Introduction
Alzheimer's disease (AD) is a progressive
neurodegenerative disorder, which is characterized by severe memory
loss and behavioral disturbances (1–3). AD
is a multifactorial disease and is affected by genetic risk
factors, aging and oxidative stresses (4,5). The
impairment of memory and cognition in patients with AD is caused by
synaptic loss, enhanced inflammatory signaling, progressive
deposition of senile plaques, neurofibrillary tangles and
neurodegeneration (6–8). Additionally, amyloid-β (Aβ) peptides,
as well as the tau protein, in the form of neurofibrillary tangles,
are implicated in the pathogenesis of AD (9). In addition to the tau-amyloid
signature, oxidative damage, neuroinflammation, widespread synaptic
loss and neuronal death are considered hallmark features of AD
(9).
With an aging population, AD is on the increase, and
there are currently no effective treatments or cures. Therefore,
the pursuit of novel disease-modifying therapeutics for AD is the
subject of intense investigation.
Salidroside is a type of traditional Chinese
medicine, which is extracted from rhodiola medical plants, named
Rhodiolasa chinensis A Bor. Salidroside has numerous
pharmacological activities, such as anti-anoxia (10), anticancer (11,12),
anti-fatigue (13), and anti-toxin
effects (14). Furthermore,
numerous studies have suggested that salidroside may also improve
cognitive functioning in disease models (15,16);
however, the mechanisms were not determined. In this study, the
potential therapeutic effect of salidroside in APPswe/PS1ΔE9 mice
and its underlying mechanisms were investigated.
Materials and methods
Experimental animals
All the experiments were performed with the approval
of the Ethics Committee of the Tianjin Medical University (Tianjin,
China). APPswe/PS1ΔE9 mice (n=20; 3 months, male, 26–30 g) and
C57BL/6J mice (n=20; 3 months, male, 23–26 g) in clean grade were
obtained from Beijing Huafukang Biological Technology company
(Beijing, China) and raised in the temperature of 25±2°C, relative
humidity 70% and in a 12-h light/dark room at with free access to
food and water. Following 1 week of adaption, all APPswe/PS1ΔE9
mice were randomly divided into either the AD model group or the
salidroside + AD model group (n=10 in each group). C57BL/6J mice
were also randomly divided into either the normal control (NC)
group or the salidroside + NC group (n=10 in each group). The mice
in the salidroside + NC and salidroside + AD model groups were
administered salidroside (30 mg/kg) orally once daily for 3
consecutive months. Following treatment, behavioral tests and
biochemical experiments were performed.
Morris water maze (MWM)
Following the 3 months of salidroside treatment, the
cognitive behavior of all of the mice was investigated using MWM
(17), which measures spatial
learning and memory ability. The animals were subjected to a daily
session of four training trials for 4 consecutive days. In each
training session, mice were placed into the pool at four different
starting points (different quadrants). The mice were then permitted
to find the platform within a maximum time period of 120 sec and
following that the mice were allowed to remain on the platform for
a further 30 sec. If a mouse was unable to find the platform within
the 120 sec time period, the mouse would then be guided to the
platform by the experimenter, and the escape latency was recorded
as 120 sec. At day 5 of spatial testing, the platform was removed
and mice were placed into water at any starting point. The time
period that the mouse remained in the quadrant where the platform
had previously been placed was then recorded.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
Detection of hippocampal neuronal apoptosis was
carried out using the TUNEL assay. The hippocampal tissue was fixed
in 4% formalin at 4°C for 48 h and then embedded in paraffin wax.
Apoptosis rates were detected using the In Situ Cell Death
Detection kit (Roche Applied Science, Rotkreuz, Switzerland)
according to the manufacturer's instructions. Apoptotic rate
changes were measured via a light microscope (Olympus Corporation,
Tokyo, Japan). Hippocampal tissue was fixed in 4% paraformaldehyde
for 48 h at 4°C and embedded into paraffin wax. In Situ Cell
Death Detection kit was used to detect the apoptosis according to
the manufacturer's instructions. The images were taken using a
light microscope (Olympus Corporation). Apoptotic rates were then
determined according to the method previously detailed by Soslow
et al (18). A positive
expression rate of 0–1% was defined as score 0 (negative), 1–10% as
score 1 (weakly positive), 10–50% as score 2 (positive), 50–80% as
score 3 (medium positive) and 80–100% as score 4 (strongly
positive). Three sections were used for each group, and five fields
were randomly selected from each section (magnification, ×400).
Superoxide dismutase (SOD) and
malondialdehyde (MDA) measurements
Following the MWM test, all of the mice were
anaesthetized, immediately sacrificed, and brain tissues were
quickly isolated. Following this, the brains were separated into
two cerebral hemispheres via incision along the midline.
Hippocampal tissue of CA1 region was then isolated from one of the
cerebral hemispheres of each animal and then immediately frozen in
liquid nitrogen for subsequent SOD and MDA testing. Hippocampal SOD
(cat. no. ab65354) and MDA (cat. no. ab65354) (both from Abcam,
Cambridge, UK) activity were then detected usinga microplate reader
(wavelength, 210 nm).
Nitrate assay
Colorimetric reaction using Griess reagent was used
to investigate the nitrate concentration in the hippocampal tissue
of the mouse. The Nitric Oxide Assay kit was purchased from Abcam,
and all procedures were performed out in accordance with the
manufacturer's instructions. The results of the hippocampal nitrate
concentrations of the different groups were expressed as µg/mg
protein.
Determination of glutathione (GSH)
concentration
Firstly, the hippocampal rat samples were
precipitated by the addition of 5% sulfosalicylic acid and
protein-free supernatant was removed by centrifugation at 2,000 × g
for 10 min. A sample of 100 µl protein-free supernatant of the cell
lysate was then added to 800 µl 0.3 mM
Na2HPO4 and 100 µl 0.04%
5,5′-dithiobis-2-nitrobenzoic acid (DTNB) in 0.1% sodium citrate at
4°C overnight. Following this, the absorbance of DTNB was monitored
using a spectrophotometer at 412 nm for 5 min. A standard curve of
GSH was established, and the sensitivity of measurement was
determined to be between 1–100 µM. The results of the GSH
concentrations in the samples were expressed as µg/mg protein.
Determination of tumor necrosis
factor-α (TNF-α) and interleukin-6 (IL-6) concentrations in tissue
homogenate by ELISA
The concentrations of TNF-α and IL-6 in the
hippocampal tissue of the mice were determined using ELISA. ELISA
kits for TNF-α (cat. no. ab46070) and IL-6 (cat. no. ab100772) were
purchased from Abcam. All procedures were performed in accordance
with the manufacturer's instructions. The results of the determined
hippocampal concentrations of TNF-α and IL-6 were expressed as
µg/mg protein.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). All data were expressed as the mean
± standard deviation of the mean. One-way analysis of variance was
used to compare differences among three or more groups, and this
was followed by Student Newman-Keuls-test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
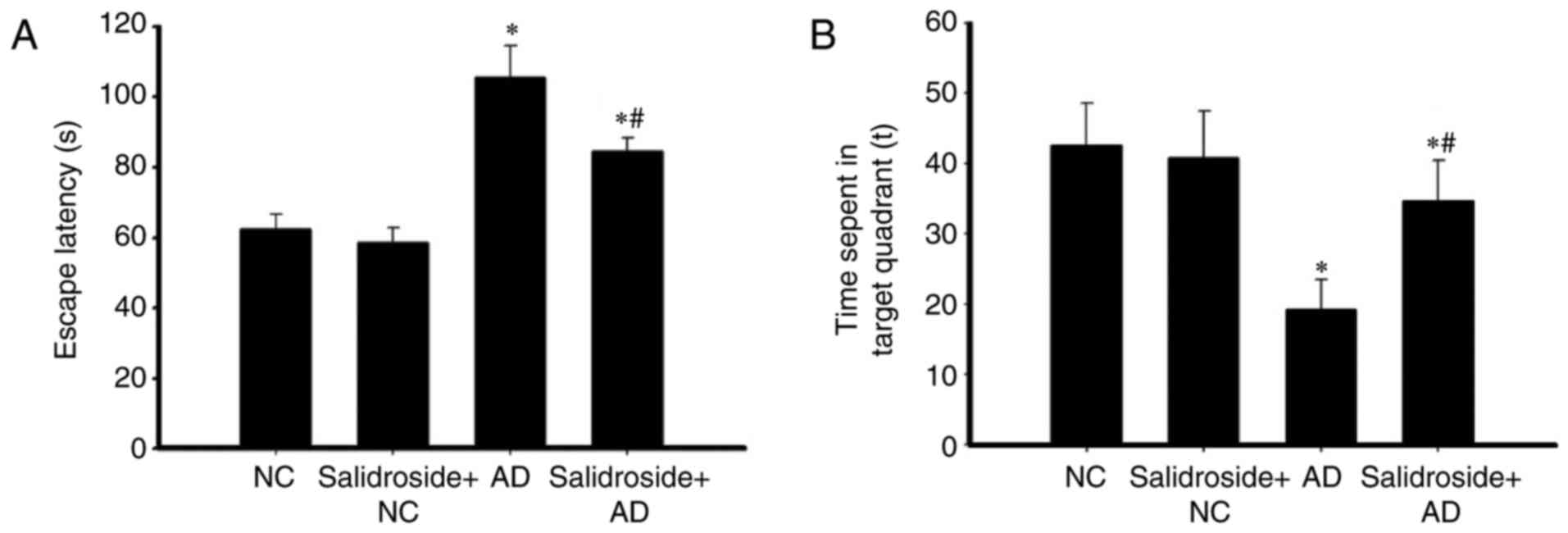
Administration of salidroside improves
the spatial learning and memory abilities of AD mice
MWM was used to investigate the effects of
salidroside administration on the learning and memory abilities of
mice. As revealed in Fig. 1A, the
escape latency time of the AD group was longer than that of the
normal group, and there was no significant difference between the
normal and the salidroside + NC groups. Furthermore, the escape
latency of the mice in the salidroside + AD model group was
significantly reduced compared with the escape latency of the AD
model group. As demonstrated in Fig.
1B, the time period that the AD model group spent in the target
quadrant was significantly less than that spent by the normal
group. Additionally, there was no significant difference in the
time period spent in the target quadrant between the NC and the
salidroside + NC groups. Furthermore, the time period that the mice
of the salidroside + AD model group spent in the target quadrant
was significantly higher than the time spent by the AD group, but
significantly less than the time spent by the NC group. Results in
the salidroside + AD group, the escape latency was significantly
shortened, the time of swim in the platform quadrant significantly
increased, suggesting that mice significantly improved learning and
memory to escape the incubation period (P<0.05; Fig. 1).
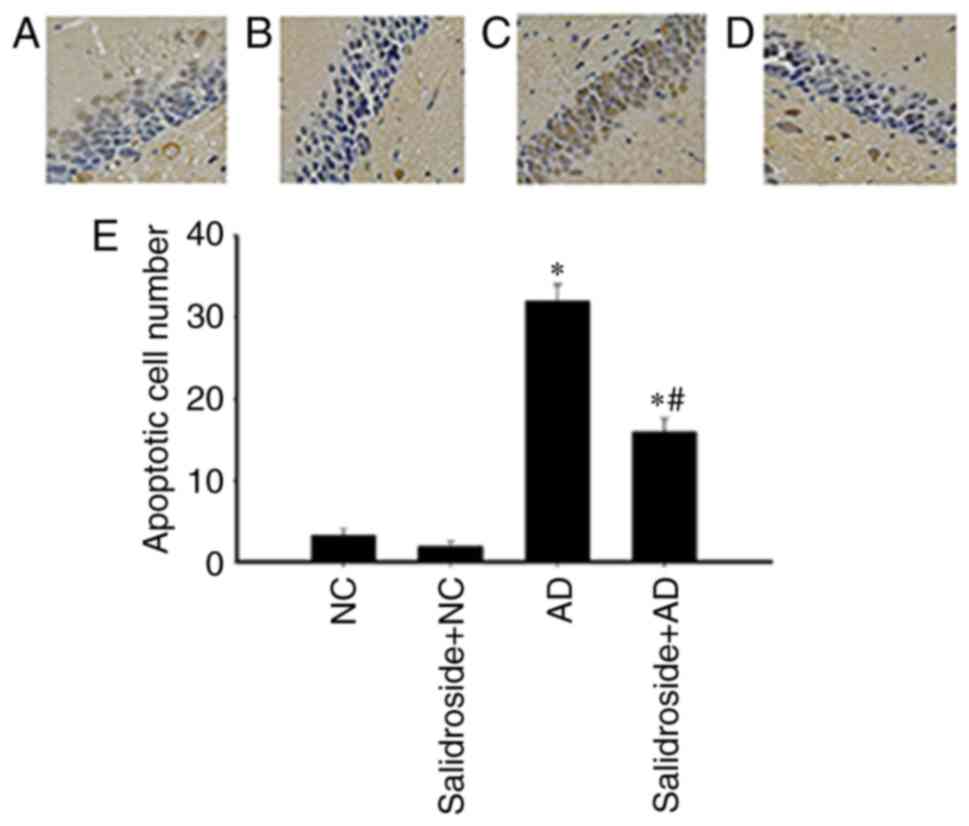
Salidroside administration mitigates
the rate of neuronal apoptosis in the CA1 hippocampal region in AD
mice
As revealed by Fig.
2, the number of neurons undergoing apoptosis in the CA1
hippocampal region was significantly increased in the AD group
compared with the other groups. However, the administration of
salidroside reduced this effect, as there was a significant
reduction in neuronal apoptotic rates between the salidroside + AD
group and the AD group.
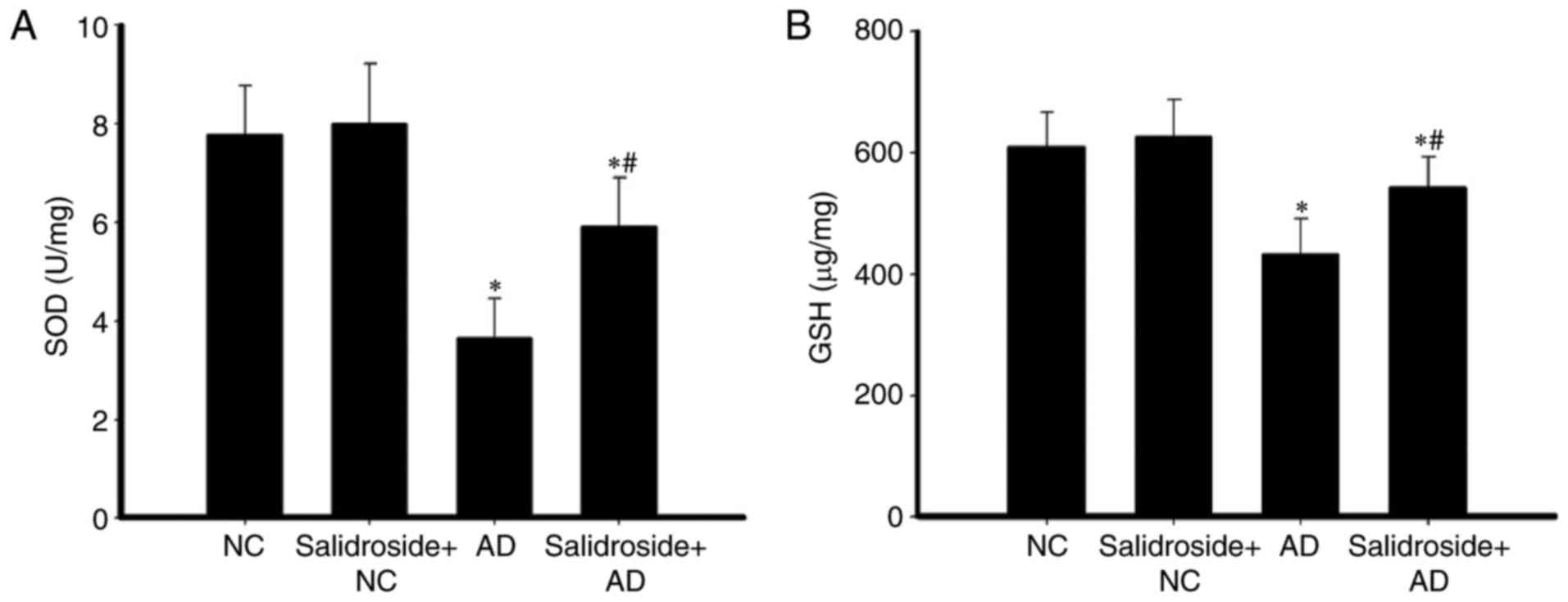
Salidroside administration increases
the concentration of GSH and the activity of SOD in the hippocampus
of AD mice
The activity of SOD and the concentration of GSH in
hippocampal tissue were investigated. As demonstrated in Fig. 3A, the relative SOD activity was
significantly decreased in the AD group compared with the NC group;
whereas SOD activity was upregulated in the salidroside + AD group
compared with the AD group. Furthermore, there was no significant
difference between the SOD activity of the NC group and of the
salidroside + NC group. Compared with the NC group, the GSH
concentration was decreased in the AD group; whereas GSH
concentration was upregulated in the salidroside + AD group
compared with the AD group. In addition, there was no significant
difference between the GSH concentration of the NC group and the
salidroside + NC group (Fig.
3B).
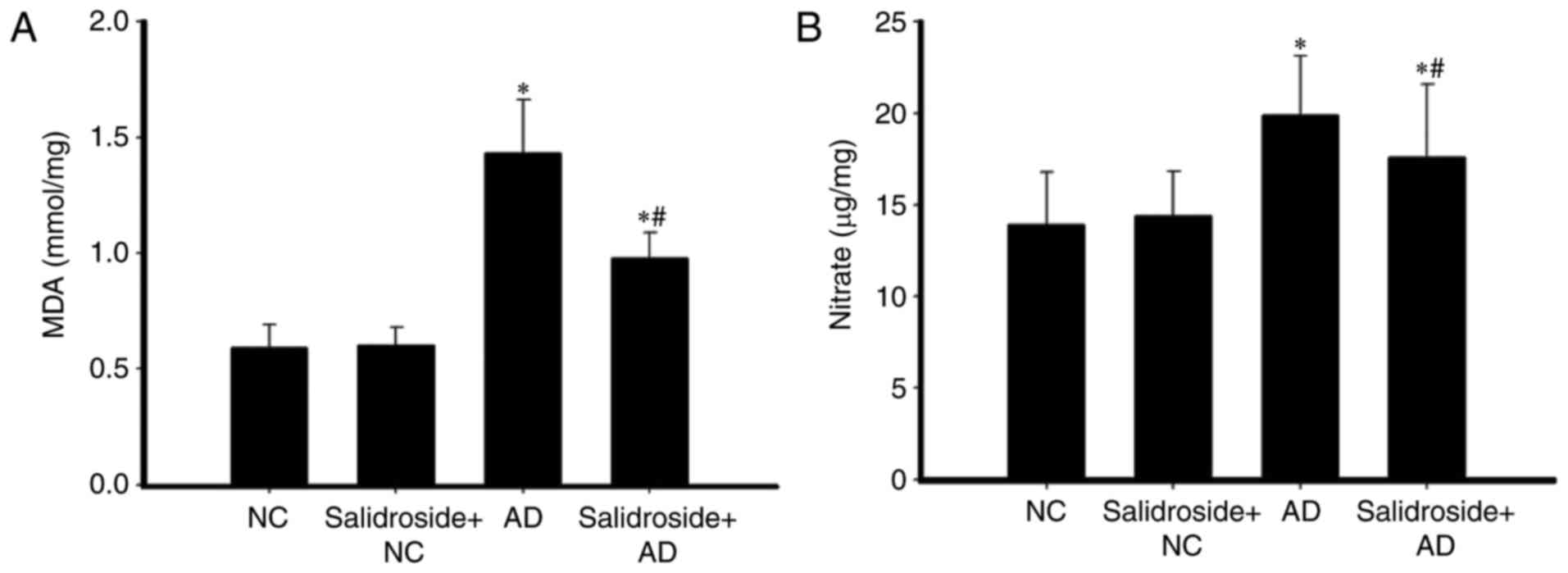
Salidroside administration reduces the
concentrations of MDA and nitrate in the hippocampus of AD
mice
The hippocampal concentrations of MDA and GSH in
mice were also investigated. As revealed in Fig. 4, in comparison with the NC group,
MDA and nitrate concentrations were both increased in the AD group;
whereas both MDA and nitrate concentrations were downregulated in
the salidroside + AD group compared with the AD group. Furthermore,
there was no significant difference between the MDA and nitrate
concentrations of the NC group and the salidroside + NC group.
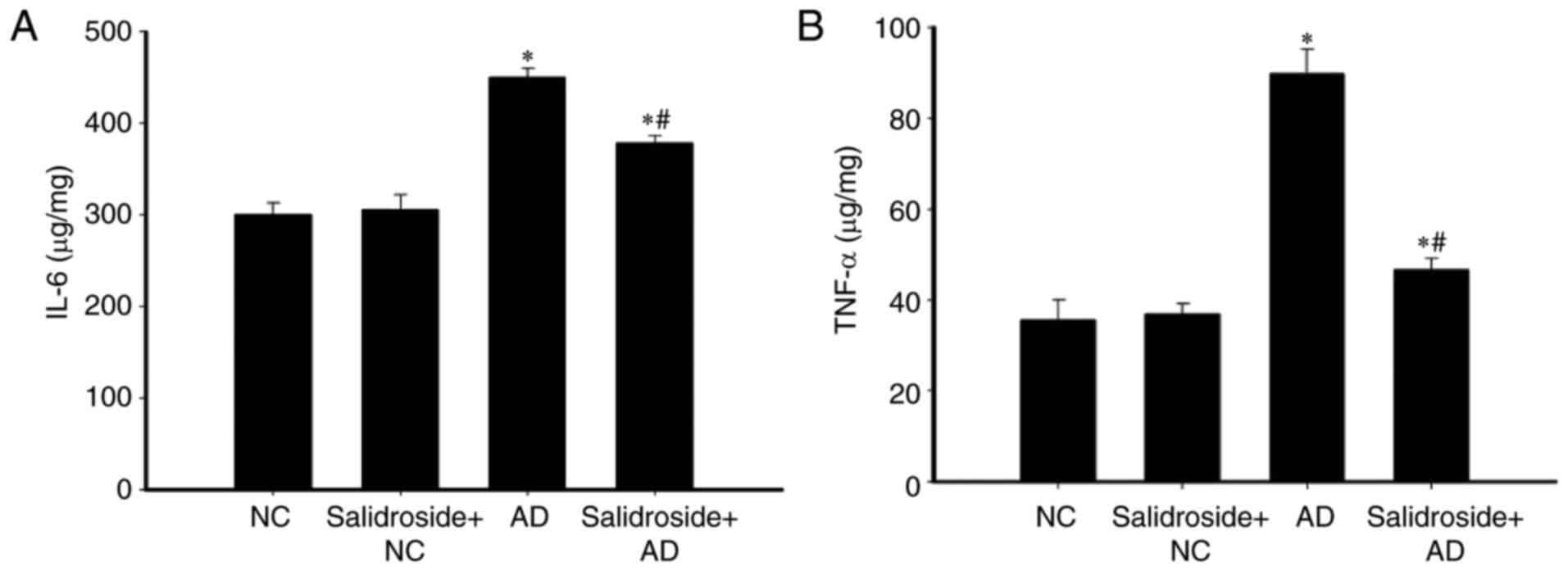
Salidroside administration decreases
the expression of TNF-α and IL-6 in the hippocampus of AD mice
The effects of salidroside administration with
regards to induced inflammation were investigated. The expressions
of TNF-α and IL-6 inflammatory factors decreased significantly in
the hippocampus of the salidroside + AD group compared with mice
belonging to the AD group (Fig.
5). There was no significant difference between the expression
levels of TNF-α and IL-6 of the NC group and the salidroside + NC
group.
Discussion
The number of people with AD worldwide is increasing
as a result of the aging population. Previous research has revealed
that 46.8 million people worldwide suffer from dementia, and
further research has indicated that this number may increase
2–3-fold by 2030 (19). AD is a
severe neurodegenerative disease of the central nervous system,
which results in learning and memory impairment. The rising rate of
senile dementia is of serious concern to global healthcare systems
and causes serious economic burdens to affected families and wider
society. AD is a neurologically complex disorder, and thus it is
likely that a combinatorial therapeutic approach will be needed for
effective treatment.
Chinese herbology has been heavily used globally and
is regarded as an importance source of medicines. Salidroside is a
component of Rhodiolarosea, and possesses medicinal
properties, such as anti-inflammatory (20,21),
antioxidative (14) and
neuroprotective effects (22). In
the present study, it was demonstrated that salidroside
administration attenuates the memory impairment exhibited in the AD
rat model.
Previous studies have reported that one of the major
brain regions affected by AD-induced neurogenesis is the
hippocampus, which is a vital brain region for learning and memory
(23). Hippocampal neuronal damage
adversely affects neural plasticity and synaptic regulation, and
thus is heavily implicated in the pathogenesis of AD (24). In the present study, it was
revealed that the rate of hippocampal neuronal apoptosis was
significantly increased in the rat AD model. Furthermore, it was
demonstrated that the rate of apoptotic neurons in the CA1
hippocampal region was clearly increased in the AD group, and that
administration of salidroside could attenuate this effect. In
addition, the results of this study suggest that the administration
of salidroside improves cognitive function in the AD model via
suppression of the rate of apoptosis of hippocampal neurons.
It has previously been reported that salidroside, a
potent antioxidant, ameliorates memory impairment via
anti-oxidative damage activity. The decline of learning and memory
function in rodents and humans has previously been linked to
excessive oxidative stress, which may lead to neuronal death. There
is a growing body of research suggesting that oxidative stress is
implicated in AD pathogenesis, along with increased levels of lipid
peroxidation, and oxidation of both DNA and protein in the brains
of AD sufferers (25,26). SOD is an important neural
antioxidant enzyme that can remove excess free radicals (27). In addition, oxidative damage may
also cause damage to components of the antioxidant system, such as
MDA (28,29). The present study demonstrated that
oxidative damage downregulated the activity of SOD and increased
MDA concentration in rat hippocampal tissue. Furthermore, it was
revealed that in the AD rat model with memory deficit, these
adverse effects were greatly attenuated when salidroside was
administered.
IL-6 is a cytokine that is secreted by T cells and
macrophages, and acts as both a pro-inflammatory and as an
anti-inflammatory in order to stimulate an immune response
(30,31). TNF-α can promote T cells to produce
a variety of inflammatory factors, and therefore trigger
inflammatory reactions (32,33).
In the present study, salidroside administration significantly
decreased the expression of TNF-α and IL-6 cytokine inflammatory
factors.
In conclusion, the present study demonstrated that
salidroside administration may improve cognitive function in AD
mice. The protective effects maybe associated with alteration of
the levels of free radicals in the hippocampus.
Acknowledgements
The present study was supported by a grant from
Natural Science Foundation of Tianjin Municipal Science and
Technology Commission (grant no. 13JCYBJC22700).
Glossary
Abbreviations
Abbreviations:
|
AD
|
Alzheimer's disease
|
|
MWM
|
Morris water maze
|
|
SOD
|
superoxide dismutase
|
|
GSH
|
glutathione
|
|
MDA
|
malondialdehyde
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-10
|
interleukin-10
|
References
|
1
|
Diwu YC, Tian JZ and Shi J: Effects of
Chinese herbal medicine Yinsiwei compound on spatial learning and
memory ability and the ultrastructure of hippocampal neurons in a
rat model of sporadic Alzheimer disease. Zhong Xi Yi Jie He Xue
Bao. 9:209–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riley KP, Snowdon DA and Markesbery WR:
Alzheimer's neurofibrillary pathology and the spectrum of cognitive
function: Findings from the num study. Ann Neurol. 5:567–577. 2002.
View Article : Google Scholar
|
|
3
|
Gong CX, Liu F, Grundke-Iqbal I and Iqbal
K: Postranslational modifications of tau protein in Alzheimer's
disease. J Neural Transm. 6:813–838. 2005. View Article : Google Scholar
|
|
4
|
Moceri VM, Kukull WA, Emanual I, van Belle
G, Starr JR, Schellenberg GD, McCormick WC, Bowen JD, Teri L and
Larson EB: Using census data and birth certificates to reconstruct
the early-life socioeconomic environment and the relation to the
development of Alzheimer's disease. Epidemiology. 12:383–389. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iqbal K and Grundke-Iqbal I:
Metabolic/signal transduction hypothesis of Alzheimer's disease and
other tauopathies. Acta Neuropathol. 109:25–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuppo EE and Arias HR: The role of
inflammation in Alzheimer's disease. Int J Biochem Cell Biol.
37:289–305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin Y, Liu Y and Huang L: Anti-apoptosis
effect of astragaloside Iv on Alzheimer's disease rat model via
enhancing the expression of Bcl-2 and Bcl-Xl. Scandinavian J Lab
Anim Sci. 37:75–82. 2010.
|
|
8
|
Chen Y, Tian Z, Liang Z, Sun S, Dai CL,
Lee MH, LaFerla FM, Grundke-Iqbal I, Iqbal K, Liu F and Gong CX:
Brain gene expression of a sporadic (icv-STZ Mouse) and a familial
mouse model (3×Tg-AD mouse) of Alzheimer's disease. PLoS One.
7:e514322012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Javed H, Khan MM, Ahmad A, Vaibhav K,
Ahmad ME, Khan A, Ashafaq M and Islam F, Siddiqui MS, Safhi MM and
Islam F: Rutin prevents cognitive impairments by ameliorating
oxidative stress and neuroinflammation in rat model of sporadic
dementia of Alzheimer type. Neuroscience. 210:340–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Liu A, Hou R, Zhang J, Jia X,
Jiang W and Chen J: Salidroside protects cardiomyocyte against
hypoxia-induced death: A HIF-1 α-activated and VEGF-mediated
pathway. Eur J Pharmacol. 607:6–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang D, Chen Y, Xu B, Ren K, He ZY, He LL,
Lei Y, Fan CM and Song XR: Development of lipid-shell and polymer
core nanoparticles with water-soluble salidroside for anti-cancer
therapy. Int J Mol Sci. 15:3373–3388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Li JZ, Lu AX, Zhang KF and Li BJ:
Anticancer effect of salidroside on A549 lung cancer cells through
inhibition of oxidative stress and phospho-p38 expression. Oncol
Lett. 7:1159–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Dong C, Huai L, Bende T, Lihua S and
Ying W: Anti-fatigue effects of salidroside in mice. J Med Colleges
PLA. 23:88–93. 2008. View Article : Google Scholar
|
|
14
|
Zhu Y, Shi Y, Wu D, Ji YJ, Wang X, Chen
HL, Wu SS, Huang DJ and Jiang W: Salidroside protects against
hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt
dependent pathway. DNA Cell Biol. 30:809–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
ZovkoKoncic M and Tomczyk M: New insights
into dietary supplements used in sport: Active substances
pharmacological and side effects. Curr Drug Targets. 14:1079–1092.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Ding Y, Zhou J, Sun X and Wang S:
The in vitro and in vivo antiviral effects of
salidroside from Rhodiolarosea L. against coxsackie virus B3.
Phytomedicine. 16:146–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: Cox-2 is expressed in human
pulmonary, colonic and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Realdon O, Rossetto F, Nalin M, Baroni I,
Cabinio M, Fioravanti R, Saibene FL, Alberoni M, Mantovani F,
Romano M, et al: Technology-enhanced multi-domain at home continuum
of care program with respect to usual care for people with
cognitive impairment: The Ability-TelerehABILITation study protocol
for a randomized controlled trial. BMC Psychiatry. 16:4252016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu S, Yu X, Hu B, Zou Y, Li J, Bo L and
Deng X: Salidroside rescued mice from experimental sepsis through
anti-inflammatory and anti-apoptosis effects. J Surg Res.
195:277–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Díaz Lanza AM, Abad Martínez MJ, Fernández
Matellano L, Recuero Carretero C, Villaescusa Castillo L, Silván
Sen AM and Bermejo Benito P: Lignan and phenylpropanoid glycosides
from Phillyrealatifolia and their in vitro anti-inflammatory
activity. Planta Med. 67:219–223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu ZQ, Zhou Y, Zeng YS, Lin YK, Li Y,
Zhong ZQ and Chan WY: Protective effects of a rhodiola crenulata
extract and salidroside on hippocampal neurogenesis against
streptozotocin-induced neural injury in the rat. PLoS One.
7:e296412012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kimura T, Hong Nguyen PT, Ho SA, Tran AH,
Ono T and Nishijo H: T-817MA, a neurotrophic agent, ameliorates the
deficits in adult neurogenesis and spatial memory in rats infused
i.c.v. with amyloid-β peptide. Br J Pharmacol. 157:451–463. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palop JJ and Mucke L: Amyloid-beta-induced
neuronal dysfunction in Alzheimer's disease: From synapses toward
neural networks. Nat Neurosci. 13:812–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arber CE, Li A, Houlden H and Wray S:
Review: Insights into molecular mechanisms of disease in
neurodegeneration with brain iron accumulation: Unifying theories.
Neuropathol Appl Neurobiol. 42:220–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y and Zhao B: Oxidative stress and
the pathogenesis of alzheimer's disease. Oxid Med Cell Longev.
2013:3165232013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pencea V, Bingaman KD, Wiegand SJ and
Luskin MB: Infusion of brain-derived neurotrophic factor into the
lateral ventricle of the adult rat leads to new neurons in the
parenchyma of the striatum, septum, thalamus and hypothalamus. J
Neurosci. 21:6706–6717. 2001.PubMed/NCBI
|
|
28
|
Kudin AP, Bimpong-Buta NY, Vielhaber S,
Elger CE and Kunz WS: Characterization of superoxide-producing
sites in isolated brain mitochondria. J Biol Chem. 279:4127–4135.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calvert JW, Yin W, Patel M, Badr A,
Mychaskiw G, Parent AD and Zhang JH: Hyperbaric oxygenation
prevented brain injury induced by hypoxia-ischemia in a neonatal
rat model. Brain Res. 951:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferguson-Smith AC, Chen YF, Newman MS, May
LT, Sehgal PB and Ruddle FH: Regional localization of the
interferon-beta 2/B-cell stimulatory factor 2/hepatocyte
stimulating factor gene to human chromosome 7p15-p21. Genomics.
2:203–208. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van der Poll T, Keogh CV, Guirao X,
Buurman WA, Kopf M and Lowry SF: Interleukin-6 gene-deficient mice
show impaired defense against pneumococcal pneumonia. J Infect Dis.
176:439–444. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
Direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tateya S, Kim F and Tamori Y: Recent
advances in obesity induced inflammation and insulin resistance.
Frontiers Endocrinol (Lausanne). 4:932013.
|