Introduction
Lung cancer is a leading cause of cancer mortality,
accounting for over 1 million deaths per year worldwide (1,2).
Lung cancer has been classified into small-cell lung cancer and
non-small cell lung cancer (NSCLC) according to their histological
types (1). NSCLC accounts for at
least 85% of all lung cancers, with increasing incidence and
mortality in developing countries (2). Radiotherapy is a common method used
in NSCLC clinical treatment, including X-rays and γ-rays, which
provide low-linear energy transfer. However, NSCLC cells
demonstrate poor response to radiotherapy due to radioresistance
(3). The radiosensitivity of NSCLC
cells is therefore one of the most important factors for improving
the curative effect of radiotherapy.
Powerful DNA damage repair systems in cancer cells
contribute to radioresistance, including the non-homologous end
joining (NHEJ) and the homologous recombination (HR) pathways
(4). The HR repair pathway only
occurs in the S and G2 phases of the cell cycle, while
the NHEJ pathway can occur in all the cell cycle phases (5). Notably, DNA repair kinetics of the
NHEJ pathway are much faster than those of the HR repair pathway
(6,7). Therefore, NHEJ is the dominant DNA
damage repair pathway in mammalian cells. Previous studies have
identified members of the phosphoinositide-3 kinase family that
participate in the NHEJ and HR pathways, including DNA-dependent
protein kinase (DNA-PK) and ataxia telangiectasia mutated (ATM),
respectively (4,8). It has been hypothesized that
inhibition of DNA-PK activity can block the NHEJ process to
increase radiosensitivity (9,10).
Cell apoptosis is another significant factor in the
process of blocking DNA damage repair pathways and is regulated by
a complex balance in signaling pathways controlling pro- and
anti-apoptotic factors (11). p73
serves a key role in apoptosis induction, encoding two types of
protein isoform: Full-length transactivating (TA) p73 and an
N-terminally truncated (DN) p73 (12,13).
TAp73 can activate the transcription of cell cycle and apoptosis
regulators, thus acting as a pro-apoptotic factor (14), while DNp73 is able to bind to DNA
and form dimers with TAp73 as a dominant negative anti-apoptotic
factor (12,15). Overexpression of DNp73 and the low
expression of TAp73 have frequently been detected in radioresistant
cancer cells (e.g., cervical cancer, breast cancer and non-Hodgkin
lymphoma), leading to activated mitochondrial effector protein
glucosyltransferases and Rab-like GTPase activators and
myotubularins domain-containing 4 (GRAMD4) to reduce the Bcl-2/Bax
ratio in mitochondria (16–18).
This suggested that increasing TAp73 and/or decreasing DNp73 may
enhance the radiosensitivity of NSCLC cells.
NU7026
(2-(4-Morpholinyl)-4H-naphtho[1,2-b] pyran-4-one) is
a novel DNA-PK inhibitor, which has been studied for the treatment
of human immunodeficiency virus and leukemia (19). In the present study, NU7026 was
used to reduce the DNA damage repair capacity and its effect on the
radiosensitivity of A549 lung cancer cells and xenograft tumors was
investigated. The present results may be useful in assessing the
clinical potential of NU7026 and may also identify the molecular
mechanisms involved in the regulation of the DNA damage response
and cell apoptosis. The present study may therefore serve as an
important supplement to our knowledge regarding the underlying
mechanisms of radiosensitivity.
Materials and methods
Cell culture and RNA interference
A549 lung cancer cells were purchased from ATCC and
cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Hyclone; GE Healthcare, Chicago, IL,
USA). The cells were incubated in 95% humidified atmosphere at 37°C
in the presence of 5% CO2 to maintain exponential cell
growth. A549 cells were plated in 60 mm dishes at a concentration
of 2.0×105 cells. On the second day, 100 nM
SignalSilence®ATM siRNA I (Cell Signaling Technology,
Inc., Danvers, MA, USA) targeting the ATM
(5′-CUAACAAACAGGUGAUAUAUU-3′) was mixed with
Lipofectamine® 2000 in serum-free DMEM medium
transfected A549 cells. The transfection controls included 100 nM
scramble siRNA (5′-UGUUACAUAAACAUGCAAUAG-3′; Takara Biotechnology
Co., Ltd., Dalian, China) to exclude the effect of non-specific
factors and treatment with Lipofectamine® 2000 alone to
exclude the effect of the transfection reagent and untransfected
controls (Cell Signaling Technology, Inc.).
DNA-PK inhibitor and irradiation
treatment
DNA-PK inhibitor NU7026 (Abcam, Cambridge, UK) was
dissolved in DMSO. 1–10×106 A549 cells were treated with
10 µM NU7026 for 30 min, prior to being exposed to 4 Gy X-rays for
3.6 min at room temperature. NU7026 was not washed until the sample
was collected. All the treatments with NU7026 were performed in the
same manner. X-rays were obtained from a Faxition 43885D X-ray
machine at 100 kVp energy. The X-ray dose was 1.1 Gy/min.
Non-irradiated A549 cells were handled in parallel with the
irradiated cells.
Colony formation assay
A549 cells (2×103 cells) were seeded in a
25-cm2 culture flask with 0, 2, 4, 6 and 8 Gy X-ray
irradiation. Similarly, A549 cells were treated with 10 µM NU7026
for 30 min followed by 4 Gy X-ray irradiation. The cells were
washed with phosphate-buffered saline (PBS), fixed with 70% ethanol
and stained with Giemsa for 5 min at room temperature 10 days
later. Colonies containing >50 cells were identified as
survivors under a stereomicroscope. Survival fraction (SF2; 2 Gy)
was calculated according to colonies.
Apoptosis analysis by Annexin V/PI
staining
Apoptosis was measured using the Annexin V-FITC
Apoptosis Detection kit (Bestbio, Shanghai, China). Briefly,
approximately 1×106 cells per experimental condition
(Control, NU7026, 4 Gy, NU7026+4 Gy, ATM siRNA, ATM siRNA+NU7026,
ATM siRNA+4 Gy and ATM siRNA+NU7026+4 Gy) were collected after
trypsinisation at 24 h post-irradiation, washed with cold PBS
twice, and resuspended with 400 µl binding buffer. After adding 5
µl of Annexin V-FITC solution and 10 µl PI (Abcam) solution, the
cells were incubated for 15 min at room temperature in the dark.
After the incubation, 10,000 cells were analyzed with the
FACSCalibur flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA)
and FlowJo version 7.6 software (FlowJo LLC, Ashland, OR, USA).
γH2AX foci immunofluorescence
The cells were seeded in a 6-well plate at a density
of 1×105 cells/well. The cells per experimental
condition were treated with 10 µM NU7026 for 30 min, prior to being
subjected to 4 Gy X-ray irradiation. At 30 min post-irradiation,
the A549 cells were fixed with 4% paraformaldehyde for 15 min, and
then treated with 0.1% Triton X-100 for 30 min and 5% BSA for 1 h
at room temperature. Subsequently, the cells were incubated with
primary monoclonal antibody anti-γH2AX (cat. no. 9718; 1:500; Cell
Signaling Technology Inc.) at room temperature for 2 h.
Subsequently, the cells were incubated at room temperature for 1 h
with IgG-fluorescein isothiocyanate (cat. no. A0562; 1:500;
Bestbio) in the presence of 1% BSA. Following the addition of 20 µl
DAPI (1.5 µg/ml) to counterstain the nuclei, γH2AX foci were
detected with a confocal microscope. When the sizes of γH2AX foci
were >0.01 µm2, the number of γH2AX foci was counted
in three random fields.
Western blot analysis
A total of 1–10×106 cells were treated
with NU7026 for 30 min at room temperature prior to 4 Gy X-ray
irradiation. At 24 h post-irradiation, A549 cells were lysed in 0.5
ml RIPA lysis buffer (Bestbio) supplemented with 1 mM PMSF
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h on ice, and
protein concentration was detected by BCA kit (Beyotime Institute
of Biotechnology). The protein was separated on sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with 10%
separating gel and 4% stacking gel (Bioworld Technology, Inc., St.
Louis, MN, USA) at 80 V for 2 h and transferred to polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) for 2 h at room temperature. The membranes were blocked for 1
h with PBS containing 5% BSA, and incubated with the corresponding
primary monoclonal antibody IgG anti-TAp73 (cat. no. 5B429;
1:1,000; Novus Biologicals, Littleton, CO, USA), DNp73 (cat. no.
38C674.2; 1:1,000; Novus Biologicals), GRAMD4 (cat. no. sc-515128;
1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA) and p53 (cat.
no. 9282; 1:1,000), Bcl-2 (cat. no. 2872; 1:1,000), Bax (cat. no.
2772; 1:1,000), γH2AX (cat. no. 9718; 1:1,000), ATM (cat. no. 2873;
1:1,000), DNA-PK (cat. no. 4620; 1:1,000) and β-actin (cat. no.
4970; 1:1,000; all Cell Signaling Technology Inc.) at 4°C
overnight. Subsequently, the membranes were washed with PBS with
Tween for 30 min at room temperature, incubated with an
HRP-conjugated goat anti-rabbit or goat anti-mouse secondary
antibody (1:5,000; Cell Signaling Technology, Inc., Dallas, TX,
USA) for 1 h at room temperature. Following 3 washes with PBS with
Tween-20 for 10 min at room temperature, a chemiluminescence kit
(Santa Cruz Biotechnology, Inc.) was used to detect proteins. The
intensity of protein was measured by AlphaView software (version
3.4.0.0729; ProteinSimple, San Jose, CA, USA).
Nude mouse xenograft model
A total of 20, seven-week-old male nude mice
(Balb/c-nu/nu) were purchased from the Institute of Laboratory
Animal Sciences, Institute of Laboratory Animal Sciences (Beijing,
China). The mice were housed at the animal research facility under
pathogen-free conditions in 40–60% humidified atmosphere at 26–28°C
for 10 h light and 14 h dark cycle. Mice were randomly allocated
into control, NU7026, 4 Gy and NU7026+4 Gy groups with 5 animals
per group and provided with standard laboratory food and tap water
ad libitum. Exponentially growing A549 cells
(2×107 cells in 100 µl) were injected subcutaneously
into the backs of the mice and tumors were visible on the 7th day.
Little adverse reactions were observed during the tumor formation.
Tumor growth was evaluated every two days by measurement of the
tumor major (a) and minor (b) axes, from which the tumor volume (V)
was calculated according to the formula: V=ab2/2. When
tumors became 10 mm in diameter and 250–300 mm3 in
volume, NU7026 (25 mg/kg, 200 µl) was administered via
intraperitoneal injection into tumor-bearing mice in the treatment
group for 30 min, prior to exposure to 4 Gy X-rays. Other mice only
received no-treatment, NU7026 treatment and 4 Gy irradiation,
respectively. Mice were sacrificed by cervical dislocation under 3%
aether after radiation treatment (day 15). The xenograft tumors
were removed through-dissection and weighting. No animals were lost
as a result of treatment or tumor progression. The study protocol
was approved by the ethics committee of the Bohai University.
Hematoxylin and eosin (H&E)
staining
One centimeter diameter tumors were fixed in 4%
paraformaldehyde solution for 12 h at room temperature and embedded
in paraffin. After cutting the paraffin into 5 µm sections, the
slides were dewaxed, rehydrated, and stained with 1% H&E at
room temperature as described previously (20).
Statistical analysis
Data are presented as the mean ± standard deviation
from ≥3 independent experiments. Several independent samples were
evaluated for statistical significance with one-way analysis of
variance followed by Turkey's test, using SPSS 11.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of DNA-PK sensitizes A549
cells to X-ray irradiation
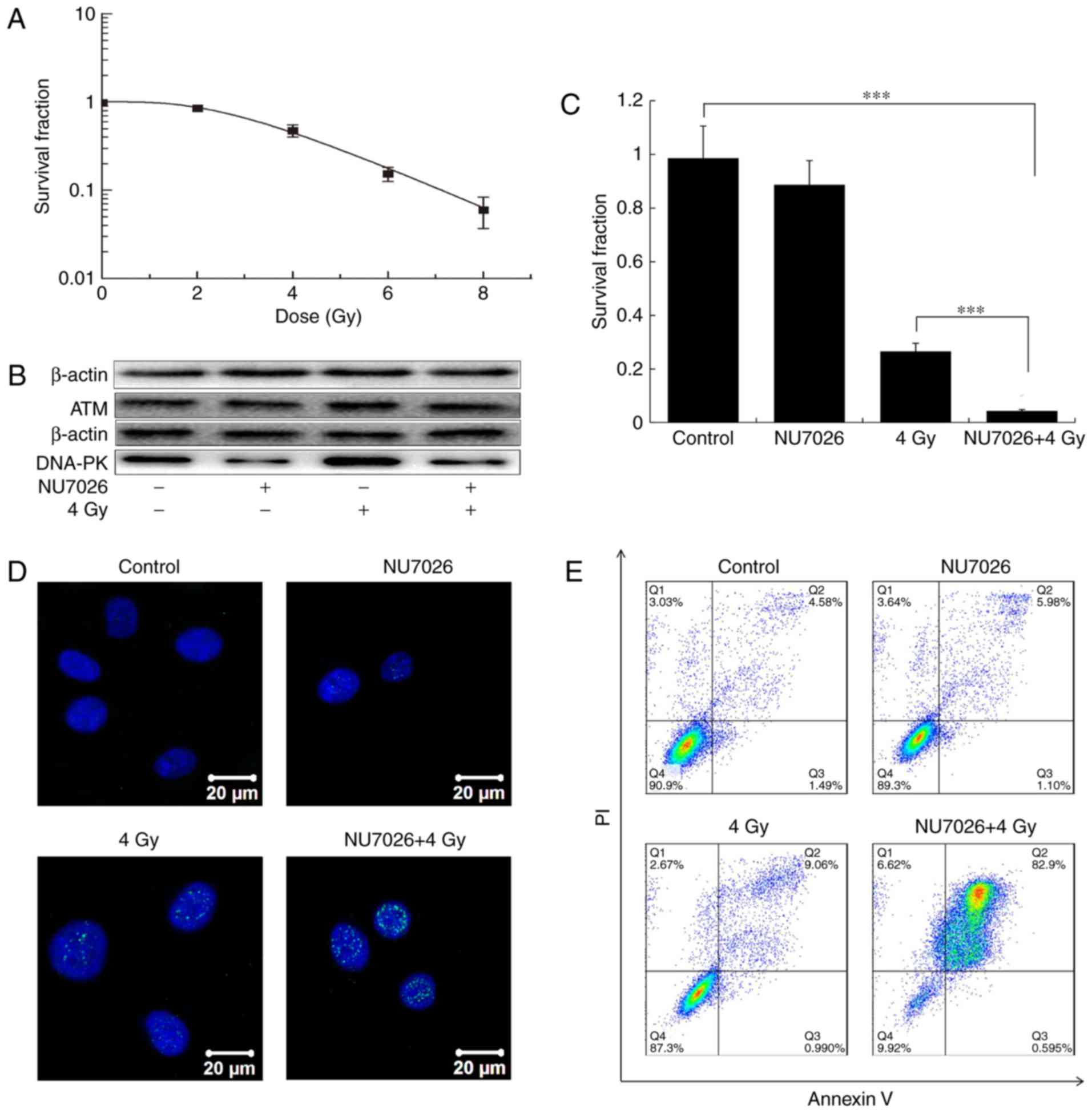
Fig. 1 shows that
the survival fractions of cells treated with 0, 2, 4, 6 and 8 Gy
for 10 days were 1.00, 0.85, 0.47, 0.15 and 0.06, respectively
(Fig. 1A). The expression of
DNA-PK was inhibited in the NU7026 or NU7026+4 Gy groups, but
DNA-PK increased in the 4 Gy group (Fig. 1B). The expression of ATM was almost
unchanged in the four groups after NU7026 treatment (Fig. 1B). The survival fraction of A549
cells after NU7026+4 Gy treatment was a 79.3% decrease relative to
4 Gy X-ray irradiation alone (P<0.001). Moreover, no
statistically significant difference was evident between control
and NU7026-treated cells (P>0.05; Fig. 1C). NU7026 increased the sensitivity
of A549 cells to X rays by 4.8-fold.
Inhibition of DNA-PK and X-ray
irradiation increases DNA damage and cell apoptosis
γH2AX foci are biomarkers of DNA double-strand
breaks that initiate the DNA damage response (21). In the present study, X-ray
irradiation significantly increased γH2AX foci (Fig. 1D). The greatest number of γH2AX
foci was observed after NU7026 and 4 Gy X-ray co-treatment,
followed by the 4 Gy irradiation-alone group. NU7026 treatment
and/or X-ray irradiation significantly increased late apoptosis
(Fig. 1E). The late apoptosis rate
in the control group was 4.58±0.2% and increased to 5.9±0.7,
9.7±0.5 and 82.5±2.6% in the NU7026, 4 Gy and NU7026+4 Gy groups,
respectively (Fig. 1E).
Inhibition of DNA-PK and X-ray
irradiation induces p73 apoptosis pathway in A549 cells
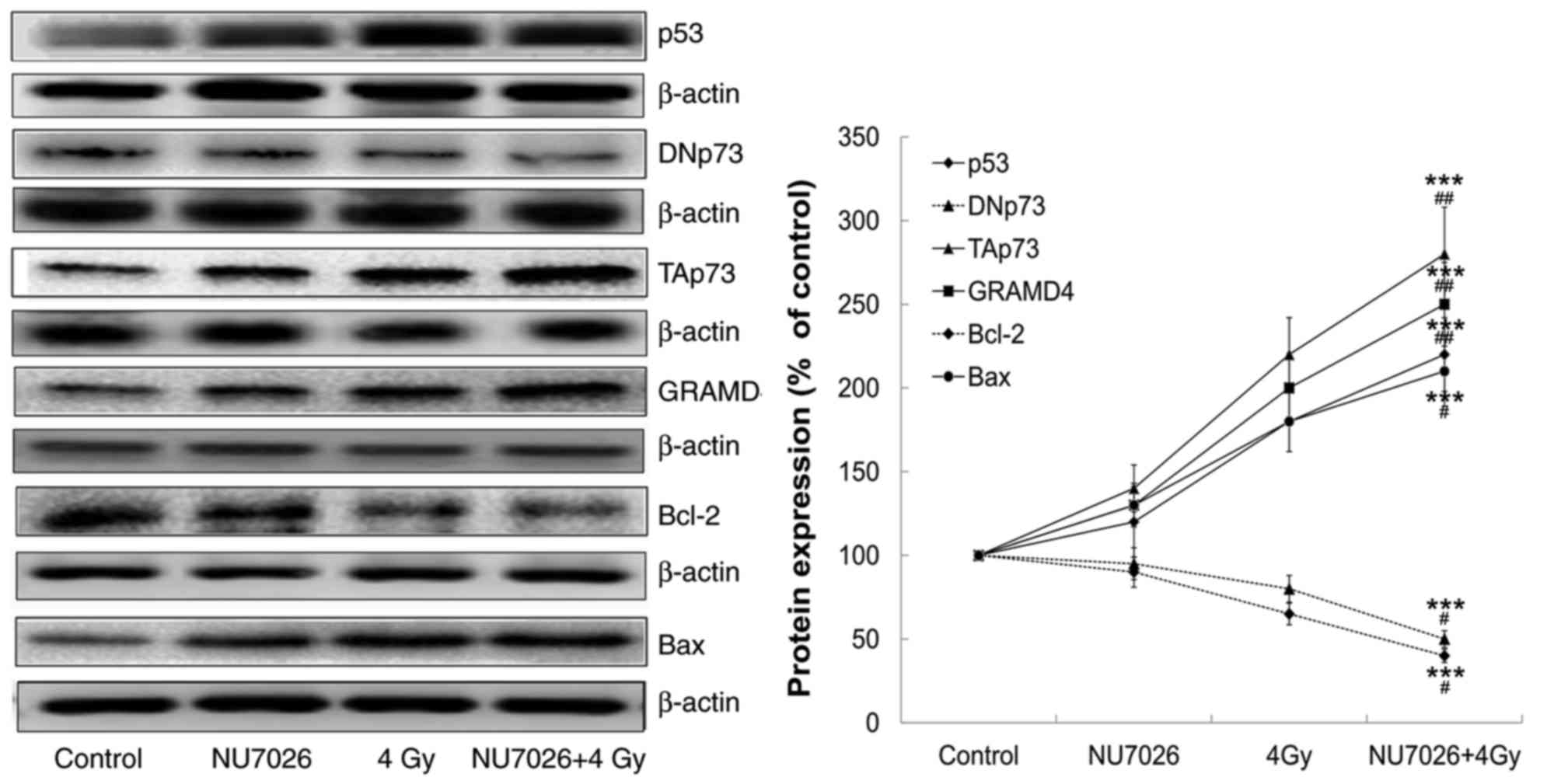
The expression of cell apoptosis regulatory proteins
was analyzed by western blot analysis and the results are shown in
Fig. 2. At 24 h post-irradiation,
NU7026 pre-treatment decreased DNp73 expression and increased p53
and TAp73 expression at the protein level. Downstream GRAMD4 was
also upregulated. Furthermore, anti-apoptotic factor Bcl-2
expression was downregulated and pro-apoptotic factor Bax
expression was upregulated.
Inhibition of DNA-PK sensitizes
xenograft tumors to X-ray irradiation
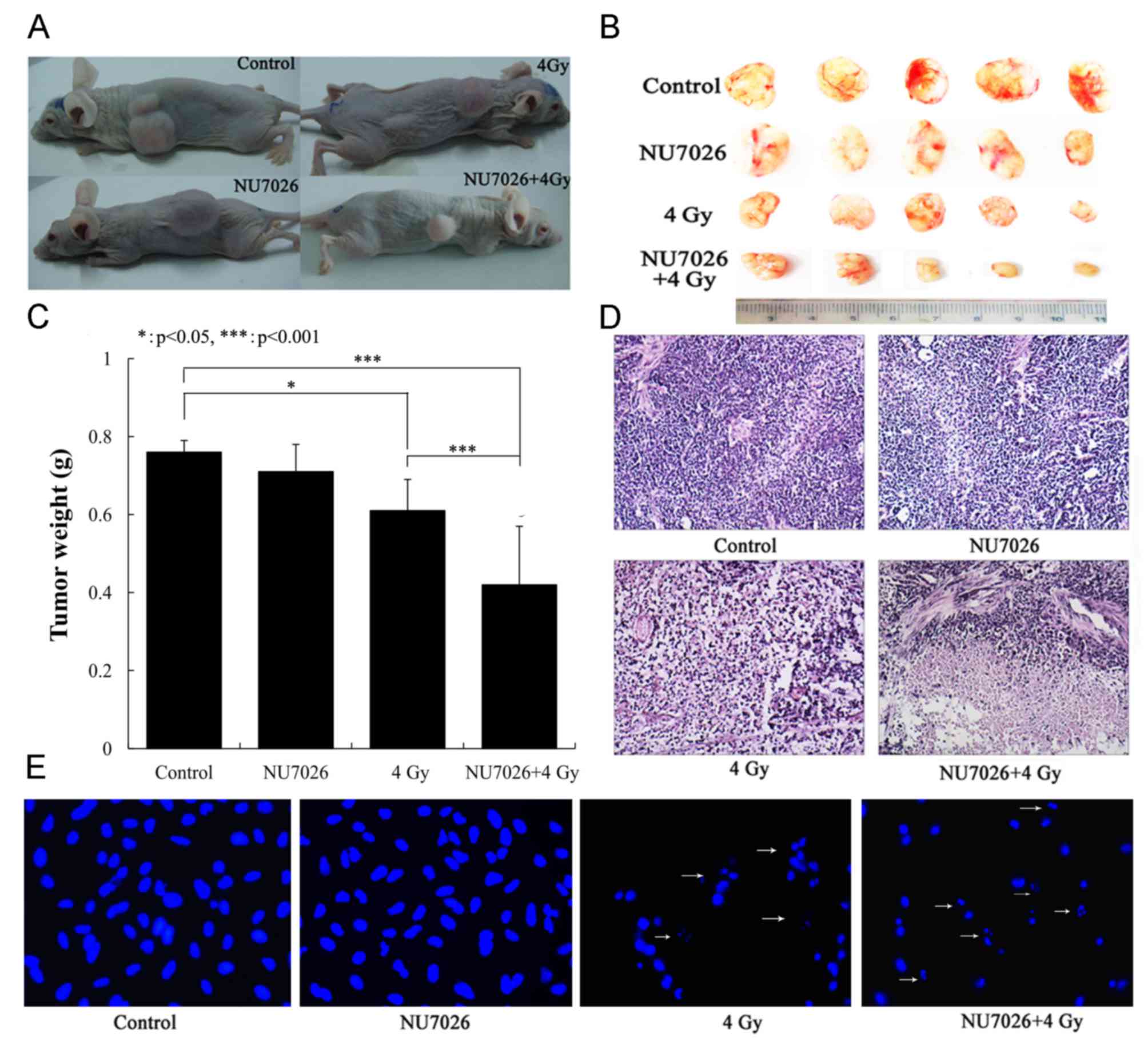
Xenograft tumor growth was recorded 15 days
post-irradiation (Fig. 3A). Little
adverse reactions of all mice were observed during tumor formation.
But with the increase of the tumor volume in the control and the
NU7026 treatment groups, the activity of nude mice decreased, the
mental state was poor with drowsiness and less exercise, and the
weight loss (6.5±1.3 and 5.8±1.1 g, respectively). The adverse
reactions of nude mice in X-ray and NU7026+X-rays treatment groups
were not present. The results indicated that each nude mouse is
loaded with one tumor. The tumor growth was not restricted in the
control group, where the mean tumor weight was 0.76±0.03 g (longest
diameter=19.19±3.27 mm, volume=1,401.24±32.32 mm3), nor
was tumor growth significantly inhibited in the NU7026 treatment
group with a mean tumor weight of 0.71±0.07 g (longest
diameter=14.78±4.65 mm, volume=1,205.75±82.55 mm3;
Fig. 3B and C). In contrast, tumor
growth was significantly inhibited in the X-ray and NU7026+X-rays
treatment groups with mean tumor weights of 0.61±0.18 g (longest
diameter=13.27±3.02 mm, volume=930.13±32.86 mm3) and
0.42±0.15 g (longest diameter=9.24±2.10 mm, volume=308.38±12.39
mm3), respectively (P<0.001; Fig. 3B and C). The tumor weight of
NU7026+4 Gy was a 31.1% decrease compared with 4 Gy X-ray
irradiation alone (P<0.001). NU7026 increased the sensitivity of
tumors to X rays by 1.5 times. The effects of NU7026+X-ray
irradiation treatment was also examined by H&E staining. The
results indicated that necrosis of tumor tissue gradually
increased, especially in the NU7026+X-ray irradiation group with
increased cytoplasm (pink; Fig.
3D) and fragmented nuclei (arrows; Fig. 3E).
ATM gene silencing promotes
NU7026/X-ray-induced inhibition of survival and apoptosis
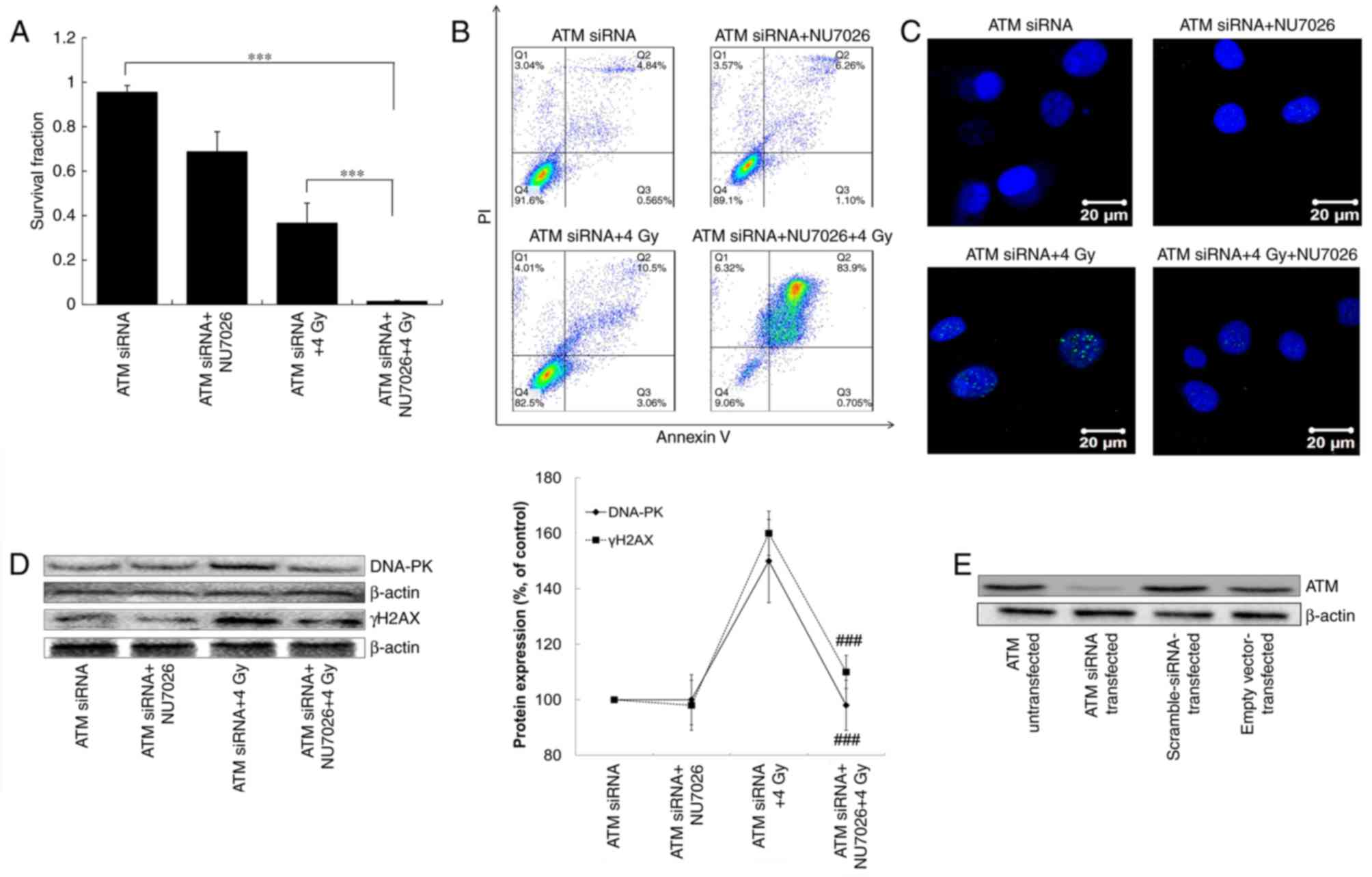
In ATM siRNA-transfected cells, NU7026 and/or X-ray
treatment decreased survival fraction (Fig. 4). The survival fraction of A549
cells after ATM siRNA+NU7026+X-ray treatment decreased by 94.1%
compared with ATM siRNA treatment (P<0.001), and decreased by
35.2% compared with ATM siRNA+X-ray treatment (P<0.001; Fig. 4A). Late apoptosis in ATM
siRNA-transfected cells was significantly increased by NU7026
treatment and/or X-ray treatment. The late apoptosis rate of the
ATM siRNA treatment group was 4.84±0.5% compared with 6.3±0.4% in
the ATM siRNA+NU7026 group, 10.8±1.6% in the ATM siRNA+X-rays group
and 84.2±1.9% in the ATM siRNA+NU7026+X-rays group (Fig. 4B). These results demonstrated that
ATM gene silencing enhanced the inhibition of survival and promoted
apoptosis induced by NU7026/X-ray treatment.
ATM gene silencing reduces the
NU7026+X-ray-induced DNA damage response
The number of γH2AX foci was increased by ATM
siRNA+4 Gy treatment compared with the ATM siRNA and ATM
siRNA+NU7026 groups. In contrast, the number of γH2AX foci was
decreased after ATM siRNA+NU7026+4 Gy treatment (Fig. 4C). These results paralleled γH2AX
protein expression in the same groups (Fig. 4D). However, the expression of
DNA-PK was almost unchanged in ATM siRNA, ATM siRNA+NU7026 and ATM
siRNA+NU7026+4 Gy groups. These results indicated that γH2AX foci
disappeared after NU7026+4 Gy treatment in ATM siRNA-transfected
A549 cells compared with normal cells. Therefore, ATM but not
DNA-PK, was involved in the NU7026+X-ray-induced DNA damage
response. When the ATM-mediated repair pathway was inhibited, cells
initiated programmed cell death. The desired effects of ATM-siRNA
transfection have been achieved, that is, the expression of ATM
protein has been reduced by western blotting in the transfected
A549 cells (Fig. 4E).
Discussion
NSCLC has a strong capacity to repair DNA damage,
which is the main reason for cancer radioresistance. DNA-PK serves
an important role in radioresistance of cancer cells as a key
kinase in NHEJ DNA damage repair (4,8).
NU7026 (DNA-PK inhibitor) has been demonstrated to enhance the
antitumor effect of X-rays against lung adenocarcinoma (10). The results of the current study
revealed that NU7026 significantly increased the radiosensitivity
of NSCLC cells exposed to X-ray irradiation by inhibiting the
growth of A549 cells and xenograft tumors. The inhibitor NU7026 may
therefore be useful as a radiosensitizing drug for
radiotherapy.
The sampling times of γH2AX protein for confocal
microscopy and other proteins for western blotting were 30 min and
24 h post-irradiation, respectively. Usually DNA damage occurs
within 30 min post-irradiation. Subsequently, cells activate the
DNA damage response pathway >30 min (5,6). DNA
damage agents can also activate the DNA damage response pathway,
which either results in DNA repair or apoptosis of cancer cells
(22–25). In the present study, the
radiosensitizing effects of NU7026 on NSCLC cells were further
investigated. The results demonstrated that inhibition of DNA-PK
increased DNA damage and initiated the ATM-dependent DNA damage
response after X-ray irradiation. It was also illustrated that
NU7026 pre-treatment activated apoptosis of NSCLC cells, indicating
that inhibition of DNA-PK could result in persistent DNA damage.
ATM is involved in activation of the downstream p73 apoptotic
pathway when DNA damage repair fails (14). Overexpression of the TAp73 isoform
directly activated pro-apoptotic factor GRAMD4 expression to induce
changes in Bcl-2 and Bax protein levels in mitochondria. Decreased
Bcl-2/Bax ratio contributes to apoptosis (11). In addition, our previous study
demonstrated that ATM knockdown effectively inhibited cell growth
and increased DNA damage and apoptosis in NSCLC cells after
co-treatment with NU7026 and X-ray irradiation (10). Therefore, combining the ATM
specific inhibitor CGK733 and DNA-PK inhibitor NU7026 may more
effectively block DNA damage repair and enhance radiosensitivity of
NSCLC cells.
Previous studies have demonstrated that DNA-PK
inhibitors can enhance the radiosensitivity of cancer cells (liver
cancer HepG2, gastric cancer N87 and leukaemia MOLT-4) by
increasing DNA damage leading to G2/M phase arrest and
induction of apoptosis (22–28).
Similarly, the present results demonstrated that a DNA-PK inhibitor
exerted radiosensitization effects on xenograft tumors in
vivo and on A549 cells in vitro. John et al
(13) demonstrated that p73 was
able to trigger apoptosis via the mitochondrial pathway by the
newly discovered pro-apoptotic mediator GRAMD4 (death-inducing
protein), which induced changes in Bcl-2 and Bax protein
expression.
A recent study has revealed that ATM-dependent DNA
repair response of cervical cancer cells were activated by
7-hydroxy-5,4′-dimethoxy-2-arylbenzofuran via causing DNA damage as
an anti-cancer agent (29).
Moreover, several cancer cell lines that lack ATM function have
enhanced sensitivity to radiotherapy and chemotherapy (10,30,31).
The function of DNA-PK and ATM is complementary since it has been
demonstrated that combined knockout of both kinases is
synthetically lethal (32).
Therefore, it could be proposed that inhibition of DNA-PK activates
the ATM-dependent DNA damage response and that ATM knockdown
increases the radiosensitivity of NSCLC cells following X-ray
irradiation.
DNA damage is a universal characteristic following
cancer cell radiotherapy. Therefore, the use of DNA repair
inhibitors alone or in combination may have great radiosensitizing
potential (10,33–37).
The key factors in the DNA damage repair pathway include PARP, ATM,
ATR, DNA-PK, Chk1 and Chk2, among others (33–39).
PARP inhibitors have been demonstrated to interfere with single
strand break (SSB) repair in HR-defective cancer cells at a safe
dose level in combination with chemotherapy and radiotherapy in
clinical trials (33). ATM and ATR
inhibitors (caffeine and KU-55933, respectively) induce
phosphorylation of p53 to promote radiosensitization, but low serum
levels and high systemic toxicity have been limiting factors in
clinical trials (34). DNA-PK
inhibitors wortmannin and LY294002 are neither specific nor
suitable for clinical use due to severe toxicity (35,36).
The pharmacokinetics of NU7026 and NU7441 (another DNA-PK
inhibitor) are still undergoing clinical analysis (37). Chk1 inhibitors (UCN-01) have
demonstrated a long half-life and decreased bioavailability,
whereas the Chk1 and Chk2 inhibitor PF-00477736 resulted in greater
inhibition of tumor growth (38,39).
Notably, the clinical development of inhibitors for PARP, ATM, ATR,
Chk1, CHk2 and DNA-PK is being actively pursued (9). In summary, inhibition of DNA-PK
activity enhanced the radiosensitivity of NSCLC cells to X-ray
radiation by inducing the ATM-dependent DNA damage response and p73
apoptosis pathway, thus elucidating mechanisms underlying the
myriad effects of DNA-PK, ATM, p73 and radiotherapy.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 31601510) and the Natural
Science Foundation of Liaoning Province of China (grant no.
20170540022).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Author's contributions
LY was a major contributor in study design, cell
tests, data analysis and writing of the manuscript. TM also was a
major contributor in study design. XY performed mice experiments.
DZ and LZ analyzed and interpreted the data. YT, SW and BW
performed cell tests. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the ethics
committee of the Bohai University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rodin D, Grover S, Xu MJ, Hanna TP, Olson
R, Schreiner LJ, Munshi A, Mornex F, Palma D and Gaspar LE:
International Association for the Study of Lung Cancer Advanced
Radiation Technology Committee: Radiotherapeutic management of
non-small cell lung cancer in the minimal resource setting. J
Thorac Oncol. 11:21–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang HJ, Kim N, Seong KM, Youn H and Youn
B: Investigation of radiation-induced transcriptome profile of
radioresistant non-small cell lung cancer A549 cells using RNA-seq.
PLoS One. 8:e593192013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhuang W, Li B, Long L, Chen L, Huang Q
and Liang Z: Induction of autophagy promotes differentiation of
glioma-initiating cells and their radiosensitivity. Int J Cancer.
129:2720–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JS, Krasieva TB, Kurumizaka H, Chen
DJ, Taylor AM and Yokomori K: Independent and sequential
recruitment of NHEJ and HR factors to DNA damage sites in mammalian
cells. J Cell Biol. 170:341–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahaney BL, Meek K and Lees-Miller SP:
Repair of ionizing radiation-induced DNA double-strand breaks by
non-homologous end-joining. Biochem J. 417:639–650. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lieber MR: The mechanism of human
nonhomologous DNA end joining. J Biol Chem. 283:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yajima H, Lee KJ, Zhang S, Kobayashi J and
Chen BP: DNA double-strand break formation upon UV-induced
replication stress activates ATM and DNA-PKcs kinases. J Mol Biol.
385:800–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang SH, Kuo TC, Wu H, Guo JC, Hsu C, Hsu
CH, Tien YW, Yeh KH, Cheng AL and Kuo SH: Perspectives on the
combination of radiotherapy and targeted therapy with DNA repair
inhibitors in the treatment of pancreatic cancer. World J
Gastroenterol. 22:7275–7288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Liu Y, Sun C, Yang X, Yang Z, Ran
J, Zhang Q, Zhang H and Wang X and Wang X: Inhibition of DNA-PKcs
enhances radiosensitivity and increases the levels of ATM and ATR
in NSCLC cells exposed to carbon ion irradiation. Oncol Lett.
10:2856–2864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di CX, Yang LN, Zhang H, An LZ, Zhang X,
Ma XF, Sun C, Wang XH, Yang R, Wu ZH and Si J: Effects of
carbon-ion beam or X-ray irradiation on anti-apoptosis ΔNp73
expression in HeLa cells. Gene. 515:208–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conforti F, Yang AL, Agostini M, Rufini A,
Tucci P, Nicklison-Chirou MV, Grespi F, Velletri T, Knight RA,
Melino G and Sayan BS: Relative expression of TAp73 and ΔNp73
isoforms. Aging (Albany NY). 4:202–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
John K, Alla V, Meier C and Putzer BM:
GRAMD4 mimics p53 and mediates the apoptotic function of p73 at
mitochondria. Cell Death Differ. 18:874–886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muppani N, Nyman U and Joseph B:
TAp73alpha protects small cell lung carcinoma cells from caspase-2
induced mitochondrial mediated apoptotic cell death. Oncotarget.
2:1145–1154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bailey SG, Cragg MS and Townsend PA:
Family friction as ΔNp73 antagonises p73 and p53. Int J Biochem
Cell Biol. 43:482–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di C, Sun C, Li H, Si J, Zhang H, Han L,
Zhao Q, Liu Y, Liu B, Miao G, et al: Diallyl disulfide enhances
carbon ion beams-induced apoptotic cell death in cervical cancer
cells through regulating Tap73/ΔNp73. Cell Cycle. 14:3725–3733.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hassan HM, Dave BJ and Singh RK: TP73, an
under-appreciated player in non-Hodgkin lymphoma pathogenesis and
management. Curr Mol Med. 14:432–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Sun C, Jin X, Li P, Ye F, Zhao T,
Gong L and Li Q: Genistein enhances the radiosensitivity of breast
cancer cells via G2/M cell cycle arrest and apoptosis.
Molecules. 18:13200–13217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cooper A, Garcia M, Petrovas C, Yamamoto
T, Koup RA and Nabel GJ: HIV-1 causes CD4 cell death through
DNA-dependent protein kinase during viral integration. Nature.
498:376–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Xue H, Cutz JC, Bayani J, Mawji
NR, Chen WG, Goetz LJ, Hayward SW, Sadar MD, Gilks CB, et al: An
orthotopic metastatic prostate cancer model in SCID mice via
grafting of a transplantable human prostate tumor line. Lab Invest.
85:1392–1404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vandersickel V, Depuydt J, Van Bockstaele
B, Perletti G, Philippe J, Thierens H and Vral A: Early increase of
radiation-induced gammaH2AX foci in a human Ku70/80 knockdown cell
line characterized by an enhanced radiosensitivity. J Radiat Res.
51:633–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiloh Y and Ziv Y: The ATM protein
kinase: Regulating the cellular response to genotoxic stress and
more. Nat Rev Mol Cell Biol. 14:197–210. 2013. View Article : Google Scholar
|
|
23
|
Yang J, Yu Y, Hamrick HE and
Duerksen-Hughes PJ: ATM, ATR and DNA-PK: Initiators of the cellular
genotoxic stress responses. Carcinogenesis. 24:1571–1580. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Durocher D and Jackson SP: DNA-PK, ATM and
ATR as sensors of DNA damage: Variations on a theme? Curr Opin Cell
Biol. 13:225–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jackson SP: DNA damage detection by DNA
dependent protein kinase and related enzymes. Cancer Surv.
28:261–279. 1996.PubMed/NCBI
|
|
26
|
Yang C, Wang Q, Liu X, Cheng X, Jiang X,
Zhang Y, Feng Z and Zhou P: NU7441 enhances the radiosensitivity of
liver cancer cells. Cell Physiol Biochem. 38:1897–1905. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tichy A, Durisova K, Salovska B, Pejchal
J, Zarybnicka L, Vavrova J, Dye NA and Sinkorova Z:
Radio-sensitization of human leukaemic MOLT-4 cells by
DNA-dependent protein kinase inhibitor, NU7441. Radiat Environ
Biophys. 53:83–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niazi MT, Mok G, Heravi M, Lee L, Vuong T,
Aloyz R, Panasci L and Muanza T: Effects of dna-dependent protein
kinase inhibition by NU7026 on dna repair and cell survival in
irradiated gastric cancer cell line N87. Curr Oncol. 21:91–96.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Zeng X, Gao C, Ming P, Zhang J,
Guo C, Zhou L, Lu Y, Wang L, Huang L, et al: A new arylbenzofuran
derivative functions as an anti-tumour agent by inducing DNA damage
and inhibiting PARP activity. Sci Rep. 5:108932015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Williamson CT, Muzik H, Turhan AG, Zamò A,
O'Connor MJ, Bebb DG and Lees-Miller SP: ATM deficiency sensitizes
mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1
inhibitors. Mol Cancer Ther. 9:347–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weston VJ, Oldreive CE, Skowronska A,
Oscier DG, Pratt G, Dyer MJ, Smith G, Powell JE, Rudzki Z, Kearns
P, et al: The PARP inhibitor olaparib induces significant killing
of ATM-deficient lymphoid tumor cells in vitro and in vivo. Blood.
116:4578–4587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arlander SJ, Greene BT, Innes CL and
Paules RS: DNA protein kinase-dependent G2 checkpoint revealed
following knockdown of ataxia-telangiectasia mutated in human
mammary epithelial cells. Cancer Res. 68:89–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen Y, Rehman FL, Feng Y, Boshuizen J,
Bajrami I, Elliott R, Wang B, Lord CJ, Post LE and Ashworth A: BMN
673, a novel and highly potent PARP1/2 inhibitor for the treatment
of human cancers with DNA repair deficiency. Clin Cancer Res.
19:5003–5015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sarkaria JN, Busby EC, Tibbetts RS, Roos
P, Taya Y, Karnitz LM and Abraham RT: Inhibition of ATM and ATR
kinase activities by the radiosensitizing agent, caffeine. Cancer
Res. 59:4375–4382. 1999.PubMed/NCBI
|
|
35
|
Rosenzweig KE, Youmell MB, Palayoor ST and
Price BD: Radiosensitization of human tumor cells by the
phosphatidylinositol3-kinase inhibitors wortmannin and LY294002
correlates with inhibition of DNA-dependent protein kinase and
prolonged G2-M delay. Clin Cancer Res. 3:1149–1156. 1997.PubMed/NCBI
|
|
36
|
Critchlow SE, Bowater RP and Jackson SP:
Mammalian DNA double-strand break repair protein XRCC4 interacts
with DNA ligase IV. Curr Biol. 7:588–598. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Davidson D, Amrein L, Panasci L and Aloyz
R: Small molecules, inhibitors of DNA-PK, targeting DNA repair and
beyond. Front Pharmacol. 4:52013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blasina A, Hallin J, Chen E, Arango ME,
Kraynov E, Register J, Grant S, Ninkovic S, Chen P, Nichols T, et
al: Breaching the DNA damage checkpoint via PF-00477736, a novel
small-molecule inhibitor of checkpoint kinase 1. Mol Cancer Ther.
7:2394–2404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sausville EA, Arbuck SG, Messmann R,
Headlee D, Bauer KS, Lush RM, Murgo A, Figg WD, Lahusen T, Jaken S,
et al: Phase I trial of 72-hour continuous infusion UCN-01 in
patients with refractory neoplasms. J Clin Oncol. 19:2319–2333.
2001. View Article : Google Scholar : PubMed/NCBI
|