protocols. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 3 min, followed by 40
cycles of 95°C for 10 sec and 55°C for 15 sec, and a final
extension at 72°C for 15 sec (12). Each sample was tested at least
three times and β-actin was used as the internal reference gene.
mRNA expression was quantified using the 2−ΔΔCq method
(13). The primers were designed
and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and
the sequences are listed in Table
I. qPCR was performed on a Roche 480II Real Time PCR System
(Roche Diagnostics, Basel, Switzerland).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| β-actin |
AGCCATGTACGTAGCCATCC |
GCTGTGGTGGTGAAGCTGTA |
| TBX18 |
TGCTGTCCCTGCTACACATC |
GCTGTAGGTCTCTGCCAAGG |
| HCN4 |
TCATCTCCTCCATCCCTGTC |
CTGGCCAGGTCATAGGTCAT |
| TBX3 |
GCTGCTGCGAACTCTCTTCT |
GAAGGTGTCGGAAACTGGAA |
| Sr |
TCATCTCAGGTGAACGCTTG |
AGATGTCCTGGATGGCAAAG |
Immunofluorescence staining
At 48 h post-transduction cells (when cells had
reached 60% confluence) were cultured on glass slides in a complete
medium (DMEM containing 15% FBS, 0.5% penicillin, 0.5% streptomycin
and 0.5% amphotericin B) for 48 h at 37°C with 5% CO2.
Cells were fixed in 100% methanol for 10 min and blocked with 5%
BSA (containing 0.3% Triton X-100 for permeabilization;
Sigma-Aldrich; Merck KGaA) for 30 min prior to incubation with HCN4
(1:200) and Sr (1:50) primary antibodies overnight at 4°C. Cells
were subsequently washed three times (5 min each time) with PBS and
incubated with FITC-goat anti-mouse secondary antibody (GB22301;
1:1,000; Shanghai Wei Biotechnology Co., Ltd.) and CY3-goat
anti-rabbit IgG (GB; GB21302; 1:1,000; Shanghai Wei Biotechnology
Co., Ltd.) at room temperature for 1 h prior to counterstaining
with DAPI for 3 min at room temperature. The prepared sample was
observed under an inverted fluorescence microscope. Images were
collected with a CCD digital camera (Canon, Inc., Tokyo, Japan).
The obtained fluorescent images were analyzed (three fields from
each sample) with Image Pro Plus version 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA) and Adobe Photoshop CS3 (Adobe Systems,
Inc., San Jose, CA, USA).
Western blot analysis
At 48 h post-transduction, proteins were extracted
from adipose stem cells by RIPA (cat. no. WB0101; Shanghai Wei
Biotechnology Co., Ltd.). Protein concentration was determined with
a bicinchoninic acid protein assay kit. Proteins (30 µg) were
separated via SDS-PAGE on a 12 and 10% gel. The separated proteins
were subsequently transferred onto a polyvinylidene difluoride
membrane and blocked with 5% BSA for 2 h at room temperature.
Following this, the membranes were incubated TBX18 (1:1,000), TBX3
(1:1,000), Sr (1:1,000), HCN4 (1:1,000) and β-actin (1:2,000;
Shanghai Wei Biotechnology Co., Ltd.) primary antibodies overnight
at 4°C. Following the addition of horseradish peroxidase-labeled
goat anti-mouse secondary antibody (1:2,000; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA), goat anti-rat secondary
antibody (1:2,000; WB0179; Shanghai Wei Biotechnology Co., Ltd.)
and goat anti-rabbit secondary antibody (1:2,000; WB0177; Shanghai
Wei Biotechnology Co., Ltd.) for 2 h at 37°C, the membrane was
reacted with ECL hypersensitive chemiluminescence kit (cat. no.
WB0164; Shanghai Wei Biotechnology Co., Ltd.). The X-ray film was
photosensitized, developed and fixed in a dark room, and relative
protein expression was calculated with Quantity One 4.6.2 software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The gray value of
the target protein band was compared with that of the β-actin band,
and the obtained gray level ratio was used as the relative
expression of the target protein.
Statistical analysis
Statistical analysis was performed with GraphPad
Prism 5.0 statistical software (GraphPad Software, Inc., La Jolla,
CA, USA). The experiments were repeated three times. Data are
presented as the mean ± standard error of the mean, n=3. Data were
statistically analyzed with one-way analysis of variance followed
by Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of BASCs and
WASCs
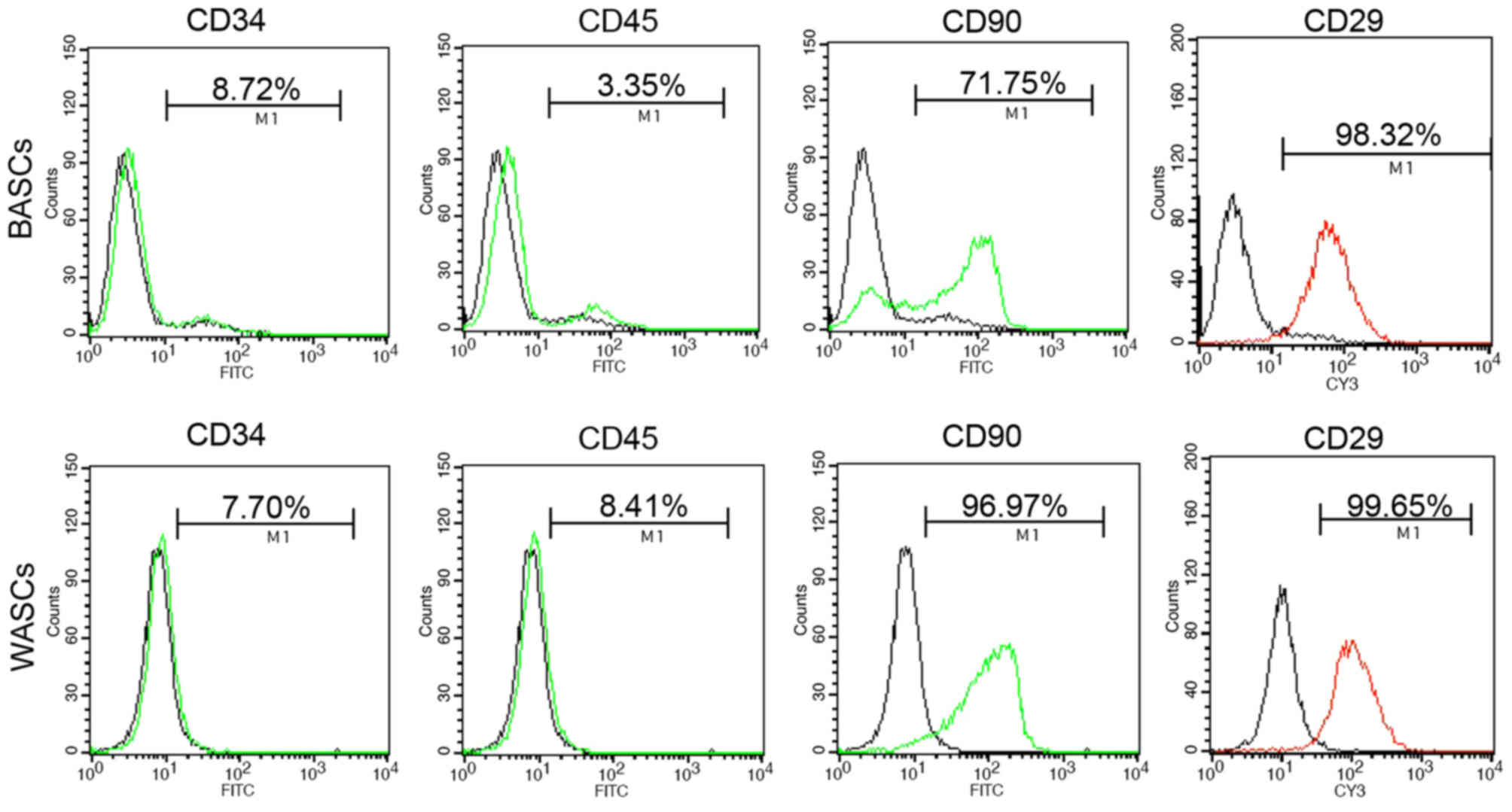
Analysis of the third generation BASC and WASC
characteristics was performed using flow cytometry analysis
(Fig. 1 and Table II). Flow cytometry detected the
absence of hematopoietic stem cell surface antigens CD34 and CD45
expression in BASCs and WASCs (Fig.
1 and Table II). However,
positive expression of mesenchymal stem cell surface antigens CD90
and CD29 was detected in both cell types (Fig. 1 and Table II).
 | Table II.Surface marker analysis of third
generation BASCs and WASCs by flow cytometry. |
Table II.
Surface marker analysis of third
generation BASCs and WASCs by flow cytometry.
| Surface marker | BASCs (%) | WASCs (%) | Positive (+) or
negative (−) |
|---|
| CD34 |
8.39±0.3 |
7.37±0.3 | − |
| CD45 |
3.15±0.1 |
8.19±0.2 | − |
| CD90 |
70.48±1.4 |
96.6±0.6 | + |
| CD29 |
97.26±0.7 |
98.3±1.3 | + |
BASCs and WASCs differentiate into
osteogenic, adipogenic and chondrogenic lineages
It is established that BASCs and WASCs are
multipotential mesenchymal stem cells. To determine the
multipotential differentiation capability of BASCs and WASCs,
passage 3 cells were induced to differentiate towards osteogenic,
adipogenic and chondrogenic lineages. Adipogenic induction
observation in BASCs and WASCs revealed cell morphology alterations
from a long, spindle shape to a quasi-spherical shape, and bright
fat drops were observed intracellularly. The amount lipid droplets
increased gradually with time to 80–90% of the cell volume and the
nuclear volume reduced or disappeared. No volume enlargement or
lipid droplet formation was observed in control cells (Fig. 2A-D). After 7 days of induction,
bright red lipid droplets were observed by Oil Red O staining,
while the same was not observed in cells without induction in both
WASCs (Fig. 2A and B) and BASCs
(Fig. 2C and D).
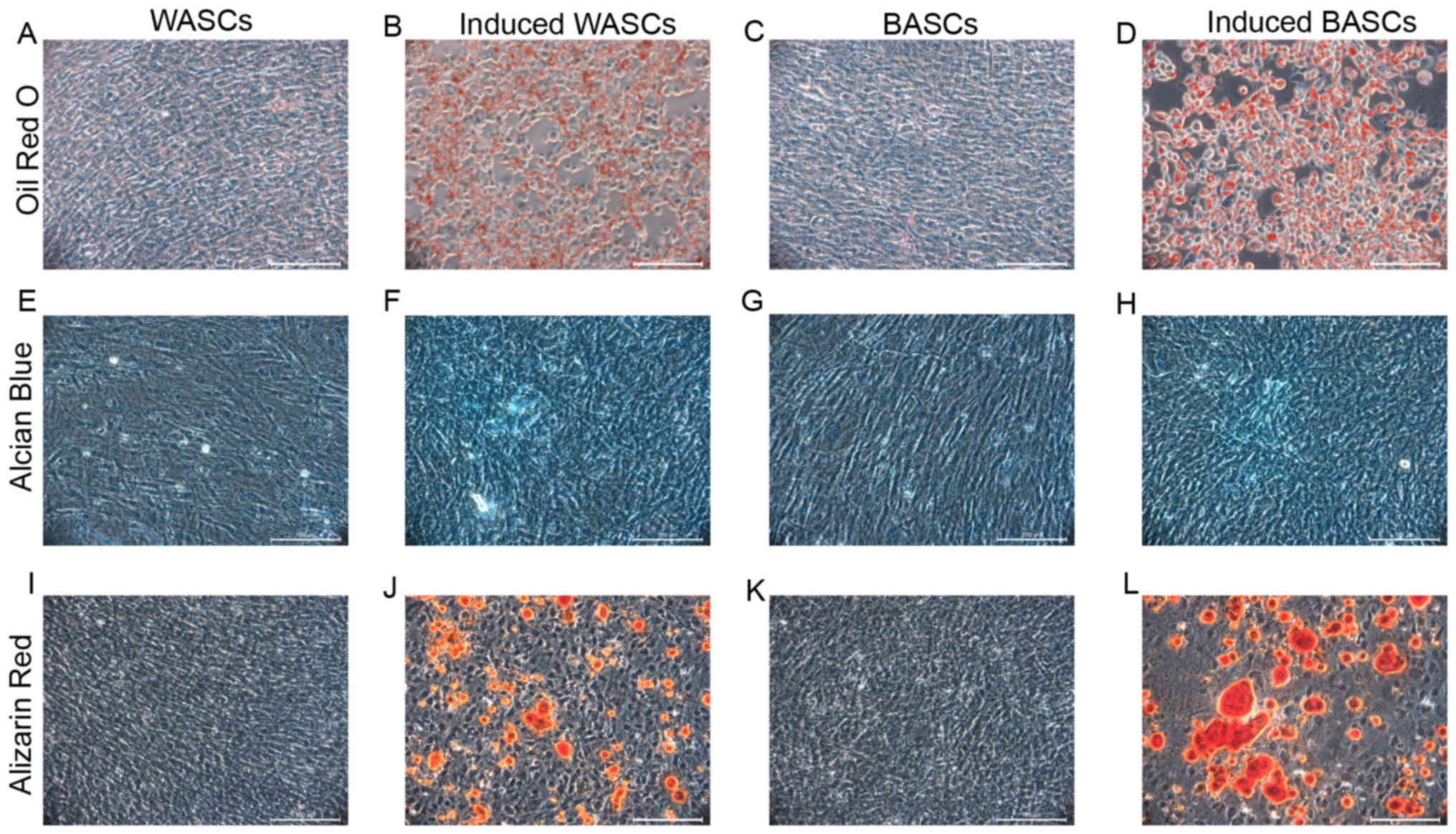 | Figure 2.Differentiation potential of WASCs and
BASCs. Cells were cultured in adipogenic, chondrogenic or
osteogenic inductive medium. (A) Control WASCs, (B)
adipogenesis-induced WASCs, (C) control BASCs and (D)
adipogenesis-induced BASCs were stained with Oil Red O 7 days
post-adipogenic induction. (E) Control WASCs, (F)
chondrogenesis-induced WASCs, (G) control BASCs and (H)
chondrogenesis-induced BASCs were stained with Alcian Blue 21 days
post-chondrogenic induction. (I) Control WASCs, (J)
osteogenesis-induced WASCs, (K) control BASCs and (L)
osteogenesis-induced BASCs were stained with alizarin red S 21 days
post-osteogenic induction. Scale bar, 100 µm. WASCs, white
adipose-derived stem cells; BASCs, brown adipose-derived stem
cells. |
Following chondrogenic differentiation induction in
BASCs and WASCs, Alcian Blue staining was performed in control and
induced BASCs and WASCs (Fig.
2E-H), which revealed chondrocyte aggregation into small cell
masses. Prussian Blue staining demonstrated the presence of light
blue-stained dense nuclei and cells around the masses grew in a
radial manner in both, indicating the potential generation of
sulfuric acid proteoglycan specific to the cartilage matrix in both
WASCs (Fig. 2F) and BASCs
(Fig. 2H). No such phenomenon was
observed in the corresponding control cells (Fig. 2E and G).
After 7 days of osteogenic induction, light
microscopy revealed enlarged cell bodies that were initially
spindle or cork-like shaped that subsequently became polygonal and
grew in multiple layers. After 14 days of induction, cell bodies
had further increased in size and had the tendency to aggregate.
Dense, overlapping cell growth was observed and cells gradually
grew around the center of the colony in a small island-like
distribution. Subsequently, small opaque calcified spots were
present. These small calcified spots gradually became larger and on
day 21, typical orange calcified nodules were observed following
alizarin red staining (Fig. 2I-L).
These observations were noted in both WASCs (Fig. 2J) and BASCs (Fig. 2L). These were not observed in the
corresponding control cells (Fig. 2I
and K).
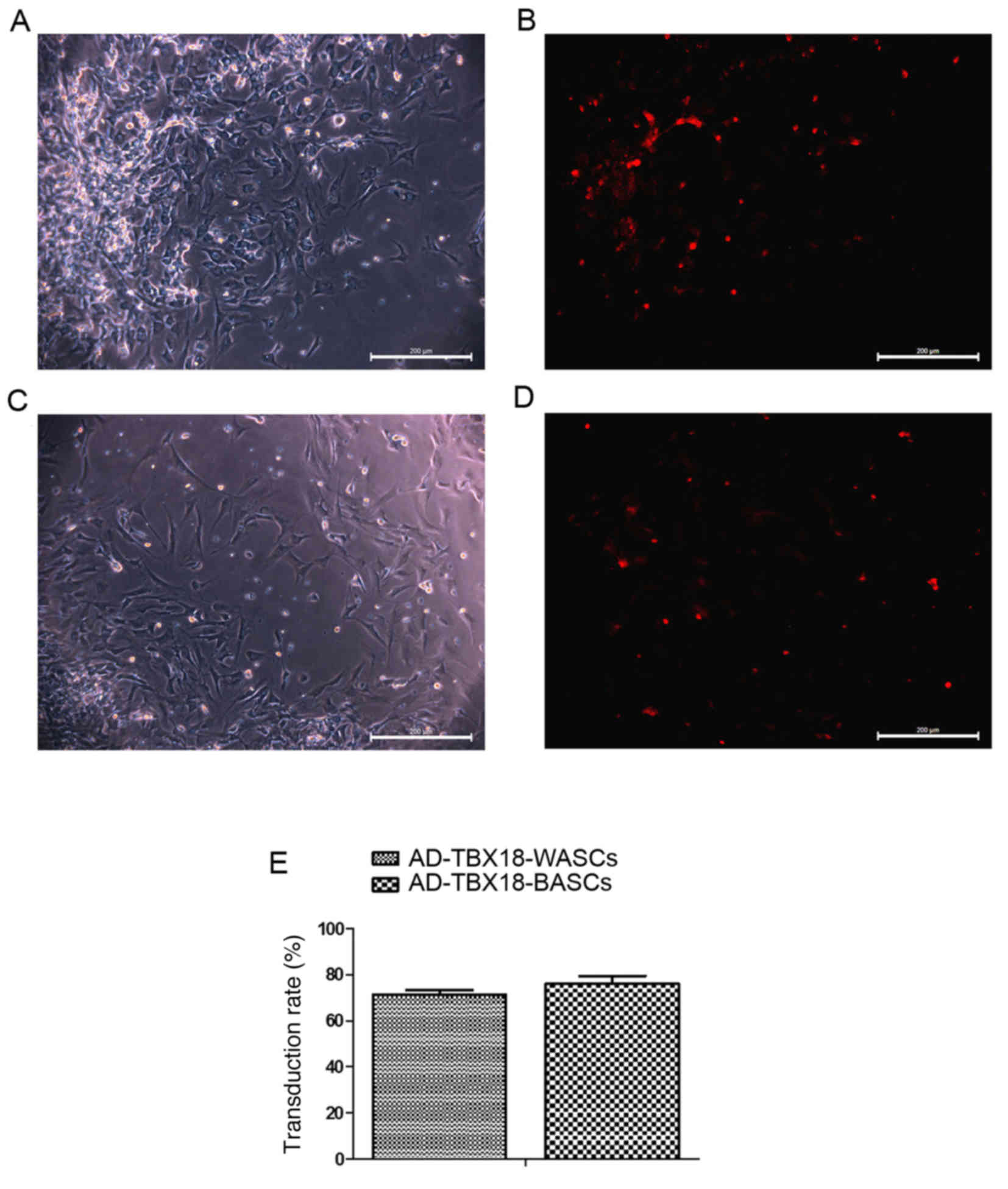
The transduction rate of BASCs and
WASCs with AD-TBX18 is not significantly different
Different MOI values were tested in BASCs and WASCs
for AD-TBX18 and AD-GFP transduction. A MOI value of 50 was
selected as it exhibited the highest cell transduction efficiency.
Following successful transduction of AD-TBX18, BASCs and WASCs
emitted red fluorescence (AD-cherry-TBX18). Transduction efficiency
was observed under an inverted microscope. Cells with red
fluorescence were densely observed in the TBX18-BASC group
(Fig. 3A and B). Fewer cells with
red fluorescence were observed in the TBX18-WASC group (Fig. 3C and D). However, no significant
difference was detected in the BASC and WASC TBX18 transduction
rate (P>0.05; Fig. 3E),
indicating that TBX18 may be stably expressed in BASCs and
WASCs.
The ultrastructure of TBX18-BASCs is
more similar to pacemaker-like cells than that of TBX18-WASCs
WASC, GFP-WASC, TBX18-WASC, BASC, GFP-BASC and
TBX18-BASC ultrastructure was observed by TEM (Fig. 4). The results revealed marked
ultrastructural differences between the WASCs and BASCs. WASCs
contained only a small number of organelles (Fig. 4A-C), whereas BASCs contained more
cytoplasm, organelles and mitochondrial cristae (Fig. 4D-F). Furthermore, a multilocular
structure was observed in BASCs, while a monolocular structure was
observed in WASCs. It was evident that the ultrastructure of BASCs
was more complex compared with that of WASCs. Thus, it may be
concluded that BASCs possess ultrastructural advantages for
differentiation into pacemaker-like cells.
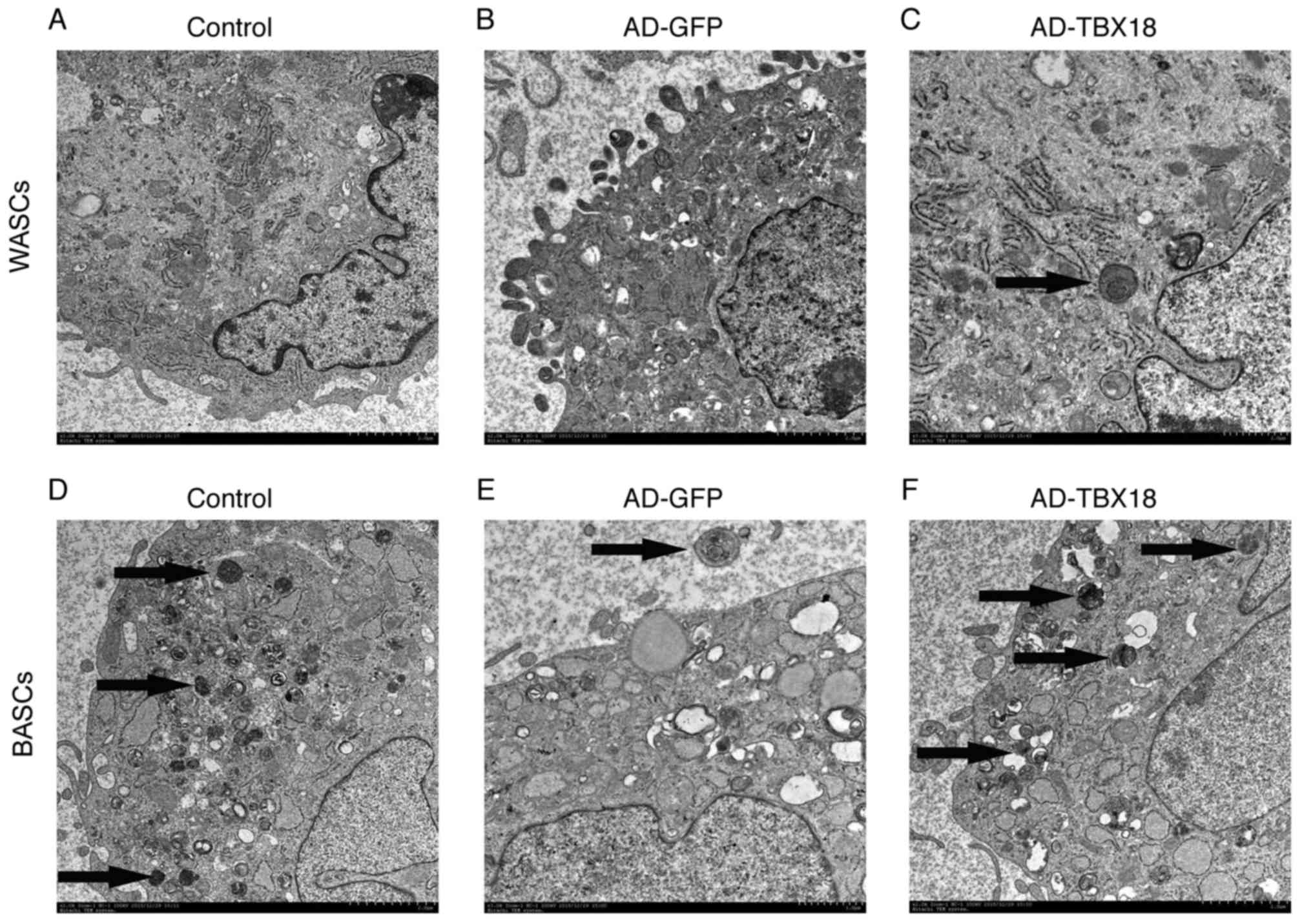 | Figure 4.Ultrastructure of BASCs and WASCs was
examined by TEM (magnification, ×10,000). Ultrastructural images of
(A) normal control WASCs, (B) control WASCs transduced with AD-GFP,
(C) WASCs transduced with AD-TBX18, (D) normal control BASCs, (E)
control BASCs transduced with AD-GFP and (F) BASCs transduced with
AD-TBX18. Images were captured by TEM. The black arrows indicate
the Golgi body. BASCs, brown adipose-derived stem cells; WASCs,
white adipose-derived stem cells; TEM, transmission electron
microscopy; AD, adenovirus; GFP, green fluorescent protein; TBX18,
T-box 18. |
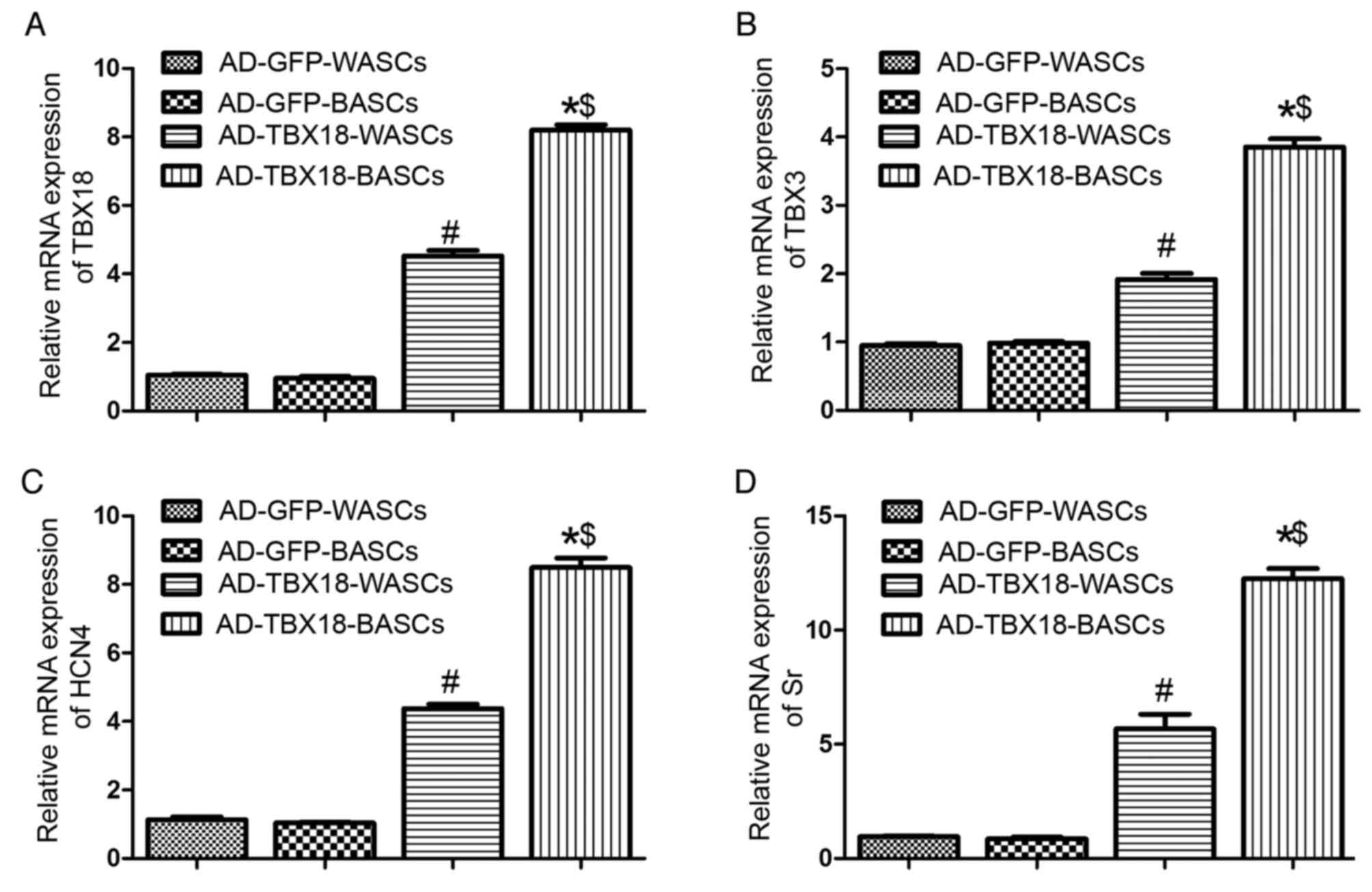
mRNA expression of pacemaker
cell-associated genes is higher in TBX18-transduced BASCs
TBX18 mRNA expression was significantly higher in
TBX18-BASCs compared with TBX18-WASCs (P<0.05; Fig. 5A). Additionally, TBX18 mRNA
expression in TBX18-transduced BASCs and WASCs was significantly
higher compared with the corresponding AD-GFP control groups
(P<0.05; Fig. 5A). No
significant difference was detected in TBX18 expression between the
GFP-BASCs and GFP-WASCs (Fig.
5A).
Furthermore, TBX3 (Fig.
5B), HCN4 (Fig. 5C) and Sr
(Fig. 5D) mRNA expression was
significantly higher in TBX18-BASCs compared with TBX18-WASCs
(P<0.05). TBX3, HCN4 and Sr mRNA expression in TBX18-BASCs and
TBX18-WASCs was significantly higher compared with the
corresponding GFP control groups (P<0.05; Fig. 5B-D). No difference in TBX3, HCN4
and Sr expression was observed between GFP-BASCs and GFP-WASCs
(Fig. 5B-D).
Immunofluorescence analysis of Sr and
HCN4 protein expression
Immunofluorescence staining results in Fig. 6 revealed that the expression of Sr
and HCN4 protein was significantly higher in TBX18-BASCs compared
with TBX18-WASCs. Additionally, Sr and HCN4 expression in
AD-TBX18-tranduced cells was significantly higher compared with
WASCs and BASCs transduced with AD-GFP. It was identified that the
positive cells for Sr of AD-GFP-BASCs (Fig. 6B) were lower (2.6±0.8%) compared
with AD-TBX18-WASCs (9.8±1.60%; P<0.05; Fig. 6C). The positive cells for Sr of
AD-TBX18-BASCs (Fig. 6D) were
23.6±2.5%, higher compared with AD-TBX18-WASCs (P<0.05; Fig. 6I). No Sr or HCN4 positive cells
were identified in AD-GFP-WASCs (Fig.
6A and E). It also was found that the positive cells for HCN4
of AD-GFP-BASCs (Fig. 6F) were
4.9±0.6%, which were lower compared with AD-TBX18-WASCs
(10.9±1.50%; P<0.05; Fig. 6G).
The positive cells for HCN4 of AD-TBX18-BASCs (Fig. 6H) were 17.5±2.1%, higher compared
with AD-TBX18-WASCs (P<0.05; Fig.
6J).
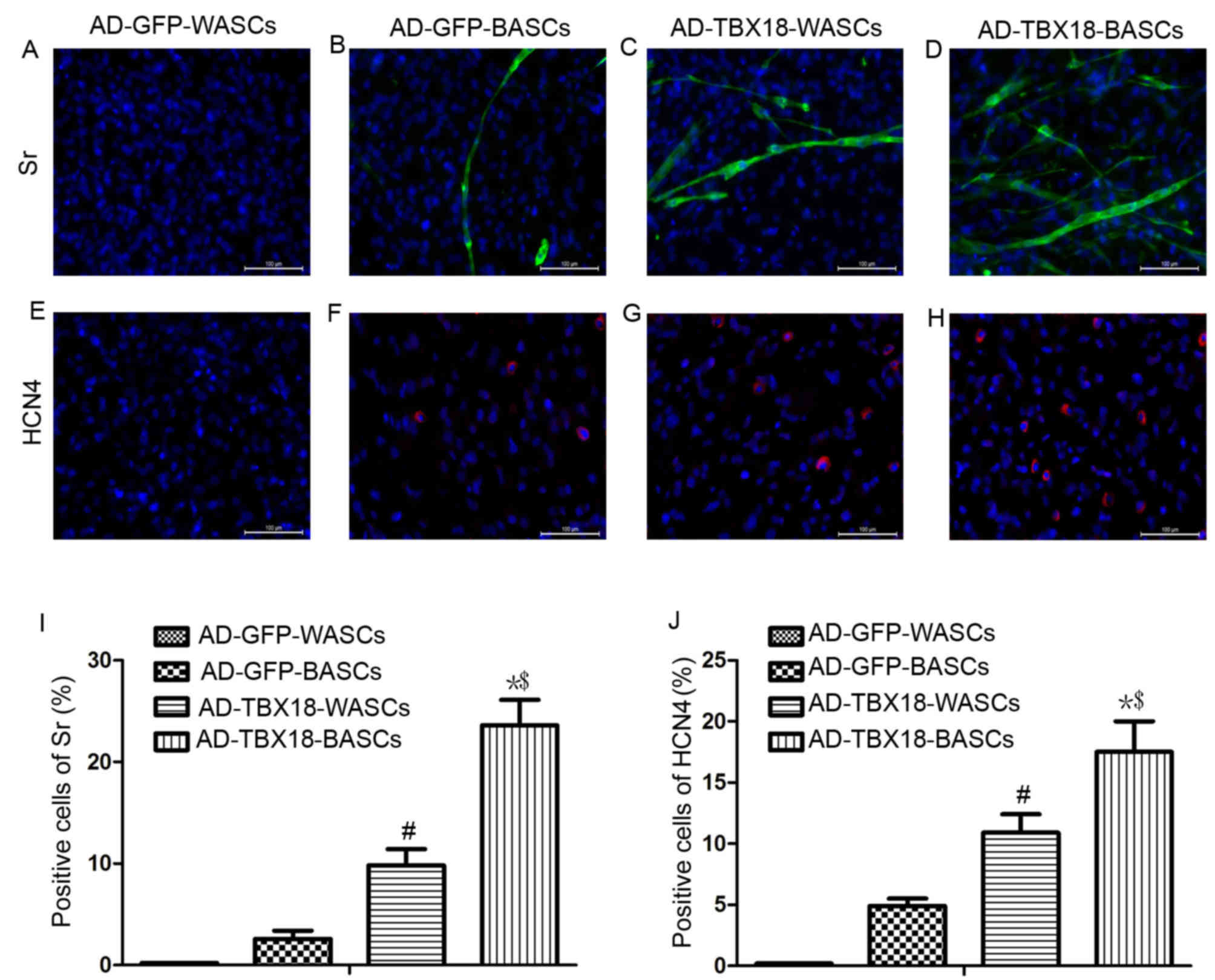 | Figure 6.Immunofluorescence staining analysis
of Sr and HCN4 expression in WASCs and BASCs following TBX18
transduction. Immunofluorescence staining of Sr in (A) control
WASCs transduced with AD-GFP, (B) control BASCs transduced with
AD-GFP, (C) WASCs transduced with AD-TBX18 and (D) BASCs transduced
with AD-TBX18. HCN4 expression was also investigated by
immunofluorescence staining in (E) control WASCs transduced with
AD-GFP, (F) control BASCs transduced with AD-GFP, (G) WASCs
transduced with AD-TBX18 and (H) BASCs transduced with AD-TBX18.
Cells were counterstained blue with DAPI to visualize the nucleus.
Scale bar, 100 µm. All significant differences of positive cells
between groups were presented as a graph; positive cells of (I) Sr
and (J) HCN4. *P<0.05 vs. AD-GFP-BASCs; #P<0.05
vs. AD-GFP-WASCs; $P<0.05 vs. AD-TBX18-WASCs. Sr,
sarcomeric α-actinin; HCN4, hyperpolarization-activated cyclic
nucleotide-gated channel 4; WASCs, white adipose-derived stem
cells; BASCs, brown adipose-derived stem cells; TBX18, T-box 18;
AD, adenovirus; GFP, green fluorescent protein. |
The expression of pacemaker
cell-associated proteins is higher in TBX18-BASCs
Western blot analysis was performed in TBX18-BASCs,
TBX18-WASCs, GFP-BASCs and GFP-WASCs to detect the expression of
TBX18, TBX3, HCN4 and Sr (Fig.
7A). Quantitative analysis revealed that TBX18 protein
expression was significantly higher in TBX18-BASCs compared with
TBX18-WASCs (P<0.05; Fig. 7B).
Additionally, TBX18 protein expression was significantly higher in
TBX18-BASCs and TBX18-WASCs, compared with the corresponding GFP
control groups (P<0.05; Fig.
5B). No significant difference in TBX18 expression was detected
between GFP-BASCs and GFP-WASCs (Fig.
7B).
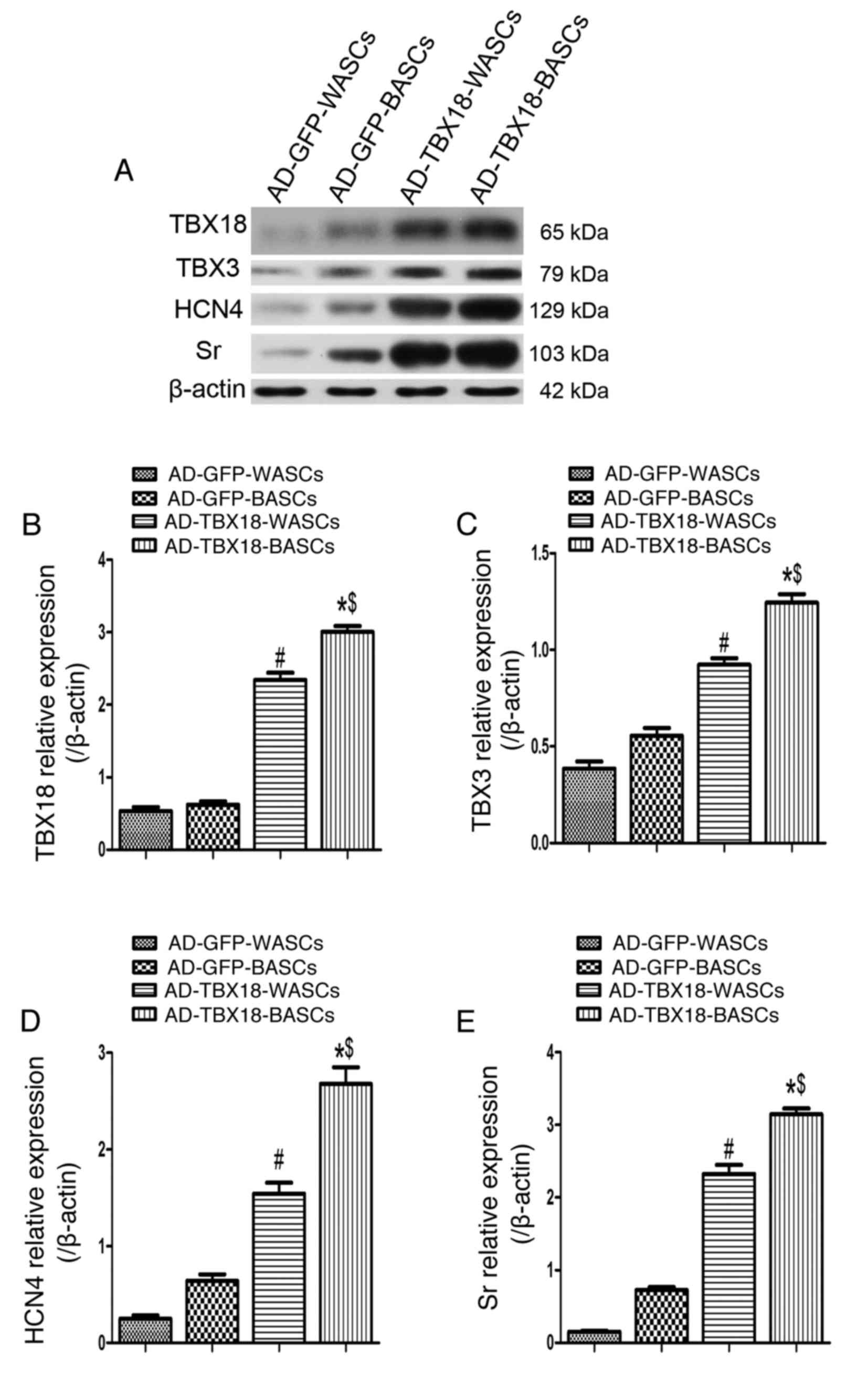 | Figure 7.Western blot analysis of pacemaker
cell-associated protein expression in BASCs and WASCs following
TBX18 transduction. (A) Representative western blot bands for
TBX18, TBX3, HCN4 and Sr in WASCs and BASCs transduced with either
AD-TBX18 or AD-GFP. Densitometric analysis was performed to
quantify the protein expression of (B) TBX18, (C) TBX3, (D) HCN4
and (E) Sr. Expression levels were normalized to β-actin. Data are
presented as the mean ± standard error of the mean n=3. *P<0.05
vs. AD-GFP-BASCs; #P<0.05 vs. AD-GFP-WASCs;
$P<0.05 vs. AD-TBX18-WASCs. BASCs, brown
adipose-derived stem cells; WASCs, white adipose-derived stem
cells; TBX, T-box; HCN4, hyperpolarization-activated cyclic
nucleotide-gated channel 4; Sr, sarcomeric α-actinin; AD,
adenovirus; GFP, green fluorescent protein. |
Furthermore, TBX3 (Fig.
7C), HCN4 (Fig. 7D) and Sr
(Fig. 7E) protein expression was
significantly higher in TBX18-BASCs compared with TBX18-WASCs
(P<0.05). TBX3, HCN4 and Sr protein expression was also
significantly higher in TBX18-BASCs and TBX18-WASCs, compared with
the corresponding GFP control groups (P<0.05; Fig. 7C-E). No significant difference in
TBX3, HCN4 and Sr expression between the GFP-BASCs and GFP-WASCs
was detected (Fig. 7C-E).
Discussion
The fitting of an electronic pacemaker for the
treatment of heart block and sinoatrial node (SAN) dysfunction has
the advantage of high reliability and low recurrence (14). However, electronic pacemakers are
not sensitive to the body's humoral regulation and are susceptible
to infection. These limitations have driven research into the
development of biological pacemakers (15). However, the development of
biological pacemakers has proven difficult due to the lack of seed
cells that can be induced to differentiate into autonomic pacemaker
cells. Compared with embryonic, induced pluripotent and adult stem
cells, ADSCs have gradually emerged as a novel choice of target
cell, owing to their easy availability, lower rejection rate and
the potential of multidirectional differentiation (16).
TBX18 controls the formation of the head region in
the developing SAN. In the present study, TBX18 was successfully
transduced into BASCs and WASCs. The results revealed that there
was no significant difference between the BASC and WASC TBX18
transduction rate, indicating that TBX18 may be stably expressed in
both cell types. It has been confirmed that TBX18 controls ~75% of
SAN head formation, but does not have much involvement in SAN tail
formation. By contrast, TBX3 is not involved in SAN head formation,
but controls SAN tail cell differentiation (6,17).
No marked morphological changes were observed in the SAN of
TBX3-deficient hearts (18). The
present study confirmed that TBX3 mRNA and protein expression in
BASCs transduced with AD-TBX18 was significantly higher compared
with in WASCS transduced with AD-TBX18. Furthermore, TEM revealed
that BASCs exhibited more complex ultrastructures compared with
WASCs. In the present study, cells in the TBX18-BASCs group in
particular had extremely similar internal structures to that of
pacemaker cells (19). In the
current study, fluorescence microscopy and western blot analysis
demonstrated that the expression of Sr, a pacemaker cell-specific
striated muscle actin, in TBX18-transduced BASCs was significantly
higher compared with in TBX18-transduced WASCs. Therefore, it was
concluded that BASCs may have a structural advantage over WASCs in
pacemaker-like cell differentiation.
HCN4, one of the major subtypes of the
hyperpolarization-activated cyclic nucleotide-gated channel family,
has been reported to be highly expressed in SAN head cells and
highly sensitive to cyclic AMP. HCN4 predominantly encodes
pacemaker current If (20–22).
If initiates the four-stage depolarization process,
which means this process underlies the ability of pacemaker cells
to autonomically regulate the heart and spontaneously produce
current. Thus, current research has primarily focused on developing
HCN4-mediated biological pacemakers (23,24).
The findings of the present study confirmed that the mRNA and
protein expression of HCN4 was significantly higher in TBX18-BASCs,
compared with the expression in TBX18-WASCs, further indicating
that BASCs may be more susceptible to pacemaker cell
differentiation. As brown adipose tissue is formed during the fetal
period, its amount decreases gradually with age (25), which limits the source available
for autologous cell transplantation. Additionally, the origins of
the brown and white adipose tissues are different, having a
different embryonic source, and adults have less brown fat, which
limits the clinical utilization of brown fat (26). Notably, an experiment in both mice
and humans revealed that white adipose tissue may be converted into
brown adipose tissue in cold environments; this may provide a basis
for the clinical use of brown fat converted from white fat
(27).
In summary, the present study confirmed that both
BASCs and WASCs transduced with TBX18 had the potential to
differentiate into pacemaker-like cells in vitro. The
results also revealed that differentiated BASCs exhibited more
pacemaker-like cell characteristics compared with differentiated
WASCs. Therefore, BASCs may be considered for use as a carrier or
platform for gene transfer therapy and may provide an effective
biological intervention method for patients with SAN disease.
However, investigations into the security, stability, persistent
functional expression and the specific mechanisms in vivo of
BASC gene transfer are required. Despite certain limitations, such
as the danger of gene transduction resulting in tumorigenesis, the
current study demonstrated that TBX18 gene transduction facilitated
the differentiation of BASCs and WASCs into pacemaker-like
myocardial cells, of which BASCs may have a higher differentiation
capability.
Acknowledgements
The authors are greatly indebted to Professor
Yu-Quan Li and Professor Xiang-Qun Yang, for their valuable
instructions and suggestions on our thesis as well as their careful
reading of the manuscript. We would like to thank colleagues for
their valuable suggestions and criticisms.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31271050 and
31170934).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQL and XQY conceived and designed the study, and
reviewed and edited the manuscript. AJS and LQ performed the
experiments. CH and XZ contributed to the data analyzes and images
processing. LQ, CH and XZ wrote the paper. All authors read and
approved the final manuscript for publication.
Ethics approval and consent to
participate
All animal experiments were approved by the ethics
committee of the Second Military Medical University (Shanghai,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cho HC and Marbán E: Biological therapies
for cardiac arrhythmias: Can genes and cells replace drugs and
devices? Circ Res. 106:674–685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kapoor N, Liang W, Marbán E and Cho HC:
Direct conversion of quiescent cardiomyocytes to pacemaker cells by
expression of Tbx18. Nat Biotechnol. 31:54–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu Y, Liu T, Song K, Fan X, Ma X and Cui
Z: Adipose-derived stem cell: A better stem cell than BMSC. Cell
Biochem Funct. 26:664–675. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taha MF and Hedayati V: Isolation,
identification and multipotential differentiation of mouse adipose
tissue-derived stem cells. Tissue Cell. 42:211–216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi YS, Dusting GJ, Stubbs S,
Arunothayaraj S, Han XL, Collas P, Morrison WA and Dilley RJ:
Differentiation of human adipose-derived stem cells into beating
cardiomyocytes. J Cell Mol Med. 14:878–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiese C, Grieskamp T, Airik R, Mommersteeg
MT, Gardiwal A, de Gier-de Vries C, Schuster-Gossler K, Moorman AF,
Kispert A and Christoffels VM: Formation of the sinus node head and
differentiation of sinus node myocardium are independently
regulated by Tbx18 and Tbx3. Circ Res. 104:388–397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Greulich F, Rudat C and Kispert A:
Mechanisms of T-box gene function in the developing heart.
Cardiovasc Res. 91:212–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Wijk B, van den Berg G, Abu-Issa R,
Barnett P, van der Velden S, Schmidt M, Ruijter JM, Kirby ML,
Moorman AF and van den Hoff MJ: Epicardium and myocardium separate
from a common precursor pool by crosstalk between bone
morphogenetic protein- and fibroblast growth factor-signaling
pathways. Circ Res. 105:431–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang M, Zhang GG, Wang T, Wang X, Tang YH,
Huang H, Barajas-Martinez H, Hu D and Huang CX: TBX18 gene induces
adipose-derived stem cells to differentiate into pacemaker-like
cells in the myocardial microenvironment. Int J Mol Med.
38:1403–1410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamada Y, Wang XD, Yokoyama S, Fukuda N
and Takakura N: Cardiac progenitor cells in brown adipose tissue
repaired damaged myocardium. Biochem Biophys Res Commun.
342:662–670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Deng ZJ, Zhou JS, Ji RJ, Zhang X,
Zhang CS, Li YQ and Yang XQ: Tbx18-dependent differentiation of
brown adipose tissue-derived stem cells toward cardiac pacemaker
cells. Mol Cell Biochem. 433:61–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karam JP, Bonafè F, Sindji L, Muscari C
and Montero-Menei CN: Adipose-derived stem cell adhesion on
laminin-coated microcarriers improves commitment toward the
cardiomyogenic lineage. J Biomed Mater Res A. 103:1828–1839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Potapova I, Plotnikov A, Lu Z, Danilo P
Jr, Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova IN, Gao J,
et al: Human mesenchymal stem cells as a gene delivery system to
create cardiac pacemakers. Circ Res. 94:952–959. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung JJ, Husse B, Rimmbach C, Krebs S,
Stieber J, Steinhoff G, Dendorfer A, Franz WM and David R:
Programming and isolation of highly pure physiologically and
pharmacologically functional sinus-nodal bodies from pluripotent
stem cells. Stem Cell Reports. 2:592–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizuno H, Tobita M and Uysal AC: Concise
review: Adipose-derived stem cells as a novel tool for future
regenerative medicine. Stem Cells. 30:804–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bakker ML, Boink GJ, Boukens BJ, Verkerk
AO, van den Boogaard M, den Haan AD, Hoogaars WM, Buermans HP, de
Bakker JM, Seppen J, et al: T-box transcription factor TBX3
reprogrammes mature cardiac myocytes into pacemaker-like cells.
Cardiovasc Res. 94:439–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoogaars WM, Engel A, Brons JF, Verkerk
AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P,
et al: Tbx3 controls the sinoatrial node gene program and imposes
pacemaker function on the atria. Genes Dev. 21:1098–1112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bleeker WK, Mackaay AJ, Masson-Pévet M,
Bouman LN and Becker AE: Functional and morphological organization
of the rabbit sinus node. Circ Res. 46:11–22. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang X, Wang G, Lin L, Lowe J, Zhang Q,
Bu L, Chen Y, Chen J, Sun Y and Evans SM: HCN4 dynamically marks
the first heart field and conduction system precursors. Circ Res.
113:399–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Biel M, Schneider A and Wahl C: Cardiac
HCN channels: Structure, function, and modulation. Trends
Cardiovasc Med. 12:206–212. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Plotnikov AN, Sosunov EA, Qu J, Shlapakova
IN, Anyukhovsky EP, Liu L, Janse MJ, Brink PR, Cohen IS, Robinson
RB, et al: Biological pacemaker implanted in canine left bundle
branch provides ventricular escape rhythms that have
physiologically acceptable rates. Circulation. 109:506–512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
DiFrancesco D and Tortora P: Direct
activation of cardiac pacemaker channels by intracellular cyclic
AMP. Nature. 351:145–147. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wainger BJ, DeGennaro M, Santoro B,
Siegelbaum SA and Tibbs GR: Molecular mechanism of cAMP modulation
of HCN pacemaker channels. Nature. 411:805–810. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dinas PC, Nikaki A, Jamurtas AZ,
Prassopoulos V, Efthymiadou R, Koutedakis Y, Georgoulias P and
Flouris AD: Association between habitual physical activity and
brown adipose tissue activity in individuals undergoing PET-CT
scan. Clin Endocrinol (Oxf). 82:147–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seale P, Kajimura S, Yang W, Chin S, Rohas
LM, Uldry M, Tavernier G, Langin D and Spiegelman BM:
Transcriptional control of brown fat determination by PRDM16. Cell
Metab. 6:38–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Boström P, Sparks LM, Ye L, Choi JH,
Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al:
Beige adipocytes are a distinct type of thermogenic fat cell in
mouse and human. Cell. 150:366–376. 2012. View Article : Google Scholar : PubMed/NCBI
|