Introduction
Hepatitis C virus (HCV) is the major cause to HCC
and liver failure worldwide, moreover, China is considered as a
high endemic area of HCV infection (1–4). HCV
which belongs to the Flaviviridae family has only a positive
single-stranded RNA genome encoding a precursor polyprotein of
about 3000 amino acid residues (5). HCV core protein plays an important
role in the regulation of cell growth and host expression of genes
that were crucial for infectivity, for instance, cell apoptosis
(6,7). After the infection of host cells by
HCV, they initiate the defense ability which named as cell
apoptosis. However, HCV core protein has evolved to inhibit the
ability of host-mediated cell apoptosis (8). It has been revealed that, apart from
forming virus, HCV core proteins can modulate gene transcription,
cell proliferation, cell apoptosis, and progression to HCC.
Generally, HCV core protein appears to exert multiple effects on
cell apoptosis which rely on the apoptotic stimuli as well as the
cell type and microenvironment (9). Though there are numerous reports
describing the functions of HCV core proteins in cellular
apoptosis, the mechanisms and impacts of these proteins in Huh-7
cell apoptosis have not so far been studied or reported.
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL), also named Apo2L, belongs to the
TNF superfamily and induces cell apoptosis typically in a variety
of transformed cells but not healthy cells (10). Moreover, it is reported that TRAIL
also induces cell apoptosis in virus-infected cells, for instance,
cells that are infected by HCV (11). Therefore, TRAIL may serve an immune
surveillance factor by selectively killing virus-infected
cells.
It is well known that the tumor promoter protein p53
plays a key role in cell apoptosis, while the functional
inactivation of p53 is often a crucial stage during tumorigenesis
(12). Through sirt1, when p53
protein is deacetylated, its DNA binding activity is impaired,
consequently leading to a reduction in p53-mediated cell apoptosis
in response to DNA damage (13,14).
BH3 interacting-domain death agonist (Bid), whose promoter has
p53-binding sites and whose expression is regulated by p53, takes
part in numberous apoptotic processes (15,16).
Be cleaved by other proteases or caspase-8, activated Bid
translocates to the mitochondrial outer membrane and leads to the
activation of Bcl-2-associated X protein (Bax)/Bak (17).
In the present study, we focus on HCV core proteins
of 3 different strains to explore the possible mechanisms and hunt
for a novel therapeutic target for HCV infection.
Materials and methods
Plasmids
The plasmids of pcDNA3.1, pcDNA3.1-C191, pcDNA3.1-NT
and pcDNA3.1-T were conserved by our lab in Laboratory of
Infectious Disease, Affiliated Hospital of Xuzhou Medical
University.
Cell culture and DNA transfection
The human hepatoma cell line Huh-7 was purchased
from ATCC. Huh-7 cells were cultured in Dulbecco Minimum Essential
Medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (both Gibco; Thermo Fisher Scientific,
Inc.) in an incubator at the temperature of 37°C and 5%
CO2.
Transfection was performed using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) as recommended by the
manufacturer. The p53-specific siRNA was purchased from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA (sc-29435). The siRNA was
transfected into cells that were cultured in 6-well plates by
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at
the concentration of 100 pmol/well, or transfected into cells in
24-well plates with HiPerFect transfection reagent (Qiagen, Inc.,
Valencia, CA, USA) at the concentration of 37.5 ng/well.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was purified from cultured Huh-7
cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), in
accordance with the manufacturer's instructions. Reverse
transcription was performed by random primer and Moloney murine
leukemia virus reverse transcriptase (Promega Corp., Madison, WI,
USA), and cDNA was used as a template for RT-PCR. RT-qPCR was
carried out using SYBR master mix (Toyobo Life Science, Osaka,
Japan) and ABI7300. PCR conditions were as followed: 95°C for 60
sec followed by 40 cycles of 95°C for 15 sec, 60°C for 15 sec, and
72°C for 45 sec. The mRNA levels of casein kinase (CK)1α, CK1β,
CK1γ, CK1δ and GAPDH (internal control) in Huh-7 cells were
measured using the following primers: CK1α (sense,
5′-AGTGGCAGTGAAGCTAGAATCT-3′ and antisense,
5′-CGCCCAATACCCATTAGGAAGTT-3′) CK1ε (sense,
5′-TCCAAAGCCGAACTCGTTGT-3′ and antisense,
5′GATGCCCAGATAAACGTCTCC-3′), CK1γ (sense,
5′-ATGGACCATCCTAGTAGGGAAAA-3′ and antisense,
5′-CACATCCTATCTTCTTGCCAACC-3′), CK1δ (sense,
5′-CAGGAGAAGAGGTTGCCATCA-3′ and antisense,
5′-CAAGCAGCAGGACGGTTTTG-3′).
Electron microscope
The Huh-7 cells from each group were collected into
different centrifuge tubes, washed with phosphate-buffered saline
(PBS; pH 7.4), carefully transferred into 0.25% glutaraldehyde
using a pipette. Then, they were incubated at 4°C overnight.
Subsequently, samples were fixed with 3% glutaraldehyde and 1%
osmiumtetroxide. The fixed samples were dehydrated with a gradient
series of acetone and embedded in Epon-812 agar (Shell Chemicals,
Deer Park, TX, USA). Finally, the embedded samples, which were
constructed into ultrathin sections by automatic semi-thin rotary
microtome (Leica, Wetzlar, Germany) and stained with uranyl
acetate/lead citrate, were observed under a Hitachi H-600IV
transmission electron microscope (Hitachi, Tokyo, Japan). Images
were captured accordingly.
FACS analysis
Apoptosis assays were performed as manufacturer's
instructions (BD Biosciences, Franklin Lakes, NJ, USA). For each
sample, 0.3×106 cells were seeded per 35-mm well and
analyzed at 18 h (for apoptosis) or 72 h (for survival) after
treatment, respectively. Annexin V-FITC/PI staining for apoptosis
assays were conducted according to the manufacturer's
protocols.
Induction of apoptosis by TRAIL
Huh-7 cells (60–70% confluent) were seeded into
6-well plates. Three days post transfection, the Huh-7 cells were
treated with recombinant human TRAIL (Perprotech, London, UK) for 2
h. Subsequently, cells were harvested, washed with PBS and stained
with Annexin V as recommended by the manufacturer. The proportion
of apoptotic cells was determined with flow cytometry.
Western blot analysis
At 24 h after the cells were harvested, the protein
expression levels were measured by WB analysis. Equal amounts of
protein samples were resolved by 10% sodium dodecyl sulfate
polyacrylamide gel (SDS-PAGE), and transferred onto a
nitrocellulose membrane (Amersham; GE Healthcare, Chicago, IL,
USA). The membranes were probed using a polyclonal antibody against
CK1α (1:1,000 ab108296; Abcam, Cambridge, UK), CK1ε (1:1,000;
sc-373912; Santa Cruz Biotechnology, Inc.), CK1γ (1:1,000;
ab64829), CK1δ (1:1,000; ab85320; both Abcam), Bid (1:1,000; 8762),
caspase-8 (1:1,000; 4927; both Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight. Thereafter, membrane was washed
with TBST for 3 times, and incubated with secondary antibody at
room temperature for 1 h. After wash for 3 times, blots were
developed with SuperSignal West Femto Maximum Sensitivity Substrate
(Thermo Fisher Scientific, Inc.). GAPDH (1:8,000; G5262;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used as an
internal control for the comparison of protein load.
Statistical analysis
The results were analyzed using GraphPad Prism 6.0
software (GraphPad Software, Inc., La Jolla, CA, USA) and are
expressed as the mean ± standard deviation of at least 3
independent experiments. Comparisons between groups were performed
using Student's t-test and one-way analysis of variance with
Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
HCV core proteins induced Huh-7 cell
apoptosis
The ratios of Annexin V/PI-positive human Huh-7
cells in different groups were quantified by flow cytometry.
Compared with Huh-7 cells that were transfected with pcDNA3.1,
total number of Annexin V-positive Huh-7 cells was significantly
increased after transfection with three different core proteins
(Fig. 1A).
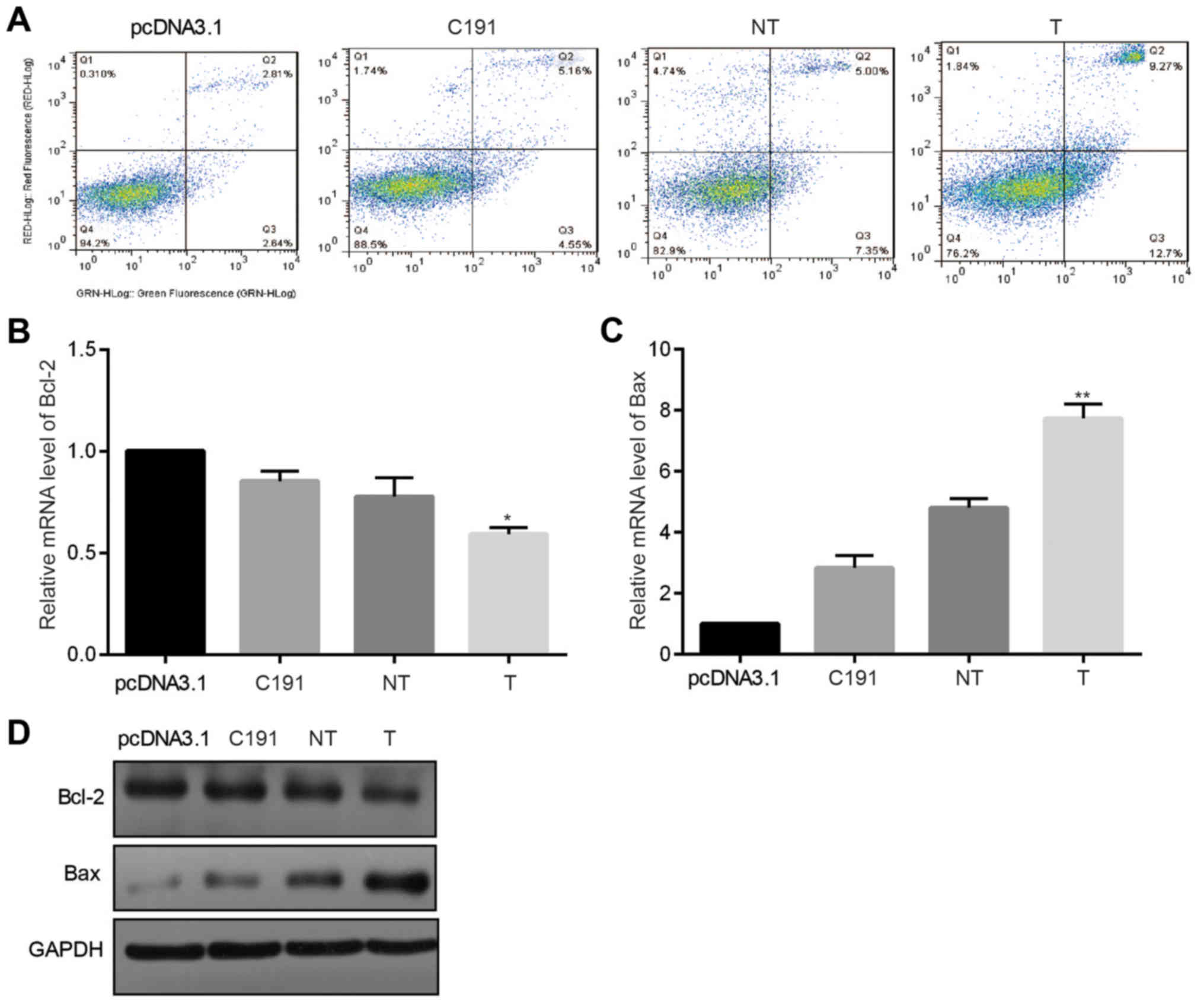 | Figure 1.Apoptotic and anti-proliferation
effects of HCV core proteins in Huh-7 cells. Huh-7 cells were
transiently transfected with three core proteins, and Huh-7 cells
that were transfected with pcDNA3.1 were used as the negative
control. (A) The ratio of Annexin V-positive Huh-7 cells was
measured by flow cytometry (n=3). HCV core proteins were
transiently transfected into Huh-7 cells. Following 48 h, mRNA
levels of (B) Bcl-2 and (C) Bax (n=5) were measured. (D)
Correspondingly, following 48 h, the protein levels of BAX and
Bcl-2 were evaluated (n=5). *P<0.05 and **P<0.01 vs.
pcDNA3.1. HCV, hepatitis C virus; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; T, core proteins derived from tumor
tissues; NT, core proteins derived from non-tumor tissues; C191,
core proteins from the HCV-J6 strain. |
Thereafter, we measured mRNA and protein levels of
BAX and B-cell lymphoma 2 (Bcl-2) in Huh-7 cells that were
transfected with three different HCV core plasmids 48 h later. As
shown in Fig. 1B-D, Bcl-2 was
significantly downregulated while BAX was significantly upregulated
at both mRNA level and protein level in Huh-7 cells in groups
transfected with core proteins than in group transfected with
pcDNA3.1.
These results exhibited that all the different HCV
core proteins induced Huh-7 cell apoptosis.
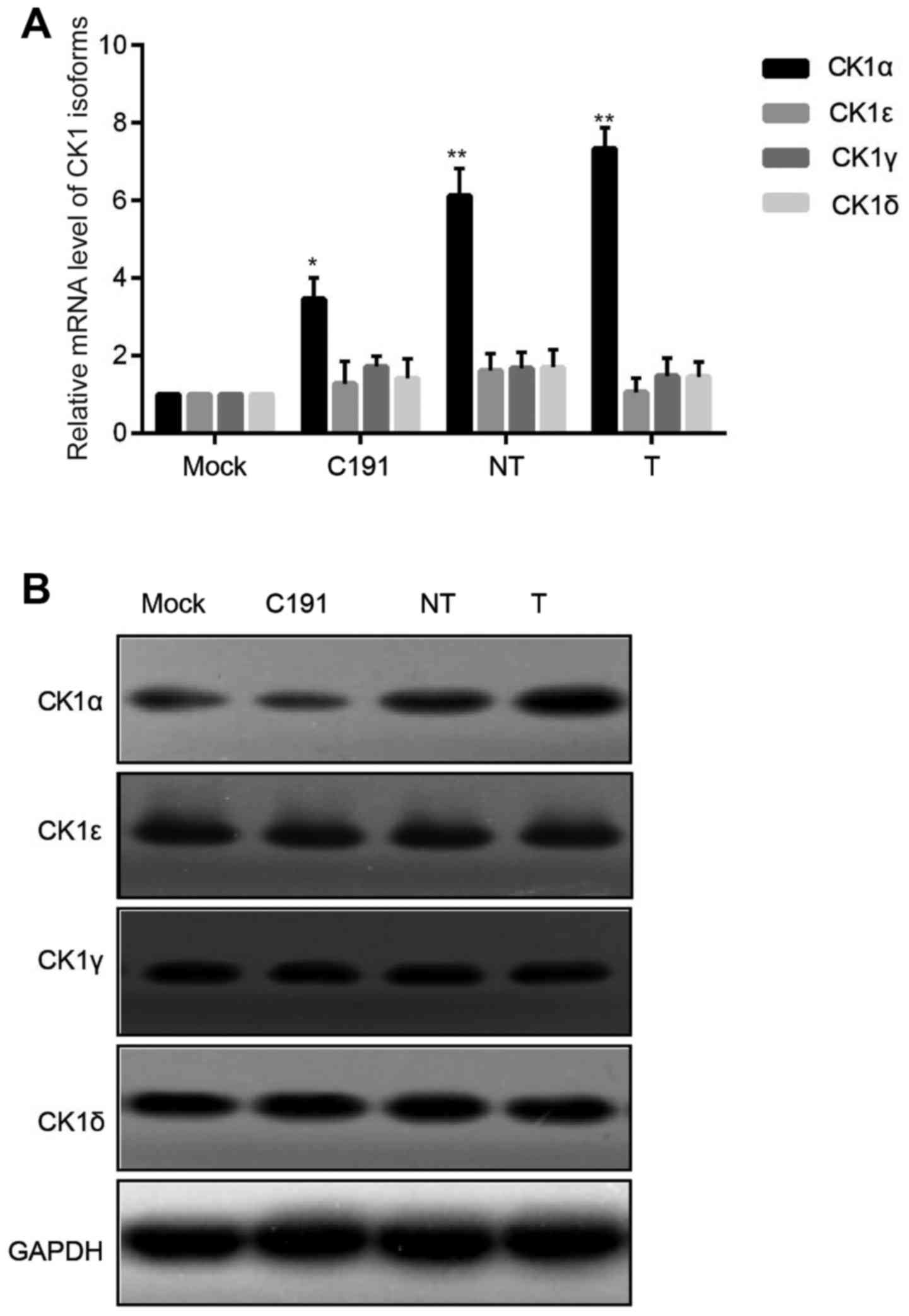
Different strains of HCV core proteins
elevated expression of CK1α in Huh-7 cells specifically
To explore the function of different strains of HCV
core proteins (T, NT, C191), we firstly examined the changes of CK1
isoforms (CK1α, CK1ε, CK1γ and CK1δ) in Huh-7 cells after
transfection with HCV core proteins. There were no statistical
significance for changes of CK1ε, CK1γ and CK1δmRNA levels, while
mRNA level of CK1α in Huh-7 cells that transfected with HCV core
protein was statistically increased compared with mock group
(Fig. 2A). Protein expression of
CK1 isoforms was in accordance with mRNA level changes (Fig. 2B).
In conclusion, CK1α played a pivotal role in
responding to the transfection of HCV core proteins into Huh-7
cells. It was unknown whether there were down-stream molecules that
could be regulated or affected by CK1α.
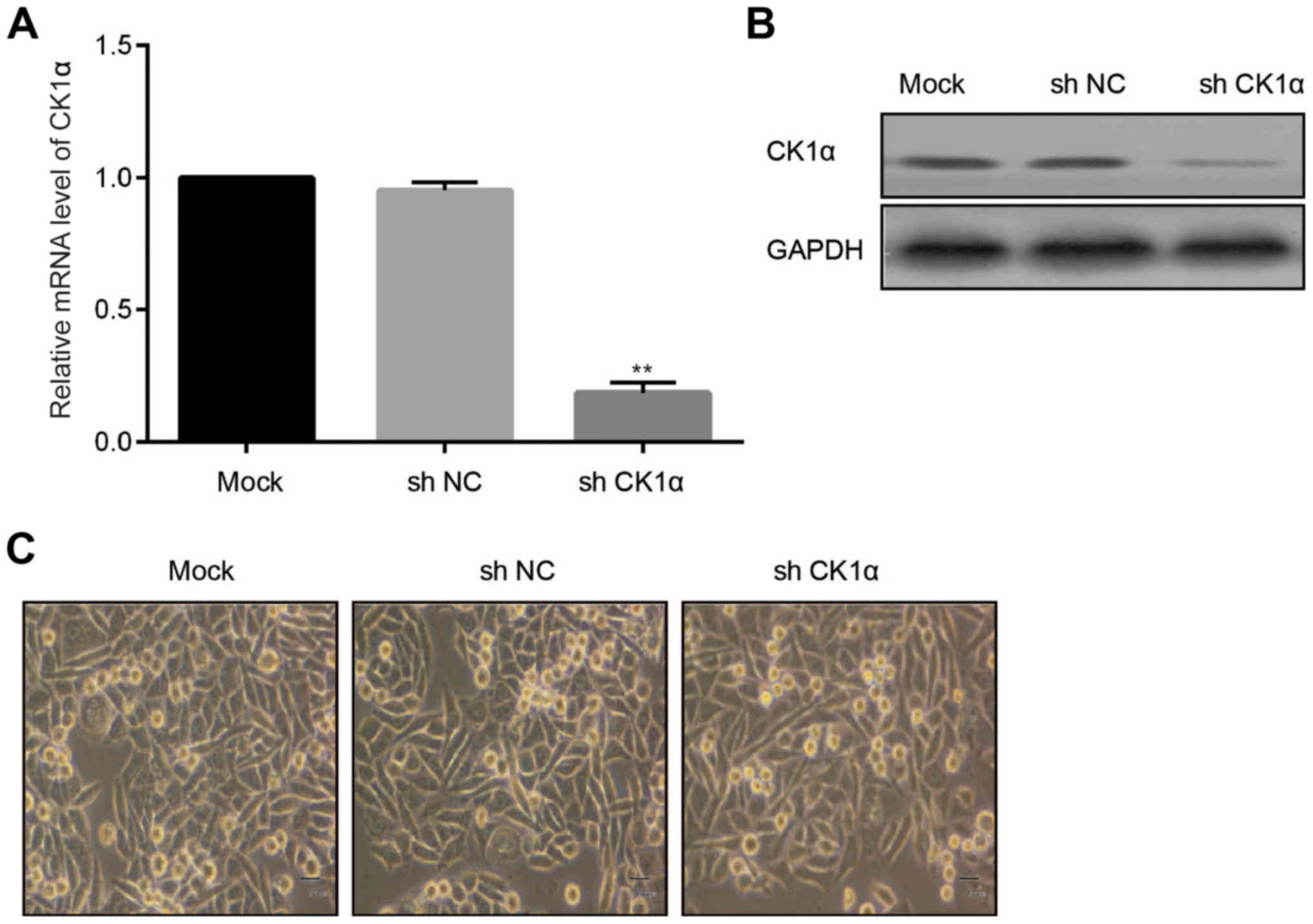
Successful construction of stable CK1α
knock-down Huh-7 cells
To investigate the function of CK1α in Huh-7 cells
which were transfected with different strains of HCV core proteins,
virus-mediated shRNA CK1α was adopted to infect Huh-7 cells.
Compared to the MOCK group and control group, mRNA level of CK1α
was statistically decreased in the CK1α known-down group (Fig. 3A). Protein level of CK1α was in
accord with changes of mRNA (Fig.
3B). There were not obvious morphological changes of Huh-7
cells among MOCK group, NC group and CK1α knockdown group (Fig. 3C).
Taken together, we obtained the stable CK1α
knock-down Huh-7 cells. We aimed to further identify the influence
of CK1α knock-down on Huh-7 cell behaviors and the related
molecules.
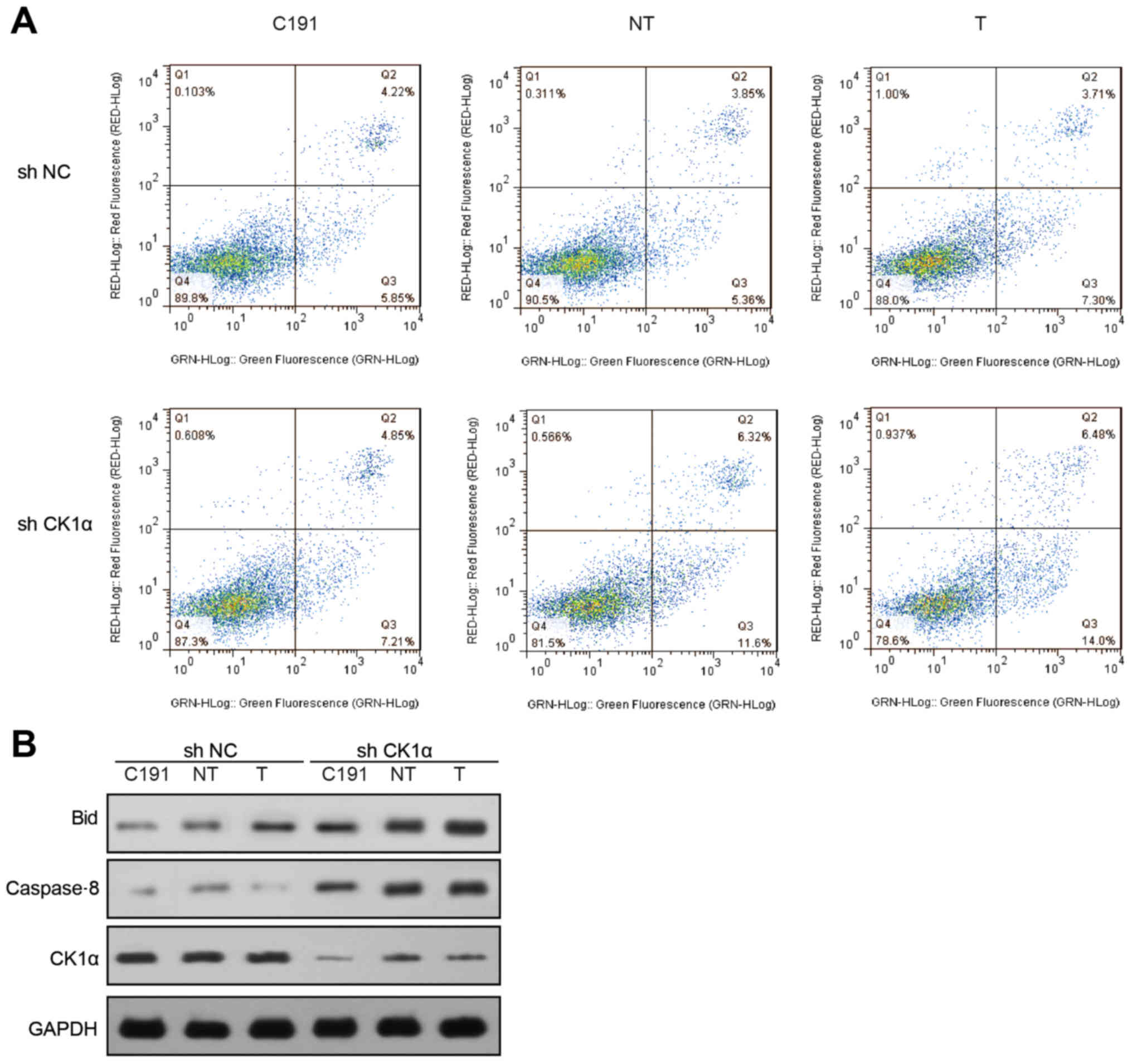
Knockdown of CK1α increased HCV core
protein-induced cell apoptosis
We transfected 3 different HCV core proteins into
non-CK1α-sileneced and CK1α-sileneced Huh-7 cells. Compared with
non-CK1α-silenced Huh-7 cells, CK1α-silenced Huh-7 cells were more
sensitive to cell apoptosis. Cell apoptotic ratio in the
non-CK1α-silenced Huh-7 cells was 14.8% (C191), 14.74% (NT) and
17.13% (T), while cell apoptosis in the CK1α-silenced group was
18.2% (C191), 24.81% (NT) and 31.11% (T), respectively (Fig. 4A).
The protein levels of Bid and caspase8 in
CK1α-silenced Huh-7 cells were notably higher than in
non-CK1α-silenced Huh-7 cells (Fig.
4B).
We could draw the conclusion that, knockdown of CK1α
upregulated HCV core protein-induced cell apoptosis. Whereas, the
corresponding molecular mechanism was still unknown.
CK1α knockdown upregulated HCV core
protein levels and TRAIL-induced cell apoptosis
Since Trail could induce virus-infected cell
apoptosis, we further tested whether HCV core protein-transfected
CK1α-sileneced Huh-7 cells were sensitive to TRAIL. Flow cytometry
revealed that, in non-CK1α-silenced Huh-7 cells transfected with 3
different HCV core protein, the cell apoptosis ratio was 24.35%
(C191), 26.6% (NT) and 31.65% (T), which was significantly lower
than that in CK1α-silenced group was 24.9% (C191), 46.6% (NT) and
50.77% (T) (Fig. 5A).
 | Figure 5.Knockdown of CK1α increased different
HCV core proteins and TRAIL-induced cell apoptosis. (A) Cell
apoptosis was tested by flow cytometry in control cells and CK1α
knockdown cells that were induced by TRAIL (A). (B) Western blot
analysis for the expression of Bid, p53 and PCNA in control and
CK1α knockdown cells. (C) CHIP-PCR amplification demonstrated
binding of p53 to the promoter regions of human Bid in Huh-7 cells.
PCR was performed in input, p21-positive controls and IgG negative
controls. (D) The quantitative PCR method was carried out for
detecting the CHIp. TRAIL, tumor necrosis factor-related
apoptosis-inducing ligand; HCV, hepatitis C virus; CK1, casein
kinase 1; CHIP-PCR, chromatin immunoprecipitation-polymerase chain
reaction; Bid, BH3 interacting-domain death agonist; NC, negative
control; T, core proteins derived from tumor tissues; NT, core
proteins derived from non-tumor tissues; C191, core proteins from
the HCV-J6 strain; PCNA, proliferating cell nuclear antigen. |
To investigate the corresponding mechanism, western
blot analysis was adopted and exhibited that protein level of Bid
in CK1α-silenced Huh-7 cells was obviously higher than in
non-CK1α-silenced Huh-7 cells which was tightly associated with HCV
core proteins of different strains. p53 that translocated to
nuclear was also increased (Fig.
5B), indicating that translocation of p53 to nuclear initiated
the transcription of Bid.
CHIp-PCR amplification demonstrated that p53 bound
to the promoter regions of human Bid gene in Huh-7 cells (Fig. 5C).
We further measured the amount of Bid promoter
precipitated by p53 in CK1α-silenced Huh-7 cells with RT-qPCR, and
found that it was significantly higher than in non-CK1α-silenced
Huh-7 cells which was also correlated with HCV core proteins of
different strains (Fig. 5D).
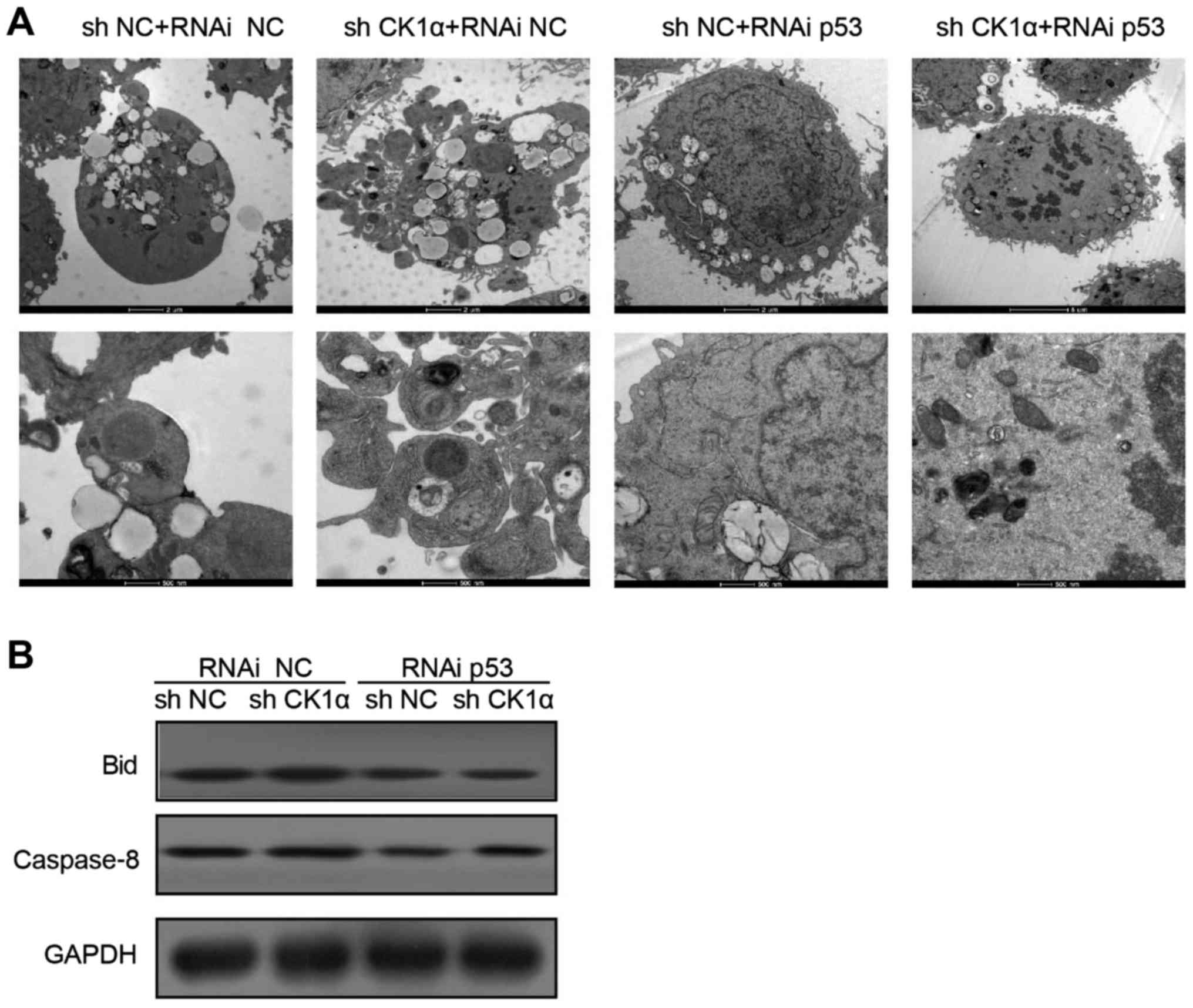
Knockdown of p53 decreased HCV core
protein and TRAIL-induced cell apoptosis
To verify the function of p53 in TRAIL-induced cell
apoptosis in the CK1α-sileneced Huh-7 cells that were transfected
with HCV core proteins, we further transfected p53 siRNA or control
siRNA into CK1α-sileneced Huh-7 cells which were transfected with T
core protein. Results were measured by electron microscope,
indicating that knockdown of p53 decreased HCV core protein and
TRAIL-induced cell apoptosis (Fig.
6A). The protein levels of Bid and caspase-8 in CK1α-silenced
Huh-7 cells that transfected with p53 siRNA were significantly
lower than in control group (Fig.
6B).
In sum, we demonstrates that HCV core proteins
sensitize host cells to TRAIL-induced cell apoptosis by activating
CK1α-P53-Bid dependent pathway.
Discussion
As the major cause of HCC and liver failure, HCV
seriously affected the health of people worldwide (1–4).
Therefore, it is urgent to explore effective treatments for HCV
infection. In the past several years, the role of HCV core proteins
in cell apoptosis has been debated, whereas, the mechanisms and
impacts of HCV core proteins in Huh-7 cell apoptosis have not been
reported yet (18). The research
data showed that HCV core proteins function as both promoter and
suppressor during the process of apoptosis. In the present study,
we aimed to provide an improved understanding of the effects of HCV
core proteins on Huh-7 cell apoptosis, and we studied 3 different
HCV strains to explore the corresponding possible mechanisms for
the first time.
As acknowledged, CK1 was an ubiquitously
serine/threonine protein kinase, furthermore, human CK1 was
reported to take part in the controlling of cell differentiation as
well as cell proliferation (19,20).
In current study, we found that all of the 3 HCV core proteins of
different strains significantly upregulated the ratio of apoptotic
Huh-7 cells, and they affected CK1α in Huh-7 cells specifically,
which were in consistent with previous studies.
As p53 acting as a direct transcriptional activator
of the apoptosis related genes (12). In current study, we also found that
knockdown of CK1α increased different HCV core protein-induced cell
apoptosis and enhanced p53 translocating to nucleus, which were in
line with a former reported research which demonstrated that CK1α
knockdown activated p53 in cultured cells thus inducing obvious
cell death (21).
Whereas, under physiological circumstances, CK1α
exhibits a more complex function during the process of tumor
development (22). A recent mouse
model with tissue-specific knockout of CK1α in the gut revealed
that CK1α depletion triggered p53 activation, induced cellular
senescence and inhibited cell invasion significantly (23). CK1α depletion, nevertheless,
likewise led to stabilization of oncoprotein β-catenin, augmented
cell proliferation and activation of DNA damage signals. The in
vivo results consequently agree with the role of CK1α in
inhibiting p53 function via interacting with MDMX, but p53
activation after CK1α depletion may affect additional mechanisms,
for instance, oncogenic stress and DNA damage signaling
pathways.
Because there were p53-binding sites in the promoter
region of Bid, consequently, the expression of Bid was modulated by
p53, and Bid participated in apoptotic processes (15,16).
Be cleaved by caspase-8, activated Bid could lead to the activation
of Bax/Bak (17). We carried out
experiments to test their expression changes, and results revealed
that during the progression of transfecting HCV core proteins, the
expression levels of both Bid and caspase-8 were obviously
upregulated. By CHIp assay, we found that the amount of Bid
promoter which was associated with HCV core proteins of different
strains was much higher in CK1α-silenced Huh-7 cells than in
non-CK1α-silenced Huh-7 cells. Different HCV core proteins of
different strains showed different abilities to induce cell
apoptosis.
As acknowledged, TRAIL induced cell apoptosis in
various transformed cells and HCV infected cells (10,11).
Therefore, TRAIL might selectively kill virus-infected cells. If
HCV core protein enhances TRAIL-induced cell apoptosis,
HCV-infected cells should preferentially be eliminated. To address
the aforementioned question, we studied the impact of TRAIL on
CK1α-silenced Huh-7 cells that were transfected with 3 different
HCV core proteins. The results showed that CK1α played a crucial
role in the aforementioned progression.
Therefore, it can be concluded from our findings
that 3 different HCV core proteins induced Huh-7 cell apoptosis via
the CK1α-p53-Bid signaling pathway, with CK1α acting as a key
factor.
Whereas, there was only one cell line used in the
present study, we will use more than one cell line as a model for
the in vitro studies to confirm results in our future
work.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by the
Natural Science Foundation of China (grant no. 81371867) and the
Grant from Jiangsu Health Administration of China (grant no.
bl-2014033).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SS and CL carried out the experiments and analyzed
the data. MD analyzed the data, and XY designed the experiments,
obtained funding and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Z, Xing WG, Zhang YH, Zhang Q, Long
XS, Zhang GY, Wu H and Jiang Y: Study on the epidemiology and HCV
genotype distribution of HIV/HCV co-infection among HIV infected
blood donors in China. Zhonghua Gan Zang Bing Za Zhi. 14:464–465.
2006.(In Chinese). PubMed/NCBI
|
|
2
|
Rekha RD, Amali AA, Her GM, Yeh YH, Gong
HY, Hu SY, Lin GH and Wu JL: Thioacetamide accelerates
steatohepatitis, cirrhosis and HCC by expressing HCV core protein
in transgenic zebrafish Danio rerio. Toxicology. 243:11–22.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colomino Iborra M, Sancho-Tell Aguilera V
and Haym Berenguer M: Development of hepatocellular carcinoma (HCC)
in peginterferon and ribavirin HCV-infected cirrhotic treated
patients. Rev Esp Enferm Dig. 98:794–795. 2006.PubMed/NCBI
|
|
4
|
Zhang L, Zhang D, Chen W, Zou X and Ling
L: High prevalence of HIV, HCV and tuberculosis and associated risk
behaviours among new entrants of methadone maintenance treatment
clinics in Guangdong Province, China. PLoS One. 8:e769312013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang C, Wu N, Liu J, Ge Q, Huang Y, Ren
Q, Feng Q and He G: HCV subtype characterization among injection
drug users: Implication for a crucial role of Zhenjiang in HCV
transmission in China. PLoS One. 6:e168172011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SK, Park SO, Joe CO and Kim YS:
Interaction of HCV core protein with 14-3-3epsilon protein releases
Bax to activate apoptosis. Biochem Biophys Res Commun. 352:756–762.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quan J, Fan XG, Hu GL, Li N and Tan DM:
The influence of HCV core protein and apoptosis on cellular
telomerase activities. Zhonghua Gan Zang Bing Za Zhi.
12:4242004.(In Chinese). PubMed/NCBI
|
|
8
|
Honda A, Hatano M, Kohara M, Arai Y,
Hartatik T, Moriyama T, Imawari M, Koike K, Yokosuka O, Shimotohno
K and Tokuhisa T: HCV-core protein accelerates recovery from the
insensitivity of liver cells to Fas-mediated apoptosis induced by
an injection of anti-Fas antibody in mice. J Hepatol. 33:440–447.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hahn CS, Cho YG, Kang BS, Lester IM and
Hahn YS: The HCV core protein acts as a positive regulator of
fas-mediated apoptosis in a human lymphoblastoid T cell line.
Virology. 276:127–137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brost S, Zimmermann A, Koschny R, Sykora
J, Stremmel W, Schirmacher P, Walczak H and Ganten TM: Hepatocyte
expression of TRAIL pathway regulators correlates with
histopathological and clinical parameters in chronic HCV infection.
Pathol Res Pract. 210:83–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yano Y, Hayashi Y, Nakaji M, Nagano H, Seo
Y, Ninomiya T, Yoon S, Wada A, Hirai M, Kim SR, et al: Different
apoptotic regulation of TRAIL-caspase pathway in HBV- and
HCV-related hepatocellular carcinoma. Int J Mol Med. 11:499–504.
2003.PubMed/NCBI
|
|
12
|
Wawryk-Gawda E, Chylinska-Wrzos P,
Lis-Sochocka M, Chłapek K, Bulak K, Jędrych M and Jodłowska-Jędrych
B: P53 protein in proliferation, repair and apoptosis of cells.
Protoplasma. 251:525–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshimura M, Ishizawa J, Ruvolo V, Dilip
A, Quintás-Cardama A, McDonnell TJ, Neelapu SS, Kwak LW, Shacham S,
Kauffman M, et al: Induction of p53-mediated transcription and
apoptosis by exportin-1 (XPO1) inhibition in mantle cell lymphoma.
Cancer Sci. 105:795–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan F, Xie Q, Wu J, Bai Y, Mao B, Dong Y,
Bi W, Ji G, Tao W, Wang Y and Yuan Z: MST1 promotes apoptosis
through regulating Sirt1-dependent p53 deacetylation. J Biol Chem.
286:6940–6945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song G, Wang W and Hu T: p53 facilitates
BH3-only BID nuclear export to induce apoptosis in the irrepairable
DNA damage response. Med Hypotheses. 77:850–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wiseman A: p53 protein or BID protein
select the route to either apoptosis (programmed cell death) or to
cell cycle arrest opposing carcinogenesis after DNA damage by ROS.
Med Hypotheses. 67:296–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fischer B, Coelho D, Dufour P, Bergerat
JP, Denis JM, Gueulette J and Bischoff P: Caspase 8-mediated
cleavage of the pro-apoptotic BCL-2 family member BID in
p53-dependent apoptosis. Biochem Biophys Res Commun. 306:516–522.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Qu A, Han X and Wang Y: HCV core
protein represses the apoptosis and improves the autophagy of human
hepatocytes. Int J Clin Exp Med. 8:15787–15793. 2015.PubMed/NCBI
|
|
19
|
Beyaert R, Vanhaesebroeck B, Declercq W,
Van Lint J, Vandenabele P, Agostinis P, Vandenheede JR and Fiers W:
Casein kinase-1 phosphorylates the p75 tumor necrosis factor
receptor and negatively regulates tumor necrosis factor signaling
for apoptosis. J Biol Chem. 270:23293–23299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Izeradjene K, Douglas L, Delaney AB and
Houghton JA: Casein kinase I attenuates tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis by
regulating the recruitment of fas-associated death domain and
procaspase-8 to the death-inducing signaling complex. Cancer Res.
64:8036–8044. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Li C, Pan Y and Chen J: Regulation
of p53-MDMX interaction by casein kinase 1 alpha. Mol Cell Biol.
25:6509–6520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gross SD, Loijens JC and Anderson RA: The
casein kinase Ialpha isoform is both physically positioned and
functionally competent to regulate multiple events of mRNA
metabolism. J Cell Sci. 112:2647–2656. 1999.PubMed/NCBI
|
|
23
|
Elyada E, Pribluda A, Goldstein RE,
Morgenstern Y, Brachya G, Cojocaru G, Snir-Alkalay I, Burstain I,
Haffner-Krausz R, Jung S, et al: CKIα ablation highlights a
critical role for p53 in invasiveness control. Nature. 470:409–413.
2011. View Article : Google Scholar : PubMed/NCBI
|