Introduction
The immune-mediated diseases including pathogens,
atherosclerosis, autoimmunity, sel-tolerance, graft-versus-host
disease (GVHD) and cancer have increased with socio-economic. As
professional antigen presenting cells (APCs), Dendritic cells (DCs)
are central regulators of innate and adaptive immune responses.
Since their discovery by Steinman RM in 1973 (1), DCs have been demonstrated as key
components in immune response directed against cancer cells,
pathogens, allergens and autoantigens (2). During the past decades, increasingly
evidences have certified that DCs activate the immune responses and
result in immune-related diseases. Atherosclerosis (AS) is a
chronic inflammatory disease of vessel wall. DCs can be observed in
arterial vessels of AS. Some studies have demonstrated that DCs can
take up lipids (3,4), control cholesterol homeostasis
(5) and enhance T-cell activation
in AS (6). Our previous studies
have addressed the role of DCs in GVHD. MicroRNA let-7i-5p
was upregulated in mature DCs (mDCs), and
let-7i-5p-inhibitor depressed the maturation of DCs
(7). In another study, we have
demonstrated that let-7i-5p regulated DCs maturation through
targeting IL-10 via the JAK1-STAT3 signal pathway (8). Moreover, transfusion
let-7i-5p-inhibitor DCs can prolong cardiac allograft
survival in a rat heart transplantation model (8).
DCs have two different functions depending on their
maturation status. MDCs with high levels of major
histocompatibility complex (MHC) and costimulatory molecules have
been identified as potent immune activators of Ag-specific immune
response, while, immature DCs (imDCs) expressed low expression of
MHC and costimulatory molecules that maintain immune homeostasis in
the steady state (9,10). IDCs recognize ‘danger’-associated
signals including pathogen-associated molecular patterns (PAMPs)
and damage-associated molecular patterns (DAMPs) through pattern
recognition receptors (PPRs), such as Toll-like receptors (TLRs)
and NOD-like receptors. IDCs response to these signals and change
to mDCs (11). MDCs show the high
status of activation and capable of promoting T cell polarization
toward to type 1 T helper (Th1), Th2, Th17 or regulatory T (Treg)
cells (7,12). Therefore, dynamic regulation of DCs
maturation is essential for controlling immune-induced
diseases.
The high-throughput platforms for analysis of gene
expression, such as gene microarray technology and high-throughput
sequence, have been used for more than ten years. These
high-throughput analyses are broadly used as effective methods for
obtaining the pathology-associated changes in the transcriptome
level. A large number of gene expression profiling studies have
been performed and covered thousands of differentially expressed
genes (DEGs) in pathways, biological process or molecular
functions. However, comparative analysis of DEGs is limited because
of tissue or sample heterogeneity in independent studies.
Therefore, the integrated bioinformatics methods combining with
different expression profiling are used to identify hub genes in
many diseases. The integrated bioinformatics methods are widely
performed in cancer. Guo et al (13), found that potential candidate
biomarkers for diagnosis, drug targets and prognosis in colorectal
cancer. Dong et al (14),
analyzed two expression profiles and explored five hub genes as
critical biomarkers of osteosarcoma. However, the interactions
among DEGs and the pathways in DCs remain unclear.
In this investigation, we assayed four microarray
datasets including GSE52894, GSE72893, GSE75938 and GSE77969 from
Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/), from which there
are total of 14 mDCs samples and 14 imDCs samples available. Gene
expression profiles of mDCs were compared with imDCs to identify
the DEGs. Subsequently, the DEGs were screened using DAVID
Bioinformatics Resources 6.8 for Gene Ontology (GO) and pathway
enrichment analysis. Furthermore, we identified ten hub genes
(ISG20, IFITM1, HLA-F, IRF1, USP18, IFI44L, GBP1, IFI35, IFI27,
IFI6) through protein-protein interaction (PPI) and modular
analysis. Our results suggested that data mining and integration
could be a useful method to understand the mechanism and
development of DCs in immune-induced diseases.
Materials and methods
Microarray data information
The gene expression profiles of GSE52894, GSE72893,
GSE75938 and GSE77969 were downloaded from GEO (https://www.ncbi.nlm.nih.gov/geo/geo2r/). The gene
expression profiles GSE52894 were measured with GPL10558 platforms
(Illumina HumanHT-12 v4.0 expression beadchip) and included sixteen
samples from four monDCs types (immature, mature, tolerogenic and
LPS-tolerogenic). The microarray data of GSE72893 was based on
GPL10558 platforms (Illumina HumanHT-12 v4.0 expression beadchip)
and consisted of four imDCs samples, four mature DCs samples and
two Treg-conditioned DCs samples. GSE75938 was based on GPL15207
platforms (Affymetrix Human Gene Expression Array). The GSE75938
dataset contained 14 samples, including three sets of monocytes,
derived imDCs and macrophage, mature DCs and macrophage. GSE77969
which was based on GPL13667 platforms (Affymetrix Human Genome U219
Array) consisted of three imDCs samples and three mature DCs
samples. We chose imDCs and mature DCs samples from these 4
datasets for integrated analysis in the present study.
Identification of DEGs
GEO2R (www.ncbi.nlm.nih.gov/geo/geo2r/) is an interactive web
tool in order to identify genes that are differentially expressed
across experimental conditions using the GEOquery and limma R
packages from the Bioconductor project. We screened DEGs between
imDCs and mature DCs in these four datasets by GEO2R. The P-value
was adjusted for the correction of false positive results when
using the Benjamini and Hochberg false discovery rate method by
default. According to other studies, we used log transformation to
identify DEGs with adjusted P-value <0.01 and |logFC|>1.
Gene ontology and pathway enrichment
analysis
The GO and Kyoto Encyclopedia of Genes and Genomes
(KEGG) of candidate DEGs were analyzed by DAVID Bioinformatics
Resources 6.8 (david.ncifcrf.gov//tools.jsp). DAVID is a website for
high-throughput functional annotation analysis. A P-value <0.05
was set as the cut-off criterion.
PPI network and module selection
We used search tool for the retrieval of interacting
genes 10.5 (STRING, https://string-db.org/) to evaluate the interactive
relationships among DEGs. The combined score >0.4 was set as the
cut-off criterion. PPI networks were constructed and analyzed using
Cytoscape v3.5.1 software. The plug-in molecular complex detection
(MCODE) was performed to screen modules of PPI network in
Cytoscape. The criteria were set as follows: degree cutoff=2, node
score cutoff=0.2, k-core=2, max. depth=100. MCODE scores >3 and
number of nodes >4.
Generation of human monocyte-derived
DCs
Human peripheral blood was isolated from healthy
volunteers by buffy coats and DCs were generated as previously
described (15). Briefly,
peripheral blood mononuclear cells (PBMCs) were isolated using
Ficoll-Hypaque centrifugation (TBDscience, China) according to the
manufacturer's instructions. The project was approved by the
Clinical Research Ethics Committee of the Second Affiliated
Hospital of Harbin Medical University and written informed consent
was obtained from all volunteers. PBMCs were seeded at a
concentration of 6×106 cells per well in RMPI 1640
(Hyclone; GE Healthcare, Chicago, IL, USA) media with 10% fetal
bovine serum (ScienCell Research Laboratories, Inc., San Diego, CA,
USA), penicillin (100 U/ml; Beyotime Institute of Biotechnology,
Haimen, China) and streptomycin (100 ug/ml; Beyotime Institute of
Biotechnology) for 2 h at 37°C. Then, non-adherent cells were
removed by washing. The adherent cells were cultured with human
recombinant IL-4 (50 ng/ml; PeproTech, Inc., Rocky Hill, NJ, USA)
and GM-CSF (100 ng/ml; PeproTech, Inc.) in order to obtain immature
monocyte-derived dendritic cells (Mo-DCs). The half of medium was
changed every other day. Cells were stimulated with
lipopolysaccharides (LPS) (200 ng/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) on 6 days for 24 h for inducing maturation
DCs.
Reverse transcription-quantitative
transcriptase polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagents (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and the firststrand complementary DNAs (cDNAs) were
synthesized with a Transcriptor First Strand cDNA Synthesis kit
(Roche Diagnostics, Basel, Switzerland) according to the
manufacturer's instructions. The RT reactions were carried out for
60 min at 50°C and 5 min at 85°C. The PCR protocol consisted of 40
cycles of 10 sec at 95°C, 30 sec at 60°C and 30 sec at 72°C using
Bestar Sybr-Green qPCR Master Mix (DBI Bioscience, Germany). All
reactions were measured in triplicate. The primers used for RT-qPCR
were in Table I. The expression of
hug genes relative to ACTH were analyzed using the 2-ΔΔCq
method. All the data were given in terms of relative mRNA
expression level as means ± SD (standard deviation). P<0.05 was
considered to indicate a statistically significant difference.
 | Table I.List of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
List of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Name | Sense sequence
(5′-3′) | Antisense sequence
(5′-3′) |
|---|
| ISG20 |
GCTTGCCTTTCAGGAGCTG |
ATCACCGATTACAGAACCCG |
| IFITM1 |
CCTCTTCTTGAACTGGTGCTGTCTG |
CGTCGCCAACCATCTTCCTGTC |
| HLA-F |
TGATCTCCGCAGGGTAGAAG |
AATGGGAAGGAGACGCTACA |
| IRF1 |
ATCCTTGTTGATGTCCCAGC |
GACCCTGGCTAGAGATGCAG |
| USP18 |
TCAGGACAGCACGACTTCAC |
CGGAACTTCGGTCCCAG |
| IFI44L |
TTCCATGTCAATCTTGTTGTCAC |
TTTCTGTCTCCAAACCGTGG |
| GBP1 |
GCAGAACTAGGATGTGGCCT |
AACAAGCTGGCTGGAAAGAA |
| IFI35 |
CCCACAGCCTCATCTTGAGT |
TCTGAAGCCTCAGCTCTTGC |
| IFI27 |
CCACAACTCCTCCAATCACA |
GCCTCTGCTCTCACCTCATC |
| IFI6 |
GTGGCAGCAGCGTCGTCATAG |
GGCTACTCCTCATCCTCCTCACTATC |
| ACTB |
CGTGGACATCCGCAAAGA |
GAAGGTGGACAGCGAGGC |
MicroRNA regulatory analysis
MiRTarBase is the experimentally validated
microRNA-target interactions database. We used miRTarBase release
6.0 to identify target genes among DEGs specifically for important
microRNAs in DCs. The microRNA-targets regulatory network was
constructed for DCs by Cytoscape.
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc., La Jolla,
CA, USA) was used to analyze the data. All data are expressed as
the mean ± standard deviation. For three independent experiments, a
two-tailed Student's t-test was used to evaluate the differences
between imDC and mDC. P<0.05 was considered to indicate a
statistically significant difference
Results
Identification of DEGs in mDCs
The raw data file of GES52894, GSE72893, GSE75938
and GSE77969 were uploaded to GEO2R to screen DEGs between immature
and mature DCs. 14 imDCs samples and 14 mDCs samples were analyzed.
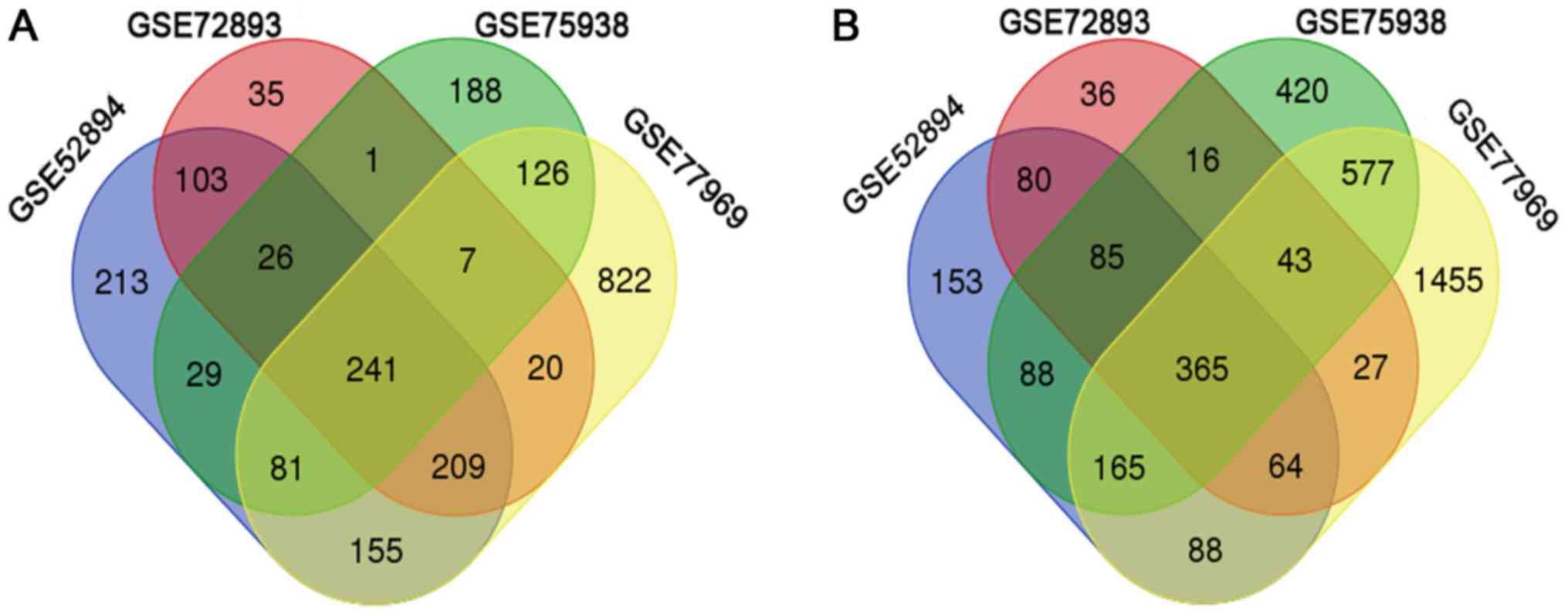
A total of 1057, 642, 699 and 1661 DEGs were up-regulated in the
GES52894, GSE72893, GSE75938 and GSE77969 datasets (Fig. 1A). In addition, 1088, 716, 1759 and
2784 DEGs were down-regulated in GES52894, GSE72893, GSE75938 and
GSE77969 datasets, respectively (Fig.
1B). After integrated bioinformatical analysis, 596 DEGs were
identified from the four profile datasets (data not shown),
including 241 upregulated genes and 365 downregulated genes in the
mDCs compared to imDCs (Fig.
1).
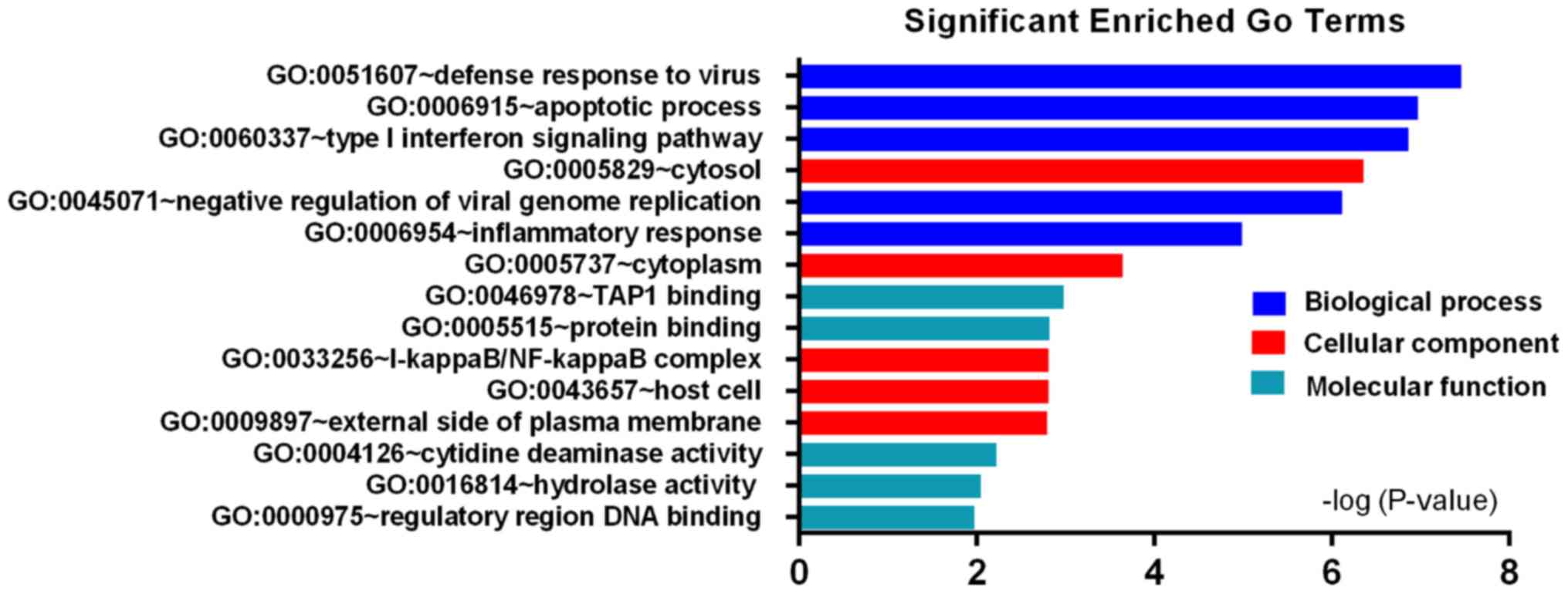
DEGs gene ontology enrichment
analysis
To explore the possible functional annotation and
pathway enrichment of DEGs, we respectively uploaded the
upregulated genes and downregulated genes to online software DAVID.
The top five terms enriched were selected in each category
according to P-value. GO biological processes (BP) analysis showed
that the upregulated DEGs were significantly enriched in defense
response to virus, apoptotic process, type I interferon signaling
pathway, negative regulation of viral genome replication and
inflammatory response (Fig. 2),
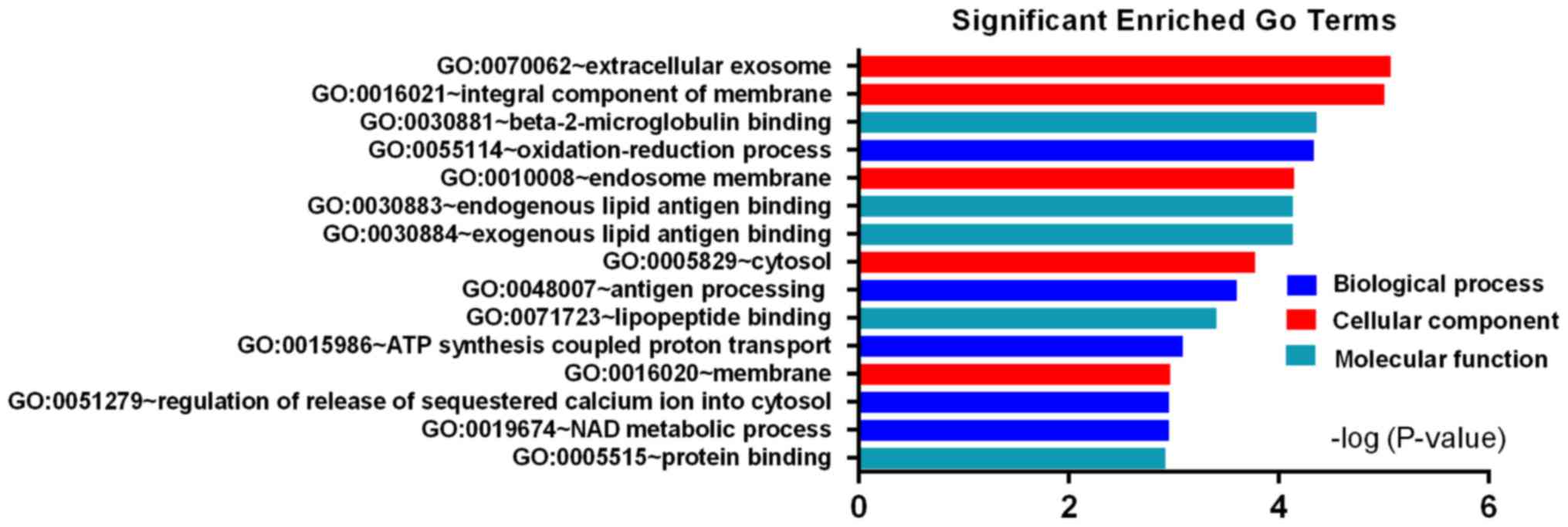
while the downregulated DEGs were enriched in oxidation-reduction
process, antigen processing, ATP synthesis coupled proton
transport, regulation of release of sequestered calcium ion into
cytosol and NAD metabolic process (Fig. 3). For cell component (CC), the
upregulated DEGs were enriched in cytosol, cytoplasm,
I-kappaB/NF-kappaB complex, host cell and external side of plasma
membrane (Fig. 2); The
downregulated DEGs were enriched in extracellular exosome, integral
component of membrane, endosome membrane, cytosol and membrane
(Fig. 3). In addition, the
molecular function (MF) of upregulated DEGs were mainly associated
with TAP1 binding, protein binding, cytidine deaminase activity,
hydrolase activity and regulatory region DNA binding (Fig. 2), while the downregulated DEGs were
involved in beta-2-microglobulin binding, endogenous and exogenous
lipid antigen binding, lipopeptide binding and protein binding
(Fig. 3). These results showed
that the DEGs were involved in the regulation of immune
response.
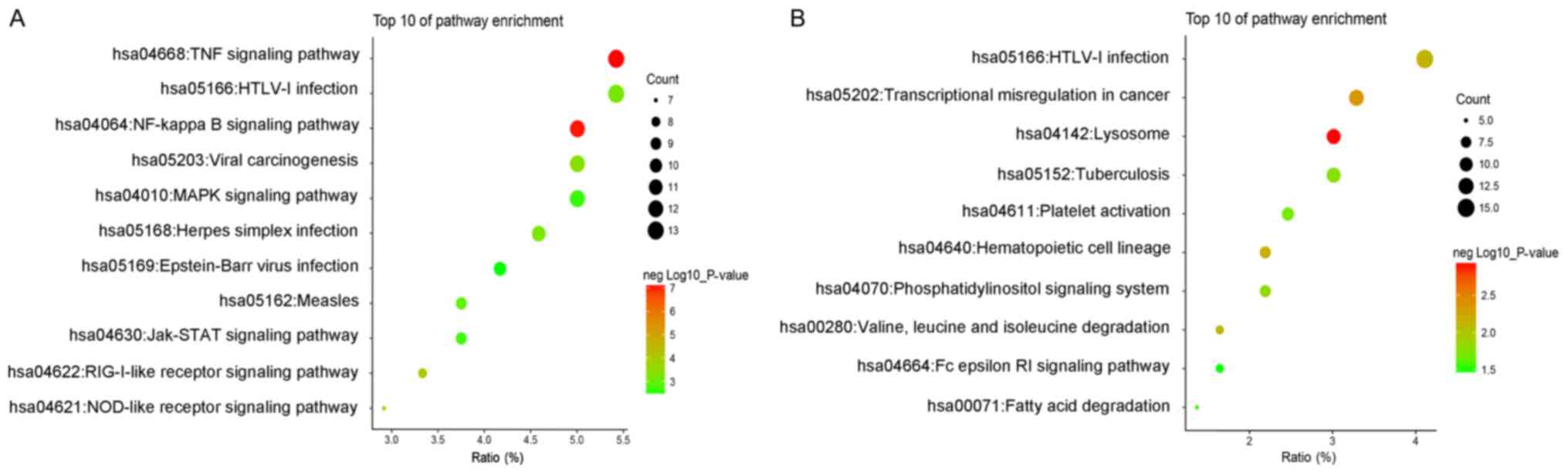
KEGG pathway analysis
The most significantly enriched pathways of the
upregulated and downregulated DEGs were shown in Fig. 4. The upregulated DEGs mainly
enriched in TNF signaling pathway, NF-kappa B signaling pathway,
RIG-I-like receptor signaling pathway, NOD-like receptor signaling
pathway and viral carcinogenesis (Fig.
4A). The down-regulated DEGs were enriched in lysosome,
transcriptional misregulation in cancer, hematopoietic cell
lineage, HTLV–I infection and valine, leucine and isoleucine
degradation (Fig. 4B). These
enrichment analysis suggested that the regulation and function of
DEGs was mainly in response to inflammatory.
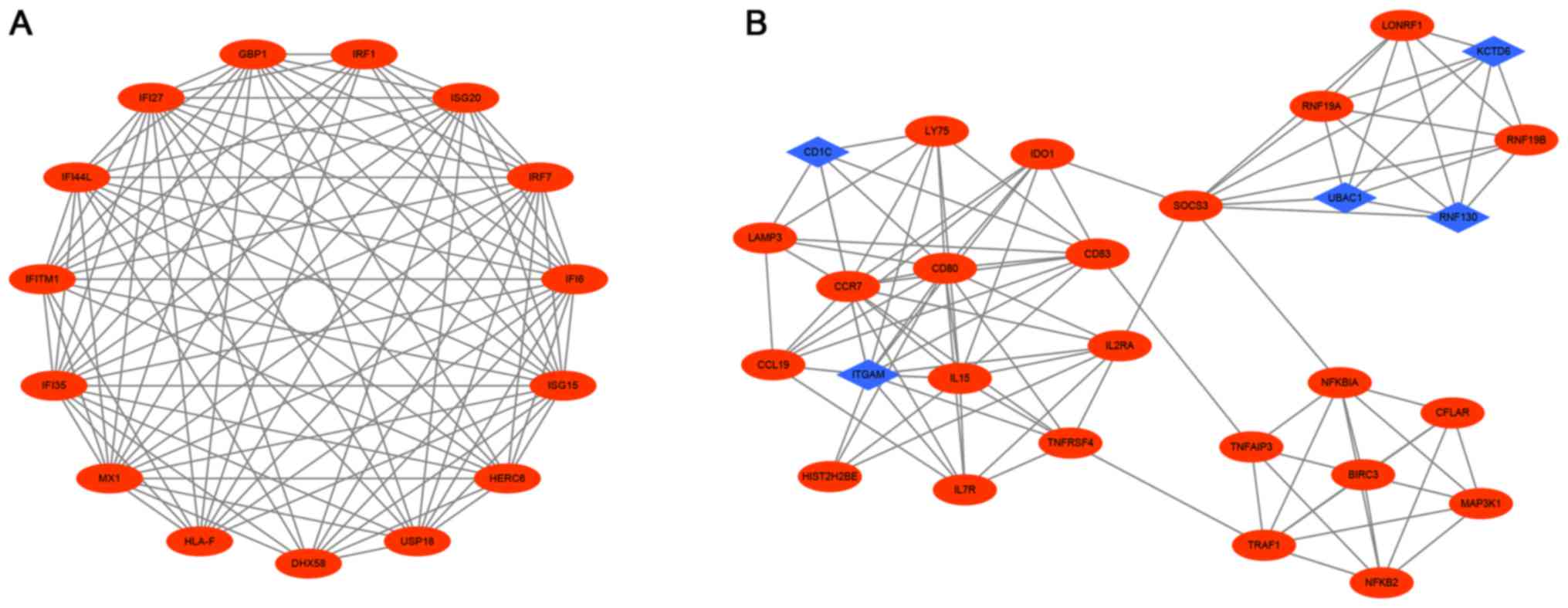
PPI network construction and modules
selection
To understand cellular functions and biological
processes, we constructed the PPI network of DEGs using STRING and
Cytoscape software. The PPI network consisted of 606 nodes and 1632
edges (data not shown). The top 10 hub genes with higher degrees
were screened including ISG20, IFITM1, HLA-F, IRF1, USP18,
IFI44L, GBP1, IFI35, IFI27, IFI6.Then, we verified the
expression of the top 10 hub genes in mDCs by RT-qPCR. These 10 hub
genes were upregulated in mDCs compared with imDCs (Fig. 5A-J). Moreover, the top 2
significant modules were selected from the DEGs PPI network using
plug-ins MCODE (Fig. 6A and B).
Module 1 included 15 nodes and 93 edges and module 2 had 28 nodes
and 101 edges. Furthermore, functional and pathway enrichment
analysis of genes in these two modules were performed using DAVID.
The results showed that Module 1 were mainly associated with type I
interferon signaling pathway, defense response to virus, regulatory
region DNA binding and RIG-I-like receptor signaling pathway, while
genes in Module 2 were mainly enriched in inflammatory response,
apoptotic process, immune response, NF-kappa B signaling pathway
and TNF signaling pathway (Table
II). These results indicate that the hub genes which we
screened from the PPI network play an important role in the immune
response of DCs.
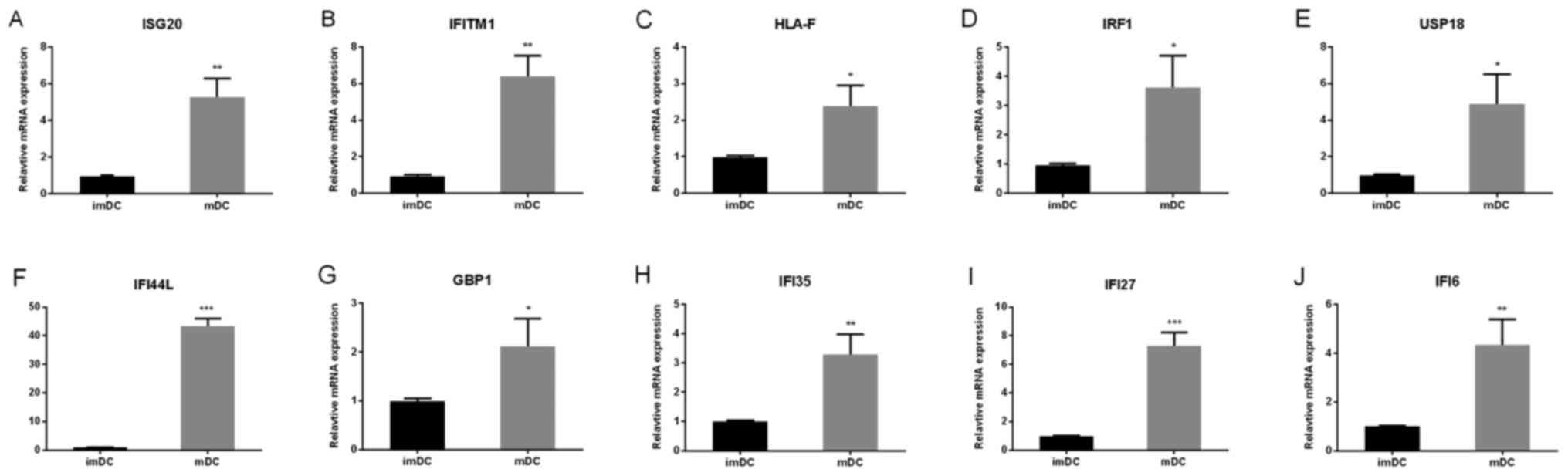 | Figure 5.Top 10 hub genes were detected by
reverse transcription-quantitative polymerase chain reaction. The
expression of the top 10 hub genes, (A) ISG20, (B) IFITM1, (C)
HLA-F, (D) IRF1, (E) USP18, (F) IFI44L, (G) GBP1, (H) IFI35, (I)
IFI27 and (J) IFI6 were upregulated in mDCs. The data are presented
as the mean ± standard deviation of 3 independent experiments.
*P<0.05, **P<0.01 and ***P<0.001, vs. the imDCs group.
DCs, dendritic cells; mDCs, mature DCs; imDCs, immature DCs; ISG20,
interferon-stimulated gene of 20 kDa protein; IFITM1, interferon
induced transmembrane protein 1; HLA-F, human leukocyte antigen F;
IRF1, interferon regulatory factor 1; USP18, ubiquitin-specific
peptidase 18; IFI44L, interferon-induced protein 44-like; GBP1,
Guanylate-binding protein 1; IFI35, interferon-induced protein 35;
IFI27, interferon-a-inducible protein 27; IFI6,
interferon-α-inducible protein 6. |
 | Table II.Functional and pathway enrichment
analysis of the genes in modules. |
Table II.
Functional and pathway enrichment
analysis of the genes in modules.
| Category | Term | Gene function | Gene count | P-value |
|---|
|
|---|
| A, Module 1 |
|---|
|
|---|
|
GOTERM_BP_DIRECT | GO:0060337 | Type I interferon
signaling pathway | 10 |
1.86×1019 |
|
GOTERM_BP_DIRECT | GO:0051607 | Defense response to
virus | 8 |
2.52×1011 |
|
GOTERM_BP_DIRECT | GO:0009615 | Response to
virus | 5 |
1.66×106 |
|
GOTERM_BP_DIRECT | GO:0045071 | Negative regulation
of viral genome replication | 4 |
4.48×106 |
|
GOTERM_BP_DIRECT | GO:0060333 |
Interferon-γ-mediated signaling
pathway | 4 |
2.55×105 |
|
GOTERM_CC_DIRECT | GO:0005829 | Cytosol | 8 | 0.006784 |
|
GOTERM_MF_DIRECT | GO:0000975 | Regulatory region
DNA binding | 2 | 0.00991 |
|
GOTERM_MF_DIRECT | GO:0005525 | GTP binding | 3 | 0.03921 |
| KEGG_PATHWAY | hsa04622 | RIG-I-like receptor
signaling pathway | 3 |
9.92×104 |
|
| B, Module
2 |
|
|
GOTERM_BP_DIRECT | GO:0006954 | Inflammatory
response | 8 |
1.70×106 |
|
GOTERM_BP_DIRECT | GO:0006915 | Apoptotic
process | 8 |
2.38×105 |
|
GOTERM_BP_DIRECT | GO:0006955 | Immune
response | 7 |
4.54×105 |
|
GOTERM_BP_DIRECT | GO:0032735 | Positive regulation
of interleukin-12 production | 3 |
7.30×104 |
|
GOTERM_BP_DIRECT | GO:0010803 | Regulation of tumor
necrosis factor-mediated signaling pathway | 3 | 0.001053 |
|
GOTERM_CC_DIRECT | GO:0009897 | External side of
plasma membrane | 5 |
2.22×104 |
|
GOTERM_CC_DIRECT | GO:0005829 | Cytosol | 12 | 0.003709 |
|
GOTERM_CC_DIRECT | GO:0009986 | Cell surface | 5 | 0.006877 |
|
GOTERM_CC_DIRECT | GO:0005737 | Cytoplasm | 14 | 0.017334 |
|
GOTERM_MF_DIRECT | GO:0004842 | Ubiquitin-protein
transferase activity | 6 |
1.05×104 |
|
GOTERM_MF_DIRECT | GO:0016874 | Ligase
activity | 6 |
6.21×104 |
|
GOTERM_MF_DIRECT | GO:0008270 | Zinc ion
binding | 8 | 0.001191 |
|
GOTERM_MF_DIRECT | GO:0031624 | Ubiquitin
conjugating enzyme binding | 2 | 0.044943 |
| KEGG_PATHWAY | hsa04064 | NF-κB signaling
pathway | 7 |
7.96×108 |
| KEGG_PATHWAY | hsa04668 | TNF signaling
pathway | 7 |
2.61×107 |
| KEGG_PATHWAY | hsa04060 | Cytokine-cytokine
receptor interaction | 6 |
3.10×104 |
| KEGG_PATHWAY | hsa04640 | Hematopoietic cell
lineage | 4 | 0.00151 |
| KEGG_PATHWAY | hsa05166 | HTLV-I
infection | 5 | 0.004594 |
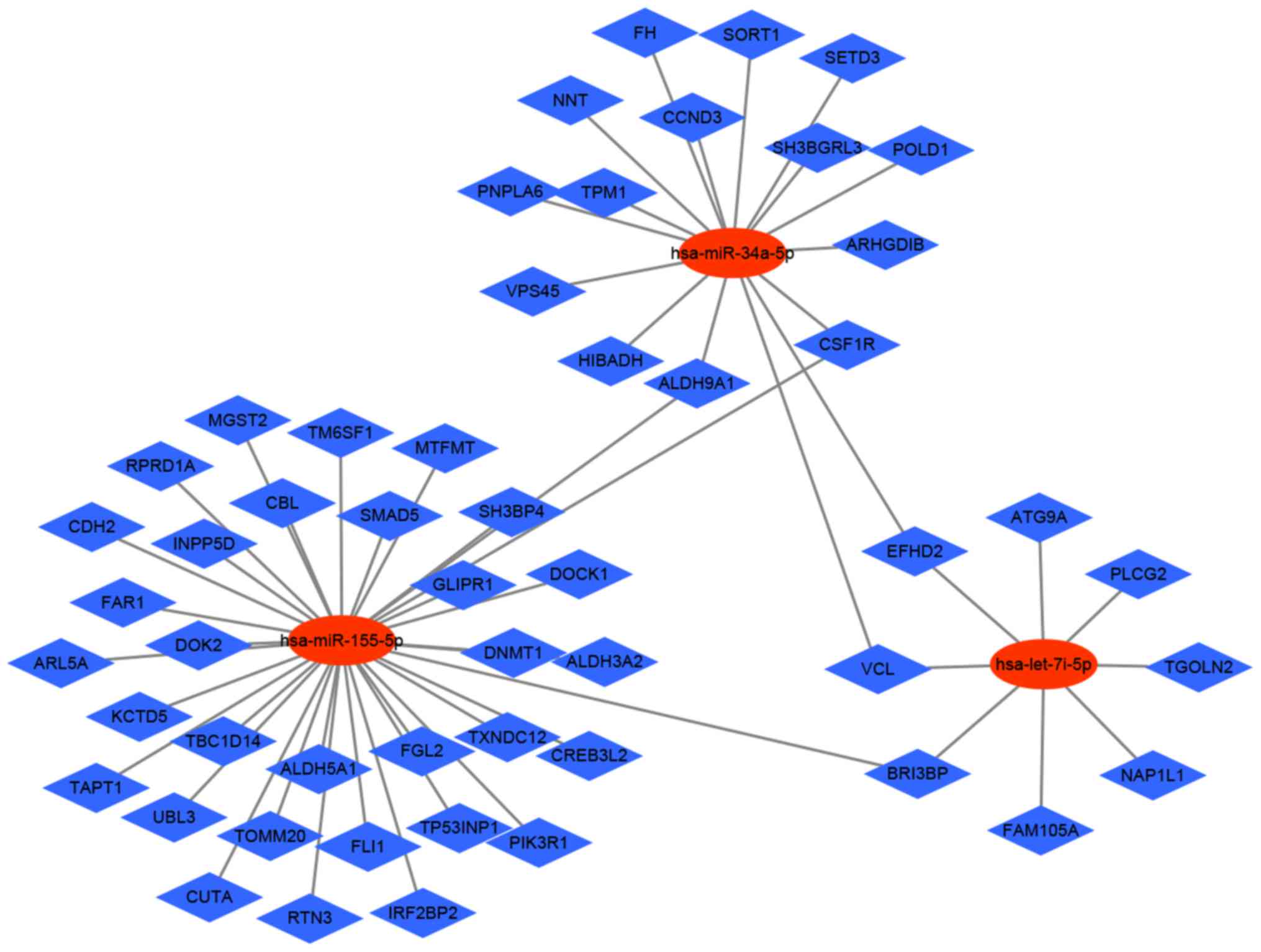
MicroRNA regulatory network
analysis
MicroRNAs (miRNAs) inhibit gene expressing by
binding to complementary 3′-untranslated regions of mRNA and
degrading specific target mRNA. A growing body of evidence has
demonstrated that miRNAs play an important role in the immune
response of DCs (16). In a
previous study, we demonstrated that let-7i-5p depress
maturation of DCs via targeting SOCS1 (7). MiR-155-5p is a key negative
regulator controlling IL-1β and other inflammatory cytokines
produced during activation of DCs (17). Several studies have demonstrated
that miR-34a-5p impact on the maturation of DCs (18,19).
Therefore, we selected let-7i-5p, miR-155-5p and
miR-34a-5p for further analysis. We identified miRNAs-mRNA
network from the downregulated DEGs because miRNAs suppress the
target gene expression. The miRNAs-DEGs regulatory network of mDCs
included 63 pairs of regulatory relationship combined with 3 miRNAs
and 58 regulatory genes (Fig. 7).
Through the construction of miRNAs-DEGs network, we screened the
DEGs can provide new ideas for the treatment of immune response in
DCs.
Discussion
Many diseases, such as atherosclerosis, GVHD,
cancer, rheumatic diseases, are caused by abnormal function of the
immune system. DCs play a central role in immune-induced diseases,
the dynamic control state of DCs maturation may be a key factor for
these diseases. Therefore, understanding the regulation mechanism
of DCs maturation is essential to intervene the progress of
diseases. Numerous studies have been conducted to reveal the
mechanism of DCs maturation, but most studies focus on a single
genetic event. In the present study, we screened four gene
expression profiles (GSE52894, GSE72893, GSE75938 and GSE77969) and
deeply analyzed these datasets using bioinformatics methods. A
total of 596 DEGs were identified, consisting of 241 upregulated
genes and 365 downregulated genes in mDCs. Function annotation and
KEGG pathway enrichment analysis showed that the upregulated DEGs
were mainly enriched in apoptotic process, type I interferon
signaling pathway, inflammatory response, TNF signaling pathway and
NF-kappa B signaling pathway; while the downregulated DEGs were
mainly involved in oxidation-reduction process, antigen processing,
regulation of release of sequestered calcium ion into cytosol,
lysosome and HTLV–I infection. This is consistent with the
knowledge that the NF-kappa B signaling pathway, TNF signaling
pathway and type I interferon signaling pathway are the main
pathway for mDCs (20–24). These results indicated that the
DEGs were mainly focused on the immune response. Therefore,
monitoring these signaling pathways may suppress the maturation of
DCs.
Based on the PPI network, we finally identified 10
hub genes: ISG20, IFITM1, HLA-F, IRF1, USP18, IFI44 L, GBP1,
IFI35, IFI27 and IFI6. They were maybe the therapeutic
molecular targets for immune-induced diseases. ISG20 was
interferon stimulated exonuclease gene 20 which is involved in
defense response to virus and type I interferon signaling pathway.
DCs play a crucial role in presenting viral antigens and inducing
adaptive immune responses that eliminate the virus (25). Most studies have demonstrated that
ISG20 can suppress virus replication. Leong et al
(26), reported that ISG20
can selectively degrade HBV RNA and blocks replication of
infectious HBV particles. It has also demonstrated that
ISG20 may represent a maker in chronic hepatitis B patients,
which was associated with a favorable response to IFN-α therapy
(27). Moreover, Zahoor et
al (28) reported that
ISG20 was upregulated in mDCs treated with HIV-1 Vpr. Thus,
we supposed that the ISG20 may play an important role in
immune-induced diseases. The second hub gene interferon induced
transmembrane protein 1 (IFITM1) is a member of the
IFN-inducible transmembrane protein family. IFITM1 controls
proliferation, homotypic adhesion in lymphocytes and metastasis
(29,30). Overexpression of IFITM1 can
negatively regulate cell growth (31). A wide range of viruses can be
inhibited by IFITM1 through immune responses, such as
hepatitis C virus, hepatitis B virus, H5N1 virus and HIV (32). Zhang et al (33), reported that IFITM1 was
significantly increased in human mo-DCs treated with dengue virus.
It has also reported that IFITM1 was one of the up-regulated
interferon-inducible antiviral proteins in LPS-stimulation DCs
(34). These results suggested
that IFITM1 in this study induced maturation of DCs. Human
leukocyte antigen F (HLA-F), a non-classical MHC molecule,
is expressed on proliferating lymphoid and monocyte cells as a
protective molecule in a novel pathway for Ag cross-presentation
(35,36). Goodridge et al (35), showed that HLA-F may act in
an immunoregulatory capacity centered on inflammatory response.
HLA-F were up-regulated in many immune-induced diseases,
such as coronary heart disease (37), SLE (38). Intriguingly, our study for the
first time has documented that HLA-F was up-regulated during
maturation of DCs. However, the precise function of HLA-F in
DCs remains elusive, we need to completely decipher the mechanism
of HLA-F in DCs. Interferon regulatory factor 1
(IRF-1), a kind of transcription factors, is the first
discoverable member of the interferon regulatory factor family
(39). The level of IRF-1
was regulated by various stimuli including IFN (type I and II),
cytokines, double-stranded RNA. IRF-1 plays an important
role in many physiological and pathological aspects, such as
proinflammatory injury, development of immune system, autoimmunity
and viral infection (39). The
tick-borne encephalitis virus, a leading cause of viral
encephalitis, can inhibit DCs maturation by diminished the protein
of IRF-1 and nuclear localization (23). NF-κB activated by TNF-α could
increase the expression of IRF-1 and induced the maturation
of DCs marker CD25, CD40 (40).
Interestingly, IRF-1 can regulate the expression of other
hub genes, ISG20, USP18 and GBP1 (41–43).
Thus, IRF-1 may exert an enormous function on regulating
maturation of DCs. Ubiquitin-specific peptidase 18 (USP18),
a member of USP family, is a negative regulator of type I and type
III interferon signaling (44).
USP18 regulates various immunological processes, including
autoimmune diseases, pathogen control and cancer development. Cong
et al (45), demonstrated
that USP18 can promote DCs development. USP18−/− mice
bone marrow-derived DCs was reduced because of high expression of
GM-CSF signaling inhibitors SOCS1/SOCS3 (45). In addition, lack of USP18
reduced the number of DCs and enhanced the expression of MHC I and
the costimulatory molecular CD80 (46). Guanylate-binding protein 1
(GBP1) was associated with the control of immune innate
response to foreign antigens (47). Thomas has demonstrated that
GBP1 DNA was isolated from a human genomic library and
mapped to human chromosome 1 (48). Kim et al (47), showed that GBP1 contributed
to vascular dysfunction in chronic inflammatory diseases by
inhibiting the proliferation and migration of endothelial
progenitor cells. GBP1 was up-regulated in stimulated T-cell
treated with phytohaemagglutinin (49). GBP1, a classical mature DCs
biomarker, was up-regulated throughout maturation of DCs (50). The mechanism of USP18 and
GBP1 in maturation DCs remains unknown.
In the present study, we found that four interferon
inducible proteins, IFI44L, IFI35, IFI27 and IFI6,
were up-regulated in mDCs. IFI44L, IFI35, IFI27 and
IFI6 were key regulatory targets in immune-induced diseases,
such as SLE, rheumatoid arthritis and myelofibrosis (51–53).
Interestingly, IFI44L was the highest up-regulated genes
among the ten hub genes validated by RT-qPCR. Aida demonstrated
that IFI44L and IFI27 were up-regulated in human DCs
treated with HIV-1 Vpr (28). In
other studies, IFI27 was up-regulated in DCs with different
stimuli (54,55). However, the biological function of
IFI35 and IFI6 in DCs were unclear. Thus, further
researches are needed to understand the mechanism of four
interferon inducible proteins in DCs. Taken together, these data
suggested that these ten hub genes were up-regulated and highly
connected. The hub genes may be involved in the regulation of
maturation of DCs via the checkpoint mechanism.
Module analysis of the PPI network revealed that
module 1 contained the ten hub genes, suggesting that these ten hub
genes had close interaction and together determined the key pathway
during the maturation of DCs. Functional and pathway enrichment
analysis of genes in module 1 and 2 was mainly involved in type I
interferon signaling, NF-kappa B signaling pathway, TNF signaling
pathway. Studies have demonstrated that type I interferon
signaling, NF-kappa B signaling pathway, TNF signaling pathway play
an important role in mDCs (56–58).
Thus, we propose that these molecular pathways in mDCs might
represent the promising candidates for pharmacologic evaluation and
for therapeutic intervention in immune-induced diseases.
In conclusion, we have identified 596 DEGs, 606
nodes, 1632 edges and 10 hub genes by multiple cohorts profile
datasets and integrated bioinformatical analysis in maturation DCs.
The 10 hub genes significantly enriched in several pathways,
including type I interferon signaling, NF-kappa B signaling
pathway, TNF signaling pathway. Our study provided a set of
candidate target genes for future investigation into the molecular
mechanisms and biomarkers of mDCs. These findings could
significantly improve our understanding of DCs in immune-induced
diseases.
Acknowledgements
The authors also would like to thank Dr. Yuanyuan
Wei (China Pharmaceutical University, Jiangsu, China) for the
excellent technical support and for critically reviewing the
manuscript.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81670373, 81330033,
81670459 and 81771946), The Natural Science Foundation of
Heilongjiang Province of China (grant no. H2015048), and Key
Laboratory of Myocardial Ischemia, Harbin Medical University,
Ministry of Education, Heilongjiang Province, China (grant nos.
KF201715, KF201716 and KF201717).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and JW and BY conceived and designed the study;
SL, ML, HZ and QY performed the experiments; and YZ, XZ, YS and MZ
analyzed the data. YZ and XZ wrote the paper, and YS, JW and BY
revised the manuscript and gave final approval of the version to be
published.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Research Ethics Committee of the Second Affiliated Hospital of
Harbin Medical University (Heilongjiang, China), and written
informed consent was obtained from all participants.
Consent for publication
Written informed consent was obtained from all
volunteers for the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DCs
|
dendritic cells
|
|
mDCs
|
mature DCs
|
|
imDCs
|
immature DCs
|
|
DEGs
|
differentially expressed genes
|
|
GVHD
|
graft-versus-host disease
|
|
APCs
|
antigen presenting cells
|
|
AS
|
atherosclerosis
|
|
MHC
|
major histocompatibility complex
|
|
PAMPs
|
pathogen-associated molecular
patterns
|
|
DAMPs
|
damage-associated molecular
patterns
|
|
PPRs
|
pattern recognition receptors
|
|
TLRs
|
toll-like receptors
|
|
Treg
|
regulatory T
|
|
NPC
|
nasopharyngeal carcinoma
|
|
GEO
|
gene expression omnibus
|
|
GO
|
gene ontology
|
|
PPI
|
protein-protein interaction
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
STRING
|
Search Tool for the Retrieval of
Interacting Genes
|
|
MCODE
|
Molecular Complex Detection
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
Mo-DCs
|
monocyte-derived dendritic cells
|
|
LPS
|
lipopolysaccharides
|
|
miRNAs
|
microRNAs
|
References
|
1
|
Steinman RM and Cohn ZA: Identification of
a novel cell type in peripheral lymphoid organs of mice. I.
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Austyn JM: Dendritic cells in the immune
system-history, lineages, tissues, tolerance and immunity.
Microbiol Spectr. 4:2016.doi: 10.1128/microbiolspec. PubMed/NCBI
|
|
3
|
Gautier EL, Huby T, Saint-Charles F,
Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum
JL, Chapman MJ and Lesnik P: Conventional dendritic cells at the
crossroads between immunity and cholesterol homeostasis in
atherosclerosis. Circulation. 119:2367–2375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stoneman V, Braganza D, Figg N, Mercer J,
Lang R, Goddard M and Bennett M: Monocyte/macrophage suppression in
CD11b diphtheria toxin receptor transgenic mice differentially
affects atherogenesis and established plaques. Circ Res.
100:884–893. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paulson KE, Zhu SN, Chen M, Nurmohamed S,
Jongstra-Bilen J and Cybulsky MI: Resident intimal dendritic cells
accumulate lipid and contribute to the initiation of
atherosclerosis. Circ Res. 106:383–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansson GK and Hermansson A: The immune
system in atherosclerosis. Nat Immunol. 12:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M, Liu F, Jia H, Zhang Q, Yin L, Liu
W, Li H, Yu B and Wu J: Inhibition of microRNA let-7i depresses
maturation and functional state of dendritic cells in response to
lipopolysaccharide stimulation via targeting suppressor of cytokine
signaling 1. J Immunol. 187:1674–1683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Jin X, Liu X, Liu X, Zhang M, Liu
W, Li Z, Han N, Tan M, Chi D, et al: MicroRNA let-7i regulates
dendritic cells maturation targeting interleukin-10 via the Janus
kinase 1-signal transducer and activator of transcription 3 signal
pathway subsequently induces prolonged cardiac allograft survival
in rats. J Heart Lung Transplant. 35:378–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Audiger C, Rahman MJ, Yun TJ, Tarbell KV
and Lesage S: The importance of dendritic cells in maintaining
immune tolerance. J Immunol. 198:2223–2231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waisman A, Lukas D, Clausen BE and Yogev
N: Dendritic cells as gatekeepers of tolerance. Semin Immunopathol.
39:153–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motran CC, Ambrosio LF, Volpini X, Celias
DP and Cervi L: Dendritic cells and parasites: From recognition and
activation to immune response instruction. Semin Immunopathol.
39:199–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malinova D, Fritzsche M, Nowosad CR, Armer
H, Munro PM, Blundell MP, Charras G, Tolar P, Bouma G and Thrasher
AJ: WASp-dependent actin cytoskeleton stability at the dendritic
cell immunological synapse is required for extensive, functional T
cell contacts. J Leukoc Biol. 99:699–710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Y, Bao Y, Ma M and Yang W:
Identification of key candidate genes and pathways in colorectal
cancer by integrated bioinformatical analysis. Int J Mol Sci.
18:pii: E722. 2017. View Article : Google Scholar
|
|
14
|
Dong B, Wang G, Yao J, Yuan P, Kang W, Zhi
L and He X: Predicting novel genes and pathways associated with
osteosarcoma by using bioinformatics analysis. Gene. 628:32–37.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marzaioli V, Hurtado-Nedelec M, Pintard C,
Tlili A, Marie JC, Monteiro RC, Gougerot-Pocidalo MA, Dang PM and
El-Benna J: NOX5 and p22phox are 2 novel regulators of human
monocytic differentiation into dendritic cells. Blood.
130:1734–1745. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smyth L, Boardman D, Tung S, Lechler R and
Lombardi G: MicroRNAs affect dendritic cell function and phenotype.
Immunology. 144:197–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ceppi M, Pereira P, Dunand-Sauthier I,
Barras E, Reith W, Santos MA and Pierre P: MicroRNA-155 modulates
the interleukin-1 signaling pathway in activated human
monocyte-derived dendritic cells. Proc Natl Acad Sci USA.
106:2735–2740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang A, Yang Y, Chen S, Xia F, Sun D,
Fang D, Xiong S, Jin L and Zhang J: MiR-34a promotes DCs
development and inhibits their function on T cell activation by
targeting WNT1. Oncotarget. 8:17191–17201. 2017.PubMed/NCBI
|
|
19
|
Kurowska-Stolarska M, Alivernini S,
Melchor EG, Elmesmari A, Tolusso B, Tange C, Petricca L, Gilchrist
DS, Di Sante G, Keijzer C, et al: MicroRNA-34a dependent regulation
of AXL controls the activation of dendritic cells in inflammatory
arthritis. Nat Commun. 8:158772017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chae CS, Kim GC, Park ES, Lee CG, Verma R,
Cho HL, Jun CD, Yoo YJ and Im SH: NFAT1 regulates systemic
autoimmunity through the modulation of a dendritic cell property. J
Immunol. 199:3051–3062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Riol-Blanco L, Delgado-Martín C,
Sánchez-Sánchez N, Alonso-C LM, Gutiérrez-López MD, Del Hoyo GM,
Navarro J, Sánchez-Madrid F, Cabañas C, Sánchez-Mateos P and
Rodríguez-Fernández JL: Immunological synapse formation inhibits,
via NF-kappaB and FOXO1, the apoptosis of dendritic cells. Nat
Immunol. 10:753–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
deLuca Summers L and Gommerman J:
Fine-tuning of dendritic cell biology by the TNF superfamily. Nat
Rev Immunol. 12:339–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robertson SJ, Lubick KJ, Freedman BA,
Carmody AB and Best SM: Tick-borne flaviviruses antagonize both
IRF-1 and type I IFN signaling to inhibit dendritic cell function.
J Immunol. 192:2744–2755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prete F, Catucci M, Labrada M, Gobessi S,
Castiello MC, Bonomi E, Aiuti A, Vermi W, Cancrini C, Metin A, et
al: Wiskott-Aldrich syndrome protein-mediated actin dynamics
control type-I interferon production in plasmacytoid dendritic
cells. J Exp Med. 210:355–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neyt K and Lambrecht B: The role of lung
dendritic cell subsets in immunity to respiratory viruses. Immunol
Rev. 255:57–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leong C, Funami K, Oshiumi H, Mengao D,
Takaki H, Matsumoto M, Aly HH, Watashi K, Chayama K and Seya T:
Interferon-stimulated gene of 20 kDa protein (ISG20) degrades RNA
of hepatitis B virus to impede the replication of HBV in vitro and
in vivo. Oncotarget. 7:68179–68193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu X, Qin B, Ma Q, Yang C, Gong XY and
Chen LM: Differential expression of ISG20 in chronic hepatitis B
patients and relation to interferon-alpha therapy response. J Med
Virol. 85:1506–1512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zahoor M, Xue G, Sato H and Aida Y:
Genome-wide transcriptional profiling reveals that HIV-1 Vpr
differentially regulates interferon-stimulated genes in human
monocyte-derived dendritic cells. Virus Res. 208:156–163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu F, Xie D, Ng SS, Lum CT, Cai MY, Cheung
WK, Kung HF, Lin G, Wang X and Lin MC: IFITM1 promotes the
metastasis of human colorectal cancer via CAV-1. Cancer Lett.
368:135–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Narayana SK, Helbig KJ, McCartney EM, Eyre
NS, Bull RA, Eltahla A, Lloyd AR and Beard MR: The
interferon-induced transmembrane proteins, IFITM1, IFITM2 and
IFITM3 inhibit hepatitis C virus entry. J Biol Chem.
290:25946–25959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang G, Xu Y, Chen X and Hu G: IFITM1
plays an essential role in the antiproliferative action of
interferon-gamma. Oncogene. 26:594–603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li K, Jia R, Li M, Zheng YM, Miao C, Yao
Y, Ji HL, Geng Y, Qiao W, Albritton LM, et al: A sorting signal
suppresses IFITM1 restriction of viral entry. J Biol Chem.
290:4248–4259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Sze DM, Yung BY, Tang P, Chen WJ,
Chan KH and Leung PH: Distinct expression of interferon-induced
protein with tetratricopeptide repeats (IFIT) 1/2/3 and other
antiviral genes between subsets of dendritic cells induced by
dengue virus 2 infection. Immunology. 148:363–376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishii K, Kurita-Taniguchi M, Aoki M,
Kimura T, Kashiwazaki Y, Matsumoto M and Seya T: Gene-inducing
program of human dendritic cells in response to BCG cell-wall
skeleton (CWS), which reflects adjuvancy required for tumor
immunotherapy. Immunol Lett. 98:280–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goodridge JP, Burian A, Lee N and Geraghty
DE: HLA-F and MHC class I open conformers are ligands for NK cell
Ig-like receptors. J Immunol. 191:3553–3562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dulberger CL, McMurtrey CP, Hölzemer A,
Neu KE, Liu V, Steinbach AM, Garcia-Beltran WF, Sulak M, Jabri B,
Lynch VJ, et al: Human leukocyte antigen f presents peptides and
regulates immunity through interactions with NK cell receptors.
Immunity. 46:1018–1029.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zidi I, Kharrat N, Abdelhedi R, Hassine
AB, Laaribi AB, Yahia HB, Abdelmoula NB, Abid L, Rebai A and Rizzo
R: Nonclassical human leukocyte antigen (HLA-G, HLA-E and HLA-F) in
coronary artery disease. Hum Immunol. 77:325–329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jucaud V, Ravindranath MH, Terasaki PI,
Morales-Buenrostro LE, Hiepe F, Rose T and Biesen R: Serum
antibodies to human leucocyte antigen (HLA)-E, HLA-F and HLA-G in
patients with systemic lupus erythematosus (SLE) during disease
flares: Clinical relevance of HLA-F autoantibodies. Clin Exp
Immunol. 183:326–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dou L, Liang HF, Geller DA, Chen YF and
Chen XP: The regulation role of interferon regulatory factor-1 gene
and clinical relevance. Hum Immunol. 75:1110–1114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu Y, Park-Min K, Yarilina A and Ivashkiv
L: Regulation of STAT pathways and IRF1 during human dendritic cell
maturation by TNF-alpha and PGE2. J Leukoc Biol. 84:1353–1360.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gongora C, Degols G, Espert L, Hua T and
Mechti N: A unique ISRE, in the TATA-less human Isg20 promoter,
confers IRF-1-mediated responsiveness to both interferon type I and
type II. Nucleic Acids Res. 28:2333–2341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Briken V, Ruffner H, Schultz U, Schwarz A,
Reis LF, Strehlow I, Decker T and Staeheli P: Interferon regulatory
factor 1 is required for mouse Gbp gene activation by gamma
interferon. Mol Cell Biol. 15:975–982. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Potu H, Sgorbissa A and Brancolini C:
Identification of USP18 as an important regulator of the
susceptibility to IFN-alpha and drug-induced apoptosis. Cancer Res.
70:655–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Honke N, Shaabani N, Zhang DE, Hardt C and
Lang KS: Multiple functions of USP18. Cell Death Dis. 7:e24442016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cong XL, Lo MC, Reuter BA, Yan M, Fan JB
and Zhang DE: Usp18 promotes conventional CD11b+ dendritic cell
development. J Immunol. 188:4776–4781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Honke N, Shaabani N, Zhang DE, Iliakis G,
Xu HC, Häussinger D, Recher M, Löhning M, Lang PA and Lang KS:
Usp18 driven enforced viral replication in dendritic cells
contributes to break of immunological tolerance in autoimmune
diabetes. PLoS Pathog. 9:e10036502013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim BH, Shenoy AR, Kumar P, Das R, Tiwari
S and MacMicking JD: A family of IFN-γ-inducible 65-kD GTPases
protects against bacterial infection. Science. 332:717–721. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Strehlow I, Lohmann-Matthes ML and Decker
T: The interferon-inducible GBP1 gene: Structure and mapping to
human chromosome 1. Gene. 144:295–299. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Haudek-Prinz V, Klepeisz P, Slany A, Griss
J, Meshcheryakova A, Paulitschke V, Mitulovic G, Stöckl J and
Gerner C: Proteome signatures of inflammatory activated primary
human peripheral blood mononuclear cells. J Proteomics. 76:150–162.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jin P, Han T, Ren J, Saunders S, Wang E,
Marincola FM and Stroncek DF: Molecular signatures of maturing
dendritic cells: Implications for testing the quality of dendritic
cell therapies. J Transl Med. 8:42010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao M, Zhou Y, Zhu B, Wan M, Jiang T, Tan
Q, Liu Y, Jiang J, Luo S, Tan Y, et al: IFI44L promoter methylation
as a blood biomarker for systemic lupus erythematosus. Ann Rheum
Dis. 75:1998–2006. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Weix J, Häupl T, Raio L, Villiger P and
Förger F: The physiologic increase in expression of some type I
IFN-inducible genes during pregnancy is not associated with
improved disease activity in pregnant patients with rheumatoid
arthritis. Transl Res. 161:505–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Skov V, Larsen TS, Thomassen M, Riley CH,
Jensen MK, Bjerrum OW, Kruse TA and Hasselbalch HC: Whole-blood
transcriptional profiling of interferon-inducible genes identifies
highly upregulated IFI27 in primary myelofibrosis. Eur J Haematol.
87:54–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tang B, Shojaei M, Parnell G, Huang S,
Nalos M, Teoh S, O'Connor K, Schibeci S, Phu AL, Kumar A, et al: A
novel immune biomarker IFI27 discriminates between influenza and
bacteria in patients with suspected respiratory infection. Eur
Respir J. 49:16020982017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xi Y, Troy N, Anderson D, Pena OM, Lynch
JP, Phipps S, Bosco A and Upham JW: Critical role of plasmacytoid
dendritic cells in regulating gene expression and innate immune
responses to human rhinovirus-16. Front Immunol. 8:13512017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Veenhuis R, Freeman ZT, Korleski J, Cohen
LK, Massaccesi G, Tomasi A, Boesch AW, Ackerman ME, Margolick JB,
Blankson JN, et al: HIV-antibody complexes enhance production of
type I interferon by plasmacytoid dendritic cells. J Clin Invest.
127:4352–4364. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Meng Y, Chen C, Liu Y, Tian C and Li H:
Angiotensin II regulates dendritic cells through activation of
NF-κB/p65, ERK1/2 and STAT1 pathways. Cell Physiol Biochem.
42:1550–1558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu J, Eastman A, Flaczyk A, Neal LM, Zhao
G, Carolan J, Malachowski AN, Stolberg VR, Yosri M, Chensue SW, et
al: Disruption of early tumor necrosis factor alpha signaling
prevents classical activation of dendritic cells in lung-associated
lymph nodes and development of protective immunity against
cryptococcal infection. Mol Biol. 7:e005102016.
|