Introduction
Heat shock protein 27 (HSP27) is a differential
protein isolated from silicosis tissue (1). HSP27 belongs to a small molecular
weight HSP family, whose proteins contain a conservative C-terminal
domain, termed the α-lens protein domain. Previous studies revealed
that HSP27 may antagonize oxidative stress, inhibit cell apoptosis
and act as a chaperone in inflammatory reactions, cell signal
transduction, cell differentiation, and proliferation by regulating
actin cytoskeleton structure (2–7).
HSP27 is involved in the epithelial-mesenchymal transition (EMT) in
cancer (8) and in the development
and progression of organ fibrosis (9). However, previous studies have also
revealed that HSP27 may exert dual regulation during EMT in organ
fibrosis (9,10). Transforming growth factor-β1
(TGF-β1) is an important transforming growth factor associated with
fibrosis in vitro and in vivo (11,12).
A previous study by the authors revealed that TGF-β1 may induce
A549 human alveolar type II epithelial cells to differentiate into
myofibroblasts (13). In order to
investigate the effect of HSP27 on the differentiation of A549
human alveolar type II epithelial cell line into myofibroblasts and
collagen synthesis, the current study used liposome transfection to
transfect A549 human alveolar type II epithelial cell line and
determined the optimal liposome:plasmid ratio and the best
interference sequence. Lipofectamine® 2000 was used in
the current study as the protocol was established, safe and
reliable with high transfection efficacy. Following successful
transfection of the HSP27 interfering plasmid, the effect on
collagen expression during TGF-β1-induced differentiation of A549
human alveolar type II epithelial cells into myofibroblast was
examined.
Materials and methods
Reagents and materials
The following reagents were used in the study: A549
human alveolar type II epithelial cell line derived from human lung
carcinoma (cat. no. TCHu150, Cell Bank of Chinese Academy of
Science, Shanghai, China); high-glucose Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA);
10% fetal bovine serum (A15-101, PAA, New Bedford, Massachusetts,
USA; www.openfos.com/supply/3298452-PAA-LAB-in-New-Bedford-MA/);
TGF-β1 (cat. no. 100-21; Peprotech, Inc., Rocky Hill, NJ, USA),
mouse anti-HSP27 monoclonal antibody (cat. no. ab114067; Abcam,
Cambridge, UK), rabbit polyclonal anti-type I/type III collagen
antibody (cat. nos. BA0325/0326, GeneTex, Inc., Irvine, CA, USA),
Platinum® SYBR®-Green qPCR SuperMix-UDG kit
(cat. no. C11733038, Invitrogen; Thermo Fisher Scientific, Inc.),
Lipofectamine® 2000 transfection reagent (1.5 ml/U;
Invitrogen; Thermo Fisher Scientific, Inc.); HSP27-interfering kit
including fragments A, B, C and D (Shanghai GenePharma Co., Ltd.,
Shanghai, China); Opti-MEM medium (500 ml/bottle, Gibco; Thermo
Fisher Scientific, Inc.); and polyclonal anti-GAPDH antibody (cat.
no. sc-25778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Cell culture and experimental
groups
Specific HSP27 gene fragment was introduced into
A549 cells by transfection. Cells were divided into three groups:
i) Blank plasmid control group; ii) blank plasmid + TGF-β1 group;
and iii) HSP27-interfering plasmid + TGF-β1 group.
Transfection efficacy determined by
flow cytometry
Cells were seeded into 10 cm diameter dishes and
transfected at 90–100% confluency. Initially, two aliquots of 3 ml
Opti-MEM medium was added into two 5 ml Eppendorf micro test tubes,
Lipofectamine® 2000 and HSP27 plasmid were added
individually into the two tubes and mixed with OPTI-MEM medium. A
series of Lipofectamine® 2000 volumes (20, 25, 30, 20,
and 20 µl) and HSP27 plasmid (8, 8, 8, 9 and 10 µg) were
correspondingly tested. The reagents were individually incubated at
37°C for 10 min prior being mixed and incubated at 37°C for an
additional 20 min. The transfection mixture was added into dishes
with 15 ml OPTI-MEM and mixed by gentle shaking. After 72 h
incubation at 37°C, cells were trypsinized with 0.25% trypsin,
harvested, and centrifuged at 201 × g on 4°C for 5 min.
Transfection efficacy of each combination of
Lipofectamine® 2000 and plasmid was determined using
fluorescent intensity via flow cytometry.
Examination by laser confocal
microscopy
Cells were seeded at 6×103 cells/well in
the small dish for confocal observation. HSP27 plasmid and
Lipofectamine® 2000 were added at 8 µg:20 µl ratio for
transfection as aforementioned. The fluorescence marker green
fluorescent protein (GFP) in the plasmid cells were observed under
laser confocal microscope at a magnification of ×800.
Screen optimal interfering HSP27
plasmid by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
Cells were seeded at a density of 6×103
cells/well in 6-well plate and transfected when cells reached
90–100% confluency. For transfection, 500 µl OPTI-MEM medium was
added into 1.5 ml Eppendorf tube and 12 tubes were prepared for 6
transfection groups, including 4 different HSP27 interfering
sequences, HSP27 positive control and negative control plasmids; 5
µl Lipofectamine® 2000 transfection reagent or the
plasmid was mixed with OPTI-MEM and incubated for 10 min. Then the
transfection reagent mixture was added into plasmid/OPTI-MEM
mixture and incubated at 37°C for 20 min. RNA was isolated with
TRIzol (Invitrogen, Thermo Fisher Scientific, Inc.) after 72 h and
the concentration and purity of total RNA was measured with
deionized water as control by micro nucleic acid analyzer. The
primer of target gene was designed and synthesized (Invitrogen;
Thermo Fisher Scientific, Inc.). HSP27: Forward,
5′-GCTTCACGCGGAAATACACG-3′ and reverse, 5′-GTGATCTCGTTGGACTGCGT-3′;
β-actin forward, 5′-GTCACCTTCACCGTTCCAGTTTT-3′ and reverse,
5′-CTTAGTTGCGTTACACCCTTTCTT-3′. qPCR amplification was performed as
follows: Pre-denaturation at 95°C for 15 sec followed by 40 cycles
at 60°C for 20 sec. And data were analyzed to determine the optimal
HSP27 interfering sequence by the method of ∆∆Cq (14).
Immunoblotting
Protein was isolated from cells with RIPA (Beijing
Solarbio Science & Technology Co., Ltd. Beijing, China)
following the designated transfection period. After determining the
protein concentration by BCA assay, 50 µl samples per lane were
loaded on 10% SDS-PAGE. The blots were subjected to standard
transfer methods and PVDF membranes were blocked with 10% BSA
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and then incubated
with primary antibodies (HSP27, type I and III collagen all at
1:200 dilution and GAPDH at 1:100 dilution) at 4°C overnight. The
membranes were incubated with goat anti-rabbit IgG-AP (BA1011;
Boster Biological Technology, Pleasanton, CA, USA) and goat
anti-mouse IgG-AP (BA1010; Boster Biological Technology) secondary
antibodies diluted at 1:5,000 at 37°C for 2 h and then with
RCIP/NBT chromogenic reagent for 1 min. The optical density of
protein band was determined by ImageJ software (National Institutes
of Health, Bethesda, MD, USA). Relative expression of protein was
calculated by the ratio of optical density of target protein band
to internal control band. Statistical analysis was performed on
relative protein expression.
Statistical analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis which included Bartlett's,
Brown-Forsythe and Levene's tests, to test for equal variance
followed by Student-Newman-Keuls multiple-range test and one-way
analysis of variance was used to compare differences between
groups.
Results
Transfection efficacy of
Lipofectamine® 2000 and HSP27 assessed by flow
cytometry
Five different combinations of HSP27 plasmid and
transfection reagent Lipofectamine® 2000 were assessed,
including 8 µg:20 µl; 8 µg:25 µl; 8 µg:30 µl; 9 µg:20 µl; 10 µg:20
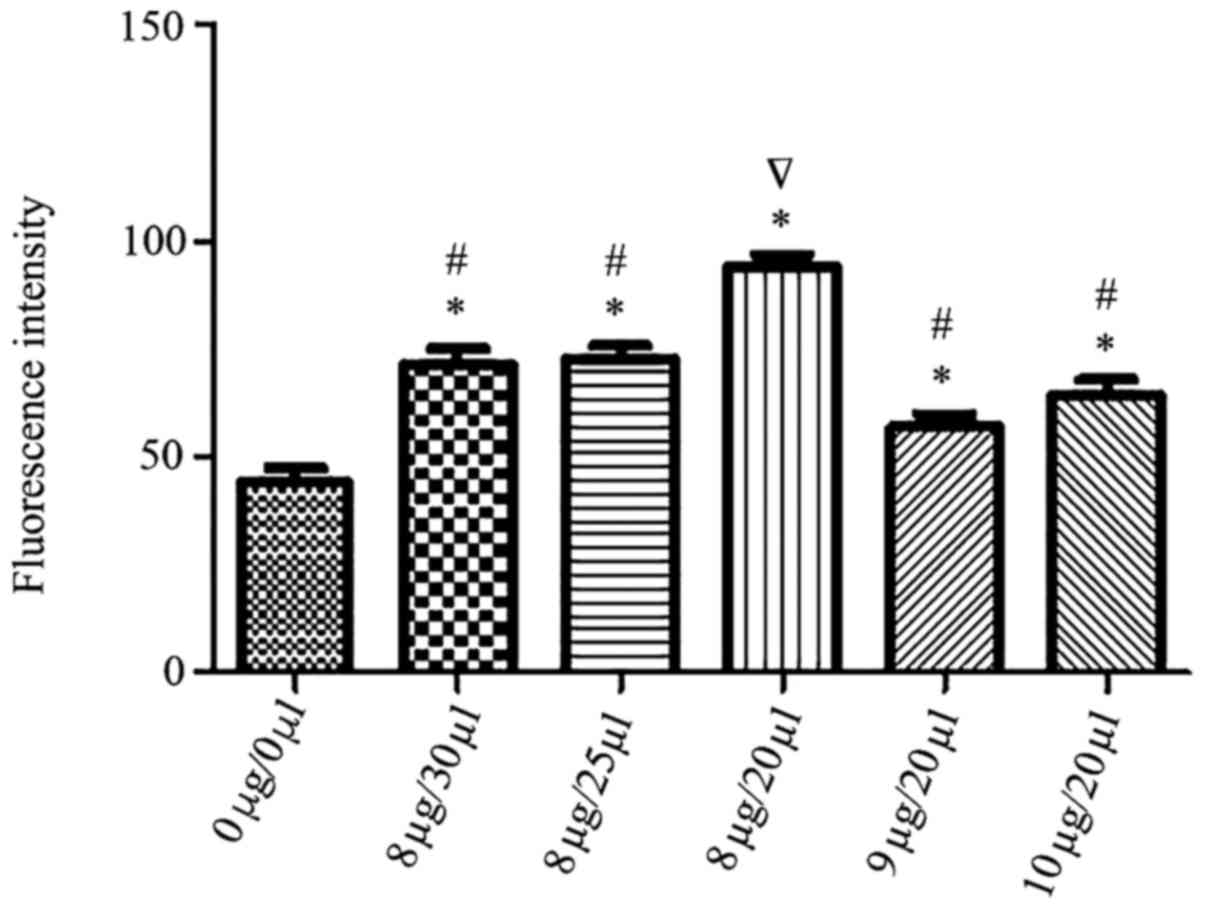
µl. Flow cytometry data indicated in Fig. 1 revealed that transfection efficacy
was 83, 76, 76, 70, 74%, respectively. The 8 µg:20 µl ratio led to
a 83% transfection efficiency and was considered to be the optimal
liposome to plasmid ratio and used for subsequent experiments.
Laser scanning confocal microscope
detection for the optimal ratio of Lipofectamine® 2000
and HSP27 with best transfection efficacy
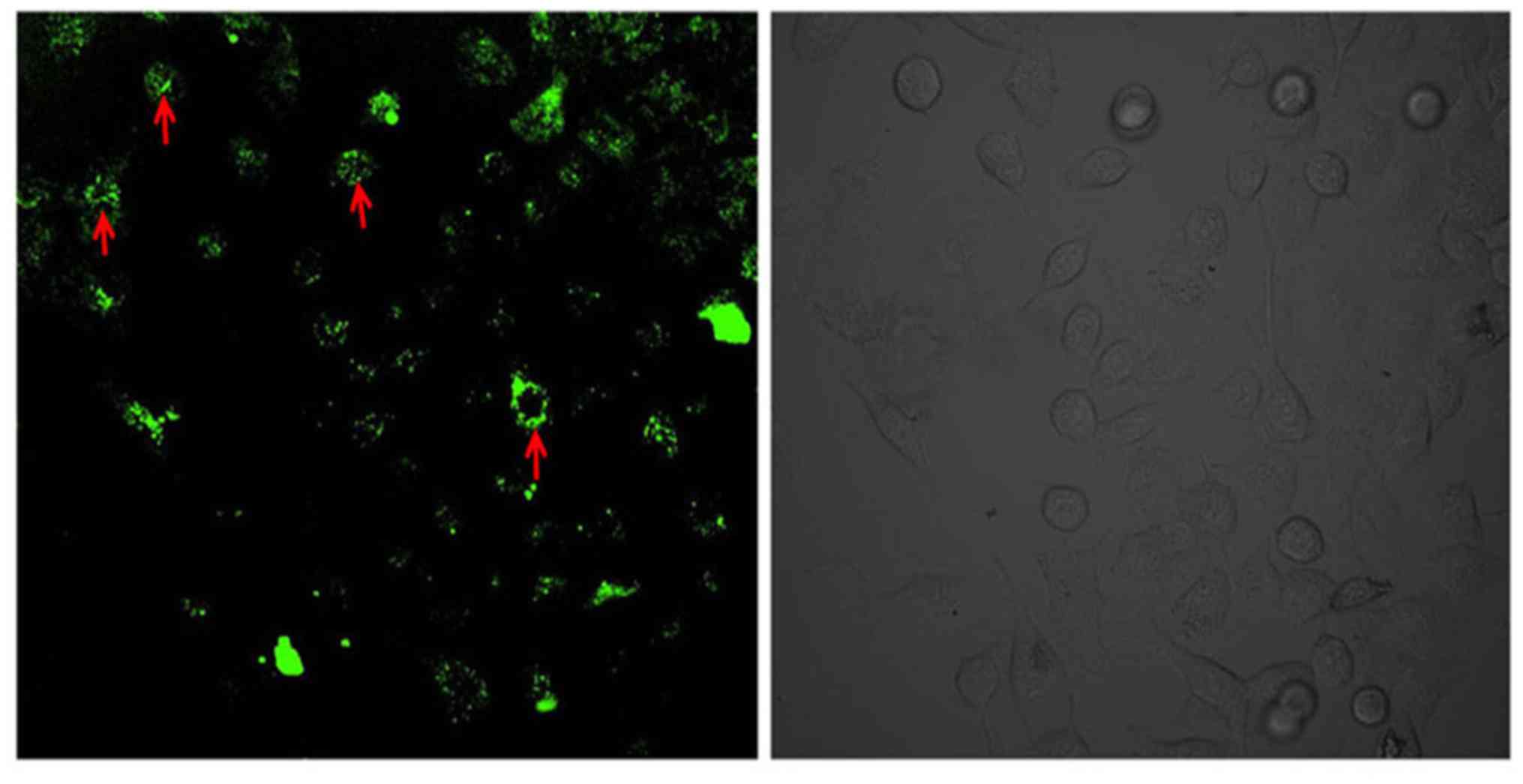
Flow cytometry results were confirmed with
microscopy and after successful transfection at 8 µg:20 µl of
HSP27: Lipofectamine® 2000, GFP positive expression was
visible in the cytoplasm (Fig.
2).
Optimal interfering HSP27 plasmid
identified using RT-qPCR
Data from the RT-qPCR are presented in Fig. 3 and indicated that the interference
effect of the four different sequences was 48, 55, 59 and 30% with
respect to the positive control group and 48, 54, 58 and 30%
relative to negative control group. The fourth interfering sequence
(D) achieved the most efficient gene silencing of HSP27 with 70% of
the HSP27 gene being silenced, which was statistically significant
compared with positive control and negative control group.
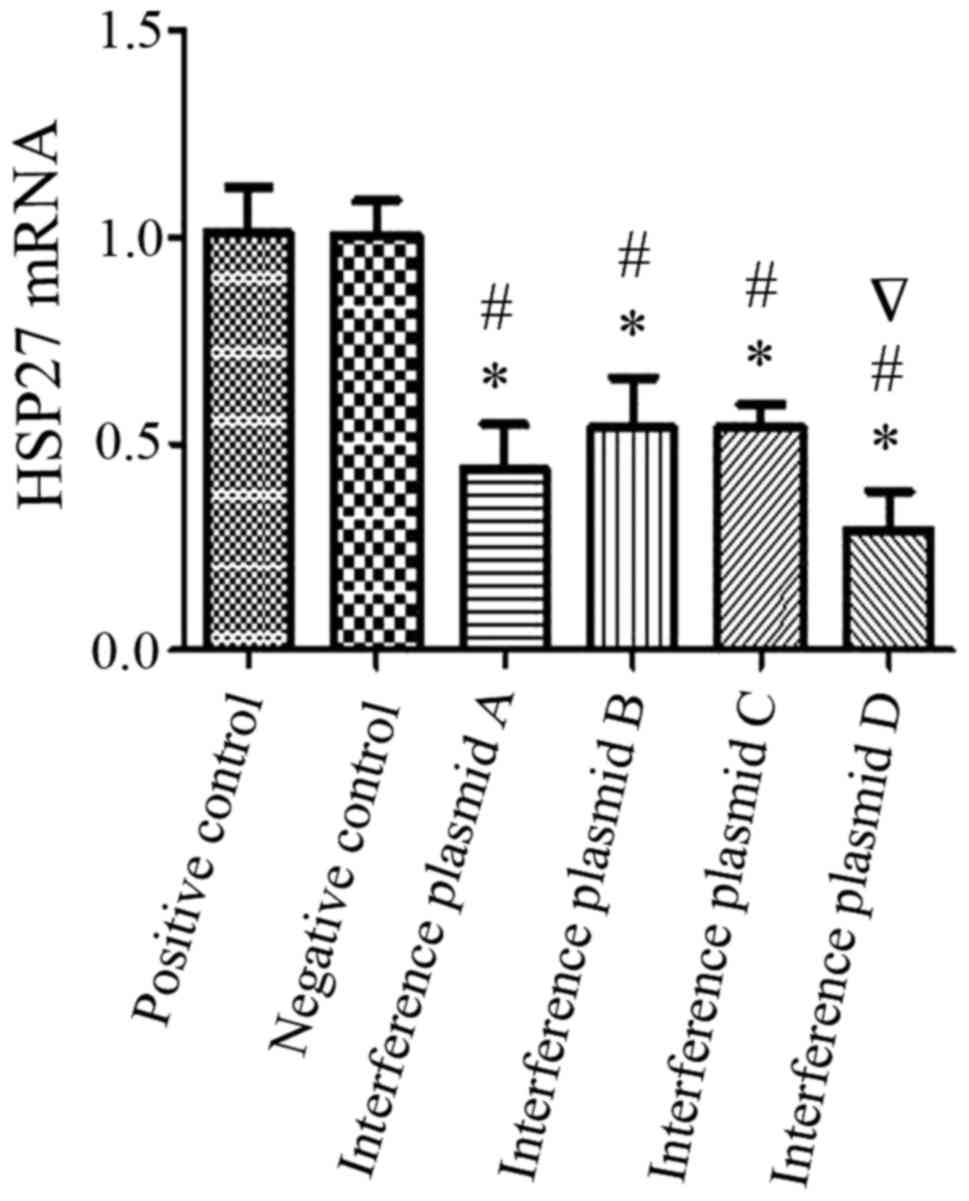 | Figure 3.Comparison of silencing efficacy with
four different interference fragments in alveolar type II
epithelial cells. The optimal ratio of plasmid to transfection
reagent was used (8 µg:20 µl) and four different interference
plasmids (A, B, C, D) were transfected into A549 alveolar type II
epithelial cells. HSP27 mRNA expression was detected using reverse
transcription-quantitative polymerase chain reaction. The
interference effect of each interference plasmid (A, B, C, D) was
48, 55, 59 and 30% when compared with the positive control group,
and 48, 54, 58 and 30% when compared with the negative control
group. From the four plasmids, plasmid D had the highest gene
silencing effect with 70% HSP27 gene silencing. *P<0.05 vs.
positive control, #P<0.05 vs. negative control.
∇Indicates most efficient interference fragment plasmid.
HSP27, heat shock protein 27. |
Transfection efficacy and the
expression of type I and III collagen
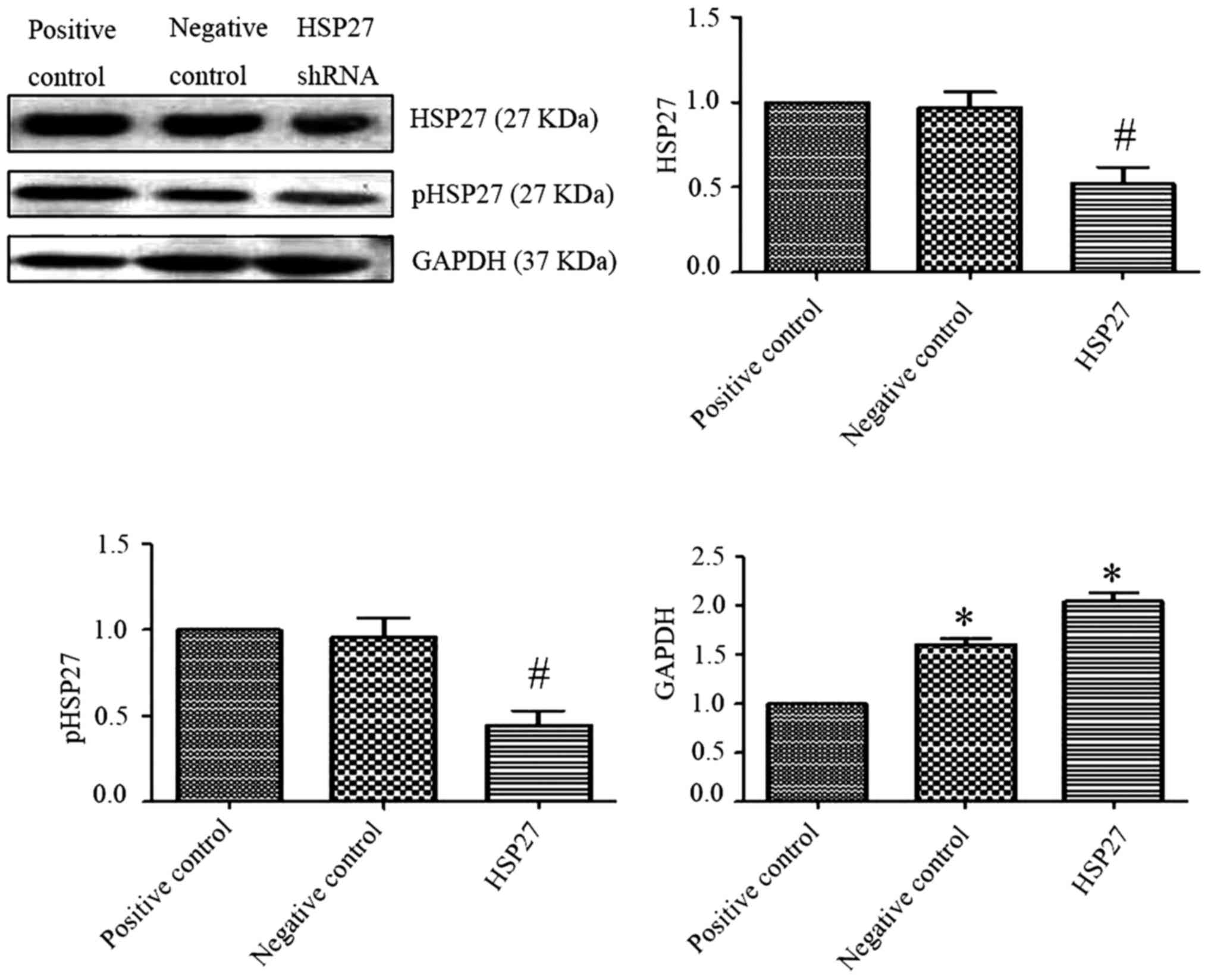
Data from western blot experiments revealed that
compared with the HSP27 negative control group, the expression of
HSP27 and phosphorylated HSP27 in transfection of HSP27-interfering
plasmid group was downregulated (Fig.
4; P<0.05). The difference was statistically significant
compared with the transfection of GAPDH in the positive control
group, the expression of GAPDH was significantly upregulated in
transfected with HSP27 interfering plasmid and the HSP27 negative
control group (P<0.05). This indicated that the transfection of
HSP27 interference plasmid was successful. Additionally, findings
from the western blot analysis revealed that compared with the
blank plasmid transfection group, the expression of HSP27 and
pHSP27 increased, which was accompanied by the increased expression
of type I and III collagen in blank plasmid group, which was
induced with TGF-β1 (Fig. 5),
which was statistically significant (P<0.05). The expression of
HSP27 and pHSP27 was significantly downregulated along with the
reduced expression of type I and III collagen in the TGF-β1-induced
HSP27-interfering plasmid group, compared with the TGF-β1-induced
blank plasmid transfection group (P<0.05).
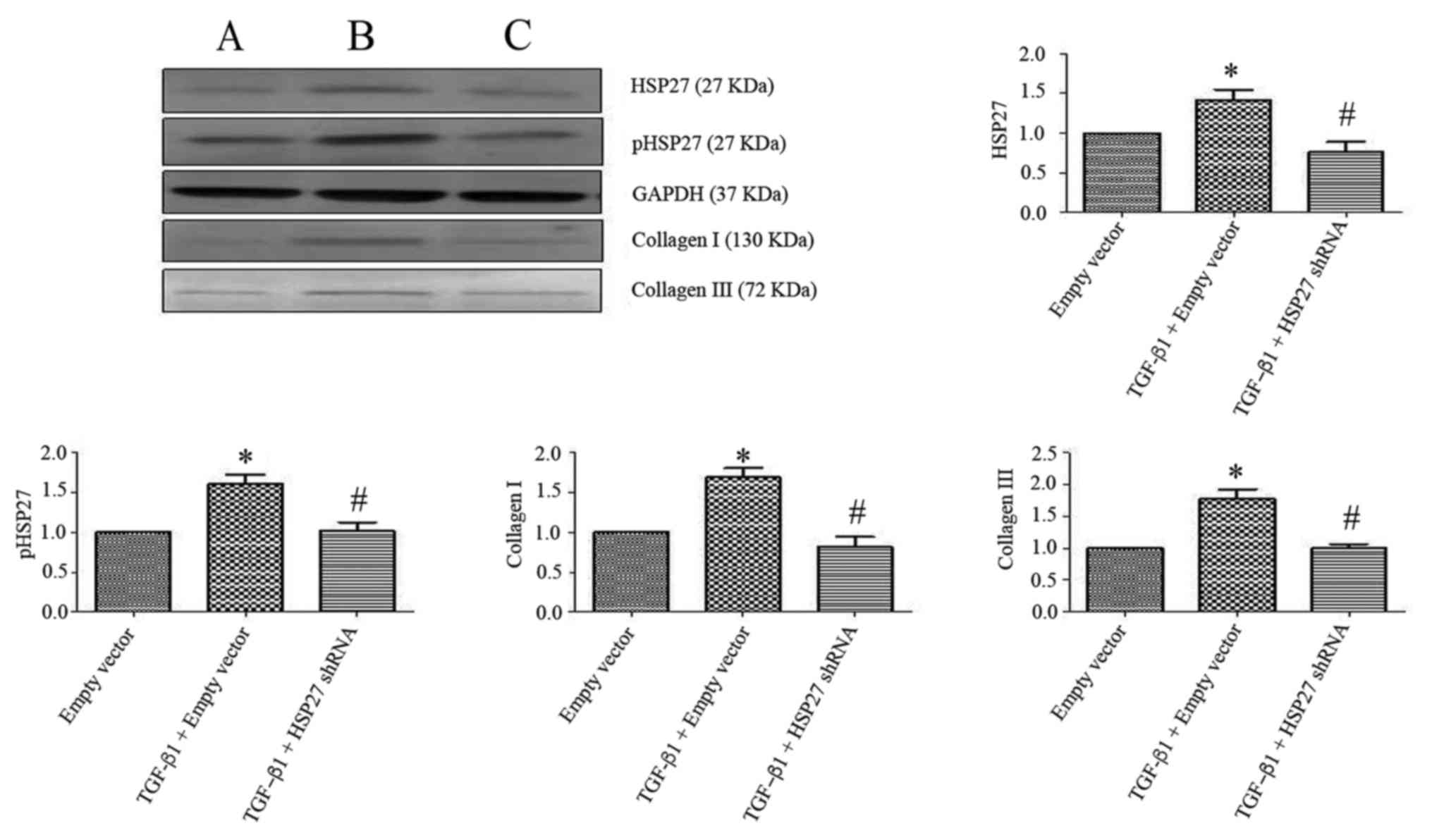 | Figure 5.Effect of HSP27 interfering plasmid
on the expression of type I and III collagen in TGF-β1-induced
alveolar type II epithelial cell. Western blotting revealed that
compared with the empty vector transfection group, the expressions
of HSP27, pHSP27, and type I and III collagen were upregulated in
TGF-β1-induced empty vector transfection group. Following
transfection with an HSP27-interfering plasmid, cells were treated
with TGF-β1. The expression levels of HSP27, pHSP27, type I and III
collagen were downregulated in HSP27-interfering plasmid group when
compared with the TGF-β1-induced empty vector group. (A) Vector
transfection group. (B) TGF-β1-induced empty vector transfection
group. (C) HSP27-interfering plasmid group. The difference was
statistically significant. P<0.05. TGF-β1, transforming growth
factor-β1; HSP27, heat shock protein 27; p-HSP27,
phosphorylated-HSP27; shRNA, short hairpin RNA. |
Discussion
Cationic liposome is a promising non-virus gene
vehicle (15,16), whose membrane property confers
various advantages in application terms of therapy and study of
gene transfection, including maintaining the biological activity of
genes, protecting genes from degradation by lysosomes. It is also
non-immunogenic, easy to apply, with high repeatability and it is
naturally degraded (17–25). The current study used
Lipofectamine® 2000 to transfect A549 human alveolar
type II epithelial cell line with HSP27-interfering plasmid; the
procedure was easy, safe and reliable with high transfection
efficacy, and met the requirement of subsequent experiments.
Therefore, this method provides a reference in the application of
Lipofectamine® 2000-mediated transfection of other cell
lines.
HSP27 is a small molecular weight protein in heat
shock protein family (26).
Previous studies have previously reported that HSP27 is involved in
the tumor cell EMT (27–32). In breast cancer, tumor cells that
undergo EMT exhibit a high expression of HSP27 protein, which
indicated that HSP27 protein may be closely associated with breast
cancer cell EMT and may be involved in tumor migration and drug
resistance (32). When MET-5A
pleural mesothelial cells were stimulated in vitro with
TGF-β1, the expression of HSP27 was significantly upregulated and
accompanied by the increased expression of α-smooth muscle actin
(α-SMA) and reduced the expression of E-cadherin, whereas cell
morphology changed from flat cobblestones to long spindle-shapes.
When HSP27-specific inhibitor OGX-427 (antisense oligonucleotides)
or HSP27 siRNA transfection were used the increased expression of
HSP27 and α-SMA protein by TGF-β stimulation was effectively
reduced (9). Additionally, the
downregulation of E-cadherin expression was also inhibited and cell
morphology was maintained as flat or round-like. This indicated
that HSP27 may be important for fibrosis and involved in pleural
mesothelial cell EMT (9). In
NRK52E tubular epithelial cells, TGF-β1 stimulation led to the
upregulation of HSP27 expression, accompanied by the upregulation
of α-SMA and vimentin, as well as fibronectin expression and led to
downregulation of E-cadherin expression. This suggested that HSP27
has a protective role in tubular epithelial cell EMT (10). Additionally, HSP27 had a protective
effect on myocardial cells in ischemic heart disease and
adriamycin-induced myocardial injury due to this anti-apoptotic
effect (33,34). Therefeore, it is important to
investigate the effect of HSP27 on A549 human alveolar type II
epithelial cell EMT. Extracellular matrix (ECM) deposition is a
direct cause of fibrogenesis (35–39).
Type I and III collagen are essential ECM components that reflect
the differentiation extent of myofibroblasts (40–43).
In our previous studies, we found that 5 ng/ml TGF-β1 was able to
successfully induce the transition of A549 human alveolar type II
epithelial cells to myofibroblasts, accompanied with upregulation
of type I and III collagen expression (13). In A549 cells transfected with
HSP27-interfering plasmid, the expression of type I and III
collagen was downregulated by TGF-β1 stimulation, which suggested
that HSP27 is a factor that may induce fibrosis in the transition
of TGF-β1-induced A549 human alveolar type II epithelial cells to
myofibroblasts. The present study provided the experimental basis
and theoretical evidence for further investigation of the effect of
HSP27 in vivo on EMT in silicosis or fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
National Natural Science Foundation of China (grant no. 81302395)
and Science and Technology Research Projects in the Colleges and
Universities of Hebei Province (grant no. QN2016147).
Availability of data and materials
The analyzed data generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
FY conceived and designed the experiments. HJD, XMG,
HBP and XLH performed the experiments. HJD, HX and JW analyzed the
data. FY wrote the manuscript and HJD provided support on
reagents/materials/analysis tools.
Ethics approval and consent to
participate
A549 human alveolar type II epithelial cell line
derived from human lung carcinoma purchased from Cell Bank of
Chinese Academy of Science, Shanghai, China which was received
ethics approval. The manuscript involves no other human
participants, human data or human tissue.
Consent for publication
All authors consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu H, Xue X, Du S, Li S, Sun Y, Yuan Y,
Deng H, Wei Z, Wang R and Yang F: Comparative proteomic analysis on
anti-fibrotic effect of N-acetyl-seryl-aspartyl-lysyl-proline in
rats with silicosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za
Zhi. 32:561–567. 2014.(In Chinese). PubMed/NCBI
|
|
2
|
Tang S, Chen H, Cheng Y, Nasir MA, Kemper
N and Bao E: Expression profiles of heat shock protein 27 and
αB-crystallin and their effects on heat-stressed rat myocardial
cells in vitro and in vivo. Mol Med Rep. 13:1633–1638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song IS, Kang SS, Kim ES, Park HM, Choi
CY, Tchah H and Kim JY: Heat shock protein 27 phosphorylation is
involved in epithelial cell apoptosis as well as epithelial
migration during corneal epithelial wound healing. Exp Eye Res.
118:36–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao M, Ding JX, Zeng K, Zhao J, Shen F,
Yin YX and Chen Q: Heat shock protein 27: A potential biomarker of
peritoneal metastasis in epithelial ovarian cancer? Tumour Biol.
35:1051–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zembron-Lacny A, Ziemann E, Zurek P and
Hübner-Wozniak E: Heat shock protein 27 response to wrestling
training in relation to the muscle damage and inflammation. J
Strength Cond Res. 31:1221–1228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang S, Yang S, Vlantis AC, Liu SY, Ng EK,
Chan AB, Wu J, Du J, Wei W, Liu X, et al: Expression of antioxidant
molecules and heat shock protein 27 in thyroid tumors. J Cell
Biochem. 117:2473–2481. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carper SW, Rocheleau TA, Cimino D and
Storm FK: Heat shock protein 27 stimulates recovery of RNA and
protein synthesis following a heat shock. J Cell Biochem.
66:153–164. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schweiger T, Nikolowsky C, Starlinger P,
Traxler D, Zimmermann M, Birner P, Hegedüs B, Dome B, Bergmann M,
Mildner M, et al: Stromal expression of heat-shock protein 27 is
associated with worse clinical outcome in patients with colorectal
cancer lung metastases. PLoS One. 10:e01207242015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wettstein G, Bellaye PS, Kolb M, Hammann
A, Crestani B, Soler P, Marchal-Somme J, Hazoume A, Gauldie J,
Gunther A, et al: Inhibition of HSP27 blocks fibrosis development
and EMT features by promoting Snail degradation. FASEB J.
27:1549–1560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vidyasagar A, Reese S, Acun Z, Hullett D
and Djamali A: HSP27 is involved in the pathogenesis of kidney
tubulointerstitial fibrosis. Am J Physiol Renal Physiol.
295:F707–F716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun YC, Liang Q, Qian KL, Xiao L, Liu Q
and Shi XF: Effect of TGF-b1 siRNA-mediated silencing on Smad
proteins in hepatic fibrosis rats. Zhonghua Gan Zang Bing Za Zhi.
20:289–293. 2012.(In Chinese). PubMed/NCBI
|
|
12
|
Cheng J, Wang M, Ma H, Li H, Ren J and
Wang R: Adiponectin inhibits oxidative stress and modulates TGF-b1
and COL-1 expression via the AMPK pathway in HSC-T6 cells. Zhonghua
Gan Zang Bing Za Zhi. 23:69–72. 2015.(In Chinese). PubMed/NCBI
|
|
13
|
Deng H, Yang F, Xu H, Sun Y, Xue X, Du S,
Wang X, Li S, Liu Y and Wang R: Ac-SDKP suppresses
epithelial-mesenchymal transition in A549 cells via HSP27
signaling. Exp Mol Pathol. 97:176–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harigai T, Kondo M, Isozaki M, Kasukawa H,
Hagiwara H, Uchiyama H and Kimura J: Preferential binding of
polyethylene glycol-coated liposomes containing a novel cationic
lipid, TRX-20, to human subendthelial cells via chondroitin
sulfate. Pharm Res. 18:1284–1290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Negishi Y, Endo-Takahashi Y, Matsuki Y,
Kato Y, Takagi N, Suzuki R, Maruyama K and Aramaki Y: Systemic
delivery systems of angiogenic gene by novel bubble liposomes
containing cationic lipid and ultrasound exposure. Mol Pharm.
9:1834–1840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arisaka M, Takano K, Negishi Y, Arima H
and Aramaki Y: Involvement of lipid rafts in macrophage apoptosis
induced by cationic liposomes. Arch Biochem Biophys. 508:72–77.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bailey AL and Cullis PR: Membrane fusion
with cationic liposomes: effects of target membrane lipid
composition. Biochemistry. 36:1628–1634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bajoria R, Sooranna S and Chatterjee R:
Effect of lipid composition of cationic SUV liposomes on
materno-fetal transfer of warfarin across the perfused human term
placenta. Placenta. 34:1216–1222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barenholz Y, Bombelli C, Bonicelli MG, di
Profio P, Giansanti L, Mancini G and Pascale F: Influence of lipid
composition on the thermotropic behavior and size distribution of
mixed cationic liposomes. J Colloid Interface Sci. 356:46–53. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bianco A, Napolitano R, Bonadies F, Celona
D, Ortaggi G and Cametti C: Liposomes from a new chiral cationic
lipid based on iridoidic template. Natl Prod Res. 21:1221–1227.
2007. View Article : Google Scholar
|
|
22
|
Chen T, Wang RT, Wang Z, Lu TL and Zhao W:
Construction and evaluation of non-specific targeting cationic
polymer lipid liposomes. Yao Xue Xue Bao. 45:359–364.
2010.PubMed/NCBI
|
|
23
|
de Paula Rigoletto T, Silva CL, Santana
MH, Rosada RS and de la Torre LG: Effects of extrusion, lipid
concentration and purity on physico-chemical and biological
properties of cationic liposomes for gene vaccine applications. J
Microencapsul. 29:759–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Zhen Y, Ma X, Wei B, Li S and Wang
N: Mannosylated and lipid A-incorporating cationic liposomes
constituting microneedle arrays as an effective oral mucosal HBV
vaccine applicable in the controlled temperature chain. Colloids
Surf B Biointerfaces. 126:520–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang N, Wang T, Zhang M, Chen R, Niu R and
Deng Y: Mannose derivative and lipid A dually decorated cationic
liposomes as an effective cold chain free oral mucosal vaccine
adjuvant-delivery system. Eur J Biopharm. 88:194–206. 2014.
View Article : Google Scholar
|
|
26
|
Vidyasagar A, Wilson NA and Djamali A:
Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic
target. Fibrogenesis Tissue Repair. 5:72012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Research on the efficacy of Celastrus
Orbiculatus in suppressing TGF-β1-induced epithelial-mesenchymal
transition by inhibiting HSP27 and TNF-α-induced NF-κB/Snail
signaling pathway in human gastric adenocarcinoma. BMC Complement
Altern Med. 14:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cordonnier T, Bishop JL, Shiota M, Nip KM,
Thaper D, Vahid S, Heroux D, Gleave M and Zoubeidi A: Hsp27
regulates EGF/β-catenin mediated epithelial to mesenchymal
transition in prostate cancer. Int J Cancer. 136:E496–E507. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen SF, Nieh S, Jao SW, Liu CL, Wu CH,
Chang YC, Yang CY and Lin YS: Quercetin suppresses drug-resistant
spheres via the p38 MAPK-Hsp27 apoptotic pathway in oral cancer
cells. PLoS One. 7:e492752012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mizutani H, Okano T, Minegishi Y, Matsuda
K, Sudoh J, Kitamura K, Noro R, Soeno C, Yoshimura A, Seike M and
Gemma A: HSP27 modulates epithelial to mesenchymal transition of
lung cancer cells in a Smad-independent manner. Oncol Lett.
1:1011–1016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shiota M, Bishop JL, Nip KM, Zardan A,
Takeuchi A, Cordonnier T, Beraldi E, Bazov J, Fazli L, Chi K, et
al: Hsp27 regulates epithelial mesenchymal transition, metastasis
and circulating tumor cells in prostate cancer. Cancer Res.
73:3109–3119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei L, Liu TT, Wang HH, Hong HM, Yu AL,
Feng HP and Chang WW: Hsp27 participates in the maintenance of
breast cancer stem cells through regulation of
epithelial-mesenchymal transition and nuclear factor-κB. Breast
Cancer Res. 13:R1012011. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bernard Y, Ribeiro N, Thuaud F, Türkeri G,
Dirr R, Boulberdaa M, Nebigil CG and Désaubry L: Flavaglines
alleviate doxorubicin cardiotoxicity: Implication of Hsp27. PLoS
One. 6:e253022011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Won YW, Kim JK, Cha MJ, Hwang KC, Choi D
and Kim YH: Prolongation and enhancement of the anti-apoptotic
effects of PTD-Hsp27 fusion proteins using an injectable
thermo-reversible gel in a rat myocardial infarction model. J
Control Release. 144:181–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen Y, Miao N, Xu J, Gan X, Xu D, Zhou L,
Xue H, Zhang W and Lu L: Metformin prevents renal fibrosis in mice
with unilateral ureteral obstruction and inhibits ang II-induced
ECM production in renal fibroblasts. Int J Mol Sci. 17:2016.
View Article : Google Scholar
|
|
36
|
Minton K: Extracellular matrix:
Preconditioning the ECM for fibrosis. Nat Rev Mol Cell Biol.
15:766–767. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li SB and Jia YJ: Interactions between ECM
and HSC cells in hepatic fibrosis. Sheng Li Ke Xue Jin Zhan.
45:462–464. 2014.(In Chinese). PubMed/NCBI
|
|
38
|
Corona BT, Wu X, Ward CL, McDaniel JS,
Rathbone CR and Walters TJ: The promotion of a functional fibrosis
in skeletal muscle with volumetric muscle loss injury following the
transplantation of muscle-ECM. Biomaterials. 34:3324–3335. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chun TH: Peri-adipocyte ECM remodeling in
obesity and adipose tissue fibrosis. Adipocyte. 1:89–95. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi MC, Cheung KK, Li X and Cheing GL:
Pulsed electromagnetic field (PEMF) promotes collagen fibre
deposition associated with increased myofibroblast population in
the early healing phase of diabetic wound. Arch Dermatol Res.
308:21–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Al-Qattan MM, Abd-Elwahed MM, Hawary K,
Arafah MM and Shier MK: Myofibroblast expression in skin wounds is
enhanced by collagen III suppression. Biomed Res Int.
2015:9586952015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang HS, Zhu LL, Zhang Z, Chen H, Chen Y
and Dai YT: Estradiol attenuates the TGF-β1-induced conversion of
primary TAFs into myofibroblasts and inhibits collagen production
and myofibroblast contraction by modulating the Smad and Rho/ROCK
signaling pathways. Int J Mol Med. 36:801–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mia MM and Bank RA: The pro-fibrotic
properties of transforming growth factor on human fibroblasts are
counteracted by caffeic acid by inhibiting myofibroblast formation
and collagen synthesis. Cell Tissue Res. 363:775–789. 2016.
View Article : Google Scholar : PubMed/NCBI
|