Introduction
Pulmonary fibrosis is a refractory pulmonary disease
that significantly affects lung-associated functions (1). Pulmonary fibrosis is also a type of
severe interstitial lung disease that has been associated with a
progressive loss of lung function; in addition, relatively higher
mortality rates have been observed in clinical settings compared
with pulmonary contusion (2).
Investigations have revealed that pulmonary fibrosis is caused by
various factors, including inflammation and breathing disorders
(3). A systematic review and
meta-analysis demonstrated the association between the severity of
breathing disorders, and the aggregation of extracellular matrix
components and inflammation-associated injury (4). A reported increase in the incidence
and mortality rates of pulmonary fibrosis has been associated with
the development of severe acute respiratory syndrome (5). Therefore, understanding the potential
signaling mechanism underlying pulmonary fibrosis is essential to
understand the progression of this disease.
Long non-coding RNAs (lncRNAs) are associated with
numerous human diseases via the regulation of different signal
pathways within cells (6–8). A previous analysis of lncRNA as a
competing endogenous RNA and its association with protein-coding
genes has indicated potential associations among lncRNAs, microRNAs
(miRNAs) and mRNAs in pulmonary fibrosis (9), which may be applied to future
investigations into the treatment of this disease. In addition, Wu
et al (10) reported that
miRNA-489 could inhibit silica-induced pulmonary fibrosis by
targeting myeloid differentiation response 88 and mothers against
decapentaplegic homolog 3, which are negatively regulated by
lncRNA-CHRF (10). Studies
regarding lncRNA polymorphisms are of increasing interest to
scientists and pathologists, and may aid the development of lung
disease-associated therapies (11–14).
Therefore, investigations into the potential roles of lncRNAs are
crucial in understanding human pulmonary diseases.
Evidence has revealed that the lncRNA, prostate
cancer-associated transcript 29 (lncRNAPCAT29), constitutes a
tumor-suppressive factor within numerous cell types (11); however, the role of lncRNAPCAT29 in
the progression of pulmonary fibrosis has yet to be analyzed. In
the present study, the role of lncRNAPCAT29 in the progression of
pulmonary fibrosis and its underlying mechanism were investigated.
Additionally, the involvement of lncRNAPCAT29 in suppressing
pulmonary fibroblast proliferation and ameliorating inflammation in
silica-induced pulmonary fibrosis were analyzed.
Materials and methods
Statement of ethics
Animal procedures were conducted in accordance with
humane animal care standards. Experimental protocols were approved
by the Ethics Committee of the Affiliated Hospital of Shandong
University of Traditional Chinese Medicine (Jinan, China).
Animals
Specific pathogen-free C57BL/6 male mice (age, 4–6
weeks of age; body weight, 26–32 g) were purchased from the
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Mice
were fed under pathogen-free conditions (23±1°C; relative humidity,
50±5%) and were maintained under a 12-h light/dark cycle with free
access to food and water. To establish a mouse model of pulmonary
fibrosis, mice were instilled with 50 mg/kg silica (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) in 0.05 ml sterile saline
intratracheally or with 0.05 ml sterile saline intratracheally
(n=30/group) (12). Mice were
sacrificed, and lungs were harvested and stored at −80°C
immediately after treatment.
Cell culture and reagents
Pulmonary fibroblasts were isolated from
experimental mice treated with 50 mg/kg silica or sterile saline.
Lung tissues were sectioned to ~1 mm3 and were digested
with 0.25% trypsin for ~12 h at 4°C. Cells were then cultured in
Minimum Essential Medium (MEM; Sigma-Aldrich; Merck KGaA)
supplemented with 10% fetal calf serum (FCS; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a 37°C humidified atmosphere
containing 5% CO2. Subsequently, cells were filtered via
100 µm nylon filters to remove undigested tissue.
Endogenous overexpression of
TGF-β1
Pulmonary fibroblasts (1×107) were
isolated from experimental mice prior to treatment (n=5) and were
cultured in MEM supplemented with 10% FCS. Cells were grown to 85%
confluence and were subsequently transfected with pedue12.4-TGF-β1
(TGF-β1, 100 pmol; GenBank: GQ338152.1; Invitrogen; Thermo Fisher
Scientific, Inc.) or pedue12.4 (Control, 100 pmol; Invitrogen;
Thermo Fisher Scientific, Inc.) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Stable TGF-β1
overexpression within pulmonary fibroblasts was identified using a
GS screening system (BD Biosciences, Franklin Lakes, NJ, USA)
according to the manufacturer's protocol (13).
Endogenous overexpression of miRNA-221
or lncRNAPCAT29
Pulmonary fibroblasts were grown to 85% confluence
and were subsequently transduced with 100 pmol
plentivirus-miRNA-221 (miRNA-221, 5′-GUGUAGUCCACCACUAGCUAGC-3′), or
100 pmol plentivirus-lncRNAPCAT29 (lncRNAPCAT29,
5′-AUCUCGACGUGCGGUUACUCUA-3′), 100 pmol plentivirus-lncRNA vector
(5′-UUAGGCUGAGUAGCUUGAA-3′) or 100 pmol scramble miRNA
(5′-CAUGUAAGCGGAUUGCA-3′) using a lentiviral vector system (System
Biosciences, Palo Alto, CA, USA) according to the manufacturer's
protocol. All miRNA sequences were supplied by Invitrogen; Thermo
Fisher Scientific, Inc. After 48 h transduction, stable expression
of miRNA-221 and lncRNAPCAT29 within pulmonary fibroblast cells
were identified as stated in a previous report (14). In additon,
plentivirus-lncRNAPCAT29-transduced cells were then transduced with
miRNA-221 using Lipofectamine RNAiMax reagent (Invitrogen; Thermo
Fisher Scientific, Inc.).
Small interfering-RNA (siRNA) for
miRNA-221 or TGF-β1 knockdown
siRNA sequences targeting miRNA-221 or TGF-β1 gene
sequences were designed and synthesized by Invitrogen (Thermo
Fisher Scientific, Inc.). The siRNA oligonucleotide sequences were
as follows: si-TGF-β1 5′-AGCTTCTGTCCGGATCTAA-3′; si-miRNA-221
5′-GTGTAGTCCACCACTAGCTAGC-3′ or si-Vector (Control)
5′-ACGTAGATCCTTCAGCACC-3′. The siRNAs were transfected into
pulmonary fibroblast cells for further analysis using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to a
recent study (15).
LncRNAPCAT29-overexpressed fibroblast cells were also transefected
with siRNA sequences targeting miRNA-221.
LncRNAPCAT29-overexpressed cells were also treated with si-TGF-β1
and pedue12.4-TGF-β1. After 48 h transfection, cells were used for
further analysis.
Proliferation assay
Transfected/transduced pulmonary fibroblasts
(1×103) exhibiting stable expression of each condition
were seeded in a 96-well plate for 48 h in triplicate. Following
incubation at 37°C, 20 µl MTT (5 mg/ml) in PBS solution was added
to each well and incubated for 4 h at 37°C. The medium (100 µl) was
removed and 100 µl dimethyl sulfoxide was added to the wells to
solubilize the crystals. The optical density was measured using an
ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 450
nm.
Migration assay
Prior to incubation for 48 h at 37°C in a Matrigel
Invasion Chamber (BD Biosciences), according to the manufacturer's
protocol, treated cells were suspended at a density of
1×105 in 500 µl serum-free MEM (Sigma-Aldrich; Merck
KGaA). Migration of transfected pulmonary fibroblasts was analyzed
in ≥3 randomly-selected fields of each membrane via light
microscopy using ImageJ software (version 2.2; National Institutes
of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was obtained from pulmonary fibroblasts
isolated from experimental mice using an RNAeasy Mini kit (Qiagen,
Inc., Valencia, CA, USA). Expression levels of PCAT29 in cells were
measured via Verso One-Step RT-qPCR kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and RT-qPCR conditions were performed as descibed
previously (16). Forward and
reverse primers were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc.). PCAT29 forward, 5′-TTTATGCTTGAGCCTTGA-3′ and
reverse, 5′-CTTGCCTGAAATACTTGC-3′; β-actin (control) forward,
5′-GTGGGCGCCCAGGCACCA-3′ and reverse,
5′-CTCCTTAATGTCACGCACGATTT-3′); miRNA-221,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGGGGTATT-3′; and
tRNAthr 5′-CTCAACTGGTGTCGTGGA-3′. Relative mRNA
expression alterations were calculated by 2−ΔΔCq
(17). Results are expressed as
the n-fold compared with the control.
Western blot analysis
Protein was extracted from treated cells using
radioimmunoprecipitation assay buffer (M-PER reagent for cells;
Thermo Fisher Scientific, Inc.), followed by homogenization at 4°C
for 10 min. Protein concentration was measured using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). A total of 20 µg protein was electrophoresed via 12.5%
SDS-PAGE and subsequently transferred to nitrocellulose membranes.
Blocking buffer (5% milk) was applied to membranes for 2 h at 37°C
prior to incubation with primary antibodies at 4°C overnight. The
primary antibodies used in the immunoblotting assays included:
TGF-β1 (1:200; ab92486), matrix metalloproteinase (MMP) 3 (1:1,000;
ab53015), tumor necrosis factor-α (TNF-α; 1:500; ab6671),
interleukin-1β (IL-1β; 1:500; ab200478), MMP9 (1:500; ab73734), RAS
protein activator like 1 (RASAL1; 1:500; ab214321), extracellular
signal-regulated kinase 1/2 (ERK 1/2; 1:1,000; ab32537),
phosphorylated (p)-Thr202/Tyr204 ERK1/2 (1:500; ab214362),
fibronectin (FN; 1:500; ab2413), extracellular matrix collagen I
(CLAI; 1:500; ab34710), NEDD4 binding protein 2 (N4bp2; 1:500;
ab102634), plexin A4 (Plxna4; 1:500; ab39350) and β-actin (1:500;
ab8227; all Abcam, Cambridge, UK). Horseradish peroxidase
(HRP)-conjugated anti-rabbit immunoglobulin G (IgG; 1:5,000; cat.
no. 1706515; Bio-Rad Laboratories, Inc.) was applied for 2 h at
37°C and bands were detected using WesternBright ECL
Chemiluminescent HRP Substrate (Advansta, Inc., Menlo Park, CA,
USA).
Immunohistochemical staining
Lung tissues were obtained from experimental mice
following treatment. Tissues were fixed with 4% paraformaldehyde
for 12 h at 4°C, paraffin-embedded lung tissue sections (4 µm) were
prepared and epitope retrieval was performed using Tris-HCl buffer
(AP-9005-050; Thermo Fisher Scientific, Inc.) for 30 min at 37°C
for further analysis. The paraffin-embedded sections were treated
with hydrogen peroxide (3%) for 15 min and subsequently blocked
with a regular blocking solution for 20 min at 37°C. The sections
were subsequently incubated with rabbit anti-mouse PCAT29 antibody
[1:500; Q7L5N7; Baiqi Biotechnology (Suzhou Co.. Ltd., Suzhou,
China) at 4°C for 12 h]. Sections were washed three times and
incubated with HRP-conjugated anti-rabbit IgG (1:10,000; cat. no.
1706515; Bio-Rad Laboratories, Inc.) for 1 h at 37°C. Tissues
sections were observed in six randomly selected fields under a
confocal microscope (LSM780; Carl Zeiss AG, Oberkochen, Germany).
Densitometric semi-quantification of the immunoblot data was
performed using Quantity-One software version 4.2 (Bio-Rad
Laboratories, Inc.).
Statistical methods
Data are expressed as the mean ± standard error of
the mean. Unpaired data were analyzed by Student's t-test.
Comparisons of data between multiple groups were analyzed using
one-way analysis of variance followed by Tukey's honest significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
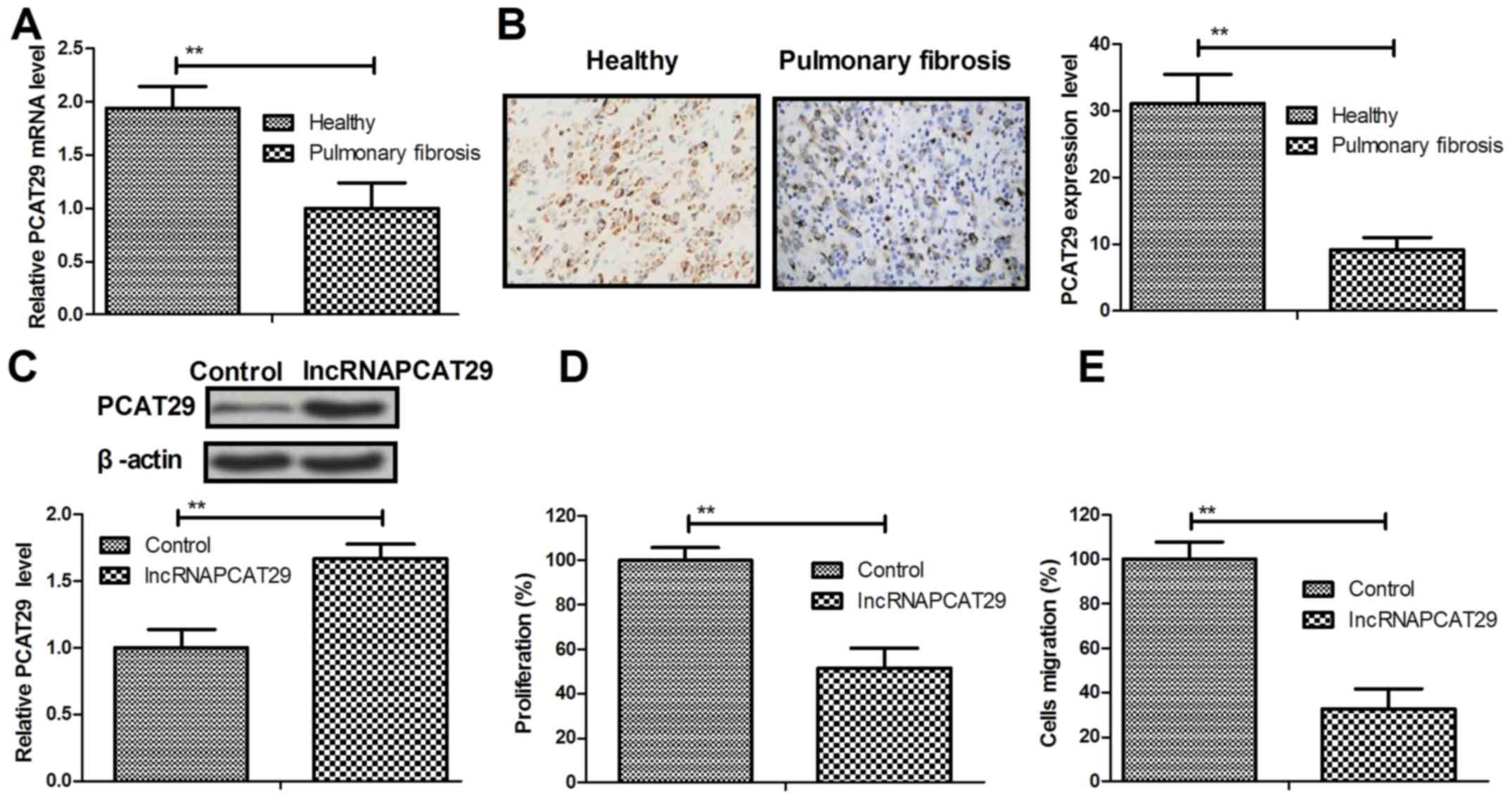
lncRNAPCAT29 inhibits pulmonary
fibroblast proliferation and migration in vitro
Expression levels of lncRNAPCAT29 in pulmonary
fibroblasts isolated from healthy and silica-induced mouse models
of pulmonary fibrosis were analyzed. The results of the present
study demonstrated that PCAT29 was significantly downregulated in
pulmonary fibroblasts from the pulmonary fibrosis group, as
determined by RT-qPCR and immunohistochemistry (Fig. 1A and B). lncRNAPCAT29 transfection
increased PCAT29 protein expression levels in pulmonary fibroblasts
(Fig. 1C). In addition,
significant inhibition of pulmonary fibroblast cell proliferation
was observed (Fig. 1D). The
results also suggested that migration of pulmonary fibroblasts was
downregulated in response to lncRNAPCAT29 overexpression (Fig. 1E). Collectively, these results
indicated that lncRNAPCAT29 is downregulated in pulmonary fibrosis,
whereas lncRNAPCAT29 overexpression may inhibit pulmonary
fibroblast proliferation and migration in vitro.
lncRNAPCAT29 inhibits differentiation
by targeting TGF-β1 in pulmonary fibroblasts
In the present study, the potential molecular
mechanism of lncRNAPCAT29-associated inhibition of pulmonary
fibroblast proliferation was analyzed. Overexpression of miRNA-221
(pmiRNA-221) was associated with increased expression levels of
miRNA-221, whereas silenced miRNA-221 (Si-miRNA-221) was associated
with reduced expression of miRNA-221 within pulmonary fibroblasts
(Fig. 2A and B). lncRNAPCAT29
transfection was observed to suppress differentiation of pulmonary
fibroblast cells, as determined by reduced levels of MMP3 and MMP9
(Fig. 2C). miRNA-221 was also
significantly upregulated due to lncRNAPCAT29 transfection
(Fig. 2D). pmiRNA-221 was
associated with reduced TGF-β1 expression levels within pulmonary
fibroblasts (Fig. 2E),
additionally western blot analysis indicated that lncRNAPCAT29
inhibited TGF-β1 expression; whereas, Si-miRNA-221 expression
inhibited lncRNAPCAT29-suppressed (Si221-29) TGF-β1 expression
within pulmonary fibroblasts (Fig.
2F). Collectively, these results suggested that lncRNAPCAT29
inhibited fibroblast differentiation via affecting the
miRNA-221-regulated TGF-β1 signaling pathway in pulmonary
fibroblasts.
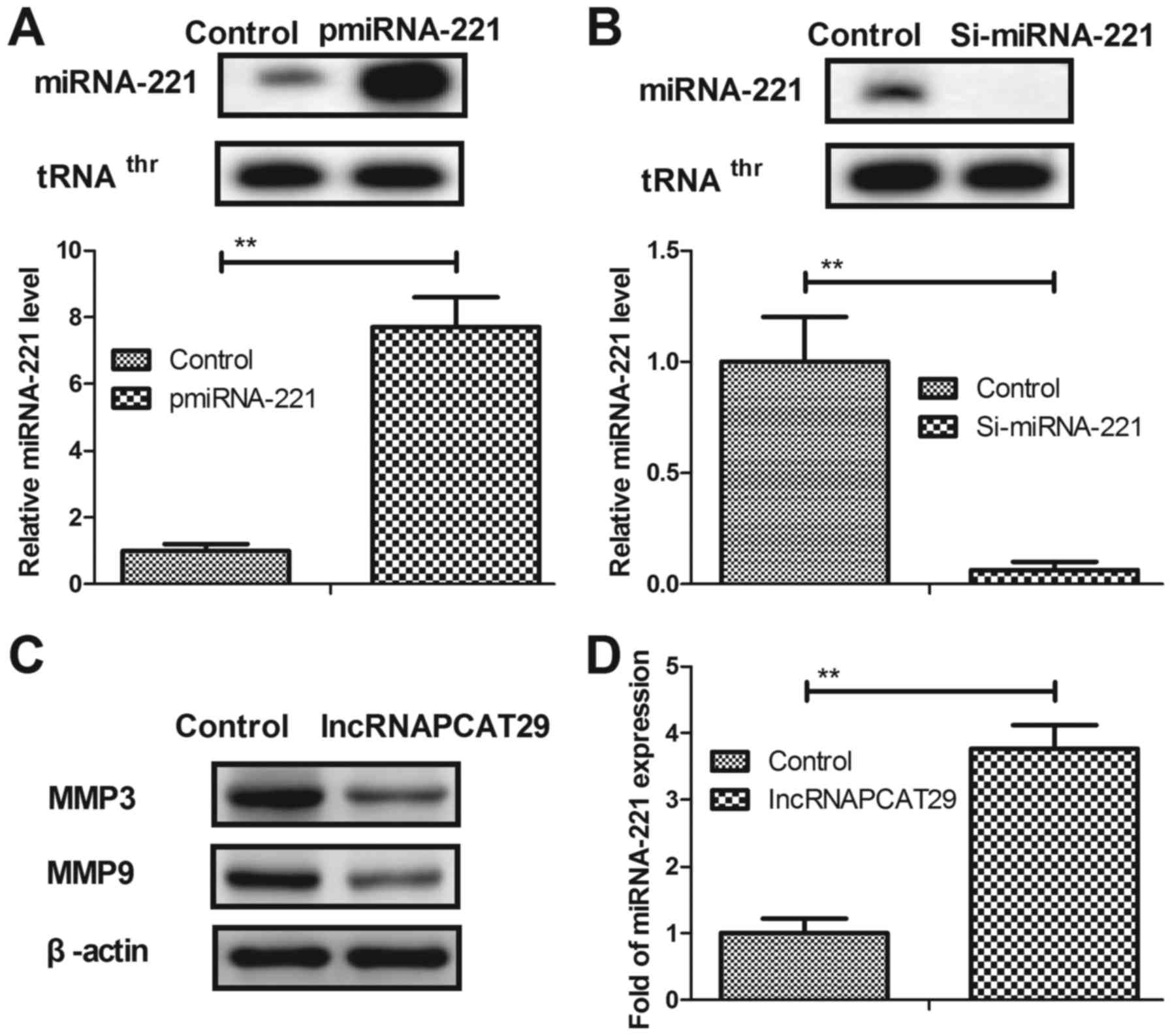 | Figure 2.lncRNAPCAT29 inhibits fibroblast
differentiation by targeting TGF-β1 within pulmonary fibroblasts.
(A) miRNA-221 overexpression increased miRNA-221 expression levels
within pulmonary fibroblasts determined by RT-qPCR. (B) miRNA-221
silencing decreased miRNA-221 expression levels within pulmonary
fibroblasts determined by RT-qPCR. (C) lncRNAPCAT29 transfection
suppressed pulmonary fibroblast differentiation, as determined by
decreasing levels of MMP3 and MMP9. (D) miRNA-221 expression levels
were significantly upregulated by lncRNAPCAT29 transfection. (E)
miRNA-221 overexpression suppressed TGF-β1 expression levels within
pulmonary fibroblast cells. (F) lncRNAPCAT29 inhibited TGF-β1
expression levels and miRNA-221 downregulation inhibited this
suppression within pulmonary fibroblasts. Results were expressed as
the mean ± standard deviation of three independent experiments.
**P<0.01, vs. the control group (scramble miRNA). lncRNAPCAT29,
long non-coding RNA prostate cancer-associated transcript 29;
miRNA-221, microRNA-221; MMP, matrix metalloproteinase; pmiRNA-221,
miRNA-221 overexpression; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Si-miRNA-221,
miRNA-221 silencing; Si221-29, miRNA-221 silencing and lncRNAPCAT29
overexpression; TGF-β1, transforming growth factor-β1. |
lncRNAPCAT29 inhibits the expression
of inflammatory cytokines by targeting the TGF-β1-mediated
RASAL1/ERK1/2 signal pathway
As shown in Fig.
3A, TGF-β1 overexpression markedly upregulated TGF-β1 mRNA
expression compared with in the control (pedu12.4-transduced group)
pulmonary fibroblast cells. In addition, it was observed in the
present study that inflammatory cytokines, including TNF-α and
IL-1β were downregulated within pulmonary fibroblasts following
lncRNAPCAT29 transfection; however, this downregulation was
abrogated by TGF-β1 overexpression (pTGF-β1) (Fig. 3B). TGF-β1 knockdown (Si-TGF-β1)
also inhibited the expression of TNF-α and IL-1β within pulmonary
fibroblast cells (Fig. 3C).
Expression levels of RASAL1, ERK1/2 and p-ERK1/2 were downregulated
in lncRNAPCAT29-transfected pulmonary fibroblasts, whereas pTGF-β1
eradicated lncRNAPCAT29-inhibited (lncRNA-pTGF-β1) RASAL1 and
ERK1/2 expression, as well as ERK1/2 phosphorylation (Fig. 3D). Si-TGF-β1 also decreased RASAL1,
ERK1/2 expression and ERK1/2 phosphorylation (Fig. 3E). The results also demonstrated
that pTGF-β1 inhibited lncRNA-pTGF-β1-associated downregulation of
MMP3 and MMP9 expression, whereas Si-TGF-β1 inhibited MMP3 and MMP9
expression (Fig. 3F and G). In
addition, the expression levels of CLAI and FN were significantly
decreased due to lncRNAPCAT29 transfection; however, this
inhibition was abrogated by pTGF-β1. Conversely, Si-TGF-β1
suppressed CLAI and FN expression (Fig. 3H and I). Collectively, these
results suggested that lncRNAPCAT29 may inhibit inflammatory
cytokines expression by targeting the RASAL1/ERK1/2 signal pathway
in pulmonary fibroblasts.
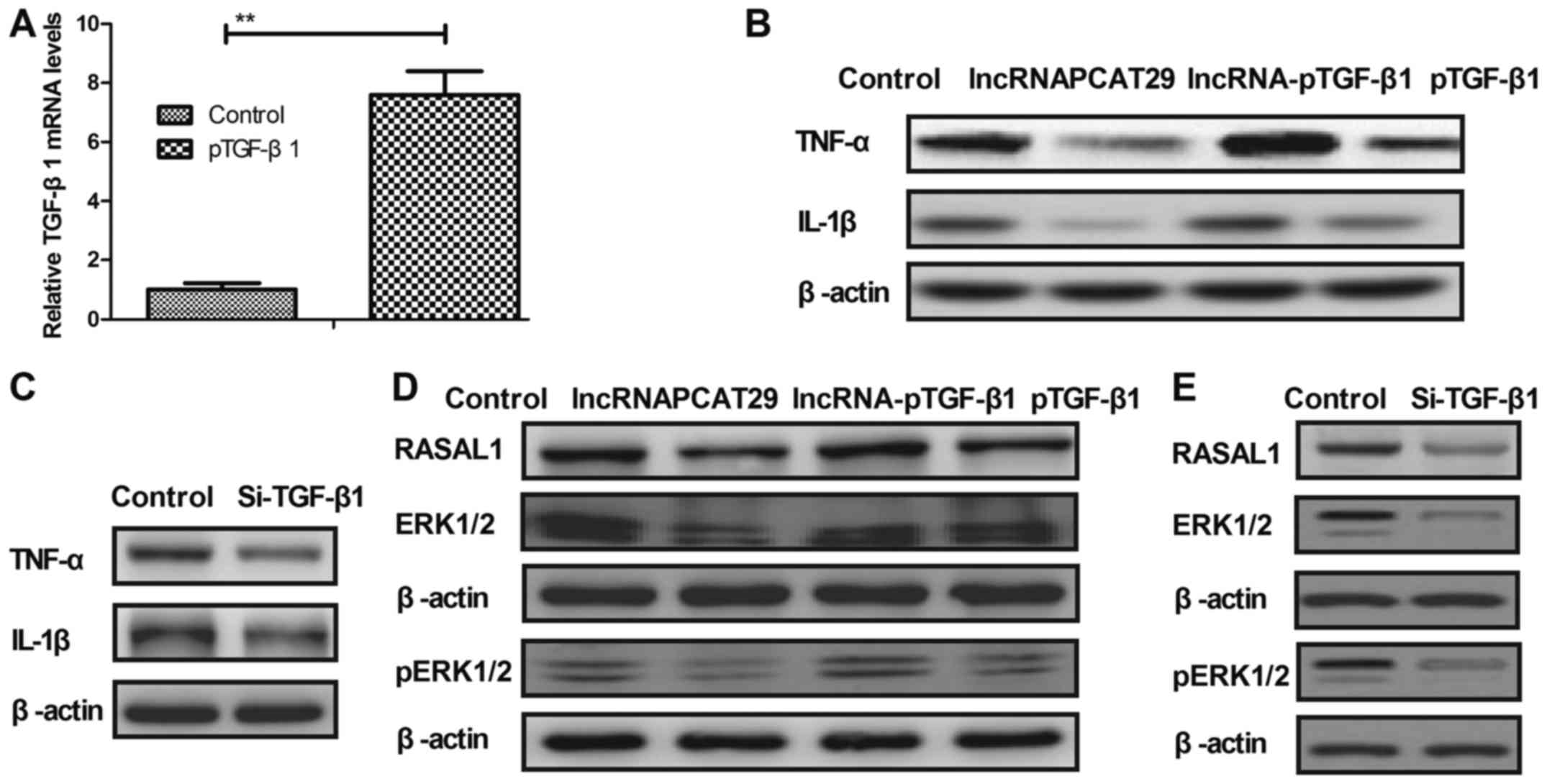 | Figure 3.lncRNAPCAT29 inhibits inflammatory
cytokine expression by targeting the TGF-β1-mediated RASAL1/ERK1/2
signaling pathway within pulmonary fibroblasts. (A) TGF-β1
transduction upregulated TGF-β1 mRNA expression compared with in
the control pulmonary fibroblast cells. Control, pVector. (B)
lncRNAPCAT29 inhibited expression of the inflammatory cytokines,
TNF-α and IL-1β; TGF-β1 overexpression abrogated
lncRNAPCAT29-mediated downregulation of TNF-α and IL-1β in
pulmonary fibroblasts. Control, pVector. (C) TGF-β1 downregulation
inhibited the expression of the inflammatory cytokines TNF-α and
IL-1β within pulmonary fibroblasts. Control, Si-Vector. (D) Effects
of TGF-β1 overexpression on lncRNAPCAT29 transfection-inhibited
RASAL1, ERK1/2 expression and ERK1/2 phosphorylation. Control,
pVector. (E) TGF-β1 downregulation decreased RASAL1 and ERK1/2
expression, and ERK1/2 phosphorylation within pulmonary
fibroblasts. Control, Si-Vector. (F) TGF-β1 overexpression blocked
lncRNAPCAT29-induced downregulation of MMP3 and MMP9 expression
within pulmonary fibroblasts. Control, pVector. (G) TGF-β1
downregulation inhibited MMP3 and MMP9 expression within pulmonary
fibroblasts. Control, Si-Vector (H) TGF-β1 overexpression abolished
lncRNAPCAT29-induced downregulation of CLAI and FN expression
within pulmonary fibroblasts. Control, pVector. (I) TGF-β1
downregulation inhibited CLAI and FN expression within pulmonary
fibroblasts. Control, Si-Vector **P<0.01 vs. the control group.
The results are expressed as the mean ± standard deviation of three
independent experiments. CLAI, extracellular matrix collagen I; ERK
1/2, extracellular signal-regulated kinases 1/2; FN, fibronectin;
IL-1β, interleukin-1β; lncRNAPCAT29, long non-coding RNA prostate
cancer-associated transcript 29; pTGF-β1, transforming growth
factor-β1 overexpression; lncRNA-pTGF-β1, lncRNAPCAT29
overexpression and TGF-β1 overexpression; MMP, matrix
metalloproteinase; RASAL 1, RAS protein activator like 1;
Si-TGF-β1, TGF-β1 downregulation; TNF-α, tumor necrosis
factor-α. |
lncRNAPCAT29 inhibits the expression
of N4bp2 and Plxna4, which are regulated by TGF-β1 within pulmonary
fibroblasts
The expression levels of N4bp2 and Plxna4 are
associated with pulmonary fibrosis; therefore, the effects of
lncRNAPCAT29 on N4bp2 and Plxna4 expression in pulmonary
fibroblasts were investigated. miR-221 knockdown or TGF-β1
overexpression significantly increased N4bp2 and Plxna4 expression
in pulmonary fibroblasts (Fig. 4A and
B). As presented in Fig. 4C and
D, lncRNAPCAT29 transfection inhibited N4bp2 and Plxna4
expression, which was eliminated by miR-221 overexpression
(lncRNA-miR-221) or pTGF-β1 expression. Collectively, these results
suggested that lncRNAPCAT29 may inhibit the expression levels of
N4bp2 and Plxna4, which are regulated by the miRNA-221-inhibited
TGF-β1 pathway within pulmonary fibroblasts.
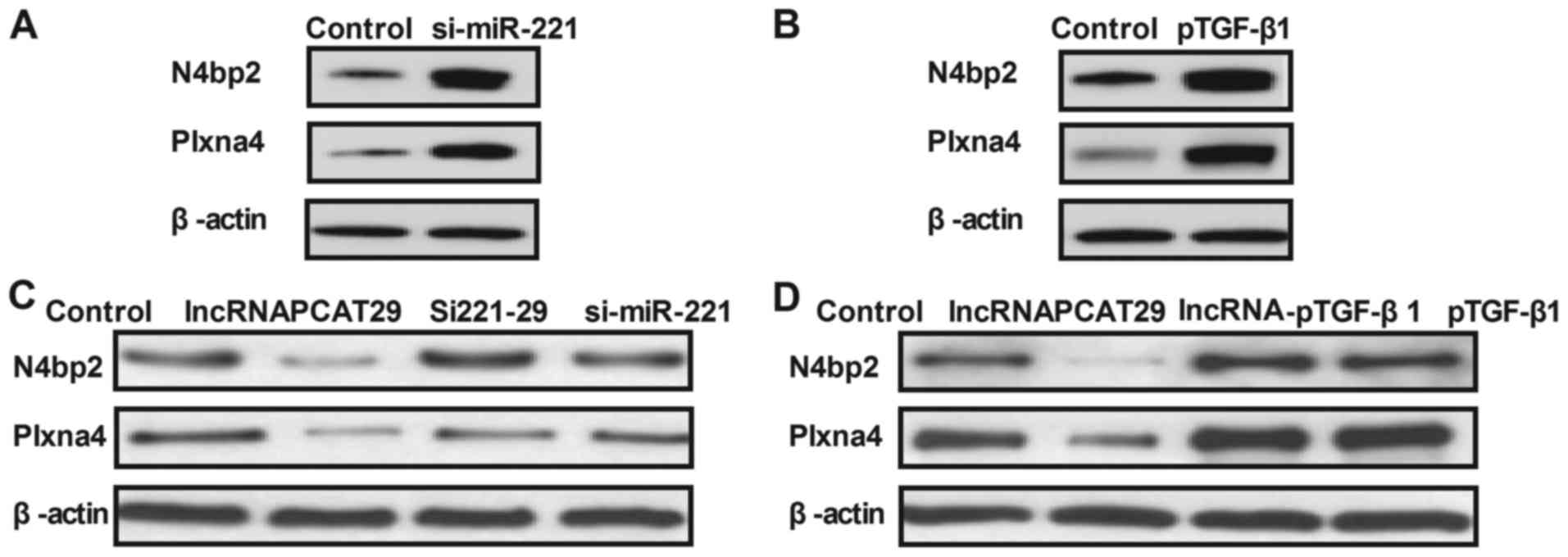 | Figure 4.lncRNAPCAT29 inhibits TGF-β1-regulated
N4bp2 and Plxna4 expression levels in pulmonary fibroblasts. (A)
miR-221 knockdown increased N4bp2 and Plxna4 expression levels
within pulmonary fibroblasts. Control, scramble miRNA. (B) TGF-β1
overexpression increased N4bp2 and Plxna4 expression levels within
pulmonary fibroblasts. Control, pVector. (C) miR-221 knockdown
abolished lncRNAPCAT29-decreased N4bp2 and Plxna4 expression levels
within pulmonary fibroblasts. Control, scramble miRNA. (D) TGF-β1
overexpression abolished lncRNAPCAT29-decreased N4bp2 and Plxna4
expression levels within pulmonary fibroblasts. Control, pVector.
Results were expressed as the mean ± standard deviation of three
independent experiments. lncRNAPCAT29, long non-coding RNA prostate
cancer-associated transcript 29; Si221-29, miRNA-221 silencing and
lncRNAPCAT29 overexpression; lncRNA-pTGF-β1, lncRNAPCAT29
overexpression and TGF-β1 overexpression; si-miR-221, miRNA-221
silencing; N4bp2, NEDD4 binding protein 2; Plxna4, Plexin-A4;
pTGF-β1, TGF-β1 overexpression; TGF-β1, transforming growth
factor-β1. |
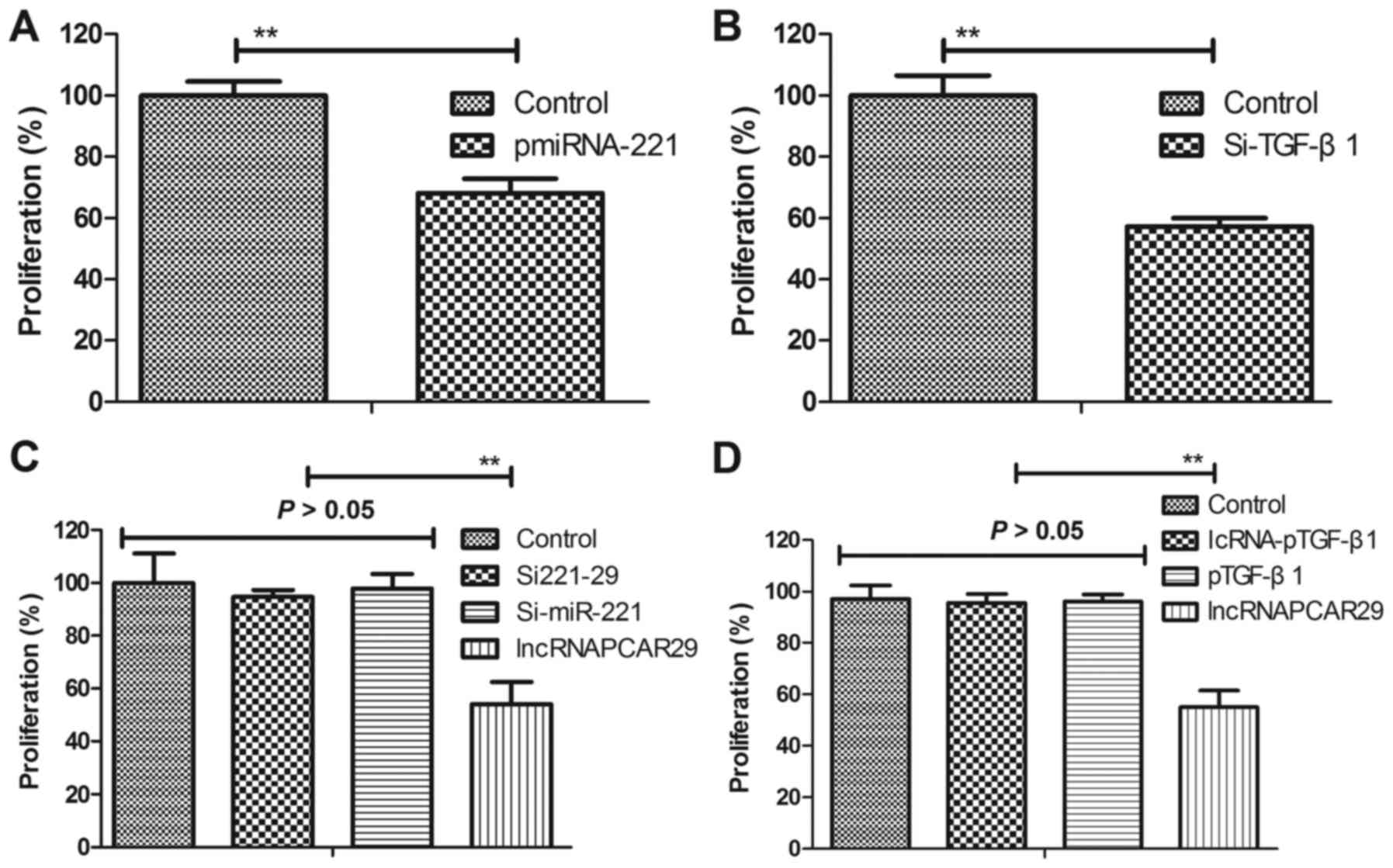
lncRNAPCAT29 regulates the growth of
pulmonary fibroblasts via the miRNA-221-mediated TGF-β1 signaling
pathway
In the present study, the effects of miRNA-221 and
TGF-β1 on pulmonary fibroblast growth were analyzed. miRNA-221
upregulation (pmiRNA-221) or TGF-β1 knockdown (Si-TGF-β1) inhibited
pulmonary fibroblast proliferation (Fig. 5A and B). In addition, the results
demonstrated that miRNA-221 downregulation or TGF-β1 overexpression
abolished lncRNAPCAT29-suppressed proliferation of pulmonary
fibroblasts (Fig. 5C and D).
Collectively, these results suggested that lncRNAPCAT29 regulated
growth of pulmonary fibroblasts via the miRNA-221-mediated TGF-β1
signal pathway.
Discussion
Pulmonary fibrosis is an intractable lung disease
characterized by the accumulation of collagen, injury to the
overlying epithelium and fibroblast differentiation (18). Increasing evidence has suggested
that lncRNAs are associated with human fibrotic diseases via
regulation of cellular signal pathways (9,19).
In addition, a recent study has reported a novel epigenetic cascade
of renal fibrogenesis via TGF-β1-induced epigenetic aberrations of
miRNAs and DNA methyltransferase (20). In addition, miRNA-221 promoted
fibrosis in cystic fibrosis airway epithelial cells (21). In the present study, the
associations between lncRNA, miRNA and pulmonary fibrosis were
investigated. The results demonstrated that lncRNAPCAT29 expression
was reduced within pulmonary fibroblasts isolated from
silica-induced mouse models. IncRNAPCAT29 transfection inhibited
pulmonary fibroblast differentiation by targeting the
TGF-β1-mediated RASAL1/ERK1/2 signal pathway, which is regulated by
miR-221.
PCAT29 is a potential target for prostate cancer
therapy and is regarded as the first androgen receptor-repressed
lncRNA (22). In the present
study, it was demonstrated that lncRNAPCAT29 is downregulated
within pulmonary fibroblasts of silica-induced pulmonary fibrotic
mice, which may regulate the proliferation and differentiation of
pulmonary fibroblasts. A recent study indicated that miRNA-221
expression levels may be elevated within cystic fibrosis airway
epithelial cells, which may affect the expression of
transcriptional regulators via regulating the expression of
activating transcription factor 6 (21). Therefore, the potential target of
lncRNAPCAT29 within pulmonary fibroblasts was investigated. Results
of the present study indicated that lncRNAPCAT29 transfection
increased expression levels of miRNA-221 and decreased TGF-β1
expression within pulmonary fibroblasts.
TGF-β1 overexpression is associated with the
progression of pulmonary fibrosis (23). TGF-β1 has been regarded as a
therapeutic target for pulmonary fibrosis due to TGF-β1-associated
genes or signals that restore extracellular matrix homeostasis
(24). In the present study, it
was reported that miRNA-221 transduction decreased TGF-β1
expression within pulmonary fibroblasts, which was associated with
the suppression of pulmonary fibrosis (25). However, TGF-β1 overexpression
eliminated the effects of lncRNAPCAT29-inhibition on
differentiation of pulmonary cytokines and associated inflammation.
Therefore, lncRNAPCAT29 may serve a role in regulating the growth
of pulmonary fibroblasts via the miRNA-221-inhibited TGF-β1 signal
pathway.
RASAL1 is a key protein associated with renal
fibrosis and hepatic stellate cell proliferation (26,27).
Research has revealed that Paridis Rhizoma saponins attenuate liver
fibrosis in rats by downregulating expression of the RASAL1/ERK1/2
signaling pathway (28). As
reported in the present study, lncRNAPCAT29 inhibited the
RASAL1/ERK1/2 signaling pathway within pulmonary fibroblasts;
therefore, lncRNAPCAT29 may have contributed to the suppression of
pulmonary fibrosis development. Studies have indicated that ERK
inhibitors decrease the expression levels of MMP2 and MMP9 in
alveolar epithelial cells, which may be a potential target for the
treatment of lung fibrosis (29,30).
Additionally, upregulated expression and activity of MMP9 has been
reported in bleomycin-induced pulmonary fibrosis (31). In the present study, lncRNAPCAT29
transfection was associated with decreased levels of MMP3 and MMP9
expression, which suppressed pulmonary fibroblast cell
differentiation. Analysis into the potential mechanism underlying
the effects of lncRNAPCAT29 indicated that RASAL1 and ERK1/2
expression levels were reduced, mediated by the miRNA-221-inhibited
TGF-β1 signaling pathway. lncRNAPCAT29 was also demonstrated to
inhibit the expression of N4bp2 and Plxna4, which was regulated by
the miRNA-221-inhibited TGF-β1 pathway within pulmonary
fibroblasts. However, further investigation into the numerous
molecules within the RASAL1/ERK1/2 pathway is required.
In conclusion, the results of the present study
demonstrated the potential role of lncRNAPCAT29 in the progression
of pulmonary fibrosis as well as the potential underlying
mechanism. Findings revealed that lncRNAPCAT29 overexpression is
associated with improvements in pulmonary fibrosis; lncRNAPCAT29
exerted key functions in silica-induced pulmonary fibrosis via the
miR-221-TGF-β1-regulated RASAL1/ERK1/2 signal pathway (Fig. 6). The present study has provided
novel insights into understanding the complex molecular mechanisms
of certain miRNAs and the lncRNA-mediated RASAL1/ERK1/2 signaling
pathway in silica-induced pulmonary fibrosis. These findings may
contribute to the development of lncRNA-associated therapy for the
treatment of pulmonary fibrosis. However, the in vivo
efficacy of lncRNAPCAT29 treatment has yet to be investigated.
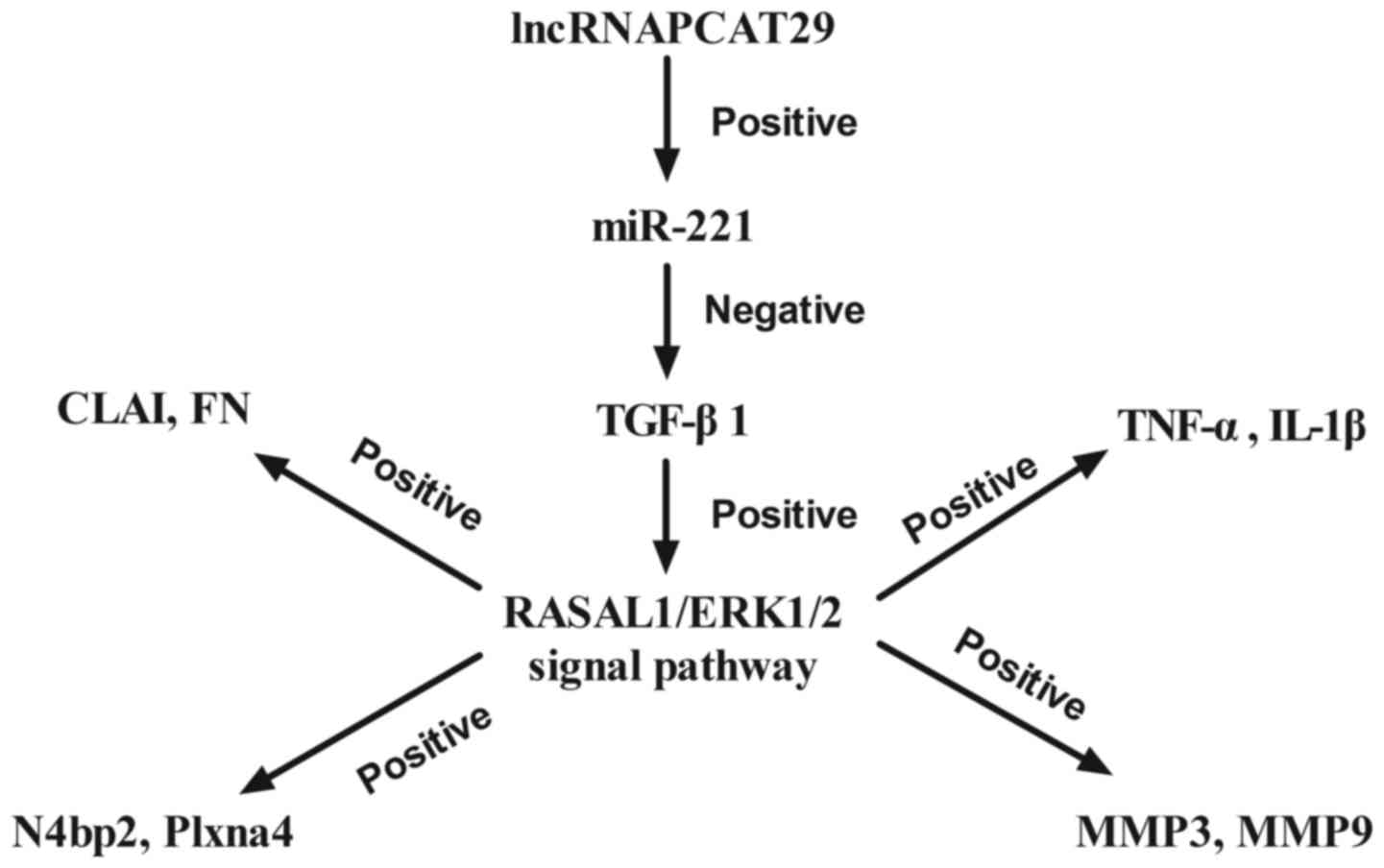 | Figure 6.Schematic diagram of the signaling
pathways mediated by lncRNAPCAT29. CLAI, extracellular matrix
collagen I; ERK 1/2, extracellular signal-regulated kinases 1/2;
FN, fibronectin; IL-1β, interleukin-1β; lncRNAPCAT29, long
non-coding RNA prostate cancer-associated transcript 29; N4bp2,
NEDD4 binding protein 2; MMP, matrix metalloproteinase; miR-221,
microRNA-221; RASAL 1, RAS protein activator like 1; TGF-β1,
transforming growth factor-β1; TNF-α, tumor necrosis factor-α. |
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL performed the experiments in this study. SG
designed the experiments. HX analyzed the data generated in this
study.
Ethics approval and consent to
participate
Experimental protocols were approved by the Ethics
Committee of the Affiliated Hospital of Shandong University of
Traditional Chinese Medicine (Jinan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vaidya S, Hibbert CL, Kinter E and Boes S:
Identification of key cost generating events for idiopathic
pulmonary fibrosis: A systematic review. Lung. 195:1–8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Renzoni E, Srihari V and Sestini P:
Pathogenesis of idiopathic pulmonary fibrosis: Review of recent
findings. F1000prime Rep. 6:692014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rozanski C and Mura M: Multi-dimensional
indices to stage idiopathic pulmonary fibrosis: A systematic
review. Sarcoidosis Vasc Diffuse Lung Dis. 31:8–18. 2014.PubMed/NCBI
|
|
4
|
Upala S and Sanguankeo A: Severity of
breathing disorder during sleep is not correlated with idiopathic
pulmonary fibrosis: A systematic review and meta-analysis. QJM.
109:1412016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koerner-Rettberg C and Ballmann M:
Colistimethate sodium for the treatment of chronic pulmonary
infection in cystic fibrosis: An evidence-based review of its place
in therapy. Core Evid. 9:99–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Figueroa T, Boumart I, Coupeau D and
Rasschaert D: Hyperediting by ADAR1 of a new herpesvirus lncRNA
during the lytic phase of the oncogenic Marek's disease virus. J
Gen Virol. 97:2973–2988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huarte M: RNA. A lncRNA links genomic
variation with celiac disease. Science. 352:43–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cabianca DS, Casa V and Gabellini D: A
novel molecular mechanism in human genetic disease: A DNA
repeat-derived lncRNA. RNA Biol. 9:1211–1217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song X, Cao G, Jing L, Lin S, Wang X,
Zhang J, Wang M, Liu W and Lv C: Analysing the relationship between
lncRNA and protein-coding gene and the role of lncRNA as ceRNA in
pulmonary fibrosis. J Cell Mol Med. 18:991–1003. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Q, Han L, Yan W, Ji X, Han R, Yang J,
Yuan J and Ni C: miR-489 inhibits silica-induced pulmonary fibrosis
by targeting MyD88 and Smad3 and is negatively regulated by lncRNA
CHRF. Sci Rep. 6:309212016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakurai K, Reon BJ, Anaya J and Dutta A:
The lncRNA DRAIC/PCAT29 locus constitutes a tumor-suppressive
nexus. Mol Cancer Res. 13:828–838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han R, Ji X, Rong R, Li Y, Yao W, Yuan J,
Wu Q, Yang J, Yan W, Han L, et al: MiR-449a regulates autophagy to
inhibit silica-induced pulmonary fibrosis through targeting Bcl2. J
Mol Med (Berl). 94:1267–1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Renshaw A and Elsheikh TM: A validation
study of the Focalpoint GS imaging system for gynecologic cytology
screening. Cancer Cytopathol. 121:737–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL,
Zhu XQ, Liu DY, Chen J, Xue Q, Zhou HJ, et al: Lentiviral-mediated
miRNA against osteopontin suppresses tumor growth and metastasis of
human hepatocellular carcinoma. Hepatology. 48:1834–1842. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aguileta MA, Rojas-Rivera D, Goossens V,
Estornes Y, Van Isterdael G, Vandenabeele P and Bertrand MJ: A
siRNA screen reveals the prosurvival effect of protein kinase A
activation in conditions of unresolved endoplasmic reticulum
stress. Cell Death Differ. 23:1670–1680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao S, Wang J and Xiao N: MicroRNAs as
noninvasive biomarkers in bladder cancer detection: A diagnostic
meta-analysis based on qRT-PCR data. Int J Biol Markers.
31:e276–e285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kotecha J, Atkins C and Wilson A: Patient
confidence and quality of life in idiopathic pulmonary fibrosis and
sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 33:341–348.
2016.PubMed/NCBI
|
|
19
|
Fu N, Niu X, Wang Y, Du H, Wang B, Du J,
Li Y, Wang R, Zhang Y, Zhao S, et al: Role of LncRNA-activated by
transforming growth factor beta in the progression of hepatitis C
virus-related liver fibrosis. Discov Med. 22:29–42. 2016.PubMed/NCBI
|
|
20
|
Yin S, Zhang Q, Yang J, Lin W, Li Y, Chen
F and Cao W: TGFbeta-incurred epigenetic aberrations of miRNA and
DNA methyltransferase suppress Klotho and potentiate renal
fibrosis. Biochim Biophys Acta. 2017. View Article : Google Scholar
|
|
21
|
Oglesby IK, Agrawal R, Mall MA, McElvaney
NG and Greene CM: miRNA-221 is elevated in cystic fibrosis airway
epithelial cells and regulates expression of ATF6. Mol Cell
Pediatr. 2:12015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malik R, Patel L, Prensner JR, Shi Y, Iyer
MK, Subramaniyan S, Carley A, Niknafs YS, Sahu A, Han S, et al: The
lncRNA PCAT29 inhibits oncogenic phenotypes in prostate cancer. Mol
Cancer Res. 12:1081–1087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee CM, Park JW, Cho WK, Zhou Y, Han B,
Yoon PO, Chae J, Elias JA and Lee CG: Modifiers of TGF-β1 effector
function as novel therapeutic targets of pulmonary fibrosis. Korean
J Int Med. 29:281–290. 2014. View Article : Google Scholar
|
|
24
|
Kang HR, Lee JY and Lee CG: TGF-beta1 as a
therapeutic target for pulmonary fibrosis and COPD. Exp Rev Clin
Pharmacol. 1:547–558. 2008. View Article : Google Scholar
|
|
25
|
Xu YD, Hua J, Mui A, O'Connor R,
Grotendorst G and Khalil N: Release of biologically active
TGF-beta1 by alveolar epithelial cells results in pulmonary
fibrosis. Am J Physiol Lung Cell Mol Physiol. 285:L527–L539. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao Y: Hypermethylation of RASAL1: A key
for renal fibrosis. EBio Med. 2:7–8. 2015.
|
|
27
|
Tao H, Huang C, Yang JJ, Ma TT, Bian EB,
Zhang L, Lv XW, Jin Y and Li J: MeCP2 controls the expression of
RASAL1 in the hepatic fibrosis in rats. Toxicology. 290:327–333.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong Y, Han YQ, Wang YZ, Gao JR, Li YX,
Liu Q and Xia LZ: Paridis Rhizoma Sapoinins attenuates liver
fibrosis in rats by regulating the expression of RASAL1/ERK1/2
signal pathway. J Ethnopharmacol. 192:114–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Xu X, Geng J, Huang X, Jiang D, Zhu
M and Dai H: Role and underlying mechanism of IGF-I/ERK signaling
pathway in lung fibrosis. Zhonghua Yi Xue Za Zhi. 95:1615–1618.
2015.(In Chinese). PubMed/NCBI
|
|
30
|
Leask A: MEK/ERK inhibitors:
Proof-of-concept studies in lung fibrosis. J Cell Commun Signal.
6:59–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zuo WL, Zhao JM, Huang JX, Zhou W, Lei ZH,
Huang YM, Huang YF and Li HG: Effect of bosentan is correlated with
MMP-9/TIMP-1 ratio in bleomycin-induced pulmonary fibrosis. Biomed
Rep. 6:201–205. 2017. View Article : Google Scholar : PubMed/NCBI
|