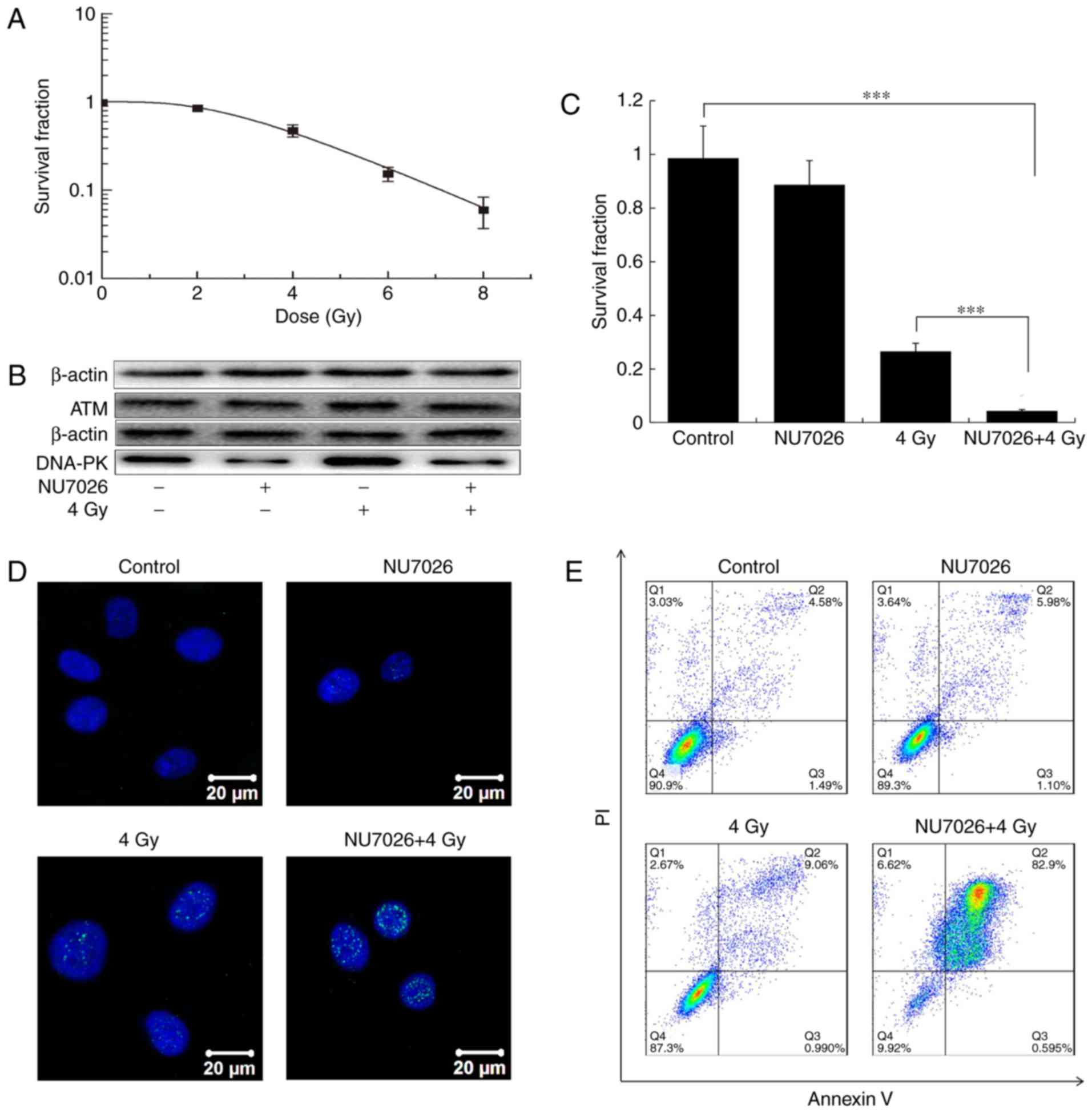
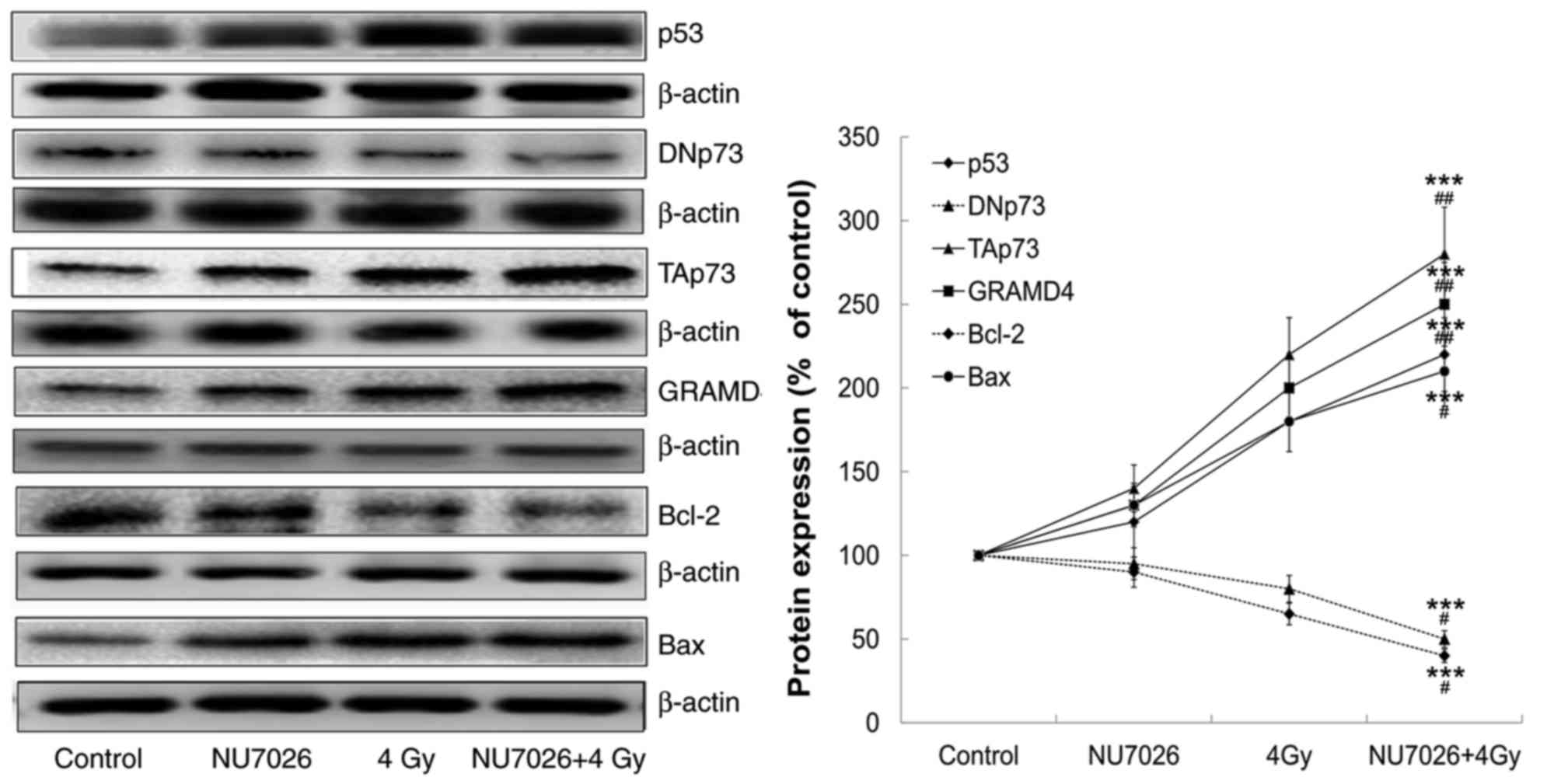
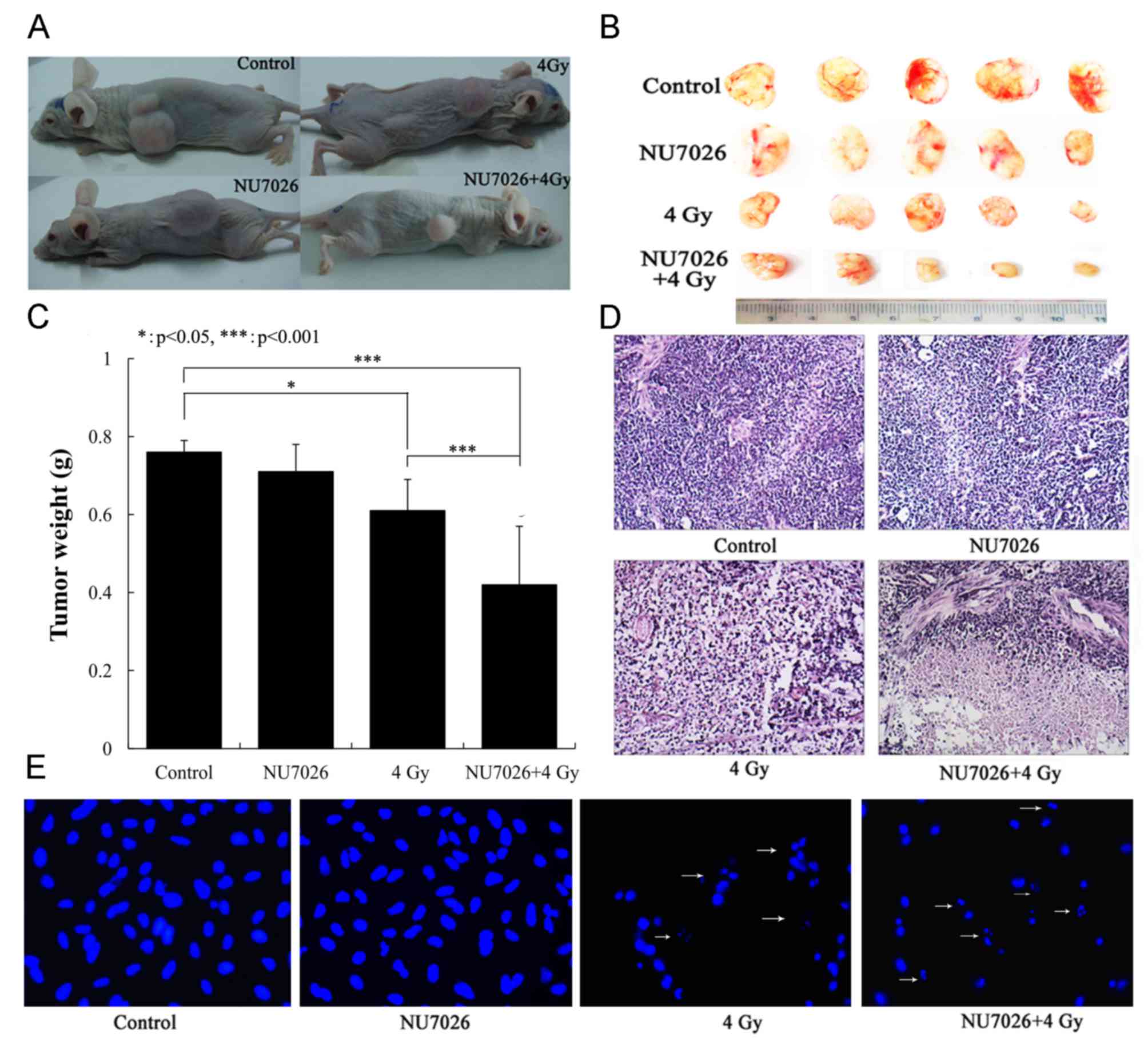
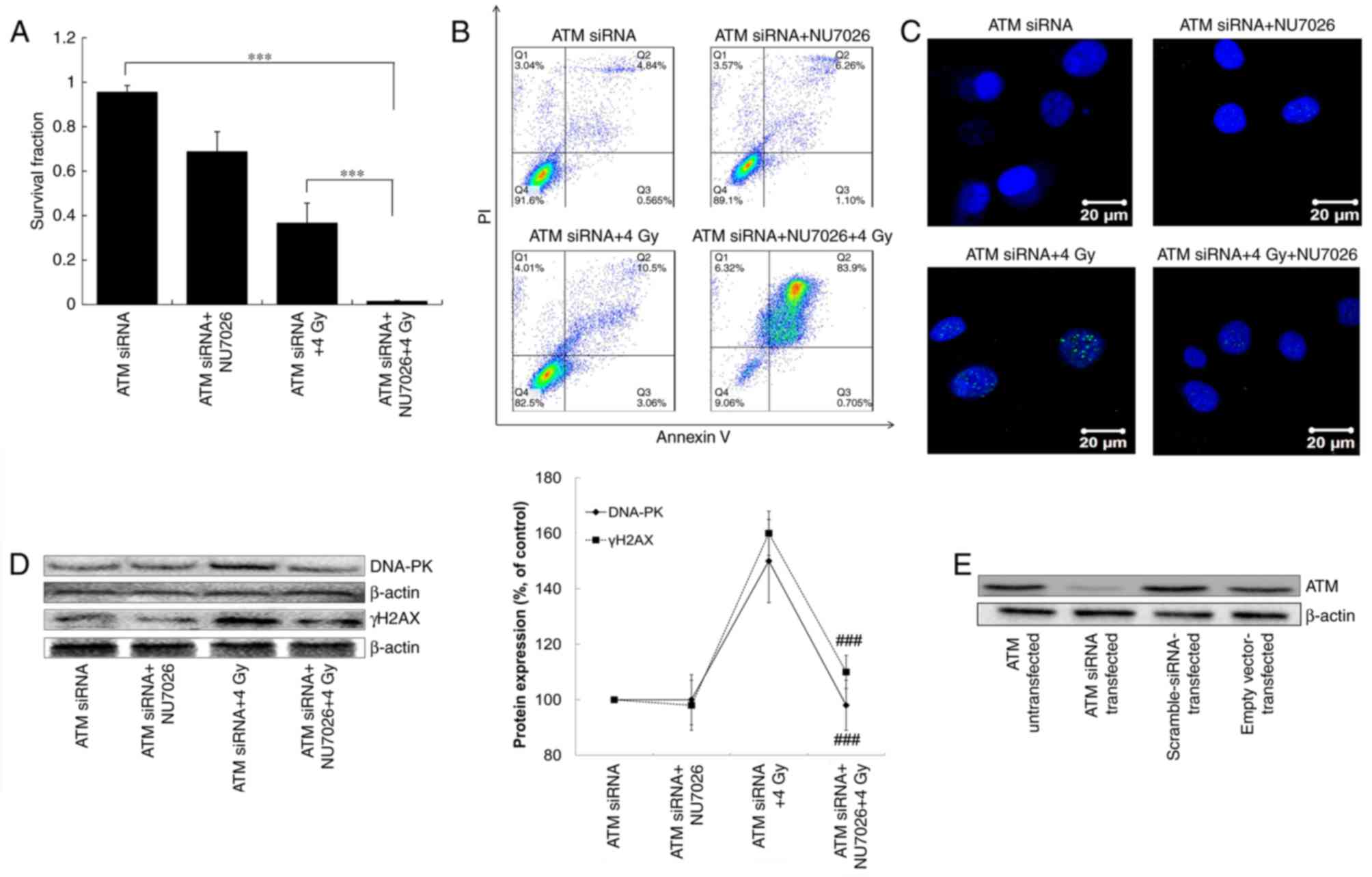
|
1
|
Rodin D, Grover S, Xu MJ, Hanna TP, Olson
R, Schreiner LJ, Munshi A, Mornex F, Palma D and Gaspar LE:
International Association for the Study of Lung Cancer Advanced
Radiation Technology Committee: Radiotherapeutic management of
non-small cell lung cancer in the minimal resource setting. J
Thorac Oncol. 11:21–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang HJ, Kim N, Seong KM, Youn H and Youn
B: Investigation of radiation-induced transcriptome profile of
radioresistant non-small cell lung cancer A549 cells using RNA-seq.
PLoS One. 8:e593192013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhuang W, Li B, Long L, Chen L, Huang Q
and Liang Z: Induction of autophagy promotes differentiation of
glioma-initiating cells and their radiosensitivity. Int J Cancer.
129:2720–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JS, Krasieva TB, Kurumizaka H, Chen
DJ, Taylor AM and Yokomori K: Independent and sequential
recruitment of NHEJ and HR factors to DNA damage sites in mammalian
cells. J Cell Biol. 170:341–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahaney BL, Meek K and Lees-Miller SP:
Repair of ionizing radiation-induced DNA double-strand breaks by
non-homologous end-joining. Biochem J. 417:639–650. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lieber MR: The mechanism of human
nonhomologous DNA end joining. J Biol Chem. 283:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yajima H, Lee KJ, Zhang S, Kobayashi J and
Chen BP: DNA double-strand break formation upon UV-induced
replication stress activates ATM and DNA-PKcs kinases. J Mol Biol.
385:800–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang SH, Kuo TC, Wu H, Guo JC, Hsu C, Hsu
CH, Tien YW, Yeh KH, Cheng AL and Kuo SH: Perspectives on the
combination of radiotherapy and targeted therapy with DNA repair
inhibitors in the treatment of pancreatic cancer. World J
Gastroenterol. 22:7275–7288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Liu Y, Sun C, Yang X, Yang Z, Ran
J, Zhang Q, Zhang H and Wang X and Wang X: Inhibition of DNA-PKcs
enhances radiosensitivity and increases the levels of ATM and ATR
in NSCLC cells exposed to carbon ion irradiation. Oncol Lett.
10:2856–2864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di CX, Yang LN, Zhang H, An LZ, Zhang X,
Ma XF, Sun C, Wang XH, Yang R, Wu ZH and Si J: Effects of
carbon-ion beam or X-ray irradiation on anti-apoptosis ΔNp73
expression in HeLa cells. Gene. 515:208–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conforti F, Yang AL, Agostini M, Rufini A,
Tucci P, Nicklison-Chirou MV, Grespi F, Velletri T, Knight RA,
Melino G and Sayan BS: Relative expression of TAp73 and ΔNp73
isoforms. Aging (Albany NY). 4:202–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
John K, Alla V, Meier C and Putzer BM:
GRAMD4 mimics p53 and mediates the apoptotic function of p73 at
mitochondria. Cell Death Differ. 18:874–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muppani N, Nyman U and Joseph B:
TAp73alpha protects small cell lung carcinoma cells from caspase-2
induced mitochondrial mediated apoptotic cell death. Oncotarget.
2:1145–1154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bailey SG, Cragg MS and Townsend PA:
Family friction as ΔNp73 antagonises p73 and p53. Int J Biochem
Cell Biol. 43:482–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di C, Sun C, Li H, Si J, Zhang H, Han L,
Zhao Q, Liu Y, Liu B, Miao G, et al: Diallyl disulfide enhances
carbon ion beams-induced apoptotic cell death in cervical cancer
cells through regulating Tap73/ΔNp73. Cell Cycle. 14:3725–3733.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hassan HM, Dave BJ and Singh RK: TP73, an
under-appreciated player in non-Hodgkin lymphoma pathogenesis and
management. Curr Mol Med. 14:432–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Sun C, Jin X, Li P, Ye F, Zhao T,
Gong L and Li Q: Genistein enhances the radiosensitivity of breast
cancer cells via G2/M cell cycle arrest and apoptosis.
Molecules. 18:13200–13217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cooper A, Garcia M, Petrovas C, Yamamoto
T, Koup RA and Nabel GJ: HIV-1 causes CD4 cell death through
DNA-dependent protein kinase during viral integration. Nature.
498:376–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Xue H, Cutz JC, Bayani J, Mawji
NR, Chen WG, Goetz LJ, Hayward SW, Sadar MD, Gilks CB, et al: An
orthotopic metastatic prostate cancer model in SCID mice via
grafting of a transplantable human prostate tumor line. Lab Invest.
85:1392–1404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vandersickel V, Depuydt J, Van Bockstaele
B, Perletti G, Philippe J, Thierens H and Vral A: Early increase of
radiation-induced gammaH2AX foci in a human Ku70/80 knockdown cell
line characterized by an enhanced radiosensitivity. J Radiat Res.
51:633–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiloh Y and Ziv Y: The ATM protein
kinase: Regulating the cellular response to genotoxic stress and
more. Nat Rev Mol Cell Biol. 14:197–210. 2013. View Article : Google Scholar
|
|
23
|
Yang J, Yu Y, Hamrick HE and
Duerksen-Hughes PJ: ATM, ATR and DNA-PK: Initiators of the cellular
genotoxic stress responses. Carcinogenesis. 24:1571–1580. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Durocher D and Jackson SP: DNA-PK, ATM and
ATR as sensors of DNA damage: Variations on a theme? Curr Opin Cell
Biol. 13:225–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jackson SP: DNA damage detection by DNA
dependent protein kinase and related enzymes. Cancer Surv.
28:261–279. 1996.PubMed/NCBI
|
|
26
|
Yang C, Wang Q, Liu X, Cheng X, Jiang X,
Zhang Y, Feng Z and Zhou P: NU7441 enhances the radiosensitivity of
liver cancer cells. Cell Physiol Biochem. 38:1897–1905. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tichy A, Durisova K, Salovska B, Pejchal
J, Zarybnicka L, Vavrova J, Dye NA and Sinkorova Z:
Radio-sensitization of human leukaemic MOLT-4 cells by
DNA-dependent protein kinase inhibitor, NU7441. Radiat Environ
Biophys. 53:83–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niazi MT, Mok G, Heravi M, Lee L, Vuong T,
Aloyz R, Panasci L and Muanza T: Effects of dna-dependent protein
kinase inhibition by NU7026 on dna repair and cell survival in
irradiated gastric cancer cell line N87. Curr Oncol. 21:91–96.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Zeng X, Gao C, Ming P, Zhang J,
Guo C, Zhou L, Lu Y, Wang L, Huang L, et al: A new arylbenzofuran
derivative functions as an anti-tumour agent by inducing DNA damage
and inhibiting PARP activity. Sci Rep. 5:108932015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Williamson CT, Muzik H, Turhan AG, Zamò A,
O'Connor MJ, Bebb DG and Lees-Miller SP: ATM deficiency sensitizes
mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1
inhibitors. Mol Cancer Ther. 9:347–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weston VJ, Oldreive CE, Skowronska A,
Oscier DG, Pratt G, Dyer MJ, Smith G, Powell JE, Rudzki Z, Kearns
P, et al: The PARP inhibitor olaparib induces significant killing
of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood.
116:4578–4587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arlander SJ, Greene BT, Innes CL and
Paules RS: DNA protein kinase-dependent G2 checkpoint revealed
following knockdown of ataxia-telangiectasia mutated in human
mammary epithelial cells. Cancer Res. 68:89–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen Y, Rehman FL, Feng Y, Boshuizen J,
Bajrami I, Elliott R, Wang B, Lord CJ, Post LE and Ashworth A: BMN
673, a novel and highly potent PARP1/2 inhibitor for the treatment
of human cancers with DNA repair deficiency. Clin Cancer Res.
19:5003–5015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sarkaria JN, Busby EC, Tibbetts RS, Roos
P, Taya Y, Karnitz LM and Abraham RT: Inhibition of ATM and ATR
kinase activities by the radiosensitizing agent, caffeine. Cancer
Res. 59:4375–4382. 1999.PubMed/NCBI
|
|
35
|
Rosenzweig KE, Youmell MB, Palayoor ST and
Price BD: Radiosensitization of human tumor cells by the
phosphatidylinositol3-kinase inhibitors wortmannin and LY294002
correlates with inhibition of DNA-dependent protein kinase and
prolonged G2-M delay. Clin Cancer Res. 3:1149–1156. 1997.PubMed/NCBI
|
|
36
|
Critchlow SE, Bowater RP and Jackson SP:
Mammalian DNA double-strand break repair protein XRCC4 interacts
with DNA ligase IV. Curr Biol. 7:588–598. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Davidson D, Amrein L, Panasci L and Aloyz
R: Small molecules, inhibitors of DNA-PK, targeting DNA repair and
beyond. Front Pharmacol. 4:52013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blasina A, Hallin J, Chen E, Arango ME,
Kraynov E, Register J, Grant S, Ninkovic S, Chen P, Nichols T, et
al: Breaching the DNA damage checkpoint via PF-00477736, a novel
small-molecule inhibitor of checkpoint kinase 1. Mol Cancer Ther.
7:2394–2404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sausville EA, Arbuck SG, Messmann R,
Headlee D, Bauer KS, Lush RM, Murgo A, Figg WD, Lahusen T, Jaken S,
et al: Phase I trial of 72-hour continuous infusion UCN-01 in
patients with refractory neoplasms. J Clin Oncol. 19:2319–2333.
2001. View Article : Google Scholar : PubMed/NCBI
|