Introduction
Thyroid cancer incidence is rapidly increasing in
the USA, with an estimated annual diagnosis rate of 56,870 people
and an annual mortality rate of 2,010 cases in 2017 (1). Papillary thyroid carcinoma (PTC)
accounts for the majority of thyroid cancers and it generally
exhibits favorable prognosis (2,3).
However, the recurrence rate is approximately 15% in 3 years
(4). Following thyroidectomy in
local invasion and distant metastasis cases, radioiodine ablation
(131I) is the primary treatment for PTC. Nevertheless,
some patients may fail to respond to radioiodine ablation therapy
due to the loss of radioiodine aggregate ability in thyroid
follicular cells (5). Therefore,
determining the molecular mechanisms underlying radioiodine
ablation refractory may aid the identification of novel therapeutic
targets and consequently increase the survival of patients with PTC
in the future.
La ribonucleoprotein domain family member (LARP)7 is
a member of the LARP family, that contains four La
domain-containing RNA-binding proteins (LARP1, 4, 6, and 7), and it
modulates metabolism (6). LARP7
functions as a potential suppressor in breast cancer and gastric
carcinoma (6,7). The role of LARP7 in PTC remains to be
elucidated. Therefore, the function and mechanism of LARP7 with
regards to PTC should be determined.
The sonic hedgehog (SHH) signaling pathway is a
highly conserved system; it is involved in proliferation,
migration, and invasion of PTC. The association between SHH
signaling and radioiodine ablation refractory remains unclear.
Therefore, further investigation of the SHH signaling pathway
mechanism in PTC radioiodine ablation refractory is required.
The present study aimed to evaluate the potential
tumor-suppressive properties of LARP7 in the PTC, LARP7 expression
in non-neoplastic and neoplastic PTC tissues. Additionally, the
effects of LARP7 expression on iodide uptake and the sodium/iodide
symporter (NIS) expression in PTC cell lines was investigated. The
potential mechanisms through which LARP7 increased the iodide
uptake of PTC, including the SHH signaling pathway and NIS, were
also investigated.
Materials and methods
Cell culture and tissue
collection
The human Nthy-ori3-1, and PTC cell lines, TPC-1 and
BCPAP were obtained from the American Type Culture Collection
(Manassas, VA, USA). Cell lines were authenticated by short-tandem
repeat profiling performed by BMR Genomics (Padua, Italy). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone;
GE Healthcare, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Hyclone; GE Healthcare) in a 95% humidified atmosphere
with 5% CO2 at 37°C. The recombinant human Shh
N-terminal peptide (rhSHH; R&D system, Minneapolis, MN, USA)
was added to the culture medium. Human PTC specimens and their
adjacent normal thyroid tissues (30 pairs) were collected from 30
patients (9 males and 21 females; age 27–63 years) who underwent
surgery according to an approved human protocol at the Weifang
People's Hospital (Weifang, China), between January 2016 and
December 2016. All patient materials were obtained with written
informed consent.
Lentivirus-mediated LARP7
overexpression in PTC cells
A LARP7 overexpression plasmid and control vector
were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). During cell transfection, the cells (10,000/well) were seeded
in 6-well plates and were subsequently cultured until 40–60%
confluence. Transfection was performed with a
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. The transfection mixture was replaced with a medium
containing 10% FBS after 6–8 h. The expression level of LARP7 in
the transfected cells was validated via western blot analysis 7
days post-transfection.
Cell viability assay
Cell viability was examined by Cell Counting Kit-8
(CCK8) assay (Beyotime Institute of Biotechnology, Haimen, China).
Cells overexpressing LARP7 and control were cultured overnight.
Subsequently, cells were trypsinized and seeded at a density of
3,000 cells/well in a 96-well plate with or without 131I
(0.5 ml serum-free DMEM containing 0.1 µCi Na131I) for
12 h at 37°C. Following culture for the indicated time (0, 24, 48
and 72 h), 10 µl CCK8 was added into each well. The resulting
mixtures were incubated at 37°C for 3 h. Subsequently the
absorbance of each well was examined using a Multi-skan MK3
spectrophotometer at a wavelength of 450 nm.
Colony formation assay
Cells transfected with LARP7 and control (200
cells/well) were plated in 6-well plates with or without
131I. Following 1 week of culture, colonies were fixed
with methanol and stained with 0.1% crystal violet (Beyotime
Institute of Biotechnology) for 20 min at 37°C, and the images of
the stained colonies were captured using a CKX41 light microscope.
The number of the colonies that had migrated through the pores was
quantified by randomly counting 10 independent visual fields using
the images.
Cell apoptosis analysis
Cell apoptosis was assessed using flow cytometry
with staining of the cells using an Annexin V/propidium iodide (PI)
kit (Nanjing Keygen Biotech, Co, Ltd., Nanjing, Jiangsu, China).
Briefly, cells were collected and washed twice in ice cold PBS. The
washed cells (2×105) were resuspended in 100 µl binding
buffer (included in the kit), and stained with 5 µl Annexin V and 5
µl PI. Following incubation for 15 min in the dark at room
temperature, flow cytometry was performed. A flow cytometer
(Cytomics FC 500 MPL; Beckman Coulter, Inc., Brea, CA, USA) was
utilized to evaluate the apoptotic levels in each sample according
to the manufacturer's protocol. Data were analyzed using ModFit LT
3.0 (Verity Software House, Inc., Topsham, ME, USA).
Western blot analysis
The expression levels of LARP7, sonic hedgehog
(SHH), protein patched homolog 1 (PTCH1), glioma-associated protein
1 (GLI1) and sodium-iodide symporter (NIS) proteins were analyzed
via western blot assay using the following primary antibodies:
Mouse polyclonal LARP7 (1:200; cat. no. sc515209), SHH (1:200; cat.
no. sc1194), PTCH1 (1:200; cat. no. sc23929), GLI1 (1:200; cat. no.
sc20687) (all from Santa Cruz Biotechnology, Inc.), mouse
polyclonal NIS (1:200; cat. no. ab101084; Abcam, Cambridge, MA,
USA) overnight at 4°C. Normalization was performed by blotting the
same samples with an antibody against mouse monoclonal β-actin
(1:800; cat. no. AA128; Beyotime Institute of Biotechnology)
overnight at 4°C. Radioimmunoprecipitation assay buffer (Thermo
Fisher Scientific, Inc.) was used to extract total protein from
cell lines. Protein concentration of whole cell lysates was
assessed using the Pierce™ Bicinchonic Acid Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of proteins
(30 µg) from the lysates of the cells were subjected to
electrophoresis via 10% SDS-PAGE (Beyotime Institute of
Biotechnology) at 80 V for 30 min then 100 V for 1.5 h, and were
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Following blocking in 5% skimmed
milk for 60 min at room temperature, the membranes were then
incubated with the aforementioned diluted primary antibodies.
Following incubation with peroxidase-coupled secondary antibody
(1:2,000; cat. no. A0216; Beyotime Institute of Biotechnology) at
37°C for 2 h, specific bands were visualized with enhanced
chemiluminescence reagent (Nanjing KeyGen Biotech Co., Ltd.) on an
autoradiographic film. Images were analyzed using ImageJ Software
(National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cells was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Complementary DNA was synthesized from
500 ng of total RNA, reaction mixture (20 µl) containing 1 µg of
total RNA was reverse transcribed to cDNA using a PrimeScript RT
Reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocol. RT-qPCR
was performed using a SYBR Premix Ex Taq (Takara Bio, Inc. Ostu,
Japan) according to the manufacturer's protocols. The RT-qPCR
conditions were applied for detecting mRNAs: 95°C for 30 sec,
followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C
for 30 sec. The β-actin was used as internal controls for the
detection. The primer sequences used are presented in Table I. The mRNA relative expression
levels were calculated using 2−ΔΔCq and normalized to
the reference (8).
 | Table I.Sequences of all primers used in the
present study. |
Table I.
Sequences of all primers used in the
present study.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| SHH |
CCCAATTACAACCCCGACATC |
TCACCCGCAGTTTCACTCCT |
| PTCH1 |
TGAGACTGACCACGGCCTG |
ACCCTCAGTTGGAGCTGCTTC |
| GLI1 |
AGGGCTGCAGTAAAGCCTTCA |
CTTGACATGTTTTCGCAGCG |
| NIS |
GCGTGGCTCTCTCAGTCAA |
GCGTCCATTCCTGAGCTG |
| β-actin |
GATCATTGCTCCTCCTGAGC |
ACTCCTGCTTGCTGATCCAC |
Iodine uptake assay
Cells were cultured in 24-well plates
(105 cells/0.5 ml) and treated with or without 0.5 µg/ml
rhSHH (R&D Systems, Inc.) for 24 h, subsequently, radioiodine
uptake assays were performed. Cells were washed twice with 0.5 ml
PBS and subsequently incubated with 0.5 ml of serum-free DMEM/F-12,
which contained µCi carrier-free Na131I (0.1 m) (Atomic
Hi-tech Radiation Co., Ltd., Beijing, China), with or without
perchlorate (100 µM) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Perchlorate was used as a NIS-competitive inhibitor for
radioiodine uptake. Following 1-h incubation at 37°C, the
radioiodine-containing medium was removed and cells were rinsed
twice with 1 ml PBS. Subsequently, cells in each well were lysed
with NaOH and washed twice with PBS. The cell-associated
radioactivity of the collected lysed cells was measured using a
gamma counter.
Animal studies
All experiments involving animals were approved by
the Animal Care and Welfare Committee of Weifang Medical University
(Weifang, China). Lv-control or Lv-LARP7 (1×108/ml)
(Santa Cruz Biotechnology, Inc.) BCPAP cells (1.0×106)
were subcutaneously injected into the right flanks of 4-week-old
BALB⁄c athymic nude mice (n=12, female, weight range; 20–25 g)
(Laboratory Animal Center of Yangzhou University, Yangzhou, China)
and maintained in a specific-pathogen free environment with
constant humidity (45–50%) and constant temperature (25–27°C) under
a 12 h light/dark cycle with free access to food and water. These
nude mice were randomly divided into two groups (6 mice per group)
(9). Tumor volume (mm3)
was calculated every 3 days over 3 weeks using the following
formula: V=0.5 × length × width2. Tumors were collected
and imaged at 3 weeks following inoculation.
Immunohistochemistry
Paraffin-embedded sections of tumor tissue (4 µm
thick) were deparaffinized in xylene, rehydrated via graded alcohol
solutions, blocked in methanol containing 3% hydrogen peroxide for
10 min at room temperature, and then incubated with mouse
anti-human anti-Ki67 antibody (1:200; cat. no. sc-23900; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. Following rinsing with PBS
solution, biotinylated goat anti-rabbit serum IgG (1:2,000; cat.
no. ab64256; Abcam) was used as secondary antibodies and
streptavidin peroxidase complex reagent were applied for 1 h at
room temperature. Finally, the sections were incubated in a 3,
3′-diaminobenzidine solution at room temperature for 10 min and
then counterstained with hematoxylin for 3 min at room temperature.
Ten randomly selected visual fields per section were examined under
a light microscope to evaluate the Ki67 expression.
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). All experiments were
performed in triplicate. Unless otherwise indicated, the data were
evaluated as mean ± standard deviation. Differences between two
groups were assessed using two-tailed Student's t-test. Data of
more than two groups were using one way analysis of vaiance with
post hoc test by Tukey's test. P<0.05 was considered to indicate
a statistically significant difference.
Results
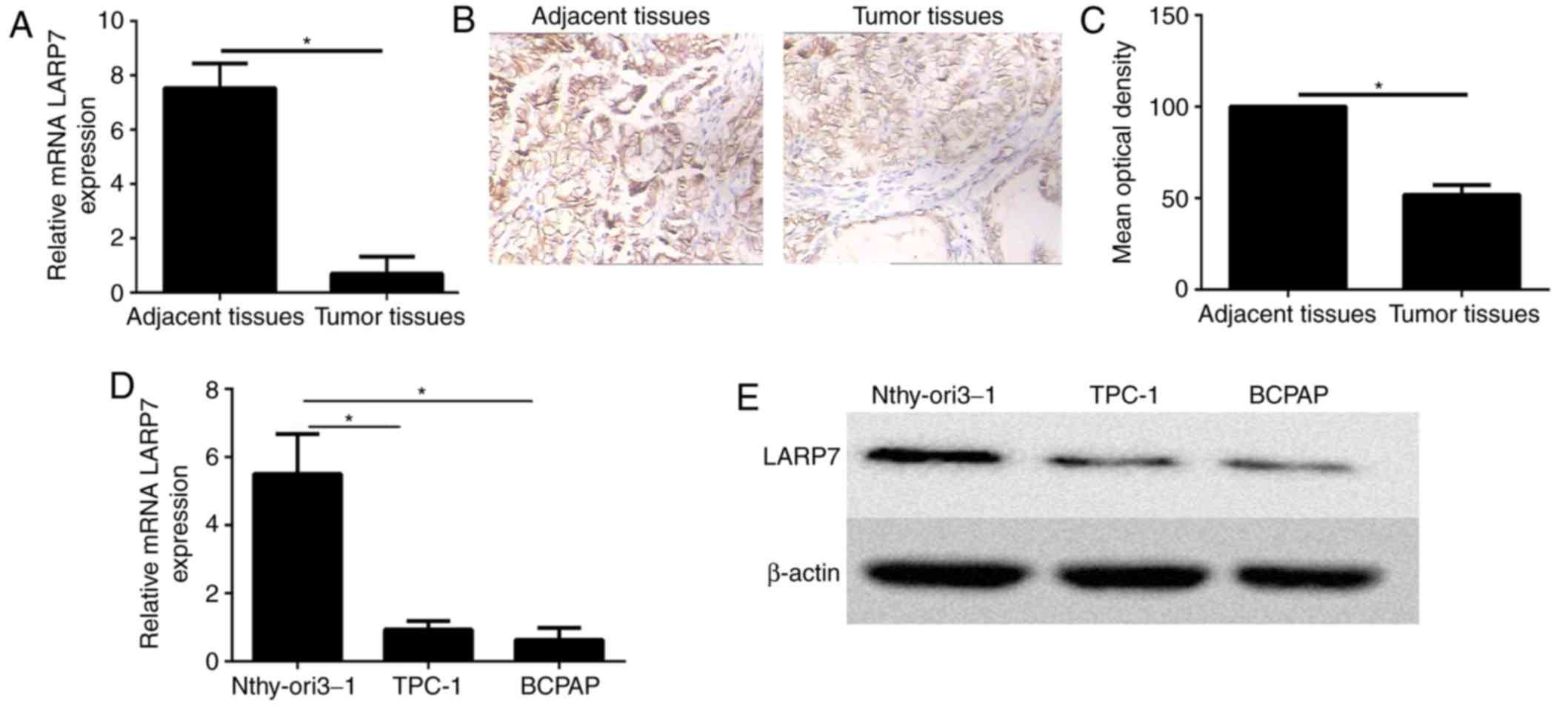
LARP7 expression is significantly
downregulated in papillary thyroid carcinoma tissues and cells
LARP7 expression levels were detected in 30 paired
PTC tissues and their corresponding non-neoplastic thyroid tissues
by RT-qPCR. The expression level of LARP7 was significantly reduced
in PTC tissues when compared with the non-neoplastic thyroid
tissues (Fig. 1A).
Immunohistochemistry demonstrated that LARP7 was overexpressed in
adjacent normal tissues compared with the PTC samples
(representative images from 30 pairs; Fig. 1B and C). Furthermore, LARP7 mRNA
and protein expression levels were significantly higher in the
human thyroid cell line Nthy-ori3-1 cell line when compared with
the PTC cell lines using RT-qPCR and western blot analysis
(Fig. 1D and E, respectively).
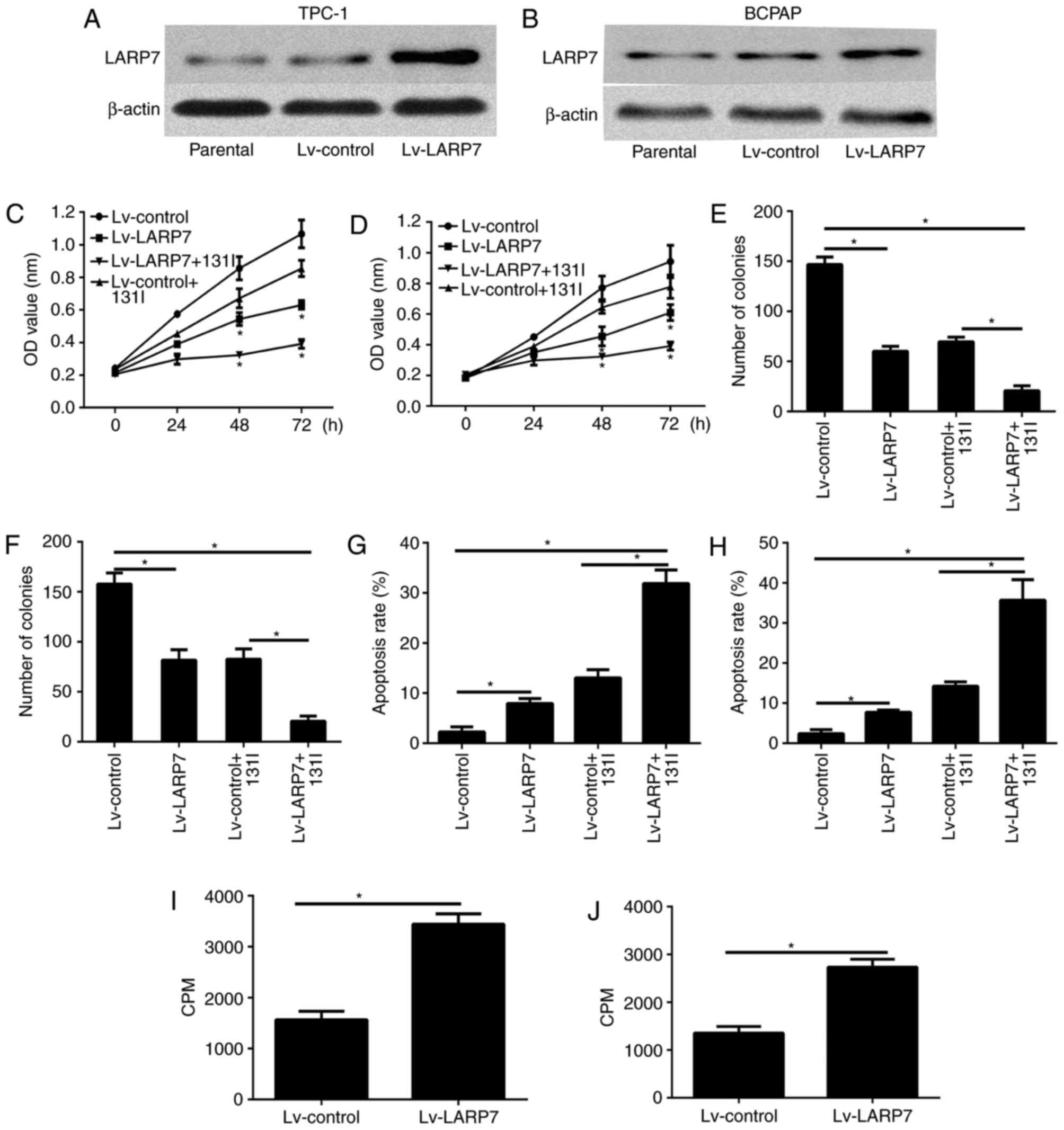
Overexpression of LARP7 reduces cell
proliferation, induces cell apoptosis and enhances radioiodine
uptake in vitro
In order to investigate the effect of LARP7 in PTC
cells, LARP7 was overexpressed in the TPC-1 and BCPAP cells. The
expression level of LARP7 was confirmed by western blotting
(Fig. 2A and B). The cell
viability of the TPC-1 and BCPAP cells was examined using the CCK8
assay 24, 48 and 72 h after incubation. LARP7 overexpression
significantly abrogated the viability of both cell lines (Fig. 2C and D). It is of note that LARP7
overexpression significantly increased the inhibitory effect on
cell viability, which was reduced following 131I
treatment (Fig. 2C and D). The
colony formation assay revealed that LARP7 overexpression inhibited
cell proliferation (Fig. 2E and
F), and the combination of LARP7 overexpression with
131I treatment led to a reduced number of colonies
compared with LARP7 overexpression or 131I alone
(Fig. 2E and F). In addition, flow
cytometry analysis was used to determine the effect of LARP7
overexpression on cell apoptosis. The findings demonstrated that
LARP7 overexpression promoted the apoptosis of PTC; however, the
combination of LARP7 overexpression with 131I treatment
led to a greater apoptotic rate of PTC compared with LARP7
overexpression or 131I alone (Fig. 2G and H). The cell iodine uptake
ability was investigated further and as demonstrated in Fig. 2I and J, as radioiodine uptake was
markedly increased in TPC-1 and BCPAP cell lines.
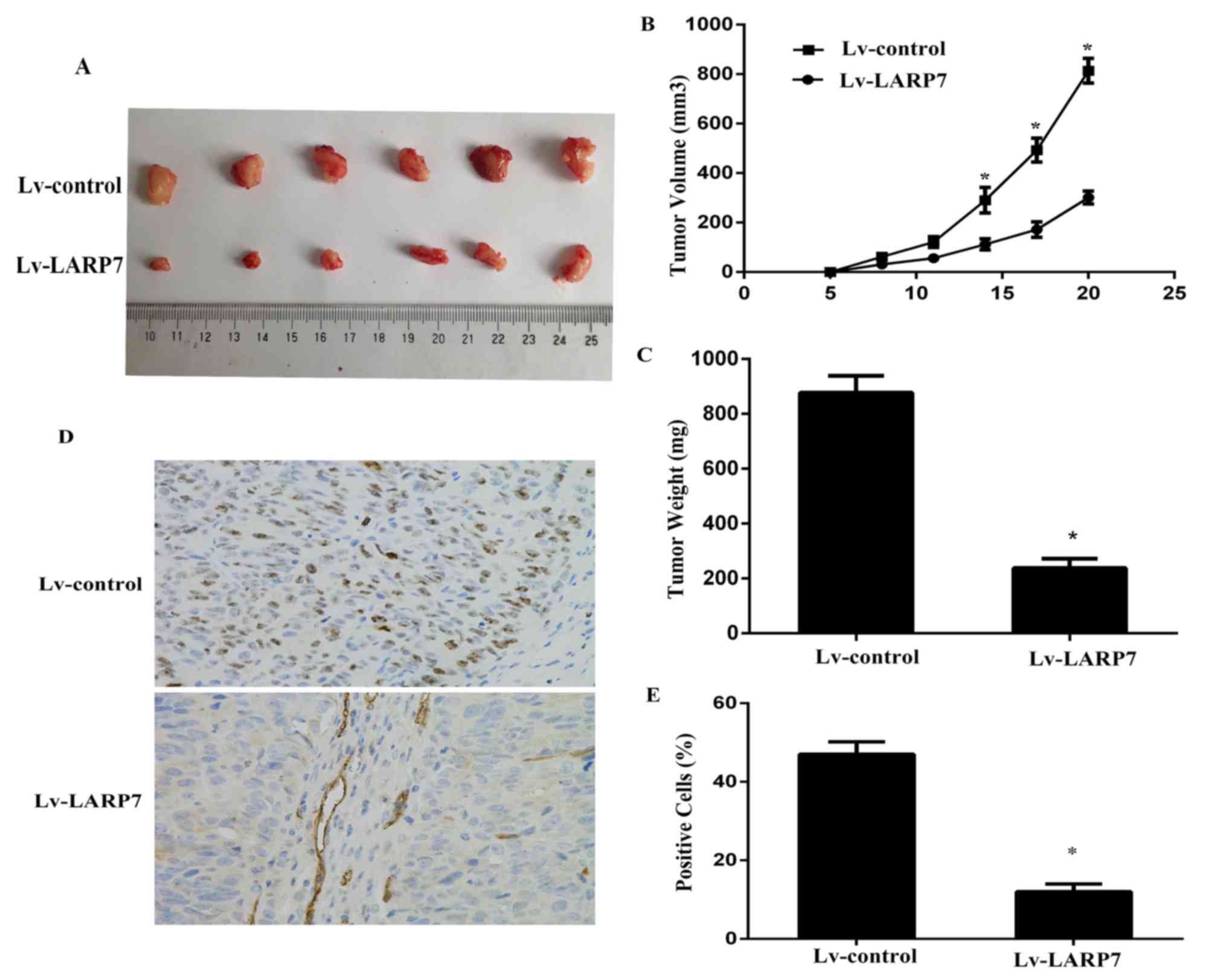
Overexpression of LARP7 inhibits tumor
growth in vivo
The present study investigated the association of
LARP7 and tumor progression in vivo using subcutaneously
transplanted Lv-control or Lv-LARP7 BCPAP cells into BALB⁄c athymic
nude mice (n=6/group). Tumors were removed and imaged 3 weeks
following cell implantation (Fig.
3A). Results revealed that LARP7 overexpression significantly
inhibited tumorigenicity (Fig. 3B and
C). Histological analysis of the proliferation index revealed
that Lv-LARP7 tumors had a significantly lower number of Ki67
positive cells compared with the Lv-control tumors (Fig. 3D and E). These findings revealed
that LARP7 significantly inhibited the tumor growth in
vivo.
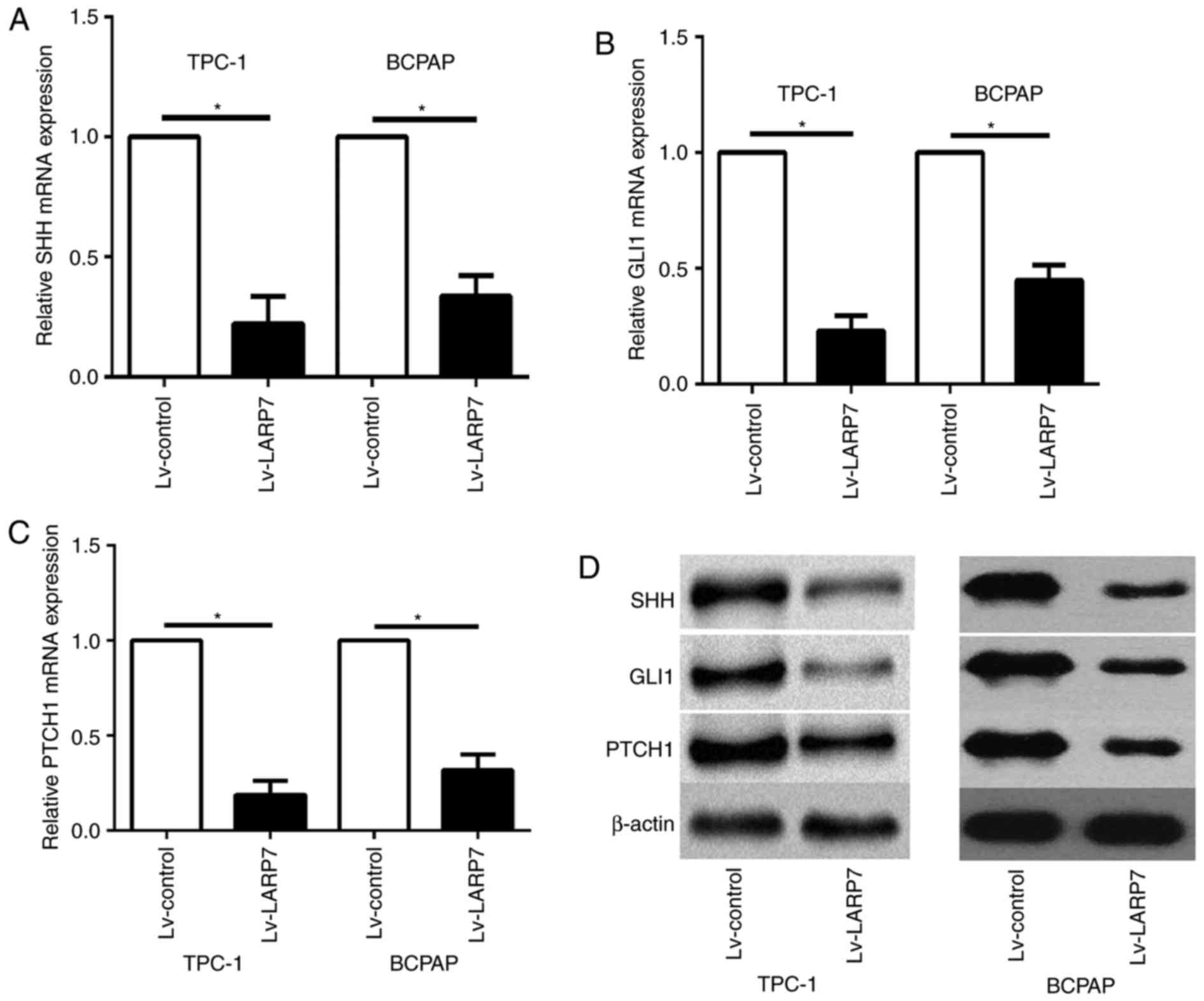
LARP7 regulates SHH signaling pathway
in PTC cells
A recent study revealed that the loss of LARP7
expression contributed to cancer progression and metastasis
(5). In cancer cells, one of the
primary signaling pathways associated with cancer progression and
metastasis is the SHH signaling pathway (10). The present study investigated
whether LARP7 is involved in regulating the SHH signaling pathway
in PTC cells. Expression levels of the principal components of the
SHH signaling pathway in LARP7-overexpressing TPC-1 and BCPAP cells
were analyzed. Overexpression of LARP7 inhibited the expression
levels of SHH pathway-associated genes, including SHH, PTCH1 and
GLI1 in TPC-1 and BCPAP cell lines at the mRNA and protein levels
(Fig. 4).
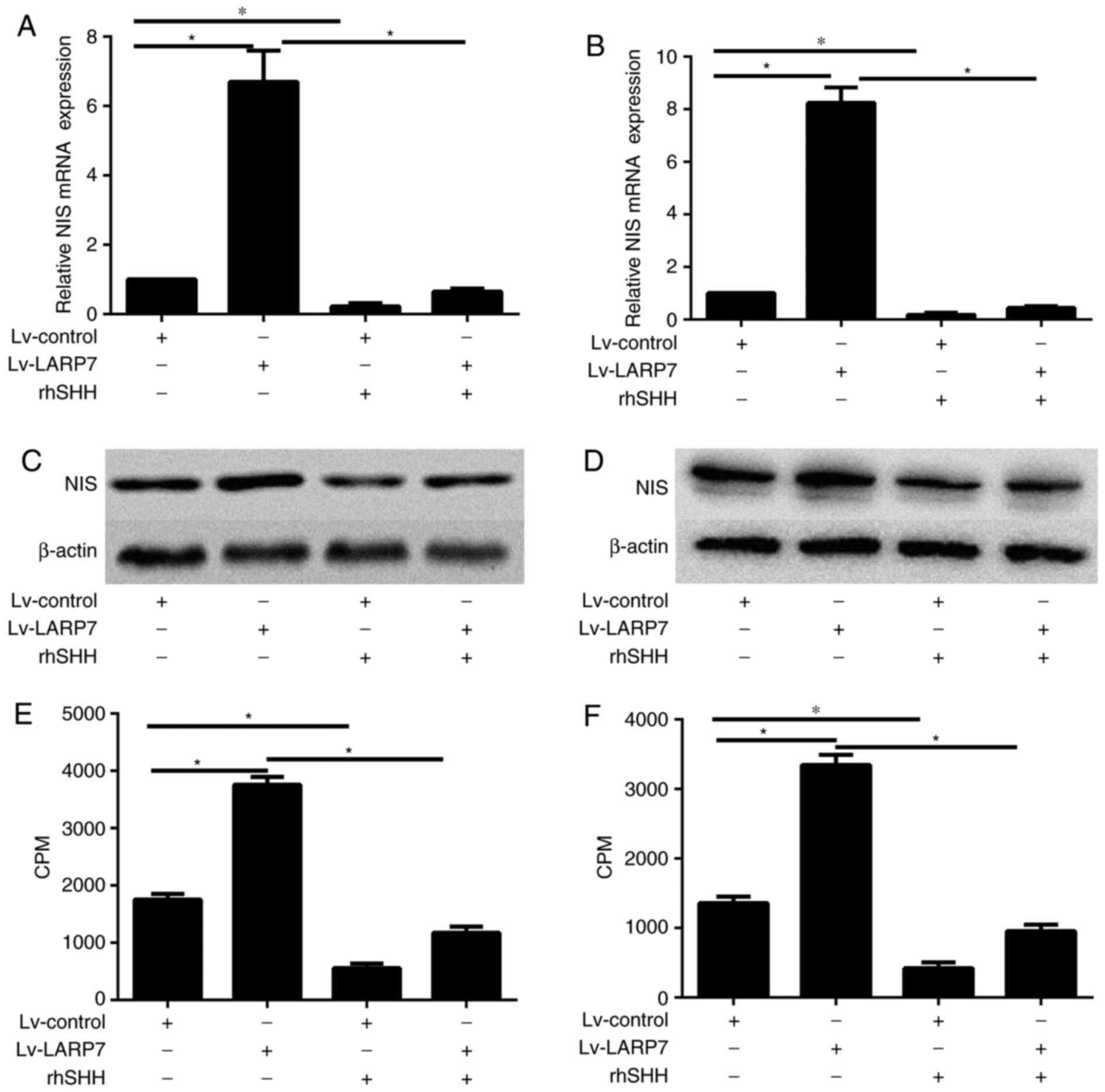
LARP7 increases cell iodine uptake
ability through SHH signaling pathway-mediated expression of NIS in
PTC cells
The present study investigated the mechanism through
which LARP7 increases the iodine uptake ability of PTC cells, the
expression level of NIS in LARP7 overexpressing cells and control
TPC-1 and BCPAP cells were also assessed. The mRNA and protein
expression levels in LARP7-overexpressing PTC cells were
upregulated compared with control cells, as determined by RT-qPCR
and western blot analysis (P<0.05; Fig. 5A-D). In order to determine whether
LARP7 increased cell iodine uptake ability via the SHH signaling
pathway, TPC-1 and BCPAP cells were treated with rhSHH. rhSHH
treatment reduced NIS mRNA and protein expression levels in both
cell lines (P<0.05; Fig. 5A-D).
These findings raveled that the SHH signaling pathway may regulate
NIS expression. As presented in Fig.
5E and F, radioiodine uptake was significantly reduced in TPC-1
and BCPAP cells treated with rhSHH. Subsequently, TPC-1 and BCPAP
cells overexpressing LARP7 and control cells were treated with
rhSHH and RT-qPCR and western blot analysis demonstrated that LAPR
overexpression combined with led to rhSHH treatment led to reduced
NIS mRNA and protein expression levels (P<0.05; Fig. 5A-D). Radioiodine uptake assay
indicated that treatment with rhSHH significantly reduced the
radioiodine uptake ability of TPC-1 and BCPAP cell lines, which was
previously increased by LARP7 overexpression alone (P<0.05;
Fig. 5E and F).
Discussion
PTC, with a rapidly increasing morbidity in the USA,
is a common endocrine malignancy. In PTC, oncogene aberrant
deregulation contributes to tumor progression (10). However, the role of tumor
suppressors involved in cellular processes associated with
tumorigenesis and therapy in PTC remains to be elucidated.
Furthermore, the majority of patients with PTCs exhibit no
alterations in commonly implicated oncogenes (3). Therefore, genes and mechanisms that
currently remain unidentified might also have critical roles in
papillary thyroid carcinogenesis. In the present study, LARP7 was
downregulated in PTC tissues and cell lines. In addition, in
vitro data demonstrated that LARP7 inhibited the viability and
proliferation and increased the radioiodine uptake ability of PTC
cells. Therefore, LARP7 might have an important role in
tumorigenesis and survival of PTC.
Following thyroidectomy, radioiodine ablation is the
most important auxiliary treatment for PTC. This technology is
commonly used to delete the residual tissues following
thyroidectomy (4). Furthermore,
the metastatic lesions, which cannot be removed by surgery, may be
removed using this method. However, a few cases become refractory
to radioiodine therapy (5). The
loss of radioactive iodine (RAI) uptake inversely correlates with
survival. For patients with RAI refractory disease, few treatment
options are available because these tumors are generally resistant
to external radiation and conventional chemotherapy (11). The side effects of 131I
therapy are more tolerable than those of external radiation,
conventional chemotherapy, or small-molecule inhibitors (12). However, at present, no novel
treatment has been demonstrated to improve overall survival despite
improved progression-free survival in some patients with RAI
refractory disease (12).
Accordingly, strategies to restore and increase thyroidal RAI
accumulation for patients with RAI refractory disease are
clinically important. NIS is an intrinsic plasma membrane protein,
which offers thyroid cells the distinct function to uptake,
concentrate iodine (12). Patients
with low expression of NIS may be pre-disposed to an
iodine-refractory metastatic disease, which may lead to treatment
failure (13,14). NIS expression in thyroid cancer
cells is regulated by the binding of transcription factors, such as
paired box 8 and micro RNAs (miRs), to the NIS upstream enhancer
(13). Small interfering RNAs may
be used to knockdown molecular targets reducing NIS expression or
function (15,16). Anti-miRs may be potential
candidates to increase TSH-stimulated RAI accumulation in thyroid
cancer cells when critical miRs regulating NIS expression or
function are identified (14).
LARP7 is a member of the LARP family that contains
four La domain-containing RNA-binding proteins. LARP7 may bind to
and stabilize 7SK small nuclear RNA (17). LARP7 is also involved in
transcription elongation controlled by RNA Polymerase II (17). Consequently, the 7sk-LARP7 complex
suppresses the positive transcription elongation factor b (P-TEFb)
complex, which is the ubiquitous and principal promoter of general
mRNA elongation and processing (18–20).
In breast cancer, LARP7 regulates breast cancer
epithelial-mesenchymal transition and metastasis, thereby
suggesting a novel therapeutic option to treat metastatic breast
cancer by blocking P-TEFb activation (6). However, the mechanism of LARP7
suppression in tumor progression, which is independent of P-TEFb
remains to be elucidated. The role of LARP7 in PTC should also be
determined. The present study demonstrated that LARP7 expression
was significantly downregulated in PTC tissues and cells compared
with the adjacent normal thyroid tissues. Furthermore, it was
revealed that LARP7 overexpression reduced cell viability and
proliferation, increased the apoptotic rate, and induced cell
radioiodine uptake in TPC-1 and BCPAP cells. These findings
suggested a potential role of LARP7 in regulating PTC biological
activity, which to the best of our knowledge was not previously
reported. The present study also established a lentiviral-mediated
LARP7 overexpression model and demonstrated that LARP7
overexpression significantly increased NIS expression, which
coincided with increased radioiodine uptake ability.
Previous studies demonstrated that the SHH signaling
pathway has an important role in the development and progression of
PTC (21–23). The SHH signaling pathway is
initiated by the binding of the secreted SHH ligand to PTCH1, which
relieves the PTCH1-mediated repression of smoothened (SMO). SMO
triggers the downstream signaling cascade inducing nuclear
translocation of GLI1, activating the transcription of SHH target
genes, including PTCH1 (24). The
association of the SHH signaling pathway and radioiodine uptake
ability remains to be elucidated. In the present study, rhSHH
significantly reduced mRNA and protein levels of NIS and SHH
pathway-associated genes, such as PTCH1 and GLI1 in PTC cells.
Radioiodine uptake was also significantly reduced in TPC-1 and
BCPAP cell lines. These findings indicated that the SHH signaling
pathway may regulate radioiodine uptake ability.
Additionally, the present study aimed to investigate
the hypothesis that LARP7 overexpression increases NIS expression
and the radioiodine uptake ability of PTC cells through the SHH
signaling pathway. Firstly, it was demonstrated that the LARP7
overexpression significantly reduced the mRNA and protein levels of
SHH pathway-associated genes in PTC cells. In addition, rhSHH
reduced LARP7-induced overexpression of NIS. Finally, radioiodine
uptake assay demonstrated that treatment with rhSHH significantly
reduced the radioiodine uptake ability of TPC cells which was
previously increased following LARP7 overexpression.
These findings support the hypothesis that LARP7
inhibits SHH signaling pathway and enhances the therapeutic effects
of radioiodine. However, the present study has some limitations.
Due to the low expression level of LARP7 in TPC-1 and BCPAP cells,
expression of LARP7 was not suppressed using small interfering RNA.
Therefore, the association of LARP7 and PTC was not further
demonstrated using knockdown of the LARP7 expression. In the
future, immortal human follicular thyroid cell lines Nthy-ori3-1
may employed, with upregulated expression levels of LARP7 than PTC
cell lines. Furthermore, the present study did not investigate the
association between LARP7 and follicular thyroid cancer and
anaplastic thyroid cancer, which is also resistant to radioiodine
ablation, which requires investigation in future studies (4). Providing that LARP7 overexpression
may enhance the therapeutic effect of radioiodine follicular and
anaplastic thyroid cancers, LARP7 may be considered as a novel
target of alternative therapeutic approaches for thyroid
cancer.
In conclusion, LARP7 may have inhibited the
proliferation and increased the radioiodine uptake of PTC cells by
regulating the SHH signaling pathway. Therefore, LARP7 may be a
novel target for the development of alternative therapeutic
approaches for PTC.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Foundation of
Shandong People and Family Planning Commission (grant no.
2015WSA07008).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW conceived and designed the experiments. YS wrote
and revised the manuscript. XS conducted all experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang People's Hospital (Weifang, China). All
patients provided written informed consent.
Consent for publication
All patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopes JP and Fonseca E: BRAF gene mutation
in the natural history of papillary thyroid carcinoma: Diagnostic
and prognostic implications. Acta Med Port. 24 Suppl 4:S855–S868.
2011.(In Portuguese).
|
|
3
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lupoli R, Cacciapuoti M, Tortora A, Barba
L, Verde N, Romano F, Vastarella M, Fonderico F, Masone S, Milone
M, et al: Clinical outcome in differentiated thyroid carcinoma and
microcarcinoma. Int J Surg. 12 Suppl 1:S148–S151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer, . Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL,
Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al: Revised
American Thyroid Association management guidelines for patients
with thyroid nodules and differentiated thyroid cancer. Thyroid.
19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji X, Lu H, Zhou Q and Luo K: LARP7
suppresses P-TEFb activity to inhibit breast cancer progression and
metastasis. Elife. 3:e029072014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng Y, Jin Z, Agarwal R, Ma K, Yang J,
Ibrahim S, Olaru AV, David S, Ashktorab H, Smoot DT, et al: LARP7
is a potential tumor suppressor gene in gastric cancer. Lab Invest.
92:1013–1019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YH, Sui YN, Yan K, Wang LS, Wang F
and Zhou JH: BRD4 promotes pancreatic ductal adenocarcinoma cell
proliferation and enhances gemcitabine resistance. Oncol Rep.
33:1699–1706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo T, Asa SL and Ezzat S: Epigenetic
dysregulation in thyroid neoplasia. Endocrinol Metab Clin North Am.
37(389–400): ix2008.
|
|
11
|
Schlumberger M and Sherman SI: Approach to
the patient with advanced differentiated thyroid cancer. Eur J
Endocrinol. 166:5–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sherman SI: Targeted therapies for thyroid
tumors. Mod Pathol. 24 Suppl 2:S44–S52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kogai T and Brent GA: The sodium iodide
symporter (NIS): Regulation and approaches to targeting for cancer
therapeutics. Pharmacol Ther. 135:355–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung JK and Cheon GJ: Radioiodine therapy
in differentiated thyroid cancer: The first targeted therapy in
oncology. Endocrinol Metab (Seoul). 29:233–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma S, Wang Q, Ma X, Wu L, Guo F, Ji H, Liu
F, Zhao Y and Qin G: FoxP3 in papillary thyroid carcinoma induces
NIS repression through activation of the TGF-β1/Smad signaling
pathway. Tumour Biol. 37:989–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang C, Zhang ML, Zhao QZ, Xie QP, Yan
HC, Yu X, Wang P and Wang Y: LncRNA-SLC6A9-5:2: A potent sensitizer
in 131I-resistant papillary thyroid carcinoma with PARP-1
induction. Oncotarget. 8:22954–22967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bayfield MA, Yang R and Maraia RJ:
Conserved and divergent features of the structure and function of
La and La-related proteins (LARPs). Biochim Biophys Acta.
1799:365–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barboric M, Lenasi T, Chen H, Johansen EB,
Guo S and Peterlin BM: 7SK snRNP/P-TEFb couples transcription
elongation with alternative splicing and is essential for
vertebrate development. Proc Natl Acad Sci USA. 106:7798–7803.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swarbreck D, Wilks C, Lamesch P, Berardini
TZ, Garcia-Hernandez M, Foerster H, Li D, Meyer T, Muller R, Ploetz
L, et al: The arabidopsis information resource (TAIR): Gene
structure and function annotation. Nucleic Acids Res. 36:(Database
Issue). D1009–D1014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bres V, Yoh SM and Jones KA: The
multi-tasking P-TEFb complex. Curr Opin Cell Biol. 20:334–340.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williamson AJ, Doscas ME, Ye J, Heiden KB,
Xing M, Li Y, Prinz RA and Xu X: The sonic hedgehog signaling
pathway stimulates anaplastic thyroid cancer cell motility and
invasiveness by activating Akt and c-Met. Oncotarget.
7:10472–10485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu X, Ding H, Rao G, Arora S, Saclarides
CP, Esparaz J, Gattuso P, Solorzano CC and Prinz RA: Activation of
the sonic hedgehog pathway in thyroid neoplasms and its potential
role in tumor cell proliferation. Endocr Relat Cancer. 19:167–179.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heiden KB, Williamson AJ, Doscas ME, Ye J,
Wang Y, Liu D, Xing M, Prinz RA and Xu X: The sonic hedgehog
signaling pathway maintains the cancer stem cell self-renewal of
anaplastic thyroid cancer by inducing snail expression. J Clin
Endocrinol Metab. 99:E2178–E2187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the sonic hedgehog signaling pathway: Review
of smoothened and GLI inhibitors. Cancers (Basel). 8:E222016.
View Article : Google Scholar : PubMed/NCBI
|