Introduction
As the common end stage of all progressive chronic
kidney diseases, renal interstitial fibrosis is defined as an
excessive infiltration of leucocytes and deposition of
extracellular matrix, leading to the impairment of renal function,
destruction of kidney structure and vicious progression to renal
failure (1). Research advances
regarding this progressive disease have been made; however,
patients with end-stage renal disease tend to be dialysis-dependent
(2).
Increasing efforts to investigate novel therapeutic
strategies to hinder the development of renal interstitial fibrosis
have been conducted. In vivo and in vitro studies
have suggested that bone marrow-derived mesenchymal stem cell
(BMSC)-based therapy produced significant renoprotective effects by
reducing renal infiltration of inflammatory cells,
glomerulosclerosis and fibrogenesis (3,4). The
therapeutic property was mainly attributed to the paracrine effect
and immunomodulatory response (5,6);
however, allogenic MSC injection may also lead to various problems,
including tumorigenesis, maldifferentiation and immune
incompatibility (7–10).
Therefore, MSC-conditioned media (MSC-CM), which is
rich in cytokines secreted by MSCs, is of primary research
interest. Evidence has confirmed the protective effects of MSC-CM
in various models (11,12); inhibition of apoptosis,
inflammation, cell proliferation and epithelial-mesenchymal
transition are all potential mechanisms (9). B cell-dependent immune responses were
previously reported to serve a vital role throughout the renal
fibrotic process; however, research focusing on the involvement of
MSC-CM in this immunoregulatory pattern is limited (13).
In the present study, the model of unilateral
ureteral obstruction (UUO) was employed and the therapeutic role of
MSC-CM in renal fibrosis was investigated. MSC-CM treatment was
demonstrated to attenuate renal fibrosis by hindering B
cell-dependent immune responses following UUO in the present
study.
Materials and methods
MSC isolation and preparation of
MSC-CM
Primary MSCs were isolated from C57BL/6J mice (n=60,
male; 8 weeks of age, 20–25 g) by flushing the femurs, and were
cultured with Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Mice were maintained in air-filtered units at
21±2°C and 50±15% relative humidity under a 12 h light/dark cycle
during the entire experiment. Mice were provided with rodent food
and sterile water ad libitum. MSC-CM was prepared as
described previously (14). MSC
plates (80% confluence) were incubated with serum-free DMEM
low-glucose for 24 h at 37°C. Supernatants from each plate were
then collected and centrifuged at 500 × g at 4°C for 5 min. For
each animal, 300 µl of conditioned medium generated by
approximately 5.0×106 cells were injected
intraperitoneally (i.p.). The present study was approved by the
ethics committee of the People's Hospital of Rizhao (Rizhao,
China).
UUO and injection of MSC-CM
All C57BL/6J mice (male, 8 weeks of age) were
purchased from the Model Animal Research Center of Nanjing
University (Nanjing, China). Mice were divided into four
experimental groups: Sham-operated and PBS treated (Sham + PBS),
sham-operated and MSC-CM treated (Sham + MSC-CM), UUO model and PBS
treated (UUO + PBS), and UUO model and MSC-CM treated (UUO +
MSC-CM) mice (n=12 for each group). Surgical UUO or a sham
operation was performed as previously described (15) for UUO and sham groups,
respectively. Briefly, the mice were carefully anesthetized using
sodium pentobarbital. Following satisfactory anesthesia, a 1 cm
ventral incision was made at the abdominal midline, and the left
ureter and kidney were dissociated, and the left proximal ureter
was exposed and ligated at two separate locations; sham-operated
mice underwent identical exposure without ligation. The incision
was then closed using polypropylene sutures, and the mice were
allowed to recover from anesthesia. Following surgery, animals
received MSC-CM (300 µl) or PBS (300 µl) i.p., which was repeated 3
days later as previously described (16,17).
Flow cytometry and histological assessments were performed at days
3 and 14 following surgery, respectively. Prior to collection of
tissue, mice were fasted overnight and anesthetized with 1.5%
pentobarbital sodium (60 mg/kg i.p.; Shanghai XiTang Biotechnology
Co., Ltd., Shanghai, China). All anaesthetized mice underwent
thorax opening and heart exposure. Following the conduction of
circulatory system perfusion with heparinized PBS, mice were
sacrificed and then the left kidneys were removed for further
analysis.
Histological analysis
The left kidney of each mouse was collected and
fixed in 10% formaldehyde as previously described (13). Masson's trichrome and Sirius Red
staining procedures (at 25–28°C for 20 min) were performed on 6 µm
sections of these paraffin-embedded kidneys for the evaluation of
the severity of tubulointerstitial fibrosis. To determine the
accumulation of monocytes/macrophages and B lymphocytes, sections
of the kidney were also used for immunohistochemical analysis with
the following antibodies: Anti-F4/80 (1:200; ab6640; Abcam,
Cambridge, UK) and anti-B220 (1:100; ab64100; Abcam) antibodies.
Sections were blocked with 5% bovine serum albumin (Sangon Biotech,
Shanghai, China) at 25–28°C for 20 min and then incubated with the
primary antibodies overnight at 4°C. Following incubation with
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:1,000; ab6734; Abcam) at 25–28°C for 20 min, sections were
incubated with 3,3′-diaminobenzidine. The diaminobenzidine reaction
was conducted for ~5 min at 25–28°C. For each section, 10
non-consecutive visual fields were randomly selected and captured
with a light microscope (magnification, ×400, Olympus Corporation,
Tokyo, Japan). Images were quantitatively analyzed using Image-Pro
Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Flow cytometry
Kidney samples were prepared as previously described
(13). Briefly, all anaesthetized
mice underwent thorax opening. The heart was exposed and a
circulatory system perfusion with heparinized PBS was conducted.
Renal cortex tissues were then immediately collected, minced and
placed into RPMI 1640 medium (Thermo Fisher Scientific, Inc.)
containing 40 mg/ml Liberase TM (Roche Diagnostics, Basel,
Switzerland) and 8.5 U/ml DNase I (Roche Diagnostics) for 40 min at
37°C. Cells were washed with serum-free RPMI 1640 medium and
resuspended in FACS buffer (BD Biosciences, Franklin Lakes, USA)
following the addition of red blood cell lysis buffer (BD
Biosciences) to exclude erythrocytes. To quantitatively analyze the
number of leucocytes, the single cell suspensions were labelled
with anti-B220-allophycocyanin (1:100; 17-0452-81; Thermo Fisher
Scientific, Inc.) and anti-CD19-phycoerythrin (1:100; 557399; BD
Pharmingen; BD Biosciences) for 30 min in the dark at 4°C prior to
washing with FACS buffer. Multicolour flow cytometry was performed
using a flow cytometer (FACSAriaIII; BD Biosciences) and data was
analyzed using FlowJo software version 7.6 (FlowJo LLC, Ashland,
OR, USA).
Preparation of cDNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from harvested kidneys was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufactures' protocols. RT was performed
using a First-strand cDNA Synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. The
PCR cycling conditions were as follows: Predenaturation at 95°C for
10 min, 40 cycles at 95°C for 15 sec, 60°C for 1 min and 72°C for
20 sec, and a final extension at 60°C for 5 min. qPCR was performed
with Power SYBR® Green PCR Master Mix in a StepOne
system (Applied BioSystems; Thermo Fisher Scientific, Inc.). The
sequences of the primers for CC chemokine ligand-2 (CCL-2) were as
follows: 5′-TTAAAAACCTGGATCGGAACCAA-3′ (forward) and
5′-GCATTAGCTTCAGATTTACGGGT-3′ (reverse). Gene expression levels
were normalized with GAPDH [5′-GGTGAAGGTCGGTGTGAACG-3′ (forward)
and 5′-CTCGCTCCTGGAAGATGGTG-3′ (reverse)] and data were quantified
and analyzed with StepOne software v2.1 (Thermo Fisher Scientific,
Inc.).
Western blotting
Renal tissue samples were used for the extraction of
protein and homogenized in ProteoJET Mammalian Cell Lysis Reagent
(Fermentas; Thermo Fisher Scientific, Inc.). Total proteins were
quantified using the bicinchoninic acid method. Western blotting
was performed as previously described (18). Proteins (30 µg/lane) were separated
by 10% SDS-PAGE. The protein was transferred to a polyvinylidene
floride membrane and blocked with 5% skimmed milk for 2 h at
25–28°C. Tumor necrosis factor (TNF)-α (1:1,000; ab66579, Abcam),
interleukin (IL)-1β (1:1,000; sc-7884, Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), intercellular adhesion molecule 1 (ICAM-1;
1:1,000; ab179707, Abcam), IL-6 (1:1,000; ab208113, Abcam) were
incubated with the membrane overnight at 4°C. Horseradish
peroxidase-labelled goat anti-rabbit secondary antibody (1:5,000;
cat. no. 7074; Cell Signaling Technology, Inc., Danvers, MA, USA)
was added for incubation at room temperature for 1 h, followed by
addition of an enhanced chemiluminescence luminous fluid (Beyotime
Institute of Biotechnology, Haimen, China) at room temperature for
3 min. The gel was photographed using a gel imaging system. β-actin
(1:2,000; cat. no. 4970; Cell Signaling Technology, Inc.) was used
as a house-keeping reference. Densitometric analysis of the western
blot results was performed with Image J version 1.48 software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were analyzed using SPSS software, version 15.0
for Windows (SPSS Inc., Chicago, IL, USA). Results are presented as
the mean ± standard deviation. The statistical analysis for the
determination of differences in the measured properties between
groups was accomplished using one-way analysis of variance followed
by a Turkey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
MSC-CM treatment attenuates
UUO-induced fibrosis and renal inflammation
Masson's trichrome and Sirius Red staining was
performed to assess fibrotic alterations induced by UUO. The
blue-stained (Masson's trichrome-positive) and red-stained (Sirius
Red-positive) areas, associated with collagen deposition, were
markedly increased in UUO mice compared with sham-operated groups.
Notably, treatment with MSC-CM effectively protected the injured
kidney from tubulointerstitial fibrosis following UUO, compared
with the UUO + PBS group (Fig.
1).
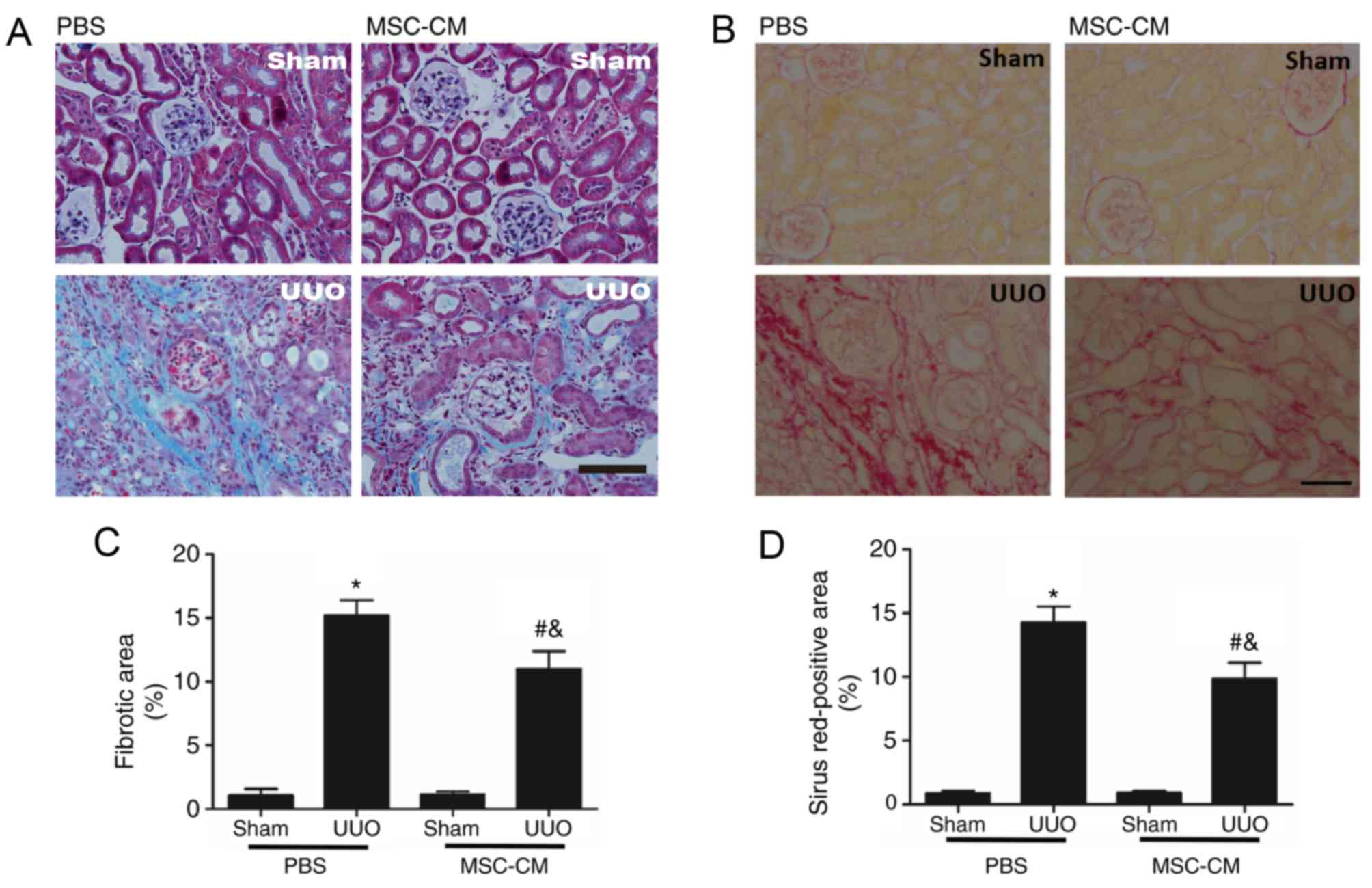 | Figure 1.MSC-CM treatment attenuates
UUO-induced renal fibrosis. (A) Representative images of renal
Masson's trichrome-stained sections in Sham + PBS, Sham + MSC-CM,
UUO + PBS, and UUO + MSC-CM mice 2 weeks following surgery.
Collagen is visible as the blue color. Scale bar=50 µm. (B)
Representative images of Sham + PBS, Sham + MSC-CM, UUO + PBS and
UUO + MSC-CM mice 2 weeks following surgery. Collagen is visible as
the red stain. Scale bar=50 µm. (C) Quantitative analysis of renal
Masson's trichrome-stained sections. (D) Quantitative analysis of
Sirius red-stained renal sections. *P<0.05 vs. Sham + PBS group;
#P<0.05 vs. Sham + MSC-CM group;
&P<0.05 vs. UUO + PBS group. Data are presented
as the mean ± standard deviation (n=6–8). MSC-CM, mesenchymal stem
cell-conditioned media; Sham + PBS, sham-operated and PBS treated;
Sham + MSC-CM, sham-operated and MSC-CM treated; UUO, unilateral
ureteral obstruction; UUO + PBS, UUO model and PBS treated; UUO +
MSC-CM, UUO model and MSC-CM treated. |
Throughout the procession of UUO-associated
fibrogenesis, local inflammatory responses serve a vital role.
Therefore, the expression levels of numerous key proinflammatory
factors 14 days following the UUO operation were analyzed via
western blotting. Statistical analysis revealed that MSC-CM
treatment significantly decreased renal expression of TNF-α, IL-1β,
IL-6 and ICAM-1 following UUO compared with the UUO + PBS group. In
addition, the expression levels of TNF-α, IL-1β, IL-6 and ICAM-1
were significantly lower in the sham groups compared with in the
UUO groups (Fig. 2).
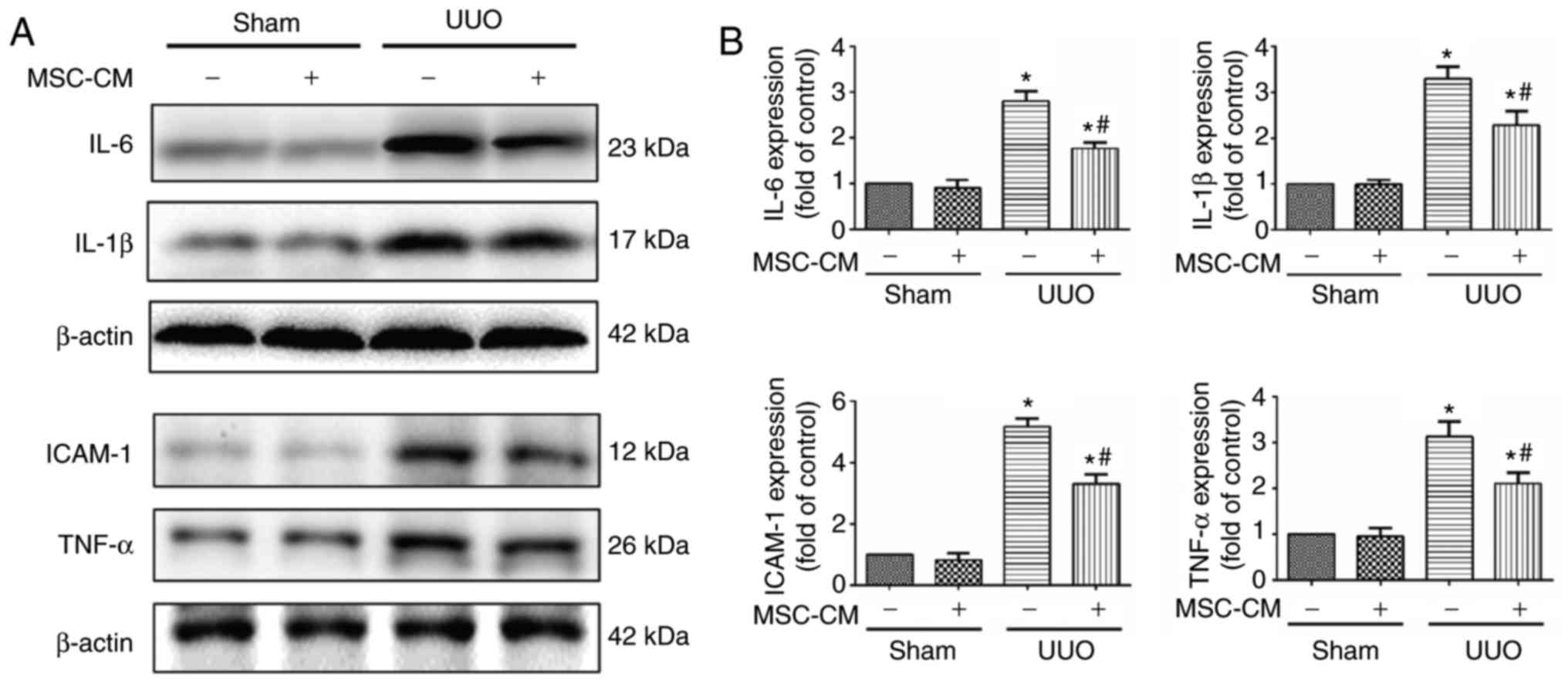 | Figure 2.MSC-CM treatment ameliorates
UUO-induced renal inflammation. (A) Representative western blotting
images and (B) quantitative analysis of the expression levels of
key proinflammatory factors in Sham + PBS, Sham + MSC-CM, UUO +
PBS, and UUO + MSC-CM mice 3 days following surgery. *P<0.05 vs.
Sham + PBS group; #P<0.05 vs. UUO + PBS group.
ICAM-1, intercellular adhesion molecule 1; IL, interleukin; TNF-α,
tumor necrosis factor-α; MSC-CM, mesenchymal stem cell-conditioned
media; Sham + PBS, sham-operated and PBS treated; Sham + MSC-CM,
sham-operated and MSC-CM treated; UUO, unilateral ureteral
obstruction; UUO + PBS, UUO model and PBS treated; UUO + MSC-CM,
UUO model and MSC-CM treated. |
MSC-CM treatment reduces intrarenal
infiltration of monocytes/macrophages following UUO
Monocytes/macrophages are a primary source of
proinflammatory factors, and their influx into the renal
interstitium is deemed one of the typical features of kidney
injury. Analysis of the immunohistochemical results revealed no
alterations in the number of infiltrated F4/80-positive cells
between PBS and MSC-CM-treated sham mice. Notably, MSC-CM-treated
ones revealed a significant reduction in monocytic infiltration
into the kidney 3 days following UUO surgery (Fig. 3A and B).
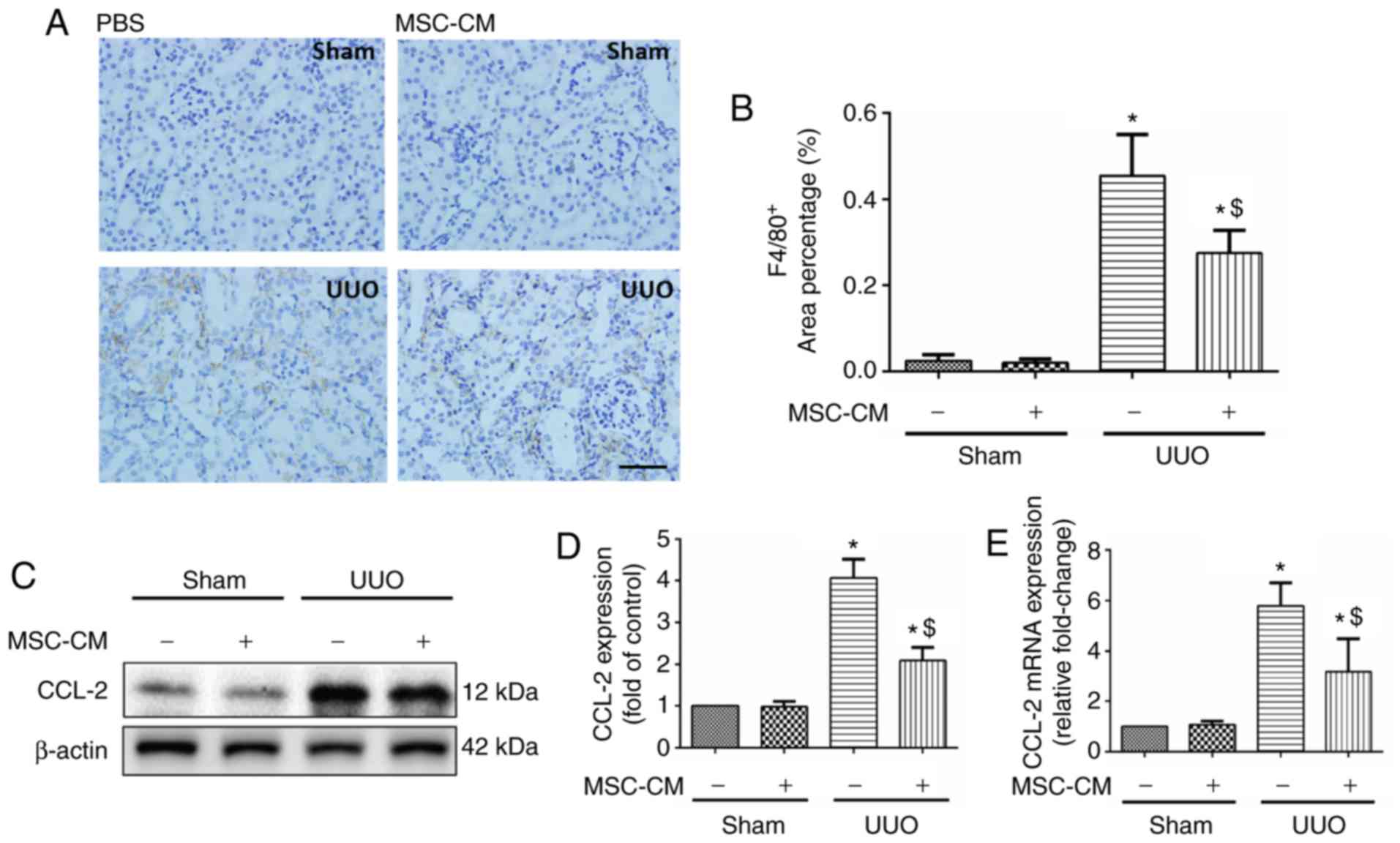 | Figure 3.MSC-CM treatment alters chemotactic
responses following UUO. (A) Representative images and (B)
quantitative analysis of F4/80 staining in Sham + PBS, Sham +
MSC-CM, UUO + PBS and UUO + MSC-CM mice 3 days following surgery.
Scale bar=50 µm. Data are presented as the mean ± standard
deviation (n=7–8). (C) Representative western blotting images and
(D) quantitative analysis for CCL-2, 3 days following surgery. Data
are presented as the means ± standard deviation (n=6). (E) Reverse
transcription-quantitative polymerase chain reaction analysis of
CCL-2 mRNA in kidneys from Sham + PBS, Sham + MSC-CM, UUO + PBS and
UUO + MSC-CM mice 3 days following surgery. Data are presented as
the means ± standard deviation (n=6). *P<0.05 vs. Sham + PBS
group; $P<0.05 vs. UUO + PBS group. CCL-2, CC
chemokine ligand-2; MSC-CM, mesenchymal stem cell-conditioned
media; Sham + PBS, sham-operated and PBS treated; Sham + MSC-CM,
sham-operated and MSC-CM treated; UUO, unilateral ureteral
obstruction; UUO + PBS, UUO model and PBS treated; UUO + MSC-CM,
UUO model and MSC-CM treated. |
MSC-CM treatment decreases the
expression of local CCL-2 in UUO kidneys
CCL-2, also known as MCP-1, is a member of CC-family
chemokines. Several studies have reported that CCL-2 serves a
critical role in the mobilization and relocation of inflammatory
cells in response to UUO injury (13,19,20).
To investigate the potential mechanisms associated with the effects
of MSC-CM, the expression levels of CCL-2 were analyzed via western
blotting and RT-qPCR. As revealed by western blotting, UUO resulted
in the upregulation of CCL-2, which was attenuated by MSC-CM
treatment (Fig. 3C and D). This
result was further verified by RT-qPCR (Fig. 3E).
MSC-CM treatment alleviates mature B
lymphocyte infiltration into the kidneys following UUO
B lymphocytes (B220+ and/or
CD19+ cells) are a primary source of renal CCL-2
following UUO, particularly during the early stages. Mice were
sacrificed for immunohistochemistry and flow cytometry 3 days
following surgery. According to the immunohistochemical analysis,
obstructed kidneys suffered greater infiltration of
B220+ B lymphocytes compared with sham-operated groups.
This effect was markedly inhibited by MSC-CM treatment (Fig. 4A and B). Additionally, the results
of flow cytometry demonstrated that staining B cells with both
anti-B220 and anti-CD19 antibodies, produced a similar effect
(Fig. 4C and D).
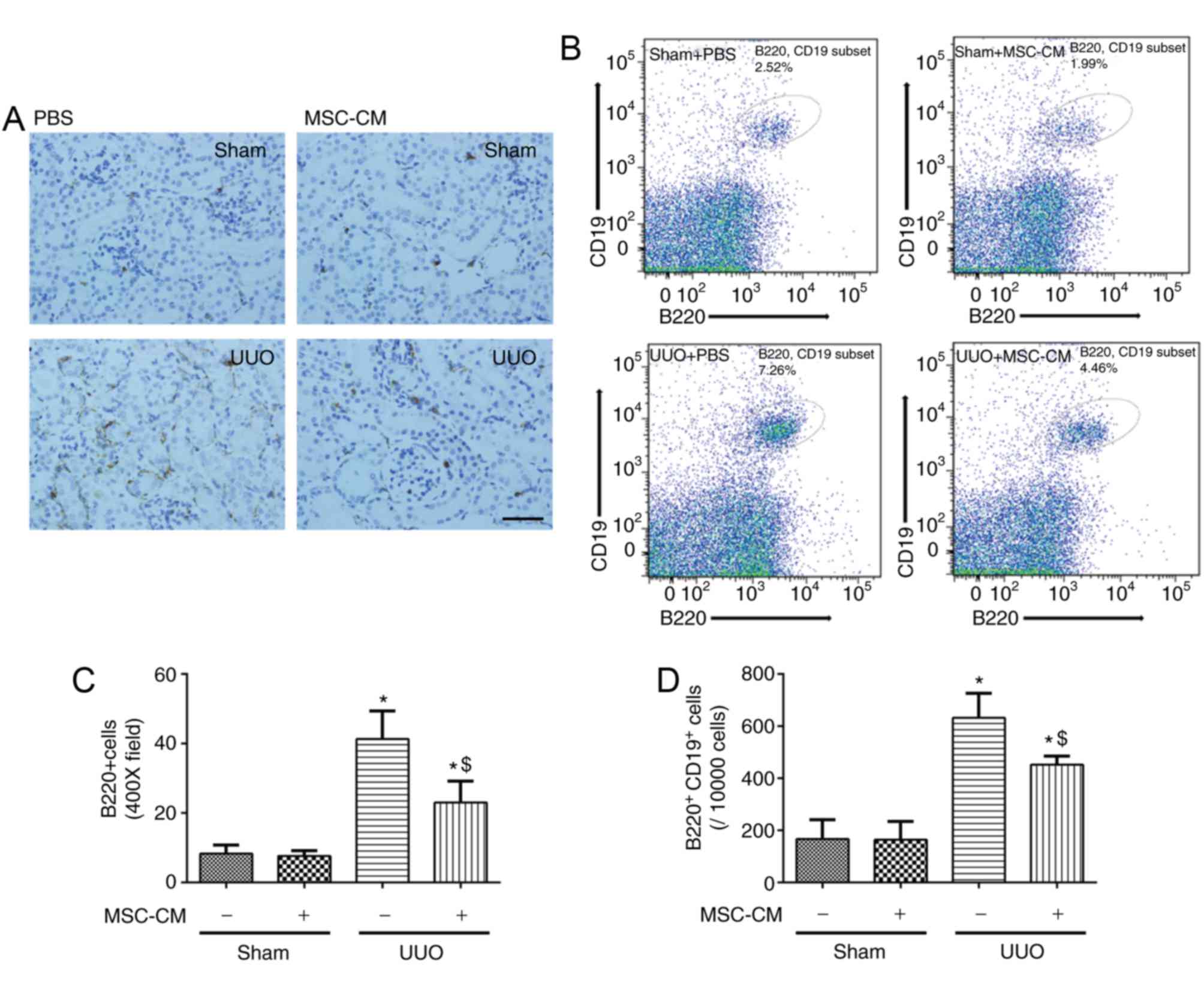 | Figure 4.MSC-CM treatment alleviates mature B
lymphocyte infiltration into the kidneys following UUO. (A)
Representative images of Sham + PBS, Sham + MSC-CM, UUO + PBS and
UUO + MSC-CM mice 3 days following surgery. Scale bar=50 µm. Data
are presented as the mean ± standard deviation (n=6–7). (B)
Representative flow cytometry images of renal
B220+CD19+ B lymphocyte cytometry of Sham +
PBS, Sham + MSC-CM, UUO + PBS and UUO + MSC-CM mice 3 days
following surgery. (C) Quantitative analysis of B220 staining and
(D) quantitative analysis of renal B220+CD19+
B lymphocyte cytometry. Data are presented as the means ± standard
deviation (n=6). *P<0.05 vs. Sham + PBS group;
$P<0.05 vs. UUO + PBS group. MSC-CM, mesenchymal stem
cell-conditioned media; Sham + PBS, sham-operated and PBS treated;
Sham + MSC-CM, sham-operated and MSC-CM treated; UUO, unilateral
ureteral obstruction; UUO + PBS, UUO model and PBS treated; UUO +
MSC-CM, UUO model and MSC-CM treated. |
Discussion
Previous studies have reported the beneficial
effects of MSC-CM administration in modulating renal expression of
cytokines associated with inflammation and cell proliferation.
Immune cells, including monocytes/macrophages, B and T cells, and
mast cells, are also considerable participants in the initiation
and progression of renal fibrosis (21–23);
however, it is unclear whether MSC-CM exerts an extensive impact on
the regulation of intrarenal infiltration of lymphocytes following
UUO. The present study revealed a protective effect of MSC-CM in
attenuating fibrosis. In this process of treatment, the
mobilization of monocytes was efficiently weakened by MSC-CM
administration.
Abundant evidence has established that excess
inflammation is an essential part of host defense mechanisms
following tissue injury (24). By
producing various inflammatory cytokines, monocytes/macrophages
serve a considerable role in the process of post-injury
fibrogenesis. Once induced, the progression of the fibrotic process
may increase over time, and established fibrosis can seldom be
reversed. Therefore, it is a great challenge to intervene from the
initial phase and throughout the whole process of the disease. For
UUO mice, MSC-CM treatment led to a reduced infiltration of renal
monocytes/macrophages, and a decreased expression of
pro-inflammatory factors in damaged kidneys, suggesting that MSC-CM
may have protected against fibrotic progression by rebalancing the
inflammatory response following UUO, particularly at the early
stages of pathogenesis.
The reduction of monocytic infiltration and
proinflammatory cytokines is most likely a consequence that is
associated with the B cell-mediated immune responses. The present
study demonstrated that MSC-CM treatment attenuated the
upregulation of CCL-2 induced by UUO. CCL-2, also known as monocyte
chemotactic protein 1, is ubiquitously expressed in a large variety
of cell types, including smooth muscle cells, tubular cells,
podocytes, mesangial cells and infiltrated leucocytes (25). Via the interaction with CC
chemokine receptor-2, CCL-2 mediates the transmigration and influx
of inflammatory monocytes into impaired kidneys (26,27).
It has previously been demonstrated that early-stage infiltrated B
cells are one of the major producers of CCL-2 in the damaged renal
tissue (13). The results of the
present study revealed that obstructed kidneys exhibited increased
incidence of mature B lymphocytic infiltration compared with the
sham-operated groups. This effect was significantly alleviated by
MSC-CM treatment. These results reinforced the suggestion that
MSC-CM may be a potent regulator and option for the treatment of
renal immuno-imbalance and interstitial fibrosis following acute
injury.
In conclusion, the present study suggested that the
administration of MSC-CM ameliorated the intrarenal infiltration of
proinflammatory monocytes/macrophages, the level of inflammation
associated with renal fibrosis and subsequent fibrotic progression
in mice subjected to UUO. The therapeutic effect of MSC-CM may
possibly be attributed to the reduced recruitment of B lymphocytes
and expression of CCL-2.
Acknowledgements
We are grateful for the assistance of physicians
from the Departments of Laboratory Medicine and Nephrology, the
People's Hospital of Rizhao (Rizhao, China).
Funding
The present study was supported by Discipline
Development Fund from the People's Hospital of Rizhao.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and QW produced the main manuscript text and
prepared all the figures and tables. JP participated in the
production of the manuscript and contributed to the research
design. XS and WL participated in data analysis. All the authors
discussed and agreed on the results, and read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the People's Hospital of Rizhao (Rizhao, China).
Consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 7:684–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X and Dai C: Advances in understanding
and management of residual renal function in patients with chronic
kidney disease. Kidney Dis (Basel). 2:187–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bianco P, Cao X, Frenette PS, Mao JJ,
Robey PG, Simmons PJ and Wang CY: The meaning, the sense and the
significance: Translating the science of mesenchymal stem cells
into medicine. Nat Med. 19:35–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monsel A, Zhu YG, Gennai S, Hao Q, Liu J
and Lee JW: Cell-based therapy for acute organ injury: Preclinical
evidence and ongoing clinical trials using mesenchymal stem cells.
Anesthesiology. 121:1099–1121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gnecchi M, Zhang Z, Ni A and Dzau VJ:
Paracrine mechanisms in adult stem cell signaling and therapy. Circ
Res. 103:1204–1219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duffield JS and Bonventre JV: Kidney
tubular epithelium is restored without replacement with bone
marrow-derived cells during repair after ischemic injury. Kidney
Int. 68:1956–1961. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong JO, Han JW, Kim JM, Cho HJ, Park C,
Lee N, Kim DW and Yoon YS: Malignant tumor formation after
transplantation of short-term cultured bone marrow mesenchymal stem
cells in experimental myocardial infarction and diabetic
neuropathy. Circ Res. 108:1340–1347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kunter U, Rong S, Boor P, Eitner F,
Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T,
Villa L, et al: Mesenchymal stem cells prevent progressive
experimental renal failure but maldifferentiate into glomerular
adipocytes. J Am Soc Nephrol. 18:1754–1764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
da Silva AF, Silva K, Reis LA, Teixeira VP
and Schor N: Bone Marrow-derived mesenchymal stem cells and their
conditioned medium attenuate fibrosis in an irreversible model of
unilateral ureteral obstruction. Cell Transplant. 24:2657–2666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Koppen A, Joles JA, van Balkom BW, Lim
SK, de Kleijn D, Giles RH and Verhaar MC: Human embryonic
mesenchymal stem cell-derived conditioned medium rescues kidney
function in rats with established chronic kidney disease. PLoS One.
7:e387462012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang B, Cheng X, Wang H, Huang W, la Ga
Hu Z, Wang D, Zhang K, Zhang H, Xue Z, Da Y, et al: Mesenchymal
stem cells and their secreted molecules predominantly ameliorate
fulminant hepatic failure and chronic liver fibrosis in mice
respectively. J Transl Med. 14:452016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohnishi S, Sumiyoshi H, Kitamura S and
Nagaya N: Mesenchymal stem cells attenuate cardiac fibroblast
proliferation and collagen synthesis through paracrine actions.
FEBS Lett. 581:3961–3966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han H, Zhu J, Wang Y, Zhu Z, Chen Y, Lu L,
Jin W, Yan X and Zhang R: Renal recruitment of B lymphocytes
exacerbates tubulointerstitial fibrosis by promoting monocyte
mobilization and infiltration after unilateral ureteral
obstruction. J Pathol. 241:80–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aslam M, Baveja R, Liang OD,
Fernandez-Gonzalez A, Lee C, Mitsialis SA and Kourembanas S: Bone
marrow stromal cells attenuate lung injury in a murine model of
neonatal chronic lung disease. Am J Respir Crit Care Med.
180:1122–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nilsson L, Madsen K, Krag S, Frøkiær J,
Jensen BL and Nørregaard R: Disruption of cyclooxygenase type 2
exacerbates apoptosis and renal damage during obstructive
nephropathy. Am J Physiol Renal Physiol. 309:F1035–F1048. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kay AG, Long G, Tyler G, Stefan A,
Broadfoot SJ, Piccinini AM, Middleton J and Kehoe O: Mesenchymal
stem cell-conditioned medium reduces disease severity and immune
responses in inflammatory arthritis. Sci Rep. 7:180192017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ooi YY, Dheen ST and Tay SS: Paracrine
effects of mesenchymal stem cells-conditioned medium on microglial
cytokines expression and nitric oxide production.
Neuroimmunomodulation. 22:233–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao F, Wang Y, Li S, Wang Z, Liu C and Sun
D: Inhibition of p38 mitogen-activated protein kinases attenuates
renal interstitial fibrosis in a murine unilateral ureteral
occlusion model. Life Sci. 167:78–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watatani H, Maeshima Y, Hinamoto N,
Yamasaki H, Ujike H, Tanabe K, Sugiyama H, Otsuka F, Sato Y and
Makino H: Vasohibin-1 deficiency enhances renal fibrosis and
inflammation after unilateral ureteral obstruction. Physiol Rep.
2:pii: e12054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma W, Tao L, Wang X, Liu Q, Zhang W, Li Q,
He C, Xue D, Zhang J and Liu C: Sorafenib inhibits renal fibrosis
induced by unilateral ureteral obstruction via inhibition of
macrophage infiltration. Cell Physiol Biochem. 39:1837–1849. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bohle A, Wehrmann M, Bogenschütz O, Batz
C, Vogl W, Schmitt H, Müller CA and Müller GA: The long-term
prognosis of the primary glomerulonephritides. A morphological and
clinical analysis of 1747 cases. Pathol Res Pract. 188:908–924.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eardley Sean K and Cockwell P: Macrophages
and progressive tubulointerstitial disease. Kidney Int. 68:437–455.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chevalier RL, Forbes MS and Thornhill BA:
Ureteral obstruction as a model of renal interstitial fibrosis and
obstructive nephropathy. Kidney Int. 75:1145–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tesch GH: MCP-1/CCL2: A new diagnostic
marker and therapeutic target for progressive renal injury in
diabetic nephropathy. Am J Physiol Renal Physiol. 294:F697–F701.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haller H, Bertram A, Nadrowitz F and Menne
J: Monocyte chemoattractant protein-1 and the kidney. Curr Opin
Nephrol Hypertens. 25:42–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Connor T, Borsig L and Heikenwalder M:
CCL2-CCR2 signaling in disease pathogenesis. Endocr Metab Immune
Disord Drug Targets. 15:105–118. 2015. View Article : Google Scholar : PubMed/NCBI
|


















