Introduction
Malignant melanoma (MM), primarily caused by
melanophore cancerization and hyperplasia, can occur in the skin,
mucous membranes and central nervous system (1,2). MM
is a common type of malignant tumor in dermatology with a high
malignancy and incidence. The median survival rate of patients with
MM is only 18 months, and it is the leading cause of skin malignant
tumor-associated mortality around the world (3,4). The
occurrence of MM has ethnic and regional features, being higher in
the European and American countries. It is the cause of skin
cancer-associated mortality in developed countries (5,6). In
the Asian population, primary cutaneous melanoma accounts for
50–70% of cases, where the primary pathological type is entigo
maligna melanoma, followed by superficial invasive melanoma and
nodular MM (7,8). Previously, the incidence of MM was
low in China; however, the incidence of MM has gradually increased
following changes to lifestyle. MM is characterized as highly
malignant and readily metastasized, and has a poor prognosis
(9).
The pathogenetic mechanism of MM is complex and
remains to be fully elucidated. Multiple factors are associated
with the induction of MM, including genetics, physics, chemistry,
family history and long-term sun exposure (10). Following detailed investigations of
the mechanism, current treatment methods for MM include
chemotherapy and molecular target therapy. The aim of molecular
target therapy is to interpose MM proliferation and mutation from
the molecular level (11,12). Although multiple molecular
anticancer drugs for MM have been examined, their curative effect
remains poor. Tumor angiogenesis is important in the occurrence and
development of MM; therefore, targeting angiogenesis is important
for the treatment of MM (13). As
an important proangiogenic factor of tumor growth and metastasis,
VEGF can promote neovascularization and increase vascular
permeability (14). Gefitnib is a
novel target drug against VEGF (15). However, the effect and mechanism of
gefitinib in MM remain to be fully elucidated. Therefore, the
present study aimed to investigate the effect of gefitinib on MM
cell proliferation and invasion, and the associated mechanism.
Materials and methods
Main instruments and reagents
The MM A375 cell line was purchased from the
American Type Culture Collection Cell Bank (ATCC; Mannasas, VA,
USA). DMEM, FBS, and penicillin-streptomycin were obtained from
Hyclone; GE Healthcare Life Sciences (Logan, UT, USA). Dimethyl
sulfoxide and MTT powder were purchased from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Enzyme-EDTA was from
Sigma-Aldrich; Merck Miilipore (Darmstadt, Germany). The caspase-3
activity detection kit and PVDF membrane were from Pall Life
Sciences (Ann Arbor, MI, USA). EDTA was purchased from Hyclone; GE
Healthcare Life Sciences. The reagents associated with western blot
analysis were from Beyotime Institute of Biotechnology (Haimen,
China). ECL reagent was from GE Healthcare Life Sciences. Rabbit
anti-human VEGF (cat. no. 2463) and AKT (cat. no. 4691) monoclonal
antibodies, and mouse anti-rabbit horseradish peroxidase
(HRP)-tagged IgG secondary antibody (cat. no. 5127) were from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The Transwell
chamber was from Corning Inc. (Corning, NY, USA). The ABI 7700 Fast
fluorescence quantitative PCR reaction apparatus was from Applied
Biosystems; Thermo Fisher Scientific, Inc. The RNA extraction kit
and reverse transcription kit were from Axygen Biosceiences (Union
City, CA, USA). Other common reagents were purchased from Sangon
Biotech Co., Ltd. (Shanghai, China). The Labsystem version 1.3.1
microplate reader was from Bio-Rad Laboratories, Inc. (Hercules,
CA, USA).
MM A375 cell culture and grouping
The A375 cell line stored in liquid nitrogen was
thawed in a 37°C water bath and centrifuged at 300 × g for 3 min at
room temperature. The cells were then resuspended in 1 ml medium
and cultured in a 50 ml flask at 37°C and 5% CO2 for
24–48 h. The cells were passaged every 2–3 days and were used for
experiments in the logarithmic phase at passages 2–8. The cells
were divided into three groups, including the control, 5 µM
gefitinib group and 10 µM gefitinib group. The cells in the
treatment two groups were treated with gefitinib for 48 h at
37°C.
MTT assay
The A375 cells in the logarithmic phase were seeded
into 96-well plate at 5×l03/well for 24 h. The cells
were divided into control and gefitinib groups with three
replicates, which were cultured for 48 h. Subsequently, the plate
was treated with 20 µl 5 g/l MTT solution and incubated for 4 h at
37°C. Following removal of the supernatant, 150 µl DMSO was added
to the plate for 10 min and read at 570 nm to calculate the
proliferation rate.
Transwell assay
The Transwell chamber was coated with 50 mg/l
Matrigel at 1:5 for 24 h and then air dried at 4°C. A total of 500
µl DMEM containing 10% FBS were added to the lower chamber, and 100
µl tumor cell suspension in FBS-free medium was added to the upper
chamber with three replicates. The cells in the control were
cultured in a Transwell chamber without Matrigel. After 48 h, the
chamber was washed in PBS and fixed in ice ethanol. Following
staining with crystal violet, the cells on the lower membrane were
counted under a light microscope (BX43; Olympus Corporation, Tokyo,
Japan). All experiments were repeated three times.
Detection of caspase-3 activity
Caspase 3 activity was detected using a kit
according to the manufacturer's instructions. The cells were
digested in enzyme and centrifuged at 600 g and 4°C for 5 min. The
cells were then placed on ice for 15 min and centrifuged at 20,000
g and 4°C for 5 min. Following the addition of 2 mM Ac-DEVD-pNA,
the sample was read at 405 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the A375 cells using
TRIzol and reverse transcribed into cDNA. The primers used were
designed by Primer 6.0 software (Premier Biosoft, Palo Alto, CA,
USA) and synthetized by Invitrogen; Thermo Fisher Scientific, Inc.
(Table I). qPCR was performed in a
total volume of 20 µl, including 10 µl SYBR Green qPCR Super mix,
0.5 µl forward primer (10 µM), 0.5 µl reverse primer (10 µM), 5 µl
cDNA and 4 µl sterile water. The reaction conditions were as
follows: 55°C for 1 min, followed by 35 cycles of 92°C for 30 sec,
58°C for 45 sec, and 72°C for 35 sec. GAPDH was used as internal
reference. The 2−ΔΔCq method (16) was applied to calculate relative
expression levels.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| GADPH |
AGTACCAGTCTGTTGCTGG |
TAATAGACCCGGATGTCTGGT |
| VEGF |
ATCCTTATCTCTGTGTGGAACTTTGTG | CTCCCTCTCAGCG
CTCACAGCTTGCTG |
| AKT |
TATCTCTCTGTCTCCCACAGAAGTC |
TACTTACCTCGCATGGGGTAATTTGG |
Western blot analysis
The cells were lysed in RIPA buffer (150 mM NaCL, 1%
NP-40, 0.1% SDS, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 1 mM PMSF,
1.5 mM EDTA and 1 mM NaVanadate) on ice for 15–30 min and
ultrasonicated for 5 sec four times to extract protein. Following
centrifugation at 10,000 × g and 4°C for 15 min, the protein was
moved to a new Ep tube and store at −20°C. The protein was
separated by 10% SDS-PAGE and transferred onto a PVDF membrane.
Following blocking in 5% skim milk for 2 h, the membrane was
incubated in VEGF primary antibody at 1:1,000 and AKT primary
antibody at 1:2,000 overnight at 4°C. The membrane was then
incubated with secondary antibody at 1:2,000 for 30 min at room
temperature and washed with PBST. Finally, the membrane was treated
with chemiluminescent agent for 1 min, and underwent X-ray imaging.
The protein image processing system and Quantity One software
version 4.6 (Bio-Rad Laboratories, Inc.) were used for data
analysis. All experiments were repeated four times.
Statistical analysis
All statistical analyses were performed on SPSS 11.5
software (SPSS, Inc., Chicago, IL, USA). Measurement data are
presented as the mean ± standard deviation. One-way analysis of
variance was used for comparison of means. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of gefitinib on melanoma cell
proliferation
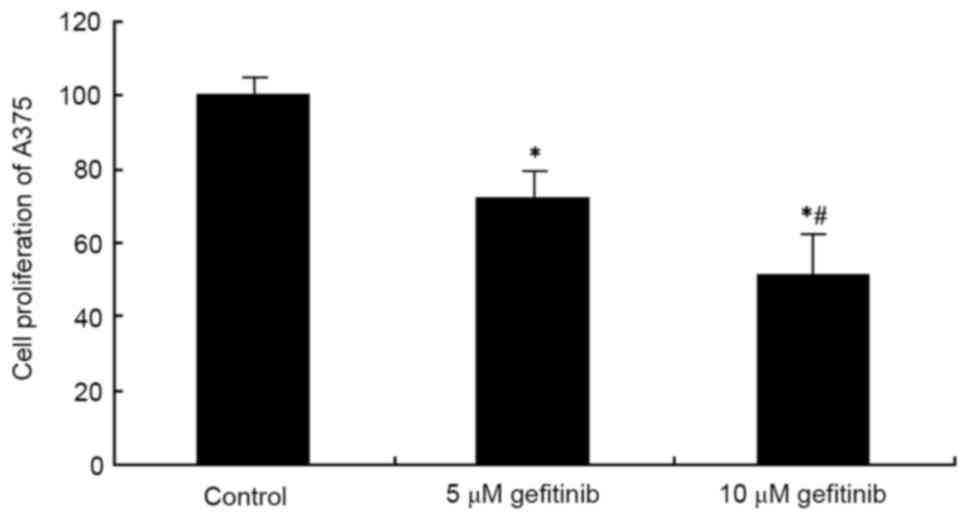
An MTT assay was used to examine the effect of
gefitinib on A375 cell proliferation. The results showed that
gefitinib treatment for 48 h significantly suppressed A375 cell
proliferation, compared with the control (P<0.05). Following an
increase in dose, the tumor cell-suppressing effect was more marked
(P<0.05; Fig. 1). These results
suggested that gefitinib inhibited abnormal proliferation of the MM
cells.
Effects of gefitinib on MM cell
invasion
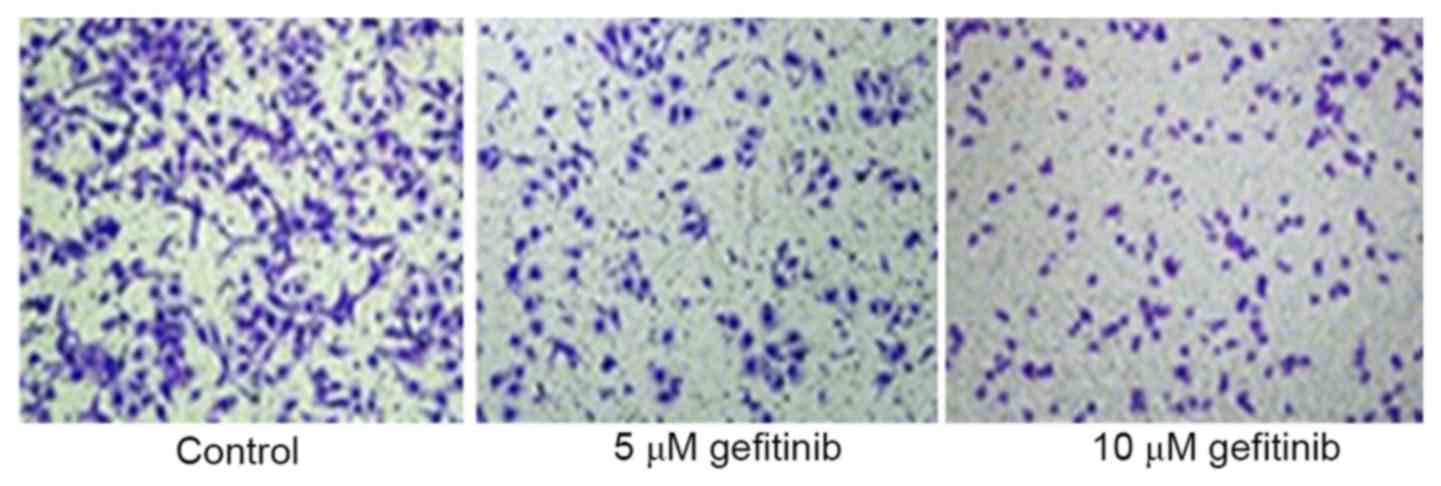
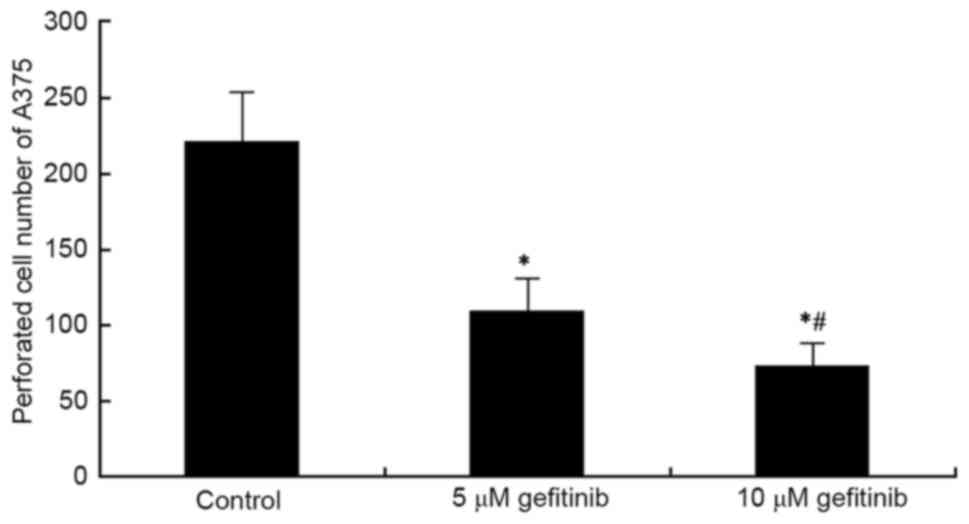
A Transwell assay was used to determine the effect
of effect on the invasive ability of A375 cells. It was revealed
that gefitinib treatment for 48 h markedly inhibited A375 cell
invasion, compared with that in the control (P<0.05). Following
an increase in dose, gefitinib had a more marked suppressive effect
on tumor cell invasion (P<0.05; Figs. 2 and 3). These results indicated that gefitinib
affected MM cell invasive ability.
Effect of gefitinib on the activity of
caspase-3 in MM cells
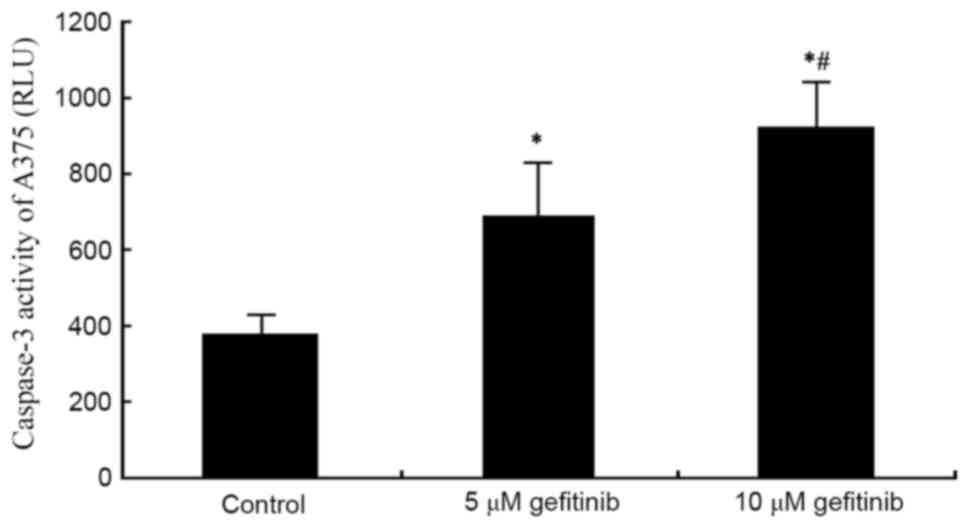
A caspase 3 activity detection kit was used to
measure the effect of gefitinib on the activity of caspase-3 in the
A375 cells. The results demonstrated that gefitinib treatment for
48 h significantly increased the activity of caspase-3 in the A375
cells (P<0.05). Following an increase of dose, gefitinib exerted
a more marked promoting effect on the activity of caspase-3
(P<0.05; Fig. 4). These results
suggested that gefitinib promoted MM cell apoptosis by enhancing
the activity of caspase-3.
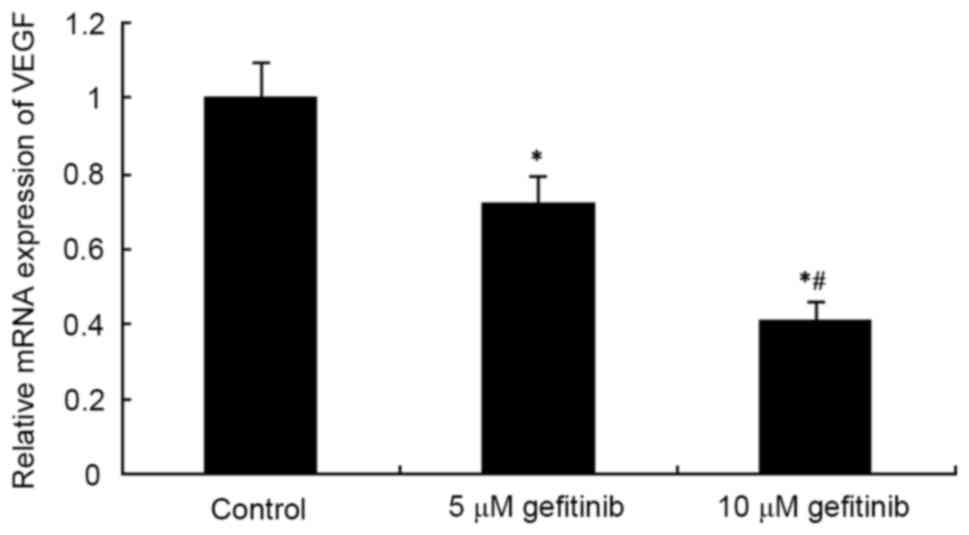
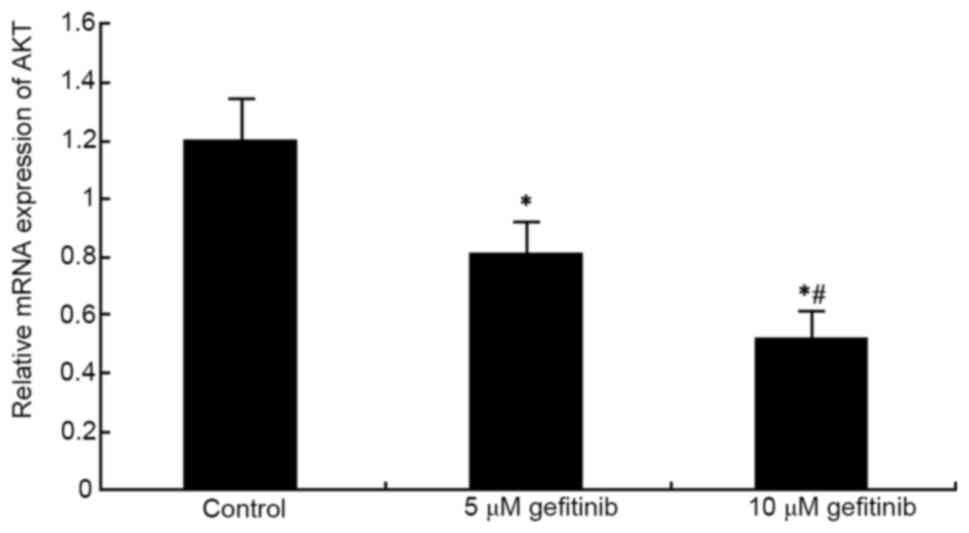
Effects of gefitinib on the mRNA
expression of VEGF and AKT in MM cells
RT-qPCR analysis was used to determine the effect of
gefitinib on the mRNA expression of VEGF and AKT mRNA in A375
cells. The results showed that gefitinib treatment for 48 h
markedly decreased the mRNA expression of VEGF in the A375 cells
(P<0.05). Following an increase in dose, gefitinib exerted a
higher suppressive effect on VEGF (P<0.05; Fig. 5). In addition, gefitinib treatment
for 48 h significantly reduced the mRNA expression of AKT in the
A375 cells (P<0.05). An increase in dose also resulted in an
increased suppressive effect on AKT (P<0.05; Fig. 6).
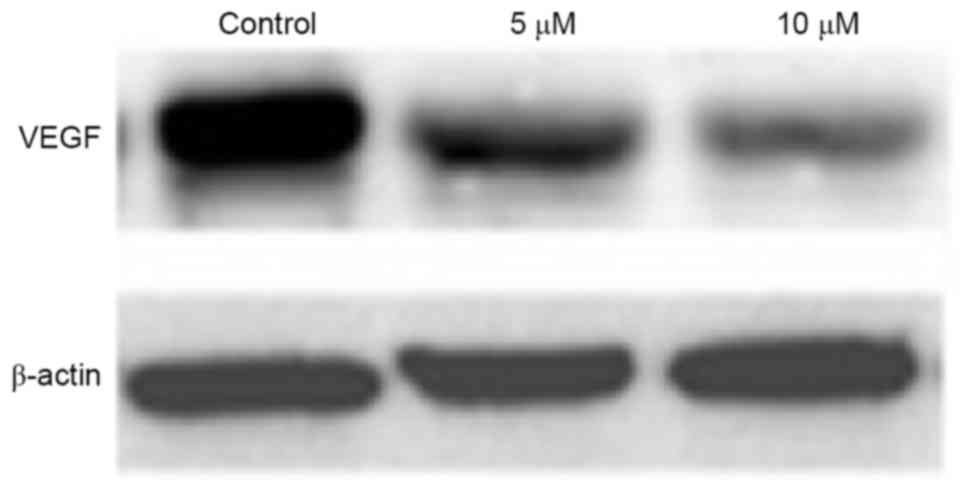
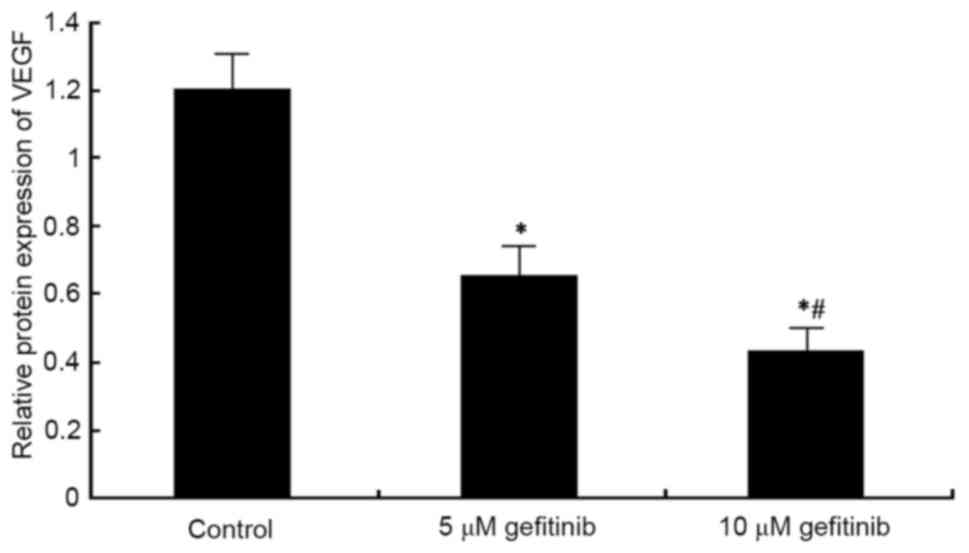
Effect of gefitinib on the protein
expression of VEGF in MM cells
Western blot analysis was performed to detect the
effect of gefitinib on the protein expression of VEGF in A375
cells. It was found that, similar to the mRNA expression of VEGF,
gefitinib treatment for 48 h weakened the protein expression of
VEGF in A375 cells (P<0.05). Following dose elevation, gefitinib
exerted a higher suppressive effect on VEGF (P<0.05; Figs. 7 and 8).
Effect of gefitinib on the protein
expression of AKT in MM cells
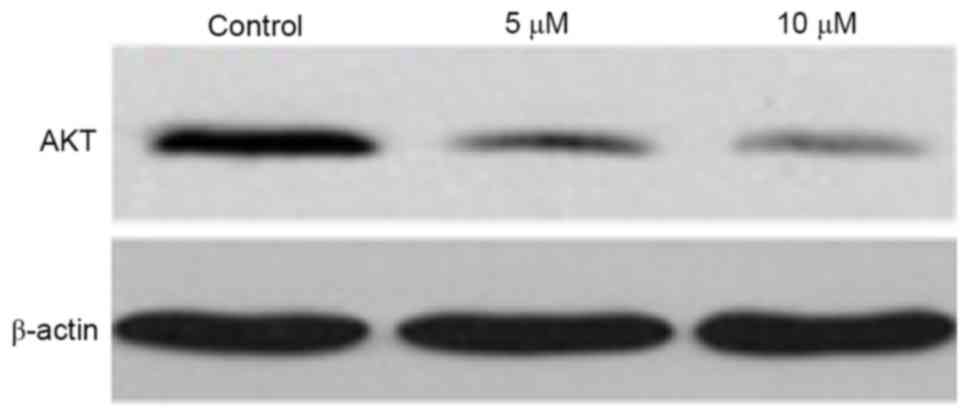
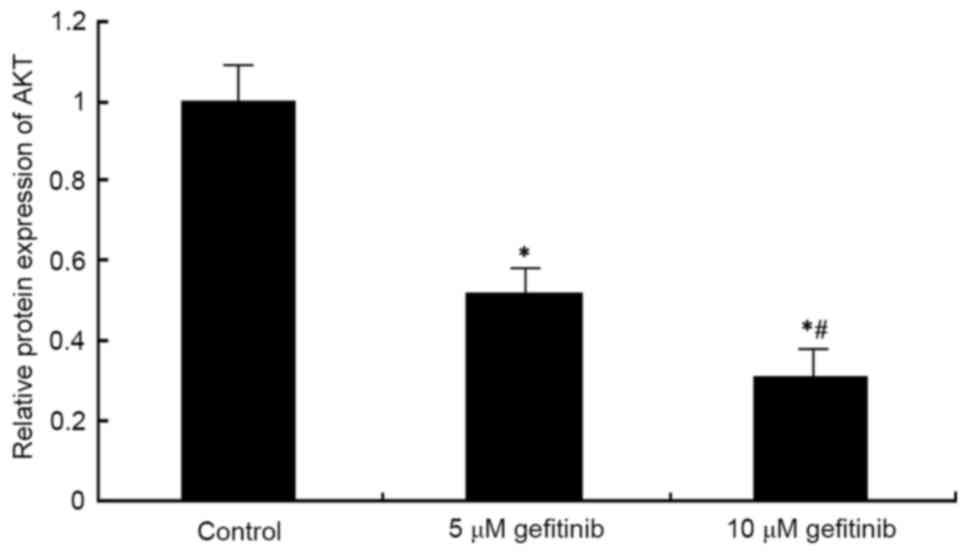
Western blot analysis was used to determine the
effect of gefitinib on the protein level of AKT in A375 cells. It
was found that, similar to the mRNA expression of AKT, gefitinib
treatment for 48 h decreased the protein expression of AKT in the
A375 cells (P<0.05). Following dose elevation, gefitinib exerted
a higher suppressive effect on AKT (P<0.05; Figs. 9 and 10).
Discussion
The incidence of MM has gradually increased over
time. Due to its lack of apparent symptoms in the early stage and
its ability to metastasize, the majority of patients present with
metastasis at diagnosis, leading to poor surgical outcome. In
addition, chemotherapy drug resistance leads to MM treatment
inefficiency (17). The present
study showed that molecular target drugs have certain curative
effects on MM. Therefore, identifying suitable molecular target
drugs to inhibit MM-associated pathways is likely to improve the
survival rates and prognosis of patients with MM (18).
As one of the most important proangiogenic factors,
VEGF is expressed in endothelial cells. It can promote vascular
endothelial cell proliferation, differentiation, migration and
movement, and form vessel structures by enhancing blood vessel
permeability and degrading extracellular matrix (19). VEGF can promote neovascularization
in tumorigenesis (20). The
binding of VEGF to VEGF receptor, synergized with angiogenin-2, can
facilitate lymphatic vessel hyperplasia surrounding the tumor to
ensure that new capillaries can provide nutrition for the tumor and
promote tumor metastasis (21). It
has been shown that the protein kinase AKT is an important molecule
involved in various biological behaviors of cells; for example, the
overexpression of AKT promotes MM metastasis (22). In the present study, MM cells were
treated with gefitinib targeting VEGF, and its effect and mechanism
were analyzed. The results showed that gefitinib suppressed MM cell
proliferation and inhibited cell invasive ability in a
dose-dependent manner. Gefitinib promoted tumor cell apoptosis by
enhancing the activity of caspase-3. Analysis of the mechanism
confirmed that gefitinib suppressed the mRNA and protein expression
of VEGF and AKT, suggesting that gefitinib may reduce the
occurrence and development of MM through the VEGF/AKT pathway.
In conclusion, the present study confirmed that
gefitinib suppressed MM cell proliferation and invasion in
vitro through regulating the VEGF/AKT signaling pathway. These
results indicate a potential molecular target and theoretical basis
for the treatment of MM.
References
|
1
|
Whiteman DC, Green AC and Olsen CM: The
growing burden of invasive melanoma: Projections of incidence rates
and numbers of new cases in six susceptible populations through
2031. J Invest Dermatol. 136:1161–1171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soura E, Eliades PJ, Shannon K, Stratigos
AJ and Tsao H: Hereditary melanoma: Update on syndromes and
management: Emerging melanoma cancer complexes and genetic
counseling. J Am Acad Dermatol. 74:411–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen H, Cai Y, Liu Y, He J, Hu Y, Xiao Q,
Hu W and Ding K: Incidence, surgical treatment, and prognosis of
anorectal melanoma from 1973 to 2011: A population-based SEER
analysis. Medicine (Baltimore). 95:e27702016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter JH, Deddens JA, Spaulding NR IV,
Lucas D, Colligan BM, Lewis TG, Hawkins E, Jones J, Pemberton JO,
Douglass LE and Graff JR: Phosphorylation of eIF4E serine 209 is
associated with tumour progression and reduced survival in
malignant melanoma. Br J Cancer. 114:444–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oba J, Nakahara T, Hashimoto-Hachiya A,
Liu M, Abe T, Hagihara A, Yokomizo T and Furue M: CD10-equipped
melanoma cells acquire highly potent tumorigenic activity: A
plausible explanation of their significance for a poor prognosis.
PLoS One. 11:e01492852016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soura E, Eliades PJ, Shannon K, Stratigos
AJ and Tsao H: Hereditary melanoma: Update on syndromes and
management: Genetics of familial atypical multiple mole melanoma
syndrome. J Am Acad Dermatol. 74:395–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu X, Cao Z and Zhu H: Capsule endoscopy
in the diagnosis of an exophytic gastrointestinal stromal tumor in
the small intestine of a young adult woman: A case report. Mol Clin
Oncol. 4:268–270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khoja L, Atenafu EG, Ye Q, Gedye C,
Chappell M, Hogg D, Butler MO and Joshua AM: Real-world efficacy,
toxicity and clinical management of ipilimumab treatment in
metastatic melanoma. Oncol Lett. 11:1581–1585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferreira AK, Pasqualoto KF, Kruyt FA,
Palace-Berl F, Azevedo RA, Turra KM, Rodrigues CP, Ferreira AC,
Salomόn MA, de Sá PL Junior, et al: BFD-22 a new potential
inhibitor of BRAF inhibits the metastasis of B16F10 melanoma cells
and simultaneously increased the tumor immunogenicity. Toxicol Appl
Pharmacol. 295:56–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You Z, Zhou Y, Guo Y, Chen W, Chen S and
Wang X: Activating transcription factor 2 expression mediates cell
proliferation and is associated with poor prognosis in human
non-small cell lung carcinoma. Oncol Lett. 11:760–766. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Chen YW, Zhang L, Gong XG, Zhou Y
and Shang DJ: Melanoma cell surface-expressed phosphatidylserine as
a therapeutic target for cationic anticancer peptide,
temporin-1CEa. J Drug Target. 24:548–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogawara K, Shiraishi T, Araki T, Watanabe
T, Ono T and Higaki K: Efficient anti-tumor effect of photodynamic
treatment with polymeric nanoparticles composed of polyethylene
glycol and polylactic acid block copolymer encapsulating
hydrophobic porphyrin derivative. Eur J Pharm Sci. 82:154–160.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Sun B, Liu Y, Zhang D, Liu Z, Zhao
X, Gu Q, Han C, Dong X, Che N, et al: Linearly patterned programmed
cell necrosis induced by chronic hypoxia plays a role in melanoma
angiogenesis. J Cancer. 7:22–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai YW, Wang SW, Chang CH, Liu SC, Chen
YJ, Chi CW, Chiu LP, Chen SS, Chiu AW and Chung CH: Butein inhibits
metastatic behavior in mouse melanoma cells through VEGF expression
and translation-dependent signaling pathway regulation. BMC
Complement Altern Med. 15:4452015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amin DN, Bielenberg DR, Lifshits E,
Heymach JV and Klagsbrun M: Targeting EGFR activity in blood
vessels is sufficient to inhibit tumor growth and is accompanied by
an increase in VEGFR-2 dependence in tumor endothelial cells.
Microvasc Res. 76:15–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wheatley K, Wilson JS, Gaunt P and Marsden
JR: Surgical excision margins in primary cutaneous melanoma: A
meta-analysis and Bayesian probability evaluation. Cancer Treat
Rev. 42:73–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gershenwald JE and Guy GP Jr: Stemming the
rising incidence of melanoma: Calling prevention to action. J Natl
Cancer Inst. 108:djv3812015.PubMed/NCBI
|
|
19
|
Zhang ZQ, Han YZ, Nian Q, Chen G, Cui SQ
and Wang XY: Tumor invasiveness, not lymphangiogenesis, is
correlated with lymph node metastasis and unfavorable prognosis in
young breast cancer patients (≤35 years). PLoS One.
10:e01443762015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naruse T, Yanamoto S, Yamada SI, Takahashi
H, Matsushita Y, Imayama N, Ikeda H, Shiraishi T, Fujita S, Ikeda
T, et al: Immunohistochemical study of vascular endothelial growth
factor-C/vascular endothelial growth factor receptor-3 expression
in oral tongue squamous cell carcinoma: Correlation with the
induction of lymphangiogenesis. Oncol Lett. 10:2027–2034. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Li HG, Wen JM, Peng TS, Zeng H and
Wang LY: Expression of CD44v3, erythropoietin and VEGF-C in gastric
adenocarcinomas: Correlations with clinicopathological features.
Tumori. 100:321–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho JH, Robinson JP, Arave RA, Burnett WJ,
Kircher DA, Chen G, Davies MA, Grossmann AH, VanBrocklin MW,
McMahon M and Holmen SL: AKT1 activation promotes development of
melanoma metastases. Cell Rep. 13:898–905. 2015. View Article : Google Scholar : PubMed/NCBI
|