Introduction
As one of the three predominant malignant tumors of
the female reproductive system, ovarian cancer is difficult to
diagnose at its onset. It has been reported that >70% of
patients with ovarian cancer have already reached a median or
advanced phase of the disease when they consult a doctor (1). The mortality rate of ovarian cancer
ranks second among all cancers of the female reproductive system
(2). Therapy for ovarian cancer is
based on surgical treatment supplemented by chemotherapy. Although
chemotherapy may effectively combat cancer in the early stages of
treatment, the majority of patients eventually experience cancer
recurrence in the postoperative stage, and thus the 5-year survival
rate for ovarian cancer is only 44% (3). Therefore, it is important to
investigate novel therapeutic methods that may help improve the
quality of treatment and the survival rate of patients with ovarian
cancer.
With the recent advances in biomedical research,
biotherapies, including gene therapy and immunotherapy, have
attracted attention due to their great potential compared with
current treatment strategies, including surgical treatment,
radiotherapy and chemotherapy. However, being a novel therapeutic
method, gene therapy also faces problems. Viral genetic
transporters exhibit a high efficiency but high toxicity to cells,
whereas non-viral vectors are comparatively safe but inefficient
(4). With the development of
ultrasound and associated imaging technology, ultrasound
microvesicles as contrast agents have helped make notable progress
in gene therapy. When microvesicles are applied as contrast agents,
they may function as genetic vectors, following the principles of
cavitation and sonoporation; this improves gene transfection in
tissues or cells and may achieve the goal of effective cancer
treatment (4–6).
Targeting protein for Xklp2 (TPX2) is a
microtubule-associated protein (7). The expression of TPX2 is influenced
by the cell cycle. The gene product appears during the G1-S stage
and disappears following the completion of mitosis. Possessing a
key role in the regulation of mitosis, TPX2 controls microtubule
assembly and spindle stability in cooperation with Aurora-A kinase
and Eg5 kinesin (8,9). Furthermore, it serves a role in the
formation of spindle apparatus and in chromosome segregation
(9,10). A number of research studies have
provided evidence that TPX2 is overexpressed in numerous types of
tumors, including lung, hepatic, colon, pancreatic and salivary
gland cancer, which suggests a probable association of TPX2 with
oncogenesis, or at least with certain associated malignancies
(11–15). Overexpression of TPX2 results in
the amplification of centrosomes and in DNA polyploidy (16). During interphase, TPX2 is
preferentially located in the nucleus (9). Recently, TPX2 expression has been
regarded as a marker for the diagnosis and prognosis of
malignancies in a number of types of cancer (9,11,12,17).
The present study explored the effects of TPX2
silencing in combination with two other treatments, microvesicles
and/or ultrasonic radiation, on the ovarian cancer cell line SKOV3,
to investigate the potential of this silencing phenomenon as an
inhibiter of migration and invasion of ovarian cancer cells.
Materials and methods
Cells and recombinant plasmid
The cell line SKOV3 was obtained from the Cell Bank
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) contained with
10% fetal calf serum (Beijing Transgen Biotech Co., Ltd., Beijing,
China) at 37°C in a 5% CO2 incubator. The medium was
changed every 2 days. Cell passage cultivation was commenced when
cells grew to 90% confluence. Recombinant plasmids of small
interfering (si)RNA-TPX2 were provided by Shanghai Sangong
Pharmaceutical Co., Ltd. (Shanghai, China). The sequences of
siRNA-TPX2 were 5′-GGAUGAUAUUAACCUGUUATT-3′ and
5′-UAACAGGUUAAUAUCCUCCTT-3′.
Grouping and treatment
The SKOV3 cells were randomly divided into 5groups:
Control, siRNA-TPX2, siRNA-TPX2 + microvesicles (M), siRNA-TPX2 +
ultrasonic irradiation (UI), and siRNA-TPX2 + M + UI. The
microvesicles used in the present study were purchased from Cold
Spring Biotech Corp. (Tapei, Taiwan). Except for the control, cells
in all other groups were transfected with a recombinant plasmid,
siRNA-TPX2, by different means, including pure transfection and
transfection assisted with microvesicles and/or ultrasonic
irradiation. Cells in the siRNA-TPX2 group were transfected with
plasmids using Lipofectamine® 2000 reagent (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Cells in the siRNA-TPX2 + M group were transfected with 4 µg
plasmid and 200 µl microvesicles (10%). In the siRNA-TPX2 + UI
group, cells were transfected with 4 µg plasmid, followed by
treatment with UI at a frequency of 2.0 MHz and mechanical index of
0.28 for 30 sec. In the siRNA-TPX2 + M + UI group, cells were
transfected with a mixture of 4 µg plasmid and 200 µl microvesicles
(10%), followed by treatment with ultrasonic radiation with the
same parameters as above for 30 sec. Following transfection, cells
were incubated in 5% CO2 at 37°C for 48 h prior to
subsequent experiments.
Detection of cell viability
Cell viability in each group and the control was
detected using a Cell Counting kit 8 assay (CCK8; Beyotime
Institute of Biotechnology, Haimen, China). Cells (3×104
cells/ml) were seeded into 96-well plates with 100 µl added per
well; the plates were incubated at 37°C in the presence of 5%
CO2 for 4 h. Cells were then supplemented with 10 µl CCK
reagent in each well, then placed in an incubator with 5%
CO2 at 37°C for 3 h. The optical density of cells from
each group was measured at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Detection of cell migration and
invasion
Transwell inserts (Corning Incorporated, Corning,
NY, USA) were used to detect cell migration and invasion. For the
migration assay, cells (5×105 cells/ml) cultured in
serum-free medium from each group were added to the upper chamber,
and fetal bovine serum (Beijing Transgen Biotech Co., Ltd.)
containing DMEM (Thermo Fisher Scientific, Inc.) was added to the
lower chamber. All the cells were left to migrate for 24 h. The
migrated cells were fixed in 95% alcohol, stained with 0.1%
hexamethyl pararosaniline (Beyotime Institute of Biotechnology,
Haimen, China) for 20 min at room temperature, and counted under an
upright metallurgical microscope (magnification, ×100; Leica
Microsystems GmbH, Wetzlar, Germany). The experiment was repeated 3
times for each group. For invasion detection, upper chambers of
Transwell were specifically coated with Matrigel. The rates of cell
migration and invasion in all groups were calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells were seeded into 6-well plates at a density of
2×106 cells/well. Total RNA was extracted using TRIzol
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. The concentration of extracted RNA was read using a UV
spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA was
synthesized using: A reverse transcriptase, a poly (A) polymerase
and tagged oligo (dT) primers in a 20 µl reaction volume for
RT-qPCR (miScript; Qiagen, Inc., Valencia, CA, USA) according to
the manufacturer's protocol. Each RT-qPCR contained 2 µl of cDNA,
400 nM of each sense and antisense primer, and 2× SYBR-Green PCR
Master Mix (Takara Bio, Inc., Otsu, Japan). The reaction mixture
was incubated at 37°C for 60 min, 85°C for 5 min and 4°C for 5 min.
The amplification assay was performed using ABI 7500 (Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR was carried out by
activating the DNA polymerase at 95°C for 10 min, followed by 40
cycles of two-step PCR (95°C for 15 sec and 60°C for 45 sec). GAPDH
was used as the internal control to monitor the efficiency of
RT-qPCR. All primers in the present study were designed by Shanghai
Sangon Biotech Co., Ltd. (Shanghai, China). The specific primer
sequences for each gene were as follows: 5′TCACAGCTCACTGCAGCCTT3′
and 5′CTCTGGGAGGCCAAGATGGG3′ for epithelial cadherin (E-cadherin;
product, 196 bp); 5′GCCAAGTTCTTCGCCTGCAT3′ and
5′AGCTGGACCAGTCGAAACCC3′ for metalloproteinase inhibitor 2 (TIMP-2;
product, 168 bp); 5′CGAGACCGAGTCGCTCAAGT3′ and
5′GCCATCCTCCTCGCCTTCTT3′ for metastasis associated 1 (MTA1;
product, 169 bp); 5′AGGACTACGACCGCGACAAG3′ and
5′TGTGGTCGCACACCACATCT3′ for matrix metallopeptidase 2 (MMP2;
product, 173 bp) and 5′CGGGAAACTGTGGCGTGATG3′ and
5′ATGACCTTGCCCACAGCCTT3′ for GAPDH (product, 87 bp). Each reaction
was run in triplicate. The 2−ΔΔCq method was performed
for the quantification of gene expression data (18).
Gelatin zymography assay
Cells were centrifuged for 10 min at 310 × g at 4°C
to remove suspended cells from all treatments. The supernatant from
each was then resolved via 8% SDS-PAGE containing 1% gelatin. The
gel was incubated in a renaturing buffer (2.5% Triton X 100) for 30
min at room temperature, followed by incubation for a further 30
min in a developing buffer (Tris base, 50 mM; Tris HCl, 0.2M; NaCl,
0.2M; CaCl2, 5 mM; and Brij-35, 0.02%) with gentle
agitation. The gel was subsequently incubated in developing buffer
at 37°C overnight and stained with Coomassie R-250 (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for >2.5 h with gentle agitation
at room temperature. The samples were viewed using the LI-COR
Odyssey® CLx infrared imaging system (LI-COR
Biosciences, Lincoln, NE, USA) and analyzed using ImageQuant™ TL
(version number 7.0; GE Healthcare, Chicago, IL, USA).
Western blot analysis
Cells were seeded into 6-well plates at a density of
2×106 cells/well. Cells were harvested and washed twice
with phosphate buffer solution and lysed in ice-cold
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) with freshly mixed 0.01% protease inhibitor
phenylmethane sulfonyl fluoride (Beyotime Institute of
Biotechnology). The mixture was then incubated for 30 min on ice.
Cell lysate was centrifuged at 10,000 × g for 5 min at 4°C. The
supernatants containing 20–30 µg protein were collected, and
protein concentration was determined using a BCA protein assay kit
(Thermo Fisher Scientific, Inc.). Samples were then subjected to
10% SDS-PAGE, and transferred onto a nitrocellulose membrane (Merck
& Co., Inc., Kenilworth, NJ, USA). The membranes were blocked
using 5% non-fat milk in Tris buffered saline containing 0.5%
Tween-20 for 1 h at room temperature. Membranes were incubated with
the following primary specific antibodies at 4°C for 6 h and then
at room temperature for 4 h: Anti-E-cadherin (cat. no. ab76055;
1:1,000), anti-TIMP-2 (cat. no. ab1828; 1:1,000), anti-MTA1 (cat.
no. ab71153; 1:2,000), anti-MMP2 (cat. no. ab92536; 1:1,000),
anti-phosphorylated-P-38 (phosphorylated Y182; cat. no. ab47363;
1:800); anti-P-38 (cat. no. ab170099; 1:1,000);
anti-phosphorylated-JNK1 + JNK2 + JNK3 (phosphorylated T183 + T183
+ T221; cat. no. ab179461; 1:1,000); anti-JNK1 + JNK2 + JNK3 (cat.
no. ab8245; 1:1,000) and anti-GAPDH (cat. no. ab8245; 1:2,000; all
Abcam, Cambridge, MA, USA). The membranes were then incubated with
the following secondary antibodies at room temperature for 1 h:
Goat anti-mouse IgG H&L (cat. no. ab6789; 1:2,000), goat
anti-rabbit IgG H&L (cat. no. ab6721; 1:2,000) and donkey
anti-goat IgG H&L (cat. no. ab6885; 1:2,000; all Abcam). Blots
were visualized via enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.). Densitometry of the bands was determined using
ImageJ software (version 1.48; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All statistical analyses were performed using
SigmaStat 2.0 for Windows (Systat Software Inc., San Jose, CA,
USA). Data were expressed as the mean ± standard deviation.
Comparisons of multiple treatment groups to controls were performed
using one-way analysis of variance followed by Tukey and Bonferroni
post-hoc tests. Differences among groups were evaluated through
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of microvesicles
Microvesicles added to the siRNA-TPX2 + M and
siRNA-TPX2 + M + UI groups were clearly identified via an upright
metallurgical microscope (magnification, ×400). The grain size of
these microvesicles was 1,071±469.04 µm. Their average electric
potential was 11.35±2.11 µA (Fig.
1A).
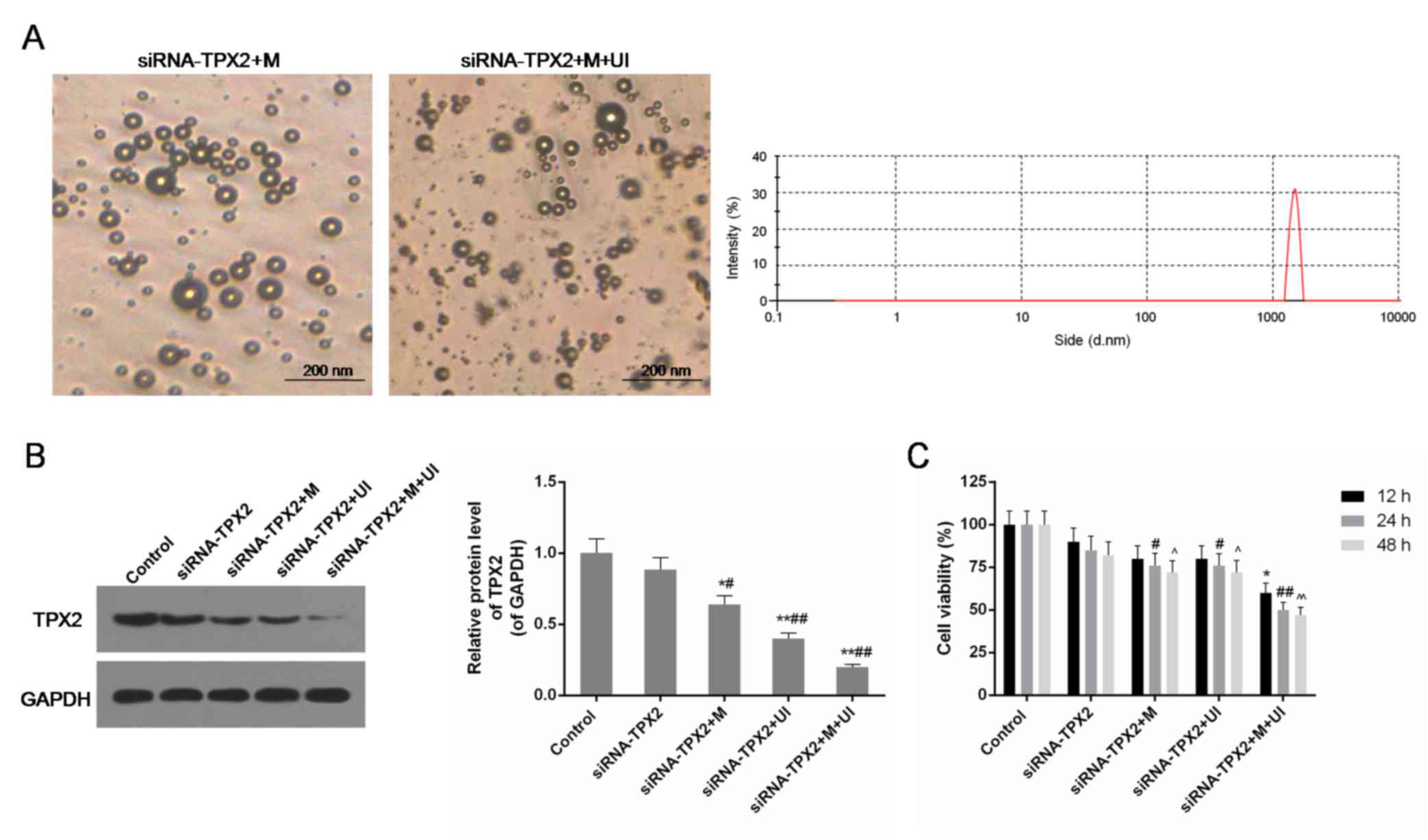 | Figure 1.Identification of microvesicles, and
the expression level of TPX2 and cell viability in the control
group, siRNA-TPX2 group, siRNA-TPX2 + M group, siRNA-TPX2 + UI
group and siRNA-TPX2 + M + UI group. (A) M were clearly identified
in the siRNA-TPX2 + M group (left) and the siRNA-TPX2 + M + UI
group (right), with a 1071±469.04 nm grain size and an 11.35±2.11
mA average electric potential. (B) The protein expression level of
TPX2 was substantially inhibited in M-and/or UI-mediated TPX2
silencing, particularly in siRNA-TPX2 + M + UI group. Data are
presented as the mean ± standard deviation (n=3). *P<0.05 and
**P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. siRNA-TPX2. (C) Cell viability was
decreased in M-and/or UI-mediated TPX2 silencing, particularly in
the siRNA-TPX2 + M + UI group. Data are presented as the mean ±
standard deviation n=3. *P<0.05 vs. control (12 h);
#P<0.05 and ##P<0.01 vs. control (24
h); ^P<0.05 and ^^P<0.01 vs. control
(48 h). siRNA, small interfering RNA; M, microvesicles; UI,
ultrasonic irradiation; TPX2, targeting protein for Xklp2. |
Inhibition of TPX2 expression
The amount of TPX2 protein in each group and the
control was detected by western blotting. This additionally
provided information on the transfection efficiency of the
siRNA-TPX2 plasmid under different conditions. In the siRNA-TPX2
group, no significant decrease in TPX2 protein levels was observed
compared with the control group. In the siRNA-TPX2 + M and
siRNA-TPX2 + UI groups, TPX2 was less expressed compared with the
siRNA-TPX2 group (P<0.05 and P<0.01, respectively). The
combination of ultrasonic radiation and microvesicles significantly
inhibited the expression of TPX2 to one-fifth of the expression in
control SKOV3 cells (P<0.01; Fig.
1B).
Cell viability was negatively affected
by TPX2 silencing
Cell viability in the siRNA-TPX2, siRNA-TPX2 + M,
siRNA-TPX2 + UI, and siRNA-TPX2 + M + UI groups clearly decreased
when the TPX2 gene was silenced. The reduction in cell
proliferation due to TPX2 silencing and its joint action with
microvesicles and/or UI occurred in a time-dependent manner. The
number of cells in the siRNA-TPX2 + M, siRNA-TPX2 + UI, and siRNA +
M + UI groups treated for 24 and 48 h was significantly different
compared with the control (P<0.05 or P<0.01; Fig. 1C). The treatment time of 24 h was
selected for further experiments.
Cell migration and invasion
inhibited
Cell migration and invasion of SKVO3 cells were
detected via Transwell assay. In the siRNA-TPX2 group, cell
migration and invasion were decreased to 82.21±8.14% and
79.02±8.52%, respectively, compared with normal SKOV3 cells. When
TPX2 silencing was combined with microvesicles and ultrasonic
irradiation, cell migration and invasion were further inhibited
(P<0.05 or P<0.01), particularly in the siRNA-TPX2 + M + UI
group, with only 41.46±4.34% of cell migration and 37.64±4.04% of
cell invasion retained in comparison with the control (P<0.01;
Fig. 2).
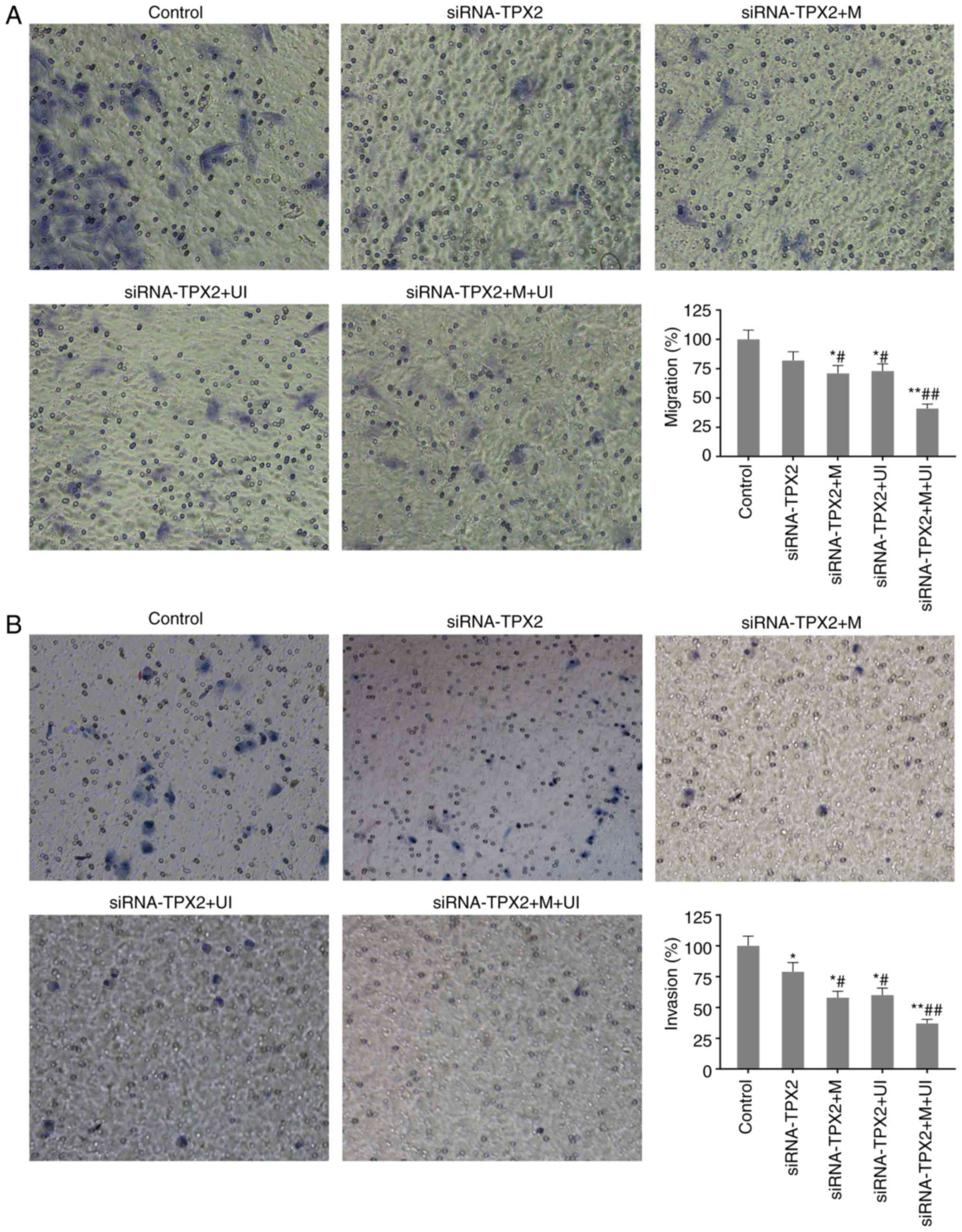 | Figure 2.Migration and invasion of SKOV3 in the
control group, siRNA-TPX2 group, siRNA-TPX2 + M group, siRNA-TPX2 +
UI group and siRNA-TPX2 + M + UI group. (A) Cell migration was
clearly inhibited in M-and/or UI-mediated TPX2 silencing,
particularly in the siRNA-TPX2 + M + UI group. (B) Cell invasion
was markedly inhibited in M-and/or UI-mediated TPX2 silencing,
particularly in the siRNA-TPX2 + M + UI group. Invaded cells were
counted under an inverted microscope (magnification, ×100). Data
are presented as the mean ± standard deviation n=3. *P<0.05 and
**P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. siRNA-TPX2. siRNA, small interfering
RNA; M, microvesicles; UI, ultrasonic irradiation; TPX2, targeting
protein for Xklp2. |
Expression of E-cadherin and TIMP-2 is
upregulated, while that of MTA1 and MMP2 is downregulated
The production of mRNA and proteins of E-cadherin,
TIMP-2, MTA1 and MMP2 from their respective genes was detected by
means of RT-qPCR and western blotting (Fig. 3). These variables (the level of
mRNA and proteins) for these four genes were similar (Fig. 3A-C and E). In the siRNA-TPX2 group,
the genes of E-cadherin and TIMP-2 were increasingly expressed,
while those for MTA1 and MMP2 were decreasingly expressed upon TPX2
silencing. The differences in expression of all the genes, however,
were observed to be insignificant between treatments and control.
Silencing of TPX2 in combination with microvesicles and/or
ultrasonic irradiation significantly strengthened the alterations
in the mRNA and protein expression levels of E-cadherin, TIMP-2,
MTA1 and MMP2. In the siRNA-TPX2 + M + UI group, the expression of
E-cadherin and TIMP-2 almost doubled, while that of MTA1 and MMP2
was reduced to less than one-quarter compared with the expression
in siRNA-TPX2 group (P<0.01; Fig.
3A-E). The results of the gelatin zymography assay for MMP2
activity were consistent with the expression of MMP2 (Fig. 3F).
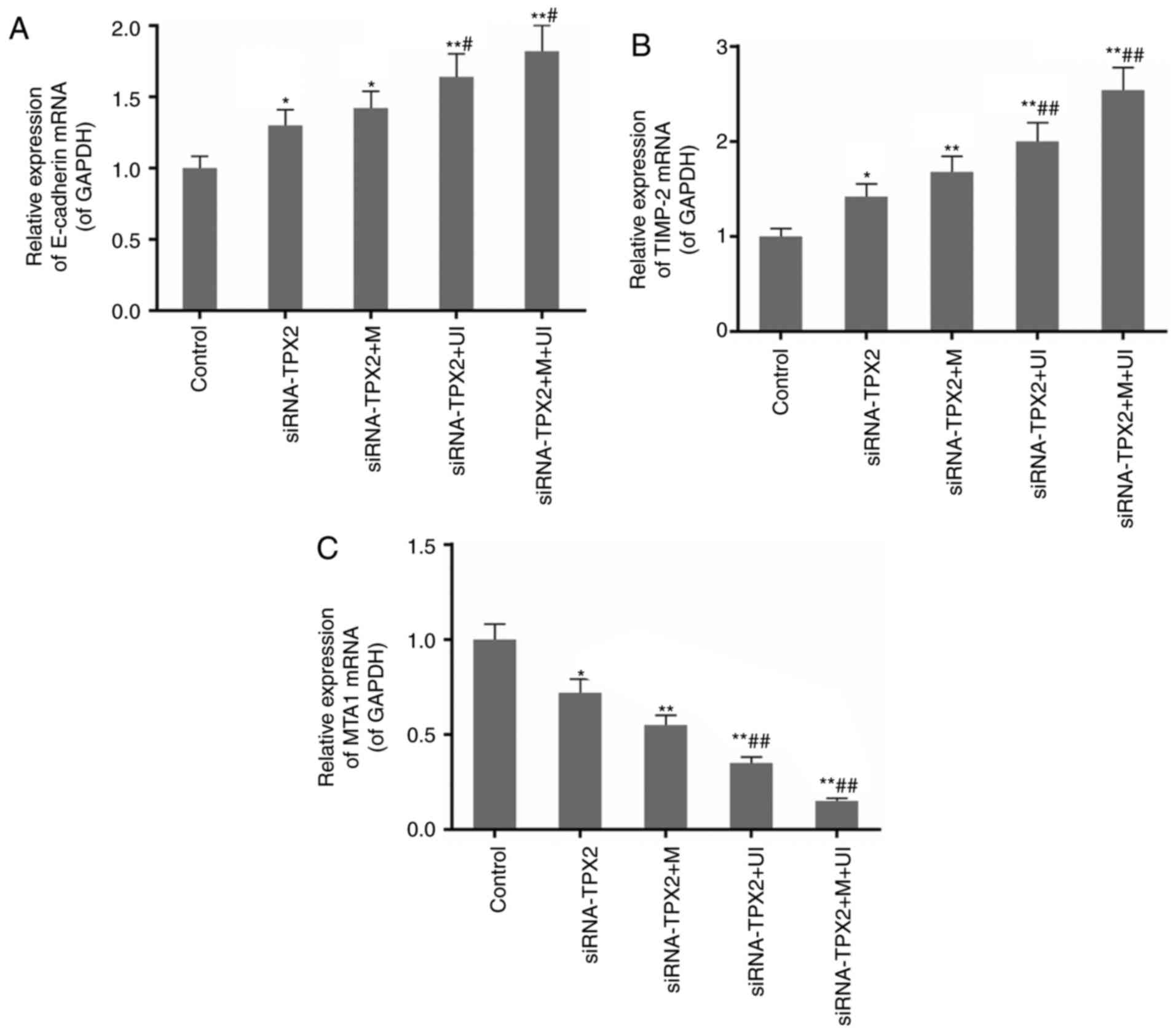 | Figure 3.Expression levels of E-cadherin,
TIMP-2, MTA1 and MMP2 in the control group, siRNA-TPX2 group,
siRNA-TPX2 + M group, siRNA-TPX2 + UI group and siRNA-TPX2 + M + UI
group. The expression of (A) E-cadherin and (B) TIMP-2 mRNA was
upregulated by M-and/or UI-mediated TPX2 silencing, particularly in
the siRNA-TPX2 + M + UI group. (C) The expression of MTA1 mRNA was
downregulated by M- and/or UI-mediated TPX2 silencing, particularly
in the siRNA-TPX2 + M + UI group. (D) The protein expression levels
of E-cadherin and TIMP-2 were upregulated, while the levels of MTA1
and MMP2 were downregulated by M-and/or UI-mediated TPX2 silencing,
particularly in the siRNA-TPX2 + M + UI group. (E) The expression
of MMP2 mRNA and (F) the activity of MMP2 were downregulated by M-
and/or UI-mediated TPX2 silencing, particularly in the siRNA-TPX2 +
M + UI group. Data are presented as the mean ± standard deviation
n=3. *P<0.05 and **P<0.01 vs. control; #P<0.05
and ##P<0.01 vs. siRNA-TPX2. E-, epithelial; TIMP-2,
metalloproteinase inhibitor 2; MTA1, metastasis associated 1; MMP2,
matrix metallopeptidase 2; siRNA, small interfering RNA; M,
microvesicles; UI, ultrasonic irradiation; TPX2, targeting protein
for Xklp2. |
Phosphorylation levels of p38 and JNK
are restricted
The protein levels of phosphorylated p38 and JNK
were observed to decrease through RT-qPCR analysis in the
siRNA-TPX2 group compared with control SKOV3 cells. These levels
were even lower in the siRNA-TPX2 + M, siRNA-TPX2 + UI and
siRNA-TPX2 + M + UI groups (P<0.05 or P<0.01), particularly
when ultrasonic irradiation and microvesicles were combined to
mediate the RNA silencing of TPX2 (P<0.01). p38 and JNK levels
were not affected, which resulted in marked decreases in the ratios
of phosphorylated to un-phosphorylated proteins in microvesicles-
and/or ultrasonic irradiation-mediated TPX2 silencing, particularly
in the siRNA-TPX2 + M + UI group (P<0.01; Fig. 4).
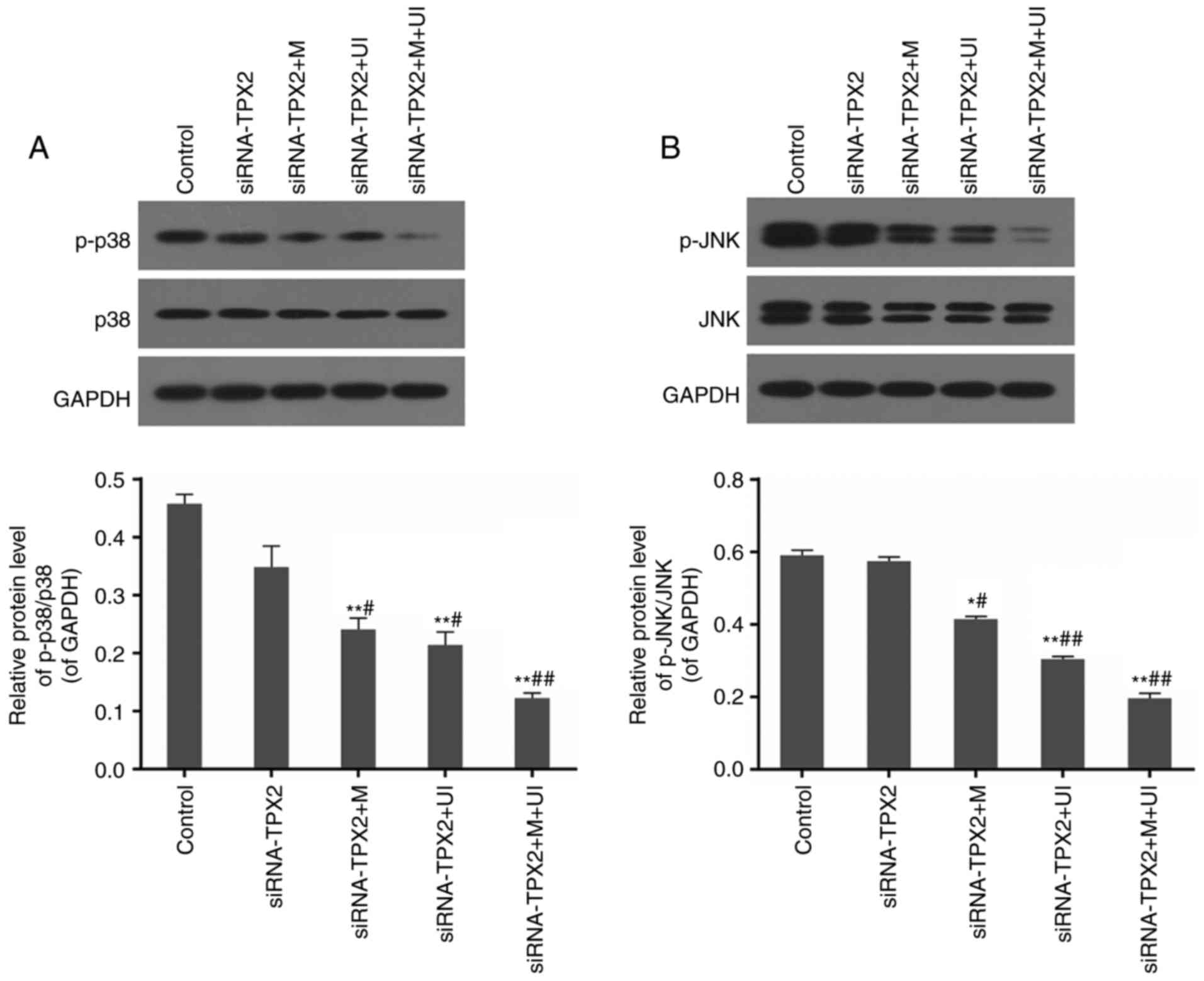 | Figure 4.Protein levels of p-p38, p38, p-JNK
and JNK in the control group, siRNA-TPX2 group, siRNA-TPX2 + M
group, siRNA-TPX2 + UI group and siRNA-TPX2 + M + UI group. (A) The
phosphorylation of p38 was inhibited by M-and/or UI-mediated TPX2
silencing, particularly in the siRNA-TPX2 + M + UI group. (B) The
phosphorylation of JNK was inhibited by M- and/or UI-mediated TPX2
silencing, particularly in the siRNA-TPX2 + M + UI group. Data are
presented as the mean ± standard deviation n=3. *P<0.05 and
**P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. siRNA-TPX2. p-, phosphorylated; JNK,
c-Jun N-terminal kinase; siRNA, small interfering RNA; M,
microvesicles; UI, ultrasonic irradiation; TPX2, targeting protein
for Xklp2. |
Discussion
Malignant tumor cells are characterized by
uncontrolled cell growth and an aberrant ability to differentiate
and infiltrate the body, which enables them to cause metastasis.
Cell migration and invasion are two of the primary causes of
mortality in patients with cancer (11,19).
A number of research studies have provided evidence that TPX2
silencing may decrease cell viability and proliferation in numerous
types of cancer, including colon, cervical and hepatic cancers
(12,13,15).
It has additionally been reported that siRNA-TPX2 induces cellular
apoptosis in hepatoma cells, and markedly decreases the growth and
weight of already-developed xenograft tumors in nude mice; these
findings suggest that a reduction in TPX2 levels may be a potential
way of treating cancer (12,17,19–21).
Although viral vectors are known to possess high transfection
efficiency, they have poor targeting ability and low security
(19,20). Therefore, improving the
transfection efficiency of non-viral vectors is crucial to improve
the curative effects of these plasmids and, thus, reduce associated
complications in the treatment of cancer.
Microvesicles are a type of non-invasive vector,
whose destruction by UI may generate high-energy shockwaves or
microjets in the endothelial cell membrane, which subsequently
induce shear stress so as to increase membrane permeability
(5). The increase in permeability
possibly results from the formation of temporary holes in the cell
plasma or nuclear membranes. These non-lethal holes allow
extracellular macromolecules and particles into cells, an effect
termed sonoporation, which promotes the delivery of genes or drugs
into the cells by microvesicles. Rapid shock administered to
microvesicles in cell membranes facilitates the transport of DNA
through the cell membranes (4).
Ultrasonic waves monitor whether the microvesicle-based contrast
agent has arrived at the targeting tissue in real-time; it is
possible to use the wave to break up the microvesicle at a specific
time and place in order to improve the curative effect (6).
The present study investigated the transfection
efficiency of the plasmid siRNA-TPX2 when mediated by ultrasonic
irradiation and microvesicles in SKOV3 cells, in order to evaluate
its effect on growth and apoptosis in cancer cells. It was
identified that conventional transfection with the siRNA-TPX2
plasmid only slightly downregulated the expression of TPX2 so as to
reduce cell viability, and inhibit cell migration and invasion. The
results demonstrated the inhibitory effect of TPX2 silencing on the
growth of SKOV3 cells; however, the efficiency of transfection was
not significant without the provision of other techniques.
Introduction of microvesicles and ultrasonic irradiation was
observed to markedly improve the transfection efficiency of
siRNA-TPX2 into the cells, and the expressed TPX2 level was reduced
to almost one-fifth of that in normal control cells. With weak
expression of TPX2 in siRNA-TPX2 + M + UI group, cell viability was
markedly inhibited, and cell migration and invasion were
significantly reduced by over one-half in comparison with that in
the siRNA-TPX2 group.
Calcium-dependent adhesions (cadherin) are a class
of Ca2+-dependent transmembrane proteins, including
three classical subtypes: E (epithelial) -, N (neural) -, and VE
(vascular endothelial) -cadherins (22,23).
E-cadherins, which mediate Ca2+-dependent cell adhesion
in epithelial tissues, are associated with cell recognition,
differentiation, morphogenesis and tumor suppression (24,25).
Previous studies have identified that weak or no expression of
E-cadherin is associated with metastasis and infiltration of
various malignant tumors, including primary ovarian, esophagus,
cervical and lung cancer (26,27).
MMPs and their inhibitors (TIMPs) serve important roles in
extracellular matrix turnover (28). For example, MMP2, together with
MMP9, is able to degrade the most plentiful component of the
basement membrane, type IV collagen. Degradation of the basement
membrane allows cancer cells to migrate out of the tumor, resulting
in metastases. MTA1, a member of the MTA family, is an integral
component of the nucleosome remodeling and histone deacetylation
complexes. In the early 1990s, it was reported to be overexpressed
in highly metastatic cells compared with non-metastatic cells
(29). The MTA1 protein suppresses
the expression of numerous tumor suppressor genes and, therefore,
facilitates cell migration and invasion (30). The present study noted that TPX2
silencing slightly increased the expression of E-cadherin and
TIMP2, and decreased that of MMP2 and MTA1, so as to improve cell
adhesion and provide protection against membrane degradation. With
the application of microvesicles and ultrasonic radiation during
the process of siRNA-TPX2 transfection, the effects of TPX2
silencing on the expression of these genes were much stronger. In
the siRNA-TPX2 + M + UI group, E-cadherin and TIMP-2 were more
highly expressed, while MMP2 and MTA1 were more weakly expressed
than in other groups, which led to an improved inhibition of cell
migration and invasion.
Mitogen-activated protein kinases (MAPKs), a series
of serine/threonine protein kinases, are essential signaling
components that convert external or internal stimuli to a cell
reaction or signaling pathway. These MAPK signaling pathways make
up an intricate network of signaling cascades, which regulate a
variety of physiological processes, including cell migration
(31). The primary function of
MAPKs is the phosphorylation of phospholipases, transcription
factors and cytoskeletal proteins to conduct extracellular signals
into and across the cells. As two primary MAPK signal pathway
members, JNKs and p38 regulate cell proliferation, differentiation
and survival rates in addition to the migration of specific types
of cells (32,33). The results of the present study
indicated a decrease in the protein expression levels of
phosphorylated p38 and JNK when TPX2 was silenced, meaning that p38
and JNK pathways were restricted in M- and/or UI-mediated TPX2
silencing, particularly in the siRNA-TPX2 + M + UI group. In this
group, the effect of TPX2 silencing was more pronounced compared
with the other groups. The combined action of microvesicles and
ultrasonic irradiation improved the transfection effect of
siRNA-TPX2 to inhibit the phosphorylation of p38 and JNK.
The observations of the present study suggested that
targeted inactivation of TPX2 may possess therapeutic benefits in
the treatment of ovarian cancer by upregulating E-cadherin and
TIMP2, and downregulating MMP2 and MTA1; it also helped inhibit the
phosphorylation of p38 and JNK. The combined action of
microvesicles and ultrasonic irradiation was demonstrated to
markedly improve the transfection efficiency of the siRNA-TPX2
plasmid to control cell migration and invasion in ovarian
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
DH and MW designed the study. CY and JC performed
the experiments. DH was the major contributor in the writing of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
committee of Sir Run Run Shaw Hospital, School of Medicine,
Zhejiang University (Hangzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: Current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frenkel V, Kimmel E and Iger Y:
Ultrasound-induced cavitation damage to external epithelia of fish
skin. Ultrasound Med Biol. 25:1295–1303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dijkmans PA, Juffermans LJ, Musters RJ,
van Wamel A, ten Cate FJ, van Gilst W, Visser CA, de Jong N and
Kamp O: Microbubbles and ultrasound: from diagnosis to therapy. Eur
J Echocardiogr. 5:245–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao ZG, Fain HD and Rapoport N: Controlled
and targeted tumor chemotherapy by micellar-encapsulated drug and
ultrasound. J Control Release. 102:203–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tonon G, Wong KK, Maulik G, Brennan C,
Feng B, Zhang Y, Khatry DB, Protopopov A, You MJ, Aguirre AJ, et
al: High-resolution genomic profiles of human lung cancer. Proc
Natl Acad Sci USA. 102:9625–9630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Castro Perez I and Malumbres M: Mitotic
stress and chromosomal instability in cancer: The case for TPX2.
Genes Cancer. 3:721–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neumayer G, Belzil C, Gruss OJ and Nguyen
MD: TPX2: Of spindle assembly, DNA damage response, and cancer.
Cell Mol Life Sci. 71:3027–3047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wittmann T, Wilm M, Karsenti E and Vernos
I: TPX2, A novel xenopus MAP involved in spindle pole organization.
J Cell Biol. 149:1405–1418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma Y, Lin D, Sun W, Xiao T, Yuan J, Han N,
Guo S, Feng X, Su K, Mao Y, et al: Expression of targeting protein
for xklp2 associated with both malignant transformation of
respiratory epithelium and progression of squamous cell lung
cancer. Clin Cancer Res. 12:1121–1127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei P, Zhang N, Xu Y, Li X, Shi D, Wang Y,
Li D and Cai S: TPX2 is a novel prognostic marker for the growth
and metastasis of colon cancer. J Transl Med. 11:3132013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Guo W and Kan H: TPX2 is a
prognostic marker and contributes to growth and metastasis of human
hepatocellular carcinoma. Int J Mol Sci. 15:18148–18161. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shigeishi H, Ohta K, Hiraoka M, Fujimoto
S, Minami M, Higashikawa K and Kamata N: Expression of TPX2 in
salivary gland carcinomas. Oncol Rep. 21:341–344. 2009.PubMed/NCBI
|
|
15
|
Chang H, Wang J, Tian Y, Xu J, Gou X and
Cheng J: The TPX2 gene is a promising diagnostic and therapeutic
target for cervical cancer. Oncol Rep. 27:1353–1359.
2012.PubMed/NCBI
|
|
16
|
Gruss OJ, Wittmann M, Yokoyama H,
Pepperkok R, Kufer T, Sillje H, Karsenti E, Mattaj IW and Vernos I:
Chromosome-induced microtubule assembly mediated by TPX2 is
required for spindle formation in HeLa cells. Nat Cell Biol.
4:871–879. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang B, Jia C, Huang Y, He H, Li J, Liao
H, Liu X, Liu X, Bai X and Yang D: TPX2 level correlates with
hepatocellular carcinoma cell proliferation, apoptosis and EMT. Dig
Dis Sci. 60:2360–2372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Warner SL, Stephens BJ, Nwokenkwo S,
Hostetter G, Sugeng A, Hidalgo M, Trent JM, Han H and Von Hoff DD:
Validation of TPX2 as a potential therapeutic target in pancreatic
cancer cells. Clin Cancer Res. 15:6519–6528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miwa T, Kokuryo T, Yokoyama Y, Yamaguchi J
and Nagino M: Therapeutic potential of targeting protein for Xklp2
silencing for pancreatic cancer. Cancer Med. 4:1091–1100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satow R, Shitashige M, Kanai Y, Takeshita
F, Ojima H, Jigami T, Honda K, Kosuge T, Ochiya T, Hirohashi S and
Yamada T: Combined functional genome survey of therapeutic targets
for hepatocellular carcinoma. Clin Cancer Res. 16:2518–2528. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klezovitch O and Vasioukhin V: Cadherin
signaling: Keeping cells in touch. F1000Res. 4:5502015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Bennett SA and Wang L: Role of
E-cadherin and other cell adhesion molecules in survival and
differentiation of human pluripotent stem cells. Cell Adh Migr.
6:59–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Faleiro-Rodrigues C, Macedo-Pinto I,
Pereira D and Lopes CS: Prognostic value of E-cadherin
immunoexpression in patients with primary ovarian carcinomas. Ann
Oncol. 15:1535–1542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oda H and Takeichi M: Evolution:
Structural and functional diversity of cadherin at the adherens
junction. J Cell Biol. 193:1137–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carico E, Atlante M, Bucci B, Nofroni I
and Vecchione A: E-cadherin and alpha-catenin expression during
tumor progression of cervical carcinoma. Gynecol Oncol. 80:156–161.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernebro E, Bendahl PO, Dictor M, Persson
A, Fernö M and Nilbert M: Immunohistochemical patterns in rectal
cancer: Application of tissue microarray with prognostic
correlations. Int J Cancer. 111:921–928. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brun JL, Cortez A, Lesieur B, Uzan S,
Rouzier R and Daraï E: Expression of MMP-2, −7, −9, MT1-MMP and
TIMP-1 and −2 has no prognostic relevance in patients with advanced
epithelial ovarian cancer. Oncol Rep. 27:1049–1057. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xue Y, Wong J, Moreno GT, Young MK, Côté J
and Wang W: NURD, a novel complex with both ATP-dependent
chromatin-remodeling and histone deacetylase activities. Mol Cell.
2:851–861. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y and Wang XF: Post-transcriptional
regulation of MTA family by microRNAs in the context of cancer.
Cancer Metastasis Rev. 33:1011–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan JL, Trevithick JE and Hynes RO:
Fibronectin/integrin interaction induces tyrosine phosphorylation
of a 120-kDa protein. Cell Regul. 2:951–964. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Genet Dev. 12:14–21. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rincon M and Davis RJ: Regulation of the
immune response by stress-activated protein kinases. Immunol Rev.
228:212–224. 2009. View Article : Google Scholar : PubMed/NCBI
|


















